Abstract
The consumption of processed meat has been associated with non-cardia gastric cancer, but evidence regarding a possible role of red meat is more limited. This study aims to quantify the association between meat consumption, namely white, red and processed meat, and the risk of gastric cancer, through individual participant data meta-analysis of studies participating in the ‘Stomach cancer Pooling (StoP) Project’.
Data from 22 studies, including 11,443 cases and 28,029 controls, were used. Study-specific odds ratios (ORs) were pooled through a two-stage approach based on random-effects models. An exposure-response relationship was modelled, using one and two-order fractional polynomials, to evaluate the possible non-linear association between meat intake and gastric cancer.
An increased risk of gastric cancer was observed for the consumption of all types of meat (highest vs. lowest tertile), which was statistically significant for red (OR: 1.24; 95%CI: 1.00–1.53), processed (OR: 1.23; 95%CI: 1.06–1.43) and total meat (OR: 1.30; 95%CI: 1.09–1.55). Exposure-response analyses showed an increasing risk of gastric cancer with increasing consumption of both processed and red meat, with the highest OR being observed for an intake of 150 g/day of red meat (OR: 1.85; 95%CI: 1.56–2.20).
This work provides robust evidence on the relation between the consumption of different types of meat and gastric cancer. Adherence to dietary recommendations to reduce meat consumption may contribute to a reduction in the burden of gastric cancer.
Introduction
Gastric cancer incidence has long been falling and is expected to decline in the next years. 1 Nevertheless, mortality rates remain high, placing gastric cancer as the third cause of oncological death worldwide.2
Dietary patterns have been associated with the risk of gastric cancer,3 but the role of specific food groups is generally less clear.4 Meat can be an important part of a balanced diet since it provides essential nutrients, such as proteins, amino acids, vitamins and other micronutrients.5, 6 However, it can also represent a source of compounds, such as heterocyclic amines, polycyclic aromatic hydrocarbons, N-nitroso compounds and heme iron, which have the potential to increase the risk of cancer.7, 8 In 2015, the International Agency for Research on Cancer classified processed meat (smoked and salted goods) as “carcinogenic to humans” and the “consumption of red meat” as “probably carcinogenic to humans” based essentially on data for colorectal cancer.9 More recently, the World Cancer Research Fund (WCRF) revised its Report on Diet, Nutrition, Physical Activity and Stomach Cancer, and concluded that there was suggestive evidence supporting a probable causal link between processed meat and non-cardia cancers,10 but for red meat no pooled analyses were available in that revision.10
The aim of this study is to quantify the association between meat consumption, namely white, red and processed meat, and the risk of gastric cancer, through individual participant data meta-analysis of studies participating in the ‘Stomach cancer Pooling (StoP) Project’.
Material and Methods
Study population
This study is based on the second release of the StoP Project dataset, which included 30 case-control studies, or case-control analyses nested within cohort studies, for a total of 14,016 cases of gastric cancer (9,247 men, 4,769 women) and 33,704 controls (20,352 men and 13,352 women). The StoP Project aims at examining the role of several lifestyles and genetic determinants in the etiology of gastric cancer through pooled analyses of individual-level data, after central collection and validation of the original data sets. For each study, a completion of a study description form providing information on the study characteristics was asked. Investigators who agreed to participate provided a signed DTA and, thereafter, the complete original data set; investigators not wishing to share the complete data set were invited to provide a set of core variables including, among others, age, sex, education/social class, smoking habits, family history of gastric cancer, selected variables, as well as markers of H. pylori infection, whenever available. In addition to the data sets, the original questionnaires and any useful information to help with data handling (codebooks, labels, etc.) was collected from the participating studies, to optimize data harmonization. All data were harmonized at the pooling center, according to a pre-specified format; the whole body of information was divided into several sections (e.g. sociodemographic characteristics, smoking habits, lifetime alcohol use, physical activity, etc.), and, for each topic, a project codebook was created reporting which variables were present in each study, their names and codes. The data for the core variables were standardized among studies, as well as for the variables for selected topics of interest. The StoP Project received ethical approval from the University of Milan Review Board (reference 19/15 on 01/04/2015), and detailed information on the overall aims and methods has been given elsewhere.11
For the present analyses, individual-level data from 22 studies with information on meat intake was used, including 11,443 cases and 28,029 controls, from Brazil (two studies),12, 13 Canada,14 China (two studies),15, 16 Greece,17 Iran,18 Italy (four studies),19–22 Japan (three studies),23–25 Mexico (three studies),26–28 Portugal,29 Russia,30 Spain (two studies),31, 32 and the USA.33
Variables defining the exposure
All studies assessed the participants’ dietary habits through food frequency questionnaires (FFQ) focusing on diet in periods of one, two, three or five years before diagnosis (for cases), onset of disease or hospital admission (for hospital-based controls) or recruitment (for population-based controls). Twelve of the included studies reported that the questionnaire used was previously validated by comparison with multiple 24-hour recall interviews and/or diet records (Supplementary Table 1).These FFQs included between 15 and 147 individual food and beverage items frequently consumed in each country (Supplementary Table 1).
Food items identified as “chicken”, “turkey” and “rabbit” were classified as white meat. Those identified as “beef”, “pork”, other non-poultry meat (e.g. “lamb”), as well as mammalian offals (such as “liver”) were considered red meat. All meat items that had undergone some form of transformation (salting, curing, smoking, fermentation or other processes to enhance flavor or improve preservation) including those identified as “sausages”, “bacon”, “ham”, “cold cuts”, “croquettes” and “hot dogs” were classified as processed meat, regardless of including white or red meat.34
Statistical analysis
Study-specific frequency of consumption of each food item or group was converted into g/day, according to the information available in each questionnaire or country specific dietary standards (Brazilian35 studies), and categorized into tertiles on the basis of the study-specific distribution in the controls.
To quantify the associations between gastric cancer and white, red, processed, and total meat intake, both two- and one-stage modeling approaches were used.36
For the two-stage analysis, the association between meat intake and gastric cancer was first assessed by estimating the odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) for each study, using multivariable unconditional logistic regression models. Considering that the proportion of missing data was low, a complete case approach was adopted. Models included, when available and applicable, terms for sex, age (5-year age groups: <40;40–44; …; 70–74; ≥75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 11 to 20 cigarettes/day; >20 cigarettes/day), alcohol drinking (never, low: ≤12 g of ethanol/day, intermediate: >12 to 47 g of ethanol/day, high: >47 g of ethanol/day), fruits and vegetables consumption (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies), race/ethnicity (“White”, “Black/African American”, “Asian”, “Hispanic/Latino”, “Other”), body mass index (BMI) categories (<18.5, 18.5–25, 25–30, >30 kg/m2) and family history of gastric cancer (Supplementary Table 2). Then, summary (pooled) effects estimates were computed as weighted averages of each study’s ORs, using random-effects models.37 This was performed for the comparison between the second and third study-specific tertile, with the first as the reference category.
Heterogeneity between studies was tested using the Q test statistics and quantified using I2, i.e. the percentage of the total variation across studies that is due to heterogeneity rather than chance.38
Stratified analyses were carried out to investigate the effect of high consumption of each type of meat across strata of selected covariates, including sex, age, geographic area of the studies, socioeconomic status, smoking status, alcohol drinking, BMI categories, family history of gastric cancer, but also according to and type of controls (hospital-based, population-based), cancer anatomical location (cardia, non-cardia) and histological type (intestinal, diffuse and undifferentiated, as defined by the Lauren classification). For the analyses according to cancer subsite and histological type we used multinomial logistic regression models to estimate the ORs for each type of cancer separately (i.e., cardia and non-cardia or intestinal and diffuse). The difference between groups was assessed through the Q test for heterogeneity.38 Sensitivity analyses were performed by excluding one study at a time, and by defining the same categories of exposure for all studies: initially using tertiles of the distribution of meat consumption in all controls as cut-offs to define the categories; then, since the maximum amounts of intake recommended by the WCRF are 300 g/week for red meat and 50 g/week for processed meat, using cut-offs that describe intakes of less than half of the recommended intake, between half and the recommended amount, or more than the recommended amount, resulting in three categories of exposure. Additionally, adjusted and unadjusted estimates for total energy intake, as well as for H. pylori infection status were compared, among studies with information on these variables.
