Abstract
Background
Urgent neurosurgical interventions for pediatric patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are rare. These cases pose additional stress on a potentially vulnerable dysregulated inflammatory response that can place the child at risk of further clinical deterioration. Our aim was to describe the perioperative course of SARS-CoV-2–positive pediatric patients who had required an urgent neurosurgical intervention.
Methods
We retrospectively analyzed pediatric patients aged ≤18 years who had been admitted to a quaternary children's hospital with a positive polymerase chain reaction test result for SARS-CoV-2 virus from March 2020 to October 2021. The clinical characteristics, anesthetic and neurosurgical operative details, surgical outcomes, and non-neurological symptoms were collected and analyzed.
Results
We identified 8 SARS-CoV-2–positive patients with a mean age of 8.83 years (median, 8.5 years; range, 0.58–18 years). Of the 8 patients, 6 were male. All children had had mild or asymptomatic coronavirus disease 2109. The anesthetic and surgical courses for these patients were, overall, uncomplicated. All the patients had been admitted to a specialized isolation unit in the pediatric intensive care unit for cardiopulmonary and neurological monitoring. The use of increased protective personal equipment during anesthesia and surgery did not impede a successful neurosurgical operation.
Conclusions
SARS-CoV-2–positive pediatric patients with minimal coronavirus disease 2019–related symptoms who require urgent neurosurgical interventions face unique challenges regarding their anesthetic status, operative delays due to SARS-CoV-2 polymerase chain reaction testing, and requirements for additional protective personal equipment. Despite these clinical challenges, the patients in our study had not experienced adverse postoperative consequences, and no healthcare professional involved in their care had contracted the virus.
Key words: Arteriovenous malformation, COVID-19, Hydrocephalus, Intracranial hemorrhage, Pediatric intensive care unit, Pediatric neurosurgery, Shunt
Abbreviations and Acronyms: AVM, Arteriovenous malformation; COVID-19, Coronavirus disease 2019; CSF, Cerebrospinal fluid; CT, Computed tomography; ETV, Endoscopic third ventriculostomy; EVD, Externalized ventriculostomy drain; MIS-C, Multisystem inflammatory syndrome in children; PAPR, Powered air-purifying respirator; PCR, Polymerase chain reaction; PICU, Pediatric intensive care unit; PPE, Personal protection equipment; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VA, Ventriculoatrial; VPS, Ventriculoperitoneal shunt
Introduction
Coronavirus disease 2019 (COVID-19) is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has caused an ongoing worldwide pandemic. The burden of severe disease and mortality has been greatest for older adults (aged >60 years) and those with underlying comorbid medical conditions, including cardiovascular disease, obesity, poorly controlled diabetes, and immunocompromised conditions.1, 2, 3 Acute respiratory failure has been the most common complication of COVID-19 in adults. In contrast, children will generally experience a mild illness that infrequently leads to hospital admission and medical intervention.4, 5, 6 As of October 2021, >6 million children in the United States alone have tested positive for COVID-19.3 , 7 In the pediatric population, the most common symptoms have been fever, cough, runny nose, diarrhea, sore throat, and vomiting.4 , 8 However, it has been increasingly recognized that COVID-19 can lead to neurological manifestations such as encephalopathy, central nervous system inflammation, and stroke.9 , 10 Pediatric patients with COVID-19 are especially susceptible to developing a systemic inflammatory syndrome, termed “multisystem inflammatory syndrome in children” (MIS-C), which can result in cardiovascular, respiratory, gastrointestinal, mucocutaneous, hematologic, and neurological compromise.11 Although rare, some children have developed intracranial hypertension that resolved with supportive medical care.12 Thus, a risk exists that patients with COVID-19 could have neurological symptoms due to the virus that can confound the perioperative neurological assessment of pediatric neurosurgical patients. Furthermore, it is unknown whether general anesthesia and neurosurgical intervention places infected children at greater risk of developing neurological and/or cardiorespiratory complications from SARS-CoV-2, highlighting the need for additional data from this population.
