ABSTRACT
The xyloglucan endotransglucosylase/hydrolase (XTH) genes in Arabidopsis thaliana (L.) Heynh. form part of a group of mechano-stimulated genes and play an important role in abiotic stress tolerance. Mining the RNAseq transcriptomic database of 40,430 potato (Solanum tuberosum L.) genes based on functional annotation and homology search, our objective was to discover potentially homologous XTH genes. A Gene Ontology-based XTH homology search and functional annotation discovered, from among the 33 A. thaliana (AtXTH) and 25 tomato (Solanum lycopersicum L.) (SlXTH) XTH genes, 35 gene sequences corresponding to 20 AtXTH genes and 40 gene sequences corresponding to 21 SlXTH genes, respectively. Thirteen sequences corresponding to 11 putative XTH genes in potato, named as StXTH after SlXTH genes, were significantly up- or down-regulated in response to ultrasound. These putative StXTH genes in potato can serve for future functional genetic analyses.
KEYWORDS: Abiotic stress, mechanostimulus, potato, putative homology, transcriptome, ultrasound, xyloglucan endotransglucosylase/hydrolase
Introduction
Plants are constantly exposed to environmental effects and stresses from which they cannot escape but which can modify their growth and development. Therefore, the ability of plants to perceive and respond to different environmental signs is of evolutionary importance.1
Sound vibration (SV), including sound and ultrasound (US), is one group of physical stimuli originating from the surrounding environment.2,3 Depending on the frequency and intensity of SV and US, they can positively or negatively affect different biological functions of plants, such as germination, the cell cycle, shoot, root and callus growth and development, signal transduction systems, activities of various enzymes and plant hormones, and gene expression.4-7 Natural SVs produced by birds (chirping), bees (buzzing) and other animals cause different changes in plant gene expression patterns8,9 and accelerate seed germination rates,10 Collins and Foreman (2001)11 using different frequencies (500, 5,000, 6,000, 12,000 and 14,000 Hz) of sound vibration on common bean (Phaseolus vulgaris L.) observed frequency-specific responses in which beans showed maximum growth at 5000 Hz. Qin et al. (2003)12 found improved growth in Chinese cabbage (Brassica rapa L.) and cucumber (Cucumis sativus L.) in response to 20,000 Hz. Our group has worked on trying to better understand the biochemical and transcriptomic changes that take place in in vitro potato (Solanum tuberosum L.) explants exposed to ultrasound (US; 20 min; 35 kHz; 70 W) transmitted by air (air-based ultrasonication, AB-US) or liquid (liquid-based ultrasonication by piezoelectric ultrasound generator, PE-US). Those studies5,7,13 concluded that both AB-US and PE-US-generated US waves represent abiotic stresses and that both US treatments modified the gene transcription of the in vitro potato plants. Changes in the transcription profile of both ultrasonicated plant materials resulted in modified and stimulated growth response.
Mechanostimulation-induced genes of plants play a role in the response to sound waves or US.4,8 Almost three decades ago, five “touch” (TCH) genes (TCH1-TCH5) were discovered in Arabidopsis thaliana (L.) Heynh. ecovar Colombia in response to rain, wind and touch, which the authors then attributed to genes related to calmodulin.14 As neatly summarized by Braam (2005),15 several experiments conducted over more than a decade elucidated that TCH1 encodes a calmodulin protein, CAM2, TCH2 encodes a calmodulin-like (CML) protein, CML24, TCH3 encodes CML12, while TCH4 encodes a xyloglucan endotransglucosylase/hydrolase, XTH22. Johnson et al. (1998)16 observed strong induction of TCH4 (XTH) which modified the cell wall after exposure to sound vibrations (SVs) at 50 Hz, 30 min. Curiously, no additional studies after the 1990 study were ever conducted on TCH5, which is not listed on NCBI as a gene. XTHs are cell wall-loosening enzymes, thereby affecting cell expansion and growth17 and also play a role in a plant’s adaptation to abiotic stresses.18 Lee et al. (2005)19 found that 589 genes out of 22,810 genes on an Affimetrix microchip were touch-inducible or 2.58% of the genome, whereas 171 genes (0.75%) were down-regulated in response to touch. In their study of the 33 XTH genes, only four (XTH17, XTH22, XTH25, and XTH31) were significantly over-expressed in response to touch. A. thaliana Col−0 seedlings, in response to sound stimulus, showed strong up-regulation of XTH22.20 These studies indicate that XTH genes, including the TCH4 gene, form part of a wider subset of mechanostimulation-induced genes in A. thaliana.
Saladié et al. (2006)21 identified 25 tomato (Solanum lycopersicum L. cv. Alisa Craig) XTH genes (SlXTH1-25) (http://labs.plantbio.cornell.edu/XTH) but they did not study its expression intensity change in response to mechanostimuli, such as touch or SV. However, the phylogenetic analyses of A. thaliana and tomato XTHs based on full-length protein sequences revealed four distinct clades. Considering that potato and tomato are closely related species belonging to the same genus of Solanum, it is of importance to assess the similarity and potential homology for SlXTHs, as well.
