Abstract
As our life expectancy increases, specific medical conditions appear, and new challenges are met in terms of global health. Frailty has become a medical and scientific concept to define pathologies where inflammation, depressed immune system, cellular senescence, and molecular aging converge. But more importantly, frailty is the ultimate cause of death that limits our life span and deteriorates health in an increasing proportion of the world population. The difficulty of tackling this problem is the combination of factors that influence frailty appearance, such as stem cells exhaustion, inflammation, loss of regeneration capability, and impaired immunomodulation. To date, multiple research fields have found mechanisms participating in this health condition, but to make progress, science will need to investigate frailty with an interdisciplinary approach. This article summarizes the current efforts to understand frailty from their processes mediated by inflammation, aging, and stem cells to provide a new perspective that unifies the efforts in producing advanced therapies against medical conditions in the context of frailty. We believe this approach against frailty is particularly relevant to COVID‐19, since people in a state of frailty die more frequently due to the hyperinflammatory process associated with this infection.
Keywords: aging, COVID‐19, frailty, immunomodulation, inflammation, mesenchymal stem cell, MSC, regeneration, stem cell exhaustion
Frailty induced inflammation feedbacks disrupting the immune system, tissue repair, and homeostasis. Stem cell exhaustion is one process that mediates frailty reducing immunomodulatory secreted factors, the number of immune cells, and repair capabilities as limited source of stem new differentiating cells. The compromise of these three processes favors chronic inflammation processes that feedback on frailty features through oxidative metabolism and a cytokine cascade. Warm color represents inflammation intensity.
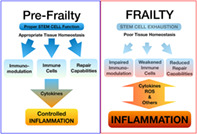
Significance statement.
In recent years, the authors have assisted with important advances in the understanding of stem cell exhaustion as a wide mechanism of disease responsible for age‐related health deterioration. On the other hand, the stem cell role in immunomodulation has emerged as one of the most promising effects of stem cell therapy targeting the inflammatory process. These two separate topics are now emerging under a common theme and need to be addressed as a whole. The present study intends to put in perspective the common ground among these concepts and offers a future perspective of the next steps in the search for therapeutic approaches to frailty.
1. INTRODUCTION
There is no doubt that infection with SARS‐CoV‐2, the coronavirus responsible for COVID‐19, causes a high mortality rate in the elderly, especially among men presenting age‐related comorbidities. 1 , 2 This has been easily justified by age‐related pathologies, which are inherently associated with systemic and subclinic chronic inflammation (inflammaging) and acquired immune system impairment (immunosenescence). These age‐related mechanisms follow four age‐related medical events that are associated with increased mortality 3 : (a) systemic inflammation, (b) a blunted acquired immune system, (c) downregulation of ACE2, and (d) accelerated molecular aging. These are all well‐established correlations that we unfortunately do not understand enough to develop specific and more efficient therapies. In an effort to understand these factors, the frailty concept was coined. Frailty (from the Latin “fragilis” means “easily broken”) is defined as a clinical syndrome in which several of the following criteria are present: rapid weight loss, self‐reported exhaustion, weakness (measured by grip strength), slow walking speed, and limited physical activity. 4 However, the “frailty” term was used first by Vaupel and colleagues 5 as a way to “measure” the variability in the risk of death among people of the same age. 6 From a more mechanistic point of view, frailty begins when aged individuals deteriorate following what some have called the seven pillars of aging 7 or the nine hallmarks of aging. 8 These can be summarized as genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These aging processes are interconnected and thus some can induce others. In this review, we focus on stem cell exhaustion, a well described process during regeneration but less clear regarding frailty. We also discuss stem cell therapies as one of the few modern strategies that has promised to tackle this stem cell dysfunction, not only as a therapy for frailty but also related emerging diseases like COVID‐19.
2. MESENCHYMAL STEM CELLS, MORE THAN A TRADEMARK
Since the discovery of pluripotency by Thompson and colleagues in early human blastocyst stem cells (embryonic stem cells, ESCs) and their abilities to differentiate into the three blastodermic lineages, 9 there has been an explosion in stem cell research for therapeutic strategies and even the emergence of a new discipline, regenerative medicine, considered by some as a new medical field called Regenology. 10 This race for stem cell applications has been a “roller coaster” with more wishes than clinic realities, but after 20 years of research, the field is finally obtaining solid results through several approaches. After the discovery of ESC pluripotency, the field also turned to the adult stem cells (ASCs), tissue specific stem cells that show a more restricted lineage. These cells respond to tissue damage through life by proliferation and differentiation, suffering telomere attrition, and finally reduction of the proliferative capacity. Inside this group, hematopoietic stem cells (HSCs), the origin of hematopoietic lineages and mesenchymal stem cell (MSC), and the origin of mesodermal lineages are the most investigated and have more clinical applications under development. A third strategy arose from induced pluripotent stem cells (iPSCs), which also show the ability to differentiate into several lineages but are obtained from differentiated adult somatic cells through activation of Yamanaka's transcription factors: Oct4, Sox2, Klf4, and Myc. 11
Among all the ASC types, MSCs were the first to be described and used for regenerative studies, although, their applications have always been a subject of an intense debate and controversy. 12 They were described and named by Caplan 13 , 14 when HSC were the only adult stem cells known to science, but controversy began even from the moment in which the term MSC was coined. Nowadays, the acronym MSC can also mean “marrow/multipotent/mesodermal, stromal/stem, cells,” according to criteria of location, power, embryonic origin, morphological features, and so forth. Finally, Caplan chose the name “mesenchymal stem cell” because mesenchyme is a tissue characterized by weakly associated unpolarized cells with an abundant extracellular matrix, and for their in vitro clonability and multipotency. 15 Even so, recently, Caplan proposed to adapt the meaning of MSC to “medicinal signaling cell,” saving the widely used acronym but providing to these controversial cells a new clinical feature from its paracrine function by which these cells are able to repair and immunomodulate upon injury or inflammation through their secretory signals. 16 Nevertheless, mesenchymal stromal/stem cell are the most widely used.
