Abstract
Droplet-based microfluidic technology has enabled the production of emulsions with high monodispersity in sizes ranging from a few to hundreds of micrometers. Taking advantage of this technology, attempts to generate monodisperse emulsion drops with high drug loading capacity, ordered interfacial structure, and multi-functionality have been made in the cosmetics industry. In this article, we introduce the practicality of the droplet-based microfluidic approach to the cosmetic industry in terms of innovation in productivity and marketability. Furthermore, we summarize some recent advances in the production of emulsion drops with enhanced mechanical interfacial stability. Finally, we discuss the future prospects of microfluidic technology in accordance with consumers' needs and industrial attributes.
I. INTRODUCTION
Emulsions have been applied in numerous ways from traditional food, pharmaceutical, and cosmetic industries to technology-oriented biomedical and sensor industries.1–7 Therefore, it is important to understand recent research trends regarding the development of a diverse emulsion system. To date, conventional emulsification methods commonly used in industries such as paddle mixing, simple homogenization, and high-pressure homogenization have been focusing only on the technical aspect of simply mixing two immiscible oil and water phases with the aid of a surfactant. The emulsions produced by using conventional emulsification in a batch not only have small drop sizes ranging from nanoscale to several micrometers, but also broad size distributions, which makes them useful for large-scale production of stable emulsions with small drop sizes. If an emulsion with the same droplet size is uniformly dispersed in another liquid phase and has a sufficient size for direct observation, its applicability is very high in the cosmetic industry. Due to these advantages, many studies have been conducted for designing uniform sized emulsion drops based on a microfluidic technology, employing various surface-active substances such as low molecular surfactants,8–10 polymeric surfactants,11–13 and colloidal particles.14–17 For visual enjoyment that comes from the appearance of emulsion products, several cosmetic companies have attempted to generate monodisperse emulsions while varying their size, shape, and texture.18–25 The size of emulsions produced by a microfluidic device can be controlled from several to hundreds of micrometers, depending on a variety of parameters such as flow rates, fluid viscosity, additives, and geometry of microfluidic channels. The shape of emulsion drops can be manipulated by adjusting the wettability of two immiscible liquids or by using a geometric combination of microfluidic channels. These parameters enable production of multiple emulsions, multi-layered emulsions, or Janus-type emulsions. These emulsions may contain several active components or both hydrophilic and hydrophobic ingredients in one drop. Thanks to the multiphase structure, they cannot only release the drug gradually but also provide a different sense of use when applied to the skin. Despite these advantages, improving their structural stability and productivity remains a challenge. In addition, by forming a particle–polymer composite film at the oil–water interface of the emulsion, buckling and wrinkle of the interface can be induced to give rise to unique textures. Such emulsion droplets even further increase their cosmetic usefulness if they can encapsulate and stabilize skin-effective ingredients against external stimuli, such as heat, pH, light, and oxidation.26 For this, the emulsion interface should have sufficient mechanical resistance to hinder the occurrence of mass transfer. In addition, large-scale production of emulsions must be implemented in one device system to achieve substantial marketability in the cosmetic market.27–29 To meet these requirements, a more advanced droplet-based microfluidic technology that generates monodisperse emulsion drops with an ordered interfacial structure within a single device system should be developed.30–33
In this Perspective, a review and summary of recent researches on droplet-based microfluidics and their application in the cosmetic industry has been covered. In the first part, we succinctly describe recent advances in the development of microfluidic devices for mass production of uniform emulsions. Subsequently, novel approaches to strengthen the emulsion interfaces have been introduced. We also discuss technological strategies that allow monodisperse emulsions to have commercially available values for cosmetic formulations. This short article provides essential information for future research studies toward cosmetic applications of well-engineered emulsion systems.
