ABSTRACT
Roots grow asymmetrically, sometimes helically, around their growth direction likely to facilitate environmental sensing. We recently demonstrated that nitrate deficiency induces root coiling on horizontal surface through nitrate transporter/sensor NRT1.1 and PIN2- and AUX-mediated polar auxin transport. Here, we show that nitrate deficiency or NRT1.1 loss-of-function induces differential distribution of PIN2 between the future concave and concave sides in root epidermal cells. Treatment with pharmacological drugs suggests that enhanced endocytosis at the future convex side leads to reduced plasma membrane (PM) association of PIN2. A reduction of PIN2 at the PM would maintain a low auxin response to further enhance endocytosis at the convex side, leading to root coiling.
KEYWORDS: Nitrate, asymmetric root growth, endocytosis, auxin, BFA
Plant roots grow asymmetrically, sometimes helically, around their growth direction likely to facilitate environmental sensing and to increase plant fitness. Asymmetric root growth (ARG) is best studied in root responses to gravity and mechanical stresses. Gravitropism enables deep rooting for more efficient nutrient absorption while thigmotropism allows roots to be away from obstacles and therefore to avoid harsh growth conditions. However, asymmetry of root growth was also observed at the absence of gravity or touch,1 a behavior named circumnutation, i.e. the helical movement that all plant organs produce around the growth direction.2–4
ARG is developmentally regulated.5,6 ARG in general involves microtubule (MT) reorganization. Mutations at MT plus end-binding proteins EB17 and SKU6/SPIRAL1,8 or variants of α-tubulin,9 cause ARG. By contrast, several genetic factors, including two seven-transmembrane proteins, the MILDEW RESISTANCE LOCUS O (MLO) family members,10,11 and the heterotrimeric G proteins XLG3 and AGB1,12 are involved in ARG in a gravity- or touch-independent way. ARG requires polar auxin transport,1,5,10–13 most importantly through the activities of auxin carriers, such as PIN-FORMED2 (PIN2) and AUXIN RESISTANT 1 (AUX1).14–20
We recently showed that nitrate deficiency resulted in a strong ARG in Arabidopsis. Nitrate deficiency caused root coiling on horizontal plates, which is inhibited by functional loss of PIN2 and AUX1. In addition, suppression of ARG by nitrate is mediated by the nitrate transporter/sensor NRT1.1.21
Because either nitrate deficiency or NRT1.1 loss-of-function resulted in an asymmetric distribution of PIN2 at the PM, i.e. lower signals at the convex than at the concave,21 we wondered whether it was a result of asymmetrically enhanced endocytosis of PIN2, as reported previously during gravitropic responses.14,22,23 To test this hypothesis, we analyzed PIN2:GFP plants by using confocal laser scanning fluorescence microscopy (CLSM) and pharmacological treatments. BRASSINOSTEROID INSENSITIVE1:YFP (BRI1:YFP) plants was also included as a control since BRI1 is constitutively internalized without showing asymmetry at the PM.24 Brefeldin A (BFA) is a fungal toxin that inhibits exocytic trafficking and causes aggregation of endosomes.25 Cycloheximide (CHX), an inhibitor of de novo protein synthesis, was applied (50 µM) during BFA treatment (50 µM) to exclude the influence of the newly synthesized proteins. After being transferred to horizontally placed medium, PIN2:GFP showed a significantly reduced intensity at the PM of the future convex on 0 N medium (Supplemental Methods), compared with that on 7 N medium (Figure 1a, c). By contrast, there was no significant difference of BRI1:YFP intensity at the PM in all samples (Figure 1b, e). Treatment of BFA resulted in the accumulation of PIN2:GFP into so-called “BFA compartments” (Figure 1a), i.e. cytoplasmic aggregates containing both trans-Golgi network/early endosomes and the Golgi.25 The intensity ratio of PIN2:GFP between BFA compartment-trapped and PM-associated was significantly increased at the future convex side compared with that at the future concave on 0 N medium but not 7 N medium (Figure 1d), suggesting asymmetric endocytosis upon nitrate deficiency. The differential effect of nitrate deficiency on endocytosis is not specific on PIN2 because the intensity ratio of BRI1:YFP between BFA compartment-trapped and PM-associated was also significantly increased at the future convex side compared with that at the future concave on 0 N medium but not 7 N medium (figure 1f).
