Abstract
Experimental cell models are indispensable for clarifying the pathophysiology of coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, and for developing therapeutic agents. To recapitulate the symptoms and drug response of COVID‐19 patients in vitro, SARS‐CoV‐2 studies using physiologically relevant human embryonic stem (ES)/induced pluripotent stem (iPS) cell‐derived somatic cells and organoids are ongoing. These cells and organoids have been used to show that SARS‐CoV‐2 can infect and damage various organs including the lung, heart, brain, intestinal tract, kidney, and pancreas. They are also being used to develop COVID‐19 therapeutic agents, including evaluation of their antiviral efficacy and safety. The relationship between COVID‐19 aggravation and human genetic backgrounds has been investigated using genetically modified ES/iPS cells and patient‐derived iPS cells. This review summarizes the latest results and issues of SARS‐CoV‐2 research using human ES/iPS cell‐derived somatic cells and organoids.
Keywords: COVID‐19, human ES cells, human iPS cells, organoids, SARS‐CoV‐2
To recapitulate the symptoms and drug response of COVID‐19 patients in vitro, SARS‐CoV‐2 studies using physiologically relevant human embryonic stem/induced pluripotent stem cell‐derived somatic cells and organoids are ongoing. They are being used to investigate SARS‐CoV‐2 cell tropism, to develop COVID‐19 therapeutic agents, and to examine the relationship between COVID‐19 aggravation and human genetic backgrounds.
Significance statement.
COVID‐19 and SARS‐CoV‐2 are dominating discussion in the scientific community and the news. Although many clinical trials are underway worldwide, basic research on SARS‐CoV‐2 entry and replication and identification of the best drug targets is lacking. Therefore, this study introduces the human pluripotent stem cell‐derived cells and organoids available for research on SARS‐CoV‐2, with consideration to their strengths and weaknesses. This overview will help researchers select suitable human pluripotent stem cell‐derived cells and organoids for SARS‐CoV‐2 studies. Thus, this review will provide valuable information to accelerate drug discovery for COVID‐19.
1. INTRODUCTION
As of June 10, 2021, the number of coronavirus disease 2019 (COVID‐19) patients is approximately 173 million, and the number of deaths is approximately 3.74 million. Although pneumonia and acute respiratory distress syndrome are widely recognized as symptoms of COVID‐19, many symptoms of extrapulmonary organs are also known. These symptoms include cardiac arrhythmias, myocardial ischemia, diarrhea, stroke, acute kidney injury, and hyperglycemia. Elucidation of the complex pathophysiology of COVID‐19 and the development of therapeutic agents are essential for stopping this pandemic. While several COVID‐19 vaccines are now available, progress in therapeutic agents has been slower. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the cause of COVID‐19, uses its spike (S) protein to enter host cells. Because the viral S protein binds to angiotensin converting enzyme 2 (ACE2) on the host cell surface, cells expressing ACE2 are susceptible to SARS‐CoV‐2 infection. Accordingly, SARS‐CoV‐2 has high infectivity in primates, including humans, rhesus monkeys, and cynomolgus monkeys, but low infectivity in wild‐type mice, 1 , 2 , 3 , 4 limiting the animal species that can be used for experiments. Moreover, it is ethically difficult to use these animals in large quantities. Therefore, physiologically relevant human embryonic stem (ES)/induced pluripotent stem (iPS) cell‐derived somatic cells and organoids are being developed as cell models for SARS‐CoV‐2 infection. Organoids are three‐dimensional structures that can be generated from somatic stem cells 5 or human ES/iPS cells. 6 The derived somatic cells and organoids have closer cellular and organ functions to primary cells than other cell lines commonly used in in vitro SARS‐CoV‐2 research, such as Vero, Calu‐3, and Caco‐2, suggesting that they more accurately reproduce the pathophysiology of COVID‐19 and drug effects. 7 Although the infection efficiency of SARS‐CoV‐2 in Vero, Calu‐3, and Caco‐2 cells, it is difficult to reproduce the cellular and organ responses due to SARS‐CoV‐2 infection. This review introduces the latest findings and issues of SARS‐CoV‐2 research using human ES/iPS cell‐derived somatic cells and organoids.