II. MICROFLUIDIC TECHNOLOGIES FOR COSMETIC INDUSTRY
A. Microfluidic large-scale production of emulsions
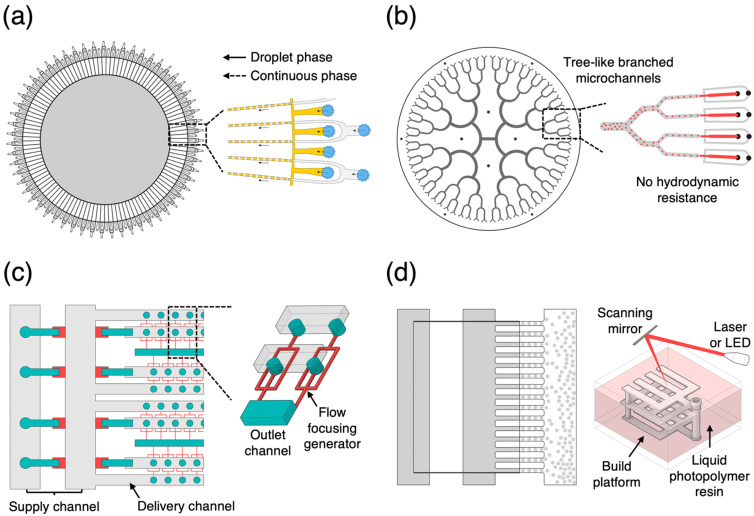
Microfluidic emulsification is of great interest in industries because it allows precise control over the shape, size, and compartment of monodisperse liquid drops suspended in an immiscible liquid continuous phase.34–37 In fact, such well-designed emulsion drops produced in a single microfluidic channel are mostly obtainable in lab-scale fabrication processes, thus making it very challenging to produce emulsions with sufficient commerciality. For decades, various efforts have been made to develop microfluidic technologies for the mass production of emulsions, as summarized in Table I. The methods and materials have their own advantages and disadvantages in mass production of uniform emulsions, while determining the emulsion type, dimension, and interface property. In the early 2000s, there were attempts to increase the number of replicated modules, which face the primitive problem of connecting a large number of accessories such as tubes, connectors, and pumps.38 To address this problem, microfluidic researchers have proposed a distribution microchannel system. Nisisako and Torri reported the mass production of monodisperse emulsion droplets with a coefficient of variation below 1.3% by using a microchannel module with 128 cross-junctions arranged circularly on a chip [Fig. 1(a)].39 They were able to show that biphasic Janus emulsion droplets, employing modified modules of co-flow junctions with Y-shaped channels, could be successfully generated. The common design for distributing microchannels is a tree-like branched microchannel network [Fig. 1(b)].40 This microchannel evenly and symmetrically divided fluids into branched microchannels starting from one set of inlets without any hydrodynamic resistance of each microchannel.
TABLE I.
Comparison and characteristics of drop-based microfluidic technologies.
| Applied technique | Device material | Large-scale production | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Micro-capillary assembly | Glass | Poor |
|
|
20 and 22 |
| Photo/soft-lithography | Polydimethylsiloxane (PDMS) | Good |
|
|
41 and 42 |
| 3D printing | PEG diacrylate methacrylate photoresist | Excellent |
|
|
46 and 47 |
FIG. 1.
Microfluidic distribution microchannel designs for mass production of monodisperse emulsion. (a) Circular array of droplet generators. Reproduced with permission from Nisisako and Torri, Lab Chip 8(2), 287 (2008). Copyright 2008 Royal Society of Chemistry. (b) Tree-like distribution microchannels. Reproduced with permission from Conchouso et al., Lab Chip 14(16), 3011 (2014). Copyright 2014 Royal Society of Chemistry. (c) Ladder geometry microchannels. Reproduced with permission from Jeong et al., Lab Chip 15(23), 4387 (2015). Copyright 2015 Royal Society of Chemistry. (d) 3D printed microchannels with parallel droplet generators. Reproduced with permission from Au et al., Lab Chip 14(7), 1294 (2014). Copyright 2014 Royal Society of Chemistry.
More recently, challenging attempts have been made to manipulate soft materials to implement the geometry of distribution microchannels via photo- and soft-lithography.41,42 The distribution microchannel with a ladder-like geometry has been commonly used for the mass production of monodisperse emulsions [Fig. 1(c)]. These ladder-like microchannels are two- or three-dimensionally arrayed to fabricate the multi-layered microfluidic device. To fabricate this type of microfluidic device, soft materials, including photo-polymerizable polydimethylsiloxane or polyurethane precursors, were employed in conjunction with the soft-lithography technique. Using this chemical material engineering approach, Jeong et al. demonstrated that three-dimensional (3D) microchannels with 1000 parallel flow-focusing generators with a ladder geometry were able to increase the emulsion production scale up to 1.5 l h−1.43 The 3D printing technique has made it possible to directly produce a user-designed microfluidic device [Fig. 1(d)].44–47 Femmer et al. developed a parallelized microfluidic flow-focusing device with 28-droplet generators using digital light processing, which generates monodisperse emulsion drops with a diameter of 500 μm at a production level to 3 l h−1.45 These results highlight that mass production of monodisperse simple emulsion droplets through the use of such advanced fabrication technologies is no longer a major issue.
