Abstract
Spoligotyping has been suggested as a screening test in multistep genotyping of Mycobacterium tuberculosis strains. Relying on restriction fragment length polymorphism (RFLP) analysis with IS6110 (IS6110 RFLP analysis) as a “gold standard,” we performed a comparative evaluation of spoligotyping and ligation-mediated PCR (LMPCR), a recently described PCR-based typing method, as rapid screening tests for fingerprinting of 158 M. tuberculosis strains collected in Verona, Italy. LMPCR seemed to be comparable to spoligotyping in terms both of feasibility with rapidly extracted DNA and of generation of software-analyzable images. Moreover, LMPCR grouped considerably fewer strains than spoligotyping (38 versus 67%) and was found to reduce the cluster overestimation rate (26.3 versus 58%) and to give a better discriminatory index (0.992 versus 0.970) compared to spoligotyping. In our geographical region, where there was no evidence of clustered strains carrying fewer than six IS6110 copies, LMPCR was found to be more discriminatory than spoligotyping. We also evaluated two models of three-step typing strategies, involving the use of spoligotyping and LMPCR as screening methods and IS6110 RFLP analysis as a further supporting test. LMPCR proved to be a more effective first-step test than spoligotyping, significantly reducing the need for subtyping. LMPCR should be considered an alternative to spoligotyping as a rapid screening method for M. tuberculosis fingerprinting, particularly in areas with a low prevalence of M. tuberculosis strains carrying few copies of IS6110.
Mycobacterial strain typing by means of molecular methods has become an important instrument for tuberculosis surveillance, control, and prevention (25). The insertion sequence IS6110 has great utility for the identification of restriction fragment length polymorphisms (RFLPs) (13, 24) in Mycobacterium tuberculosis isolates, since the element is usually present in multiple copies and in different locations within the genome (20, 21). RFLP analysis with IS6110 (IS6110 RFLP analysis) has been standardized through the use of PvuII as the restriction enzyme for genomic digestion and of a sequence located on the 3′ side of IS6110 as the probe (23). This standardized IS6110 RFLP analysis is considered to be the reference method for M. tuberculosis fingerprinting worldwide (1, 4, 6).
IS6110 RFLP analysis, albeit highly discriminatory, is laborious, requiring many technical steps and several micrograms of chromosomal DNA (22, 23). Moreover, some M. tuberculosis strains cannot be discriminated by this method if they lack IS6110 or have low IS6110 copy numbers (27).
To avoid the lengthy delay in obtaining the typing results and to enhance the discriminatory power for low-copy-number isolates, alternative PCR-based methods have been developed (5, 11, 14, 16, 17, 22).
In this context, spoligotyping (9) has recently aroused great interest. This method is based on the detection of polymorphisms in the chromosomal direct repeat (DR) locus, which contains a variable number of short DR sequences interspersed with nonrepetitive spacers. It was found to be easy, rapid, and suitable for use in the computer-assisted analysis of many molecular patterns at the same time, thus allowing its use in large-scale epidemiological surveys (2, 3).
However, spoligotyping has shown a discriminatory ability lower than that of IS6110 RFLP analysis with the exception of that for M. tuberculosis isolates with low IS6110 copy numbers (1–3, 6, 18). This method is currently proposed as the initial screening step in a multistep typing strategy for epidemiological studies (2, 19). Whenever different spoligotypes are identified, these always correspond to different IS6110 RFLP patterns, whereas grouped spoligotypes require confirmation by subtyping. The latter may be carried out by IS6110 RFLP analysis either directly or after an intermediate analysis by an IS6110-based PCR method in order to further limit the use of IS6110 RFLP analysis (2). Amplification-based methods with other genetic markers, like double-repetitive-element PCR, have also been proposed as second-step tests to integrate the spoligotyping screening analysis (19).