Figure 1.
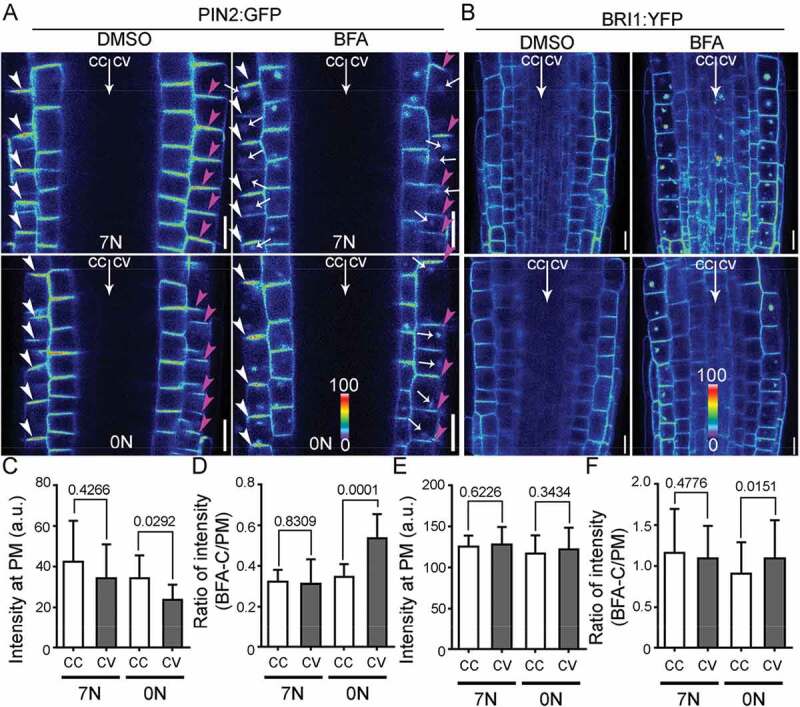
Nitrate deficiency induces differential dynamic distribution of PIN2 in horizontally placed roots.
(A‐B) CLSM of a PIN2:GFP (A) or BRI1:YFP (B) root at elongation zone. Roots of 4 DAG seedlings were transferred to 7 N or 0 N medium for 2 days and then treated with 50µM CHX for 30 min, and then stained with FM4‐64 before imaging (DMSO), or roots of 4 DAG seedlings were transferred to 7 N or 0 N medium for 2 days and then treated with 50 µM CHX for 30 min, pulse‐labeled with FM4‐64, and then incubated with 50µM CHX/50 µM BFA for 45 min before imaging (BFA). The GFP channel images are displayed in pseudocolors, covering the full range of measured values within each dataset. cc, concave; cv, convex. Arrowheads point at the PM of root epidermal cells; arrows at BFA compartments. (C‐F) Quantification of fluorescence intensity at the PM (C, E) or intensity ratio between BFA compartments and the PM (D, F) of PIN2:GFP (C,D) or BRI1:YFP (E, F). a.u., arbitrary fluorescence unit. Results are means ± standard deviation (SD). In total, 30‐40 cells from four independent experiments involving 15 roots in each genetic background or treatment were analyzed. P values between concave (cc) and convex (cv) are shown in the bar graphs (Unpaired t‐test). Bars = 10 μm.
Functional loss of NRT1.1 caused the same differential endocytosis on PIN2 as that caused by nitrate deficiency (Figure 2). Examination of PIN2 distribution at the PM in PIN2:GFP;chl1-5 plants on 7 N medium showed that PM-association of PIN2 was reduced at the future concave side (Figure 2a, c). Upon BFA treatment, significantly higher ratio between BFA compartment-associated and PM-associated PIN2 signals was detected at the future concave side (Figure 2b, d), suggesting differential endocytosis in root epidermal cells by NRT1.1 loss-of-function.
