Abstract
The LUCINDA Trial (Leuprolide plus Cholinesterase Inhibition to reduce Neurologic Decline in Alzheimer’s) is a 52 week, randomized, placebo-controlled trial of leuprolide acetate (Eligard) in women with Alzheimer’s disease (AD). Leuprolide acetate is a gonadotropin analogue commonly used for hormone-sensitive conditions such as prostate cancer and endometriosis. This repurposed drug demonstrated efficacy in a previous Phase II clinical trial in those women with AD who also received a stable dose of the acetylcholinesterase inhibitor donepezil (Bowen et al., 2015). Basic biological, epidemiological and clinical trial data suggest leuprolide acetate mediates improvement and stabilization of neuropathology and cognitive performance via the modulation of gonadotropin and/or gonadotropin-releasing hormone signaling. LUCINDA will enroll 150 women with mild-moderate AD who are receiving a stable dose of donepezil from three study sites in the United States. Cognition and function are the primary outcome measures as assessed by the Alzheimer’s Disease Assessment Scale-Cognitive Subscale. Blood and MRI biomarkers are also measured to assess hormonal, inflammatory and AD biomarker changes. We present the protocol for LUCINDA and discuss trial innovations and challenges including changes necessitated by the covid-19 pandemic and study drug procurement issues.
1. Introduction
Alzheimer’s Disease (AD) has a significant economic, social and emotional impact on virtually every stratum of our society. In the United States alone, 5.4 million, or 1 of every 9 people over age over age 65, are currently suffering from AD (www.alz.org). Currently approved medications for AD have limited effects in slowing disease progression.1 Recent failures of anti-amyloid medication trials2 highlight the critical need for new models of understanding and treating AD. Here, we describe the rationale, methods and implementation of the LUCINDA (Leuprolide plus Cholinesterase Inhibition to reduce Neurologic Decline in Alzheimer’s) Trial. Premised on the Cell Cycle Theory of aging and AD3, 4 and supported by robust preclinical evidence and prior human trials,5, 6 LUCINDA trial aims to repurpose the injectable medication leuprolide acetate for AD.
1.1. Study Rationale
Leuprolide is an analogue of gonadotrophin-releasing hormone (GnRH), a ten amino acid peptide that is synthesized and secreted from neurons in the anterior hypothalamus. GnRH is secreted in a pulsatile fashion into the hypophysial portal bloodstream at the median eminence and is carried to the pituitary gland where it binds to GnRH receptors on gonadotrope cells and signals the synthesis of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). When given chronically, GnRH analogues like leuprolide disrupt the pulsatile release of GnRH, thereby resulting in the downregulation of FSH and LH synthesis and secretion, and the suppression of gonadal sex hormone (estrogen and testosterone) production in men and pre-menopausal women. Leuprolide is commonly used to treat prostate cancer, in fertility regimens, to treat severe endometriosis, to shrink uterine fibroids and to treat precocious puberty in children.7 For these conditions, leuprolide’s suppression of circulating sex hormones is considered the primary mechanism of action. In post-menopausal women, leuprolide decreases LH and FSH but has no measurable effect on the already low concentration of ovarian hormones.8
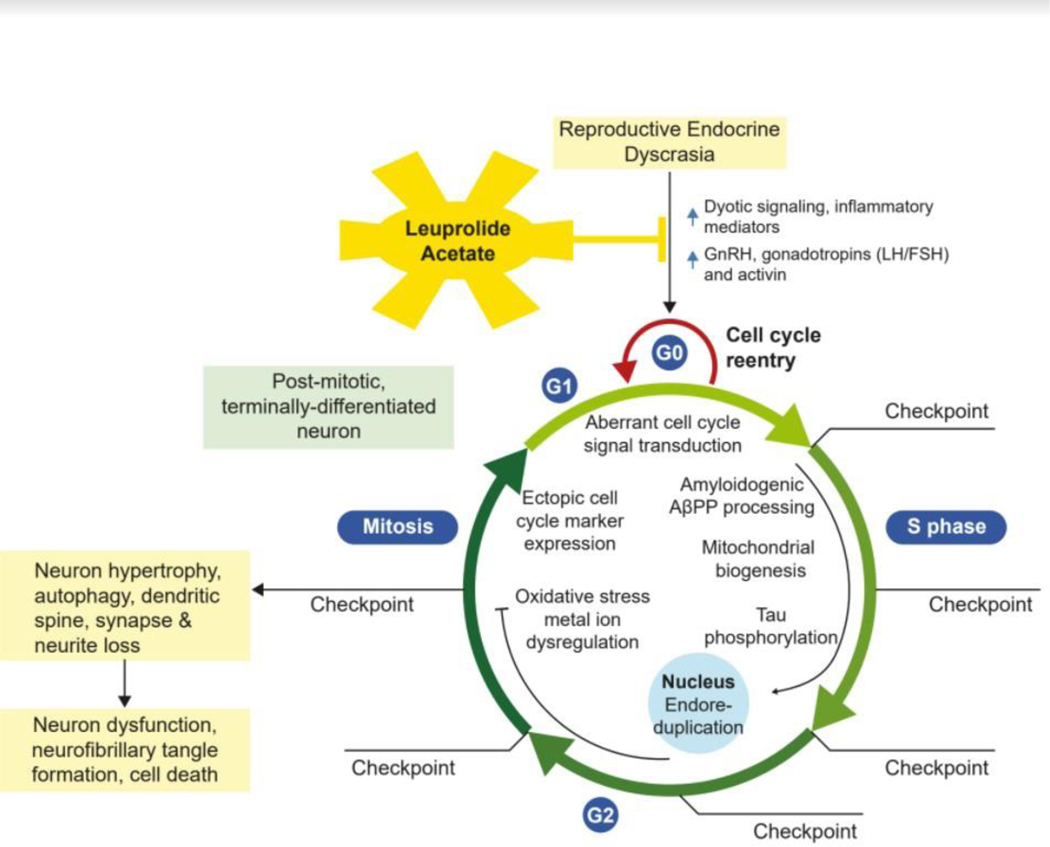
While LH is important for normal brain structure and function when the HPG axis is in balance, the endocrine dyscrasia that results with aging (marked elevations in LH signaling but loss of sex steroid signaling) is a driver of neurodegeneration 4, 5 According to the Cell Cycle Theory of Aging and AD, elevated levels of LH, a powerful mitogen,9–11 in the absence of normal circulating sex steroids, are a signal that initiates the abortive re-entry of post-mitotic, terminally differentiated, pyramidal neurons in the hippocampus and neocortex into an abortive mitotic cell cycle3, 4 as shown in Figure 1. Extensive preclinical validation work has demonstrated that decreasing LH improves cognitive performance and decreases amyloid deposition and tau phosphorylation in multiple animal models of AD.4, 5, 12–25
Figure 1. Leuprolide Acetate Action on Neuronal Cell Cycle.
