Clinical Implications.
The association between asthma and COVID-19–related outcomes is influenced by asthma severity. Although it is theorized that inhaled corticosteroids impart a protective effect against severe COVID-19, their impact on asthma and COVID-19 is frequently confounded by asthma severity.
The ongoing COVID-19 pandemic continues to represent a major threat to public health, with poor outcomes associated with obesity, age, male sex, Black race, tobacco use, and comorbidities.1 The impact of asthma on COVID-19 severity is controversial and confounded by asthma severity, T2 inflammation, and inhaled corticosteroids (iCS).2, 3, 4, 5 A recent analysis of electronic health records (EHRs) from 61,338 patients with COVID-195 found that adults with active asthma, but not those with inactive asthma, were at higher risk for COVID-19–related hospitalization and admission to the intensive care unit (ICU).4 Of note, active asthma was not associated with increased COVID-19–related mortality.5
To understand the effect of asthma severity on COVID-19–related outcomes, we analyzed data from Cleveland Clinic’s COVID-19 Research Registry (CCCRR) between April 1, 2020 and March 31, 2021.6 Patients aged less than 18 years or with an extremely large body mass were excluded (see Figure E1 in this article’s Online Repository at www.jaci-inpractice.org). We considered patients to have active asthma if they were prescribed an asthma medication or had asthma-related International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) billing codes listed in the EHR within 1 year of the positive COVID-19 result (Table I ).5 We defined severe asthma based on Global Initiative for Asthma steps 4 and 5 (Table II ) for those treated with high-dose iCS equivalent to or exceeding 800 μg/d of budesonide. We then used the most recent ICD-10-CM codes to stratify asthma severity into mild (J45.20; J45.30), moderate (J45.40), or severe (J45.50).7
Table I.
Demographics, clinical characteristics, and outcomes of patients with and without airway disease, stratified by asthma disease status and asthma therapy
| Variable∗ |
No asthma |
Inactive asthma† |
Short-acting β-agonist |
Low-dose iCS |
Low-dose iCS-LABA |
High-dose iCS-LABA |
Triple therapy§ |
Chronic oral corticosteroid |
P¶ |
|---|---|---|---|---|---|---|---|---|---|
| n | 62,042 | 3,890 | 3,828 | 877 | 761 | 363 | 93 | 115 | |
| Demographics | |||||||||
| Age, y (median [IQR]) | 50.0 [34.3-64.1] | 43.9 [29.6-58.5] | 45.2 [32.3-58.8] | 48.5 [35.8-59.8] | 53.0 [41.1-63.7] | 53.4 [43.3-65.0] | 58.3 [42.9-68.5] | 52.8 [44.8-63.2] | <.001 |
| Female sex | 33,115 (53.4) | 2,424 (62.3) | 2,670 (69.7) | 617 (70.4) | 523 (68.7) | 241 (66.4) | 67 (72.0) | 91 (79.1) | <.001 |
| Body mass index, kg/m2 (median [IQR]) | 29.1 [25.1-34.0] | 29.9 [25.5-35.8] | 31.6 [26.6-37.6] | 32.1 [27.2-38.5] | 32.5 [27.5-38.3] | 33.1 [28.4-39.5] | 32.3 [26.8-39.3] | 32.6 [27.5-37.6] | <.001 |
| Race | <.001 | ||||||||
| Black | 10,684 (17.2) | 829 (21.3) | 920 (24.0) | 186 (21.2) | 151 (19.8) | 81 (22.3) | 24 (25.8) | 21 (18.3) | |
