Abstract
Modulating disease-relevant protein-protein interactions (PPIs) using pharmacological tools is a critical step toward the design of novel therapeutic strategies. Over the years, however, targeting PPIs has proven a very challenging task owing to the large interfacial areas. Our recent efforts identified possible novel routes for the design of potent and selective inhibitors of PPIs using structure-based design of covalent inhibitors targeting Lys residues. In this present study, we report on the design, synthesis, and characterizations of the first Lys-covalent BH3 peptide that has a remarkable affinity and selectivity for hMcl-1 over the closely related hBfl-1 protein. Our structural studies, aided by X-ray crystallography, provide atomic level details of the inhibitor interactions that can be used to further translate these discoveries into novel generation, Lys-covalent pro-apoptotic agents.
Keywords: Mcl-1, Bcl-2, covalent drugs, Lys covalent, sulfonyl fluorides
Graphical Abstract
Introduction
The identification of novel pharmacological tools or even therapeutics targeting protein-protein interactions has long been regarded as a very challenging task, which requires detailed structural information, innovative and meticulous biophysics-guided design, and iterative optimizations. To this end, we previously developed a variety of novel ligand discovery strategies including HTS by NMR and related approaches, 1–5 while we more recently focused on strategies to derive covalent PPIs antagonists.6–8 In this present study, we targeted Mcl-1, an anti-apoptotic Bcl-2 protein. 9–11 Our earlier studies in this field included the derivation of semi-synthetic pan-Bcl-2 antagonists, which have been used to address the role of Mcl-1 in various cellular assays. 12–19 A major goal of our research is to derive potent and selective pharmacological tools that can be used to dissect the role of each of the Bcl-2 proteins in apoptosis and cancer resistance, and that can be additionally used as stepping stones for the design of future therapeutic agents. While this goal may appear arduous on many levels, we recently developed innovative strategies to covalently target Lys residues to help overcome the challenge of deriving potent and selective antagonists of PPIs, 20–24 and we deployed this Lys-covalent binding strategy here to target hMcl-1.
Despite Mcl-1 being one of the most frequently amplified genes in cancer and a major chemoresistance factor, the development of clinical Mcl-1 inhibitors is not as advanced as for Bcl-2 inhibitors. Only very recently, potent and selective small molecule antagonists of Mcl-1 have been reported, 25–28 and others are under investigation for the treatment for Mcl-1–driven cancers. 28–32 Given the potential translational hindrances that these inhibitors may encounter, it is always judicious to pursue alternative strategies for advancing additional novel agents for both basic and clinical research. Moreover, recent efforts in the development of novel therapeutic agents in oncology suggest that covalent irreversible inhibitors present both pharmacodynamics and pharmacokinetics advantages over reversible compounds. 33–36 This may be particularly advantageous in targeting Mcl-1 given that its reversible interactions with its endogenous binding partners (pro-apoptotic Bcl-2 proteins such as BIM) are mediated by high affinities (low nanomolar) interactions. Hence, this present study aims at deriving novel, potent and selective Lys-covalent BH3-based Mcl-1 targeting agents.
Results
Design and synthesis of Mcl-1 covalent BH3 peptides
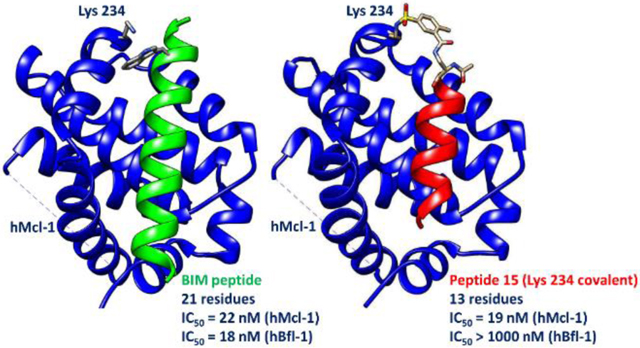
Recently we derived potent Cys-covalent Bfl-1 inhibitors based on a BIM BH3 peptide (Table 1) that was derivatized with a mild electrophile to react with a unique Cys residue present on the BH3 binding pocket of hBfl-1.8, 37 In these studies, the distance between the γ-carbon of the side chain of Trp 147 and the sulfur atom of hBfl-1 Cys 55 was noted to be 3.8 Å in the X-ray structure (PDB ID 2VM6),8, 37 and therefore represented an ideal place for the placement of a mild Michael acceptor. Our considerations in designing covalent peptides followed the general strategies we previously reported, where we targeted the ubiquitin ligase SIAH with a peptide derived by the protein Sip.7 Recently, we have studied the reactivity of aryl-fluorosulfates22 and aryl-sulfonyl fluorides20–21, 23–24 as possible electrophiles to target surface Lys, Tyr, or His residues at protein-protein interfaces. In this present study, we examined the X-ray crystal structures of hMcl-1 in complex with various BH3 peptides, so as to design possible covalent agents. Close inspection of the structure of hMcl-1 in complex with a BIM peptide (PDB ID 2NL9) revealed that no cysteine residues are present within the BH3 binding pocket. However, we identified Lys 234 as a possible residue for covalent modification, and notably, Lys 234 in hMcl-1 occupies an equivalent position to Cys 55 in hBfl-1 (Figure 1).
Table 1.
Identification of minimal binding peptides derived from BIM BH3 peptide. All peptides are amidated at the C-terminus and present a free N-terminus, except for the BIM peptide and peptides 10 and 12 that are acetylated. IC50 values were determined from dose response DELFIA displacement assay measurements as described in the experimental section. Errors represent duplicate measurements. Hse = L-homo-Serine; Nle = L-norleucine; Abu = 2-aminoburytic acid.
| Compound ID | Sequence | MW | DELFIA IC50 (nM) |
|---|---|---|---|
| hMcl-1 | |||
| BIM(146–166) | IWIAQELRRIGDEFNAYYARR | 2682 | 22 ± 1 |
| 1 | AIAQALRRIGDAF | 1400 | ~2,200 |
| 2 | AIAQALRRIGDAY | 1416 | >3,600 |
| 3 | AIAQALRRIGDAR | 1409 | >10,000 |
| 4 | AIAQALRRIGDAL | 1366 | 1,200 |
| 5 | AIAQALRRIGDA(Nle) | 1366 | ~1,600 |
| 6 | AIAQ(Abu)LRRIGDAF | 1414 | ~1,300 |
| 7 | AIAQVLRRIGDAF | 1428 | ~1,800 |
| 8 | AIAQLLRRIGDAF | 1442 | ~1,800 |
| 9 | AIAQ(Hse)LRRIGDAF | 1430 | ~3,000 |
| 10 | AIAEALRRIGDAF | 1433 | 261 ± 14 |
| 11 | AIAEQLRRIGDAF | 1460 | 313 ± 9 |
| 12 | AIAEQLRRIGDRF | 1585 | 570 ± 16 |
Figure 1.