Perhaps before abandoning its mesenchymal name, it would be convenient to look deeper into the mesenchymal area of the human body, because even if MSCs are called differently, their function still happens in that specific tissue environment. This widely located compartment is able to reach and expand virtually through the whole body and its ubiquitous presence provides a place for the immune system to act and propagates signals that trigger the inflammatory response.
The mesenchyme is generated as an embryonic tissue, with features of loose connective, with an abundant and hydrated extracellular matrix made of thin fibers and well‐spread unspecialized cells. It is mesodermal in origin and gives rise to cardiovascular, musculoskeletal, mesothelial, and lymphatic systems. Thus, if we try to understand the mechanisms that rule the immunological response, we must understand its physiopathological context through its wide distribution in the organism.
3. MESENCHYME AND INFLAMMATION
Inflammation (from Latin: inflammatio: kindling or setting on fire) is a common manifestation of many diseases. In fact, it is an unspecific response against environmental aggressions. Interestingly, inflammation only happens in vascularized connective tissues with the goal of isolating and removing harmful stimuli and inducing damaged tissue repair. It is considered an innate immune mechanism that works similarly in most situations in contrast with the adaptive immune response, specific to each situation. 17
Our current medical knowledge indicates that aging correlates with chronic systemic inflammation, even if it does not always show clinical manifestations. This has been called inflammatory aging and it is accompanied by a deterioration of the immune system, also called immunosenescence. 3 , 18 Thus, aging mediates an alteration of the previously described “mesenchymal territory” that predisposes to chronic inflammation by failure of the mechanisms that maintain the homeostatic state of early ages. Interestingly, this happens more often in men than women, 19 which correlates with the higher mortality rates of males in medical diseases, such as COVID‐19. 2
At the molecular level, MSCs are known to mediate inflammation; however, two MSC phenotypes can switch between proinflammatory and anti‐inflammatory regulation. So, a key aspect of MSC use in inflammation is to correctly manipulate MSCs to favor an anti‐inflammatory effect. A possible approach is explored by Mounayar and colleagues showing that PI3K and STAT1 signaling can balance MSCs into an anti‐inflammatory state in response to IFNγ by inducing indoleamine 2,3‐dioxygenase (IDO 20 ). Other homeostasis factors like Toll‐like receptor (TLR4) or AKT‐independent FOXO3 signaling pathways have shown similar participation in the MSC2 state induction. 21 , 22 This less explored aspect of the MSC induced anti‐inflammatory phenotype could hide the key to the successful use of MSCs against inflammation.