Figure 2.
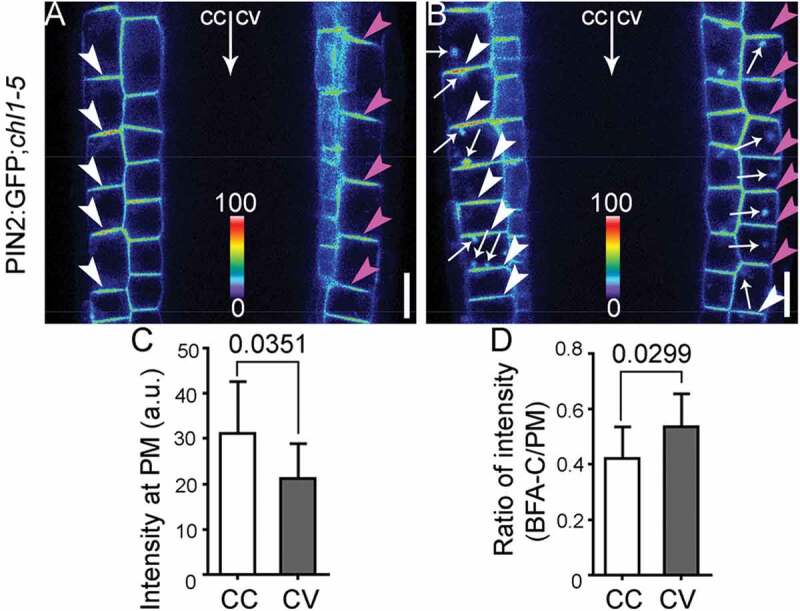
Chl1‐5 shows differential dynamic distribution of PIN2 in horizontally placed roots.
(A‐B) CLSM or a PIN2:GFP;chl1‐5 root upon DMSO (A) or BFA (B) treatment. The GFP channel images are displayed in pseudocolors, covering the full range of measured values within each dataset. cc, concave; cv, convex. Arrowheads point at the PM of root epidermal cells; arrows at BFA compartments. (C‐D) Quantification of fluorescence intensity at the PM (C) or intensity ratio between BFA compartments and the PM (D) of PIN2:GFP;chl1‐5. a.u., arbitrary fluorescence unit. Results are means ± SD. In total, 30‐40 cells from four independent experiments involving 15 roots in each genetic background or treatment were analyzed. P values between concave (cc) and convex (cv) are shown in the bar graphs (Unpaired t‐test). Bars = 10 μm.
We show that nitrate deficiency or NRT1.1 loss-of-function resulted in enhanced internalization of PIN2, indicative of enhanced endocytosis at the future convex side. The asymmetry of PIN2 endocytosis is likely resulted from a general reduction of endocytosis at the future convex side26,27 because the dynamic localization of BRI1:YFP were also affected by nitrate deficiency. Auxin inhibits endocytosis,23 and thus the future concave side with higher auxin responses21 would have reduced endocytosis, leading to PIN2 asymmetry. On the other hand, the asymmetric distribution of PIN2 would further stabilize the established auxin asymmetry and thus maintain differential endocytosis between the two sides, leading to root coiling.
Supplementary Material
Acknowledgments
We thank Prof. Yong Wang for chl1-5. This work was supported by Natural Science Foundation of China (31771558 and 31970332 to S.L.).
Funding Statement
This work was supported by the National Natural Science Foundation of China [31970332]; National Natural Science Foundation of China [31771558].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
The supplemental data for this article can be accessed here.
