Abstract
Objectives
To investigate the relationship between COVID-19 full vaccination (two completed doses) and possible arthritis flare.
Methods
Patients with rheumatoid arthritis (RA) were identified from population-based electronic medical records with vaccination linkage and categorised into BNT162b2 (mRNA vaccine), CoronaVac (inactive virus vaccine) and non-vaccinated groups. The risk of possible arthritis flare after vaccination was compared using a propensity-weighted cohort study design. We defined possible arthritis flare as hospitalisation and outpatient consultation related to RA or reactive arthritis, based on diagnosis records during the episode. Weekly prescriptions of rheumatic drugs since the launch of COVID-19 vaccination programme were compared to complement the findings from a diagnosis-based analysis.
Results
Among 5493 patients with RA (BNT162b2: 653; CoronaVac: 671; non-vaccinated: 4169), propensity-scored weighted Poisson regression showed no significant association between arthritis flare and COVID-19 vaccination ((BNT162b2: adjusted incidence rate ratio 0.86, 95% Confidence Interval 0.73 to 1.01); CoronaVac: 0.87 (0.74 to 1.02)). The distribution of weekly rheumatic drug prescriptions showed no significant differences among the three groups since the launch of the mass vaccination programme (all p values >0.1 from Kruskal-Wallis test).
Conclusions
Current evidence does not support that full vaccination of mRNA or inactivated virus COVID-19 vaccines is associated with possible arthritis flare.
Keywords: COVID-19, arthritis, rheumatoid, epidemiology, vaccination, outcome assessment, health care
Key message.
What is already known about this subject?
Fear of arthritis flare after vaccination could introduce vaccine hesitancy.
To date, there are no analytical studies on COVID-19 vaccination and arthritis flare among patients with rheumatoid arthritis (RA).
What does this study add?
Current cohort study showed no evidence of increased risk of possible arthritis flare among patients with RA who were fully vaccinated with mRNA or inactivated virus COVID-19 vaccines.
How might this impact on clinical practice or future developments?
Individuals with RA should be encouraged to receive the vaccine against COVID-19.
Real-world COVID-19 vaccine safety surveillance should continue to provide more robust evidence on the association between arthritis flare and COVID-19 vaccines with direct disease activity tests and consideration of immunomodulated medications.
Introduction
Vaccine is an effective public health measurement to control the global COVID-19 pandemic. Patients with rheumatoid arthritis (RA) are twofold more vulnerable to infections that result in hospitalisation and impaired quality of life.1 With consideration to the benefits of vaccination outweighing the risks, the European Alliance of Associations for Rheumatology (EULAR)2 recommends that patients with RA should receive COVID-19 vaccines without needing major adjustment to their ongoing treatment regimens. However, one of the major barriers to vaccine uptake among patients with RA is the fear of arthritis flare despite non-relevant evidence from landmark trials and few case reports in the post marketing.3
Understanding the association between arthritis flare and vaccination is important to overcome vaccine hesitancy. Currently, the Hong Kong (HK) Government Vaccination Programme provides two authorised COVID-19 vaccines: CoronaVac (inactivated virus vaccine; recommended vaccination interval 28 days) and BNT162b2 (mRNA vaccine; recommended vaccination interval 21 days). Since the launch of the vaccination programme on 23 February 2021, more than 8 million doses have been administered with close safety monitoring. In this study, we analysed the territory-wide electronic medical records (EMRs) database and aimed to investigate the population-level risk of possible arthritis flare following full vaccination based on two technology platforms.
Method
Data sources
We analysed population-based EMRs from the Hospital Authority (HA) with linked vaccination records from the Department of Health (DH) of the HK Government.4 HA provides publicly funded health services to around 7 million HK residents. The EMRs database managed by the HA holds centralised medical records from 42 public hospitals with high population coverage, representativeness and coding accuracy.5 6 This study linked the EMRs with the vaccination records of all HK residents ≥16 years old who ever used the HA service. We used de-identified and non-reversible series numbers for the record linkage to protect patient privacy.