2. SARS‐CoV‐2‐TARGET CELLS AND ORGANS
The main symptoms of COVID‐19 are manifested in the respiratory system, but many cases of multiple organ failure have been reported, 8 , 9 , 10 indicating many organs are affected by SARS‐CoV‐2 infection. Accordingly, human ES/iPS cell‐derived somatic cells and organoids have been used to study the infection in several of these organs (Table 1).
TABLE 1.
SARS‐CoV‐2 infection experiments using human embryonic stem/induced pluripotent stem (ES/iPS) cell‐derived somatic cells and organoids
| Organ model | Original cell or tissue | Cell composition | Permissiveness to SARS‐CoV‐2 infection | Effective drugs or compounds | Ref. | |
|---|---|---|---|---|---|---|
| Respiratory | Human alveolar epithelial type 2 alveolospheres | Human lung tissues | Alveolar epithelial type 2 cells | + | IFN‐α, IFN‐γ | 11 |
| Human alveolar epithelial type 2 alveolospheres a | Human iPS cells | Alveolar epithelial type 2 cells | + | Remdesivir, Camostat | 12 | |
| Human airway epithelial cells a | Human lung tissues | Ciliated cells | + | − | 13 | |
| Goblet cells | + | |||||
| Human lung alveolospheres | Human lung tissues | Alveolar epithelial type 2 cells | + | Remdesivir, IFN‐β, Hydroxychloroquine | ||
| Alveolar epithelial type 1 cells | Unknown | |||||
| Human airway organoids | Human ES cells | Ciliated cells | + | Remdesivir, Camostat, SARS‐CoV‐2 antibody CB6 | 14 | |
| Basal cells | − | |||||
| Club cells | + | |||||
| Goblet cells | − | |||||
| Human alveolar organoids | Human ES cells | Alveolar epithelial type 2 cells | + | Remdesivir, SARS‐CoV‐2 antibody CB6 | ||
| Alveolar epithelial type 1 cells | − | |||||
| Human airway organoids a | Human lung tissues | Ciliated cells | + | − | 15 | |
| Human alveolar epithelial type 2 cells | Human iPS cells | Alveolar epithelial type 2 cells | + | − | 16 | |
| Human airway epithelial cells | Human iPS cells | Ciliated cells | + b | Remdesivir | 17 | |
| Goblet cells | ||||||
| Club cells | ||||||
| Basal cells | ||||||
| Pulmonary neuroendocrine cells | ||||||
| Human small airway organoids a | Human lung tissue | Ciliated cells | + | − | 18 | |
| Club cells | + | |||||
| Goblet cells | − | |||||
| Human organoid‐derived bronchioalveolar model a | Human lung tissue | Alveolar epithelial type 2 cells | + | IFN‐λ1 | ||
| Alveolar epithelial type 1 cells | + | |||||
| Basal cells | − | |||||
| Pulmonary neuroendocrine cells | − | |||||
| Tuft cells | Unknown | |||||
| ACE2‐overexpressed human lung organoids | Human lung tissue | − | + | Camostat | 19 | |
| Human 3D alveolar type 2 cell cultures (h3ACs) | Human lung tissue | Alveolar epithelial type 2 cells | + | Camostat | 20 | |
| 3D cultures of human bronchial cells (h3BCs) | Human lung tissue | Airway cells including basal cells and secretory cells | + b | ‐ | ||
| Human lung organoids | Human ES cells | Alveolar epithelial type 2 cells | + | Imatinib, Mycophenolic acid, Quinacrine dihydrochloride, Chloroquine | 21 | |
| Alveolar epithelial type 1 cells | Unknown | |||||
| Stromal cells | ||||||
| Pulmonary neuroendocrine cells | ||||||
| Airway epithelial cells | ||||||
| Proliferating cells | ||||||
| Fibroblasts | ||||||
| Human bronchial organoids a | Cryopreserved adult human bronchial epithelial cells | Basal cells | + | Camostat | 22 | |
| Ciliated cells | Unknown | |||||
| Goblet cells | ||||||
| Club cells | ||||||
| Heart | Human cardiomyocytes | Human iPS cells | Cardiomyocytes | + | Remdesivir, N‐acetyl‐L‐leucyl‐L‐leucyl‐L‐methionine, recombinant human ACE2 protein, ACE2 neutralizing antibody | 23 |
| Human cardiospheres | Human iPS cells | Cardiomyocytes | + | − | ||