Design of Lys covalent Mcl-1 agents. A) Ribbon representation of hMcl-1 (cyan) in complex with a BIM derived BH3 peptide (red; PDB ID 2NL9). The side chains of hMcl-1 Lys 234 and BIM Trp 147 are displayed. B) Ribbon representation of hBfl-1 (magenta) in complex with a BIM derived BH3 peptide (green; PDB ID 2VM6). The side chains of hBfl-1 Cys 55 and BIM Trp 147 are displayed. C) Ribbon representation of hBfl-1 (marron) in complex with a covalent BIM derived peptide (magenta; PDB ID 6RJP). The covalent bond was accomplished replacing Trp 147 with a L-Dap(chloro-acetamide) that reacted with the thiol of Cys 55. The figure was prepared using Chimera.38
These observations suggested that proper derivatizations at Trp 147 of BIM peptides with aryl-fluorosulfates or sulfonyl-fluorides may result in possible covalent agents targeting hMcl-1 Lys 234. To engineer selectivity in the resulting compounds, we first synthesized and tested a variety of BIM related peptides, in order to identify a minimal peptide region with sufficient affinity (low to sub-micromolar) for Mcl-1 (Table 1). The goal of this first step is to discover and then render such agents as very potent against and specific for hMcl-1, by the introduction of a properly juxtaposed Lys-covalent warhead. These studies, also based on previous structure-activity relationships39–40 and the X-ray structure of BIM BH3 peptides in complex with Mcl-1, suggested subtle modifications (for example introducing a glutamic in position 150 of the BIM derived peptide)39–40 that led to peptides 10–12 as possible suitable template molecules for the introduction of the Lys-targeting sulfonyl fluoride, in lieu of the N-terminal Ala residue. Peptide 12 was preferred due to its increased solubility. These shorter peptides like compound 12, while retaining some binding affinity for Mcl-1, have also lost affinity for Bfl-1 (Table 2), as predicted by the preferences of essential amino-acids sidechains which are slightly different for binding to Bfl-1.8
Table 2.
Sequences of the covalent agents targeting hMcl-1. All peptides are amidated at the C-terminus and acetylated at the N-terminus. 4FSB = 4-sulfonyl-fuoride benzamide; 3FSB = 3-sulfonyl-fuoride benzamide; 2Me,3FSB = 2-methyl, 5-sulfonyl-fuoride benzamide; 2MeO,5FSB = 2-methoxy, 5-sulfonyl-fuoride benzamide; the chemical structures of these electrophiles are reported. IC50 values were determined from dose response DELFIA displacement assay measurements as described in the experimental section. Errors represent duplicate measurements.
| Compound ID | Sequence and electrophile | MW | DELFIA IC50 (nM) | |
|---|---|---|---|---|
| hMcl-1 | hBfl-1 | |||
| BIM(146–166) | IWIAQELRRIGDEFNAYYARR | 2681 | 22 ± 1 | 18 ± 1 |
| 12 | AIAEQLRRIGDRF | 1585 | 570 ± 16 | >10,000 |
| 13 | Dap(4-FBS)IAEQLRRIGDRF
|
1785 | 94 ± 18 | >5,000 |
| 14 | Dap(3-FBS)IAEQLRRIGDRF
|
1785 | 23 ± 4 | >400 |
| 15 | Dap(2Me,5FSB)IAEQLRRIGDRF
|
1799 | 19.1 ± 0.6 | >1,000 |
| 16 | Dap(2MeO,5FSB)IAEQLRRIGDRF
|
1815 | 23.6 ± 0.1 | >250 |
Hence, to further improve the affinity for compound 12 for Mcl-1 via a possible covalent interaction with Lys 234, we first introduced a L-di-amino-propionic acid (L-Dap) in lieu of its N-terminal Ala residue, and its side chain amine was subsequently coupled with various fluoro-sulfonyl-benzoic acids (compounds 13-16; Table 2). Incorporation of the sulfonyl fluorides was possible by using an ivDde protected L-Dap (L-di-amino propionic acid) as we reported recently. 20–21, 23–24 The electrophiles in agents 13-16 were chosen based on our recent studies aimed at identifying “warheads” that presented the proper balance between buffer stability and reactivity. 20–21 Accordingly, aqueous stability studies at pH = 7.5 using 1D 1H NMR indicated that the half-life for compounds 15 or 16 was >> 3 hr 30 min for each agent at room temperature (supplementary Figure S5). In particular, compound 15 remained > 80% intact after 3 hr 30 min in aqueous buffer, while compound 16 remained nearly completely intact under the same experimental conditions (supplementary Figure S5).
Next, to determine which of these agents could interact covalently with Lys 234 in the BH3 binding site of hMcl-1, we performed a set of biochemical and biophysical assays as described below.
First, we performed dose-response measurements in a Dissociation-Enhanced Lanthanide Fluorescence Immunoassay (DELFIA) displacement assay as we described previously,8 which was designed to quantify the ability of each tested agent to compete for the binding of a reference biotinylated BIM peptide. The assay was also used to determine the selectivty of the agents for the most closely related Bcl-2 protein, namely the anti-apoptotic protein hBfl-1 (Table 2). While IC50 values for covalent inhibitors are time dependent, and thus not suitable for quantitative evaluations, we could initially qualitatively compare IC50 values obtained from dose-response curves measured at a given pre-incubation time (2 h) of test agent and protein target (Table 2). From these studies, we noted that agents derivatized with various sulfonyl fluorides were all very potent in displacing the reference BH3 peptide (see experimental session) from hMcl-1, but not from hBfl-1 (Figure 2). This was expected, given that the agents were designed to target Lys 234 in hMcl-1 that corresponds to Cys 55 in hBfl-1 (Figure 1), and aryl-sulfonyl fluorides do not react irreversibly with Cys residues. The agents seemed dramatically more potent against hMcl-1 when the sulfonyl-fluoride was placed in meta position, presumably having a more optimal geometry to covalently interact with Lys 234. This appears to be fortunate, as our recent studies on benzamide-sulfonyl fluoride reactivities revealed that meta sulfonyl fluoride-benzamides, which are also methyl or methoxyl substituted, presented a more balanced reactivity, as compared to para and unsubstituted counterparts (Table 2). 20
Figure 2.
Agents 15 and 16 are potent hMcl-1 covalent antagonists. A) Dose response DELFIA displacement assay curves comparing the ability of minimal BH3 peptides 12 (black curve), with covalent agents 15 (red curve) and 16 (green curve), to displace a reference full length BH3 peptide at a given incubation time (see experimental session). B) Right panel, SDS gel electrophoresis of hMcl-1 collected in the absence or presence of agents 15 or 16, as indicated. The same experiment was also conducted with hBfl-1 and reported in the right panels. The gels were obtained by incubating hMcl-1 or hBfl-1 (10 μM) with 100 μM of the indicated agent for 2h at room temperature in buffer (25 mM TRIS pH 7.5, 150 mM NaCl, 1 mM DTT).
Second, covalent adduct formation could be simply monitored by SDS polyacrylamide gel electrophoresis (PAGE) of hMcl-1 measured in the absence or presence of various test agents. Covalent adduct formation was evident by a band shift, due to the increased MW of the covalent complex (Figure 2). As a control, and under the same experimental conditions, no band shift was observed for hBfl-1, further corroborating the selectivity of the agents for hMcl-1, as anticipated by the DELFIA data (Figure 2; Table 2).
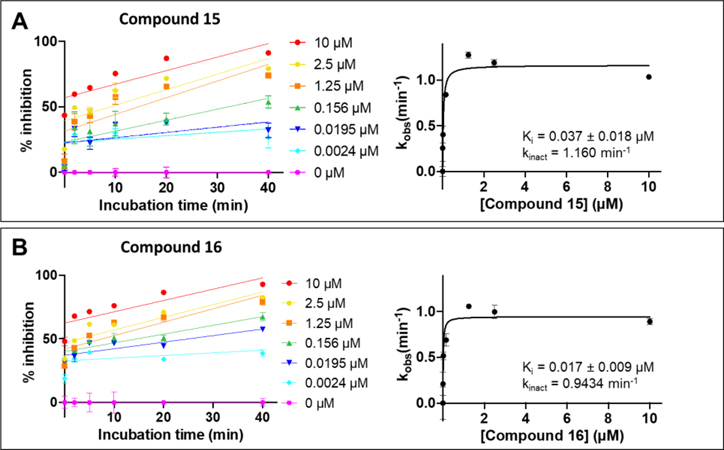
For compounds 15 and 16 we also performed kinetics analysis using the displacement DELFIA assay at various concentration of agents and at different incubation times (Figure 3). For compound 15 we found Ki = 0.037 ± 0.018 μM and kinact = 1.16 min−1, while for compound 16 we found Ki = 0.017 ± 0.009 μM and kinact = 0.943 min−1, indicating that the agents react rapidly and efficiently with the target, as predicted by the qualitative assays.
