Abstract
Background.
Despite the risk of new persistent opioid use after cardiac surgery, postdischarge opioid use has not been quantified and evidence-based prescribing guidelines have not been established.
Methods.
Opioid-naive patients undergoing primary cardiac surgery via median sternotomy between January and December 2019 at 10 hospitals participating in a statewide collaborative were selected. Clinical data were linked to patient-reported outcomes collected at 30-day follow-up. An opioid prescribing recommendation stratified by inpatient opioid use on the day before discharge (0, 1–3, or ≥4 pills) was implemented in July 2019. Interrupted time-series analyses were performed for prescription size and postdischarge opioid use before (January to June) and after (July to December) guideline implementation.
Results.
Among 1495 patients (729 prerecommendation and 766 postrecommendation), median prescription size decreased from 20 pills to 12 pills after recommendation release (P < .001), while opioid use decreased from 3 pills to 0 pills (P < .001). Change in prescription size over time was +0.6 pill/month before and −0.8 pill/month after the recommendation (difference = −1.4 pills/month; P = .036). Change in patient use was +0.6 pill/month before and −0.4 pill/month after the recommendation (difference = −1.0 pills/month; P = .017). Pain levels during the first week after surgery and refills were unchanged. Patients using 0 pills before discharge (n = 710) were prescribed a median of 0 pills and used 0 pills, while those using 1 to 3 pills (n = 536) were prescribed 20 pills and used 7 pills, and those using greater than or equal to 4 pills (n = 249) were prescribed 32 pills and used 24 pills.
Conclusions.
An opioid prescribing recommendation was effective, and prescribing after cardiac surgery should be guided by inpatient use.
Opioid overprescribing after surgery is common.1–6 A larger opioid prescription size has been associated with higher opioid use6 and an increased risk of new persistent opioid use 3 to 6 months after surgery in previously opioid-naive patients.8 New persistent opioid use has been reported in 3% to 8% of patients after minor and major surgery,9–11 over 10% of cancer patients undergoing curative surgery,12 and up to 17% of patients undergoing cardiothoracic surgery.8,13–15 In addition, overprescribing has been shown to increase the risk of opioid overdose in patients’family members through opioid diversion.16
Despite high rates of new persistent opioid use and large prescription sizes at discharge,8,14,17 patient-reported opioid use after cardiac surgery has not been described and evidence-based prescribing guidelines have not been established. With the sharp rise of opioidrelated drug overdoses17 prompting the declaration of the opioid crisis as a public health emergency, considerable efforts to reduce opioid use through evidence-based prescribing guidelines after general surgery procedures have been largely successful.1,5,18–20 However, many of these guidelines were established for outpatient procedures, and may not translate to cardiac surgery because patients undergo median sternotomy and spend a median 6 to 12 days in the hospital, depending on the pro-cedure.21 A single-center study of inpatient general surgery found that inpatient opioid use before discharge was associated with postdischarge use and could be utilized to guide prescribing through an individualized approach,22 which may be similarly beneficial in cardiac surgery patients. At present, appropriate opioid prescription quantities after cardiac surgery remain unknown, and the relationship between inpatient and postdischarge use has not been characterized. Over 300,000 cardiac surgery procedures are performed annually, and these procedures confer among the highest published rates of new persistent opioid use. Therefore, establishing evidence-based opioid prescribing recommendations should be prioritized to guide responsible prescribing and optimize patient care.
This prospective, multicenter collaboration was organized to (1) define opioid prescribing and patient-reported use after cardiac surgery, (2) assess inpatient opioid use as a predictor of postdischarge opioid use, (3) establish opioid prescribing recommendations for opioid-naive patients, and (4) evaluate the implementation of statewide recommendations by analyzing postintervention prescribing and patient-reported outcomes (PROs). We hypothesized that implementing recommendations would decrease both opioid prescribing and use and that incorporating inpatient opioid use would allow for more personalized prescribing.
Patients and Methods
This study was deemed exempt by the University of Michigan Institutional Review Board (HUM00156194).
Data Source
Clinical data were collected through the Michigan Society of Thoracic and Cardiovascular Surgeons(MSTCVS) Quality Collaborative, developed in 2001 as a cardiac surgeon–led quality collaborative embedded in the MSTCVS, including all 33 nonfederal hospitals perform-ing cardiac surgery in Michigan. The Collaborative receives standardized harvest files sent by each of the hospitals to The Society of Thoracic Surgeons national database.
Opioid naivite was defined as not using opioids at time of admission for surgery. PROs including postoperative pain scores, number of pills used postdischarge, number of days using an opioid, refills, and use of adjuncts were captured in an 11-item questionnaire prospectively administered at 30-day clinical follow-up (Supplemental Appendix 1). Inpatient opioid use during the 24-hour calendar day before discharg and prescription informa-tion were collected through chart review by data management team members at each hospital. Although perioperative patient instructions were not uniform across all 10 centers, each patient receiving a opioid prescription at all 10 centers reviewed the Opioid Start Talking form (Supplemental Appendix 2) with a provider according to state law.