A one-stage strategy of analysis was used to assess the significance of a linear trend, by considering the variables defining meat intake as continuous,39 and to model the functional form of the relation between the daily amount (g) of meat consumed (continuous) and gastric cancer risk. The latter was accomplished through first- and second-order fractional polynomial models, including study center and the core variables used in the main analysis as covariates; this family of models includes the predefined set of power terms P={−2;−1;−0.5;0;1;0.5;2;3}, where P=0 means Log(X) and the linear model has P=1. The best-nonlinear fitting model, i.e., the one minimizing the model difference with respect to the linear model, was selected.40
Data availability
The data that support the findings of this study are available from the Stomach cancer Pooling (StoP) Project but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Steering Committee of the StoP Project.
Results
Tables 1 and 2 describe the main characteristics of the 11,443 cases and 28,029 controls considered for the present analysis. The median meat intake among controls ranged between 0.3 and 38.7 g/day for white meat, between 21.4 and 99.5 g/day for red meat and between 0.0 and 26.9 g/day for processed meat; gastric cancer cases generally presented higher levels of consumption, particularly for red meat and among studies from the Americas (median intake: 61.3 g/day) (Table 1). Compared to controls, cases had higher proportions of men (65.1 vs 59.4%) and individuals who were older (45.6 vs 39.8% over 65 years of age), with low socioeconomic status (50.4 vs 36.8%), current smokers (29.9 vs 25.9%) or alcohol drinkers (59.2 vs 51.9%) (Table 2).
Table 1.
Median and interquartile range (grams per [g/] day) of white, red and processed meat intake, by case-control status and study.
| Cases (n=11,443) | Controls (n=28,029) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Median (P25-P75) g/day | N | % | Median (P25-P75) g/day | |||||
|
| ||||||||||
| White | Red | Processed | White | Red | Processed | |||||
|
| ||||||||||
| Study center | ||||||||||
| America | 2,324 | 20.3 | 20.0 (9.0–48.6) | 61.3 (32.4–100.0) | 13.9 (4.0–27.6) | 7,261 | 25.9 | 16.2 (9.7–48.6) | 56.2 (31.4–88.6) | 11.9 (3.2–24.8) |
| Brazil 113 | 226 | 2.0 | 20.0 (20.0–50.0) | 78.0 (48.0–100.0) | 0.0 (0.0–26.5) | 226 | 0.8 | 20.0 (20.0–50.0) | 99.5 (50.0–100.0) | 0.0 (0.0–26.5) |
| Brazil 212 | 93 | 0.8 | 50.0 (20.0–50.0) | 85.0 (50.0–100.0) | 0.0 (0.0–26.5) | 186 | 0.7 | 20.0 (20.0–50.0) | 50.0 (20.0–100.0) | 0.0 (0.0–26.5) |
| Canada 14 | 1,171 | 10.2 | 16.2 (16.2–48.6) | 71.4 (40.0–104.8) | 19.1 (8.9–33.7) | 5,019 | 17.9 | 16.2 (16.2–48.6) | 63.8 (39.0–97.2) | 14.7 (4.7–26.6) |
| Mexico 126 | 248 | 2.2 | 26.9 (9.0–26.9) | 38.6 (21.8–64.7) | 7.6 (2.8–15.1) | 478 | 1.7 | 26.9 (9.0–26.9)) | 30.3 (14.5–54.9) | 6.6 (2.3–12.7) |
| Mexico 228 | 220 | 1.9 | 29.1 (29.1–29.1) | 48.6 (32.2–81.4) | 14.9 (6.9–28.2) | 752 | 2.7 | 29.1 (9.7–29.1)) | 43.4 (26.5–71.2) | 12.4 (4.1–24.4) |
| Mexico 327 | 234 | 2.0 | 8.9 (4.5–26.8) | 42.4 (16.8–77.5) | 6.4 (2.3–12.9) | 468 | 1.7 | 8.9 (4.5–26.8) | 36.7 (12.1–71.3) | 5.8 (2.1–10.0) |
| USA 133 | 132 | 1.2 | 1.4 (0.0–6.7) | 42.2 (21.2–83.9) | 15.7 (4.5–31.9) | 132 | 0.5 | 0.3 (0.0–3.3) | 25.8 (7.1–50.3) | 6.1 (1.3–18.3) |
| Asia | 5,054 | 44.2 | 10.5 (3.5–10.5) | 26.6 (12.6–38.9) | 1.8 (0.0–5.3) | 10,826 | 38.6 | 10.5 (3.5–10.5) | 26.6 (12.6–37.8) | 1.7 (0.0–5.3) |
| China 215 | 182 | 1.6 | 0.4 (0.0–3.3) | 25 (10.4–42.9) | 0.8 (0.0–12.5) | 403 | 1.4 | 2.5 (0.3–6.9) | 36.9 (14.6–68.8) | 0.4 (0.0–14.3) |
| China 316 | 692 | 6.1 | 5.0 (2.2–10.0) | 31.9 (15.9–59.5) | 5.0 (1.4–10.0) | 686 | 2.5 | 6.7 (3.3–13.3) | 40.0 (19.3–66.2) | 3.6 (1.1–10.0) |
| Iran 118 | 216 | 1.9 | 21.0 (21.0–50.0) | 50 (21.0–250.0) | 0.0 (0.0–0.0) | 393 | 1.4 | 21.0 (21.0–50.0) | 35.5 (21.0–50.0) | 0.0 (0.0–0.0) |
| Japan 124 [1] | 1,260 | 11.1 | 27.0 (18.5–42.5) | 3,914 | 14,0 | 27.0 (17.5–42.5) | ||||
| Japan 223 | 2,551 | 22.3 | 10.5 (3.5–10.5) | 26.6 (12.6–37.8) | 1.8 (0.0–5.3) | 5,127 | 18.4 | 10.5 (3.5–10.5) | 26.6 (12.6–37.8) | 1.8 (0.0–5.3)1 |
| Japan 325 | 153 | 1.3 | 6.7 (2.3–14.7) | 41.7 (28.3–61.2) | 4.3 (1.5–10.3) | 303 | 1.1 | 7 (2.3–15.0) | 45.8 (25.8–71.4) | 5.2 (2.0–11.2) |
| Europe | 4,065 | 35.5 | 25.7 (14.3–42.9) | 49.1 (28.1–66.8) | 21.4 (10.7–38.3) | 9,942 | 35.5 | 21.6 (14.3–35.2) | 38.1 (21.4–58.7) | 21.4 (10.7–34.6) |
| Greece17 | 110 | 1.0 | 14.3 (14.3–28.6) | 44.5 (28.6–71.4) | 0.0 (0.0–1.7) | 100 | 0.4 | 14.3 (8.8–28.6) | 42.9 (28.6–57.1) | 0.0 (0.0–1.7) |
| Italy 119 | 769 | 6.7 | 28.6 (14.3–28.6) | 46.4 (32.1–71.4) | 28.6 (17.9–39.3) | 2,081 | 7.4 | 28.6 (14.3–28.6) | 46.4 (28.6–60.7) | 25.0 (14.3–35.7) |
| Italy 220 | 230 | 1.2 | 14.3 (7.1–14.3) | 25 (17.9–32.1) | 14.3 (10.7–17.9) | 547 | 2.0 | 14.3 (7.1–14.3) | 21.4 (14.3–28.6) | 14.3 (10.7–17.9) |
| Italy 321 | 133 | 1.2 | NA | NA | 23.8 (12.2–41.6) | 400 | 1.4 | NA | NA | 21.4 (21.4–57.1) |
| Italy 422 | 1,016 | 8.9 | 27.1 (14.5–40) | 55.4 (36.0–77.4) | 28.3 (15.0–48.6) | 1,159 | 4.1 | 28.6 (14.6–38.6) | 48.7 (33.1–69.3) | 23.8 (12.2–41.6) |
| Portugal29 | 633 | 5.5 | 42.8 (25.1–59.4) | 51.4 (25.7–59.4) | 8.6 (1.4–18.6) | 1,600 | 5.7 | 33.7 (17.1–59.4) | 51.4 (25.7–77.1) | 8.7 (3.1–18.6) |
| Russia30 | 446 | 3.9 | 21.4 (13.4–42.9) | 48.3 (28.1–77.7) | 23.2 (10.7–39.7) | 607 | 2.2 | 21.4 (6.7–42.9) | 40.3 (20.1–64.3) | 17.1 (7.4–35.4) |
| Spain 131 | 330 | 2.9 | 20.7 (14.5–30.5) | 32.0 (20.5–50.6) | 32.6 (20.5–55.6) | 2,993 | 10.7 | 18.9 (12.7–26.6) | 26.5 (15.5–42.9) | 26.9 (15.5–42.1) |
| Spain 232 | 398 | 3.5 | 37.6 (25.4–44.3) | 53.8 (24.6–60.5) | 24.8 (15.8–31.8) | 455 | 1.6 | 38.7 (32.2–44.3) | 53.8 (24.2–60.5) | 24.8 (14.3–30.1) |
NA – not available; P25-P75 – percentile 25- percentile 75; USA – United States of America
Only total meat available.