| White | 41,570 (67.0) | 2,556 (65.7) | 2,464 (64.4) | 602 (68.6) | 545 (71.6) | 260 (71.6) | 61 (65.6) | 81 (70.4) | |
| Hispanic ethnicity | 2,916 (4.7) | 135 (3.5) | 134 (3.5) | 29 (3.3) | 22 (2.9) | NA | NA | NA | <.001 |
| Smoking history | <.001 | ||||||||
| Current | 3,714 (6.0) | 303 (7.8) | 316 (8.3) | 51 (5.8) | 28 (3.7) | 18 (5.0) | NA | NA | |
| Past | 11,707 (19.0) | 816 (21.1) | 888 (23.2) | 192 (21.9) | 209 (27.5) | 97 (26.8) | 31 (33.3) | 36 (31.3) | |
| Pack-years, n (median [IQR]) | 10.0 [3.9-23.0] | 7.5 [2.0-16.0] | 7.5 [2.5-18.0] | 7.5 [2.5-19.0] | 8.0 [4.5-20.0] | 9.0 [3.0-20.5] | 10.0 [2.4-16.5] | 5.0 [0.90-13.75] | <.001 |
| Respiratory symptoms | |||||||||
| Cough | 28,193 (45.4) | 2,017 (51.9) | 2,248 (58.7) | 499 (56.9) | 471 (61.9) | 228 (62.8) | 53 (57.0) | 71 (61.7) | <.001 |
| Dyspnea | 13,674 (22.0) | 1,019 (26.2) | 1,478 (38.6) | 362 (41.3) | 285 (37.5) | 150 (41.3) | 45 (48.4) | 56 (48.7) | <.001 |
| Comorbidities | |||||||||
| Allergic rhinitis | 4,382 (7.1) | 648 (16.7) | 964 (25.2) | 249 (28.4) | 326 (42.8) | 153 (42.1) | 24 (25.8) | 44 (38.3) | <.001 |
| Diabetes | 7,723 (12.4) | 492 (12.6) | 650 (17.0) | 129 (14.7) | 142 (18.7) | 76 (20.9) | 25 (26.9) | 21 (18.3) | <.001 |
| Hypertension | 17,244 (27.8) | 1,175 (30.2) | 1,359 (35.5) | 319 (36.4) | 327 (43.0) | 164 (45.2) | 49 (52.7) | 47 (40.9) | <.001 |
| Coronary artery disease | 3,700 (6.0) | 212 (5.4) | 238 (6.2) | 47 (5.4) | 54 (7.1) | 37 (10.2) | NA | 13 (11.3) | <.001 |
| Heart failure | 2,418 (3.9) | 121 (3.1) | 183 (4.8) | 28 (3.2) | 33 (4.3) | 29 (8.0) | NA | NA | <.001 |
| Cancer history | 5,100 (8.2) | 302 (7.8) | 320 (8.4) | 82 (9.4) | 80 (10.5) | 39 (10.7) | 13 (14.0) | 13 (11.3) | <.001 |
| Immunosuppressive disease | 3,493 (5.6) | 218 (5.6) | 274 (7.2) | 73 (8.3) | 52 (6.8) | 31 (8.5) | 11 (11.8) | 14 (12.2) | <.001 |
| Medications | |||||||||
| Nonsteroidal anti-inflammatory drugs | 7,149 (11.5) | 585 (15.0) | 760 (19.9) | 149 (17.0) | 131 (17.2) | 54 (14.9) | 13 (14.0) | 25 (21.7) | <.001 |
| Angiotensin converting enzyme inhibitors | 4,962 (8.0) | 248 (6.4) | 315 (8.2) | 77 (8.8) | 54 (7.1) | 37 (10.2) | NA | NA | <.001 |
| Angiotensin receptor blockers | 3,296 (5.3) | 219 (5.6) | 271 (7.1) | 76 (8.7) | 86 (11.3) | 43 (11.8) | 14 (15.1) | 13 (11.3) | <.001 |
| Intranasal corticosteroids | 9,294 (15.0) | 883 (22.7) | 1,470 (38.4) | 327 (37.3) | 486 (63.9) | 213 (58.7) | 43 (46.2) | 75 (65.2) | <.001 |
iCS, inhaled corticosteroids; IQR, interquartile range; LABA, long-acting β-agonist; NA, not available.
Data are presented as n (%) for categorical variables unless otherwise noted.
Data for high-dose iCS without LABA are not reported owing to the small number of patients (n = 21). Data with less than 10 patients are listed as NA.
Asthma medications used to differentiate between active and inactive asthma are short acting beta agonists, leukotriene receptor antagonist, iCS, LABA, and long-acting muscarinic antagonist (LAMA).
Triple therapy = iCS + LAMA + LABA.
Group means were compared using Kruskal-Wallis test.
Table II.