B. Structuring emulsion interfaces
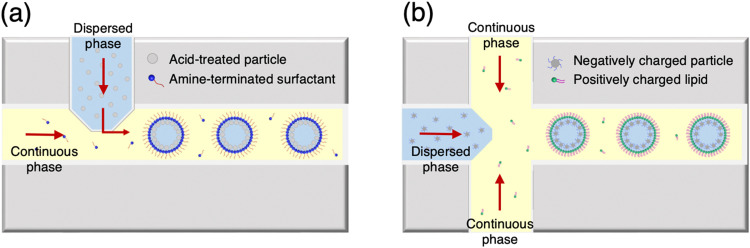
Conventional emulsions are thermodynamically unstable, thus eventually coarsening the drops into a phase-separated mixture.48 In order to improve the stability of an emulsion system, surfactants have been appropriately incorporated considering their hydrophilic–lipophilic balance and molecular geometric features while following the Bancroft rule.49–51 In general emulsion systems, surfactants adsorb spontaneously to the oil–water interface, which lowers the interfacial tension to form a physical barrier, thus preventing coalescence between drops. In a microfluidic channel, the adsorption kinetics of surfactants is important and plays a role in determining the mechanical properties of the emulsion interface.52 The surfactants used in droplet-based microfluidics must adsorb rapidly to the emulsion droplets to prevent coalescence between droplets, but they should simultaneously lower the interfacial tension only to a level at which jetting does not occur. Because it is difficult to meet these requirements at once by using a low molecular weight surfactant, approaches using polymeric surfactants or colloidal surfactants with a bulky structure have been developed (Fig. 2).53–56 When preparing cosmetic emulsions using microfluidics, the surfactant must be carefully selected. In fact, the surfactants that can be used in cosmetic formulations are very limited, as those with excellent surface activity may not only pollute the environment, but are also highly likely to cause skin irritation.57 Ionic surfactants [e.g., sodium lauryl sulfate (SLS) and cetrimonium bromide (CTAB)] or polyethylene glycol (PEG) derivatives surfactants (e.g., Span series and Tween series) are good examples. For these reasons, many consumers are negatively concerned about the use of chemical surfactants in the development of cosmetic products.
FIG. 2.
Strategy for engineering emulsion interface during microfluidic emulsification. Schematic illustration of emulsions stabilization by using (a) silica nanoparticle/surfactant and (b) silica nanoparticle/lipid surfactant compositions.
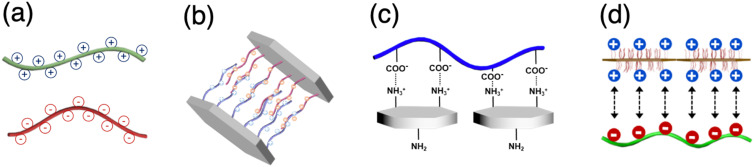
Surfactant-free emulsification is becoming a hot trend in the cosmetics industry. If a stable emulsion can be prepared without using a surfactant, both environmental and skin safety issues can be resolved. For this purpose, a variety of types of colloidal particles such as silica,58,59 clay,60,61 polymeric particles,62,63 and organic fibrous materials64,65 can be considered emulsifiers for emulsion stabilization. Although such materials are effective for solidifying the emulsion interface with a monolayer, interfacial coverage cannot be perfect because of the generation of interstitial defects due to the geometry of the particles. A promising solution to this challenge is to take advantage of interfacial coacervation that fills the defect dimension while mechanically strengthening the interface. The interfacial coacervation is induced between oppositely charged materials, that is, polymer–polymer,66–69 colloid–colloid,70,71 colloid–polymer,72,73 or colloid–nanofiber74,75 (Fig. 3). This technique provides a useful methodology for surfactant-free emulsion stabilization in droplet-based microfluidics.
FIG. 3.