Study design and population
This was a retrospective cohort study among patients with RA. Risk of possible arthritis flare was compared among vaccine recipients and non-vaccinated individuals. Based on the International Classification of Diseases Ninth version, Clinical Modification (ICD-9-CM) diagnosis (online supplemental table 1), we identified the RA cohort from the EMRs, excluding patients who had cancer or other autoimmune diseases to avoid cohort contamination. We matched each vaccine recipient with non-vaccinated individuals by age and sex using maximum ratio matching and assigned the vaccination date as the pseudo index date for non-vaccinated individuals (controls). Individuals with completed two-dose vaccination and their matched controls were followed up from the date of second dose vaccination or the age-sex matched pseudo index date until the occurrence of interested outcome, death or the end date of data availability (31 July 2021), whichever was earlier. The record linkage, matching procedure and cohort identification is illustrated in online supplemental figure 1.
annrheumdis-2021-221571supp001.pdf (51KB, pdf)
Outcome measurements
After vaccination, any specialist outpatient clinic (SOPC) consultation or hospitalisation related to RA or reactive arthritis was considered a proxy of arthritis flare. Primary outcome is a recorded diagnosis of RA or reactive arthritis from inpatient or SOPC settings. Secondary outcome is a relevant diagnosis at inpatient setting as the proxy of severe arthritis flare.
Statistical analysis
To balance the patient characteristics among groups (CoronaVac, BNT162b2 and non-vaccinated), we used multi-group Inverse Probability Treatment Weighting method and weighted variables including age, sex, medical history and health service utilisation since 2018 and the recent 90 days of medication use. We applied Poisson regression to estimate the adjusted incidence rate ratio (IRR) with 95% Confidence Interval (CI) using the non-vaccination group as reference. Fisher’s exact test was used to examine the association between delayed second dose (defined as interdose interval more than 42 days, which is the maximum dose interval used in BNT162b2 clinical trials)7 and the occurrence of flare.
In addition, we analysed the weekly prescription pattern of rheumatoid drugs (online supplemental table 2) between 23 February (the start date of mass vaccination programme) and 31 July 2021, hypothesising that the prescription volume of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids would increase sharply if there was a significant arthritis flare in the study cohort. Number of prescriptions (per-patient) and proportion of each drug category (NSAIDs, corticosteroids, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and biological/target synthetic disease-modifying antirheumatic drugs (b/tsDMARDs)) among CoronaVac, BNT162b2 and non-vaccinated groups were compared using Kruskal-Wallis test.
Patient and public involvement
This study used de-identified electronic medical records and was conducted without patient and public involvement.
Results
We obtained 3 983 529 records of HA active patients with affirmed vaccination status. Following the cohort selection procedure, 5493 patients with RA (BNT162b2: 653; CoronaVac: 671 and non-vaccinated individuals: 4169) were included. Compared with non-vaccinated individuals, vaccine recipients were younger and less likely to have pre-existing chronic diseases. After weighting, all variables were well balanced with a standardised mean difference smaller than 0.2 (table 1).8 9 Median interdose interval was 21 days (IQR 21–23) for BNT162b2 and 28 days (IQR 28–29) for CoronaVac recipients. Delaying the second dose was very uncommon for both vaccine groups (BNT162b2: 0.5%; CoronaVac: 0.8%).
Table 1.