| Human cardiac cells | Human iPS cells | Cardiomyocytes | + | ACE2 neutralizing antibody, Aloxistatin, Remdesivir, IFN‐β, Apilimod, Bafilomycin, Z‐FY(tBu)‐DMK | 24 | |
| Cardiac fibroblasts | − | − | ||||
| Endothelial cells | − | − | ||||
| Human cardiomyocytes | Human iPS cells | Cardiomyocytes | + | Berzosertib, Remdesivir | 25 | |
| Human cardiomyocytes | Human iPS cells | Cardiomyocytes | + | − | 16 | |
| Human cardiac cells | Human ES and iPS cells | Cardiomyocytes | + | − | 26 | |
| Smooth muscle cells | − | − | ||||
| Human cardiac organoids | Human ES and iPS cells | Cardiomyocytes | + b | INCB054329 (BET inhibitor) | 27 | |
| Epicardial cells | ||||||
| Fibroblasts/pericytes | ||||||
| Endothelial cells | ||||||
| Human cardiomyocytes | Human ES cells | Cardiomyocytes | + | − | 28 | |
| Intestine | Human intestinal organoids | Human ES cells | Enterocytes | + | Remdesivir, EK1 | 29 |
| Enteroendocrine cells | + | |||||
| Paneth cells | + | |||||
| Goblet cells | − | |||||
| Human colonic organoids | Human ES cells | Enterocytes | + | Imatinib, Mycophenolic acid, Quinacrine dihydrochloride | 21 | |
| Goblet cells | + | |||||
| Transit‐amplifying cells | + | |||||
| Enteroendocrine cells | + | |||||
| Intestinal stem cells | + | |||||
| Human intestinal organoids | Human iPS cells | Enterocytes | + b | − | 30 | |
| Paneth cells | ||||||
| Kidney | Human kidney organoids | Human ES cells | Proximal tubules | + | Human recombinant soluble ACE2 protein | 31 |
| Human kidney organoids | Human ES cells | Proximal tubular epithelial cells | + b | Human recombinant soluble ACE2 | 32 | |
| Podocytes | ||||||
| Blood vessels | Human blood vessel organoids | Human iPS cells | Endothelial cells | + b | Human recombinant soluble ACE2 | 32 |
| Pericytes | ||||||
| Human endothelial cells | Human ES cells | Endothelial cells | − | − | 28 | |
| Brain | Human dopaminergic neurons | Human ES cells | Dopaminergic neurons | + | − | 28 |
| Human cortical neurons | Human ES cells | Cortical neurons | − | − | 28 | |
| Human brain organoids | Human iPS cells | Neural progenitors/outer radial glia | + b | Anti‐ACE2 antibodies, cerebrospinal fluid from a COVID‐19 patient | 33 | |
| Intermediate progenitor/interneurons | ||||||
| Neurons | ||||||
| Cortical neurons | ||||||
| Human cerebral organoids a | Human ES cells | Choroid plexus epithelial cells | + | − | 34 | |
| Neuronal progenitor cells | − | − | ||||
| Neurons | − | − | ||||
| Glial cells | − | − | ||||
| Human brain cells | Human iPS cells | Neurons | − | − | 35 | |
| Astrocytes | − | − | ||||
| Microglia | − | − | ||||
| Human cortical organoids | − | − | − | |||
| Human hippocampal organoids | − | − | − | |||
| Human hypothalamic organoids | − | − | − | |||
| Human midbrain organoids | − | − | − | |||
| Human choroid plexus organoids | Choroid plexus epithelial cells | + | − | |||
| Human microglia | Human ES cells | Microglial cells | − | − | 28 | |
| Pancreas | Human pancreatic endocrine cells | Human ES cells | Alpha cells | + | − | 28 |
| Beta cells | + | − | ||||
| Delta cells | − | − | ||||
| Primary human islets | Human pancreatic organs | Beta cells | + | − | ||
| Alpha cells | + | − | ||||
| Acinar cells | Unknown | − | ||||
| Ductal cells | − | |||||
| Mesenchymal cells | − | |||||
| Poly‐peptide cells | − | |||||
| Delta cells | − | |||||
| Endothelial cells | − | |||||
| Immune cells | − | |||||
| Liver | Human liver organoids | Human iPS cells | − | + | − | 28 |
| Human adult hepatocyte organoids | Human liver tissue | Hepatocytes | + | − | ||
| Human adult cholangiocyte organoids | Human liver tissue | Cholangiocytes | + | − | ||
| Blood | Human macrophages | Human ES cells | Macrophages | − | − | 28 |
Air‐liquid interface (ALI) culture.