Figure 3. Characterization of the kinetics of binding to hMcl-1 by peptide 15 and 16.
Kinetics measurements for agents 15 (panel A) and 16 (panel B). Percent inhibition were measured using the DELFIA assay at the indicated concentrations and at various time points. Curve fitting of the observed inhibition as function of the compound concentration provided Ki and kinact, as reported.
Finally, we could measure direct covalent adduct formation by mass spectrometry analysis. The data revealed a MW for the protein alone of 18,763 Da, while after incubation with compound 15 a MW of 20,544 Da was observed (supplementary Figure S1), that accurately corresponds to the predicted mass of the covalent adduct, while no significant additional peaks corresponding to the free protein, or to possible multiple adducts, were observed. These data identify compounds 15 and 16 as potent and selective, covalent agents for hMcl-1.
Structural characterization of covalent agents in complex with hMcl-1.
To further elucidate the binding conformation of the covalent agent to hMcl-1, and to more unambiguously identify the targeted Lys residue, we also determined the crystal structure of compound 15 in complex with hMcl-1. A summary of structural parameters is reported in Table S1. The general structure of the complex is like the previously determined hMcl-1 in complex with BH3 peptides (Figure 4A), with a monomer of hMcl-1 superimposing with PDB code 2NL9 at 0.8 Å. As predicted by modeling studies, the N-terminal residue of compound 15 formed a covalent bond with the side chain of Lys 234, as observed by a contiguous electron density (Figures 4B, C). This agrees with SDS gel electrophoresis, mass spectrometry, and kinetics data, all of which strongly support covalent adduct formation between these two molecules. In the final refined model, the density and placement of covalently bound peptide 15 in the hMcl-1 structure was well resolved in its entirety (supplementary Figure S2). The general peptide-hMcl-1 interactions are well maintained in the crystal structure, in which the amphipathic helical peptide inserts into the peptide-binding pocket with its hydrophobic side chains buried in the pocket, while the peptide-only Asp residue makes a salt bridge with hMcl-1 residue Arg 263. These interactions are typically well conserved between BH3 peptides and Mcl-1 as well as in other BH3/Bcl-2 proteins complexes.
Figure 4.
Crystal structure of hMcl-1 in complex with compound 15 (PDB ID 6VBX). A) Ribbon representation of hMcl-1 (gray) in complex with compound 15 (Table 2; green). The side chains of the agent are also displayed. B) The 2FoFc map of the bound compound 15 contoured at 1.5 sigma. C) Close-up view of the covalent sulfonamide bond resulting from the reaction of the sulfonyl fluoride of compound 15 and the side chain amine of Lys 234.
Cellular activity of Mcl-1 Lys-234 covalent agents.
To preliminarily assess the cellular properties of Mcl-1 Lys-234 covalent agents 15 or 16 we performed a series of assays with the non-small cell lung cancer cell line A549, that overexpresses Mcl-1.11 Given the covalent nature of the binding, we expected that the agent could stabilize Mcl-1 from thermal denaturation in cell.41–44 Indeed, in vitro experiments indicated that compound 15 displayed a remarkable denaturation thermal shift of 23 °C for the hMcl-1 construct used for biochemical and crystallization studies (supplementary Figure S4). Hence, in a first experiment we treated A549 cells with compound 16, and induced a brief heat shock, followed by western blot analysis of the supernatant after cell lysis. However, we could not observe a rescue of protein levels when heated cells were pretreated with compound 16 (supplementary Figure S4). However, it is known that BH3 peptides could also trigger proteasomal degradation of Mcl-145 and this phenomenon could go at odds with the expected ligand induced protection to thermal denaturation in cell. Indeed, treatment of A549 cells with compound 16 resulted in a decrease in Mcl-1 protein levels that can be rescued by the proteasome inhibitor carfilzomib (Figure 5). Moreover, a small band shift is appreciable in the treated cells due to the slightly increased molecular weight of the covalent adduct. Albeit this shift was more evident with the SDS PAGE experiment due to the smaller MW of the protein construct used for the in vitro studies compared to the full length wild-type protein in cell. The small band shift is perhaps more appreciable in a second experiment where we included the agent to A549 cell lysates and when a smaller amount of protein was loaded to the gel (Figure 5). These preliminary data suggest that the agent can covalently interact with Mcl-1 also in the more complex cellular milieu causing a proteasome dependent Mcl-1 degradation.
Figure 5.
Cellular studies with compound 16 and A549 non-small cell lung cancer cells. A) A549 cells were treated for 2 h with compound 16 or with compound 16 after being pre-treated with 1 μM carfilzomib for 30 min. B)Densitometry analysis of the western blot of panel A) suggests that Mcl-1 levels relative to β-tubulin were significantly decreased with compound 16 treatment, and the decreases was rescued by carfilzomib pre-treatment C) A549 cell lysates were spiked with DMSO or 100 μM Compound 16 for 1 h. Covalent adduct was detected between Compound 16 and Mcl-1 as appreciable by a small band shift compared to those of DMSO-treated alone due to the increased MW of the complex. D) Densitometry analysis of the western blot in panel A), highlighting the Mcl-1 fold-increase relative to the DMSO treated bands (with or without carfilzomib). Error bars are a result of biological replicates of two independent experiments (**p<0.01, as determined by a one-way ANOVA using Dunnett post-test analysis, calculated significance with GraphPad Prism version 8).
Altogether, the data reported in this manuscript, including these preliminary cellular studies, the X-ray structure of the complex, and binding and kinetics data, could form the basis for future improved versions of these agents perhaps deploying cell penetrating sequences and/or hydrocarbon stapling strategies to further improve their cellular efficacy.
Discussion and conclusions
Recent efforts in oncology research for the past several years have revealed that covalent irreversible inhibitors present significant pharmacodynamics and pharmacokinetics advantages over reversible compounds.21, 33–36 This can be typified by several irreversible, agents that have been recently approved targeting protein kinases. However, these agents invariably target an exposed Cys residue in proximity to the binding site of the target enzyme. Unfortunately, most drug targets do not present a Cys in proximity to their binding site, as Cys is a relatively rare amino acid in proteins. Hence, strategies that can expand the druggable proteome for covalent drugs by targeting other residues could have, in principle, a major impact in the development of novel effective therapeutics in oncology and for other indications. The success of the approach in targeting Cys is undoubtedly due mostly to the discovery that certain electrophiles based on acryl amides possess the proper balance between Cys-reactivity and stability in buffer, plasma, and in vivo to be used as effective “warheads”. Recently, several studies, 46–53 including those from our laboratory,6, 20–22 have identified aryl-fluorosulfates or aryl-sulfonyl fluorides as chemical moieties that could function as possible electrophiles for targeting Lys, Tyr, or His residues. Stability and cell permeability studies suggested that these moieties could present a proper balance of stability in buffer and in cell to be used as pharmacological tools or could even in the future be introduced into therapeutic agents. Such covalent agents could play perhaps an even more impactful role in targeting protein-protein interactions for which it is notoriously difficult to derive potent and selective agents.