Model of aberrant cell cycle re-entry initiated by endocrine dyscrasia (low sex hormones but elevated GnRH, LH/FSH and activin signaling) following menopause/andropause. The abortive reactivation of the cell cycle in a post-mitotic, terminally differentiated neuron drives endoreduplication, neuron hypertrophy, amyloid deposition, neurofibrillary tangle formation, autophagy, dendritic spine, synapse and neuron loss, neuron dysfunction, and ultimately cell death. Leuprolide acetate suppresses post-reproductive elevations in GnRH and LH/FSH, and thereby blocks the re-entry of neurons into the cell cycle, preventing neurodegeneration.
In addition to its LH-lowering effect, leuprolide may also have anti-inflammatory effects. Although the role of inflammation in AD is complex, there is strong evidence that peripheral inflammation is associated with AD progression 26, 27 and inflammation remains an active therapeutic target in AD.28, 29 Anti-inflammatory effects of GnRH analogues,30–35 noted in animal studies and human studies of other diseases will be evaluated in this trial.
1.1.1. Human evidence for leuprolide as a treatment for AD
A clinical trial of leuprolide for AD was initially motivated by anecdotal experience of patients with advanced AD who improved dramatically after receiving leuprolide as treatment for prostate cancer.36–38 A small European clinical trial showed cognitive improvement in male AD patients treated with leuprolide for prostate cancer.6 In a second trial, a phase II, 48-week, double-blind, placebo-controlled, dose-ranging study,4 a total of 109 women with mild to moderate AD were randomized to low dose leuprolide (11.25 mg), high dose leuprolide (22.5 mg) or placebo injections every 12 weeks. At the 48 week endpoint, there were no statistically significant differences in primary efficacy parameters: the Alzheimer’s Disease Assessment Scale–Cognitive Subscale39 (ADAS-Cog) and Alzheimer’s Disease Cooperative Study - Clinical Global Impression of Change40 (ADCS-CGIC) although there was a trend in favor of the high dose leuprolide group. However, in a pre-specified subgroup analysis of patients taking the AChEI donepezil (72% of patients) there was a statistically significant benefit in the high dose leuprolide group compared to both the low dose and placebo groups. While this trial did not succeed in showing efficacy in the primary analysis of the total study population, results of the planned secondary subgroup analyses showed cognitive and clinical improvement in women treated with high dose leuprolide who were already using an AChEI when compared to women treated with AChEI alone. The purpose of the LUCINDA trial is to further investigate this positive interaction between leuprolide and AChEIs in the treatment of AD. All LUCINDA participants are required to be taking a stable dose of the AChEI donepezil.
1.1.2. Leuprolide and donepezil synergy
While the mechanism of synergy between leuprolide and donepezil remains uncertain, we posit three possibilities: a general role of cholinergic mechanisms in neuroplasticity, with adequate cholinergic tone required for neural repair and reorganization,41 an effect on inflammation42 and/or an effect on kisspeptin, a hypothalamic peptide that is the key regulator of GnRH pulsatile secretion43 and which also affects cholinergic transmission.44 The latter two of these possibilities will be evaluated in exploratory analyses in LUCINDA.
2. Methods
2.1. Study Design
The LUCINDA trial is a three site, randomized, placebo-controlled double-blind study to assess the effect of a 48-week regimen of leuprolide acetate (Eligard, 22.5 mg subcutaneous injection every 12 weeks) compared to placebo on cognition, and blood and neuroimaging biomarkers in women (n=150) with AD or Mild Cognitive Impairment (MCI) due to AD as defined by current research criteria45, 46 who are also taking the AChEI donepezil. The three sites are: Weill Cornell Medicine, University of Miami, and University of Wisconsin – Madison. Each site Principal Investigator (PI) is also multiple PI for the NIH grant supporting this project. This project is approved by the BRANY (Biomedical Research Alliance of New York) single IRB.
2.2. Study Endpoints
The Primary Study Endpoint is change in cognition from baseline to post-treatment as measured by the ADAS-Cog.39 The ADAS-Cog is a standard measure of cognition commonly used in AD therapeutic trials.
Secondary efficacy / covariate endpoints are Alzheimer’s Disease Cooperative Study – Activities of Daily Living47 (ADCS-ADL; a caregiver interview to assess functioning), ADCS-CGIC40 (a clinician-administered semi-structured interview with both the subject and caregiver [separately] to assess clinically meaningful global change), NPI-Q48 (a caregiver questionnaire assessing psychiatric symptoms) and Burden Inventory (BI; a caregiver questionnaire assessing the burden of caring for the subject.)49 These tests were used in the prior trial of leuprolide for AD,4 which LUCINDA aims to replicate and extend. In addition, LUCINDA includes the Brief Pain Inventory,50 and the Repeatable Battery for the Assessment of Neuropsychological Status51 (RBANS) to more carefully assess cognition. Pain is being measured because GnRH analogues improve pain and mobility in elderly patients with rheumatoid arthritis,32 and decreased pain could be associated with improved function independent of cognitive benefit.