References
- 1.Piconese S, Tronelli G, Pippia P, Migliaccio F.. Chiral and non-chiral nutations in Arabidopsis roots grown on the random positioning machine. J Exp Bot. 2003;54:1–4. doi: 10.1093/jxb/erg206. [DOI] [PubMed] [Google Scholar]
- 2.Migliaccio F, Fortunati A, Tassone P.. Arabidopsis root growth movements and their symmetry: progress and problems arising from recent work. Plant Signal Behav. 2009;4:183–190. doi: 10.4161/psb.4.3.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliaccio F, Piconese S.. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6:561–565. doi: 10.1016/S1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- 4.Migliaccio F, Tassone P, Fortunati A. Circumnutation as an autonomous root movement in plants. Am J Bot. 2013;100:4–13. doi: 10.3732/ajb.1200314. [DOI] [PubMed] [Google Scholar]
- 5.Oliva M, Dunand C. Waving and skewing: how gravity and the surface of growth media affect root development in Arabidopsis. New Phytol. 2007;176:37–43. doi: 10.1111/j.1469-8137.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- 6.Roy R, Bassham DC. Root growth movements: waving and skewing. Plant Sci. 2014;221-222:42–47. doi: 10.1016/j.plantsci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Bisgrove SR, Lee YR, Liu B, Peters NT, Kropf DL. The microtubule plus-end binding protein EB1 functions in root responses to touch and gravity signals in Arabidopsis. Plant Cell. 2008;20:396–410. doi: 10.1105/tpc.107.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sedbrook JC, Ehrhardt DW, Fisher SE, Scheible WR, Somerville CR. The Arabidopsis SKU6/SPIRAL1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell. 2004;16:1506–1520. doi: 10.1105/tpc.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida T, Kaneko Y, Iwano M, Hashimoto T. Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2007;104:8544–8549. doi: 10.1073/pnas.0701224104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Noir S, Kwaaitaal M, Hartmann HA, Wu MJ, Mudgil Y, Sukumar P, Muday G, Panstruga R, Jones AM, et al. Two seven-transmembrane domain MILDEW RESISTANCE LOCUS O proteins cofunction in Arabidopsis root thigmomorphogenesis. Plant Cell. 2009;21:1972–1991. doi: 10.1105/tpc.108.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bidzinski P, Noir S, Shahi S, Reinstadler A, Gratkowska DM, Panstruga R. Physiological characterization and genetic modifiers of aberrant root thigmomorphogenesis in mutants of Arabidopsis thaliana MILDEW LOCUS O genes. Plant Cell Environ. 2014;37:2738–2753. doi: 10.1111/pce.12353. [DOI] [PubMed] [Google Scholar]
- 12.Pandey S, Monshausen GB, Ding L, Assmann SM. Regulation of root-wave response by extra large and conventional G proteins in Arabidopsis thaliana. Plant J. 2008;55:311–322. doi: 10.1111/j.1365-313X.2008.03506.x. [DOI] [PubMed] [Google Scholar]
- 13.Qi B, Zheng H. Modulation of root-skewing responses by KNAT1 in Arabidopsis thaliana. Plant J. 2013;76:380–392. doi: 10.1111/tpj.12295. [DOI] [PubMed] [Google Scholar]
- 14.Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 15.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433(7021):39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 16.Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci U S A. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennnett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. Embo J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett MJ. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GTS, Bhalerao R, Bennett MJ. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 21.Chai S, Li E, Zhang Y, Li S. NRT1.1-mediated nitrate suppression of root coiling relies on PIN2- and AUX1-mediated auxin transport. Front Plant Sci. 2020;11:671. doi: 10.3389/fpls.2020.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 23.Paciorek T, Zazimalova E, Ruthardt N, Petrasek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 24.Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, Chory J, de Vries SC. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1). Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam SK, Cai Y, Tse YC, Wang J, Law AH, Pimpl P, Chan HY, Xia J, Jiang L. BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. Plant J. 2009;60:865–881. doi: 10.1111/j.1365-313X.2009.04007.x. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa IC, Zourelidou M, Willige BC, Weller B, Schwechheimer C. D6 PROTEIN KINASE activates auxin transport-dependent growth and PIN-FORMED phosphorylation at the plasma membrane. Dev Cell. 2014;29:674–685. doi: 10.1016/j.devcel.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Kleine-Vehn J, Huang F, Naramoto S, Zhang J, Michniewicz M, Offringa R, Friml J. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell. 2009;21:3839–3849. doi: 10.1105/tpc.109.071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