In our previous AB-US and PE-US studies,5,7 we found some significantly differentially expressed genes (DEGs) that could not be functionally categorized by gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Therefore, we were interested to mine these DEGs of S. tuberosum L. genes to assess whether there might be similar or homologous sequences to XTH genes of A. thaliana (AtXTH) and/or S. lycopersicum (SlXTH), and thus putative XTH genes in potato, i.e., StXTH genes.
Methods
RNA-seq datasets originated from two different experimental studies5,7 were used for bioinformatics analysis. In both studies, single-node-stem explants cut from 4-week-old in vitro plantlets of potato (Solanum tuberosum L. cv. Desirée) were ultrasonicated with 35 kHz and 70 W US for 20 min. AB-US was applied to explants immediately after placing them horizontally onto solid hormone-free MS22 medium and they were ultrasonicated in an ultrasonication unit where the ultrasound was transmitted by air, as described in Dobránszki et al.5,13 In the case of PE-US, single-node explants were placed into beakers surrounding by distilled water and containing liquid hormone-free MS medium. After ultrasonication in an Elmasonic X-tra 30 H ultrasonicator (ElmaSchmidbauer GmbH, Singen, Germany)7 explants were cultured on the same medium and under the same in vitro growing conditions (63.5 μmol m−2 s−1, 16-h photoperiod, 22 ± 2°C, for 4 weeks) as for AB-US.5,7,13 Samplings were made five times during the 4-week-long subculture, at 0 hour (h), i.e. immediately after the ultrasonication, and then at 24 h, 48 h, 1 week (w) and 4 weeks after the US treatment.
RNA-seq datasets
In the present study, we used our previous RNA-seq datasets gained from our earlier studies.5,7,23 The RNA-seq projects were uploaded to the Gene Expression Omnibus (GEO) under GSE123176, GSE123037 and GSE135294. The RNA-seq raw FASTQ files were uploaded as GSM3498049, GSM3498050, GSM3498051, GSM3498052, GSM3498053, GSM3498054, GSM3498055, GSM3498056, GSM3498057, GSM3498058, GSM3494235, GSM3494236, GSM4002842, GSM4002843, GSM4002844, GSM4002845, GSM4002846, GSM4002847, GSM4002848, GSM4002849, GSM4002850 and GSM4002851 onto the Sequence Read Archive (SRA).
Bioinformatic analysis
The raw FASTQ files were trimmed using TrimGalore v0.5.0 (https://github.com/FelixKrueger/TrimGalore) with one addition, i.e., 10 bp was removed from the 5′ end of the reads.24 HISAT225 was used for alignment to the Solanum tuberosum L. reference genome (SolTub 3.0: https://plants.ensembl.org/Solanum_tuberosum/Info/Index). Alignments were quantitated in SeqMonk v1.42.0 (https://github.com/s-andrews/SeqMonk). The quantitated values were reads per million reads of input and were log2 transformed. The χ2 probe was used to detect significant DEGs based on the changes in intensity of gene expression between any two pairs of control (non-ultrasonicated) and ultrasonicated samples.
Functional annotation and homology search
The XTH homology search was conducted with the Gene Ontology (GO) Annotation workflow in OmicsBox v1.1.164.26 A local blast database was established in OmicsBox with the AtXTH1-33 genes (Suppl. Table 1) from the NCBI RefSeq protein database (Release 95, https://www.ncbi.nlm.nih.gov/refseq/) based on Lee et al. (2005).19 A local blast was performed with Blast+ v2.9.0 (NCBI] using blastX (E-value = 1.0E-10; number of blast hits = 10; blast description annotator included; word size = 3 with low complexity filter; HSP length cutoff = 33) on the SolTub 3.0 (EMBL_EBI Assembly ID: GCA_000226075.1) genome [40,430 genes). The blastX options, E-values and BitScore settings were based on Pearson (2013).27 InterProScan v5.36–75.028 was employed on the EMBL-EBI servers with the following families, domains, sites and repeats (CDD, HAMAP, HMMPanther, HMMPfam, HMMPIR, FPrintScan, ProfileScan, HMMSmart, HMMTigr, PatternScan), structural domains (Gene3D, SFLD, SuperFamily) and other sequence features (Coils, MobiDBLite, Phobius, SignalPHMM, TMHMM). GO mapping was performed on the putative sequences with the 2019.07 database. Annotation mapping was performed with annotation CutOff (55 score), GO weight (5), GO was filtered by taxonomy (green plants, 33090 taxa, Viridiplantae), the E-value-hit-filter was 1.0E-10, an HSP-hit coverage cutoff of zero, and a hit filter of 500. GO-Slim was run on the putative sequences with the Plant slim file from the GO-Website (http://geneontology.org/). We used GO-EnzymeCode Mapping in OmicsBox to map the existing GO terms to enzyme codes. EggNOG Mapper v1.0.3 with EggNOG v5.0.029,30 was used to predict orthology for functional annotation while avoiding the transfer of annotations from paralogs. EggNOG mapper results were merged into the NCBI results with an E-value cutoff value of 1.0E-10 and a BitScore cutoff value of 60 (Suppl. Table 1).