However, to the best of our knowledge none of the PCR-based typing methods has so far been directly compared to spoligotyping with regard to its feasibility and discriminatory power as a rapid screening method. Recently, an IS6110-based PCR (26) was found to be more discriminatory than spoligotyping as a first-line test when used in the analysis of small groups of epidemiologically linked isolates, but its use for large-scale screening is discouraged because of nonspecific priming problems.
Prod’hom et al. (15) recently described a ligation-mediated PCR (LMPCR) method for the amplification of a flanking sequence located on the 5′ side of IS6110. This method gives rise to molecular patterns with well-resolved bands, seems to be both technically simple and reproducible, and was proposed as a molecular epidemiological tool. No data are available on the discriminatory ability of LMPCR compared to those of other PCR methods (particularly spoligotyping), and there is not yet any experience with its use as a part of a multistep typing strategy.
Relying on IS6110 RFLP analysis as the “gold standard,” we performed a comparative evaluation of LMPCR and spoligotyping as screening typing methods for clinical M. tuberculosis isolates and we evaluated the possible use of LMPCR in a multistep genotyping strategy.
MATERIALS AND METHODS
Mycobacterial strains.
The 158 M. tuberculosis strains analyzed in this study were collected from 156 patients at the Laboratory of Microbiology of the Ospedale Maggiore in Verona, Italy, between 1 January 1996 and 31 December 1997. This laboratory serves as a mycobacteriological reference center for the city of Verona. Each strain corresponded to a single patient with tuberculosis, including two patients with relapses of pulmonary tuberculosis. The strains were isolated on Lowenstein-Jensen medium. The identification of the strains as M. tuberculosis was based on standard microbiological tests and was confirmed by a DNA-RNA hybridization technique (AccuProbe; Gen-Probe Incorporated, San Diego, Calif.).
DNA fingerprinting by PCR-based methods.
All the strains were analyzed by spoligotyping and LMPCR. The DNA of each strain was extracted by transferring some colonies from the Lowenstein-Jensen medium in 150 μl of Tris-EDTA buffer and heating at 80°C for 30 min with no further DNA purification, as described previously for spoligotyping (2).
(i) Spoligotyping.
Spoligotyping was performed as described elsewhere (9). Briefly, the DR region was amplified by PCR with oligonucleotide primers derived from the DR sequence. The labelled PCR product was used as a probe to hybridize with 43 synthetic spacer oligonucleotides attached to a carrier membrane (Isogen Bioscience B.V., Maarsen, The Netherlands).
(ii) LMPCR.
LMPCR was carried out as described by Prod’hom et al (15). Briefly, 17 μl of the heat-treated cell suspension was added to a SalI enzyme solution in order to digest the genomic DNA of M. tuberculosis. Then, after visual control on 0.8% agarose gels, the digestion products were ligated to an asymmetrical, double-stranded linker. This linker was constructed by annealing two nonphosphorylated oligonucleotides by incubation at temperatures that decreased from 80 to 4°C (1°C each min). After ligation, T4 DNA ligase was heat inactivated. The samples were then digested with SalI for 15 min to cleave any remaining restriction sites resulting from partial genomic digestion or regeneration through ligation. Template DNA was amplified as described above, and the PCR products were separated in 2.5% agarose gels and photographed with a Polaroid MP-4 Land Camera. A study strain was included in every procedure as an internal standard to check the reproducibilities of the fingerprints.
IS6110 RFLP fingerprinting.
All the strains included in clusters by one or both of the two PCR-based typing methods were subjected to standard IS6110 RFLP fingerprinting. DNA extraction, Southern blotting, hybridization, and detection were performed as described previously (23).
Computer-assisted analysis of fingerprints.
The molecular patterns obtained by the three typing methods were submitted to computer-assisted analysis to detect the clustered strains.
The software-assisted evaluation of spoligotypes was done with GelCompar software, version 3.1b (Applied Maths, Kortrijk, Belgium), as described previously (2). Briefly, a transmission scanner was used to record the autoradiographic images, and the software classified the strains as grouped if their patterns were scored as identical. These spoligotypes were definitively considered to be clustered after checking by direct visual control.