Combination of materials for interfacial coacervation. (a) Polymer–polymer. (b) Colloid nanoparticle–colloid nanoparticle. Reproduced with permission from Hwang et al., ACS Appl. Mater. Interfaces 13(6), 7664 (2021). Copyright 2021 American Chemical Society. (c) Polymer–colloid nanoparticle. (d) Colloid–nanofibrous material. Reproduced with permission from Cho et al., Langmuir 37(13), 3828 (2021). Copyright 2021 American Chemical Society.
III. OUTLOOK
In this paper, recent studies on monodisperse emulsion production via droplet-based microfluidics have been summarized in terms of application to the cosmetic industry. It is definite that monodisperse emulsions produced by using droplet-based microfluidics can provide useful dermatological benefits to the consumers of cosmetic products. First of all, thanks to the uniform and large droplets, the emulsion produced can provide visual pleasure in addition to the sense of smell and touch of existing cosmetics. In the case of preparing a monodisperse emulsion with a diameter of 1 mm, it is possible to enjoy the visual effect of the giant droplets with the naked eyes. In contrast, normal emulsions with a broad particle size distribution appear milky because the droplets are larger than the wavelength of light.76 In addition, the materials that form the emulsion interface should have both technical and esthetic properties. Texture is one of the critical factors that consumers rely on to determine the quality of cosmetic products. Therefore, emulsion-based cosmetic formulations should not only be free of foreign substances but also not cause an unfavorable finish feel when applied to the skin. From the perspective of performance and applicability, technological advances in engineering emulsion interfaces are strongly required. Colloidal materials used as substitutes for emulsifiers must not only form a rigid emulsion interface mechanically but also be very safe for the skin.77–79 For a better sense of use, it is necessary to have the properties of appropriate adhesion to the skin. The hydrophobic modification of colloidal materials is a key strategy for manipulating skin adhesion while controlling their wettability against the emulsion interface.80,81 The fabrication of amphiphilic Janus colloidal particles is also a promising method for engineering emulsion interfaces.82,83 The use of fibrous soft materials with the proper amount of hydrophobic moieties can also create a geometrically defect-free emulsion interface without the aid of additional surfactants.84 These approaches will pave the way for the development of new types of emulsion systems in the future for the cosmetics market.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant No. NRF-2019R1A2C1086383).
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts of interest to disclose.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Muschiolik G., Curr. Opin. Colloid Interface Sci. 12(4–5), 213 (2007). 10.1016/j.cocis.2007.07.006 [DOI] [Google Scholar]
- 2.Schemberg J., Grodrian A., Römer R., Gastrock G., and Lemke K., Eng. Life Sci. 9(5), 391 (2009). 10.1002/elsc.200800127 [DOI] [Google Scholar]
- 3.Steinsapir K. D., Rootman D., Wulc A., and Hwang C., Ophthalmic Plast. Reconstr. Surg. 31(4), 263 (2015). 10.1097/IOP.0000000000000282 [DOI] [PubMed] [Google Scholar]