Sequence alignment and phylogenetic tree
Full-length amino acid sequences of StXTH, SlXTH and AtXTH were obtained from the genome sequence of S. tuberosum (SolTub 3.0 and NCBI RefSeq protein database; Suppl. Table 1), S. lycopersicum (SL3.0; Suppl. Table 1) and A. thaliana (TAIR10: GCA_000001735.1; Suppl. Table 1), respectively. For the phylogenetic tree, we used the 11 putative StXTH genes which were verified with the RNAseq dataset. The amino acid sequence alignment of StXTHs, SlXTHs and AtXTHs was compiled using Multiple Sequence Comparison by Log-Expectation (MUSCLE: https://www.ebi.ac.uk/Tools/msa/muscle/). The phylogenetic tree was constructed by the Maximum Likelihood statistical method (ML31), Nearest-Neighbor-Interchange (NNI32) heuristic method and neighbor-joining initial tree with 5000 bootstrap replications using MEGA X.33 The amino acid substitution model was optimized by MEGA X based on the Bayesian Information Criterion (BIC) scores (Suppl. Tables 2 and 334). The BIC is a criterion for model selection, where the lowest BIC score is preferred.35 Based on the BIC scores, we used the WAG+G + F substitution model (WAG: Whelan and Goldman; G: gamma distributed rates; F: frequencies), which had the lowest BIC score for the ML analysis.
RNA-seq dataset validation with RT-qPCR
Total RNA was isolated from 120 samples (three biological and three technical replicates from each AB-US and PE-US samples from different sampling time) using Direct-zol kit (Zymo Research, Irvine, CA, USA) with TRIzol reagent based on the manufacturer’s protocol. We used microcapillary electrophoresis with Implen n50 nanophotometer (Implen, Munich, Germany), agarose gel electrophoresis and fragment analysis with Agilent Bioanalyzer 2100 system (Agilent, Santa Clara, CA, USA) to quality control (QC) of total RNA. The cDNA was synthetized from 120 ng total RNA with FIREScript RT cDNA Synthesis MIX (Solis BioDyne, Tartu, Estonia). RT-qPCR was performed with the 5x HOT FIREPol EvaGreen qPCR Supermix (Solis BioDyne, Tartu, Estonia) on the ABI 7300 real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The RNA-seq dataset was validated by RT-qPCR with gene-specific primers (Suppl. Table 4) using 13 gene sequences of the 11 predicted StXTH genes (PGSC0003DMG400024755, PGSC0003DMG400021398, PGSC0003DMG400026189, PGSC0003DMG400017299, PGSC0003DMG400021877, PGSC0003DMG400017298, PGSC0003DMG400014823, PGSC0003DMG400013975, PGSC0003DMG400007854, PGSC0003DMG400001809, PGSC0003DMG400014024, PGSC0003DMG400000408, PGSC0003DMG400004109). The Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reference gene was selected for RT-qPCR as normalization based on Tang et al. (2017)36 and our previous studies.5,7 RT-qPCR primers were designed with CLC Main Workbench 7.9.2 (Qiagen, Hilden, Germany). Logarithmic fold change was used at the RT-qPCR analysis based on the 2−ΔΔCt method. Spearman and Pearson correlation coefficients were calculated in Microsoft Office 365 Excel (Microsoft, Redmond, WA, USA).
Results
Among the 40,430 S. tuberosum genes, a total of 38 gene sequences were found that corresponded to 20 AtXTH genes (AtXTH1, AtXTH2, AtXTH3, AtXTH4, AtXTH5, AtXTH7, AtXTH8, AtXTH9, AtXTH10, AtXTH15, AtXTH16, AtXTH22, AtXTH23, AtXTH24, AtXTH25, AtXTH28, AtXTH30, AtXTH31, AtXTH32, and AtXTH33) and 21 SlXTH genes (SlXTH2, SlXTH3, SlXTH4, SlXTH5, SlXTH6, SlXTH7, SlXTH8, SlXTH9, SlXTH10, SlXTH11, SlXTH12, SlXTH13, SlXTH14, SlXTH15, SlXTH16, SlXTH18, SlXTH19, SlXTH20, SlXTH23, and SlXTH25) from among the 33 A. thaliana and 25 S. lycopersicum XTH genes studied and which may be putatively homologous genes in S. tuberosum (Suppl. Table 1). Based on sequence mining, two putatively homologous sequences (different sequences for the same gene) were identified for seven XTH genes (AtXTH16, AtXTH23, AtXTH24, AtXTH28, AtXTH30, AtXTH31, and AtXTH32), three putatively homologous sequences for three XTH genes (AtXTH5, AtXTH8, and AtXTH15), and in the case of AtXTH22, four putatively homologous sequences were found (Table 1) compared to A. thaliana and S. tuberosum.