Another possible application of MSCs as an anti‐inflammatory strategy could be for autoimmune diseases. Fiorina and colleagues analyzed the immunomodulatory effect of bone marrow‐derived MSCs on autoimmune diabetes concluding that MSCs from healthy animals expressed higher levels of negative co‐stimulatory PD‐L1 signal and promoted an immune response shifted toward a Th2 cytokine profile. 23
4. THE IMMUNOMODULATORY ROLES OF MSCs IN INJURY AND INFLAMMATION: PERICYTES
Until recently, immunological equilibrium was believed to depend only on immune cells. During the last years, MSCs have emerged as a new cell type that regulates the immune response. Although unexpectedly, the paracrine immunomodulation role of MSCs is gaining strength. A MSC related cell type, the “pericyte,” has been directly associated with many MSC functions. The regenerative medicine school of thought states that mural cells from blood vessels, mesenchymal in origin, can be the same MSC in a different moment in their morphogenetic cycle. Although pericytes have been classically described histologically, their function is still not completely understood. They have been found in a number of tissues and are molecularly defined as CD146+, NG2+, CD34−, CD45−, and CD56− cells. Besides, in culture they are able to give rise to myocytes, chondrocytes, adipocytes, and osteocytes. In situ, they express MSC markers such as CD44, CD73, CD90, and CD105 and surface antigen markers and multipotency in vitro studies indicate that pericytes become MSC in culture. 24 Several studies have demonstrated pericyte participation in the regulation of HSC quiescence, 25 local neural niche in contact with the neural stem cells (NSCs) in the subventricular zone, 26 perivascular niche of several types of adult stem cells (ASCs) in liver, muscle, and other vascularized tissues, including their participation in the establishment of metastasis in melanoma, liver, lung, and bone cancer. 27 Aside from all these functions related to MSC activities we focus on their role in immunomodulation, antimicrobial and trophic action in response to tissue damage and inflammation. 27
Thanks to the establishment of pericytes in several organs as MSC repositories, da Silva et al 24 , 28 proposed a model to explain the participation of both cell types during regeneration. In homeostatic conditions, pericytes rest adjacent to blood vessels contributing as structural elements and arresting any unwanted immune response on the inside. Upon tissue damage, the resting state of pericytes is altered by changes of the niche environment through basal membrane destabilization and blood content invasion. This promotes blood clot and platelet signals that attract immune cells. This also triggers a pericytes migration from their perivascular location into a MSC activated state, which proliferates and secretes immunomodulatory and antiapoptotic signals to turn down the immune response, favoring damage control and reestablishing perivascular integrity and the basal membrane. These MSCs then follow three possible courses: differentiation to restore lost tissues, apoptosis, or return to their pericyte vigilant state adjacent to the restored endothelium. 28 This MSC behavior is coined by some as “injured drugstores,” 29 and promotes a trophic and immunomodulatory activity in situ. The regenerative microenvironment induces proliferation, angiogenesis, decreases apoptosis, and the scarring process, 30 four core actions that define repair and avoid fibrosis. At the same time, these so‐called “medicinal stem cells‐MSCs” regulate the immune response modulating the acquired immune response from pro‐inflammatory to anti‐inflammatory cytokine producers and modulating the innate immune response by suppressing the cytotoxic response of natural killer cells and altering the capacity of antigen recognition of dendritic cells. 29 During recent years, accumulating evidence has confirmed these hypotheses about the MSC immunomodulatory function. Thus, some suggest that low levels of inflammation induce a proinflammatory MSC phenotype (MSC type 1) that activates the innate immune response (monocytes into M1 macrophages) while excessive inflammatory signals induce an anti‐inflammatory MSC phenotype (type 2 MSC) inducing immunomodulation and trophic activity corresponding with the mediation of M2 macrophages. Type 1 MSCs would contribute to an early repair response while type 2 MSCs would participate later in a regenerative process. 31 , 32
In summary, the immunomodulatory effect of MSCs include inhibition of proliferation and function of T, B, dendritic cells, and natural killer cells, the polarization of monocytes to M2 macrophages, production of IL‐10, and reduction of TNF‐α, IL‐12, and antifibrotic effects 33 , 34 , 35 (Figure 1).
FIGURE 1.
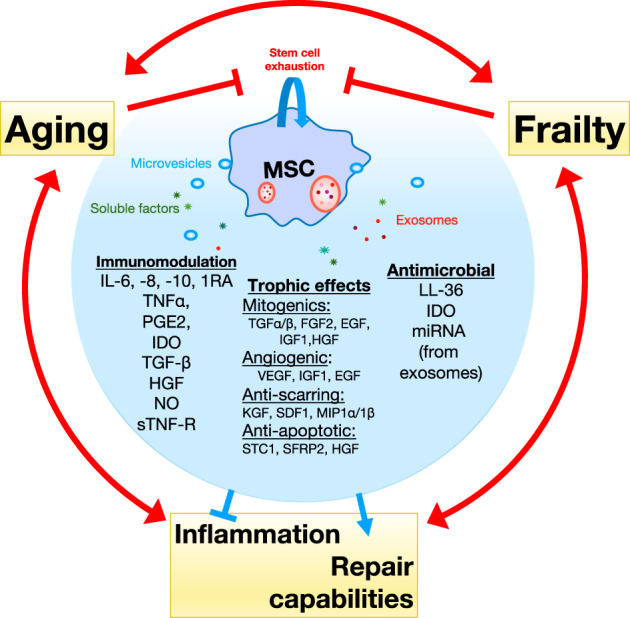
Mesenchymal stem cell (MSC) secreted factors and signals modulate the effects of aging and frailty through downregulation of inflammation and improving repair capabilities. Aging, inflammation, and frailty promote each other and reduce repair tissue capabilities including regeneration and immune system. They also reduce stem cell function as a consequence of stem cell exhaustion causing a feedback effect favoring clinical decline. MSC signals are capable of restoring the compromised homeostasis caused by aging and frailty
5. FRAILTY CONDITIONS AND ITS RELATIONSHIP WITH MSC NUMBERS
Senescence in MSCs has been documented during aging in vivo 36 and it limits MSC numbers in organisms of different ages. While the bone marrow of newborns presents an average of one MSC per 10 000 cells, this proportion decreases drastically with age to one in 2 million by the age of 80. 13 This could explain the alteration of tissue homeostasis and the occurrence of age‐related diseases. 37 MSC senescence is characterized by cell cycle arrest in G1, changes in morphology, and increases in senesce markers β‐galactosidase and SA‐α‐Fuc (senescence‐associated lysosomal α‐l‐fucosidase). Senescence also alters specific surface markers such as Stro‐1 and CD106, correlating with the number of division and donor age. 36 MSC senescence is also associated with increased reactive oxygen species (ROS). 38 Cell culture studies from people 20 to 70 years old demonstrated that older MSCs showed lower proliferation rates, less chondrogenic and osteogenic potential in favor of an increased adipogenesis, and increased signals related to senescence. 39