Did not analyze each component cell type separately.
Because respiratory failure is one of the most critical symptoms of SARS‐CoV‐2 infection, experiments using bronchial and alveolar models are especially being studied. We generated human bronchial organoids from cryopreserved human bronchial epithelial cells to reproduce the infection of SARS‐CoV‐2 in the bronchi. 22 These bronchial organoids have cellular constituents resembling basal, ciliated, goblet, and club cells, and we confirmed that some basal cells can be infected with SARS‐CoV‐2. Pei et al also performed SARS‐CoV‐2 infection experiments using airway organoids generated from human ES cells, finding ciliated and club cells are susceptible to infection. 14 Huang et al performed SARS‐CoV‐2 infection experiments using human iPS cell‐derived alveolar epithelial type 2‐like cells. 12 The expression of surfactant protein C, a critical component of lung surfactant that is expressed only in type II alveolar epithelial cells of the lung, was decreased and the nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB) signal was activated after SARS‐CoV‐2 infection.
Because myocardial damage, neuropathy, and diarrhea are frequently observed in COVID‐19 patients, 36 , 37 , 38 SARS‐CoV‐2 infection experiments using myocardial, brain, and intestinal tract models have also been performed. Perez‐Bermejo et al found that human iPS cell‐derived cardiomyocytes, but not cardiac fibroblasts or endothelial cells, can be infected by SARS‐CoV‐2. 24 Approximately 20% of the cardiomyocytes infected with SARS‐CoV‐2 showed myofibrillar fragmentation. Pellegrini et al conducted SARS‐CoV‐2 infection experiments using human ES cell‐derived cerebral organoids. 34 The virus infected choroid plexus epithelial cells but hardly infected neurons or glial cells. In addition, in human choroid plexus organoids infected with SARS‐CoV‐2, apolipoprotein J, which is a cerebrospinal fluid (CSF) component, leaked from the inside of the organoid into the medium, recapitulating destruction of the blood‐CSF barrier. Krüger et al conducted SARS‐CoV‐2 infection experiments using human ES cell‐derived intestinal organoids. 29 They found that SARS‐CoV‐2 infects enterocytes, enteroendocrine cells, and paneth cells, but hardly goblet cells. SARS‐CoV‐2 infection experiments using kidney and pancreas models have also been conducted. 28 , 32 Pancreatic alpha and beta cells differentiated from human ES cells express ACE2 and TMPRSS2 and are permissive to SARS‐CoV‐2. 28 Proximal tubular cells and podocytes differentiated from human ES cells express ACE2 and are permissive to SARS‐CoV‐2. 32
Overall, these experiments have clarified which cells are vulnerable to infection and the resulting cellular and organ damage. By utilizing multiple organ organoids, it is anticipated that the mechanisms of the multiple organ failure due to COVID‐19 will be elucidated. Furthermore, by performing long‐term culture of infected human ES/iPS cell‐derived somatic cells and organoids, it will be possible to investigate the state of organs after the virus elimination.