The analysis of the IS6110-based fingerprints (those obtained by RFLP analysis and LMPCR) was performed with the Taxotron package (Taxolab Software; Institut Pasteur, Paris, France), which includes the RestrictoScan, RestrictoTyper, Adanson, and Dendrograph programs. The procedure was similar for both typing methods. The autoradiographs of the blots probed with IS6110 and the photographs of the LMPCR gels were captured by a ScanJet II cx (Hewlett-Packard) scanner. The bands were detected with RestrictoScan, and the degree of similarity of the fingerprinting patterns was calculated as the Dice index (7) by using the RestrictoTyper program, with a linear error tolerance ranging from 3.5 to 5% in proportion to the sizes of the bands. The relationships among isolates were assessed by the unweighted pair group method of averages (10) with the Adanson program. A dendrogram was generated by the Dendrograph program, and the patterns that were identified as similar by the computer analysis were compared visually. The strains were classified as clustered if the numbers and molecular sizes of the bands were identical.
Measures of discriminatory power.
The overestimation rate (OR) of clustered strains by the PCR-based methods was defined as the percentage of clustered strains whose clonality was not supported by IS6110 RFLP analysis.
The discriminatory indices (DIs) of the typing methods, which express the probability of whether two unrelated strains are characterized as the same type, were calculated by the equation of Hunter and Gaston (8).
Although only strains grouped by one or both of the PCR-based methods were subjected to IS6110 RFLP analysis, the clustering percentage and DI of the latter were calculated by referring to the total number of strains studied and by assuming that the unique spoligotyping and LMPCR patterns of the remaining strains corresponded to unique IS6110 RFLP patterns (1–3, 9, 15).
RESULTS
LMPCR and spoligotyping included in clusters 38.6% (61 of 158) and 67.7% (107 of 158) of the strains, respectively (Table 1). Eight strains that were grouped in clusters by LMPCR were not clustered by spoligotyping, while 54 strains that were clustered by spoligotyping were not grouped by LMPCR. IS6110 RFLP analysis was performed with the 115 strains clustered by one or both of these rapid methods. The clonality by IS6110 RFLP analysis was not at variance with the clonality by LMPCR and spoligotyping for 45 strains, representing 42 and 73.7% of strains initially included in clusters by spoligotyping and LMPCR, respectively. For strains from both the patients with relapses, the three typing methods produced the same molecular patterns produced for strains from the first episodes. The IS6110 RFLP patterns contained between 6 and 19 bands (median, 9 bands). The LMPCR patterns for the same 115 strains ranged from one to eight bands (median, four bands) with molecular sizes of between 100 and 1,800 bp.
TABLE 1.
Cluster analysis and discriminatory power evaluation of the three typing methods
| Typing method | No. of patterns identified | No. of strains clustered/total no. of strains (%) | No. of clusters | Mean no. of strains/ cluster (range) | OR (%) | DI |
|---|---|---|---|---|---|---|
| Spoligotyping | 75 | 107/158 (67.7) | 22 | 4.8 (2–16) | 58.0 | 0.970 |
| LMPCR | 119 | 61/158 (38.6) | 20 | 3.0 (2–9) | 26.3 | 0.992 |
| IS6110 RFLPa | 131 | 45/158 (28.4) | 16 | 2.8 (2–8) | NDb | 0.995 |
IS6110 RFLP analysis was performed with 115 strains clustered by one or both of the PCR-based typing methods and supported the clonality of 45 strains. By IS6110 RFLP cluster analysis and DI calculation, this value was referred to the total number (n = 158) of study strains, assuming that the unique spoligotyping and LMPCR patterns of the remaining 43 strains corresponded to unique IS6110 RFLP patterns.
ND, not determined.
The OR was 58% for spoligotyping and 26.3% for LMPCR (Table 1). The DI was higher for LMPCR than for spoligotyping (0.992 versus 0.970) (Table 1).