As explained before, frailty is defined as the progressive functional decline and increased vulnerability to stress resulting from decreased physiological reserve and resilience. 40 Frailty has a high socioeconomic impact with a prevalence of 15% above 65 years of age. A frailty index (FI) has been proposed as a measurement of the multidimensional accumulative factors that lead to disability and death. 41 , 42 However, no effective therapeutic approach is available to reduce or delay the consequences of frailty. One of the best‐established facts about the mechanism of frailty is its correlation with stem cell exhaustion. Probably all endogenous adult stem cells are involved in such responsibility, but more are the MSCs located in mesodermal tissues in their network of capillaries and arterioles from which they can cover a vast and ubiquitous multi‐organ territory. The MSC/pericyte system described above decreased with age and could be necessary protection for vascularized tissues. That is why the use of MSCs is currently being explored using multiple approaches as the logical and specific therapy against the frailty effects through their ability to migrate into injured and inflamed sites, differentiate into multiple tissue specific lineages, and have immunomodulatory effects. Nonetheless, this type of therapy still needs improvement in terms of manufacturing and delivery through cell engineering to fully use the immunomodulatory properties, homing, and efficacy of MSCs. 43 , 44
6. CLINICAL TRIALS USING MSCS AGAINST FRAILTY
Many research labs have developed cell therapies in search of tissue homeostasis improvement. To date, there are more than 38 clinical trials using stem cells against the effect of aging or frailty, although there are far fewer with MSCs. Recently, randomized double blind studies showed that intravenous administration of allogeneic MSCs is safe, renders improved physical performance, and reduces inflammatory markers increased in frailty states. 45 , 46 , 47 , 48 In the first of these trials, 15 patients with mild to moderate frailty were treated with MSCs. This phase 1 study focused on safety evaluating severe adverse effects during 12 months after the injection of 20 to 200 million MSCs. Besides safety, the results showed improvements in physical activity, cognitive hallmarks, and bloodstream TNF‐α levels. In phase II (random, double blind with placebo), 30 frailty patients were injected with 100 to 200 million MSCs resulting in positive results in activity hallmarks and several immune biomarkers 6 months after the injection. 49 These studies, together with other trials, support the safety and efficacy of the intravenous injection of allogeneic MSCs from bone marrow against frailty (Table 1).
TABLE 1.
Ongoing clinical trials with MSCs against frailty or investigating their effect in stem cell exhaustion
| Title | Study type | Conditions | Status | Estimated enrollment | First posted |
|---|---|---|---|---|---|
| Clinical study of umbilical cord mesenchymal stem cells infusion for aging frailty | Phase I/II | Aging/frailty | Recruiting | 30 | March 18, 2020 |
| Human mesenchymal stem cells (LMSCs) on vaccine‐specific antibody‐ response in subjects with aging frailty | Phase I/II | Aging/frailty | Recruiting | 83 | December 6, 2016 |
| Trial to evaluate longeveron mesenchymal stem cells to treat aging frailty | Phase IIb | Aging/frailty | Active, not recruiting | 150 | May 30, 2017 |
| MSC infusion for anti‐aging and regenerative therapy | Phase I |
Aging well Regenerative Medicine |
Not yet recruiting | 100 | November 22, 2019 |
| Allogeneic human mesenchymal stem cells (hMSC) in patients with aging frailty via intravenous US delivery | Phase I/II | Frailty | Completed | 65 |
February 17, 2014 Last update March 5, 2021 |
The downside of these studies is the lack of consistency among the methodology used, with significant variations in the number of infused cells, cell origin, quality of the donor and their MSCs, and hemocompatibility, all common problems of MSC therapies. 43 Tolerance to these treatments, demonstrated in hundreds of patients in multicentric trials, and the reversion of some parameters compromised in frailty make these therapies a well‐founded hope. However, to progress in this approach, the field will need the establishment of new and consistent animal models, as well as a better and systematized diagnosis of frailty conditions through more sensible and validated biomarkers. 40 One set back of this approach has been the allograft rejection, which limits the effect of MSC treatments. Promising strategies to bypass to this problem are the use of MSCs' secretome/exosome (discussed later) or generation of an immunoprivileged site of application where MSCs can survive long enough to perform immunomodulation. 50
7. MSC THERAPIES AGAINST COVID‐19: CELLS, SECRETOME, AND EXOSOME
The SARS‐CoV‐2 pandemic has challenged the scientific communities to adapt and innovate at an accelerated rate to find effective treatments against a physiophatologic mechanisms to which we were not prepared. One of new advanced therapies explored is MSC treatment (Figure 2). COVID‐19 is mediated through an hyperinflammatory response associated with the cytokine release syndrome and is associated acute respiratory syndrome prompted the exploration of many immunomodulatory new strategies. 51 , 52 , 53 , 54 , 55 , 56 The initial therapies against the worst cases of COVID‐19, those presenting acute respiratory distress syndrome or ARDS, selectively targeted cytokines with limited results. Now we know that to get a true effect on the hyperinflammatory response we need to target a wide set of inflammatory signals and our most effective treatments have ended being classic anti‐inflammatories, such as dexamethasone. 57 The key seems to be controlling the feedback loop that transforms a normal cytokine response into a cytokine storm. This concept has taken us from limited results from early attempts to control cytokine release to modulate the adaptive immune response to avoid the secondary cytokine release.