In the Bcl-2 family proteins, two recent attempts have been reported regarding covalent molecules. A first attempt reported the design of a reversible covalent inhibitor that incorporated simultaneously a boronic acid and an aldehyde as a warhead into a previously reported indole-based Mcl-1 inhibitor.54 Because of the chosen electrophile, the covalent reaction was found to be reversible, largely losing the full potential of the covalent interaction. Indeed, the affinity and cellular activity of the agent was not significantly superior to that of the parent non-covalent molecule.54 A more recent successful attempt involved the modification of a Bcl-xL inhibitor of the class of acyl-sulfonamides with an aryl-sulfonyl fluoride to covalently target Tyr 101 in the BH3 domain-binding groove Bcl-xL. 55 We recently reported on the stability and reactivity studies of sulfonyl fluorides for use in covalent ligands targeting Lys or Tyr residues.20–23 These studies identified substituted benzamide sulfonyl fluorides as possessing a suitable balance between reactivity and stability for use in Lys/Tyr covalent pharmacological tools, and perhaps as future therapeutics. 20 The attenuated reactivity of these electrophiles, together with the inherent flexibility of the targeted Lys residue, requires a significant binding affinity (sub-micromolar) for the anchoring ligand to react efficiently. Recent work with stapled peptides derivatized with an aryl-sulfonyl fluoride also demonstrated that selected Lys-covalent agents disrupted the p53-Mdm4 interaction by 10-fold, compared to the non-covalent agents. 56
Hence, in this study, starting from a non-selective BIM peptide of 26 amino acids, we first derived a minimal peptide region that retained sub-micromolar binding affinity for hMcl-1. These studies, also guided by previously reported SAR studies and the structure of BIM in complex with hMcl-1, resulted in the selection of peptide 12 (Table 1), retaining some affinity for Mcl-1 but not for Bfl-1. Subsequently, simple structure-based considerations resulted in the design and synthesis of covalent agents 13–16 (Table 2). An array of biophysical and biochemical assays revealed that agents 15 and 16 are aqueous stable, potent and selective hMcl-1 Lys-covalent agents. Structural studies of the complex between agent 15 and hMcl-1 provided atomic resolution details on the targeted Lys residue and on the geometry of the binding agent. Finally, preliminary cellular studies suggest that compound 16 can covalently interact with Mcl-1 also in the complex cellular milieu causing Mcl-1 degradation in the non-small cell lung cancer A549 cell line.
With the resurgence of covalent agents in oncology, and recent developments on targeting Lys residues, this report provides additional critical experimental details for the design of such covalent agents targeting protein-protein interactions. It is noteworthy that agents 15 and 16 are, to our knowledge, the shortest BH3 peptides reported to date that possess low nanomolar activity against hMcl-1, and are at the same time void of any significant activity against the closely related hBfl-1 protein. Hence, we envision that such agents could be used as pharmacological tools in BH3 profiling, or through further optimizations that perhaps include using known cell permeabilization strategies,40, 57–61 be developed into potential BH3-based therapeutics.
Experimental Section
General Chemistry.
Common reagents and routine solvents were obtained by commercial sources. NMR spectra were recorded on Bruker Avance III 700 MHz equipped with a TCI cryoprobe. 1H NMR measurements in assay buffer were used for quality control and to routinely verify the concentration of the stock solutions used for all studies. Purification of the peptides was obtained using a reverse phase HPLC preparative system (JASCO) equipped with a PDA detector and a fraction collector controlled by a ChromNAV system (JASCO). For all the peptides reported, a Luna C18 10μ 10 × 250mm (Phenomenex) column was used to purify agents to > 95% purity. An Agilent LC-TOF instrument was used to obtain the high-resolution mass spectral data as reported in supplementary Table S2.
Peptide Synthesis
Peptides were synthesized by using standard solid-phase synthesis protocols. For each coupling reaction, approximately 3 eq. of Fmoc-AA, 3 eq. of HATU, 3 eq. of OximaPure, and 5 eq. of DIPEA in 1 ml of DMF were used. The coupling reaction was allowed to proceed for 1 h. Fmoc deprotection was performed by treating the resin-bound peptide with 20% piperidine in DMF twice. Peptides were cleaved from Rink amide resin with a cleavage cocktail containing TFA/TIS/water (94:3:3) for 3 h. Agents in 13–16 in Table 2 were synthesized by introducing an ivDde protected L-Dap at the N-terminus. Selective removal of the ivDde protecting group was accomplished by using 4% N2H2 (3 × 5 ml, 5 min each). The generated free amine of the L-Dap was subsequently reacted with 3 eq. of the respective benzoic acid, in presence of 3 eq. of HATU and 5 eq. of DIPEA in 1 ml of DMF. The reaction was left shaking overnight. The cleaving solution was filtered from the resin, evaporated under reduced pressure and the peptides precipitated in Et2O, centrifuged and dried in high vacuum. The crude peptides were purified by preparative RP-HPLC using a Luna C18 column (Phenomenex) and water/acetonitrile gradient (30% to 70%) containing 0.1% TFA to >95% purity (supplementary Figure S3). The final compounds were characterized by HRMS (Table S2).
Protein expression and purification, and SDS gel electrophoresis
The hMcl-1 gene (residues 172–323) was inserted into the vector pET-15b for expression into E. coli BL21. The protein was overexpressed by growing the transformed bacteria in LB medium at 37°C in presence of 100 μg/L of ampicillin until reaching an OD600 of 0.6 – 0.7, followed by induction with 1 mM IPTG and left shaking overnight at 20°C. Bacteria were collected and lysed by sonication at 4°C. The overexpressed protein containing an N-terminal His tag was purified using Immobilized Metal Ion Affinity Chromatography (IMAC) with a linear gradient of imidazole. The protein was further purified using a size exclusion chromatography with a final buffer of 25 mM TRIS pH 7.5, 150 mM NaCl, 1 mM DTT. The hBfl-1 gene (residues 1–149 and an N-terminal His tag) was inserted into a modified pET21a vector. The expression was conducted growing transformed Rosetta-gami (DE3) competent cells in LB medium at 37°C in presence of 50 μg/L of kanamycin until reaching an OD600 of 0.6 – 0.7, followed by induction with 0.1 mM IPTG and left shaking overnight at 15°C. As for hMcl-1, overexpressed protein containing an N-terminal His tag was purified using Ni2+ affinity Chromatography with a linear gradient of imidazole. The protein was further purified using a size exclusion chromatography with a final buffer of 50 mM phosphate pH 6.5, 150 mM NaCl, 1 mM DTT.
SDS-polyacrylamide gel electrophoresis (PAGE) was performed incubating either hMcl-1 or hBfl-1 at 10 μM concentration in buffer 25 mM TRIS pH 7.5, 150 mM NaCl, 1 mM DTT with or without each agent at the concentration of 100 μM. The incubation time of the protein with each peptide was 2 h at room temperature. Samples were subjected to gel electrophoresis with SDS-PAGE using the NuPAGE 12% Bis-Tris mini gels (Life Technologies), MOPS as running buffer, and were stained with SimplyBlue SafeStain (Life Technologies) according to the manufacture’s protocol.