Associations between COVID-19 outcomes and asthma
| Stratification of analyses | n | Hospitalization (OR [95% CI]) | Intensive care unit admission (OR [95% CI]) | Hospital mortality (OR [95% CI])∗ |
|---|---|---|---|---|
| By asthma therapy† | ||||
| n | 11,221 | 2,470 | 2,158 | |
| No asthma | 62,042 | 1 | 1 | 1 |
| Inactive asthma | 3890 | 1.05 (0.95-1.17) | 0.89 (0.72-1.11) | 0.78 (0.60-1.01) |
| Active asthma | ||||
| Short-acting β-agonist alone | 3828 | 1.37 (1.24-1.51) | 1.26 (1.04-1.52) | 0.80 (0.60-1.05) |
| Low-dose iCS | 877 | 1.23 (1.00-1.50) | 0.98 (0.64-1.50) | 0.63 (0.34-1.18) |
| Low-dose iCS-LABA | 761 | 1.13 (0.91-1.41) | 1.10 (0.72-1.70) | 0.70 (0.38-1.27) |
| High-dose iCS-LABA | 363 | 1.54 (1.16-2.06) | 1.21 (0.70-2.10) | 1.13 (0.57-2.23) |
| Triple therapy | 93 | 2.61 (1.16-4.26) | 1.65 (0.73-5.00) | 1.37 (0.52-3.60) |
| Chronic oral corticosteroids | 115 | 3.00 (1.60-4.70) | 2.09 (0.87-6.10) | 1.62 (0.54-4.85) |
| Anti-IgE biologic therapy | 42 | 1.60 (0.66-3.87) | NA | NA |
| Anti-IL5(Rα), IL4Rα biologic therapy | 54 | 3.31 (1.75-6.24) | NA | NA |
| By asthma severity | ||||
| n∗ | 10,262 | 2,269 | 2,054 | |
| No asthma | 62,042 | 1 | 1 | 1 |
| Active asthma (asthma severity) | ||||
| Mild | 2,496 | 1.22 (1.07-1.38) | 0.87 (0.66-1.15) | 0.92 (0.67-1.26) |
| Moderate | 1,076 | 1.37 (1.15-1.64) | 1.36 (0.98-1.90) | 0.74 (0.45-1.21) |
| Severe | 290 | 2.89 (2.15-3.88) | 1.88 (1.09-3.23) | 0.85 (0.36-2.03) |
| By asthma exacerbations‡ | ||||
| n | 1,069 | 214 | 104 | |
| Exacerbations, n | ||||
| 0 | 4,194 | 1 | 1 | 1 |
| 1 | 1,562 | 0.87 (0.73-1.04) | 0.74 (0.51-1.06) | 1.27 (0.79-2.06) |
| ≥2 | 362 | 1.09 (0.80-1.47) | 0.84 (0.46-1.54) | 1.96 (0.93-4.17)§ |
CI, confidence interval; iCS, inhaled corticosteroid; LABA, long-acting β-agonist; NA, not available; OR, odds ratio,
Asthma is stratified by disease state (active vs inactive) and therapy, by severity defined by asthma related International Classification of Diseases, Tenth Revision, Clinical Modification codes listed in medical records within 1 y of COVID-19 diagnosis, and by asthma exacerbations. Analyses were adjusted for age, sex, race, ethnicity, body mass index, smoking history, pack-years smoking, medications (nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme 2 inhibitor, angiotensin receptor blocker, and intranasal corticosteroids), comorbidities (allergic rhinitis, diabetes, hypertension, coronary artery disease, heart failure, cancer [historical or current], and immunosuppressive disease), and month of testing. We excluded 21 patients receiving high-dose iCS alone without LABA. Analyses were performed on imputed data. Data with missing dependent variables were excluded. All variables had less than 15% of missing data. Multiple imputations (five imputations) for missing variables were carried out using the MICE package in R, version 4.0.5 (R Project for Statistical Computing, Vienna, Austria); separate results were pooled using Rubin’s rules to obtain the final results.
Hospital mortality includes patients discharged to hospice.
Triple therapy: iCS + LABA + long-acting muscarinic antagonist; anti-IgE biologic = omalizumab; anti-IL5(Rα), IL4Rα biologic therapy = mepolizumab, reslizumab, benralizumab, and dupilumab,
P = .08.