Secondary Neuroimaging Biomarker Endpoints are pre-to-post treatment change in MRI-measured brain volumes (bilateral hippocampus, bilateral precuneus, bilateral posterior cingulate, total gray matter, total ventricular volume) and Arterial Spin Labeling Magnetic Resonance Imaging (ASL MRI)-measured hippocampal perfusion.
Secondary Blood Biomarker Endpoints are pre-to-post treatment change in C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR) and cytokines, in particular IL1-β, TNF-α and IL6 which are elevated in AD26 and have been shown to decrease in response to GnRH analogues.31, 32
2.3. Participants
LUCINDA will enroll 150 women with mild-moderate AD or MCI due to AD. In accord with current research criteria, subjects are diagnosed with MCI due to AD when in addition to demonstrable cognitive decline, they have biomarker evidence of AD pathology, in this case, cortical amyloid detected with Neuraceq PET.45, 46 Subjects must have a Clinical Dementia Rating (CDR) global score between .5 and 2. They must be taking a stable dose of donepezil for at least 90 days prior to enrollment. There must amyloid present in cortex based on interpretation of Neuraceq PET by a board-certified radiologist in accord with standard criteria.52 Approximately equal numbers of subjects will be enrolled at each of the three sites. All subjects and/or their study partner / caregiver will provide informed consent to participate. Full inclusion and exclusion criteria are presented in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Key Inclusion Criteria for the main study are: |
|---|
| • Age >65 |
| • Female, post-menopausal |
| • Probable AD or MCI due to AD according to NIA-AA criteria45, 53 |
| • Clinical Dementia Rating (CDR) between 0.5 and 2 |
| • Amyloid present in brain based on interpretation of Neuraceq PET by a board-certified radiologist |
| • Taking a stable dose of the AChEI donepezil for at least 90 days prior to baseline, and dosage likely to remain stable throughout the trial |
| • Stable doses of any other medication, supplement or medical food that may affect brain function |
| • MOCA >11 (out of 30) or MOCA-BLIND > 8 (out of 22) at screening visit |
| • Hachinski score54 <5 supporting clinical judgment that dementia is not of vascular origin |
| • Fluent in English |
| • Living at home or in a facility other than a nursing home with a caregiver who sees the patient at least three times a week for a total of at least 10 hours and can sign the consent form, accompany the patient on clinic visits, and participate in evaluations |
| Key Exclusion Criteria for the main study are: |
| • Presence based on exam, history or MRI of significant brain disease other than AD such as schizophrenia, epilepsy, Parkinson’s disease or large territory stroke |
| • Current substance abuse in accord with DSM V criteria |
| • Significantly depressed (Geriatric Depression Scale55 < 10) |
| • Physical or psychological MRI contraindications, or likely unable to tolerate neuroimaging |
| • Taking memantine within 90 days of baseline |
| • Taking medications known to affect serum sex hormones such as GnRH agonists or estrogen and/or progesterone for hormone replacement therapy |
| • Presence of significant systemic illness likely to interfere with participation in or completion of the study or to affect study results such as cancer within 5 years (other than non-melanoma skin cancer), autoimmune disease, recent myocardial infarction, signs/symptoms of organ failure based on history, ECG, screening laboratory and/or physical exams |
| • Receiving other investigational drugs within 30 days or 5 half-lives prior to randomization |
| • Ever treated with active or passive immunization as part of a different clinical trial for AD due to unknown alterations in systemic and brain inflammation, which may confound results |
| • Known hypersensitivity to GnRH, GnRH analogs or any of the components of Eligard |
| Criteria for amyloid-negative sub-study: |
| • same as above except amyloid absent in brain based on interpretation of Neuraceq PET |
2.3.1. Amyloid negative sub-study
In addition, up to thirty subjects diagnosed clinically with AD and meeting all study inclusion criteria except found to be amyloid negative at Neuraceq PET scanning will be randomized into a separate sub-study with procedures identical to the main study. Results from these amyloid negative subjects will be examined separately.
2.4. Recruitment and Enrollment
Patients are recruited from investigators’ clinical practices or research cohorts, letters/emails to and referrals from other clinicians, via outreach efforts in the community (e.g. health fairs, religious or social groups, assisted living or retirement communities, AD patient advocacy and caregiver support groups), posted flyers and brochures, newspaper or radio advertisements, the lucinda.weill.cornell.edu website, digital marketing (e.g. paid google search advertisements), and social media campaigns (e.g. facebook, Instagram, twitter).
2.5. Randomization
There will be a random (1:1) assignment of patients to leuprolide acetate or placebo. Randomization will be performed separately for amyloid positive (main study) and amyloid negative (sub-study) participants. Randomization for the main study and amyloid-negative sub-study will be stratified by site.
2.6. Study Drug
2.6.1. Eligard
LUCINDA uses Eligard, a polymeric matrix formulation of leuprolide acetate for subcutaneous injection designed to deliver drug at a controlled rate over a 1–6 month period. It is supplied as two separate syringes which must be mixed immediately (<30 minutes) prior to injection. Eligard is FDA approved to treat prostate cancer. As discussed in section 2.1 above, while leuprolide is used clinically to reduce sex hormone levels to treat disease such as prostate cancer and endometriosis, reduction of LH is considered the primary mechanism of leuprolide’s anti-AD effect. Eligard 22.5mg for subcutaneous administration every 3 months is supplied by the manufacturer, Tolmar Pharmaceuticals.
2.6.2. Placebo
Placebo injections consist of 0.375 cc of 0.9% sterile sodium chloride Injection administered with a 5/8 inch long 20-gauge needle (identical to the Eligard needle) on a single-use syringe.