Questionnaire responses were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the MSTCVS Coordinating Center.23 Questionnaire responses were linked to clinical data by unique Society of Thoracic Surgeons record identification numbers and verified with dates of surgery and discharge.
Patient Population
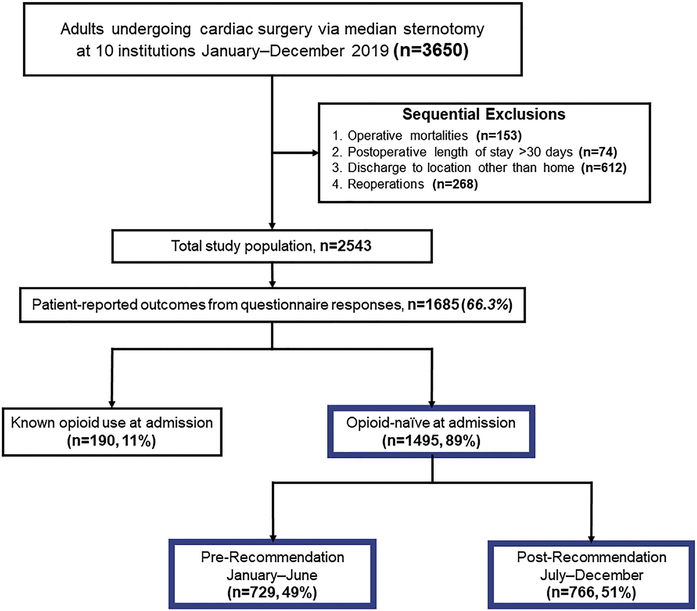
Patients undergoing cardiac surgery via median sternotomy between January and December 2019 at 10 centers participating in the MSTCVS Quality Collaborative were identified (n = 3650). From these, operative mortalities (n = 153), those with postoperative length of stay greater than 30 days (n = 74), discharge to a location other than home (n = 612), and reoperations (n = 268) were sequentially excluded. From the resulting population (n = 2543), 1685 (66%) reported PROs, including 1495 (89%) opioid-naive patients (Figure 1). Clinical characteristics, inpatient opioid use, and prescription size were collected for all patients and compared between responders and nonresponders.
Figure 1.
Patient population STROBE diagram.
Opioid Prescribing Recommendations
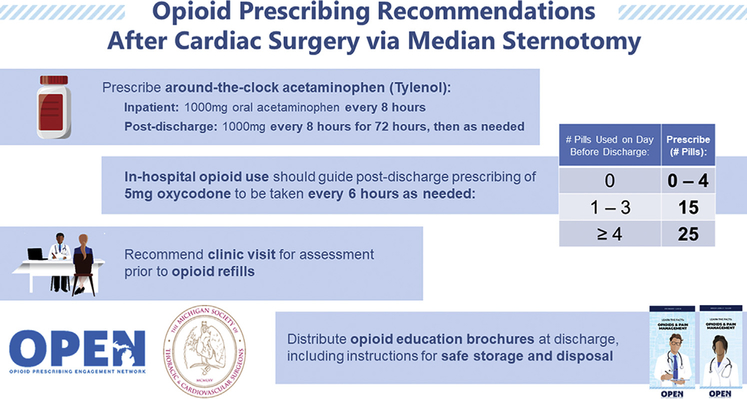
Opioid prescribing and PROs from January to March 2019 were analyzed to form an initial recommendation. The final recommendation was established through multidisciplinary input from the Michigan Opioid Prescribing Engagement Network, hospital data managers, and surgeon leaders from all 10 centers (Figure 2). This recommendation was formed in June and disseminated in early July. Throughout the 1-year study period, all 10 hospitals collected day before discharge opioid use, prescribing data, and PROs at follow-up. The prerecommendation period was defined by operative dates January 1 to June 30, while the postrecommendation period included July 1 to December 31.
Figure 2.
Opioid prescribing recommendations for opioid-naive patients undergoing cardiac surgery.
Statistical Analysis
Primary outcomes were prescription size and patient-reported, postdischarge opioid use. Secondary outcomes included inpatient opioid use before discharge, refills before follow-up, percent of patients not prescribed an opioid at discharge, and PROs including pain in the first week after surgery (with 1 denoting none, 2 denoting minimal, 3 denoting moderate, and 4 denoting severe) and use of nonopioid adjuncts.