Includes additional adjustment for therapy (short-acting β-agonist, LACA, iCS, and long-acting muscarinic antagonist).
We performed multivariable logistic regression comparing severe COVID-19 (defined by COVID-19–related hospitalization, ICU admission, or death) with asthma severity and Global Initiative for Asthma treatment steps. Regression models were adjusted for covariates a priori known to be associated with severe COVID-191 (ie, demographics, body mass index, smoking status, pack-years, comorbidities, and medications) (Table II). Adjustment for the month of testing was performed to avoid chronological bias introduced by changes in COVID-19 management.1 , 6
Table I lists clinical and demographics characteristics. Of 82,096 patients within the registry, 72,064 were included in the final analysis. Of those, 11,221 required hospitalization (15.6%), 2,470were admitted to the ICU (3.4%), and 2,158 died (3.0%). Multivariable modeling found that asthma was associated with higher hospitalization risk, with the greatest risk in biologics targeting T2 inflammation (adjusted odds ratio [OR] [95% confidence interval (CI)]: 3.31 [1.75,6.24]), chronic daily oral corticosteroids (3.00 [1.60-4.70]), high-dose iCS–long-acting β-agonist–long-acting muscarinic antagonist triple-combination inhalers (2.61 [1.16-4.26]), and severe asthma (2.89 [2.15; 3.88]). Asthmatic patients treated with short-acting β-agonists alone were at higher risk for ICU admission (1.26 [1.04-1.52]), but not death. Otherwise, we found no other significant association between asthma and critical illness or mortality (Table II). These findings were replicated in sensitivity analyses after adjustment for preexisting eosinophilia (>300 cells/μL) in a subset of 43,104 individuals (see Table E1 in this article’s Online Repository at www.jaci-inpractice.org).
Among patients with active asthma, 1,562 (25.4%) had a single exacerbation (defined by oral corticosteroid prescription <28 days), and 290 (4.7%) had frequent (two or more) exacerbations in the 12 months before COVID-19. Additional adjustments for asthma therapies found no association between asthma exacerbation and severe COVID-19 (Table II). These data suggest that COVID-19–related hospitalization is associated with severe asthma, but not asthma control, as reflected by the number of exacerbations.
In our study, increased risk for COVID-19–related hospitalization among asthmatic patients did not translate into a higher risk for ICU admission or death. To date, studies associating asthma and COVID-19 outcomes have revealed conflicting results, likely owing to differences in research methodology, data type, country of origin, the presence or absence of T2 inflammation, iCS use, and sample size.1 , 3, 4, 5 Our findings of increased risk for hospitalization are consistent with some but not all previous reports.4
Inhaled corticosteroids may impart a protective effect against severe COVID-19 by downregulating the angiotensin converting enzyme 2 receptor required for viral infection of airway epithelial cells.8 Inhaled corticosteroids have also been linked to reduced systemic IL6 in animal models of acute lung injury.9 The impact of iCS on COVID-19 outcomes varies among studies. Our analysis using CCCRR data was unable to replicate prior reports of increased risk for ICU admission or death among asthmatic patients treated with high-dose iCS or iCS–long-acting β-agonist combination therapy.1 , 3 Prospective studies of iCS and COVID-19 outcomes are ongoing and may provide more information about this important clinical question.
Our study had several limitations. Similar to other observational studies, our study might have been subject to bias introduced by measured and unmeasured confounding factors. With these real-world data extracted from EHR, it was impossible to use standardized, international consensus-based definitions to identify severe asthma. Instead, we used asthma medication use and billing codes to estimate asthma severity.7
Our study was also limited by the lack of pharmacy claim data necessary to estimate medication (re)fill adherence measures, and did not account for patients who admitted or died outside the Cleveland Clinic. All of these limitations impose an inherent risk for bias by relying on electronic records. However, the study strengths include rigorous methodologies, because CCCRR data were extracted prospectively using uniform clinical templates to standardize patient care and facilitate data extraction. Data were then verified independently by trained medical personnel.6 We aimed to improve our methods by using current asthma therapy as an additional criterion to define active asthma.5 We used two different methods to estimate asthma severity and found similar results. Finally, we adjusted our analyses for risk factors previously associated with severe COVID-19 and provided stratified analyses to test the association of asthma severity, asthma medication categories, and asthma exacerbations with COVID-19 outcomes. Although our data suggest that the risk for COVID-19–related hospitalizations is higher in severe asthma, randomized controlled trials and prospective observational studies of large asthma cohorts are needed to understand the association between asthma and COVID-19 outcomes and to study the role of iCS in COVID-19, which is a frequent confounder for asthma severity.