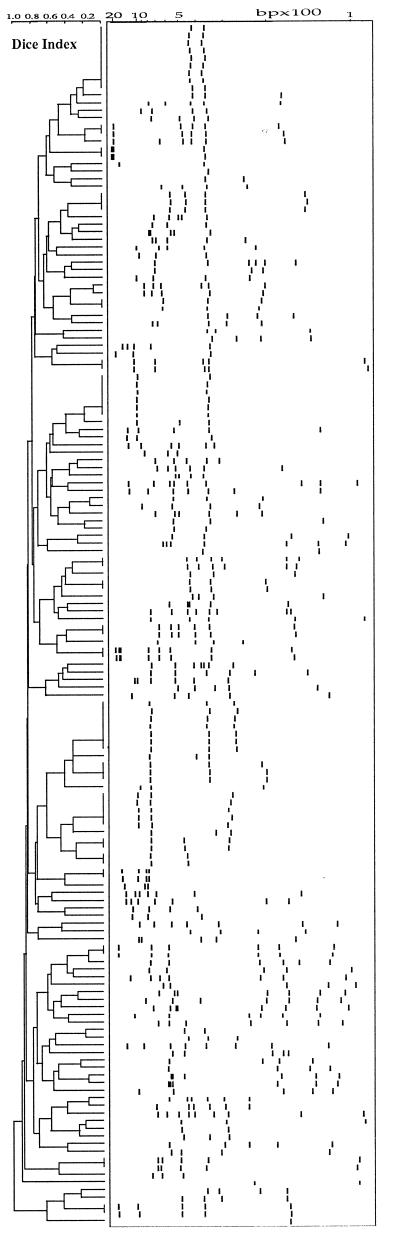
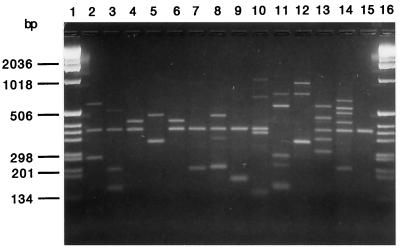
Figures 1 and 2 show the dendrograms obtained after computer-assisted analysis of the spoligotyping and LMPCR fingerprints, respectively. Figure 3 shows a representative gel view of the LMPCR patterns.
FIG. 1.
Spoligotype dendrogram generated for the 158 M. tuberculosis strains and the corresponding patterns after computer analysis with GelCompar software. The scale on the left indicates the band-based similarity coefficients.
FIG. 2.
Dendrogram and associated schematic representation of LMPCR patterns of the 158 M. tuberculosis strains after computer analysis with the Taxotron package. The scale on the left indicates the Dice index, which was used to compare the patterns with a linear tolerance error between 3.5 and 5%.
FIG. 3.
Agarose gel (2.5%) electrophoresis of amplified M. tuberculosis DNA obtained by the LMPCR method. Lanes 1 and 16, molecular size marker (1-kb ladder; Gibco); lanes 2 to 15, M. tuberculosis study strains (strains 4 and 6 were grouped in the same cluster).
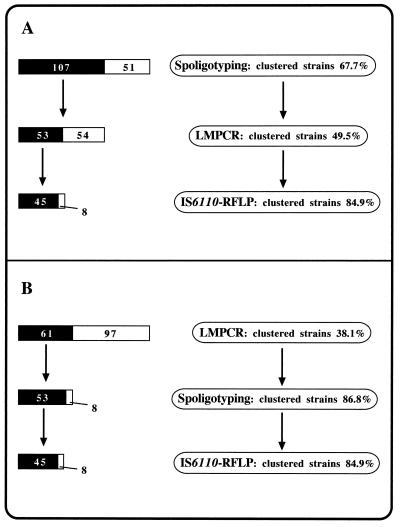
Figure 4 summarizes two models of the sequential use of the different typing methods that we used. In one model (Fig. 4A), LMPCR clustered 49.5% (53 of 107) of strains initially grouped by spoligotyping. In the other model (Fig. 4B), spoligotyping following LMPCR screening supported the grouping of 86.8% (53 of 61) of isolates clustered by LMPCR. In both cases IS6110 RFLP analysis, as a last-step test, had to be performed with 53 strains and supported the clonality of 45 (84.9%) of them. For the strains clustered by LMPCR, the eight strains differentiated by spoligotyping (Fig. 4B) did not correspond to the eight strains split by IS6110 RFLP analysis (Fig. 4A).