- 4.Byun A., Shim J., Han S. W., Kim B., Chae P. S., Shin H. S., and Kim J. W., Chem. Commun. 51(64), 12756 (2015). 10.1039/C5CC04547A [DOI] [PubMed] [Google Scholar]
- 5.Choi S.-E., Park D., Hwang H., Seo M., Lee D., Jeong U., and Kim J. W., Adv. Funct. Mater. 30, 2000431 (2020). 10.1002/adfm.202000431 [DOI] [Google Scholar]
- 6.Han S. W., Choi S.-E., Chang D. H., Lee D., Kim B., Yang H., Seo M., and Kim J. W., Adv. Funct. Mater. 29, 1805392 (2019). 10.1002/adfm.201805392 [DOI] [Google Scholar]
- 7.You I., Choi S.-E., Hwang H., Han S. W., Kim J. W., and Jeong U., Adv. Funct. Mater. 28, 1801858 (2018). 10.1002/adfm.201801858 [DOI] [Google Scholar]
- 8.Pavlovic M., Antonietti M., Schmidt B. V. K. J., and Zeininger L., J. Colloid Interface Sci. 575, 88–95 (2020). 10.1016/j.jcis.2020.04.067 [DOI] [PubMed] [Google Scholar]
- 9.Zarzar L. D., Sresht V., Sletten E. M., Kalow J. A., Blankschtein D., and Swager T. M., Nature 518(7540), 520–524 (2015). 10.1038/nature14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao C.-X., Chen D., Hui Y., Weitz D. A., and Middelberg A. P. J., ChemPhysChem 18(10), 1393–1399 (2017). 10.1002/cphc.201601334 [DOI] [PubMed] [Google Scholar]
- 11.Choi Y. H., Lee S. S., Lee D.-M., Jeong H. S., and Kim S.-H., Small 16(9), 1903812 (2020). 10.1002/smll.201903812 [DOI] [PubMed] [Google Scholar]
- 12.Czekalska M. A., Jacobs A. M. J., Toprakcioglu Z., Kong L., Baumann K. N., Gang H., Zubaite G., Ye R., Mu B., Levin A., Huck W. T. S., and Knowles T. P. J., ACS Appl. Mater. Interfaces 13(5), 6739–6747 (2021). 10.1021/acsami.0c16019 [DOI] [PubMed] [Google Scholar]
- 13.Mei L., Jin M., Xie S., Yan Z., Wang X., Zhou G., Van Den Berg A., and Shui L., Lab Chip 18(18), 2806–2815 (2018). 10.1039/C8LC00479J [DOI] [PubMed] [Google Scholar]
- 14.Sun Z., Yang C., Wang F., Wu B., Shao B., Li Z., Chen D., Yang Z., and Liu K., Angew. Chem. Int. Ed. 59(24), 9365–9369 (2020). 10.1002/anie.202001588 [DOI] [PubMed] [Google Scholar]
- 15.Xie S., Chen S., Zhu Q., Li X., Wang D., Shen S., Jin M., Zhou G., Zhu Y., and Shui L., ACS Appl. Mater. Interfaces 12(23), 26374–26383 (2020). 10.1021/acsami.0c05625 [DOI] [PubMed] [Google Scholar]
- 16.Marquis M., Alix V., Capron I., Cuenot S., and Zykwinska A., ACS Biomater. Sci. Eng. 2(4), 535–543 (2016). 10.1021/acsbiomaterials.5b00522 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki T., Li Y., Gevorkian A., and Kumacheva E., Soft Matter 14(47), 9713–9719 (2018). 10.1039/C8SM01716F [DOI] [PubMed] [Google Scholar]
- 18.Abedi S., Suteria N. S., Chen C.-C., and Vanapalli S. A., J. Colloid Interface Sci. 533, 59 (2019). 10.1016/j.jcis.2018.08.045 [DOI] [PubMed] [Google Scholar]
- 19.Nabavi S. A., Vladisavljević G. T., Bandulasena M. V., Arjmandi-Tash O., and Manović V., J. Colloid Interface Sci. 505, 315 (2017). 10.1016/j.jcis.2017.05.115 [DOI] [PubMed] [Google Scholar]
- 20.Jeyhani M., Thevakumaran R., Abbasi N., Hwang D. K., and Tsai S. S. H., Small 16(7), 1906565 (2020). 10.1002/smll.201906565 [DOI] [PubMed] [Google Scholar]
- 21.Nawar S., Stolaroff J. K., Ye C., Wu H., Nguyen D. T., Xin F., and Weitz D. A., Lap Chip 20(1), 147 (2020). 10.1039/C9LC00966C [DOI] [PubMed] [Google Scholar]