Baseline characteristics before and after multi-group inverse probability treatment weighting
| Before weighting | After weighting | |||||||
| BNT162b2 | CoronaVac | None | SMD | BNT162b2 | CoronaVac | None | SMD | |
| N | 653 | 671 | 4169 | 3893.56 | 4051.97 | 4169 | ||
| Male (N (%)) | 136 (20.8) | 194 (28.9) | 850 (20.4) | 0.132 | 681.6 (17.5) | 865.8 (21.4) | 850.0 (20.4) | 0.065 |
| Age (mean (SD)) | 55.83 (11.89) | 59.52 (11.04) | 63.97 (14.73) | 0.424 | 61.98 (12.38) | 61.60 (10.85) | 63.97 (14.73) | 0.12 |
| Comorbidities (N (%)) | ||||||||
| Asthma | 9 (1.4) | 9 (1.3) | 72 (1.7) | 0.021 | 53.6 (1.4) | 55.7 (1.4) | 72.0 (1.7) | 0.019 |
| Cerebrovascular disease | 6 (0.9) | 18 (2.7) | 230 (5.5) | 0.18 | 163.9 (4.2) | 166.0 (4.1) | 230.0 (5.5) | 0.044 |
| Chronic obstructive pulmonary disease | 12 (1.8) | 16 (2.4) | 235 (5.6) | 0.135 | 218.7 (5.6) | 264.4 (6.5) | 235.0 (5.6) | 0.025 |
| Congestive heart failure | 1 (0.2) | 2 (0.3) | 118 (2.8) | 0.153 | 120.5 (3.1) | 50.7 (1.3) | 118.0 (2.8) | 0.085 |
| Chronic renal failure | 0 (0.0) | 5 (0.7) | 76 (1.8) | 0.137 | 0.0 (0.0) | 72.9 (1.8) | 76.0 (1.8) | 0.129 |
| Dementia | 0 (0.0) | 0 (0.0) | 17 (0.4) | 0.06 | 0.0 (0.0) | 0.0 (0.0) | 17.0 (0.4) | 0.06 |
| Diabetes | 29 (4.4) | 45 (6.7) | 488 (11.7) | 0.18 | 503.8 (12.9) | 384.3 (9.5) | 488.0 (11.7) | 0.073 |
| Mild liver disease | 0 (0.0) | 1 (0.1) | 13 (0.3) | 0.056 | 0.0 (0.0) | 3.8 (0.1) | 13.0 (0.3) | 0.057 |
| Moderate-severe liver disease | 1 (0.2) | 0 (0.0) | 1 (0.0) | 0.04 | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.015 |
| Myocardial infarction | 4 (0.6) | 1 (0.1) | 48 (1.2) | 0.086 | 25.8 (0.7) | 82.8 (2.0) | 48.0 (1.2) | 0.081 |
| Peripheral vascular disease | 0 (0.0) | 1 (0.1) | 39 (0.9) | 0.1 | 0.0 (0.0) | 27.7 (0.7) | 39.0 (0.9) | 0.094 |
| Paralysis | 0 (0.0) | 1 (0.1) | 17 (0.4) | 0.065 | 0.0 (0.0) | 7.6 (0.2) | 17.0 (0.4) | 0.064 |
| Respiratory infections | 20 (3.1) | 23 (3.4) | 390 (9.4) | 0.176 | 278.5 (7.2) | 354.8 (8.8) | 390.0 (9.4) | 0.053 |
| Stroke or systemic embolism | 2 (0.3) | 7 (1.0) | 95 (2.3) | 0.121 | 73.5 (1.9) | 56.4 (1.4) | 95.0 (2.3) | 0.044 |
| Ulcers | 3 (0.5) | 14 (2.1) | 106 (2.5) | 0.116 | 80.1 (2.1) | 97.7 (2.4) | 106.0 (2.5) | 0.022 |
| Viral infections | 0 (0.0) | 2 (0.3) | 43 (1.0) | 0.104 | 0.0 (0.0) | 36.9 (0.9) | 43.0 (1.0) | 0.097 |
| Health service utilisation (N (%)) | ||||||||
| Emergency or hospital admission | 471 (72.1) | 508 (75.7) | 3464 (83.1) | 0.177 | 3185.5 (81.8) | 3327.5 (82.1) | 3464.0 (83.1) | 0.022 |
| Outpatient visits | 641 (98.2) | 665 (99.1) | 4122 (98.9) | 0.054 | 3826.2 (98.3) | 4011.8 (99.0) | 4122.0 (98.9) | 0.043 |
| Medication usage within 90 days (N (%)) | ||||||||
| Immunosuppressants | 11 (1.7) | 7 (1.0) | 134 (3.2) | 0.102 | 82.0 (2.1) | 115.4 (2.8) | 134.0 (3.2) | 0.046 |
| NSAIDs | 284 (43.5) | 295 (44.0) | 1617 (38.8) | 0.07 | 1529.5 (39.3) | 1671.0 (41.2) | 1617.0 (38.8) | 0.033 |
| Corticosteroids | 0 (0.0) | 0 (0.0) | 1 (0.0) | 0.015 | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.015 |
| b/tsDMARDs | 191 (29.2) | 187 (27.9) | 1287 (30.9) | 0.044 | 1230.7 (31.6) | 1418.6 (35.0) | 1287.0 (30.9) | 0.059 |
| csDMARDs | 486 (74.4) | 508 (75.7) | 3041 (72.9) | 0.042 | 2767.6 (71.1) | 2988.2 (73.7) | 3041.0 (72.9) | 0.04 |
| Drugs for gout | 9 (1.4) | 26 (3.9) | 120 (2.9) | 0.105 | 79.7 (2.0) | 93.1 (2.3) | 120.0 (2.9) | 0.036 |
bDMARDs, biological disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; NSAIDs, Non-steroidal anti-inflammatory drugs; SMD, standardised mean difference; tsDMARDs, target synthetic disease-modifying antirheumatic drugs.