Acknowledgments
J.G. Zein, J. Mitri, and A.H. Attaway made substantial contributions to the conception or design of the work; J.G. Zein acquired, analyzed, and interpreted the data for the work; J. Mitri, J.M. Bell, D. Lopez, R. Strauss, and A.H. Attaway contributed to drafting the work or revising it critically for important intellectual content; J.G. Zein agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part work are appropriately investigated and resolved; and J.G. Zein, J. Mitri, J.M. Bell, D. Lopez, R. Strauss, and A.H. Attaway gave the final approval of the version to be published.
Footnotes
This study was funded by National Institutes of Health–National Heart, Lung, and Blood Institute Grant K08 HL133381 to J.G. Zein.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
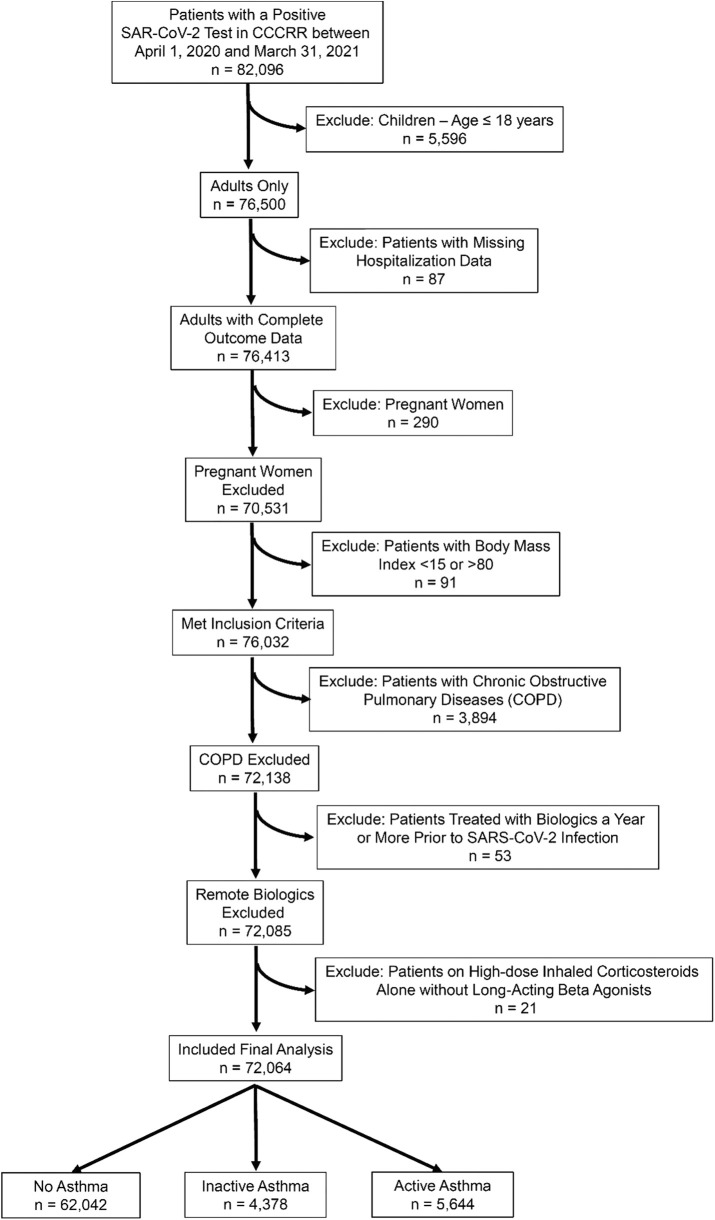
Figure E1.
Flowchart of patients in final analysis. CCCRR, Cleveland Clinic’s COVID-19 Research Registry.