Table 1.
Putatively homologous XTH genes from the Solanum tuberosum RNAseq database.
| Sequence name | E-value | Similarity mean (%) | Bit-Score | Putative homologous XTH gene in Arabidopsis thaliana1 | Putative homologous XTH in Solanum lycopersicum 1 |
|---|---|---|---|---|---|
| PGSC0003DMG400041434 | 1.11189E-22 | 63.66 | 156.4 | - | SlXTH19 |
| PGSC0003DMG400042833 | 3.41499E-36 | 65.63 | 212.2 | AtXTH1/AtXTH2 | SlXTH7 |
| PGSC0003DMG400001229 | 1.44952E-42 | 60.59 | 286.6 | AtXTH3 | SlXTH4 |
| PGSC0003DMG400002188 | 7.09171E-69 | 65.91 | 299.7 | AtXTH4-1 | SlXTH4 |
| PGSC0003DMG400024755 | 8.73328E-70 | 66.63 | 294.3 | AtXTH5-1 | SlXTH1 |
| PGSC0003DMG402010181 | 4.33809E-63 | 54.05 | 229.9 | AtXTH5-2 | SlXTH18 |
| PGSC0003DMG402010918 | 5.90652E-64 | 66.53 | 243.0 | AtXTH5-3 | SlXTH4 |
| PGSC0003DMG400021398 | 1.12747E-75 | 67.99 | 239.2 | AtXTH7 | SlXTH7 |
| PGSC0003DMG400002084 | 2.91162E-59 | 54.69 | 211.1 | AtXTH8 | SlXTH23 |
| PGSC0003DMG400024060 | 8.36804E-71 | 61.67 | 306.6 | AtXTH8 | SlXTH15-2 |
| PGSC0003DMG400011281 | 9.85939E-16 | 91.43 | 124.0 | AtXTH8 | SlXTH15-1 |
| PGSC0003DMG400026189 | 1.00497E-89 | 61.97 | 318.2 | AtXTH9-2 | SlXTH16 |
| PGSC0003DMG400005127 | 4.29487E-60 | 59.03 | 144.8 | AtXTH10-1 | SlXTH7 |
| PGSC0003DMG400017299 | 5.34064E-93 | 63.77 | 171.0 | AtXTH15 | SlXTH10 |
| PGSC0003DMG400031101 | 6.93065E-94 | 65.19 | 171.8 | AtXTH15 | SlXTH10 |
| PGSC0003DMG400021877 | 5.64726E-57 | 69.87 | 260.4 | AtXTH15 | SlXTH12 |
| PGSC0003DMG400017298 | 1.58365E-53 | 69.82 | 291.6 | AtXTH16 | SlXTH9 |
| PGSC0003DMG400004670 | 7.39946E-56 | 25812 | 260.8 | AtXTH16 | SlXTH3 |
| PGSC0003DMG401018740 | 1.26591E-40 | 63.48 | 237.7 | AtXTH22 | SlXTH13 |
| PGSC0003DMG402018740 | 1.08367E-55 | 70.22 | 284.6 | AtXTH22 | SlXTH3 |
| PGSC0003DMG400024121 | 4.36905E-23 | 72.95 | 134.0 | AtXTH22 | SlXTH10 |
| PGSC0003DMG400002309 | 2.24189E-89 | 62.1 | 161.4 | AtXTH22 | SlXTH11 |
| PGSC0003DMG400014823 | 9.64778E-106 | 67.05 | 250.8 | AtXTH23 | SlXTH3 |
| PGSC0003DMG400013975 | 2.05102E-53 | 68.65 | 251.9 | AtXTH23 | SlXTH2 |
| PGSC0003DMG400007794 | 2.54537E-61 | 61.28 | 235.3 | AtXTH24 | SlXTH9 |
| PGSC0003DMG400007854 | 9.06518E-61 | 61.11 | 236.1 | AtXTH24 | SlXTH9 |
| PGSC0003DMG400018741 | 4.50311E-57 | 83.13 | 229.6 | AtXTH25 | SlXTH20 |
| PGSC0003DMG400029503 | 2.13033E-67 | 59.27 | 319.3 | AtXTH28 | SlXTH8 |
| PGSC0003DMG400022791 | 1.19207E-67 | 57.83 | 333.6 | AtXTH28 | SlXTH8 |
| PGSC0003DMG400001809 | 5.07525E-63 | 58.3 | 337.8 | AtXTH30 | SlXTH5 |
| PGSC0003DMG400009300 | 3.39019E-56 | 59.08 | 389.8 | AtXTH30 | SlXTH5 |
| PGSC0003DMG400014024 | 1.64996E-40 | 68.94 | 161.4 | AtXTH31 | SlXTH6 |
| PGSC0003DMG400000408 | 5.57005E-46 | 70.39 | 216.9 | AtXTH31 | SlXTH6 |
| PGSC0003DMG400003432 | 1.61541E-33 | 65.02 | 256.4 | - | SlXTH6 |
| PGSC0003DMG400036031 | 3.00177E-14 | 62.97 | 236.3 | - | SlXTH6 |
| PGSC0003DMG400003434 | 5.11064E-48 | 50.48 | 117.9 | AtXTH32-1 | SlXTH6 |
| PGSC0003DMG400003866 | 2.11514E-65 | 65.16 | 271.9 | AtXTH32-2 | SlXTH14 |
| PGSC0003DMG400004109 | 3.54934E-52 | 52.48 | 297.0 | AtXTH33 | SlXTH25 |
| PGSC0003DMG400004599 | 1.57405E-11 | 60.87 | 265.6 | - | SlXTH23 |
| PGSC0003DMG400029203 | 2.54838E-12 | 72.34 | 326.8 | - | SlXTH23 |
1based on NCBI and EggNOG (St = Solanum tuberosum); different numbers after several gene names indicate orthologs or putatively homologous sequences for the same gene which were identified from S. tuberosum RNAseq data.