Bivariate comparisons utilized paired, 2-tailed t-tests or Wilcoxon rank sum tests for continuous data and chi-square testing for categorical data, as appropriate. All opioid prescription and use data were initially converted to oral morphine equivalents and quantified in 5-mg oxycodone pills. Opioid prescribing and use was quantified in median (interquartile range [IQR]) and mean ± SD number of pills. Prescription size and patient-reported use were evaluated overall and by hospital. Interrupted time-series analyses were performed comparing discharge opioid prescription size and postdischarge use prerecommendation and postrecommendation. The slope during each period represents the trend in number of pills per month, while both the differences in prescription size and postdischarge use (level change) and the difference in the trends of each before and after the recommendation release (trend change) were evaluated. The percent of patients not prescribed an opioid at discharge, opioid refill rate, and pain levels were displayed by 3-month periods to show trends related to the release of recommendations.
As in prior analyses,22 the unadjusted relationship between prescribing and postdischarge use was depicted after stratifying groups by opioid use on the day before discharge (0, 1–3, or ≥4 pills). Group definitions were consistent with the terciles of inpatient opioid use determined by the initial data used to develop the prescribing recommendation. Guideline adherence was measured by quantifying the number of patients in each tercile who were prescribed the recommended number of pills or fewer as outlined in Figure 2. Univariate analyses of postdischarge opioid use were performed with Wilcoxon rank sum tests for binary variables or else negative binomial regression. Multivariable mixed-effects negative binomial regression was performed to identify independent predictors of postdischarge opioid use. The primary exposure was inpatient opioid use during the day before discharge, while covariates included age, sex, race, ethnicity, primary insurance payor, diabetes, cerebrovascular disease, hypertension, smoking history (nonsmoker, former smoker, active smoker), peripheral vascular disease, heart failure, cancer within 5 years, depression, postoperative complication, operative status (elective, urgent, or emergent), pain during the first week after surgery, length of stay, surgery type, and hospital (random effect).
Statistical significance was defined as a P value less than .05. All analyses were performed in Stata 16.0 (Sta-taCorp, College Station, TX).
Results
Patient Demographics and Outcomes
Among 2257 eligible opioid-naive patients, respondents (n = 1495) compared with nonrespondents (n = 762) were prescribed 2 less pills at discharge, had a mean 0.5-day shorter hospital stay, and a lower proportion had a history of depression; patient demographics, clinical characteristics, and inpatient opioid use on the before discharge were otherwise similar (Supplemental Table 1). Among 1495 opioid-naive respondents across 10 centers, mean age was 64 ± 11 years and 26% were women. In total, 55% underwent isolated coronary artery bypass grafting (CABG), 30% underwent an isolated valve procedure, and 12% underwent CABG and valve procedures, most of which were elective (n = 892, 60%). A higher proportion of postrecommendation compared with prerecommendation patients had a history of hypertension, underwent isolated CABG, and were urgent rather than elective; preoperative characteristics otherwise did not statistically differ by study period (Table 1).
Table 1.
Patient Characteristics
| Variable | Overall (N = 1495) | Prerecommendation (January to June) (n = 729) | Postrecommendation (July to December) (n = 766) | P Value |
|---|---|---|---|---|
| Age y | 64 ± 11 | 63 ± 11 | 64 ± 11 | .07 |
| Female | 382 (26) | 173 (24) | 209 (27) | .12 |
| Body mass index, kg/m2 | 29.7 ± 5.7 | 29.8 ± 5.7 | 29.6 ± 5.7 | .54 |
| Race | ||||
| Caucasian | 1296 (87) | 630 (86) | 666 (87) | .84 |
| Black | 103 (7) | 54 (7) | 49 (6) | |
| Non-Caucasian, non-Black | 47(3) | 23 (3) | 24 (3) | |
| Not stated | 49(3) | 22 (3) | 27 (4) | |
| Diabetes | 555 (37) | 256 (35) | 299 (39) | .11 |
| Hypertension | 1246 (83) | 591 (81) | 655 (86) | .021 |
| Cerebrovascular disease | 310 (21) | 137 (19) | 173 (23) | .07 |
| Peripheral vascular disease | 157 (11) | 79 (11) | 78 (10) | .69 |
| Congestive heart failure | 525 (35) | 270 (37) | 255 (34) | .15 |
| Cancer within 5 y | 95 (6) | 54(7) | 41 (5) | .11 |
| Depression | 277 (19) | 128 (18) | 149 (20) | .33 |
| Smoking status | ||||
| Never smoker | 613 (41) | 307 (42) | 306 (40) | .61 |
| Former smoker | 625 (42) | 303 (42) | 322 (42) | |
| Current smoker | 256 (17) | 119 (16) | 137 (18) | |
| Procedure | ||||
| Isolated CABG | 822 (55) | 371 (51) | 451 (59) | .005 |
| Isolated valve | 446 (30) | 228 (31) | 218 (28) | |
| CABG/valve | 181 (12) | 101 (14) | 80 (10) | |
| Other | 46(3) | 29 (4) | 17 (2) | |
| Operative acuity | ||||
| Elective | 892 (60) | 456 (63) | 436 (57) | .024 |
| Urgent | 561 (38) | 249 (34) | 312 (41) | |
| Emergent | 42(3) | 24 (3) | 18 (2) | |
| Postoperative length of stay, d | 6.7 ± 3.0 | 6.9 ± 3.0 | 6.5 ± 3.0 | .027 |
| Postoperative complication | 591 (40) | 302 (41) | 289 (38) | .14 |
| Inpatient opioid use on day before discharge, in 5-mg oxycodone pills | 0.7 (0–2.4) | 0.7 (0–2.7) | 0.6 (0–2.0) | .023 |
Values are mean ± SD, n (%), or median (interquartile range).