3. COVID‐19 DRUG DEVELOPMENT
RNA‐dependent RNA polymerase inhibitors, such as Remdesivir 39 and EIDD‐2801, 40 are currently being used to inhibit the intracellular genome replication of SARS‐CoV‐2. Remdesivir has already been approved in many countries and administered to many COVID‐19 patients. Huang et al reported that the amount of intracellular viral genome decreased five magnitudes (105) in human iPS cell‐derived AT2‐like cells treated with Remdesivir. 12 Perez‐Bermejo et al showed that pretreating human iPS cell‐derived cardiomyocytes with Remdesivir also reduced the intracellular viral genome five magnitudes. 24 Remdesivir was shown to have an antiviral effect on human ES cell‐derived intestinal organoids, with the intracellular viral genome level reduced four magnitudes. 29
To inhibit the infection of SARS‐CoV‐2, a soluble recombinant protein of human ACE2, which is a SARS‐CoV‐2 receptor, has been used. Monteil et al showed that treating human iPS cell‐derived capillary organoids and human ES cell‐derived kidney organoids with human recombinant soluble ACE2 (hrsACE2) can inhibit SARS‐CoV‐2 infection. 32 Bojkova et al also reported that intracellular viral genome levels in human iPS cell‐derived cardiomyocytes are reduced by recombinant ACE2 treatment. 23 Following these findings, Aperion Biologics is conducting a phase 2 trial on human recombinant soluble ACE2.
Camostat and Nafamostat can inhibit type II transmembrane serine protease (TMPRSS2) to prevent SARS‐CoV‐2 entry. 41 We reported that Camostat treatment reduced the number of viral genome copies found in cell supernatants derived from cryopreserved human bronchial epithelial cell‐derived bronchial organoids to approximately one‐twentieth that in the untreated group. 22 Li et al also reported that Camostat pretreatment reduced the number of viral genome copies found in cell supernatants in human lung organoids derived from lung tissues to approximately one‐tenth that of the untreated group. 19 TMPRSS2 expression is regulated by androgen receptors in human prostate cancer‐derived LNCaP cells. Accordingly, treating LNCaP cells with an antiandrogen agent, enzalutamide, inhibits SARS‐CoV‐2 infection. However, since TMPRSS2 expression is not regulated by androgen receptors in lung organoids, the inhibitory effect of enzalutamide on SARS‐CoV‐2 infection has not been confirmed.
Other SARS‐CoV‐2 entry inhibitors have been discovered in drug screenings using organoids. Han et al screened for FDA‐approved drugs in human ES cell‐derived lung organoids and colonic organoids and found that Imatinib, Mycophenolic acid, and Quinacrine dihydrochloride each inhibit SARS‐CoV‐2 entry. 21 These drugs also showed antiviral effects in humanized mice with human ES cell‐derived lung xenografts.
These studies show the benefits of human ES/iPS cell‐derived somatic cells and organoids in the search for anti‐SARS‐CoV‐2 drugs. In addition, they are expected to assist in searches for anti‐inflammatory drugs. Furthermore, they can be used to evaluate the toxicity and safety of COVID‐19 therapeutic agents.
4. SEARCH FOR GENETIC FACTORS THAT INCREASE THE RISK OF COVID‐19 AGGRAVATION
About 80% of COVID‐19 patients are asymptomatic or mild, but the other 20% become severe. Various factors, such as aging, medical history, racial differences, and genetics, are predicted in COVID‐19 aggravation. 42 , 43 , 44 , 45 Genome‐wide association studies performed on mild and severe COVID‐19 patients found differences in genetic backgrounds. 46 Because human iPS cells can be established from individuals of any genetic background, they make an attractive model to study the relationship between genetic factors and COVID‐19 severity.
The mortality rate of COVID‐19 has been reported to be higher in men than in women. 42 Therefore, we examined whether the gender differences in SARS‐CoV‐2 infection efficiency can be reproduced using human ES/iPS cells 47 (Figure 1A). Because human ES/iPS cells do not express ACE2, the gene was overexpressed. As a result, the copy number of the viral genome in the cell supernatant of male‐derived ES/iPS cells was higher than that of females. Furthermore, male‐derived ES/iPS cells tended to show higher TMPRSS2 expression levels than their female counterparts, suggesting that this difference may contribute to gender differences in SARS‐CoV‐2 infection efficiency.