Table 2.
Distribution of gastric cancer cases and controls according to sex, age and other selected covariates.
| Cases | Controls | |||
|---|---|---|---|---|
| N | % | N | % | |
|
| ||||
| Sex | ||||
| Male | 7,448 | 65.1 | 16,650 | 59.4 |
| Female | 3,995 | 34.9 | 11,379 | 40.6 |
| Age | ||||
| <40 | 496 | 4.3 | 2,113 | 7.5 |
| 40–45 | 479 | 4.2 | 1,679 | 6.0 |
| 45–50 | 781 | 6.8 | 2,254 | 8.0 |
| 50–54 | 1,128 | 9.9 | 2,969 | 10.6 |
| 55–59 | 1,538 | 13.4 | 3,552 | 12.7 |
| 60–64 | 1,803 | 15.8 | 4,289 | 15.3 |
| 65–69 | 2,028 | 17.7 | 4,525 | 16.1 |
| 70–74 | 1,824 | 15.9 | 3,779 | 13.5 |
| ≥75 | 1,366 | 12.0 | 2,869 | 10.2 |
| Socioeconomic status [1] | ||||
| Low | 4,486 | 50.4 | 8,433 | 36.8 |
| Intermediate | 2,416 | 27.2 | 6,955 | 30.4 |
| High | 1,156 | 13.0 | 5,099 | 22.3 |
| Missing | 834 | 9.4 | 2,415 | 10.5 |
| History of stomach cancer in first degree relatives [2] | ||||
| No | 6,495 | 73.2 | 16,330 | 79.2 |
| Yes | 1,376 | 15.5 | 1,982 | 9.6 |
| Missing | 1,007 | 11.3 | 2,314 | 11.2 |
| Vegetables and fruit intake [3] | ||||
| Low | 3,840 | 33.6 | 8,515 | 30.4 |
| Intermediate | 3,938 | 34.4 | 9,737 | 34.7 |
| High | 3,647 | 31.8 | 9,484 | 33.8 |
| Missing | 18 | 0.2 | 293 | 1.1 |
| Total energy intake [4] | ||||
| 1st quintile | 1,120 | 17.2 | 3,123 | 19.8 |
| 2nd quintile | 1,172 | 18.4 | 3,133 | 19.9 |
| 3rd quintile | 1,228 | 18.8 | 3,135 | 19.9 |
| 4th quintile | 1,268 | 19.5 | 3,136 | 19.9 |
| 5th quintile | 1,501 | 23.1 | 3,137 | 19.9 |
| Missing | 198 | 3.1 | 72 | 0.5 |
| Body Mass Index (kg/m2) [5] | ||||
| <18.5 | 533 | 4.9 | 826 | 3.1 |
| 18.5–25 | 5,605 | 51.3 | 13,360 | 49.4 |
| 25–30 | 2,707 | 24.8 | 7,693 | 28.5 |
| >30 | 1,446 | 13.2 | 3,197 | 11.8 |
| Missing | 639 | 5.9 | 1,953 | 7.2 |
| Cigarette smoking | ||||
| Never | 4,670 | 40.9 | 12,787 | 45.7 |
| Former | 3,063 | 26.8 | 7,626 | 27.2 |
| Current (cigarettes/day) | ||||
| ≤10 | 775 | 6.8 | 2,208 | 7.9 |
| 11–20 | 1,607 | 14.1 | 3,164 | 11.3 |
| >20 | 1,026 | 9.0 | 1,867 | 6.7 |
| Missing | 281 | 2.5 | 343 | 1.2 |
| Alcohol intake | ||||
| Never | 3,810 | 37.8 | 10,803 | 43.5 |
| Low (≤12 g of ethanol/day) | 2,423 | 24.1 | 5,283 | 21.3 |
| Intermediate (>12–47 g of ethanol/day) | 2,497 | 24.8 | 5,582 | 22.4 |
| High (>47 g of ethanol/day) | 1,036 | 10.3 | 2,035 | 8.2 |
| Missing | 302 | 3.0 | 1,145 | 4.6 |
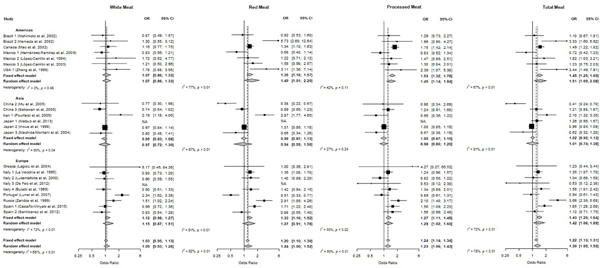
Pooled ORs and corresponding 95%CIs for the association between meat consumption and gastric cancer are presented in Table 3 and Figure 1. The odds were significantly higher for the highest versus the lowest tertile of red (OR: 1.24; 95%CI: 1.00–1.53), processed (OR: 1.23; 95%CI: 1.06–1.43) and total meat intake (OR: 1.30; 95%CI: 1.09–1.55).
Table 3.