2.6.3. Blinding Procedures
Because it was cost prohibitive to create placebo identical to Eligard, LUCINDA procedures require that study drug be obtained from the pharmacy, prepared and injected by an unblinded study staff member who plays no other role in the study. Study drug is injected in the buttock, out of view of the subject, with the study partner in a separate room.
2.7. Study procedures / evaluations
A schedule of study procedures is presented in Appendix 1. Key study procedures are described in greater detail:
2.7.1. Amyloid Positron Emission Tomography
PET scanning uses the FDA-approved tracer florbetaben (Neuraceq) which is supplied by the manufacturer, Life Molecular Imaging, to each site. PET scans are interpreted as positive or negative by a board-certified radiologist in accordance with standard guidelines.52 Amyloid positive subjects are randomized in the main study while amyloid negative subjects (stratified by site; maximum of 30 total) are randomized into an amyloid-negative sub-study with identical procedures.
2.7.2. MRI: structural and ASL sequences
MRI examinations are performed on a 3T scanner at each site using acquisition parameters based on those used for ADNI-GO 3T imaging (http://adni.loni.usc.edu/methods/documents/mri-protocols/) Regional volume measurements use Freesurfer’s longitudinal processing stream56 and mixed linear effect model57 to optimize reliability and statistical power for measuring within-subject volumetric changes over time. We will focus on the following structural MRI biomarkers associated with AD: hippocampal, posterior cingulate, precuneus, total gray matter and total ventricular volume. To image hippocampal perfusion, a functional measure similar to that obtainable using FDG PET, a pulsed ASL sequence is used.58 Average bi-hippocampal perfusion will be quantified as described previously,59, 60 and pre- to post-treatment change will be compared between treatment groups. Appropriate methods will be taken to reduce site-related differences in image acquisitions and derived volumetric and perfusion estimates.61
2.7.3. Cognitive and other assessments and modifications for remote use
Assessments used for inclusion criteria and outcome measurement include standardized scales for cognition, function, behavior/mood and other domains. Assessments and their mode of administration are listed in Table 2.
Table 2.
LUCINDA assessments and mode of administration.
| TEST | USE | INCLUSION CRITERIA | ADMINISTRATION |
|---|---|---|---|
| MOCA-Blind | Screening | >11/22 | remote |
| CDR | Screening | .5–2 (global) | remote |
| Hachinski | Screening | <5 | remote |
| Geriatric Depression Scale63 | Screening | <11 | remote |
| ADAS-cog39 | Primary Outcome | Hybrid administration | |
| RBANS | Outcome | Remote except for coding | |
| CGIC | Outcome | Remote | |
| ADCS-ADL | Outcome | Remote or in person – electronic caregiver survey | |
| NPI-Q48 | Outcome / covariate | Remote or in person – electronic caregiver survey | |
| Burden Inventory | Outcome | Remote or in person – electronic caregiver survey | |
| Brief Pain Inventory50 | Outcome / covariate | In-person | |
| Unblinding questionnaire | Covariate | Remote or in-person |
To minimize the risk of exposing our vulnerable subject population to Covid-19 during study visits, we revised our protocol to allow as many assessments as possible to be administered remotely. To ensure comparability within subject across timepoints, and across subjects, we chose modes of administration that would be accurate and appropriate even when/if risk of Covid-19 exposure diminishes. For some assessments such as the Montreal Cognitive Assessment (MOCA), a version without visual elements – the MOCA-blind62 - had already been validated and was in use as part of the National Alzheimer’s Coordinating Center Unified Data Set Telephone Cognitive Battery (naccdata.org.)
For the ADAS-cog,39 our primary outcome measure, we designed what we have termed a hybrid ADAS-cog administration involving both an on-site study coordinator and a remote tester in a separate location. According to this approach, the subject is tested in a private room in the medical center but the majority of test items are administered via teleconference by a remote tester. The on-site coordinator sets up the videoconference system (we are using Zoom) which includes an additional overhead camera so the remote tester can observe the subject’s face, hands and body. While the majority of communication is between the subject and the remote tester, the on-site coordinator presents study materials such as naming objects and paper for copying figures. Importantly, neither the subject nor the remote tester wear face masks during administration; communication between subject and tester through face masks can be challenging, especially when a subject is cognitively impaired.
Modifications to assessments motivated by Covid-19 will continue throughout the trial at all sites for all subjects, regardless of pandemic conditions.
2.7.4. Laboratory Evaluations
Routine clinical laboratory tests are be performed at each site’s clinical laboratory at baseline to exclude significant medical conditions and at each study visit to monitor subject health and safety. Other laboratory tests are be performed at the Weill Cornell Clinical and Translational Science Center Core Laboratory. Apolipoprotein E (APOE) genotyping is ascertained for all subjects. Subgroup analyses will consider APOE4 status. CRP, cytokine panel and ESR are inflammation biomarker endpoints. Determinations of serum concentrations of LH, FSH, fractionated estradiols, free and total testosterone, progesterone, sex hormone binding globulin, DHEAS, inhibin-B, kisspeptin leuprolide and donepezil will be used for covariate analyses.
2.8. Statistical Plan
2.8.1. Sample Size
Based on the prior trial of leuprolide for AD8 showing a point difference in ADAS-Cog39 of 3.12 (mean decline for leuprolide 22.5mg + AChEI = 0.18; placebo + AChEI = 3.30; effect size of .56), 150 subjects (75 per arm; 1:1 randomization) will be randomized to detect a difference in change from baseline between the two treatment groups of +/−0.46 standard deviations in this primary endpoint. Accounting for a dropout rate of approximately 20%, we would have 60 subjects per group and a detectable difference of +/−0.52 standard deviations. The study will have 80% power to demonstrate a treatment effect on ADAS-Cog39 with alpha of .05 (2-sided) based on a t-test with transformation of the data if required to meet the assumptions of the t-test.