CABG, coronary artery bypass grafting.
Mean postoperative length of stay was statistically shorter postrecommendation compared with prerecommendation (6.5 ± 3.0 days vs 6.9 ± 3.0 days; P = .027), whereas complication rate did not differ (post: n = 289 of 766 [38%] vs pre: n = 302 of 729 [41%]; P = .14). Inpatient opioid use on the day before discharge decreased from a median of 0.7 (IQR, 0–2.7) pill to 0.6 (IQR, 0–2.0) pill (P = .023) (Table 1).
Opioid Prescribing and Postdischarge Use
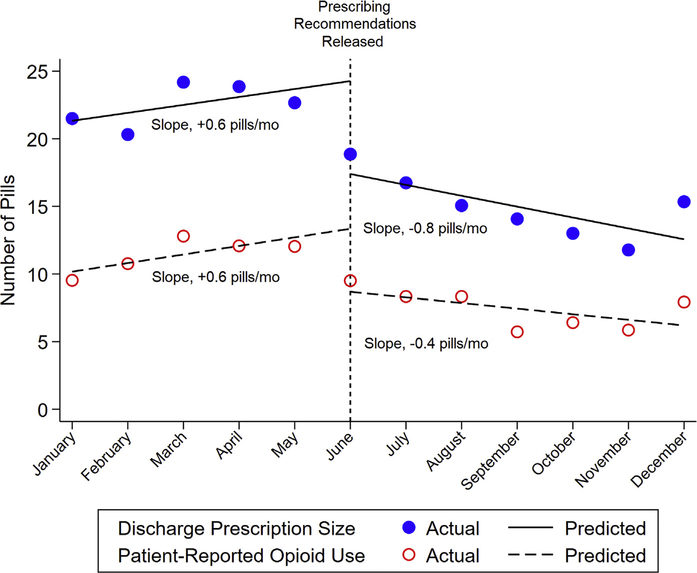
Median prescription size decreased from 20 (IQR, 0–37) pills prerecommendation to 12 (IQR, 0–24) pills postrecommendation (P < .001), while postdischarge use decreased from 3 (IQR, 0–16) pills to 0 (IQR, 0–11) pills (P < .001) and varied by hospital (Supplemental Figure 1). Interrupted time-series analyses showed a significant difference between prerecommendation and postrecommendation prescribing (P = .003) and use (P = .002). The change in prescription size over time was +0.6 pill/ month (95% confidence interval [CI], −0.2 to +1.4) before and −0.8 pill/month (95% CI, −1.8 to +0.2) after the recommendation, representing a significant difference in slope of −1.4 pills/month (95% CI, −2.7 to −0.1; P = .036). The change in postdischarge use was +0.6 pill/month (95% CI, +0.1 to +1.2) before and −0.4 pill/month (95% CI, −1.0 to +0.2) after the recommendation, representing a significant difference in slope of −1.0 pills/month (95% CI, −1.8 to −0.2 pills; P = .017) (Figure 3).
Figure 3.
Interrupted time-series analyses of opioid prescription size and patient-reported postdischarge opioid use before and after release of prescribing guidelines.
Patient-Reported Outcomes
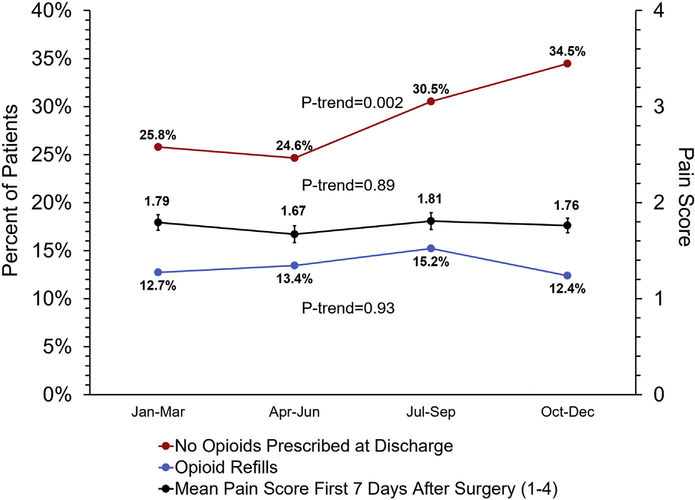
While a higher proportion of patients were not prescribed an opioid at discharge after the release of recommendations (pre: 25.2% vs post: 32.6%; P = .002), opioid refill rate (pre: 13.1% vs post: 13.8%; P for trend = .93) and patient-reported pain levels during the first week after surgery (P for trend = .89) did not significantly change (Figure 4). Additionally, patient-reported use of nonopioid adjuncts increased from 51% (n = 353 of 687) prerecommendation to 61% (n = 435 of 715) postrecommendation (P < .001). PROs before and after recommendation release varied by hospital (Supplemental Figure 2) and were collected at a median 29 (IQR, 25–36) days after discharge, which did not differ prerecommendation vs postrecommendation (29 [IQR, 25–35] days vs 29 [IQR, 25–37] days; P = .21).