Table E1.
Association of asthma therapy with COVID-19–related hospital admission, intensive care unit admission, and hospital mortality among all patients who had a positive SARS-CoV-2 test adjusting for preexisting eosinophilia
| Asthma therapy | n | Hospitalization (adjusted OR [95% CI]) (n = 7,421) | Intensive care unit admission (adjusted OR [95% CI]) (n = 1,638) | Hospital mortality (adjusted OR [95% CI]) (n = 1,431) |
|---|---|---|---|---|
| All patients, n | 43,104 | |||
| No asthma | 35,314 | 1 | 1 | 1 |
| Inactive asthma | 2,681 | 0.91 (0.80-1.04) | 0.79 (0.61-1.04) | 0.69 (0.49-0.97) |
| Active asthma | ||||
| Short-acting β-agonist monotherapy | 3,154 | 1.38 (1.24 1.54) | 1.17 (0.95-1.45) | 0.86 (0.65-1.14) |
| Low-dose iCS | 700 | 1.23 (1.00-1.57) | 0.90 (0.55-1.47) | 0.62 (0.31-1.25) |
| Low-dose iCS-LABA | 657 | 1.10 (0.87-1.38) | 1.13 (0.71-1.80) | 0.70 (0.36-1.31) |
| High-dose iCS-LABA | 321 | 1.57 (1.17-2.13) | 1.34 (0.76-2.36) | 1.34 (0.68-2.64) |
| Triple inhaler therapy | 82 | 2.47 (1.47-4.15) | 1.22 (0.48-3.14) | 1.48 (0.56-3.91) |
CI, confidence interval; iCS, inhaled corticosteroids; LABA, long-acting β-agonist; OR, odds ratio.
Preexisting eosinophilia is defined by a preexisting absolute eosinophil count of >300 cells/μL measured for 15 days or more before the date of a positive SARS-CoV-2 test. Analyses were adjusted for age, sex, race, ethnicity, body mass index, smoking history, pack-years smoking, medications (nonsteroidal anti-inflammatory drugs, angiotensin converting enzyme 2 inhibitor, angiotensin receptor blocker, and intranasal corticosteroids), comorbidities (allergic rhinitis, diabetes, hypertension, coronary artery disease, heart failure, and cancer [historical or current], and immunosuppressive disease), the month of testing, and eosinophilia.
References
- 1.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom C.I., Drake T.M., Docherty A.B., Lipworth B.J., Johnston S.L., Nguyen-Van-Tam J.S., et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultze A., Walker A.J., MacKenna B., Morton C.E., Bhaskaran K., Brown J.P., et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8:1106–1120. doi: 10.1016/S2213-2600(20)30415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh D., Halpin D.M.G. Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying? Lancet Respir Med. 2020;8:1065–1066. doi: 10.1016/S2213-2600(20)30447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B.Z., Chen Z., Sidell M.A., Eckel S.P., Martinez M.P., Lurmann F., et al. Asthma disease status, COPD, and COVID-19 severity in a large multiethnic population. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2021.07.030. 3621-8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauss R., Jawhari N., Attaway A.H., Hu B., Jehi L., Milinovich A., et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019. J Allergy Clin Immunol Pract. 2021;9:3934–3940. doi: 10.1016/j.jaip.2021.08.007. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang E., Wechsler M.E., Tran T.N., Heaney L.G., Jones R.C., Menzies-Gow A.N., et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry. Chest. 2020;157:790–804. doi: 10.1016/j.chest.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 8.Milne S., Li X., Yang C.X., Leitao Filho F.S., Hernández Cordero A.I., Yang C.W.T., et al. Inhaled corticosteroids downregulate SARS-CoV-2-related genes in COPD: results from a randomised controlled trial. Eur Respir J. 2021;58:2100130. doi: 10.1183/13993003.00130-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda K., Tsuruta M., Eom J., Or C., Mui T., Jaw J.E., et al. Acute lung injury induces cardiovascular dysfunction: effects of IL-6 and budesonide/formoterol. Am J Respir Cell Mol Biol. 2011;45:510–516. doi: 10.1165/rcmb.2010-0169OC. [DOI] [PubMed] [Google Scholar]