FIG. 4.
Two models of sequential use of the three typing methods used to analyze the 158 M. tuberculosis strains. (A) Spoligotyping is the first-step test; (B) LMPCR is the screening method. In both cases IS6110 RFLP analysis is considered the most discriminatory test, following double-step typing by the PCR-based methods. For each step, the black and white bars on the left represent the fraction of clustered isolates and the fraction of isolates with unique patterns, respectively, while the percentages of clustered strains are shown on the right.
DISCUSSION
In this study LMPCR proved to be a simple and rapid method for M. tuberculosis strain discrimination. The typing process can easily be completed in 8 to 10 h, including the time for DNA extraction, and has few technical steps. Even though high-quality mycobacterial DNA was previously thought to be critical for LMPCR (15), in this study good technical results were obtained by use of the same rapid DNA extraction procedure recommended for spoligotyping (heating a suspension of a few colonies in Tris-EDTA buffer with no further DNA purification) (2). In fact, although the difference in the median number of bands between IS6110 RFLP patterns and the corresponding LMPCR results was found to be higher than that reported by Prod’hom et al. (15) (five versus three), the rapid DNA extraction procedure did not significantly affect the feasibility of use of the LMPCR method or the reproducibilities of the molecular patterns. The bands showed the same intensities and the same molecular size ranges as those reported previously. (15). Capture of gel images by means of a scanner and computer-assisted analysis of fingerprints (Fig. 2) were possible, providing essential assistance in the evaluation of the large number of isolates. Therefore, LMPCR, like spoligotyping, seems to be useful not only for comparing selected isolates side by side in the same PCR run, as are other IS6110-based PCR methods with nonspecific priming problems (26), but also for the rapid screening of large numbers of isolates.
Furthermore, we compared the discriminatory powers of LMPCR and spoligotyping by relying on IS6110 RFLP analysis as a reference method. To the best of our knowledge this represents the first comparative evaluation of discriminatory ability between spoligotyping and another PCR-based typing method for the screening of isolates from a given geographical area. LMPCR was found to cluster considerably fewer strains than spoligotyping (38.6 versus 67.7%). The OR, considered the fraction of clustered isolates whose clonality was not supported by IS6110 RFLP analysis, appeared to be higher for spoligotyping (58%) than for LMPCR (26.3%). The spoligotyping ORs, calculated from previous studies in which the same RFLP identity criteria considered here (indistinguishable patterns) were used (1, 2, 6, 12), ranged from 31 to 58%. Our spoligotyping OR also fell in this range. Even if in some of these studies there was evidence of spoligotyping ORs lower than that in our study, the LMPCR OR (26.3%) was nevertheless below the range of values recorded for spoligotyping.
Similar evidence emerged by evaluating the DIs, the mathematical values of the probability that two unrelated strains will be characterized as the same type (8). DIs were higher for LMPCR (0.992) than for spoligotyping (0.970), further indicating LMPCR’s better discriminatory ability. Moreover, our spoligotyping DI fell in the range of values calculated from previous studies (0.933 to 0.977) (1–3, 6, 12, 18), providing an indirect methodological validation.