2.8.2. Measures to Minimize Bias
All analyses will be based on all randomized patients (intent to treat). Changes from baseline over time will be compared using mixed effects regression models that include a random effect for each subject and fixed effects of treatment group, center, and time, and include the repeated observations on each patient over time taking into account that the observations are not independent within a patient. Interactions between treatment group, center, and time will also be considered included in the models. The effects of potential confounding factors such as age, education, LH and other hormone levels, leuprolide levels, donepezil level and/or other measure of donepezil compliance (study drug compliance is ensure by injection at study visits) and baseline cognitive scores on outcomes will be explored. All variables will be transformed as necessary to meet the assumptions of the proposed analyses.
2.8.3. Primary Analysis
The primary efficacy analysis will be based on the difference between the leuprolide treated and placebo-treated group groups with respect to changes from baseline at 48 weeks in ADAS-Cog39 score. Mixed effects regression models will be used, and the primary efficacy assessment will be based on the 48-week ADAS-Cog measurement. Additional analyses will consider changes from baseline at each time point up to and including 48 weeks and sensitivity analysis based on examination of results in dropouts vs those who complete the study by treatment group among other analyses.
2.8.4. Secondary analyses
Secondary analyses will be based on the difference between the two treatment groups with respect to changes from baseline in laboratory measurements and scoring instruments (ADCS-ADL,47 ADCS-CGIC+,40 NPI-Q;48 BI,49 Brief Pain Inventory50 and the RBANS51) at 48 weeks.
2.8.5. Planned subgroup analyses
Results will be presented for specified subgroups of subjects based on relevant factors including APOE4 genotype status (APOE4 allele present or absent) and initial MOCA (high = 21–30; low = 11–20) or Blind MOCA (high = 16–22; low = 8–15) score for each of the primary and secondary outcomes. No statistical adjustments will be made for these multiple subgroup analyses.
All primary and secondary outcomes will be summarized descriptively in the amyloid negative sub-study consisting of subjects diagnosed clinically with AD but found to be amyloid negative via Neuraceq PET scanning.
2.8.6. Interim Analyses
One interim analysis for efficacy and futility will be conducted when 50% of the randomized patients could have completed 12 months of follow up based on the comparison of changes from baseline in ADAS-COG at 12 months estimated from the mixed effects regression models. This interim analysis is expected to occur at approximately 30 months after the study starts with approximately 75 patients who could have completed 12 months of follow-up.
2.8.7. Biomarker Analyses
Pre- to post-treatment change will be compared between patients treated with leuprolide versus placebo for plasma inflammatory markers (IL1-β, TNF-α, IL6, ESR, CRP) with appropriate correction for multiple comparisons. For structural MRI measures, pre- to post-treatment change in Freesurfer-measured bilateral regional or total gray matter volume, normalized to total intracranial volume, and including age and site as covariates, will be compared between treatment groups. For ASL MRI, average bi-hippocampal perfusion will be quantified as described previously,59, 60 and pre- to post-treatment change will be compared between treatment groups. Methods are similar to those of the primary analysis. Further, these results for each subject will be integrated with the primary study results and the contributions of these markers to outcome assessed.
2.8.8. Exploratory Analyses
To identify whether leuprolide has a disease-modifying effect in AD, correlations between neuroimaging biomarkers and cognitive and functional scores will be assessed. Exploratory analyses will assess pre-treatment plasma inflammatory markers as predictors of leuprolide efficacy and MRI changes, and whether plasma kisspeptin levels (which could plausibly mediate the synergy between leuprolide and donepezil44) change with treatment (vs placebo) and/or predict leuprolide efficacy.
3. Discussion
Effective therapies for treating AD are desperately needed. Treatments aimed at reducing or removing amyloid have not been successful to date.2 The LUCINDA trial is one of the first trials premised on the promising Cell Cycle Theory of Aging and AD.3, 4 It uses a safe, repurposed agent (leuprolide) which targets multiple pathophysiological mechanisms in AD. By repurposing an existing medication, in combination with a current AD treatment (donepezil), we will be able to build upon extensive previous research and development efforts, reducing the time frame and costs of making a promising therapy available to patients with AD. The LUCINDA trial protocol required solving a number of logistical and other problems. We highlight these issues and innovations with the hope that this will benefit other clinical investigators.
3.1. Amyloid negative sub-study
Thirty subjects diagnosed clinically with AD and meeting all study inclusion criteria except found to be amyloid negative at Neuraceq PET scanning will be randomized into a separate sub-study with procedures identical to the main study. The rationale for enrolling these subjects is that (1) the prior trial of leuprolide for AD,8 which met its endpoint in a prespecified subgroup, would have likely included approximately 15% amyloid negative subjects64 since amyloid PET was not performed, (2) there is preclinical evidence indicating that amyloid-negative older rodents benefit cognitively from leuprolide,35 (3) there is increasing realization that AD is highly heterogeneous, with the majority of patients diagnosed in life with AD actually having mixed pathology at autopsy65 and (4) the cost of maintaining study subjects who have already undergone MRI, PET and other screening procedures is relatively low. The rationale for not including amyloid-negative subjects in the primary study analysis is that they do not actually have AD according to current research criteria.64, 66 Enrolling these subjects in a separate sub-study was considered an appropriate strategy for addressing these issues. We suggest this could be considered for other trials of AD therapies not focused exclusively on amyloid removal.