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for gastric cancer according to tertiles of meat consumption (grams per [g/] day).
| Cases | Controls | OR (95% CI) [1] | I2 (%) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | g/day Median (P25-P75) |
N | % | g/day Median (P25-P75) |
|||
|
| ||||||||
| White meat [2] | ||||||||
| 1st tertile | 4,074 | 40.5 | 7.1 (3.3–15.4) | 9,771 | 41.2 | 7.6 (3.5–16.2) | 1 | |
| 2nd tertile | 3,697 | 36.8 | 20.9 (10.5–29.1) | 9,070 | 38.3 | 22.7 (10.5–44.3) | 1.01 (0.92–1.11) | 28.2 |
| 3rd tertile | 2,093 | 20.8 | 42.9 (25.0–59.4) | 4,578 | 19.3 | 40.9 (25.0–57.1) | 1.09 (0.93–1.26) | 56.4 |
| Missing | 186 | 1.9 | 296 | 1.3 | ||||
| P value for trend | 0.473 | |||||||
| Red meat [2] | ||||||||
| 1st tertile | 3,158 | 31.4 | 13.4 (7.1–24.2) | 8,146 | 34.4 | 14.3 (7.1–23.8) | 1 | |
| 2nd tertile | 3,922 | 39.0 | 37.8 (29.9–51.4) | 9,107 | 38.4 | 39.1 (28.8–51.4) | 1.16 (1.02–1.32) | 59.8 |
| 3rd tertile | 2,961 | 29.5 | 88.6 (64.6–113.4) | 6,446 | 27.2 | 88.6 (61.0–113.4) | 1.24 (1.00–1.53) | 82.3 |
| Missing | 9 | 0.1 | 16 | 0.1 | ||||
| P value for trend | <0.001 | |||||||
| Processed meat [3] | ||||||||
| 1st tertile | 4,060 | 39.7 | 1.8 (0.0–3.4) | 9,997 | 41.5 | 1.8 (0.0–7.1) | 1 | |
| 2nd tertile | 2,879 | 28.3 | 13.2 (5.3–21.5) | 7,315 | 30.3 | 14.3 (5.3–22.1) | 1.09 (0.94–1.26) | 63.6 |
| 3rd tertile | 2,987 | 29.3 | 37.5 (22.8–57.1) | 6,353 | 26.3 | 36.1 (23.7–52.4) | 1.23 (1.06–1.43) | 60.2 |
| Missing | 257 | 2.5 | 450 | 1.9 | ||||
| P value for trend | <0.001 | |||||||
| Total meat | ||||||||
| 1st tertile | 3,468 | 30.3 | 25.6 (16.0–57.1) | 9,524 | 34.0 | 31.9 (16.1–59.3) | 1 | |
| 2nd tertile | 3,795 | 33.2 | 65.4 (38.9–101.4) | 9,315 | 33.2 | 75.3 (39.4–101.6) | 1.14 (1.03–1.27) | 41.2 |
| 3rd tertile | 4,180 | 36.5 | 132.2 (75.3–170.0) | 9,190 | 32.8 | 126.7 (71.0–164.3) | 1.30 (1.09–1.55) | 77.9 |
| P value for trend | <0.001 | |||||||
Pooled ORs were computed using random-effects models which included, when available and applicable, terms for sex, age (5-year age groups: <40;40–44; …; 70–74; ≥75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 11 to 20 cigarettes/day; >20 cigarettes/day), alcohol drinking (never, low: ≤12g of ethanol/day, intermediate: >12 to 47g of ethanol/day, high: >47g of ethanol/day), fruits and vegetables consumption (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies), and race/ethnicity (“White”, “Black/African American”, “Asian”, “Hispanic/Latino”, “Other”), family history of gastric cancer and body mass categories (<18.5; 18.5–25; 25–30; >30 kg/m2).
No information for the study Japan 1.24
Figure 1.
Study-specific and pooled odds ratio (ORs) and corresponding 95% confidence intervals (CI) of gastric cancer risk for the highest tertile of meat (white, red, processed and total meat) consumption compared to the lowest tertile.
NA – not available; OR – Odds ratio; 95%CI – 95% confidence interval; USA – United States of America
Table 4 presents the results from the stratified analyses. For most of the strata considered, there was an increased risk of gastric cancer with a high intake of all types of meat analyzed. The only significant difference across strata was observed for processed meat, regarding the geographic area of the studies; the strongest associations were observed among the American (OR: 1.45; 95% CI: 1.15–1.84) and the European studies (OR: 1.28; 95% CI: 1.02–1.60), whereas in Asian studies there was no significant association (OR: 0.98; 95% CI: 0.80–1.20).
Table 4.
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) of gastric cancer for the highest compared to the lowest study-specific tertile of different sources of meat in strata of selected variables.
| White Meat | Red Meat | Processed Meat | Total Meat | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR (95% CI) [1] | I2 (%) | OR (95% CI) [1] | I2 (%) | OR (95% CI) [1] | I2 (%) | OR (95% CI) [1] | I2 (%) | |
|
| ||||||||
| Overall | 1.09 (0.93–1.26) | 56.4 | 1.24 (1.00–1.53) | 82.3 | 1.23 (1.06–1.43) | 60.2 | 1.30 (1.09–1.55) | 77.9 |
| Sex | ||||||||
| Men | 1.00 (0.89–1.13) | 5.4 | 1.19 (0.97–1.46) | 65.6 | 1.21 (1.05–1.38) | 26.4 | 1.23 (1.05–1.45) | 52.3 |
| Women | 1.27 (0.94–1.70) | 67.4 | 1.28 (0.96–1.69) | 69.0 | 1.29 (0.99–1.68) | 64.0 | 1.41 (1.06–1.86) | 72.4 |
| P for interaction | 0.146 | 0.690 | 0.656 | 0.421 | ||||
| Age (years) | ||||||||
| ≤55 | 1.04 (0.86–1.27) | 14.5 | 1.05 (0.78–1.42) | 62.6 | 1.39 (1.18–1.63) | 2.1 | 1.10 (0.90–1.35) | 35.0 |
| >55 to ≤65 | 1.19 (0.93–1.54) | 42.2 | 1.26 (0.95–1.66) | 60.6 | 1.19 (0.93–1.53) | 46.0 | 1.44 (1.10–1.88) | 61.0 |
| >65 | 0.97 (0.83–1.14) | 12.4 | 1.38 (1.09–1.75) | 59.9 | 1.13 (0.92–1.39) | 45.2 | 1.33 (1.07–1.67) | 58.2 |
| P for interaction | 0.389 | 0.364 | 0.265 | 0.238 | ||||
| Area | ||||||||
| Europe | 1.15 (0.88–1.52) | 72.2 | 1.27 (0.91–1.78) | 81.4 | 1.28 (1.02–1.60) | 55.3 | 1.42 (1.06–1.89) | 72.1 |
| Asia | 0.97 (0.72–1.30) | 60.0 | 0.94 (0.59–1.51) | 87.3 | 0.98 (0.80–1.20) | 27.3 | 1.01 (0.74–1.38) | 81.1 |
| Americas | 1.07 (0.86–1.33) | 0.0 | 1.49 (1.01–2.20) | 76.7 | 1.45 (1.14–1.84) | 42.4 | 1.51 (1.09–2.08) | 66.6 |
| P for interaction | 0.700 | 0.330 | 0.038 | 0.159 | ||||
| Socioeconomic status [2] | ||||||||
| Low | 1.19 (0.91–1.57) | 61.1 | 1.33 (1.04–1.70) | 61.5 | 1.25 (1.00–1.55) | 47.4 | 1.33 (1.09–1.63) | 47.6 |