FIGURE 2.
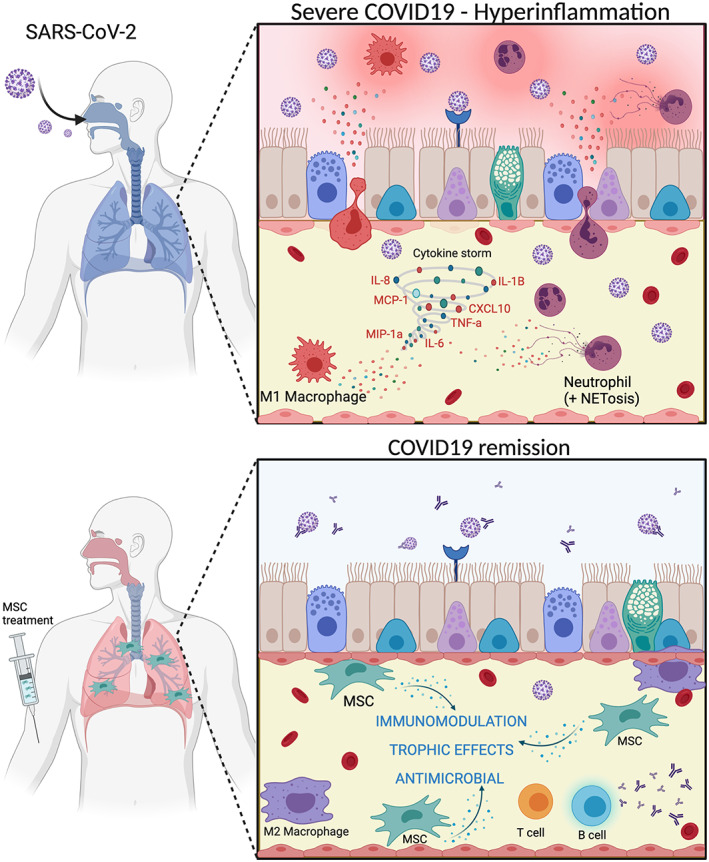
Mesenchymal stem cell (MSC) therapy is capable of reducing the hyperinflammatory response in cases of severe COVID‐19. SARS‐CoV‐2 infection causes severe COVID‐19 by inflammation associated to neutrophilia (including NETS—neutrophil extracellular traps) and pro‐inflammatory macrophagic response. MSC treatment specially targets lungs and provides of in situ and systemic immunomodulation, antimicrobial and other trophic effects (see Figure 1) supporting an anti‐inflammatory profile of macrophages and appropriate coordination of T and B cells to fight against SARS‐CoV‐2 infection. MSC mitogenic, angiogenic, and anti‐apoptotic effects drive resident cells into tissue regeneration. MSC anti‐scarring effects avoid fibrosis in lung, an adverse side effect in COVID‐19 disease
Recent results on animal model infections have shown a delay of type I interferon (IFN‐I) and the innate immune response allowing the accumulation of proinflammatory monocytes and macrophages (classically called M1) and that removal of these cells protect against lethal infection without altering viral replication. 58 On the other hand, hyperinflammation in COVID‐19 is also promoted by pyroptosis, an inflammatory form of apoptosis triggered by viral replication. This programmed cell death exacerbates the inflammatory response by the release of IL‐1B from dying cells, a situation that cannot be reversed with cellular debris removal by anti‐inflammatory (M2) macrophages, which are not produced in favor of the M1 type. 59 Besides the monocytic lineages, there are other immune cells promoting the cytokine storm. This cytokine hyperproduction, then, causes the severe phenotype through several identified mechanisms, such as formation of neutrophil extracellular nets (NETS), causing lung injury and micro thrombi, 60 or the functional exhaustion of immune cells that reduces the ability to fight the infection. 61 These results suggest that targeting the inflammatory activation of immune lineages is already a suitable strategy to tackle the hyperinflammatory response of severe COVID‐19 cases.