- 22.Guzowski J., Korczyk P. M., Jakiela S., and Garstecki P., Soft Matter 8(27), 7269 (2012). 10.1039/c2sm25838b [DOI] [Google Scholar]
- 23.Sander J. S., Isa L., Rühs P. A., Fischer P., and Studart A. R., Soft Matter 8(45), 11471 (2012). 10.1039/c2sm26700d [DOI] [Google Scholar]
- 24.Meng Q., Zhang Y., Li J., Lammertink R. G., Chen H., and Tsai P. A., Sci. Rep. 6, 26953 (2016). 10.1038/srep26953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leister N. and Karbstein H. P., Colloids Interfaces 4(1), 8 (2020). 10.3390/colloids4010008 [DOI] [Google Scholar]
- 26.Casanova F. and Santos L., J. Microencapsul. 33(1), 1 (2016). 10.3109/02652048.2015.1115900 [DOI] [PubMed] [Google Scholar]
- 27.Jeong H.-H., Issadore D., and Lee D., Korean J. Chem. Eng. 33(6), 1757 (2016). 10.1007/s11814-016-0041-6 [DOI] [Google Scholar]
- 28.Romanowsky M. B., Abate A. R., Rotem A., Holtze C., and Weitz D. A., Lab Chip 12(4), 802 (2012). 10.1039/c2lc21033a [DOI] [PubMed] [Google Scholar]
- 29.Holtze C., J. Phys. D: Appl. Phys. 46(11), 114008 (2013). 10.1088/0022-3727/46/11/114008 [DOI] [Google Scholar]
- 30.Utada A. S., Lorenceau E., Link D. R., Kaplan P. D., Stone H. A., and Weitz D. A., Science 308(5721), 537 (2005). 10.1126/science.1109164 [DOI] [PubMed] [Google Scholar]
- 31.Chu L.-Y., Utada A. S., Shah R. K., Kim J.-W., and Weitz D. A., Angew. Chem. 119(47), 9128 (2007). 10.1002/ange.200701358 [DOI] [PubMed] [Google Scholar]
- 32.Shum H. C., Kim J.-W., and Weitz D. A., J. Am. Chem. Soc. 130(29), 9543 (2008). 10.1021/ja802157y [DOI] [PubMed] [Google Scholar]
- 33.Kim S.-H. and Weitz D. A., Angew. Chem. 123(37), 8890 (2011). 10.1002/ange.201102946 [DOI] [PubMed] [Google Scholar]
- 34.Trantidou T., Friddin M. S., Salehi-Reyhani A., Ces O., and Elani Y., Lap Chip 18(17), 2488 (2018). 10.1039/C8LC00028J [DOI] [PubMed] [Google Scholar]
- 35.Doufène K., Tourné-Péteilh C., Etienne P., and Aubert-Pouëssel A., Langmuir 35(39), 12597 (2019). 10.1021/acs.langmuir.9b02179 [DOI] [PubMed] [Google Scholar]
- 36.Zhu P. and Wang L., Lab Chip 17(1), 34 (2017). 10.1039/C6LC01018K [DOI] [PubMed] [Google Scholar]
- 37.Nisisako T., Curr. Opin. Colloid Interface Sci. 25, 1 (2016). 10.1016/j.cocis.2016.05.003 [DOI] [Google Scholar]
- 38.Ehrfeld W., Hessel V., and Löwe H., Microreactors—New Technology for Modern Chemistry (Wiley-VCH, Weinheim, 2000). [Google Scholar]
- 39.Nisisako T. and Torri T., Lab Chip 8(2), 287 (2008). 10.1039/B713141K [DOI] [PubMed] [Google Scholar]
- 40.Conchouso D., Castro D., Khan S. A., and Foulds I. G., Lab Chip 14(16), 3011 (2014). 10.1039/C4LC00379A [DOI] [PubMed] [Google Scholar]
- 41.Muluneh M. and Issadore D., Lab Chip 13(24), 4750 (2013). 10.1039/c3lc50979f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji X.-H., Zhang N.-G., Cheng W., Guo F., Liu W., Guo S.-S., He Z.-K., and Zhao X.-Z., J. Mater. Chem. 21(35), 13380 (2011). 10.1039/c1jm12253c [DOI] [Google Scholar]
- 43.Jeong H.-H., Yelleswarapu V. R., Yadavali S., Issadore D., and Lee D., Lab Chip 15(23), 4387 (2015). 10.1039/C5LC01025J [DOI] [PubMed] [Google Scholar]
- 44.Au A. K., Lee W., and Folch A., Lab Chip 14(7), 1294 (2014). 10.1039/C3LC51360B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Femmer T., Jans A., Eswein R., Anwar N., Moeller M., Wessling M., and Kuehne A. J. C., ACS Appl. Mater. Interfaces 7(23), 12635 (2015). 10.1021/acsami.5b03969 [DOI] [PubMed] [Google Scholar]