| Intermediate | 0.91 (0.73–1.13) | 14.1 | 1.13 (0.81–1.58) | 66.4 | 1.25 (0.95–1.63) | 49.9 | 1.28 (0.95–1.74) | 62.5 |
| High | 1.10 (0.79–1.53) | 0.0 | 0.99 (0.57–1.71) | 62.4 | 1.34 (0.96–1.86) | 15.8 | 1.20 (0.80–1.82) | 51.6 |
| P for interaction | 0.280 | 0.540 | 0.934 | 0.903 | ||||
| Smoking status | ||||||||
| Never | 1.24 (0.97–1.59) | 63.2 | 1.29 (0.99–1.69) | 72.4 | 1.18 (0.93–1.49) | 60.1 | 1.33 (1.04–1.69) | 70.5 |
| Former | 1.01 (0.85–1.19) | 0.0 | 1.28 (1.08–1.52) | 4.3 | 1.31 (1.06–1.62) | 24.1 | 1.32 (1.14–1.53) | 0.7 |
| Current | 0.89 (0.72–1.11) | 14.3 | 1.04 (0.76–1.41) | 55.3 | 1.10 (0.93–1.30) | 0.0 | 1.08 (0.88–1.32) | 26.5 |
| P for interaction | 0.138 | 0.458 | 0.446 | 0.238 | ||||
| Alcohol drinking | ||||||||
| Non-drinker | 1.17 (0.97–1.46) | 33.4 | 1.31 (0.97–1.75) | 72.2 | 1.24 (1.02–1.50) | 32.3 | 1.34 (1.05–1.73) | 66.6 |
| Drinker | ||||||||
| Low (≤12 g/day) | 1.19 (0.88–1.59) | 50.2 | 1.10 (0.79–1.53) | 67.7 | 1.32 (0.97–1.81) | 60.5 | 1.26 (0.92–1.72) | 68.1 |
| Intermediate (>12–47 g/day) | 0.90 (0.70–1.15) | 15.3 | 1.23 (0.98–1.53) | 13.4 | 0.98 (0.75–1.28) | 32.0 | 1.20 (1.02–1.39) | 0.0 |
| High (>47 g/day) | 0.96 (0.57–1.61) | 36.5 | 1.03 (0.65–1.63) | 31.7 | 1.54 (1.12–2.12) | 0.0 | 1.42 (1.06–1.91) | 0.0 |
| P for interaction | 0.312 | 0.792 | 0.175 | 0.642 | ||||
| Controls | ||||||||
| Hospital-based[3] | 1.05 (0.87–1.26) | 17.3 | 1.51 (1.09–2.11) | 76.4 | 1.31 (1.03–1.66) | 54.0 | 1.42 (1.08–1.86) | 72.6 |
| Population-based[4] | 1.10 (0.89–1.40) | 72.5 | 1.05 (0.80–1.38) | 84.8 | 1.17 (0.96–1.44) | 68.1 | 1.20 (0.94–1.53) | 81.8 |
| P for interaction | 0.687 | 0.096 | 0.505 | 0.372 | ||||
| Site [5] | ||||||||
| Cardia | 1.30 (0.96–1.75) | 15.8 | 1.61 (0.99–2.63) | 69.0 | 1.34 (0.97–1.84) | 29.3 | 1.76 (1.15–2.70) | 59.5 |
| Non-cardia | 1.11 (0.95–1.30) | 53.8 | 1.28 (1.03–1.59) | 78.7 | 1.24 (1.03–1.49) | 68.3 | 1.34 (1.11–1.62) | 74.9 |
| P for interaction | 0.370 | 0.406 | 0.676 | 0.255 | ||||
| Histological type [6] | ||||||||
| Intestinal | 1.19 (0.91–1.55) | 48.9 | 1.67 (1.04–2.66) | 81.9 | 1.17 (0.84–1.65) | 66.2 | 1.50 (1.06–2.13) | 73.8 |
| Diffuse | 1.28 (1.03–1.58) | 11.0 | 1.34 (0.91–1.98) | 63.4 | 1.44 (1.16–1.80) | 12.4 | 1.46 (1.12–1.90) | 46.1 |
| Undifferentiated | 1.33 (0.84–2.09) | 55.3 | 1.56 (0.85–2.87) | 69.6 | 1.23 (0.87–1.74) | 50.7 | 1.57 (0.98–2.53) | 61.7 |
| P for interaction | 0.883 | 0.770 | 0.532 | 0.964 | ||||
| Vegetables and fruits intake | ||||||||
| Low | 1.04 (0.83–1.30) | 17.4 | 1.02 (0.79–1.33) | 49.9 | 1.33 (1.04–1.71) | 43.6 | 1.12 (0.90–1.38) | 39.1 |
| Intermediate | 0.97 (0.83–1.12) | 0.0 | 1.29 (1.02–1.62) | 44.3 | 1.23 (1.00–1.52) | 34.9 | 1.32 (1.07–1.63) | 44.5 |
| High | 1.23 (0.99–1.53) | 40.3 | 1.23 (0.89–1.71) | 75.8 | 1.16 (0.92–1.45) | 50.5 | 1.38 (1.02–1.88) | 75.0 |
| P for interaction | 0.188 | 0.413 | 0.711 | 0.418 | ||||
| Family history of GC [7] | ||||||||
| No | 1.12 (0.91–1.37) | 62.0 | 1.36 (1.01–1.85) | 85.2 | 1.11 (0.90–1.36) | 60.7 | 1.31 (1.04–1.67) | 79.2 |
| Yes | 1.16 (0.91–1.48) | 0.0 | 1.19 (0.68–2.07) | 77.9 | 1.30 (0.84–1.99) | 37.2 | 1.56 (0.83–2.93) | 68.9 |
| P for interaction | 0.824 | 0.671 | 0.518 | 0.626 | ||||
| Body Mass Index (BMI) (kg/m2) | ||||||||
| <18.5 | 0.56 (0.14–2.27) | 60.9 | 1.76 (0.66–4.73) | 43.7 | 0.94 (0.25–3.56) | 55.2 | 0.97 (0.62–1.53) | 0.7 |
| 18.5–25 | 1.00 (0.86–1.15) | 5.7 | 1.24 (1.01–1.52) | 43.0 | 1.15 (0.89–1.50) | 64.1 | 1.29 (1.01–1.66) | 70.2 |
| 25–30 | 1.03 (0.75–1.42) | 59.0 | 1.15 (0.84–1.58) | 61.7 | 1.46 (1.18–1.81) | 22.8 | 1.28 (1.01–1.63) | 42.3 |
| >30 | 1.50 (0.83–2.69) | 65.8 | 1.37 (0.73–2.59) | 58.9 | 1.37 (0.98–1.91) | 0.0 | 1.51 (1.02–2.22) | 36.0 |
| P for interaction | 0.485 | 0.852 | 0.530 | 0.553 | ||||
| Studies with information on total energy intake [7] | ||||||||
| Adjusting for energy | 1.02 (0.82–1.26) | 63.0 | 1.06 (0.84–1.33) | 68.9 | 1.16 (1.00–1.35) | 35.9 | 1.22 (1.06–1.41) | 35.7 |
| Not-adjusting for energy | 1.06 (0.86–1.31) | 64.4 | 1.22 (0.95–1.56) | 77.8 | 1.31 (1.10–1.56) | 54.9 | 1.36 (1.13–1.63) | 64.3 |
| Studies with information on H. pylori (HP) infection [8] | ||||||||
| Adjusting for HP | 1.22 (0.92–1.62) | 60.3 | 1.29 (0.77–2.17) | 89.7 | 1.15 (0.91–1.46) | 44.6 | 1.35 (0.93–1.96) | 82.6 |
| Not-adjusting for HP | 1.21 (0.91–1.60) | 66.0 | 1.25 (0.76–2.04) | 89.9 | 1.15 (0.88–1.49) | 61.3 | 1.28 (0.89–1.82) | 84.5 |
Pooled ORs were computed using random-effects models, which included, when available and applicable, terms for sex, age (5-year age groups: <40;40–44; …; 70–74; ≥75), socioeconomic status (low, intermediate, or high, as defined in each original study based on education, income or occupation), smoking status (never, former and current smokers of ≤10 cigarettes/day; 11 to 20 cigarettes/day; >20 cigarettes/day), alcohol drinking (never, low: ≤12 g of ethanol/day, intermediate: >12 to 47 g of ethanol/day, high: >47 g of ethanol/day), fruits and vegetables consumption (study-specific tertiles), total energy intake (study-specific quintiles), study center (for multicenter studies), and race/ethnicity (“White”, “Black/African American”, “Asian”, “Hispanic/Latino”, “Other”, family history of gastric cancer and body mass categories (<18.5; 18.5–25; 25–30; >30 kg/m2).