Given this proinflammatory mechanism and the immunomodulatory effect of MSCs, several clinical trials started under the premises of the control of cytokine release to avoid the severe conditions developed by SARS‐CoV‐2. 62 The first study started in China in January 2020, injecting 1 million MSC cells/kg in the blood stream. This very preliminary trial concluded manifesting a pulmonary function improvement, peripheral lymphocyte increase, decrease of C‐reactive protein as well as cytokine releasing cell types after 3 days of treatment in the seven treated patients. 63 Authors on the study also reported TNF‐α decrease and IL‐10 increase and concluded that MSCs seemed to inhibit hyper‐activation of the immune system.
Later, a consortium formed by the Cell Therapy Spanish Network (TerCel) performed a clinical trial using allogeneic adipogenic MSCs for 13 severe COVID‐19 patients 64 with approximately 1 million cells/kg reporting a decrease in inflammatory parameters (C‐reactive protein, IL‐6, ferritin, LDH, and D‐dimers) and a lymphocyte count increase proportional to clinical improvement. Other clinical trials rendered similar results from the use of other MSCs, such as umbilical cord blood and Wharton jelly, bone marrow, and adipose tissue (ClinicalTrials.gov; http://www.chictr.org.cn). 65 , 66
Although results from MSC therapies are promising, this strategy also presents important limitations, such as slow production, low profitability, demanding quality controls, and short expiration dates. These factors limit this kind of therapy upon the immediate need and the need of massive treatments in pandemic times. 65 , 67 , 68 , 69 An alternative to MSCs, is the use of their secretome or even only exosomes. Interestingly, MSCs are known to immunomodulate through the bioactive set of molecules that they secrete. 70 This paracrine function includes growth factors, cytokines, chemokines, extracellular components, and microvesicles that contain selective molecules of recognized anti‐inflammatory characteristics, 69 , 71 , 72 even antifibrotic signals. 35 Among all the components of the secretome mix, the exosomes are arousing special interest due to their properties that overcome the bottleneck limitations of the MSCs. Exosomes transport many of the immunomodulatory signals secreted by MSCs. Recent studies have demonstrated that MSC exosomes provide of an immunomodulatory effect similar to MSCs. 73 Studies from several research groups, including ours have demonstrated the efficacy and the immunomodulatory potential of exosomes. 74 They show specific action on macrophage activation inducing an anti‐inflammatory profile. 75 , 76 , 77 Besides, it is possible to induce the therapeutic response of the secreting MSC to produce a certain exosome profile with more anti‐inflammatory signals. 78 , 79 The use of exosomes instead of cells also avoids the risks of the later (like their tumorigenic effect) and provide logistic advantages, such as rapid scalable production and a long shelf‐life. 80 The use of exosomes is rapidly being considered a viable therapeutic strategy and 91 clinical trials are already registered at a global scale from which 12 are exosome treatments from MSCs (ClinicalTrials.gov). One of these uses allogeneic MSC exosomes for COVID‐19 cases with ARDS, injecting 24 patients with their exosome product (ExFlo) to study safety and initial efficacy. 81 Preliminary results from this study showed a reduction in hypoxic state by altered pulmonary function and immune markers recovery without adverse effects.
8. CONCLUSION
In summary, MSC research during the last decades has been a roller‐coaster of promises, controversies, and unexpected discoveries, which have changed our perspective of their potential use as a therapy for different human conditions and diseases. We believe that although the original promises have not been met, the intense dedication to their study has opened new alternatives to use their less known paracrine properties on immunomodulation and aging to find new solutions to extremely important challenges of public health, such as the increasing incidence of frailty conditions and new and unexpected hazards like COVID‐19.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
JB: conception, design and writing; ID: conception, design and writing, final approval.
ACKNOWLEDGMENTS
We thank the reviewers for their suggestions and supporting grants from J.B. and I.D.: Junta de Andalucia: CV20‐81404, UMA18‐FEDERJA‐177. Spanish Network on Cell Therapy (Red TerCel; RD16/0011/0022); Iniciativa Ingenio 2010, Consolider Program, CIBER Actions, the Instituto de Salud Carlos III; Regional Government of Andalusia (PAIDI group BIO‐217); University of Malaga (Plan Propio). Figure 2 was created with Biorender.com.