During a median follow-up of 32 days (IQR 14–72), 35 BNT162b2 recipients (crude incidence 0.45 (95% CI 0.32 to 0.62) per person-year) had RA or reactive arthritis-related hospitalisation or SOPC attendance. The number of CoronaVac recipients is 41 (crude incidence 0.45 (0.33 to 0.61) per person-year) with a median follow-up of 30 days (IQR 15–95). Receiving two doses of BNT162b2 (adjusted IRR 0.86 (95% CI 0.73 to 1.01)) or CoronaVac (adjusted IRR 0.87 (95% CI 0.74 to 1.02)) showed no significant association with arthritis flare as defined. Similarly, no significant association was detected when focusing on events identified from inpatient setting only (table 2). Delayed second dose was not associated with the occurrence of possible flare (p=0.3042 for BNT162b2; p=0.5422 for CoronaVac and p=0.1454 for overall from Fisher’s exact test).
Table 2.
Risk of flare among two-dose vaccine recipients vs unvaccinated individuals, after propensity score weighting
| N | Follow-up time (person-year) |
Crude incidence (per person-year, 95% CI) |
Adjusted IRR* (95% CI) |
P-value | |
| Primary outcome | |||||
| BNT162b2 | 35 | 78.23 | 0.45 (0.32 to 0.62) | 0.86 (0.73 to 1.01) | 0.0702 |
| CoronaVac | 41 | 91.02 | 0.45 (0.33 to 0.61) | 0.87 (0.74 to 1.02) | 0.0962 |
| None | 330 | 612.63 | 0.54 (0.48 to 0.60) | Ref | – |
| Secondary outcome | |||||
| BNT162b2 | 33 | 78.65 | 0.42 (0.29 to 0.58) | 0.96 (0.81 to 1.14) | 0.6486 |
| CoronaVac | 38 | 91.58 | 0.41 (0.30 to 0.56) | 1.03 (0.87 to 1.22) | 0.7373 |
| None | 275 | 620.26 | 0.44 (0.39 to 0.50) | Ref | – |
*Adjusted variables with standard mean difference >0.1; IRR estimated using non-vaccinated group as reference
IRR, incidence rate ratio.