Figure 4.
Percent of patients without an opioid prescription at discharge, refill rate, and patient-reported pain scores.
Stratification by Inpatient Opioid Use and Guideline Adherence
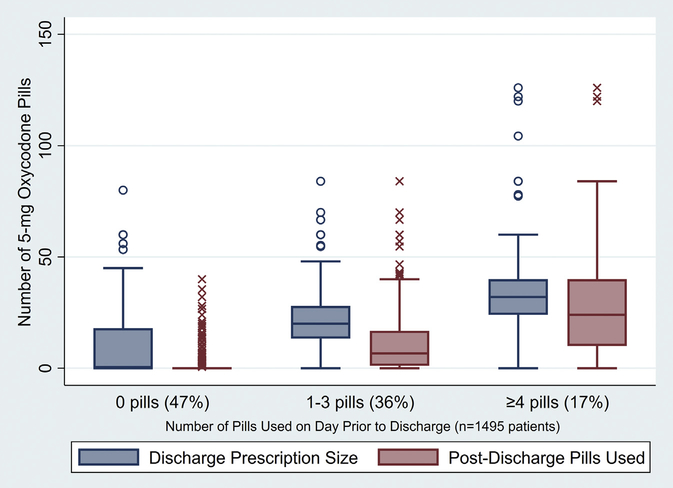
Among the 47% (n = 710 of 1495) of patients who used 0 pills on the day before discharge, nearly half (n = 316 of 710, 45%) were still prescribed a median 19 (IQR, 12–28) pills. Utilizing the same groupings of inpatient use as in the prescribing recommendations (0 pills [n = 710], 1–3 pills [n = 536], and ≥4 pills [n = 249]), both prescription size and postdischarge use increased significantly with each group of increasing inpatient use (Figure 5). Median prescription size was greater than postdischarge use for patients with inpatient use before discharge in all 3 groups: 0 pills (prescription size: 0 [IQR, 0–18] pills vs use: 0 [IQR, 0–0] pills; P < .001), 1–3 pills (prescription size: 20 [IQR, 13–28] pills vs use: 7 [IQR, 1–17] pills; P < .001), and greater than or equal to 4 pills before discharge (prescription size: 32 [IQR, 24–40] pills vs use: 24 [IQR, 10–40] pills; P < .001). Median postoperative length of stay for each group was 6 (IQR, 5–8) days, 6 (IQR, 5–7) days, and 5 (IQR, 5–7) days, respectively. Among postrecommendation patients, 52% (n = 397 of 766) were prescribed a number of opioid pills adherent to the prescribing recommendations, whereas 35% (n = 254 of 729) of prerecommendation patients were prescribed quantities adherent to the recommendation (P < .001).
Figure 5.
Opioid prescription size (blue) and patient-reported postdischarge opioid use (red) stratified by the amount of opioid use during the inpatient day before discharge (0, 1–3, or ≥4 pills).
Predictors of Postdischarge Use
Multivariable analysis indicated that inpatient opioid use the day before discharge was independently predictive of postdischarge opioid use. Additional predictors of postdischarge use included younger age, absence of cerebrovascular disease, absence of cancer within the past 5 years, patients with Medicare rather than no insurance, higher pain levels, and shorter postoperative length of stay (Table 2).
Table 2.