Becerra J, Duran I. Inflammation, a common mechanism in frailty and COVID‐19, and stem cells as a therapeutic approach. STEM CELLS Transl Med. 2021;10(11):1482–1490. 10.1002/sctm.21-0074
Funding information University of Malaga (Plan Propio); Regional Government of Andalusia; Iniciativa Ingenio 2010, Consolider Program, CIBER Actions, the Instituto de Salud Carlos III; Spanish Network on Cell Therapy, Grant/Award Number: RD16/0011/0022; Junta de Andalucia, Grant/Award Numbers: UMA18‐FEDERJA‐177, CV20‐81404
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Blagosklonny MV. From causes of aging to death from COVID‐19. Aging. 2020;12(11):10004‐10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonafè M, Prattichizzo F, Giuliani A, et al. Inflamm‐aging: why older men are the most susceptible to SARS‐CoV‐2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53(10228):33‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Franceschi C, Bonafè M, Valensin S, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244‐254. [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J, et al. Cardiovascular health study collaborative research group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146‐M156. [DOI] [PubMed] [Google Scholar]
- 5. Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439‐454. [PubMed] [Google Scholar]
- 6. Rockwood K, Howlett SE. Mech. Ageing Dev. 2019;180:107‐116. [DOI] [PubMed] [Google Scholar]
- 7. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145‐1147. [DOI] [PubMed] [Google Scholar]
- 10. Johnson S, Atala A. Regenology: time for a new specialty? Stem Cells Translational Medicine. 2019;8(1):4‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663‐676. [DOI] [PubMed] [Google Scholar]
- 12. Sipp D, Robey PG, Turner L. Clear up this stem‐cell mess. Nature. 2018;561(7724):455‐457. [DOI] [PubMed] [Google Scholar]
- 13. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641‐650. [DOI] [PubMed] [Google Scholar]
- 14. Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10(suppl 10):63‐76. [DOI] [PubMed] [Google Scholar]
- 15. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 16. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Translational Medicine. 2017;6(6):1445‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abbas AK, Lichtman AH, Pillai S. Basic immunology—functions and disorders of the immune system; Elsevier. 2016. [Google Scholar]
- 18. Fulop T, Witkowski JM, Olivieri F, Larbi A. The integration of inflammaging in age‐related diseases. Semin Immunol. 2018;40(suppl 1):17‐35. [DOI] [PubMed] [Google Scholar]
- 19. Bonafè M, Olivieri F, Cavallone L, et al. A gender‐dependent genetic predisposition to produce high levels of IL‐6 is detrimental for longevity. Eur J Immunol. 2001;31(8):2357‐2361. [DOI] [PubMed] [Google Scholar]
- 20. Mounayar M, Kefaloyianni E, Smith B, et al. PI3kα and STAT1 interplay regulates human mesenchymal stem cell immune polarization. Stem Cells. 2015;33(6):1892‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng L, Li H, Su X, et al. Chlorzoxazone, a small molecule drug, augments immunosuppressive capacity of mesenchymal stem cells via modulation of FOXO3 phosphorylation. Cell Death Dis. 2020;11(3):158‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kurte M, Vega‐Letter AM, Luz‐Crawford P, et al. Time‐dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAE. Stem Cell Res Ther. 2020;11(1):416‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiorina P, Jurewicz M, Augello A, et al. Immunomodulatory function of bone marrow‐derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 2009;183(2):993‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301‐313. [DOI] [PubMed] [Google Scholar]
- 25. Méndez‐Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell‐cell interactions. Cell Stem Cell. 2008;3(3):289‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Correa D, Somoza RA, Lin P, Schiemann WP, Caplan AI. Mesenchymal stem cells regulate melanoma cancer cells extravasation to bone and liver at their perivascular niche. Int J Cancer. 2016;138(2):417‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287‐2299. [DOI] [PubMed] [Google Scholar]
- 29. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loretelli C, Ben Nasr M, Giatsidis G, et al. Embryonic stem cell extracts improve wound healing in diabetic mice. Acta Diabetol. 2020;57(7):883‐890. [DOI] [PubMed] [Google Scholar]
- 31. Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett. 2015;168(2):136‐139. [DOI] [PubMed] [Google Scholar]
- 32. Wong SP, Rowley JE, Redpath AN, et al. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107‐120. [DOI] [PubMed] [Google Scholar]
- 33. Chow L, Johnson V, Impastato R, et al. Antibacterial activity of human mesenchymal stem cells mediated directly by constitutively secreted factors and indirectly by activation of innate immune effector cells. Stem Cells Translational Medicine. 2020;9(2):235‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pittenger MF, Discher DE, Péault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regener Med. 2019;4(1):22‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tzouvelekis A, Toonkel R, Karampitsakos T, et al. Mesenchymal stem cells for the treatment of idiopathic pulmonary fibrosis. Front Med. 2018;5:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Wu Q, Wang Y, et al. Senescence of mesenchymal stem cells. Int J Mol Med. 2017;39(4):775‐782. [DOI] [PubMed] [Google Scholar]
- 37. Rubin H. Promise and problems in relating cellular senescence in vitro to aging in vivo. Arch Gerontol Geriatr. 2002;34(3):275‐286. [DOI] [PubMed] [Google Scholar]
- 38. Jeong SG, Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun. 2015;460(4):971‐976. [DOI] [PubMed] [Google Scholar]
- 39. Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. Stem Cells Int. 2016:2152435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pahor M, Kritchevsky SB, Waters DL, et al. Designing drug trials for frailty: ICFSR Task Force 2018. J Frailty Aging. 2018;7(3):150‐154. [DOI] [PubMed] [Google Scholar]