3.2. Study Drug Procurement Issues:
While repurposing an existing medication was expected to be quicker, simpler and less expensive than developing an entirely new drug for AD, we encountered significant difficulties procuring the leuprolide formulation we had originally planned to use in this trial. This leuprolide formulation, though off-patent, was not available in generic form because of technical barriers to manufacturing. The sole manufacturer of this leuprolide formulation, which has its own AD therapies under development, was not willing to provide study drug for LUCINDA at any price. Attempting to purchase this leuprolide formulation from retail pharmacies revealed remarkable differences in price across the three study sites ranging from $300 to $6000 per dose, with significant price fluctuations over time. Realizing use of the original formulation of leuprolide was logistically and financially not feasible, we approached a different company, Tolmar Pharmaceuticals, which manufactures Eligard, a newer version of leuprolide. Tolmar agreed to provide Eligard at no cost for this trial. However, trial enrollment was delayed substantially because of the need to switch study drug formulation. This is an example of a major barrier to drug repurposing to treat AD and other human disorders, and highlights the need to incentivize cooperation from pharmaceutical companies.67
3.3. Trial modifications due to the Covid-19 pandemic
The Covid-19 pandemic shut down clinical research for months. Before enrolling the first subject, we modified LUCINDA procedures significantly to ensure that we could safely enroll subjects while minimizing infectious risk. The first LUCINDA subject was enrolled in November 2020, in the midst of the pandemic. The study visit schedule minimized the number of in-person visits to those required for PET, MRI scanning and study drug injection. We are conducting informed consent remotely in accord with the FDA’s 2016 guidance document “Use of Electronic Informed Consent: Questions and Answers Guidance for Institutional Review Boards, Investigators, and Sponsors” (81 FR 90855.) We have IRB approval to obtain remote informed consent via several methods depending on the communication technology available to the subject. Our consent form mentions specifically the risk of Covid-19 exposure during study visits, which arguably constitutes greater health risk to participants than any other trial procedures including study drug injection.
As detailed in Section 2.69, we modified study assessments for remote use whenever possible. For assessments which involve only verbal communication or filling out surveys, this was relatively simple, e.g. we converted the paper BI49 and NPI-Q,48 designed for caregiver self-administration, into redcap surveys which can be administered via any electronic device in any location. In some cases, we were able to use existing, validated instruments such as the MOCA-Blind.
For the ADAS-cog,39 our primary outcome measure, we were wary of relying upon fully remote administration. Cognitive testing via teleconference is limited by test requirements for paper or other physical test materials and by the subject/caregiver’s technological capabilities and equipment, with significant variations in testing conditions (technological and environmental) across subjects and potentially within subjects across time periods. While prior studies have shown acceptable correlation between the results of in-person and remote video teleconference ADAS-cog administration,68, 69 the correlation was less good in subjects with greater cognitive impairment and was particularly poor in the domain of language.69 This finding may reflect the ADAS-cog’s use of real objects to assess naming ability, and the fact that physical, graspable objects are processed differently by the brain than 2-D depictions of those objects.70, 71 Children perform better when naming real objects as compared to pictures of those objects,72 and it is plausible that this may also be the case for cognitively impaired subjects, though we are unaware of specific studies demonstrating this. We therefore designed a hybrid ADAS-cog administration involving both an on-site study coordinator and a remote tester in a separate location. This hybrid administration was designed to be appropriate for use even when Covid-19 is no longer a significant risk. An additional benefit of this mode of administration in multi-site studies is that it allows trained testers at one site to serve as back-up or even primary testers for other sites, regardless of physical distance.
3.4. Conclusion
We hope presenting the LUCINDA trial protocol and discussing implementation challenges and solutions will prove useful to other clinical researchers.
Acknowledgments
Funding
This work was supported by the National Institute of Aging of the National Institutes of Health, grant number R01AG057681 and by the Weill Cornell Clinical and Translational Science Center (grant UL1RR 024996.) Partial support for design (JDG) provided by UL1RR029893 from NIH/NCRR NYU+HHC Clinical Translational Science Award
Appendix 1.
Schedule of Study Visits and Procedures
| SCREENING | SCREENING PET & MRI | BASELINE 1st injection | 2nd injection | 3rd injection | 4th injection | f/u MRI | PK/PD | ||
|---|---|---|---|---|---|---|---|---|---|
| VISIT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| PROCEDURE | LOCATION | ≤31 days before baseline | ≤31 days before baseline | WEEK 0 |
WEEK 12 |
WEEK 24 |
WEEK 36 |
WEEK 48 |
WEEK 52 |
| Informed Consent | remote | X | |||||||
| Medical & Social History | remote | X | |||||||
| Review of concomitant medications | Remote or in person | X | X | X | X | X | X | ||
| CDR | Remote | X | |||||||
| MOCA-Blind | remote | X | |||||||
| Geriatric Depression Scale – Short Form63 | remote |
X | |||||||
| Hachinski Ischemia Score | remote | X | |||||||
| Physical/Neurologic Examination | In person | X | X | ||||||
| Weight & vital signs | In person | X | X | X | X | X | X | ||
| ECG | In person | X | |||||||
| Blood for B12, homocysteine, RPR, thyroid panel, HA1C, lipid panel | In person | X | |||||||
| Blood for CBC, metabolic panel | In person | X | X | X | X | X | X | ||
| Review Inclusion/Exclusion Criteria | remote | X | X | ||||||
| Multisequence MRI | In person | X | X | ||||||
| Neuraceq PET | In person | X | |||||||
| Randomization | remote | X | |||||||
| ADAS-Cog39 | hybrid | X | X | X | X | X | |||