Biochemical inhibition and kinetics measurements
Dose-response DELFIA (Dissociation Enhanced Lanthanide Fluorescence Immuno-Assay) displacement IC50 values were measured as described in our recent publication. 8
Briefly, a solution (100 μL) of biotinylated-BH3 peptide (600 ng/ml) of sequence biotin-AHA-EDIIRNIARHLAQVGDSMDR-NH2 (where AHA is amino-hexanoic acid, introduced as a spacer between the peptide and the biotin) was added to each well of 96-well streptavidin-coated plates (PerkinElmer). After a 2-h incubation, the unbound biotinylated peptide was washed 3 times with a wash solution (PerkinElmer). Subsequently, a solution of the protein and test peptide was added to the assay plates, together with a solution of Eu-N1-labeled anti-6xHis antibody (PerkinElmer) and the reaction was allowed to proceed for 2 h. Following 3 washes to remove the unbound protein-Eu-antibody complexes, 200 μL of the enhancement solution (PerkinElmer) was added to each well to release fluorescent europium from the antibody and the fluorescent readings were measured using VICTOR X5 microplate reader (excitation wavelength: 340 nm; emission wavelength: 615 nm). All the incubations were performed at RT. The final protein concentrations, previously determined by titrations,8 were 15 nM for hBfl-1, and 16 nM for hMcl-1. The final antibody concentration was 22.2 ng/well. Each well received a final DMSO concentration equal to 1%. Protein, peptide and antibody solutions were prepared in DELFIA assay buffer (PerkinElmer). Counts were normalized to control wells, which were treated with the vehicle, DMSO and reported as % inhibition. The reported IC50 values were calculated by Prism 8 (GraphPad).
Kinetics measurements were performed by incubating streptavidin-coated wells with 100 μL of 600 ng/mL biotinylated-BH3 peptide for 2 h, followed by 3 washing steps to remove unbound peptide, using a similar protocol as we recently reported.20 Briefly, each well of the 96-well plates was incubated with 16 nM of hMcl-1 and 22.2 ng Eu-N1-labeled anti-6xHis antibody (PerkinElmer) and the reaction was allowed to proceed for 2 h. A test peptide was then added to the wells and incubated for 0, 2, 5, 10, 20 and 40 min. At the end of indicated incubation times, wells were washed 3 times prior to the addition of 200 μL of enhancement solution and the fluorescent readings were taken as described previously. The observed rate constant for inhibition, kobs for each concentration was calculated from the slope of a plot of % inhibition versus incubation time. The obtained kobs values were then replotted against peptide concentration and fitted to a hyperbolic curve. Prism 8 (GraphPad) was used to calculate the inhibition constant, Ki and kinact.
X-ray crystallography
A solution of hMcl-1(172–323)-peptide 15 complex was prepared in 100 mM Tris, pH 8.0, 200 mM NaCl, and 0.5 mM DTT buffer. The complex was prepared using the hMcl-1 construct described above, but the His-tag was first cleaved by treatment with thrombin (overnight at 4 °C) and the resulting protein construct, hMcl-1(173–323), was further purified via size exclusion chromatography. Subsequently, the purified sample (0.5 mg/ml in 100 mM TRIS. pH = 8, 200 mM NaCl, 0.5 mM DTT) was treated with compound 15 at a 2:1 molar ratio, for 2 h. Excess peptide was subsequently washed using an Amicon filter with a MW cut-off of 10 kDa. Crystallization was conducted using sitting drop vapor diffusion at 4 °C, with diffraction quality crystals grown in 0.5 M potassium thiocyanate, and 0.1 M sodium acetate, pH 4.6. Crystals were frozen in 20% glycerol cryo-protectant, and data collection was conducted at the LS-CAT (21-D) beamline. A dataset was collected on a crystal that diffracted to 1.95 Å, and the diffraction data was indexed using DENZO, with the crystal belonging to the P3221 space group, and integrated and scaled with SCALEPACK (HKL2000).62 Molecular replacement for the dataset was performed using a search model based on PDB ID: 2NL9 in PHASER, PHENIX.63 The top solution was refined using rigid body refinement in REFMAC.64 Several rounds of refinement and model building were performed in the absence of peptide using COOT 65 and PHENIX. After placement of the solvent molecules, the chemical model of non-canonical N-terminal amino acid was fit into the remaining density and refined by REFMAC. The remaining parts of the peptide were built manually using COOT and refined in the structure by PHENIX. The final crystal data statistics are listed in Table S1.
Immunoblotting analyses with A549 cancer cells.
Non-small cell lung cancer cell line, A549 cells were purchased from Essen Bioscience and cultured in a Ham’s F-12 nutrient mixture with GlutaMAX-1 (Gibco) supplemented with 10% FBS, 1% PenStrep (100 U/mL penicillin and 100 μg/mL streptomycin), and 0.5 μg/mL puromycin. For the combination treatment with carfilzomib, cells were treated with DMSO, 50 μM, or 100 μM compound 16 for 2 h in the absence or the presence (pre-treating the cells for 30 min) of 1 μM carfilzomib. Lysates were collected and subjected to immunoblotting. For the spiking cell lysate assay, cells were harvested and lysed with a lysis buffer (20 mM Tris, pH 7.4, 120 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1% IGEPAL, 5 mM EDTA, supplemented with EDTA-free protease inhibitor cocktail and PhosSTOP (SigmaAldrich) for 20 min on ice. Lysates were then centrifuged, and supernatants were collected and measured for protein concentration prior to being incubated with DMSO or 100 μM Compound 16 for 1 h at 37 °C. Subsequently, the lysates were subjected to SDS-PAGE and electrophoretically transferred onto PVDF membranes. The blots were blocked with 5% nonfat milk in TBS-T and probed with primary antibodies raised against Mcl-1 (Santa Cruz Biotechnology, sc-12756) or β-tubulin (Santa Cruz Biotechnology, sc-58884) at 4 °C overnight. Membranes were then washed with TBS-T and incubated with HRP-conjugated goat anti-mouse secondary antibody (ThermoFisher Scientific). Protein bands were visualized using Clarity Western ECL kit (BIO-RAD) and imaged on a FluorChem (ProteinSimple) imaging system. Scans were processed and analyzed with AlphaView.
For the cellular thermal shift assay, one million A549 cells were plated in 6-well plates overnight and treated with 1% DMSO, 100 μM Compound 15 for 1 h. Cells were then harvested and supplemented with protease inhibitor cocktail (Sigma-Aldrich) prior to being heated at the various temperatures for 3 min. Samples were then freeze-thawed 3 times and centrifuged to collect the soluble protein fractions. The soluble proteins were subsequently subjected to immunoblotting as described above (supplementary Figure S4).
Ancillary information
The coordinates of compound 15 in complex with hMcl-1 have been deposited in the protein data bank (PDB ID 6VBX). Authors will release the atomic coordinates upon article publication.
Supplementary Material
Acknowledgements
Financial support was obtained in part by NIH grants CA168517 (to MP and JJPP), NS107479 (to MP), and CA242620 (to MP and JJP). MP holds the Daniel Hays Chair in Cancer Research at the School of Medicine at UCR. Some molecular graphics and analyses performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311.