- 41. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Verlaan S, Ligthart‐Melis GC, Wijers SLJ, et al. High prevalence of physical frailty among community‐dwelling malnourished older adults—a systematic review and meta‐analysis. J Am Med Dir Assoc. 2017;18(5):374‐382. [DOI] [PubMed] [Google Scholar]
- 43. Caplan H, Olson SD, Kumar A, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. García‐Bernal D, García‐Arranz M, Yáñez RM, Hervás‐Salcedo R, Cortés A, et al. Front Cell Dev Biol. 2021;9:650664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Couteur DG, Anderson RM, Newman AB, de Cabo R. Stem cell transplantation for frailty. J Gerontol Ser A Biol Sci Med Sci. 2017;72(11):1503‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schulman IH, Balkan W, Hare JM. Mesenchymal stem cell therapy for aging frailty. Front Nutr. 2018;5:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun XL, Hao Q‐K, Tang R‐J, et al. Frailty and rejuvenation with stem cells: therapeutic opportunities and clinical challenges. Rejuvenation Res. 2019;22(6):484‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double‐blind, placebo‐controlled clinical trial. J Gerontol Ser A. 2017;72(11):1513‐1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Florea V, Bagno L, Rieger AC, Hare JM. Attenuation of frailty in older adults with mesenchymal stem cells. Mech Ageing Dev. 2019;181:47‐58. 10.1016/j.mad.2019.111120. [DOI] [PubMed] [Google Scholar]
- 50. Ben Nasr M, Vergani A, Avruch J, Liu L, Kefaloyianni E, et al. Acta Diabetol. 2015;52(5):917‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karakike E, Giamarellos‐Bourboulis EJ. Macrophage activation‐like syndrome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yen BL, Yen ML, Wang LT, Liu KJ, Sytwu HK. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: gleaning insights for possible use in COVID‐19. Stem Cells Translational Medicine. 2020;9(10):1163‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020;NEJMoa2021436. [Google Scholar]
- 58. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe. 2016;19(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yap JKY, Moriyama M, Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID‐19. J Immunol (Baltimore, MD: 1950). 2020;205(2):307‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skendros P, Mitsios A, Chrysanthopoulou A, et al. Complement and tissue factor‐enriched neutrophil extracellular traps are key drivers in COVID‐19 immunothrombosis. J Clin Invest. 2020;130(11):6151‐6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Durand N, Mallea J, Zubair AC. Insights into the use of mesenchymal stem cells in COVID‐19 mediated acute respiratory failure. NPJ Regener Med. 2020;5(1):17‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, et al. Aging Dis. 2020;11(2):216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sánchez‐Guijo F, García‐Arranz M, López‐Parra M, et al. Adipose‐derived mesenchymal stromal cells for the treatment of patients with severe SARS‐CoV‐2 pneumonia requiring mechanical ventilation. A proof of concept study. E Clin Med. 2020;25(6490):100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID‐19: present or future. Stem Cell Rev Rep. 2020;16(3):427‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qu W, Wang Z, Hare JM, et al. Cell‐based therapy to reduce mortality from COVID‐19: systematic review and meta‐analysis of human studies on acute respiratory distress syndrome. Stem Cells Translational Medicine. 2020;9(9):1007‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC‐MSCs) as a therapeutic strategy in managing critically ill COVID‐19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71‐E83. [PubMed] [Google Scholar]
- 68. Bari E, Ferrarotti I, Saracino L, et al. Mesenchymal stromal cell secretome for severe COVID‐19 infections: premises for the therapeutic use. Cells. 2020;9(4):924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar , Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: a new paradigm towards cell‐free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46(190):1‐9. [DOI] [PubMed] [Google Scholar]
- 70. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54‐e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509‐525. [DOI] [PubMed] [Google Scholar]
- 72. Vizoso FJ, Eiro N, Cid S, Schneider J, Perez‐Fernandez R. Mesenchymal stem cell secretome: toward cell‐free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials—an ISEV position paper. J Extracell Vesicles. 2015;4(1):30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Villatoro AJ, Alcoholado C, Martín‐Astorga MC, et al. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Vet Immunol Immunopathol. 2019;208:6‐15. [DOI] [PubMed] [Google Scholar]
- 75. An JH, Li Q, Bhang D‐H, Song W‐J, Youn H‐Y. TNF‐α and INF‐γ primed canine stem cell‐derived extracellular vesicles alleviate experimental murine colitis. Sci Rep. 2020;10(1):2115‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Domenis R, Cifù A, Quaglia S, et al. Pro‐inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells‐derived exosomes. Sci Rep. 2018;8(1):13325‐13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shao M, Xu Q, Wu Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL‐6‐induced acute liver injury through miR‐455‐3p. Stem Cell Res Ther. 2020;11(1):37‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Noronha N d C, Mizukami A, Caliári‐Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell‐based therapies. Stem Cell Res Ther. 2019;10(1):131‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ragni E, Perucca Orfei C, De Luca P, et al. Inflammatory priming enhances mesenchymal stromal cell secretome potential as a clinical product for regenerative medicine approaches through secreted factors and EV‐miRNAs: the example of joint disease. Stem Cell Res Ther. 2020;11(1):165‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Phinney DG, Pittenger MF. Concise review: MSC‐derived exosomes for cell‐free therapy. Stem Cells. 2017;35(4):851‐858. [DOI] [PubMed] [Google Scholar]
- 81. Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29(12):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