- 46.Hwang Y. H., Um T., Hong J., Ahn G. N., Qiao J., Kang I. S., Qi L., Lee H., and Kim D. P., Adv. Mater. Technol. 4, 1900457 (2019). 10.1002/admt.201900457 [DOI] [Google Scholar]
- 47.Martino C., Berger S., Wootoon R. C. R., and deMello A. J., Lab Chip 14(21), 4178 (2014). 10.1039/C4LC00992D [DOI] [PubMed] [Google Scholar]
- 48.Ghosh S. and Rousseau D., Curr. Opin. Colloid Interface Sci. 16(5), 421 (2011). 10.1016/j.cocis.2011.06.006 [DOI] [Google Scholar]
- 49.Davis H. T., Colloids Surf. A 91, 9 (1994). 10.1016/0927-7757(94)02929-6 [DOI] [Google Scholar]
- 50.de Gennes P.-G., Brochard-Wyart F., and Quere D., Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves (Springer, 2004). [Google Scholar]
- 51.Lee T. Y., Choi T. M., Shim T. S., Frijns R. A. M., and Kim S.-H., Lab Chip 16(18), 3415 (2016). 10.1039/C6LC00809G [DOI] [PubMed] [Google Scholar]
- 52.Riechers B., Maes F., Akoury E., Semin B., Gruner P., and Baret J.-C., Proc. Natl. Acad. Sci. U.S.A. 113(41), 11465 (2016). 10.1073/pnas.1604307113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toor A., Lamb S., Helms B. A., and Russell T. P., ACS Nano 12(3), 2365 (2018). 10.1021/acsnano.7b07635 [DOI] [PubMed] [Google Scholar]
- 54.Sheshachala S., Grösche M., Scherr T., Hu Y., Sun P., Bartschat A., Mikut R., and Niemeyer C. M., ChemPhysChem 21(10), 1070 (2020). 10.1002/cphc.201901151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner O., Thiele J., Weinhart M., Mazutis L., Weitz D. A., Huck W. T. S., and Haag R., Lab Chip 16(1), 65 (2016). 10.1039/C5LC00823A [DOI] [PubMed] [Google Scholar]
- 56.Ki S. and Kang D.-K., Biosensors 10(11), 172 (2020). 10.3390/bios10110172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehling A., Kleber M., and Hensen H., Food Chem. Toxicol. 45(5), 747 (2007). 10.1016/j.fct.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 58.Wu F., Deng J., Hu L., Zhang Z., Jiang H., Li Y., Yi Z., and Ngai T., Colloids Surf. A 602, 125082 (2020). 10.1016/j.colsurfa.2020.125082 [DOI] [Google Scholar]
- 59.Yang T., Choi S. K., Park D., Lee Y. R., Chung C. B., and Kim J. W., Langmuir 32(50), 13403 (2016). 10.1021/acs.langmuir.6b03203 [DOI] [PubMed] [Google Scholar]
- 60.Wang J., Deng H., Sun Y., and Yang C., J. Colloid Interface Sci. 562, 529 (2020). 10.1016/j.jcis.2019.11.081 [DOI] [PubMed] [Google Scholar]
- 61.Nonomura Y. and Kobayashi N., J. Colloid Interface Sci. 330(2), 463 (2009). 10.1016/j.jcis.2008.10.063 [DOI] [PubMed] [Google Scholar]
- 62.Wei Y., Niu Z., Wang F., Feng K., Zong M., and Wu H., Mater. Sci. Eng. C 109, 110503 (2020). 10.1016/j.msec.2019.110503 [DOI] [PubMed] [Google Scholar]
- 63.Albert C., Huang N., Tsapis N., Geiger S., Rosilio V., Mekhloufi G., Chapron D., Robin B., Beladjine M., Nicolas V., Fattal E., and Agnely F., Langmuir 34(46), 13935 (2018). 10.1021/acs.langmuir.8b02558 [DOI] [PubMed] [Google Scholar]
- 64.Fujisawa S., Togawa E., and Kuroda K., Sci. Technol. Adv. Mater. 18(1), 959 (2017). 10.1080/14686996.2017.1401423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee D., Park D., Shin K., Seo H. M., Lee H., Choi Y., and Kim J. W., J. Ind. Eng. Chem. 96, 219 (2021). 10.1016/j.jiec.2021.01.018 [DOI] [Google Scholar]