FIGURE 1.
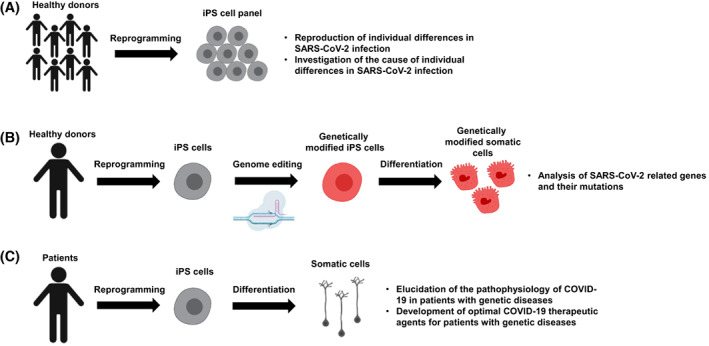
Search for genetic factors that increase the risk of COVID‐19 aggravation. A, An induced pluripotent stem (iPS) cell panel can be used to recapitulate individual differences in SARS‐CoV‐2 infection. B, Genetically modified iPS cells and their derivatives can be used to analyze SARS‐CoV‐2‐related genes. C, Patient‐derived iPS cells can be used to elucidate the pathophysiology and select effective drugs at the individual level. This figure was created using BioRender (https://biorender.com/)
Since genome editing in human ES/iPS cells is relatively efficient, functional analyses of gene mutations related to SARS‐CoV‐2 have also been performed. Dobrindt et al investigated SARS‐CoV‐2 infection in human iPS cells with a single nucleotide polymorphism (SNP) present in the FURIN gene 48 (Figure 1B). They used a CRISPR/Cas9‐based allelic conversion system to generate isogenic human iPS cells that have an SNP at the FURIN locus (rs4702) and confirmed that the expression level of FURIN and the amount of intracellular viral genome was low in human iPS cell‐derived alveolospheres and neurons with SNP rs4702. Wang et al investigated how isoforms of the apolipoprotein E (ApoE) gene affect COVID‐19 aggravation 49 (Figure 1C). The ApoE4 isoform is associated with an increased risk for Alzheimer's disease (AD), but the ApoE3 isoform is not. They generated iPS cells from an AD patient with the ApoE4 isoform and then modified the isoform to ApoE3 using CRISPR/Cas9 technology, finding that neurons derived from unmodified iPS cells were more easily infected than otherwise. In addition, SARS‐CoV‐2 infection significantly shortened the neurite length of ApoE4 iPS cell‐derived neurons, and a large number of fragmented nuclei were observed in astrocytes derived from the same iPS cells. It was also confirmed that the ApoE4 isoforms did not affect the antiviral effect of Remdesivir.
Overall, human ES/iPS cells are being used as models to study individual differences in COVID‐19 severity. Because iPS cells have been established from various populations, the study of individual differences will be accelerated by utilizing established human iPS cell panels and assist in clarifying the risks associated with the severity. Furthermore, by conducting SARS‐CoV‐2 infection experiments using iPS cells for genetic disorders, such as AD, it will be possible to elucidate the pathophysiology and select an appropriate treatment method for different genetic disorders.
5. CONCLUSION AND FUTURE PERSPECTIVES
Human ES/iPS cell‐derived somatic cells and organoids have helped identify how SARS‐CoV‐2 infects cells and causes organ failure. In addition, they have contributed to the development of many therapeutic agents, including Remdesivir and human recombinant soluble ACE2. By comparing the results obtained using these somatic cells and organoids with the results of clinical trials of COVID‐19 therapeutic agents, the clinical predictability of these models can be clarified. Future work can use these somatic cells and organoids to study the SARS‐CoV‐2 life cycle and COVID‐19 pathology and to develop safe and effective therapeutic agents. However, it is still difficult to reproduce the complex pathophysiology of COVID‐19, including cytokine storms, using these models. Immune cells, especially T cells, are known to play an important role in cytokine storms. Coculturing T cells with nonimmune cells, such as alveolar epithelial cells and vascular endothelial cells, may capture cytokine storms in a dish. Although this review introduced the application of these models to COVID‐19 drug discovery, the models are also expected to contribute to regenerative medicine for COVID‐19 patients. In particular, they can be used to generate cells that are less susceptible to SARS‐CoV‐2 infection for transplantation. With further development, human ES/iPS cell‐derived somatic cells and organoids will contribute to the eradication of COVID‐19.