Independent Predictors of Postdischarge Opioid Use After Cardiac Surgery
| Variable | Pills Taken After Discharge | Pills Taken After Discharge | Univariate P Value | Multivariable P Value |
|---|---|---|---|---|
| Agea | ||||
| <60 y (n = 510) | 5 (0–21) | 14 ± 20 | <.001 | <.001 |
| 60–69 y (n = 487) | 1 (0–14) | 9 ± 15 | ||
| ≥70 y (n = 498) | 0 (0–4) | 4 ± 9 | ||
| Cerebrovascular disease | ||||
| Yes (n = 286) | 0 (0–12) | 9 ± 15 | 0.23 | .013 |
| No (n = 1120) | 1 (0–13) | 9 ± 16 | ||
| Cancer within the past 5 y | ||||
| Yes (n = 91) | 0 (0–10) | 7 ± 13 | .24 | .049 |
| No (n = 1308) | 1 (0–13) | 9 ± 16 | ||
| Primary insurance payorb | ||||
| Medicare (reference) (n = 761) | 0 (0–9) | 7 ± 13 | Reference | |
| Self-pay/no insurance (n = 15) | 0 (0–0) | 2 ± 7 | .08 | .011 |
| Medicaid (n = 113) | 12 (0–37) | 19 ± 21 | <.001 | .50 |
| Military/other governmental (n = 35) | 0 (0–10) | 9 ± 18 | .51 | .89 |
| Commercial (n = 437) | 4 (0–17) | 11 ± 16 | .001 | .48 |
| Health maintenance organization (n = 134) | 3 (0–14) | 10 ± 15 | .09 | .61 |
| Number of pills taken on day before dischargea | ||||
| 0 (n = 710) | 0 | 2 ± 5 | <.001 | <.001 |
| 1–3 (n = 536) | 7 (1–17) | 11 ± 13 | ||
| ≥4 (n = 249) | 24 (10–40) | 28 ± 23 | ||
| Pain during the first week after surgeryb | ||||
| None (n = 80) | 0 | 2 ± 7 | Reference | |
| Minimal (n = 418) | 0 (0–3) | 4 ± 9 | .001 | .001 |
| Moderate (n = 683) | 3 (0–14) | 10 ± 15 | <.001 | <.001 |
| Severe (n = 238) | 14 (2–28) | 19 ± 22 | <.001 | <.001 |
| Length of staya | ||||
| 3–5 d (n = 615) | 3 (0–17) | 11 ± 17 | <.001 | .004 |
| 6–7 d (n = 487) | 1 (0–12) | 9 ± 15 | ||
| ≥8 d (n = 393) | 0 (0–9) | 7 ± 14 | ||
Analyzed as a continuous variable in multivariable regression
Analyzed as a categorical variable in multivariable regression.
Values are median (interquartile range) or mean ± SD. The full model is shown in in Supplemental Table 2.
Comment
In this multicenter collaboration to establish, implement, and evaluate opioid prescribing recommendations after cardiac surgery, both opioid prescribing and patient-reported use decreased significantly, while patient-reported pain levels and opioid refills remained unchanged. In addition, use of nonopioid adjuncts and the proportion of patients not prescribed an opioid at discharge increased after release of the recommendations. Collectively, these findings suggest that inpatient opioid use should guide prescribing and that the proposed opioid prescribing recommendations for opioid-naive patients undergoing cardiac surgery (Figure 2) were effective. Moreover, postoperative opioid use continued to decline, suggesting that future efforts could employ more aggressive opioid-sparing approaches.
Opioid prescribing guidelines have been successfully implemented for a wide range of procedures.5,18–20 However, many of these procedures are outpatient or involve a short hospital stay, whereas cardiac surgery patients may spend a week or more as an inpatient. Administrative claims analyses have described new persistent opioid use after cardiac7,10,13,16 and general thoracic7,9,11,12,14 procedures, and efforts have been initiated to establish thoracic surgery prescribing guidelines.4 However, the implementation of these thoracic guidelines has not been evaluated and only recommendations based on single-center expert opinion have been proposed for cardiac surgery.24 A study of inpatient general surgery concluded that inpatient opioid use before discharge should guide prescribing, rather than a single prescription quantity.22 Our study reinforces these findings in a cardiac surgery population and expands these data by implementing a stratified, 4-part prescribing and pain management recommendation across 10 hospitals. Interestingly, only 11% of patients undergoing thoracic4 and 15% of patients undergoing inpatient general surgery22 were not prescribed an opioid at discharge, vs 29% of cardiac patients in this study. However, we also found that among the 47% of patients not using opioids on the day before discharge, 45% were still prescribed an opioid. Future efforts at reductions in prescribing should focus on patients not using opioids before discharge, as this cohort appears to require few, if any, opioids after discharge.