An integrated use of different typing methods was proposed in order to overcome the technical difficulties and the limits of discrimination of each one (2, 17). Spoligotyping was suggested for screening followed by PCR-based methods or, directly, IS6110 RFLP analysis (2, 19). We evaluated two models of multistep typing strategies involving the use of the three typing methods considered in the present study. In the first (Fig. 4A) we considered spoligotyping as a first-step method (as so far suggested), while we kept LMPCR in a second-line role. LMPCR appeared to split about 50% of the isolates initially clustered by spoligotyping, significantly reducing the need for subsequent IS6110 RFLP typing. In the second model (Fig. 4B), based on the evidence of the higher discriminatory ability of LMPCR and on the feasibility of a software-assisted analysis of patterns, the IS6110-based rapid method was used for first-line screening. In this approach LMPCR showed a discriminatory ability which made further intermediate analysis by spoligotyping useless, since the latter was able to split a limited number of clustered isolates (8 of 61; 13.2%) and did not significantly limit the need for subsequent IS6110 RFLP typing. Thus, LMPCR appeared to be more effective than spoligotyping as a screening method.
However, in our study the lack of grouped strains containing few or no copies of IS6110 raises an issue to be considered. The usefulness of spoligotyping is thought to increase proportionally to the presence of clustered strains carrying low numbers of IS6110 copies (2, 9). In countries where this is an infrequent occurrence (e.g., developed countries), the better ability of LMPCR for initial discrimination should remain confirmed, while spoligotyping could still be considered a second-line method. On the other hand, in countries where these strains are largely diffused, such as in Southeast Asia (27), spoligotyping should still be considered the most suitable screening method.
In conclusion, LMPCR was found to be an easy and rapid method for M. tuberculosis genotyping and was more discriminatory than spoligotyping. We believe that LMPCR should be considered an alternative to spoligotyping for screening, particularly in countries with a low prevalence of M. tuberculosis strains carrying few copies of IS6110.
ACKNOWLEDGMENTS
We are grateful to Yves-Olivier Goguet de la Salmonière for advice on the use of GelCompar software. We thank Anne Varnerot for helpful technical assistance.
M.C. Gutiérrez was a postdoctoral fellow from the Ministerio de Educaciòn y Cultura, Madrid, Spain. This work was partly financed by the National Tuberculosis Project (grant 96/D/T50) from Istituto Superiore di Sanità, Ministero della Sanità, Rome, Italy.
REFERENCES
- 1.Díaz R, Kremer K, de Haas P E W, Gómez R I, Marrero A, Valdivia J A, van Embden J D A, van Soolingen D. Molecular epidemiology of tuberculosis in Cuba outside of Havana, July 1994–June 1995: utility of spoligotyping versus IS6110 restriction fragment length polymorphism. Int J Tuberc Lung Dis. 1998;2:743–750. [PubMed] [Google Scholar]
- 2.Goguet de la Salmoniére Y O, Li H M, Torrea G, Bunschoten A, van Embden J, Gicquel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Saunders N A, van Embden J D A, Young D B, Shaw R J. Differentiation of Mycobacterium tuberculosis isolates by spoligotyping and IS6110 restriction fragment length polymorphism. J Clin Microbiol. 1997;35:647–651. doi: 10.1128/jcm.35.3.647-651.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutiérrez M C, Vincent V, Aubert D, Bizet J, Gaillot O, Lebrun L, Le Pendeven C, Le Pennec M P, Mathieu D, Offredo C, Pangon B, Pierre-Audigier C. Molecular fingerprinting of Mycobacterium tuberculosis and risk factors for tuberculosis transmission in Paris, France, and surrounding area. J Clin Microbiol. 1998;36:486–492. doi: 10.1128/jcm.36.2.486-492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas W H, Butler W R, Woodley C L, Crawford J T. Mixed-linker polymerase chain reaction: a new method for rapid fingerprinting of isolates of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1993;31:1293–1298. doi: 10.1128/jcm.31.5.1293-1298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horgen L, Sola C, Devallois A, Goh K S, Rastogi N. Follow up of Mycobacterium tuberculosis transmission in the French West Indies by IS6110-DNA fingerprinting and DR-based spoligotyping. FEMS Immunol Med Microbiol. 1998;21:203–210. doi: 10.1111/j.1574-695X.1998.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 7.Hubalek Z. Coefficients of association and similarity, based on binary (presence-absence) data: an evaluation. Biol Rev Camb Philos Soc. 1982;57:669–689. [Google Scholar]