| ADCS-CGIC+ | remote | X | X | X | X | X | |||
| Burden Inventory, ADCS-ADL, Neuropsychiatric Inventory | Remote or in person | X | X | X | X | X | |||
| Brief Pain Inventory50 | In person | X | X | X | X | X | |||
| RBANS except coding | remote | X | X | X | |||||
| RBANS coding | In person | X | X | X | |||||
| Blood for ApoE genotyping | In person | X | |||||||
| Blood for cytokines, CRP | In person | X | X | X | |||||
| Blood for covariate analyses: estrogens, testosterone, progesterone, SHBG, inhibin, kisspeptin, donepezil | In person |
X | X | X | |||||
| Blood for covariate and PK/PD analyses: LH, FSH and leuprolide | In person | X | X | X | X | X | X | ||
| STUDY DRUG INJECTION (must occur after any blood tests or assessments scheduled for that visit) | In person | X | X | X | X | ||||
| AE assessment | Remote or in person | X | X | X | X | X | |||
| Unblinding questionnaire | Remote or in person | X |
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4 REFERENCES
- 1.Birks JS. Cholinesterase inhibitors for Alzheimer’s disease. The Cochrane Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Couteur DG, Hunter S, Brayne C. Solanezumab and the amyloid hypothesis for Alzheimer’s disease. British Medical Journal Publishing Group 2016. [DOI] [PubMed] [Google Scholar]
- 3.Herrup K. The contributions of unscheduled neuronal cell cycle events to the death of neurons in Alzheimer’s disease. Front Biosci (Elite Ed). 2012;4:2101–2109. [DOI] [PubMed] [Google Scholar]
- 4.Atwood CS, Bowen RL. The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers re-entry of post-mitotic neurons into the cell cycle, neurodysfunction, neurodegeneration and cognitive disease. Hormones and behavior. 2015;76:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atwood CS, Bowen RL. A Unified Hypothesis of Early- and Late-Onset Alzheimer’s Disease Pathogenesis. Journal of Alzheimer’s disease : JAD. 2015;47(1):33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedelec C, Ragot S, Irani J, et al. [Effects by androgen suppression with luteinizing hormone on cognitive functions in men treated for cancer of prostate]. Prog Urol. 2009;19(1):47–53. [DOI] [PubMed] [Google Scholar]
- 7.Wilson AC, Vadakkadath Meethal S, Bowen RL, et al. Leuprolide acetate: a drug of diverse clinical applications. Expert opinion on investigational drugs. 2007;16(11):1851–1863. [DOI] [PubMed] [Google Scholar]
- 8.Bowen RL, Perry G, Xiong C, et al. A clinical study of lupron depot in the treatment of women with Alzheimer’s disease: preservation of cognitive function in patients taking an acetylcholinesterase inhibitor and treated with high dose lupron over 48 weeks. Journal of Alzheimer’s Disease. 2015;44(2):549–560. [DOI] [PubMed] [Google Scholar]
- 9.Mak GK, Enwere EK, Gregg C, et al. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10(8):1003–1011. [DOI] [PubMed] [Google Scholar]
- 10.Gallego MJ, Porayette P, Kaltcheva M, et al. Trophoblastic hormones direct early human embryogenesis. Available from Nature Precedings. 2008;http://hdl.handle.net/10101/npre.2008.2671.1. [Google Scholar]
- 11.Gallego MJ, Porayette P, Kaltcheva MM, et al. Opioid and progesterone signaling is obligatory for early human embryogenesis. Stem Cells Dev. 2009;18(5):737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barron AM, Verdile G, Taddei K, et al. Effect of chronic hCG administration on Alzheimer’s-related cognition and A beta accumulation in PS1KI mice. Endocrinology. 2010;151(11):5380–5388. [DOI] [PubMed] [Google Scholar]
- 13.Berry A, Tomidokoro Y, Ghiso J, et al. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair JA, Bhatta S, McGee H, et al. Luteinizing hormone: evidence for direct action in the CNS. Hormones and behavior. 2015;76:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen RL, Verdile G, Liu T, et al. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279(19):20539–20545. [DOI] [PubMed] [Google Scholar]
- 16.Bryan KJ, Mudd JC, Richardson SL, et al. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112(4):870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnham V, Sundby C, Laman-Maharg A, et al. Luteinizing hormone acts at the hippocampus to dampen spatial memory. Hormones and Behavior. 2017;89:55–63. [DOI] [PubMed] [Google Scholar]
- 18.Casadesus G, Milliken EL, Webber KM, et al. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269(1–2):107–111. [DOI] [PubMed] [Google Scholar]
- 19.Casadesus G, Webber KM, Atwood CS, et al. Luteinizing hormone modulates cognition and amyloid-β deposition in Alzheimer APP transgenic mice. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2006;1762(4):447–452. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Li X, Yuan F, et al. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2010;69(3):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McConnell SE, Alla J, Wheat E, et al. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Hormones and behavior. 2012;61(4):479–486. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Hormones and behavior. 2010;58(5):705–713. [DOI] [PubMed] [Google Scholar]
- 24.Casadesus G, Webber K, Atwood C, et al. Luteinizing hormone mediates Alzheimer-type changes in neurons. 10th International Conference on Alzheimer’s Disease and Related Disorders 2006. [Google Scholar]
- 25.Burnham VL, Thornton JE. Luteinizing hormone as a key player in the cognitive decline of Alzheimer’s disease. Hormones and behavior. 2015;76:48–56. [DOI] [PubMed] [Google Scholar]
- 26.Swardfager W, Lanctôt K, Rothenburg L, et al. A meta-analysis of cytokines in Alzheimer’s disease. Biological psychiatry. 2010;68(10):930–941. [DOI] [PubMed] [Google Scholar]
- 27.Leung R, Proitsi P, Simmons A, et al. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PloS one. 2013;8(6):e64971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14(4):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGeer PL, Rogers J, McGeer EG. Inflammation, antiinflammatory agents, and Alzheimer’s Disease: The last 22 Years. Journal of Alzheimer’s Disease. 2016;54(3):853–857. [DOI] [PubMed] [Google Scholar]
- 30.Guzmán-Soto I, Salinas E, Hernández-Jasso I, et al. Leuprolide acetate, a GnRH agonist, improves experimental autoimmune encephalomyelitis: a possible therapy for multiple sclerosis. Neurochemical research. 2012;37(10):2190–2197. [DOI] [PubMed] [Google Scholar]