When comparing S. tuberosum L to S. lycopersicum, we identified two putatively homologous sequences for four XTH genes (SlXTH5, SlXTH8, SlXTH15 and SlXTH23), three putatively homologous sequences for four XTH genes (SlXTH3, SlXTH4, SlXTH9 and SlXTH10), and in the case of SlXTH6, five putatively homologous sequences were found (Table 1). StXTHs were named after the SlXTHs.
A total of 13 sequences corresponding to candidate 11 XTH genes were identified (Table 2) that were significantly up- or down-expressed after US treatments in different comparisons. RT-qPCR for the candidate 11 XTH genes validated RNA-seq data, indicating that all of the chosen DEGs were truly positively up- or down-regulated DEGs. A strong positive correlation (r = 0.95 and 0.98 with Spearman and Pearson correlations, respectively) between SeqMonk LFC and RT-qPCR LFC was found (Suppl. Table 4). The different comparisons correspond to the AB-US and PE-US compared to their respective controls, as well as AB-US vs PE-US comparisons, each at five sampling times, namely 0 h, 24 h, 48 h, 1 w and 4 w, corresponding to five growth stages of in vitro potato as described in Dobránszki et al.5,7,23 After the AB-US treatment, which caused a weaker stress than PE-US treatment,7 the expression intensity of five sequences differed. Up-regulation of StXTH16 and StXTH12 occurred immediately after ultrasonication (0 h), while StXTH9-1 and StXTH25 were up-regulated at 24 h. Later, at 48 h, StXTH9-1 was then down-regulated. Moreover, StXTH3 was down-regulated at 4 w (Table 2). However, the expression intensity of seven sequences belonging to six putative StXTH genes (StXTH1, StXTH3, StXTH2, StXTH6-2, StXTH7, and StXTH10) were down-regulated between 24 h and 4 w after PE-US treatment (Table 2). The most affected gene by PE-US was StXTH6-2 which was up-regulated at 4 w following its down-regulation at 24 h and 1 w. When PE-US to AB-US was compared at different sampling times, seven sequences of five putative StXTH genes (StXTH1, StXTH5, StXTH6, StXTH7, and StXTH9) were down-regulated at different sampling times (Table 2).
Table 2.
The response of the putatively homologous StXTH genes to different ultrasound treatments.