Abbreviations used:
- CETSA
cellular Thermal shift assay
- DELFIA
dissociation-enhanced lanthanide fluorescence immunoassay displacement assay
- DTT
dithiothreitol
- IMAC
Immobilized Metal Ion Affinity Chromatography
- MOPS
3-(N-morpholino)propanesulfonic acid
- TRIS
tris(hydroxymethyl)aminomethane
Footnotes
Notes: The authors declare no competing financial interest
Supporting Information: The Supporting Information is available free of charge on the ACS Publications website. Table S1 reports crystallographic parameters related to the structure of agent 15 in complex with hMcl-1(172–323); Table S2 reports HRMS data for all synthesized agents. Figure S1 reports mass spectra of hMcl-1 in the apo versus complexed form with peptide 15; Figure S2 reports a comparison of electron density maps of peptide 15 covalently bound to Lys 234 side chain of Mcl-1. Figure S3 reports HPLC traces for key compounds 15 and 16. Figure S4 reports in vitro thermal shift assay with hMcl-1 and compound 15, and cellular thermal shift assay with A549 cells and compound 15. Figure S5 reports aqueous stability studies with compounds 15 and 16. A Molecular Formula Strings file is also provided for key agents 15 and 16.
References
- 1.Baggio C; Cerofolini L; Fragai M; Luchinat C; Pellecchia M, HTS by NMR for the Identification of Potent and Selective Inhibitors of Metalloenzymes. ACS Med Chem Lett 2018, 9 (2), 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottini A; Wu B; Barile E; De SK; Leone M; Pellecchia M, High-Throughput Screening (HTS) by NMR Guided Identification of Novel Agents Targeting the Protein Docking Domain of Yoph. ChemMedChem 2016, 11 (8), 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B; Barile E; De SK; Wei J; Purves A; Pellecchia M, High-Throughput Screening by Nuclear Magnetic Resonance (HTS by NMR) for the Identification of Ppis Antagonists. Curr Top Med Chem 2015, 15 (20), 2032–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B; Zhang Z; Noberini R; Barile E; Giulianotti M; Pinilla C; Houghten RA; Pasquale EB; Pellecchia M, HTS by NMR of Combinatorial Libraries: A Fragment-Based Approach to Ligand Discovery. Chem Biol 2013, 20 (1), 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barile E; Pellecchia M, NMR-Based Approaches for the Identification and Optimization of Inhibitors of Protein-Protein Interactions. Chem Rev 2014, 114 (9), 4749–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggio C; Gambini L; Udompholkul P; Salem AF; Aronson A; Dona A; Troadec E; Pichiorri F; Pellecchia M, Design of Potent Pan-Iap and Lys-Covalent Xiap Selective Inhibitors Using a Thermodynamics Driven Approach. J Med Chem 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stebbins JL; Santelli E; Feng Y; De SK; Purves A; Motamedchaboki K; Wu B; Ronai ZA; Liddington RC; Pellecchia M, Structure-Based Design of Covalent Siah Inhibitors. Chem Biol 2013, 20 (8), 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barile E; Marconi GD; De SK; Baggio C; Gambini L; Salem AF; Kashyap MK; Castro JE; Kipps TJ; Pellecchia M, hBfl-1/hNoxa Interaction Studies Provide New Insights on the Role of Bfl-1 in Cancer Cell Resistance and for the Design of Novel Anticancer Agents. ACS Chem Biol 2017, 12 (2), 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maji S; Panda S; Samal SK; Shriwas O; Rath R; Pellecchia M; Emdad L; Das SK; Fisher PB; Dash R, Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv Cancer Res 2018, 137, 37–75. [DOI] [PubMed] [Google Scholar]
- 10.Maji S; Samal SK; Pattanaik L; Panda S; Quinn BA; Das SK; Sarkar D; Pellecchia M; Fisher PB; Dash R, Mcl-1 Is an Important Therapeutic Target for Oral Squamous Cell Carcinomas. Oncotarget 2015, 6 (18), 16623–16637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Placzek WJ; Wei J; Kitada S; Zhai D; Reed JC; Pellecchia M, A Survey of the Anti-Apoptotic Bcl-2 Subfamily Expression in Cancer Types Provides a Platform to Predict the Efficacy of Bcl-2 Antagonists in Cancer Therapy. Cell Death Dis 2010, 1, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rega MF; Wu B; Wei J; Zhang Z; Cellitti JF; Pellecchia M, Sar by Interligand Nuclear Overhauser Effects (ILOEs) Based Discovery of Acylsulfonamide Compounds Active against Bcl-X(L) and Mcl-1. J Med Chem 2011, 54 (17), 6000–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei J; Stebbins JL; Kitada S; Dash R; Zhai D; Placzek WJ; Wu B; Rega MF; Zhang Z; Barile E; Yang L; Dahl R; Fisher PB; Reed JC; Pellecchia M, An Optically Pure Apogossypolone Derivative as Potent Pan-Active Inhibitor of Anti-Apoptotic Bcl-2 Family Proteins. Front Oncol 2011, 1, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azab B; Dash R; Das SK; Bhutia SK; Shen XN; Quinn BA; Sarkar S; Wang XY; Hedvat M; Dmitriev IP; Curiel DT; Grant S; Dent P; Reed JC; Pellecchia M; Sarkar D; Fisher PB, Enhanced Delivery of Mda-7/Il-24 Using a Serotype Chimeric Adenovirus (Ad.5/3) in Combination with the Apogossypol Derivative Bi-97c1 (Sabutoclax) Improves Therapeutic Efficacy in Low Car Colorectal Cancer Cells. J Cell Physiol 2012, 227 (5), 2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dash R; Azab B; Quinn BA; Shen X; Wang XY; Das SK; Rahmani M; Wei J; Hedvat M; Dent P; Dmitriev IP; Curiel DT; Grant S; Wu B; Stebbins JL; Pellecchia M; Reed JC; Sarkar D; Fisher PB, Apogossypol Derivative Bi-97c1 (Sabutoclax) Targeting Mcl-1 Sensitizes Prostate Cancer Cells to Mda-7/Il-24-Mediated Toxicity. Proc Natl Acad Sci U S A 2011, 108 (21), 8785–8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson RS 2nd; Placzek W; Fernandez A; Ziaee S; Chu CY; Wei J; Stebbins J; Kitada S; Fritz G; Reed JC; Chung LW; Pellecchia M; Bhowmick NA, Sabutoclax, a Mcl-1 Antagonist, Inhibits Tumorigenesis in Transgenic Mouse and Human Xenograft Models of Prostate Cancer. Neoplasia 2012, 14 (7), 656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varadarajan S; Butterworth M; Wei J; Pellecchia M; Dinsdale D; Cohen GM, Sabutoclax (BI97C1) and BI112D1, Putative Inhibitors of Mcl-1, Induce Mitochondrial Fragmentation Either Upstream of or Independent of Apoptosis. Neoplasia 2013, 15 (5), 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goff DJ; Court Recart A; Sadarangani A; Chun HJ; Barrett CL; Krajewska M; Leu H; Low-Marchelli J; Ma W; Shih AY; Wei J; Zhai D; Geron I; Pu M; Bao L; Chuang R; Balaian L; Gotlib J; Minden M; Martinelli G; Rusert J; Dao KH; Shazand K; Wentworth P; Smith KM; Jamieson