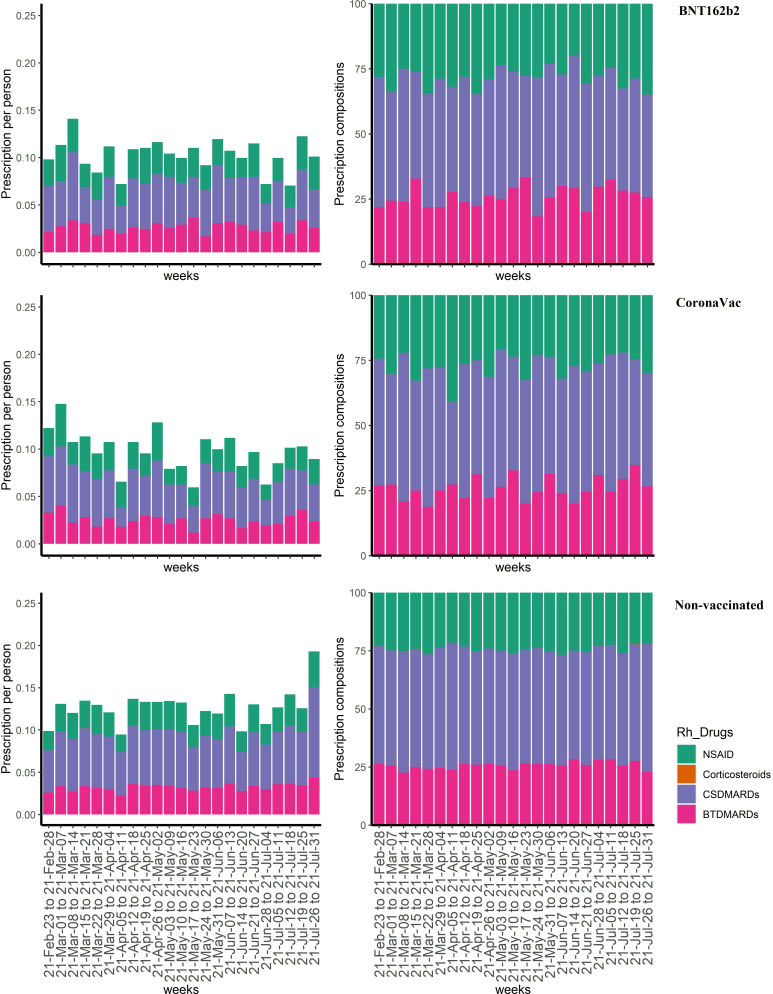
Weekly prescription of four major rheumatoid drugs were presented in figure 1. Since the launch of the COVID-19 vaccination programme in HK, weekly arthritis-related prescriptions ranged between 0.09 and 0.14 per patient. NSAIDs and corticosteroids accounted for 23%–27% of overall prescriptions. The per-patient prescription and distribution of four rheumatoid drug categories showed no significant differences among the BNT162b2 and CoronaVac recipients, and the non-vaccinated individuals (all p values >0.1 from Kruskal-Wallis test).
Figure 1.
Weekly arthritis-related prescriptions among vaccine recipients and non-vaccinated individuals, between 1 February and 31 July 2021. BTDMARDs, biological or target synthetic disease-modifying antirheumatic drugs; CSDMARDs, conventional synthetic disease-modifying antirheumatic drugs; NSAID, non-steroidal anti-inflammatory drug. Kruskal-Wallis test showed all p values >0.1 for each week comparison, indicating the distribution of arthritis-related prescriptions showed no differences among BNT162b2 recipients, CoronaVac recipients and non-vaccinated individuals.
Discussion
Using territory-wide EMRs in HK, we found that after full vaccination with BNT162b2 or CoronaVac, patients with RA did not show an increased risk of possible arthritis flare. The weekly prescription trends of major rheumatoid drugs also presented no significant differences among patients with or without vaccination. Currently, safety evidence on COVID-19 vaccine among patients with rheumatic diseases are from case reports,3 10 11 self-report surveys12 or trials among RA patients with controlled disease activities.13 Since the launch of vaccination in HK, uptake of the vaccine (approximate 24% (95% CI 22.99% to 25.25%) with full vaccination based on our study cohort) among patients with RA is gradually increasing (online supplemental figure 2), although remaining suboptimal. Findings from this study provide real-world evidence of COVID-19 vaccine safety and could potentially overcome vaccine hesitancy among patients with RA.
We acknowledge that if individuals who experienced flare after the first dose, then they would be less likely to take the second dose, which could theoretically introduce biased estimation for the current two-dose analysis. To clarify this issue, we conducted post hoc analysis to estimate the number of patients received single-dose only. We included patients who received the first-dose vaccine on or before 19 June 2021 and had no record of second dose until the study end date (31 July 2021). It would ensure at least 42-day observation period after the first dose and exclude the possibility that the second dose was scheduled beyond the study period. Although the recommended dosing interval is 21 and 28 days for BNT162b2 and CoronaVac, respectively, the HK Government allows flexibility of interval between doses for logistic or clinical reasons. Analysis of the phase III efficacy data of BNT162b2 showed it was feasible to administer the second dose from 19 to 42 days.6 Therefore, we defined an interdose interval within 42 days is acceptable. Based on the above definition, the number of subjects who received single-dose only is very small for both vaccine groups (BNT162b2: 4; CoronaVac: 7). Therefore, we anticipate the theoretical bias is neglectable and will not affect the interpretation of our current results. We also conducted a post hoc analysis to evaluate the potential effect of delayed second dose, that is, more than 42 days. Our RA cohort showed only less than 1% of the subjects received the second dose more than42 days after the first dose. Fisher’s exact test also showed no association between delayed second dose and the occurrence of flares. In summary, non-taken or delayed second dose is very uncommon in our study cohort with minimum impact to the results interpretation of current study.