| Sequence name | Putative XTH gene in Solanum lycopersicum 1 | Response to different ultrasound treatments2 |
|
|---|---|---|---|
| Up-regulation | Down-regulation | ||
| PGSC0003DMG400024755 | StXTH1 | (2.74) AB-24 h vs. PE-24 h | |
| (2.26) CPE-4 w vs. PE-4 w | |||
| PGSC0003DMG400013975 | StXTH2 | (4.06) CPE-1 w vs. PE-1 w | |
| PGSC0003DMG400014823 |
StXTH3-1 StXTH3-2 StXTH3-3 StXTH3-4 |
(3.32) CPE-4 w vs. PE-4 w | |
| (1.87) CAB-4 w vs. AB-4 w | |||
| PGSC0003DMG400001809 | StXTH5 | (1.26) AB-1 w vs. PE-1 w | |
| PGSC0003DMG400014024 | StXTH6-1 | (6.77) AB-24 h vs. PE-24 h | |
| PGSC0003DMG400000408 | StXTH6-2 | (−1.43) CPE-4 w vs. PE-4 w | (3.01) AB-24 h vs. PE-24 h |
| (1.51) AB-1 w vs. PE-1 w | |||
| (2.03) CPE-24 h vs. PE-24 h | |||
| (3.27) CPE-1 w vs. PE-1 w | |||
| PGSC0003DMG400021398 | StXTH7 | (2.66) AB-48 h vs. PE-48 h | |
| (1.66) CPE-48 h vs. PE-48 h | |||
| PGSC0003DMG400017298 | StXTH9-1 | (−2.45) CAB-24 h vs. AB-24 h | (3.86) AB-24 h vs. PE-24 h |
| (3.53) AB-1 w vs. PE-1 w | |||
| (3.86) CPE-1 w vs. PE-1 w | |||
| (4.20) CAB-48 h vs. AB-48 h | |||
| PGSC0003DMG400007854 | StXTH9-2 | (2.57) AB-24 h vs. PE-24 h | |
| PGSC0003DMG400017299 | StXTH10 | (2.54) CPE-4 w vs. PE-4 w | |
| PGSC0003DMG400021877 | StXTH12 | (−2.69) CAB-0 h vs. AB-0 h | |
| PGSC0003DMG400026189 | StXTH16 | (−2.50) CAB-0 h vs. AB-0 h | |
| PGSC0003DMG400004109 | StXTH25 | (−1.82) CAB-24 h vs. AB-24 h | |
1based on NCBI and EggNOG (St = Solanum tuberosum); different numbers after several gene names indicate orthologs or putatively homologous sequences for the same gene which were identified from S. tuberosum RNAseq data (Table 1). Names of the StXTH genes are based on the corresponding SlXTHs.
2AB, air-based US;5,23 C, control (no US treatment); h, hours; PE, liquid-based US;7 US, ultrasound; w, weeks; number in brackets indicate the change in expression intensity in the different US treatment comparisons.
h, hour; w, week
We calculated 56 different amino acid substitution models with the ML method based on the amino acid sequences of StXTH and AtXTH genes. The mtREV24 + I model had the highest BIC score (219172.5) while the WAG+G + F model had the lowest BIC score (207467.2) (Suppl. Table 2). To construct the phylogenetic tree, the substitution pattern estimation was calculated for amino acids under the WAG+G + F model (Suppl. Table 3). Based on the phylogenetic tree (Figure 1) and cluster analysis, we identified StXTH1 gene in the same cluster with SlXTH1 and close to the AtXTH5-1. StXTH2 gene was in the same cluster with SlXTH19 and SlXTH2 genes and close to the AtXTH23 gene. StXTH3-1, StXTH3-2, StXTH3-3 and StXTH3-4 were in the same cluster with SlXTH3 gene and close to the AtXTH23 gene. StXTH5 gene was in the same cluster with SlXTH25 and AtXTH33 genes. StXTH6-1 and StXTH6-2 were in the same cluster with SlXTH6 gene and close to AtXTH32-1, AtXTH32-2 and SlXTH14 genes. StXTH7 gene was in the same cluster with SlXTH7 and close to the AtXTH6 gene. StXTH9-1, StXTH9-2 and StXTH10 genes were in the same cluster with SlXTH9 and SlXTH10 genes and close to the AtXTH15 and AtXTH16 genes. StXTH12 gene was in the same cluster with SlXTH12 and close to the AtXTH15 and AtXTH16 genes. StXTH16 gene was in the same cluster with SlXTH16 and close to AtXTH9-1 and AtXTH9-2. The StXTH25 gene was in the same cluster as the SlXTH5 gene and close to the AtXTH30 gene.
Figure 1.
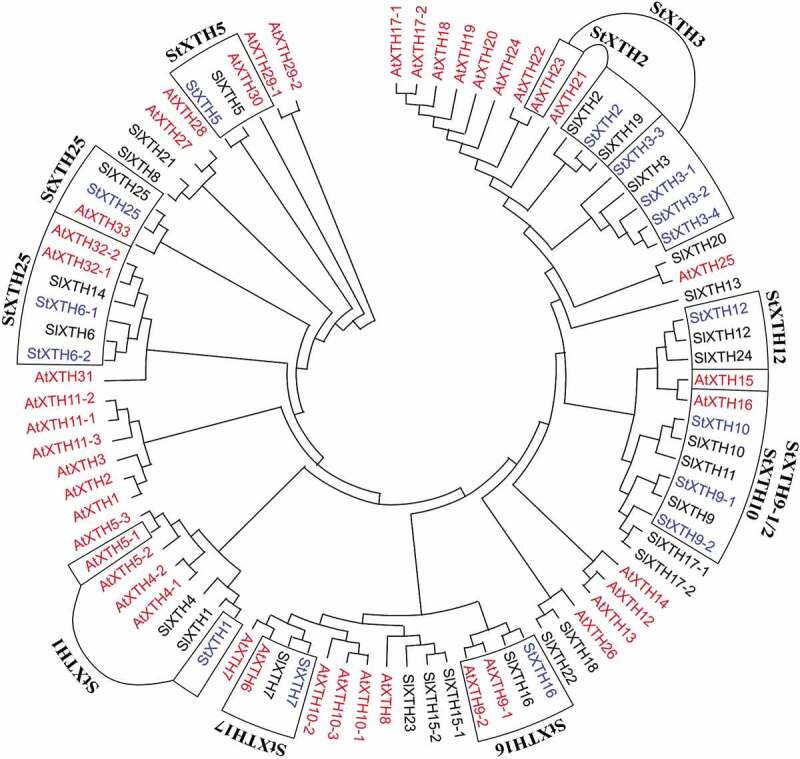
Phylogenetic tree of XTH genes from Arabidopsis thaliana (L.) Heynh. (AtXTH), Solanum lycopersicum L. (SlXTH) and putative XTH genes from Solanum tuberosum L. (StXTH). The phylogenetic tree was constructed by the neighbor-joining method with 5000 bootstrap replications using MEGA X.