CA; Morris SR; Messer K; Goldstein LS; Hudson TJ; Marra M; Frazer KA; Pellecchia M; Reed JC; Jamieson CH, A Pan-Bcl2 Inhibitor Renders Bone-Marrow-Resident Human Leukemia Stem Cells Sensitive to Tyrosine Kinase Inhibition. Cell Stem Cell 2013, 12 (3), 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan R; Ruvolo VR; Wei J; Konopleva M; Reed JC; Pellecchia M; Andreeff M; Ruvolo PP, Inhibition of Mcl-1 with the Pan-Bcl-2 Family Inhibitor (−)Bi97d6 Overcomes Abt-737 Resistance in Acute Myeloid Leukemia. Blood 2015, 126 (3), 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambini L; Udompholkul P; Salem AF; Baggio C; Pellecchia M, Stability and Cell Permeability of Sulfonyl Fluorides in the Design of Lys-Covalent Antagonists of Protein-Protein Interactions. ChemMedChem 2020, 15 (22), 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambini L; Baggio C; Udompholkul P; Jossart J; Salem AF; Perry JJP; Pellecchia M, Covalent Inhibitors of Protein-Protein Interactions Targeting Lysine, Tyrosine, or Histidine Residues. J Med Chem 2019, 62 (11), 5616–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baggio C; Udompholkul P; Gambini L; Salem AF; Jossart J; Perry JJP; Pellecchia M, Aryl-Fluorosulfate-Based Lysine Covalent Pan-Inhibitors of Apoptosis Protein (Iap) Antagonists with Cellular Efficacy. J Med Chem 2019, 62 (20), 9188–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggio C; Gambini L; Udompholkul P; Salem AF; Aronson A; Dona A; Troadec E; Pichiorri F; Pellecchia M, Design of Potent Pan-Iap and Lys-Covalent Xiap Selective Inhibitors Using a Thermodynamics Driven Approach. J Med Chem 2018, 61 (14), 6350–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baggio C; Udompholkul P; Barile E; Pellecchia M, Enthalpy-Based Screening of Focused Combinatorial Libraries for the Identification of Potent and Selective Ligands. ACS Chem Biol 2017, 12 (12), 2981–2989. [DOI] [PubMed] [Google Scholar]
- 25.Kotschy A; Szlavik Z; Murray J; Davidson J; Maragno AL; Le Toumelin-Braizat G; Chanrion M; Kelly GL; Gong JN; Moujalled DM; Bruno A; Csekei M; Paczal A; Szabo ZB; Sipos S; Radics G; Proszenyak A; Balint B; Ondi L; Blasko G; Robertson A; Surgenor A; Dokurno P; Chen I; Matassova N; Smith J; Pedder C; Graham C; Studeny A; Lysiak-Auvity G; Girard AM; Grave F; Segal D; Riffkin CD; Pomilio G; Galbraith LC; Aubrey BJ; Brennan MS; Herold MJ; Chang C; Guasconi G; Cauquil N; Melchiore F; Guigal-Stephan N; Lockhart B; Colland F; Hickman JA; Roberts AW; Huang DC; Wei AH; Strasser A; Lessene G; Geneste O, The Mcl1 Inhibitor S63845 Is Tolerable and Effective in Diverse Cancer Models. Nature 2016, 538 (7626), 477–482. [DOI] [PubMed] [Google Scholar]
- 26.Letai A, S63845, an Mcl-1 Selective Bh3 Mimetic: Another Arrow in Our Quiver. Cancer Cell 2016, 30 (6), 834–835. [DOI] [PubMed] [Google Scholar]
- 27.Li Z; He S; Look AT, The Mcl1-Specific Inhibitor S63845 Acts Synergistically with Venetoclax/Abt-199 to Induce Apoptosis in T-Cell Acute Lymphoblastic Leukemia Cells. Leukemia 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merino D; Whittle JR; Vaillant F; Serrano A; Gong JN; Giner G; Maragno AL; Chanrion M; Schneider E; Pal B; Li X; Dewson G; Grasel J; Liu K; Lalaoui N; Segal D; Herold MJ; Huang DCS; Smyth GK; Geneste O; Lessene G; Visvader JE; Lindeman GJ, Synergistic Action of the Mcl-1 Inhibitor S63845 with Current Therapies in Preclinical Models of Triple-Negative and Her2-Amplified Breast Cancer. Sci Transl Med 2017, 9 (401). [DOI] [PubMed] [Google Scholar]
- 29.Abulwerdi FA; Liao C; Mady AS; Gavin J; Shen C; Cierpicki T; Stuckey JA; Showalter HD; Nikolovska-Coleska Z, 3-Substituted-N-(4-Hydroxynaphthalen-1-Yl)Arylsulfonamides as a Novel Class of Selective Mcl-1 Inhibitors: Structure-Based Design, Synthesis, Sar, and Biological Evaluation. J Med Chem 2014, 57 (10), 4111–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruncko M; Wang L; Sheppard GS; Phillips DC; Tahir SK; Xue J; Erickson S; Fidanze S; Fry E; Hasvold L; Jenkins GJ; Jin S; Judge RA; Kovar PJ; Madar D; Nimmer P; Park C; Petros AM; Rosenberg SH; Smith ML; Song X; Sun C; Tao ZF; Wang X; Xiao Y; Zhang H; Tse C; Leverson JD; Elmore SW; Souers AJ, Structure-Guided Design of a Series of Mcl-1 Inhibitors with High Affinity and Selectivity. J Med Chem 2015, 58 (5), 2180–2194. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey HE; Fischer MA; Lee T; Gorska AE; Arrate MP; Fuller L; Boyd KL; Strickland SA; Sensintaffar J; Hogdal LJ; Ayers GD; Olejniczak ET; Fesik SW; Savona MR, A Novel Mcl-1 Inhibitor Combined with Venetoclax Rescues Venetoclax Resistant Acute Myelogenous Leukemia. Cancer Discov 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richard DJ; Lena R; Bannister T; Blake N; Pierceall WE; Carlson NE; Keller CE; Koenig M; He Y; Minond D; Mishra J; Cameron M; Spicer T; Hodder P; Cardone MH, Hydroxyquinoline-Derived Compounds and Analoguing of Selective Mcl-1 Inhibitors Using a Functional Biomarker. Bioorg Med Chem 2013, 21 (21), 6642–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer RA, Covalent Inhibitors in Drug Discovery: From Accidental Discoveries to Avoided Liabilities and Designed Therapies. Drug Discov Today 2015, 20 (9), 1061–1073. [DOI] [PubMed] [Google Scholar]
- 34.Singh J; Petter RC; Baillie TA; Whitty A, The Resurgence of Covalent Drugs. Nat Rev Drug Discov 2011, 10 (4), 307–317. [DOI] [PubMed] [Google Scholar]
- 35.Vita E, 10 Years into the Resurgence of Covalent Drugs. Future Med Chem 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T; Hatcher JM; Teng M; Gray NS; Kostic M, Recent Advances in Selective and Irreversible Covalent Ligand Development and Validation. Cell Chem Biol 2019, 26 (11), 1486–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baggio C; Udompholkul P; Gambini L; Jossart J; Salem AF; Hakansson M; Perry JJP; Pellecchia M, N-Locking Stabilization of Covalent Helical Peptides: Application to Bfl-1 Antagonists. Chem Biol Drug Des 2020, 95 (4), 412–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pettersen EF; Goddard TD; Huang CC; Couch GS; Greenblatt DM; Meng EC; Ferrin TE, Ucsf Chimera--a Visualization System for Exploratory Research and Analysis. J Comput Chem 2004, 25 (13), 1605–1612. [DOI] [PubMed] [Google Scholar]