CONFLICT OF INTEREST
A.M.B. declared an advisory role with Agendia, Eisai, Novartis, Genentech, Seattle Genetics, Pfizer, Tyme, Gliead. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.D.: conception and design, manuscript writing; Á.S.‐A., M.M.T., B.D.U., A.M.B.: manuscript writing; K.T.: conception and design, manuscript writing, final approval of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Peter Karagiannis (Kyoto University) for critical reading of the manuscript and Dr. Misaki Ouchida (Kyoto University) for drawing the graphical abstract.
Deguchi S, Serrano‐Aroca Á, Tambuwala MM, Uhal BD, Brufsky AM, Takayama K. SARS‐CoV‐2 research using human pluripotent stem cells and organoids. STEM CELLS Transl Med. 2021;10(11):1491-1499. 10.1002/sctm.21-0183
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang R‐D, Liu M‐Q, Chen Y, et al. Pathogenesis of SARS‐CoV‐2 in transgenic mice expressing human angiotensin‐converting enzyme 2. Cell. 2020;182(1):50‐58.e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winkler ES, Bailey AL, Kafai NM, et al. SARS‐CoV‐2 infection of human ACE2‐transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020;21(11):1327‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muñoz‐Fontela C, Dowling WE, Funnell SG, et al. Animal models for COVID‐19. Nature. 2020;586(7830):509‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artegiani B, Clevers H. Use and application of 3D‐organoid technology. Hum Mol Genet. 2018;27(R2):R99‐R107. [DOI] [PubMed] [Google Scholar]
- 6. Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861‐872. [DOI] [PubMed] [Google Scholar]
- 7. Takayama K. In vitro and animal models for SARS‐CoV‐2 research. Trends Pharmacol Sci. 2020;41(8):513‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. New Eng J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan W‐j, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Eng J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katsura H, Sontake V, Tata A, et al. Human lung stem cell‐based alveolospheres provide insights into SARS‐CoV‐2‐mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell. 2020;27(6):890‐904.e898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang J, Hume AJ, Abo KM, et al. SARS‐CoV‐2 infection of pluripotent stem cell‐derived human lung alveolar type 2 cells elicits a rapid epithelial‐intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962‐973.e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulay A, Konda B, Garcia G Jr, et al. SARS‐CoV‐2 infection of primary human lung epithelium for COVID‐19 modeling and drug discovery. Cell Rep. 2021;35(5):109055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pei R, Feng J, Zhang Y, et al. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS‐CoV‐2 infection. Protein Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamers MM, Mykytyn AZ, Breugem TI, et al. Human airway cells prevent SARS‐CoV‐2 multibasic cleavage site cell culture adaptation. Elife. 2021;10:e66815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Renner DM, Comar CE, et al. SARS‐CoV‐2 induces double‐stranded RNA‐mediated innate immune responses in respiratory epithelial‐derived cells and cardiomyocytes. Proc Natl Acad Sci. 2021;118(16):e2022643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin X, Riva L, Pu Y, et al. MDA5 governs the innate immune response to SARS‐CoV‐2 in lung epithelial cells. Cell Rep. 2021;34(2):108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamers MM, van der Vaart J, Knoops K, et al. An organoid‐derived bronchioalveolar model for SARS‐CoV‐2 infection of human alveolar type II‐like cells. EMBO J. 2021;40(5):e105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li F, Han M, Dai P, et al. Distinct mechanisms for TMPRSS2 expression explain organ‐specific inhibition of SARS‐CoV‐2 infection by enzalutamide. Nat Commun. 2021;12(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Youk J, Kim T, Evans KV, et al. Three‐dimensional human alveolar stem cell culture models reveal infection response to SARS‐CoV‐2. Cell Stem Cell. 2020;27(6):905‐919.e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han Y, Duan X, Yang L, et al. Identification of SARS‐CoV‐2 inhibitors using lung and colonic organoids. Nature. 2021;589(7841):270‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki T, Ito Y, Sakai Y et al. Generation of human bronchial organoids for SARS‐CoV‐2 research. BioRxiv 2020.