Importantly, patient-reported pain scores during the first week after surgery remained stable after guideline release, at a mean between 1 (none) and 2 (minimal), qualitatively lower than prior general surgery studies utilizing the same scale.19 Although opioid refill rates also did not change, the overall rate was 13.4% (hospital range, 3.4%-23.3%) (Supplemental Figure 2D), substantially higher than published rates of 0.4%,18 3.2%,5 and 6.5%22 in major general surgery series. The high overall refill rate and substantial hospital variation in conjunction with a 29% rate of patients not prescribed an opioid at discharge together highlight the wide variability in opioid requirements among cardiac surgery patients, supporting a more individualized approach to prescribing compared with other surgical procedures. Variation in these data across hospitals may also reflect differences in implementation, as well as the overall adherence to these recommendations for only 52% of patients overall. Given the effectiveness demonstrated in this study, future efforts examining the barriers and facilitators of implementation will be critical for sustainable change. For example, hospital practices in handling refill requests varied widely among collaborating centers, depending on factors such as patient driving distance to the hospital or resources to support in-person clinical assessments. The emergence of the coronavirus disease 2019 (COVID-19) pandemic could further impact and exacerbate existing barriers to in-person assessments for postoperative pain. Patients may feel hesitant or fearful to return for clinical assessment due to COVID-19 concerns. Providers may increase prescription sizes to counteract the increased barriers to in-person follow-up during the pandemic or more readily prescribe refills. These practice patterns and challenges should be continually assessed, depending on local factors including COVID-19 prevalence, hospital resources, and patient preferences.
Independent predictors of high postdischarge opioid use included younger age, shorter postoperative length of stay, higher inpatient opioid use, and higher patient-reported pain levels. Younger age was also identified as an independent predictor of postdischarge opioid use after general surgery6,22 and has additionally been identified as an independent predictor of new persistent opioid use 3 to 6 months after thoracic,7,10,12 cardiac,7,10 and other types of major elective surgery.10 Shorter length of stay as a predictor of higher postdischarge opioid use reinforces the principle that cardiac patients should not be treated uniformly, but rather stratified depending on inpatient requirements before discharge. In doing so, we identified distinct cohorts, with 47% of patients not using opioids the day before discharge and a median 0 pills after discharge, whereas patients using greater than or equal to 4 pills before discharge used a median 24 pills postdischarge (Table 2 and Figure 5). Because opioid-naive status was defined at admission, the cohort with increased opioid requirements may have had recent or remote opioid use, which may have altered their opioid tolerance and thresholds for adequate pain relief. In addition, the group with higher use could be due to a shorter length of stay, although the significance of this difference between groups (median 6 days vs 5 days) is unclear without collecting data on earlier inpatient use.
The clinical significance of a 0.1-pill decrease in the median opioid use on the da0y before discharge is unclear, but the significant trend of decreasing inpatient opioid use during the study may represent improved inpatient opioid weaning. Although not measured in this study, perioperative opioid-sparing analgesia and early inpatient opioid weaning may further reduce prescribing and postdischarge use and have been described as components of cardiac enhanced recovery after surgery pathways.25–27 Enhanced recovery after surgery programs comprehensively address perioperative management but remain difficult to formally evaluate due to inconsistent compliance and multiple competing interventions, and have not consistently addressed opioid prescribing.25–27 Because this prescribing recommendation stratifies patients by inpatient use before discharge rather than a single quantity, it could be integrated into any existing perioperative pain management program to meet the needs of a diverse spectrum of cardiac surgery patients. Finally, this recommendation should be continually reassessed through learning health system principles of quality improvement based on routine practice data.28
This study has several limitations. First, PROs are subject to recall bias. To partially mitigate this, outcomes were collected in a standardized questionnaire at a relatively consistent interval after discharge (median 29 [IQR, 25–36] days). Second, PROs were provided by 66% of the total population, which may impact the generalizability of these data. However, clinical characteristics, inpatient opioid use, and prescribing data were collected for 100% of patients, and differences between responders vs nonresponders were minimal. Furthermore, a 66% response rate compares favorably to the 56% rate reported in prior work utilizing similar methodology.19 Finally, inpatient nonnarcotic pain protocols were not standardized across hospitals during the study period and may have influenced pain management. However, the impact of differing inpatient protocols likely remained consistent before and after guideline implementation at individual centers.
In conclusion, opioid prescribing decreased by 40% and postdischarge opioid use decreased from a median of 3 to 0 pills, while pain levels and refills did not change after implementation of an opioid prescribing recommendation based on inpatient opioid use. The proposed guidelines appeared effective and should be continually reassessed through a learning health system framework.
Supplementary Material
Acknowledgments
The authors wish to acknowledge essential members of their cardiac surgical healthcare team, without whom this quality improvement effort could not have been possible, including Tara Richter, BS, MBA, Ascension Borgess Hospital; Denise Kerr, MS, NP, Ascension Providence Hospital, Southfield; Sue Meisner, BSN, RN, and Karman Beydoun, ACNP-BC, Beaumont Hospital, Dearborn; Meg Gleason, BSN, RN, Beaumont Hospital, Troy; Kathleen Wertella, BS, RHIT, Henry Ford Hospital; Donna Bonaldi-Swan, BSN, RN, Henry Ford Macomb Hospital; Jessica Hollister, RN, McLaren Greater Lansing; Anne L. Kouchoukos, BSN, RN, and Kimberlee Mason MSN, ACCNS-AG, CCRN-CSC, Mercy Health Muskegon; Mary Ryzak, BSN, RN, Michigan Medicine; and Elise Hollenbeck, BSN, RN, and Jeanne Koss, BSN, RN, Munson Medical Center. Dr Brescia is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123); Dr Likosky receives research funding from the Agency for Healthcare Research and Quality (R01HS026003 AHRQ) and National Institutes of Health (1R01HL146619-01A1 REVISED). Support for the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative is provided by the Blue Cross Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM works collaboratively with Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative, opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees.