Nevertheless, multiple factors could trigger arthritis flare, such as infection, stress and poor medication adherence.14 Flare is preventable, manageable and reversible if an appropriate regimen and dosing adjustment of DMARDs is followed. For possible flare resulting in hospitalisation, our data showed that the maximum length of stay was 6 days with no recorded registered death, indicating a satisfactory prognosis. Vaccine hesitancy is also related to the uncertainty of immunogenicity in patients with inflammatory diseases because of their immunocompromised conditions.15 16 Individuals with inflammatory disease were observed to have a higher risk of severe conditions after COVID-19 infection compared with those without inflammatory diseases.17 18 It was established that the immunogenicity of COVID-19 vaccine could achieve an acceptable threshold for protection.13 19 Combining the current evidence of safety and effectiveness, vaccination with two doses is highly recommended to achieve adequate self-protection in patients with RA.20
To the best of our knowledge, this is the first population-based analytical study with valid vaccination record linkage for COVID-19 vaccine safety monitoring among patients with RA. The study assessed the safety of two different vaccine technology platforms with relatively larger sample sizes and a longer follow-up period. Our cohort identification was based on ICD-9-CM diagnosis codes (714.xx) recorded in either inpatient or SOPC settings with clinical diagnoses made by rheumatology specialists. Furthermore, prescription data analysis showed, in our study cohort, 96% of the patients diagnosed with RA had arthritis-related prescription records (cs/b/tsDMARD, NSAIDs or corticosteroid) between 1 January 2018 and 31 July 2021 (the period of data availability), which supports the high validity of RA cohort we identified.
However, as a common drawback with EMR-based studies, information on the clinically relevant definition of flares, such as disease activity assessment (eg, Disease Activity Score-28 for Rheumatoid Arthritis) and patient-reported symptoms (eg, pain, stiffness and fever), is not available. Using arthritis-related hospital admission and SOPC consultation as a proxy of flare may underestimate the accurate occurrence. The supplementary analysis using arthritis-related prescription as a surrogate outcome of flare enables the validation of diagnosis-based outcome definition. This consistent finding further supports the non-significant association between COVID-19 vaccination and arthritis flare. Of note, almost no patients were recorded as using corticosteroids at cohort entry, indicating that those who received the vaccine were at the maintenance stage of RA with stable disease activity or in remission. The study conclusion is not entirely generalisable to patients with active RA. Our database is also restricted to patients who use the HA service. HA is the statutory body responsible for managing all the public hospitals in HK and provides a highly subsidised health service to all eligible HK residents. It is anticipated that the majority of possible flare is captured in this study, particularly severe cases resulting in hospitalisation, although we possibly missed patients consulting private rheumatologists for flare management. However, there is no evidence to show differential use of private consultants between vaccinated and unvaccinated subjects; hence, it is unlikely to affect our conclusion.
In conclusion, among patients with RA, there is no increased risk of possible flare following two doses of COVID-19 vaccination. Real-world vaccine safety surveillance with direct disease activity testing related to arthritis flare should continue to provide more robust evidence on the association.
Acknowledgments
We thank colleagues from the Drug Office of the Department of Health and the Hospital Authority for the generous provision of vaccination and clinical data. We also thank Ms Lisa Lam for proofreading the manuscript.
Footnotes
Handling editor: Josef S Smolen
Twitter: @CarlosWongHKU
Contributors: Study concept and design: XL, ICKW. Data extractions, cleaning and analysis: XT. Data validation and cross-check: PK. Data interpretation: All authors. Drafting of the manuscript: XL, XT, SHHY. Critical revision of the manuscript of significant intellectual contribution: All authors. Study supervision: ICKW. Guarantor: ICKW.