- 23. Bojkova D, Wagner JU, Shumliakivska M, et al. SARS‐CoV‐2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res. 2020;116(14):2207‐2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez‐Bermejo JA, Kang S, Rockwood SJ, et al. SARS‐CoV‐2 infection of human iPSC‐derived cardiac cells reflects cytopathic features in hearts of patients with COVID‐19. Sci Transl Med. 2021;13:eabf7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia G Jr, Sharma A, Ramaiah A, et al. Antiviral drug screen identifies DNA‐damage response inhibitor as potent blocker of SARS‐CoV‐2 replication. Cell Rep. 2021;35(1):108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchiano S, Hsiang T‐Y, Khanna A, et al. SARS‐CoV‐2 infects human pluripotent stem cell‐derived cardiomyocytes, impairing electrical and mechanical function. Stem Cell Rep. 2021;16(3):478‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mills RJ, Humphrey SJ, Fortuna PR, et al. BET inhibition blocks inflammation‐induced cardiac dysfunction and SARS‐CoV‐2 infection. Cell. 2021;184(8):2167‐2182.e2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang L, Han Y, Nilsson‐Payant BE, et al. A human pluripotent stem cell‐based platform to study SARS‐CoV‐2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27(1):125‐136.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krüger J, Groß R, Conzelmann C, et al. Drug inhibition of SARS‐CoV‐2 replication in human pluripotent stem cell–derived intestinal organoids. Cell Mol Gastroenterol Hepatol. 2021;11(4):935‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mithal A, Hume AJ, Lindstrom‐Vautrin J, et al. Human pluripotent stem cell‐derived intestinal organoids model SARS‐CoV‐2 infection revealing a common epithelial inflammatory response. Stem Cell Rep. 2021;16(4):940‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wysocki J, Ye M, Hassler L, et al. A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS‐CoV‐2 infection in human kidney organoids. J Am Soc Nephrol. 2021;32(4):795‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181(4):905‐913.e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS‐CoV‐2 in human and mouse brain. J Exp Med. 2021;218(3):e20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pellegrini L, Albecka A, Mallery DL, et al. SARS‐CoV‐2 infects the brain choroid plexus and disrupts the blood‐CSF barrier in human brain organoids. Cell Stem Cell. 2020;27(6):951‐961.e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacob F, Pather SR, Huang W‐K, et al. Human pluripotent stem cell‐derived neural cells and brain organoids reveal SARS‐CoV‐2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27(6):937‐950.e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID‐19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19. New Eng J Med. 2020;383(19):1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cox RM, Wolf JD, Plemper RK. Therapeutically administered ribonucleoside analogue MK‐4482/EIDD‐2801 blocks SARS‐CoV‐2 transmission in ferrets. Nat Microbiol. 2021;6(1):11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin J‐M, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu L, She Z‐G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077.e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID‐19 on black communities. Ann Epidemiol. 2020;47:37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Severe Covid‐19 GWAS Group . Genomewide association study of severe Covid‐19 with respiratory failure. New Eng J Med. 2020;383(16):1522‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sano E, Deguchi S, Sakamoto A, et al. Modeling SARS‐CoV‐2 infection and its individual differences with ACE2‐expressing human iPS cells. iScience. 2021;24(5):102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dobrindt K, Hoagland DA, Seah C, et al. Common genetic variation in humans impacts in vitro susceptibility to SARS‐CoV‐2 infection. Stem Cell Rep. 2021;16(3):505‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang C, Zhang M, Garcia G Jr, et al. ApoE‐isoform‐dependent SARS‐CoV‐2 neurotropism and cellular response. Cell Stem Cell. 2021;28(2):331‐342.e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