Footnotes
Dr Likosky discloses a financial relationship with Blue Cross Blue Shield of Michigan and the American Society of Extracorporeal Technology.
The Supplemental Material can be viewed in the online version of this article [10.1016/j.athoracsur.2020.11.015] on http://www.annalsthoracicsurgery.org
References
- 1.Hill MV, McMahon ML, Stucke RS, Barth RJ. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 2.Bartels K, Mayes LM, Dingmann C, et al. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiels CA, Ubl DS, Yost KJ, et al. Results of a prospective, multicenter initiative aimed at developing opioidprescribing guidelines after surgery. Ann Surg. 2018;268:457–468.. [DOI] [PubMed] [Google Scholar]
- 4.Holst KA, Thiels CA, Ubl DS, et al. Post-operative opioid consumption in thoracic surgery patients: how much is actually used? Ann Thorac Surg. 2020;109:1033–1039. [DOI] [PubMed] [Google Scholar]
- 5.Howard R, Waljee J, Brummett C, et al. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2018;153:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. November 2018:e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brescia AA, Waljee JF, Hu HM, et al. Impact of prescribing on new persistent opioid use after cardiothoracic surgery. Ann Thorac Surg. 2019;108:1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soneji N, Clarke HA, Ko DT, Wijeysundera DN. Risks of developing persistent opioid use after major surgery. JAMA Surg. 2016;151:1083–1084. [DOI] [PubMed] [Google Scholar]
- 10.Clarke H, Soneji N, Ko DT, et al. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JS-J, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol. 2017;35:4042–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brescia AA, Harrington CA, Mazurek A, et al. Factors associated with new persistent opioid usage after lung resection. Ann Thorac Surg. 2019;107:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement KC, Canner JK, Lawton JS, et al. Predictors of new persistent opioid use after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2020;160:954–963.e4. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DB, Niu J, Mitchell KG, et al. Persistent opioid use among the elderly after lung resection: a SEER-Medicare study. Ann Thorac Surg. 2020;109:194–202. [DOI] [PubMed] [Google Scholar]
- 15.Khan NF, Bateman BT, Landon JE, Gagne JJ. Association of opioid overdose with opioid prescriptions to family members. JAMA Intern Med. 2019;179:1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clement KC, Canner JK, Whitman GJR, et al. New persistent opioid use after aortic and mitral valve surgery in commercially insured patients. Ann Thorac Surg. 2020;110:829–835. [DOI] [PubMed] [Google Scholar]
- 17.Hedegaard H, Miniño AM, Warner M. Drug Overdose Deaths in the United States, 1999–2017. Accessed https://www.cdc.gov/nchs/data/databriefs/db329-h.pdf. [PubMed]
- 18.Hill MV, Stucke RS, Mcmahon ML, et al. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267:468–472. [DOI] [PubMed] [Google Scholar]
- 19.Vu JV, Howard RA, Gunaseelan V, et al. Statewide implementation of postoperative opioid prescribing guidelines. N Engl J Med. 2019;381:680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JS, Howard RA, Klueh MP, et al. The impact of education and prescribing guidelines on opioid prescribing for breast and melanoma procedures. Ann Surg Oncol. 2019;26:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez FG, Shahian DM, Kormos R, et al. The Society of Thoracic Surgeons National Database 2019 annual report. Ann Thorac Surg. 2019;108:1625–1632. [DOI] [PubMed] [Google Scholar]
- 22.Hill MV, Stucke RS, Billmeier SE, Kelly JL, Barth RJ. Guideline for discharge opioid prescriptions after inpatient general surgical procedures. J Am Coll Surg. 2018;226:996–1003.. [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overton HN, Hanna MN, Bruhn WE, et al. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JB, McConnell G, Allender JE, et al. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J Thorac Cardiovasc Surg. 2019;157:1881–1888. [DOI] [PubMed] [Google Scholar]
- 26.Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154: 755–766. [DOI] [PubMed] [Google Scholar]
- 27.Grant MC, Isada T, Ruzankin P, et al. Results from an enhanced recovery program for cardiac surgery. J Thorac Cardiovasc Surg. 2020;159:1393–1402.e7. [DOI] [PubMed] [Google Scholar]
- 28.Howard R, Vu J, Lee J, et al. A pathway for developing postoperative opioid prescribing best practices. Ann Surg. 2020;271:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.