Discussion and conclusion
In the RNAseq transcriptomic database of S. tuberosum, we detected 35 gene sequences that are putative homologs of 20 AtXTH and 21 SlXTH genes, based on sequence homology and functional annotation. Lee et al. (2005)19 found that four A. thaliana XTH genes [AtXTH17, AtXTH22, AtXTH25, and AtXTH31) were over-expressed in response to touch, while,20 detected strong over-expression of AtXTH22 in response to sound waves. Over-expression of StXTH6-2, a putative homolog of AtXTH31 in A. thaliana, was detected by 4 w after ultrasonication transmitted by liquid (PE-US), which caused more stress to plants than ultrasonication transmitted by air (AB-US).7 In contrast, none of the four genes described in earlier studies [AtXTH17, AtXTH22, AtXTH25, and AtXTH3119,20] were over-expressed in the present study, in response to mechanostimulus caused by the air-transmitted ultrasonication (AB-US), either directly after ultrasonication or later during subculture, as an after-effect. However, we detected the over-expression of four other genes (StXTH16, StXTH12, StXTH9-1, and StXTH25), potential homologues to AtXTH9, AtXTH15, AtXTH16 and AtXTH33, directly (0 h) or 24 h after ultrasonication. The up-regulation of these four genes was not observed in response to touch or sound stimulus in previous studies.19,20
Ultrasonication caused strong abiotic stress to in vitro potato plants, i.e. ultrasound transmitted via liquid, causing down-regulation of gene expression of six putative XTH genes, which was detected between 24 h and 4 w. To the best of our knowledge, down-regulation of XTH gene expression in response to touch or sound stimuli has not been reported previously.
Given the importance of XTH genes in their intricate involvement in plant mechanostimulus responses19,20 and abiotic stress tolerance in plants,18 this set of putative StXTH genes in potato and the change in their expression intensities in response to different ultrasonication treatments will be a useful dataset and a starting point for functional analyses of these sequences to determine their precise function.
Supplementary Material
Acknowledgments
The research was financed by the Higher Education Institutional Excellence Programme (NKFIH-1150-6/2019) of the Ministry of Innovation and Technology in Hungary, within the framework of the Biotechnology thematic program of the University of Debrecen. The study and submission for publication were approved by the University of Debrecen (BPTR/DEENK/0009/2019).
Funding Statement
This work was supported by the Higher Education Institutional Excellence Programme of the Ministry of Innovation and Technology in Hungary [NKFIH-1150-6/2019].