- 8.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W H. Simple method for constructing phylogenetic trees from distance matrices. Proc Natl Acad Sci USA. 1981;78:1085–1089. doi: 10.1073/pnas.78.2.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linton C J, Jalal H, Leeming J P, Millar M R. Rapid discrimination of Mycobacterium tuberculosis strain by random amplified polymorphic DNA analysis. J Clin Microbiol. 1994;32:2169–2174. doi: 10.1128/jcm.32.9.2169-2174.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.March F, Coll P, Costa R, Rodríguez P, Moreno C, Garrigó M, Prats G. Utilidad de DR, PGRS y spoligotyping en tipificación de Mycobacterium tuberculosis. Comparación con IS6110. Enferm Infecc Microbiol Clin. 1996;14:160–166. [PubMed] [Google Scholar]
- 13.Otal I, Martin C, Vincent-Lévy-Frébault V, Thierry D, Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991;29:1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel S, Wall S, Saunders N A. Heminested inverse PCR for IS6110 fingerprinting of Mycobacterium tuberculosis strains. J Clin Microbiol. 1996;34:1686–1690. doi: 10.1128/jcm.34.7.1686-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prod’hom G, Guilhot C, Gutiérrez M C, Varnerot A, Gicquel B, Vincent V. Rapid discrimination of Mycobacterium tuberculosis complex strains by ligation-mediated PCR fingerprint analysis. J Clin Microbiol. 1997;35:3331–3334. doi: 10.1128/jcm.35.12.3331-3334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross B C, Dwyer B. Rapid, simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993;31:329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sechi L A, Zanetti S, Duprè I, Delogu G, Fadda G. Enterobacterial repetitive intergenic consensus sequences as molecular targets for typing of Mycobacterium tuberculosis strains. J Clin Microbiol. 1998;36:128–132. doi: 10.1128/jcm.36.1.128-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sola C, Horgen L, Devallois A, Rastogi N. Combined numerical analysis based on the molecular description of Mycobacterium tuberculosis by four repetitive sequence-based DNA typing systems. Res Microbiol. 1998;149:349–360. doi: 10.1016/s0923-2508(98)80440-3. [DOI] [PubMed] [Google Scholar]
- 19.Sola C, Horgen L, Maïsetti J, Devallois A, Goh K S, Rastogi N. Spoligotyping followed by double-repetitive-element PCR as rapid alternative to IS6110 fingerprinting for epidemiological studies of tuberculosis. J Clin Microbiol. 1998;36:1122–1124. doi: 10.1128/jcm.36.4.1122-1124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thierry D, Brisson-Noël A, Vincent-Lévy-Frébault V, Nguyen S, Guesdon J-L, Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence IS6110 and its application in diagnosis. J Clin Microbiol. 1990;28:2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thierry D, Cave M D, Eisenach K D, Crawford J T, Bates J H, Gicquel B, Guesdon J L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990;18:188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrea G, Offredo C, Simonet M, Gicquel B, Berche P, Pierre-Audigier C. Evaluation of tuberculosis transmission in a community by 1 year of systematic typing of Mycobacterium tuberculosis clinical isolates. J Clin Microbiol. 1996;34:1043–1049. doi: 10.1128/jcm.34.5.1043-1049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R A, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen D. Utility of molecular epidemiology of tuberculosis. Eur Respir J. 1998;11:795–797. doi: 10.1183/09031936.98.11040795. [DOI] [PubMed] [Google Scholar]
- 26.Wilson S M, Gross S, Drobniewski F. Evaluation of strategies for molecular fingerprinting for use in the routine work of a mycobacterium reference unit. J Clin Microbiol. 1998;36:3385–3388. doi: 10.1128/jcm.36.11.3385-3388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen L K W, Ross B, Jackson K, Dwyer B. Characterization of Mycobacterium tuberculosis strains from Vietnamese patients by Southern blot hybridization. J Clin Microbiol. 1993;31:1615–1618. doi: 10.1128/jcm.31.6.1615-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]