- 66.Kaufman G., Boltyanskiy R., Nejati S., Thiam A. R., Loewenberg M., Dufresne E. R., and Osuji C. O., Lab Chip 14(18), 3494 (2014). 10.1039/C4LC00482E [DOI] [PubMed] [Google Scholar]
- 67.Zhang L., Cai L.-H., Lienemann P. S., Rossow T., Polenz I., Vallmajo-Martin Q., Ehrbar M., Na H., Mooney D. J., and Weitz D. A., Angew. Chem. 128(43), 13668 (2016). 10.1002/ange.201606960 [DOI] [PubMed] [Google Scholar]
- 68.Kim M., Yeo S. J., Highley C. B., Burdick J. A., Yoo P. J., Doh J., and Lee D., ACS Nano 9(8), 8269 (2015). 10.1021/acsnano.5b02702 [DOI] [PubMed] [Google Scholar]
- 69.Navi M., Kieda J., and Tsai S. S. H., Lab Chip 20(16), 2851 (2020). 10.1039/D0LC00387E [DOI] [PubMed] [Google Scholar]
- 70.Lee J. Y., Choi K. H., Hwang J., Sung M., Kim J. E., Park B. J., and Kim J. W., Chem. Commun. 56(45), 6031 (2020). 10.1039/D0CC02231D [DOI] [PubMed] [Google Scholar]
- 71.Hwang J., Sung M., Seo B., Shin K., Lee J. Y., Park B. J., and Kim J. W., ACS Appl. Mater. Interfaces 13(6), 7664 (2021). 10.1021/acsami.0c18116 [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Liu X., Zhang Z., Zhao S., Tian G., Zheng J., Wang D., Shi S., and Russell T. P., Angew. Chem. Int. Ed. 57(41), 13560 (2018). 10.1002/anie.201808888 [DOI] [PubMed] [Google Scholar]
- 73.Li J. and Stöver H. D. H., Langmuir 26(19), 15554 (2010). 10.1021/la1020498 [DOI] [PubMed] [Google Scholar]
- 74.Cho Y. S., Lee S. H., Seo H. M., Shin K., Kang M. H., Lee M., Park J., and Kim J. W., Langmuir 37(13), 3828 (2021). 10.1021/acs.langmuir.0c03082 [DOI] [PubMed] [Google Scholar]
- 75.Alison L., Rühs P. A., Tervoort E., Teleki A., Zanini M., Isa L., and Studart A. R., Langmuir 32(50), 13446 (2016). 10.1021/acs.langmuir.6b03439 [DOI] [PubMed] [Google Scholar]
- 76.Mason T. G., Wilking J. N., Meleson K., Chang C. B., and Graves S. M., J. Phys.: Condens. Matter 18(41), R635 (2006). 10.1088/0953-8984/18/41/R01 [DOI] [Google Scholar]
- 77.Marto J., Duarte A., Simões S., Gonçalves L., Gouveia L., Almeida A., and Ribeiro H., Polymers 11(1), 108 (2019). 10.3390/polym11010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marto J., Pinto P., Fitas M., Gonçalves L. M., Almeida A. J., and Ribeiro H. M., Toxicol. Appl. Pharmacol. 342, 14 (2018). 10.1016/j.taap.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 79.Tao S., Jiang H., Wang R., Yang C., Li Y., and Ngai T., Chem. Commun. 56(90), 14011 (2020). 10.1039/D0CC05690A [DOI] [PubMed] [Google Scholar]
- 80.Frelichowska J., Bolzinger M.-A., Valour J.-P., Mouaziz H., Pelletier J., and Chevalier Y., Int. J. Pharm. 368(1–2), 7 (2009). 10.1016/j.ijpharm.2008.09.057 [DOI] [PubMed] [Google Scholar]
- 81.Asfour M. H., Elmotasem H., Mostafa D. M., and Salama A. A. A., Int. J. Pharm. 534(1–2), 325 (2017). 10.1016/j.ijpharm.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 82.Kim J. W., Cho J., Cho J., Park B. J., Kim Y.-J., Choi K.-H., and Kim J. W., Angew. Chem. 128(14), 4585 (2016). 10.1002/ange.201600209 [DOI] [Google Scholar]
- 83.Haney B., Chen D., Cai L.-H., Weitz D., and Ramakrishnan S., Langmuir 35(13), 4693 (2019). 10.1021/acs.langmuir.9b00058 [DOI] [PubMed] [Google Scholar]
- 84.Seo H. M., Seo M., Shin K., Choi S., and Kim J. W., Carbohydr. Polym. 258, 117730 (2021). 10.1016/j.carbpol.2021.117730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.