Conflicts of interest
The authors declare no conflicts of interest regarding this paper.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Gagliano M, Mancuso S, Robert D.. Towards understanding plant bioacoustics. Trends Plant Sci. 2012;17:1–7. doi: 10.1016/j.tplants.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Gagliano M. Green symphonies: a call for studies on acoustic communication in plants. Behav Ecol. 2013;24:789–796. doi: 10.1093/beheco/ars206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telewski FW. A unified hypothesis of mechanoperception in plants. Am J Bot. 2006;93:1466–1476. doi: 10.3732/ajb.93.10.1466. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury M, Lim H-S, Bae H. Update on the effects of sound wave on plants. Res Plant Dis. 2014;20:1–7. doi: 10.5423/RPD.2014.20.1.001. [DOI] [Google Scholar]
- 5.Dobránszki J, Hidvégi N, Gulyás A, Teixeira da Silva JA. mRNA transcription profile of potato (Solanum tuberosum L.) exposed to ultrasound during different stages of in vitro plantlet development. Plant Mol Biol. 2019;100:511–525. doi: 10.1007/s11103-019-00876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira da Silva JA, Dobránszki J. Sonication and ultrasound: impact on plant growth and development. Plant Cell Tissue Organ Cult. 2014;117:131–143. doi: 10.1007/s11240-014-0429-0. [DOI] [Google Scholar]
- 7.Teixeira da Silva JA, Hidvégi N, Gulyás A, Tóth B, Dobránszki J. Transcriptomic response of in vitro potato (Solanum tuberosum L.) to piezoelectric ultrasound. Plant Mol Biol Rep. 2020. doi: 10.1007/s11105-020-01204-3. [DOI] [Google Scholar]
- 8.Jeong M-J, Shim C-K, Lee J-O, Kwon H-B, Kim Y-H, Lee S-K, Byun M-O, Park S-C. Plant gene responses to frequency-specific sound signals. Mol Breed. 2008;21:217–226. doi: 10.1007/s11032-007-9122-x. [DOI] [Google Scholar]
- 9.Safari M, Ghanati F, Behmanesh M, Hajnorouzi A, Nahidian B, Mina G. Enhancement of antioxidant enzymes activity and expression of CAT and PAL genes in hazel (Corylus avellana L.) cells in response to low-intensity ultrasound. Acta Physiol Plant. 2013;35:2847–2855. doi: 10.1007/s11738-013-1318-6. [DOI] [Google Scholar]
- 10.Creath K, Schwartz GE. Measuring effects of music, noise, and healing energy using a seed germination bioassay. J Altern Complementary Med. 2004;10:113–122. doi: 10.1089/107555304322849039. [DOI] [PubMed] [Google Scholar]
- 11.Collins ME, Foreman JE. The effect of sound on the growth of plants. Can Acoust. 2001;29:3–8. [Google Scholar]
- 12.Qin Y-C, Lee W-C, Choi Y-C, Kim T-W. Biochemical and physiological changes in plants as a result of different sonic exposures. Ultrasonics. 2003;41:407–411. doi: 10.1016/S0041-624X(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 13.Dobránszki J, Asboth G, Homoki D, Bíró-Molnár P, Teixeira da Silva JA, Remenyik J. Ultrasonication of in vitro potato single node explants: activation and recovery of antioxidant defence system and growth responses. Plant Physiol Biochem. 2017;121:153–160. doi: 10.1016/j.plaphy.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Braam J, Davis RW. Rain-, wind- and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell. 1990;60:357–364. doi: 10.1016/0092-8674(90)90587-5. [DOI] [PubMed] [Google Scholar]
- 15.Braam J. In touch: plant responses to mechanical stimuli. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson KA, Sistrunk ML, Polisensky DH, Braam J. Arabidopsis thaliana responses to mechanical stimulation do not require ETR1 or EIN2. Plant Physiol. 1998;116:643–649. doi: 10.1104/pp.116.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miedes E, Suslov D, Vandenbussche F, Kenobi K, Ivakov A, Van Der Straeten D, Lorences EP, Mellerowicz EJ, Verbelen J-P, Vissenberg K. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J Exp Bot. 2013;64:2481–2497. doi: 10.1093/jxb/ert107. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall H, Philippe F, Domon J-M, Gillet F, Pelloux J, Rayon C. Cell wall metabolism in response to abiotic stress. Plants. 2015;4:112–166. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee D, Polisensky DH, Braam J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol. 2005;165:429–444. doi: 10.1111/j.1469-8137.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh R, Mishra RC, Choi B, Kwon YS, Bae DW, Park S-C, Jeong M-J, Bae H. Exposure to sound vibrations lead to transcriptomic, proteomic and hormonal changes in Arabidopsis. Sci Rep. 2016;6:33370. doi: 10.1038/srep33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saladié M, Rose JKC, Cosgrove DJ, Catala C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- 22.Murashige M, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 23.Dobránszki J, Hidvégi N, Gulyás A, Tóth B, Teixeira da Silva JA. Transcription profile of potato (Solanum tuberosum L.) growing in vitro. J Plant Growth Regul. 2020a. doi: 10.1007/s00344-020-10133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucleic Acids Res. 2010;38:e131–e131. doi: 10.1093/nar/gkq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson WR. An introduction to sequence similarity (“homology”) searching. Current protocols in bioinformatics. New Jersey (NJ): John Wiley & Sons, Inc; 2013. p. 311–318. doi: 10.1002/0471250953.bi0301s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P. Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Mol Biol Evol. 2017;34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47:D309–D314. doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 32.DasGupta B, He X, Li M, Tromp J, Zhang L. Nearest neighbor interchange and related distances. In: Kao MY, editor. Encyclopedia of algorithms. New York (NY): Springer; 2016. p. 1402–1405. doi: 10.1007/978-1-4939-2864-4. [DOI] [Google Scholar]
- 33.Stecher G, Tamura K, Kumar S, Russo C. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- 35.Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 36.Tang X, Zhang N, Si H, Calderón-Urrea A.. Selection and validation of reference genes for RT-qPCR analysis in potato under abiotic stress. Plant Methods.2017;13:85. doi: 10.1186/s13007-017-0238-7 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