No information for the study Japan 2.23 As defined in each original study based on education, income or occupation.
Includes studies Brazil 1,13 Brazil 2,12 Greece,17 Italy 1,19 Italy 2,20 Italy 3,21 Japan 1,24 Japan 2,25 Japan 3,23 Mexico 3,27 Russia30 and Spain 2.32
Includes studies Canada,14 China 2,15 China 3,16 Iran 1,18 Italy 4,22 Mexico 1,26 Mexico 2,28 Portugal29 and Spain 1.31
H. pylori infection was defined using the same criteria of the original studies, according to the following serological tests: enzyme-linked immunosorbent assay (ELISA) tests (9 studies)12, 13, 18, 23, 24, 26, 27, 29, 30 to determine immunoglobulin G (IgG) antibody titers in serum, and in one study through multiplex serology.31 When anti-H. pylori serum IgG titers were assessed using an ELISA-based method, participants with borderline results were classified as testing positive for H. pylori infection.
When considering results adjusted for total energy intake, heterogeneity tended to be lower and the association remained significant for processed (OR: 1.16; 95% CI: 1.00–1.35) and total meat intake (OR: 1.22; 95% CI: 1.06–1.41). Regarding adjustment for H. pylori infection, it also contributed to lower heterogeneity, though no significant associations were observed. (Table 4). The main findings remained unchanged after further sensitivity analyses (Supplementary Tables 3 and 4 and Supplementary Figure 1), though heterogeneity decreased slightly when defining the same categories of exposure for all studies, either using the overall distribution in all controls (Supplementary Table 3) or the amounts recommended by the WCRF (Supplementary Table 4).
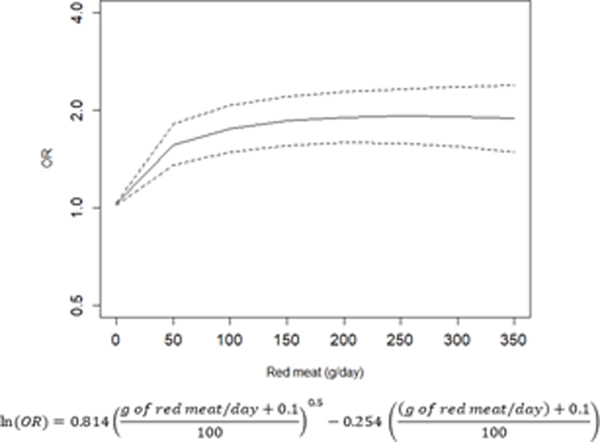
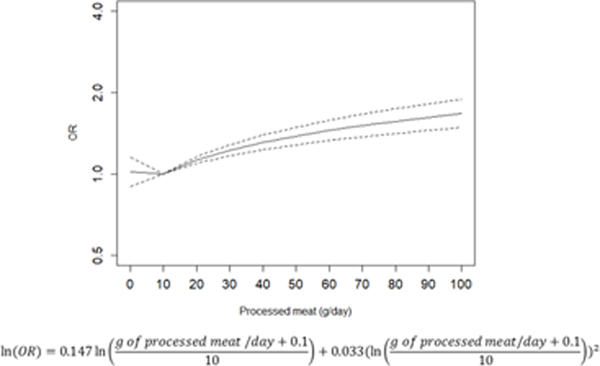
The dose-response relationships between the intake of red and processed and gastric cancer are depicted in Figure 2A and 2B, respectively. There is a trend towards increased gastric cancer risk with a higher consumption of red and processed meat, with an OR of 1.85 (95% CI: 1.56–2.20) for the consumption of 150 g/day of red meat, and an OR of 1.38 (95% CI: 1.28–1.49) for the consumption of 50 g/day of processed meat.
Figure 2.
Relation between red (A) and processed (B) meat (g/day) and risk of gastric cancer fitted by a fractional polynomial.
OR – odds ratio
Discussion
This study was based on an individual participant data approach, which constitutes the gold standard in evidence synthesis, allowing us to better quantify than previously available reports an increased risk of gastric cancer with high intakes of meat, particularly red and processed meat. This was further confirmed through the computation and graphical depiction of the exposure-response association.
Previous studies have shown positive and significant associations between high intakes of red and processed meat and gastric cancer. Compared to meta-analyses of cohort studies, our estimates are higher (Zhu et al.,41relative risk [RR] for the highest vs lowest intake of red meat 1.05 [95% CI: 0.87–1.27]; Song et al.,42 RR for the highest vs lowest intake of red meat 1.00 [95% CI: 0.82–1.20]; Zhao et al.,43 RR for the highest vs lowest intake of red meat 1.14 [95% CI: 0.97–1.34] and RR for the highest vs lowest intake of processed meat 1.23 [95% CI: 0.98–1.55]; Kim et al,44 RR for the highest vs lowest intake of red meat 1.03 [95%CI: 0.83–1.28] and RR for the highest vs lowest intake of processed meat 1.24 [95%CI: 1.04–1.47]). Nevertheless, the estimates obtained in this study are of lower magnitude than those from the previous meta-analyses of case-control studies: Zhu et al41 found RR estimates of 1.63 (95% CI: 1.33–1.99) for red meat and 1.64 (95% CI: 1.47–1.83) for processed meat from a total of 13 and 18 case-control studies, respectively; Song et al42 had a relative risk estimate of 1.59 (95% CI:1.34–1.89) for red meat with 18 studies; Zhao et al43, with 20 case-control studies for red meat and 25 for processed meat, obtained even higher estimates (red meat: 1.67 [95% CI: 1.36–2.05]; processed meat: 1.76 [95% CI: 1.51–2.05] and, most recently, Kim et al,44 with 20 case-control studies for red meat and 23 for processed meat obtained estimates similar to those from Zhao et al (red meat: 1.57 [95%CI: 1.30–1.89]; processed meat: 1.79 [95%CI: 1.51–2.12]). We have previously observed that, both smoking and alcohol drinking, the estimates obtained from the StoP Project were lower than the ones found in previous meta-analyses of case-control studies,45, 46 which is likely a reflection of the methodological strengths of individual participant data pooled analyses in terms of a more uniform strategy of analysis across studies, including control of confounding and reduction of publication bias.
The higher concentration of carcinogenic compounds such as heme iron47 and N-nitroso compounds8 present in red and processed meat contributes to explain the higher OR estimates obtained for these food groups compared with those for white meat. Furthermore, the type of processing determines the degree of carcinogenicity,48 with smoking and grilling of meats resulting in the formation of more polycyclic aromatic hydrocarbons, heterocyclic amines and other carcinogens.49 An additional hypothesis was bacterial plasmids (DNA) from meat, namely from dairy cattle, which may contribute to chronic inflammation and, in the long term, promote carcinogenesis.50
Regional differences, in particular higher estimates for American and European countries, were observed. Though previous meta-analyses have shown an increased risk of gastric cancer for higher consumptions of meat regardless of the geographic region, Asian studies tend to present the lowest estimates.41, 42, 44 The different dietary patterns between geographical regions, which determine the exposure to different amounts and types of meat, can help explain the geographic differences; as well as methodological issues of the studies included in previous meta-analyses, namely regarding control of confounding. In our study, Asian studies are those that present, for all types of meat considered, lower median values of consumption, which may contribute to the lower estimate obtained compared with the other studies where consumption is higher. Also, the variability in the detail, definition and assessment of dietary exposures across countries also contributes to the regional differences and to the high heterogeneity observed for all estimates in the present study, as well as in other investigations on dietary factors.51 Although most studies within the StoP Project used validated FFQs, and the methods used to obtain dietary information and some of the dietary items included in the questionnaires were similar among studies, there are considerable differences concerning the dietary patterns within each study. Nevertheless, stratified and sensitivity analyses did not result in significant changes in cancer risk estimates for all types of meat considered. However, we observed slight lower heterogeneity particularly when adjusting for total energy intake and when considering the same cut-offs for all studies.