- 39.Foight GW; Ryan JA; Gulla SV; Letai A; Keating AE, Designed Bh3 Peptides with High Affinity and Specificity for Targeting Mcl-1 in Cells. ACS Chem Biol 2014, 9 (9), 1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadji A; Schmitt GK; Schnorenberg MR; Roach L; Hickey CM; Leak LB; Tirrell MV; LaBelle JL, Preferential Targeting of Mcl-1 by a Hydrocarbon-Stapled Bim Bh3 Peptide. Oncotarget 2019, 10 (58), 6219–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axelsson H; Almqvist H; Seashore-Ludlow B; Lundback T, Screening for Target Engagement Using the Cellular Thermal Shift Assay - Cetsa. In Assay Guidance Manual, Markossian S; Sittampalam GS; Grossman A; Brimacombe K; Arkin M; Auld D; Austin CP; Baell J; Caaveiro JMM; Chung TDY; Coussens NP; Dahlin JL; Devanaryan V; Foley TL; Glicksman M; Hall MD; Haas JV; Hoare SRJ; Inglese J; Iversen PW; Kahl SD; Kales SC; Kirshner S; Lal-Nag M; Li Z; McGee J; McManus O; Riss T; Saradjian P; Trask OJ Jr.; Weidner JR; Wildey MJ; Xia M; Xu X, Eds. Bethesda (MD), 2004. [PubMed] [Google Scholar]
- 42.Jafari R; Almqvist H; Axelsson H; Ignatushchenko M; Lundback T; Nordlund P; Martinez Molina D, The Cellular Thermal Shift Assay for Evaluating Drug Target Interactions in Cells. Nat Protoc 2014, 9 (9), 2100–2122. [DOI] [PubMed] [Google Scholar]
- 43.Martinez Molina D; Jafari R; Ignatushchenko M; Seki T; Larsson EA; Dan C; Sreekumar L; Cao Y; Nordlund P, Monitoring Drug Target Engagement in Cells and Tissues Using the Cellular Thermal Shift Assay. Science 2013, 341 (6141), 84–87. [DOI] [PubMed] [Google Scholar]
- 44.Martinez Molina D; Nordlund P, The Cellular Thermal Shift Assay: A Novel Biophysical Assay for in Situ Drug Target Engagement and Mechanistic Biomarker Studies. Annu Rev Pharmacol Toxicol 2016, 56, 141–161. [DOI] [PubMed] [Google Scholar]
- 45.Czabotar PE; Lee EF; van Delft MF; Day CL; Smith BJ; Huang DC; Fairlie WD; Hinds MG; Colman PM, Structural Insights into the Degradation of Mcl-1 Induced by Bh3 Domains. Proc Natl Acad Sci U S A 2007, 104 (15), 6217–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F; Wang H; Li S; Bare GAL; Chen X; Wang C; Moses JE; Wu P; Sharpless KB, Biocompatible Sufex Click Chemistry: Thionyl Tetrafluoride (Sof4 )-Derived Connective Hubs for Bioconjugation to DNA and Proteins. Angew Chem Int Ed Engl 2019, 58 (24), 8029–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang B; Wu H; Schnier PD; Liu Y; Liu J; Wang N; DeGrado WF; Wang L, Proximity-Enhanced Sufex Chemical Cross-Linker for Specific and Multitargeting Cross-Linking Mass Spectrometry. Proc Natl Acad Sci U S A 2018, 115 (44), 11162–11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng Q; Woehl JL; Kitamura S; Santos-Martins D; Smedley CJ; Li G; Forli S; Moses JE; Wolan DW; Sharpless KB, Sufex-Enabled, Agnostic Discovery of Covalent Inhibitors of Human Neutrophil Elastase. Proc Natl Acad Sci U S A 2019, 116 (38), 18808–18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bongard J; Lorenz M; Vetter IR; Stege P; Porfetye AT; Schmitz AL; Kaschani F; Wolf A; Koch U; Nussbaumer P; Klebl B; Kaiser M; Ehrmann M, Identification of Noncatalytic Lysine Residues from Allosteric Circuits Via Covalent Probes. ACS Chem Biol 2018, 13 (5), 1307–1312. [DOI] [PubMed] [Google Scholar]
- 50.Liu R; Yue Z; Tsai CC; Shen J, Assessing Lysine and Cysteine Reactivities for Designing Targeted Covalent Kinase Inhibitors. J Am Chem Soc 2019, 141 (16), 6553–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettinger J; Carter M; Jones K; Cheeseman MD, Kinetic Optimization of Lysine-Targeting Covalent Inhibitors of Hsp72. J Med Chem 2019, 62 (24), 11383–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettinger J; Jones K; Cheeseman MD, Lysine-Targeting Covalent Inhibitors. Angew Chem Int Ed Engl 2017, 56 (48), 15200–15209. [DOI] [PubMed] [Google Scholar]
- 53.Wan X; Yang T; Cuesta A; Pang X; Balius TE; Irwin JJ; Shoichet BK; Taunton J, Discovery of Lysine-Targeted Eif4e Inhibitors through Covalent Docking. J Am Chem Soc 2020, 142 (11), 4960–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akcay G; Belmonte MA; Aquila B; Chuaqui C; Hird AW; Lamb ML; Rawlins PB; Su N; Tentarelli S; Grimster NP; Su Q, Inhibition of Mcl-1 through Covalent Modification of a Noncatalytic Lysine Side Chain. Nat Chem Biol 2016, 12 (11), 931–936. [DOI] [PubMed] [Google Scholar]
- 55.Mukherjee H; Su N; Belmonte MA; Hargreaves D; Patel J; Tentarelli S; Aquila B; Grimster NP, Discovery and Optimization of Covalent Bcl-Xl Antagonists. Bioorg Med Chem Lett 2019, 29 (23), 126682. [DOI] [PubMed] [Google Scholar]
- 56.Hoppmann C; Wang L, Proximity-Enabled Bioreactivity to Generate Covalent Peptide Inhibitors of P53-Mdm4. Chem Commun (Camb) 2016, 52 (29), 5140–5143. [DOI] [PubMed] [Google Scholar]
- 57.Butterworth M; Pettitt A; Varadarajan S; Cohen GM, Bh3 Profiling and a Toolkit of Bh3-Mimetic Drugs Predict Anti-Apoptotic Dependence of Cancer Cells. Br J Cancer 2016, 114 (6), 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards AL; Wachter F; Lammert M; Huhn AJ; Luccarelli J; Bird GH; Walensky LD, Cellular Uptake and Ultrastructural Localization Underlie the Pro-Apoptotic Activity of a Hydrocarbon-Stapled Bim Bh3 Peptide. ACS Chem Biol 2015, 10 (9), 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Joseph TL; Lane DP; Verma CS, Stapled Bh3 Peptides against Mcl-1: Mechanism and Design Using Atomistic Simulations. PLoS One 2012, 7 (8), e43985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muppidi A; Doi K; Ramil CP; Wang HG; Lin Q, Synthesis of Cell-Permeable Stapled Bh3 Peptide-Based Mcl-1 Inhibitors Containing Simple Aryl and Vinylaryl Cross-Linkers. Tetrahedron 2014, 70 (42), 7740–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walensky LD; Kung AL; Escher I; Malia TJ; Barbuto S; Wright RD; Wagner G; Verdine GL; Korsmeyer SJ, Activation of Apoptosis in Vivo by a Hydrocarbon-Stapled Bh3 Helix. Science 2004, 305 (5689), 1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otwinowski Z; Minor W, Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol 1997, 276, 307–326. [DOI] [PubMed] [Google Scholar]
- 63.McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ, Phaser Crystallographic Software. J Appl Crystallogr 2007, 40 (Pt 4), 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winn MD; Murshudov GN; Papiz MZ, Macromolecular Tls Refinement in Refmac at Moderate Resolutions. Methods Enzymol 2003, 374, 300–321. [DOI] [PubMed] [Google Scholar]
- 65.Emsley P; Cowtan K, Coot: Model-Building Tools for Molecular Graphics. Acta Crystallogr D Biol Crystallogr 2004, 60 (Pt 12 Pt 1), 2126–2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.