- 31.Saylor PJ, Kozak KR, Smith MR, et al. Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. The oncologist. 2012;17(2):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kåss A, Hollan I, Fagerland MW, et al. Rapid Anti-Inflammatory Effects of Gonadotropin-Releasing Hormone Antagonism in Rheumatoid Arthritis Patients with High Gonadotropin Levels in the AGRA Trial. PloS one. 2015;10(10):e0139439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanriverdi F, Silveira L, MacColl G, et al. The hypothalamic-pituitary-gonadal axis: immune function & autoimmunity. Journal of Endocrinology. 2003;176(3):293–304. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y, Cai D. Hypothalamic inflammation and GnRH in aging development. Taylor & Francis 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Li J, Purkayastha S, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappa-beta and GnRH. Nature. 2013;497(7448):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowen RL. Sex hormones, amyloid protein, and Alzheimer disease. Jama. 2001;286(7):790–791. [DOI] [PubMed] [Google Scholar]
- 37.Hannan C. Will a promising treatment for Alzheimer’s receive the attention it deserves? Milwaukee Magazine. 2015. [Google Scholar]
- 38.Valeo T. Lupron Depot slows Alzheimer’s, study shows, but it may not matter. Tampa Bay Times. 2015. [Google Scholar]
- 39.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. The American journal of psychiatry. 1984. [DOI] [PubMed] [Google Scholar]
- 40.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. Alzheimer Disease & Associated Disorders. 1997;11:22–32. [DOI] [PubMed] [Google Scholar]
- 41.McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology. 2007;74(8):1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herman A, Krawczyńska A, Bochenek J, et al. The effect of rivastigmine on the LPS-induced suppression of GnRH/LH secretion during the follicular phase of the estrous cycle in ewes. Animal reproduction science. 2013;138(3):203–212. [DOI] [PubMed] [Google Scholar]
- 43.Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Human reproduction update. 2014;20(4):485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang J, He Z, Peng Y, et al. Kisspeptin-13 enhances memory and mitigates memory impairment induced by Aβ 1–42 in mice novel object and object location recognition tasks. Neurobiology of learning and memory. 2015;123:187–195. [DOI] [PubMed] [Google Scholar]
- 45.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Disease & Associated Disorders. 1997;11:33–39. [PubMed] [Google Scholar]
- 48.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(2):233–239. [DOI] [PubMed] [Google Scholar]
- 49.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. The gerontologist. 1980;20(6):649–655. [DOI] [PubMed] [Google Scholar]
- 50.Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Annals, academy of medicine, Singapore. 1994. [PubMed] [Google Scholar]
- 51.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology. 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 52.Minoshima S, Drzezga AE, Barthel H, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. Journal of Nuclear Medicine. 2016;57(8):1316–1322. [DOI] [PubMed] [Google Scholar]
- 53.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Archives of neurology. 1975;32(9):632–637. [DOI] [PubMed] [Google Scholar]
- 55.Yesavage JA, Sheikh JI. 9/Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clinical gerontologist. 1986;5(1–2):165–173. [Google Scholar]
- 56.Reuter M, Schmansky NJ, Rosas HD, et al. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernal-Rusiel JL, Greve DN, Reuter M, et al. Statistical analysis of longitudinal neuroimage data with linear mixed effects models. Neuroimage. 2013;66:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutsaerts HJ, van Osch MJ, Zelaya FO, et al. Multi-vendor reliability of arterial spin labeling perfusion MRI using a near-identical sequence: Implications for multi-center studies. Neuroimage. 2015;113:143–152. [DOI] [PubMed] [Google Scholar]
- 59.Glodzik L, Randall C, Rusinek H, et al. Cerebrovascular Reactivity to Carbon Dioxide in Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2013;35(3):427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rusinek H, Brys M, Glodzik L, et al. Hippocampal blood flow in normal aging measured with arterial spin labeling at 3T. Magnetic Resonance in Medicine. 2011;65(1):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardoe HR, Hiess RK, Kuzniecky R. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 2016;135:177–185. [DOI] [PubMed] [Google Scholar]
- 62.Wittich W, Phillips N, Nasreddine ZS, et al. Sensitivity and specificity of the Montreal Cognitive Assessment modified for individuals who are visually impaired. Journal of visual impairment & blindness. 2010;104(6):360–368. [Google Scholar]
- 63.Brink TL. Clinical gerontology: A guide to assessment and intervention: Routledge; 2014. [Google Scholar]
- 64.Chételat G, Ossenkoppele R, Villemagne VL, et al. Atrophy, hypometabolism and clinical trajectories in patients with amyloid-negative Alzheimer’s disease. Brain. 2016;139(9):2528–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimer’s research & therapy. 2014;6(9):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landau SM, Horng A, Fero A, et al. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology. 2016;86(15):1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nature reviews Drug discovery. 2019;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 68.Carotenuto A, Rea R, Traini E, et al. Cognitive assessment of patients with Alzheimer’s disease by telemedicine: pilot study. JMIR mental health. 2018;5(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshida K, Yamaoka Y, Eguchi Y, et al. Remote neuropsychological assessment of elderly Japanese population using the Alzheimer’s Disease Assessment Scale: A validation study. Journal of telemedicine and telecare. 2020;26(7–8):482–487. [DOI] [PubMed] [Google Scholar]
- 70.Gomez MA, Skiba RM, Snow JC. Graspable objects grab attention more than images do. Psychological Science. 2018;29(2):206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marini F, Breeding KA, Snow JC. Distinct visuo-motor brain dynamics for real-world objects versus planar images. NeuroImage. 2019;195:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cocking RR, McHale S. A comparative study of the use of pictures and objects in assessing children’s receptive and productive language. Journal of Child Language. 1981;8(1):1–13. [DOI] [PubMed] [Google Scholar]