This work adds to previous evidence by providing pooled OR estimates for different types of meat, including the characterization of the exposure-response relationships. Our results provide additional evidence that adherence to the dietary recommendations to reduce meat consumption, as those from the WCRF, is likely to contribute to a reduction in the burden of gastric cancer.
Supplementary Material
Acknowledgments:
This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Project no. 21378 (Investigator Grant), by the Italian Ministry of Health (Young Researchers, GR-2011–02347943 to SB), by the Italian League for the Fight Against Cancer (LILT) and by the Associazione Industriale delle Carni e dei Salumi (ASSICA, to the Department of Clinical Sciences and Community Health, University of Milan).
This study was also funded by FEDER through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Science, Technology and Higher Education) under the Unidade de Investigação em Epidemiologia – Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01–0145-FEDER-006862; Ref. UID/DTP/04750/2013). AF (PD/BD/105823/2014) was awarded with an individual scholarship through national funding from FCT/MCTES. Individual grants attributed to ARC (SFRH/BD/102181/2014), and BP (SFRH/BPD/108751/2015) were funded by the FCT and the “Programa Operacional Capital Humano” (POCH/FSE). SM was funded under the project “NEON-PC - Neuro-oncological complications of prostate cancer: longitudinal study of cognitive decline “ (POCI-01–0145-FEDER-032358; Ref. PTDC/SAU-EPI/32358/2017).
Valentina Rosato was supported by a fellowship by the Italian Foundation for Cancer Research (FIRC #18104).
The authors thank the European Cancer Prevention (ECP) Organization for providing support for the StoP meetings.
We also thank all MCC-Spain study collaborators (CIBERESP, ISCIII, ISGlobal, ICO, University of Huelva, University of Oviedo, University of Cantabria, University of León, ibs. Granada, Instituto Salud Pública de Navarra, FISABIO, Murcia Regional Health Authority and cols)
Footnotes
No conflict of interests to disclose.
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014;50: 1330–44. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3.Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, La Vecchia C. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol 2013;24: 1450–8. [DOI] [PubMed] [Google Scholar]
- 4.Fang X, Wei J, He X, An P, Wang H, Jiang L, Shao D, Liang H, Li Y, Wang F, Min J. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51: 2820–32. [DOI] [PubMed] [Google Scholar]
- 5.De Smet S, Vossen E. Meat: The balance between nutrition and health. A review. Meat Sci 2016;120: 145–56. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Mattiuzzi C, Cervellin G. Meat consumption and cancer risk: a critical review of published meta-analyses. Crit Rev Oncol Hematol 2016;97: 1–14. [DOI] [PubMed] [Google Scholar]
- 7.Ward MH, Cross AJ, Abnet CC, Sinha R, Markin RS, Weisenburger DD. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur J Cancer Prev 2012;21: 134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hecke T, Vossen E, Hemeryck LY, Vanden Bussche J, Vanhaecke L, De Smet S. Increased oxidative and nitrosative reactions during digestion could contribute to the association between well-done red meat consumption and colorectal cancer. Food Chem 2015;187: 29–36. [DOI] [PubMed] [Google Scholar]
- 9.Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K, International Agency for Research on Cancer Monograph Working G. Carcinogenicity of consumption of red and processed meat. Lancet Oncol 2015;16: 1599–600. [DOI] [PubMed] [Google Scholar]
- 10.World Cancer Research Fund International/American Institute for Cancer Research, Continuous Update Project Report: Diet, Nutrition, Physical Activity and Stomach Cancer., 2016; Available from: wcrf.org/stomach-cancer-2016.
- 11.Pelucchi C, Lunet N, Boccia S, Zhang ZF, Praud D, Boffetta P, Levi F, Matsuo K, Ito H, Hu J, Johnson KC, Ferraroni M, Yu GP, Peleteiro B, Malekzadeh R, Derakhshan MH, Ye W, Zaridze D, Maximovitch D, Aragones N, Martin V, Pakseresht M, Pourfarzi F, Bellavia A, Orsini N, Wolk A, Mu L, Arzani D, Kurtz RC, Lagiou P, Trichopoulos D, Muscat J, La Vecchia C, Negri E. The stomach cancer pooling (StoP) project: study design and presentation. Eur J Cancer Prev 2015;24: 16–23. [DOI] [PubMed] [Google Scholar]
- 12.Hamada GS, Kowalski LP, Nishimoto IN, Rodrigues JJ, Iriya K, Sasazuki S, Hanaoka T, Tsugane S, Sao Paulo--Japan Cancer Project Gastric Cancer Study G. Risk factors for stomach cancer in Brazil (II): a case-control study among Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol 2002;32: 284–90. [DOI] [PubMed] [Google Scholar]
- 13.Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, Hanaoka T, Tsugane S, Sao Paulo--Japan Cancer Project Gastric Cancer Study G. Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol 2002;32: 277–83. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Hu J, Semenciw R, White K, Canadian Cancer Registries Epidemiology Research G. Active and passive smoking and the risk of stomach cancer, by subsite, in Canada. Eur J Cancer Prev 2002;11: 27–38. [DOI] [PubMed] [Google Scholar]
- 15.Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, Setiawan VW, Zhou XF, Ding BG, Wang RH, Zhao J, Cai L, Rao JY, Heber D, Zhang ZF. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer 2005;116: 972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setiawan VW, Yu GP, Lu QY, Lu ML, Yu SZ, Mu L, Zhang JG, Kurtz RC, Cai L, Hsieh CC, Zhang ZF. Allium vegetables and stomach cancer risk in China. Asian Pac J Cancer Prev 2005;6: 387–95. [PMC free article] [PubMed] [Google Scholar]
- 17.Lagiou P, Samoli E, Lagiou A, Peterson J, Tzonou A, Dwyer J, Trichopoulos D. Flavonoids, vitamin C and adenocarcinoma of the stomach. Cancer Causes Control 2004;15: 67–72. [DOI] [PubMed] [Google Scholar]
- 18.Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran--a population based study. Int J Cancer 2009;125: 1953–60. [DOI] [PubMed] [Google Scholar]
- 19.La Vecchia C, D’Avanzo B, Negri E, Decarli A, Benichou J. Attributable risks for stomach cancer in northern Italy. Int J Cancer 1995;60: 748–52. [DOI] [PubMed] [Google Scholar]
- 20.Lucenteforte E, Scita V, Bosetti C, Bertuccio P, Negri E, La Vecchia C. Food groups and alcoholic beverages and the risk of stomach cancer: a case-control study in Italy. Nutr Cancer 2008;60: 577–84. [DOI] [PubMed] [Google Scholar]
- 21.De Feo E, Simone B, Persiani R, Cananzi F, Biondi A, Arzani D, Amore R, D’Ugo D, Ricciardi G, Boccia S. A case-control study on the effect of Apolipoprotein E genotypes on gastric cancer risk and progression. BMC Cancer 2012;12: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer 1989;44: 611–6. [DOI] [PubMed] [Google Scholar]