Funding: Research Grant from the Food and Health Bureau, the Government of the Hong Kong Special Administrative Region (Ref. No. COVID19F01).
Competing interests: XL received research grants from Research Fund Secretariat of the Food and Health Bureau (HMRF, HKSAR), Research Grants Council Early Career Scheme RGC/ECS, HKSAR, Janssen and Pfizer; internal funding from the University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA and Amgen; personal fee from Primevigilance Ltd.; outside the submitted work. FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council, outside the submitted work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grant Council, outside the submitted work. CKHW reports the receipt of General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation, all outside the submitted work. EWYC reports honorarium from Hospital Authority, supports from the Health and Medical Research Fund (HMRF), grants from Research Grants Council (RGC, Hong Kong), grants from National Natural Science Fund of China, grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda, grants from Narcotics Division of the Security Bureau of HKSAR, grants from Innovation and Technology Commission of the Government of the HKSAR, all outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia, and also received speaker fees from Janssen and Medice in the previous 3 years. He is also independent non-executive director of Jacobson Medical in Hong Kong.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was granted by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 21-149 and UW 21-138); and the Department of Health Ethics Committee (LM 21/2021). Patient identification was all anonymised and patient consent was not required.
References
- 1. Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. 10.1093/rheumatology/kes305 [DOI] [PubMed] [Google Scholar]
- 2. Bugatti S, Balduzzi S, De Stefano L, et al. Correspondence on 'EULAR December 2020 viewpoints on SARS-CoV-2 vaccination in patients with RMDs'. Ann Rheum Dis 2021;80:e156–2. 10.1136/annrheumdis-2021-220541 [DOI] [PubMed] [Google Scholar]
- 3. Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol 2021;3:e469–70. 10.1016/S2665-9913(21)00108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. EYF W, Chui CSL, FTT L. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis 2021. 10.1016/S1473-3099(21)00451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lau WCY, Chan EW, Cheung C-L, et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 2017;317:1151–8. 10.1001/jama.2017.1363 [DOI] [PubMed] [Google Scholar]
- 6. Man KKC, Coghill D, Chan EW, et al. Association of risk of suicide attempts with methylphenidate treatment. JAMA Psychiatry 2017;74:1048–55. 10.1001/jamapsychiatry.2017.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food and Health Bureau, The Government of the Hong Kong Special Administrative Region . COMIRNATY COVID-19 mRNA vaccine (BNT162b2) 2021. Available: https://www.fhb.gov.hk/download/our_work/health/201200/e_SmPC_for_HK.pdf [Accessed 6 Oct 2021].
- 8. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacob C. Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale NJ: Lawrence Erlbaum Associates Publishers, 1988. [Google Scholar]
- 10. An Q-J, Qin D-A, Pei J-X. Reactive arthritis after COVID-19 vaccination. Hum Vaccin Immunother 2021;17:2954–6. 10.1080/21645515.2021.1920274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baimukhamedov C. Arthritis of the left elbow joint after vaccination against SARS-CoV-2 infection. Int J Rheum Dis 2021;24:1218–20. 10.1111/1756-185X.14202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbhaiya M, Levine JM, Bykerk VP, et al. Systemic rheumatic disease flares after SARS-CoV-2 vaccination among rheumatology outpatients in New York City. Ann Rheum Dis 2021;80:1352–4. 10.1136/annrheumdis-2021-220732 [DOI] [PubMed] [Google Scholar]
- 13. Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med 2021;27:1744–51. 10.1038/s41591-021-01469-5 [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 2015;11:276–89. 10.1038/nrrheum.2015.8 [DOI] [PubMed] [Google Scholar]
- 15. Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1098–9. 10.1136/annrheumdis-2021-220289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis 2020;79:1156–62. 10.1136/annrheumdis-2020-217888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 20. America College of Rheumatology . COVID-19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases 2021, 2021. Available: https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf [Accessed 10 Sept 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-221571supp001.pdf (51KB, pdf)
Data Availability Statement
No data are available.