- 23.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Hanaoka T, Tsugane S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2004;7: 46–53. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo K, Oze I, Hosono S, Ito H, Watanabe M, Ishioka K, Ito S, Tajika M, Yatabe Y, Niwa Y, Yamao K, Nakamura S, Tajima K, Tanaka H. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis 2013;34: 1510–5. [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Tajima K, Hirose K, Hamajima N, Takezaki T, Kuroishi T, Tominaga S. Epidemiological features of first-visit outpatients in Japan: comparison with general population and variation by sex, age, and season. J Clin Epidemiol 1997;50: 69–77. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Ramirez RU, Galvan-Portillo MV, Ward MH, Agudo A, Gonzalez CA, Onate-Ocana LF, Herrera-Goepfert R, Palma-Coca O, Lopez-Carrillo L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int J Cancer 2009;125: 1424–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia A, Mohar-Betancourt A, Meneses-Garcia A, Lopez-Vidal Y, Blair A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer 2003;106: 277–82. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol 1994;139: 263–71. [DOI] [PubMed] [Google Scholar]
- 29.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, Carneiro F, Barros H. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev 2007;16: 312–27. [DOI] [PubMed] [Google Scholar]
- 30.Zaridze D, Borisova E, Maximovitch D, Chkhikvadze V. Alcohol consumption, smoking and risk of gastric cancer: case-control study from Moscow, Russia. Cancer Causes Control 2000;11: 363–71. [DOI] [PubMed] [Google Scholar]
- 31.Castano-Vinyals G, Aragones N, Perez-Gomez B, Martin V, Llorca J, Moreno V, Altzibar JM, Ardanaz E, de Sanjose S, Jimenez-Moleon JJ, Tardon A, Alguacil J, Peiro R, Marcos-Gragera R, Navarro C, Pollan M, Kogevinas M, Group MC-SS. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit 2015;29: 308–15. [DOI] [PubMed] [Google Scholar]
- 32.Santibanez M, Alguacil J, de la Hera MG, Navarrete-Munoz EM, Llorca J, Aragones N, Kauppinen T, Vioque J, Group PS. Occupational exposures and risk of stomach cancer by histological type. Occup Environ Med 2012;69: 268–75. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZF, Kurtz RC, Klimstra DS, Yu GP, Sun M, Harlap S, Marshall JR. Helicobacter pylori infection on the risk of stomach cancer and chronic atrophic gastritis. Cancer Detect Prev 1999;23: 357–67. [DOI] [PubMed] [Google Scholar]
- 34.International Agency for Research on Cancer. IARC Monographs evaluate consumption of red meat and processed meat http://www.iarc.fr/en/media-centre/pr/2015/pdfs/pr240_E.pdf, 2015.
- 35.Instituto Brasileiro de Geografia e Estatística - IBGE, Pesquisa de Orçamentos Familiares 2008–2009 Tabela de Medidas Referidas para os Alimentos Consumidos no Brasil,. Ministério do Planejamento, Orçamento e Gestão,, 2011; Available from: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50000.pdf.
- 36.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017;36: 855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7: 177–88. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Horn-Ross PL, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Rodriguez C, Rohan TE, Schatzkin A, Shore R, Virtanen M, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Hunter DJ. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163: 1053–64. [DOI] [PubMed] [Google Scholar]
- 40.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. International journal of epidemiology 1999;28: 964–74. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Yang X, Zhang C, Zhu C, Tao G, Zhao L, Tang S, Shu Z, Cai J, Dai S, Qin Q, Xu L, Cheng H, Sun X. Red and processed meat intake is associated with higher gastric cancer risk: a meta-analysis of epidemiological observational studies. PLoS One 2013;8: e70955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song P, Lu M, Yin Q, Wu L, Zhang D, Fu B, Wang B, Zhao Q. Red meat consumption and stomach cancer risk: a meta-analysis. J Cancer Res Clin Oncol 2014;140: 979–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z, Yin Z, Zhao Q. Red and processed meat consumption and gastric cancer risk: a systematic review and meta-analysis. Oncotarget 2017;8: 30563–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, Park SM. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose(−)Response Meta-Analysis. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Praud D, Rota M, Pelucchi C, Bertuccio P, Rosso T, Galeone C, Zhang ZF, Matsuo K, Ito H, Hu J, Johnson KC, Yu GP, Palli D, Ferraroni M, Muscat J, Lunet N, Peleteiro B, Malekzadeh R, Ye W, Song H, Zaridze D, Maximovitch D, Aragones N, Castano-Vinyals G, Vioque J, Navarrete-Munoz EM, Pakseresht M, Pourfarzi F, Wolk A, Orsini N, Bellavia A, Hakansson N, Mu L, Pastorino R, Kurtz RC, Derakhshan MH, Lagiou A, Lagiou P, Boffetta P, Boccia S, Negri E, La Vecchia C. Cigarette smoking and gastric cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev 2018;27: 124–33. [DOI] [PubMed] [Google Scholar]
- 46.Rota M, Pelucchi C, Bertuccio P, Matsuo K, Zhang ZF, Ito H, Hu J, Johnson KC, Palli D, Ferraroni M, Yu GP, Muscat J, Lunet N, Peleteiro B, Ye W, Song H, Zaridze D, Maximovitch D, Guevara M, Fernandez-Villa T, Vioque J, Navarrete-Munoz EM, Wolk A, Orsini N, Bellavia A, Hakansson N, Mu L, Persiani R, Kurtz RC, Lagiou A, Lagiou P, Galeone C, Bonzi R, Boffetta P, Boccia S, Negri E, La Vecchia C. Alcohol consumption and gastric cancer risk-A pooled analysis within the StoP project consortium. Int J Cancer 2017;141: 1950–62. [DOI] [PubMed] [Google Scholar]
- 47.Ward HA, Gayle A, Jakszyn P, Merritt M, Melin B, Freisling H, Weiderpass E, Tjonneland A, Olsen A, Dahm CC, Overvad K, Katzke V, Kuhn T, Boeing H, Trichopoulou A, Lagiou P, Kyrozis A, Palli D, Krogh V, Tumino R, Ricceri F, Mattiello A, Bueno-de-Mesquita B, Peeters PH, Quiros JR, Agudo A, Rodriguez-Barranco M, Larranaga N, Huerta JM, Barricarte A, Sonestedt E, Drake I, Sandstrom M, Travis RC, Ferrari P, Riboli E, Cross AJ. Meat and haem iron intake in relation to glioma in the European Prospective Investigation into Cancer and Nutrition study. Eur J Cancer Prev 2018;27: 379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiang VS, Quek SY. The relationship of red meat with cancer: Effects of thermal processing and related physiological mechanisms. Crit Rev Food Sci Nutr 2017;57: 1153–73. [DOI] [PubMed] [Google Scholar]
- 49.Demeyer D, Mertens B, De Smet S, Ulens M. Mechanisms Linking Colorectal Cancer to the Consumption of (Processed) Red Meat: A Review. Crit Rev Food Sci Nutr 2016;56: 2747–66. [DOI] [PubMed] [Google Scholar]
- 50.Zur Hausen H, Bund T, de Villiers EM. Specific nutritional infections early in life as risk factors for human colon and breast cancers several decades later. Int J Cancer 2019;144: 1574–83. [DOI] [PubMed] [Google Scholar]
- 51.Boeing H Nutritional epidemiology: New perspectives for understanding the diet-disease relationship? Eur J Clin Nutr 2013;67: 424–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Stomach cancer Pooling (StoP) Project but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Steering Committee of the StoP Project.