Operative interventions for COVID-19–positive pediatric patients pose a particular set of challenges. Few studies on the anesthetic and perioperative management of pediatric patients with SARS-CoV-2 have been described compared with adults, and, as such, guidelines describing optimal care are less refined. Anesthesiologists, in particular, are likely have an increased risk of contracting COVID-19 during airway management owing to the close proximity of the patient's airway and exposure to aerosolized viral particles during airway management, including positive-pressure ventilation via a mask, endotracheal intubation, extubation, or care of a tracheostomy tube.13 , 14 A systematic review from the 2003 SARS coronavirus outbreak demonstrated that compared with healthcare workers who did not perform airway or ventilator management, those who had performed tracheal intubation had had a 6.6-fold increased risk of contracting the virus.15
Once the induction of anesthesia has begun and throughout the operative course, a risk exists for all the staff in the operating room.16 Furthermore, the pediatric patient infected with COVID-19 who requires general endotracheal anesthesia and a surgical procedure will be at risk of further stress that could exacerbate an already vulnerable and dysregulated inflammatory response, placing the child at risk of further clinical deterioration. At our institution, although elective surgeries were postponed for children with SARS-CoV-2, our pediatric neurosurgical population had had some emergent or urgent conditions that had required general endotracheal anesthesia and surgical intervention to prevent life-threatening neurological deterioration. To the best of our knowledge, only 1 specific case has been reported of a pediatric patient infected with COVID-19 who had undergone an urgent neurosurgical intervention17 and the present study is the first case series of pediatric patients who had had COVID-19 and had required urgent neurosurgical interventions.
Methods
The present retrospective observational study included pediatric patients aged ≤18 years at a quaternary children's hospital during a 20-month period (March 2020 to October 2021). The committee for the protection of human subjects institutional review board approved the present study protocol. Throughout the study period, all the patients had undergone SARS-CoV-2 polymerase chain reaction (PCR) testing from a nasopharyngeal or anterior nares source on admission. SARS-CoV-2 PCR test results were obtained within 72 hours before surgery during the study period (March 2020 to October 2021). All cases were evaluated by the attending pediatric neurosurgeon and division chief and classified as emergent, urgent, or elective, similar to the recommendations from the Brazilian Society of Pediatric Neurosurgery and an Italy pediatric hospital.18, 19, 20 Cases requiring immediate surgical intervention at admission without waiting until the next day were deemed emergent. These included hydrocephalus with altered mental status, infection, and brain or spine injury or trauma with severe neurological compromise from baseline, including lethargy and/or obtundation. Neurosurgical procedures were considered urgent if a risk of imminent neurological decline without surgical correction was present during that hospital admission, including cerebrospinal fluid (CSF) leakage, hydrocephalus, shunt malfunction, open myelomeningocele, brain or spine tumors at risk of intracranial hypertension or neurological compromise, and intracerebral hemorrhage from hemorrhagic vascular malformations. Procedures were deemed elective if not considered a neurosurgical urgent or emergent case and were rescheduled after discussion with the parents and hospital administration. Our classification system was similar to that used in other studies and was used to stratify surgical cases by clinical status and period in which they could be rescheduled (Table 1 ).18, 19, 20
Table 1.
Classification System of Pediatric Neurosurgical Procedures Stratified by Timing
| Classification | Pediatric Neurosurgical Procedure |
|---|---|
| Class 1: emergent and urgent neurosurgical procedures requiring immediate surgical treatment within 24–48 hours | |
| Shunt placement or revision for acute hydrocephalus with unstable neurological symptoms | |
| External ventricular drain placement for acute hydrocephalus with unstable neurological symptoms | |
| Exploration of penetrating spinal cord or peripheral nerve injury or cauda equina syndrome | |
| Spinal fusion for trauma and instability with neurological compromise | |
| Embolization, clipping, or coiling for ruptured vascular malformations/aneurysms | |
| Wound revision or washout for infection or CSF leakage | |
| Evacuation of epidural, subdural, intraventricular or intraparenchymal hemorrhage | |
| Decompressive hemicraniectomy for severe TBI, cerebral herniation | |
| Closure of myelomeningocele | |
| Craniotomy for epidural/subdural empyema | |
| Resection of brain or spinal tumors associated with neurological compromise | |
| Class 2: semi-elective neurosurgical treatment within 1–2 weeks | |
| Shunt placement or revision for acute hydrocephalus with stable neurological symptoms | |
| External ventricular drain placement for acute hydrocephalus with stable neurological symptoms | |
| Resection of brain or spinal tumors associated with increased risk of neurological compromise | |
| Spinal fusion for trauma and instability with increased risk of neurological compromise | |
| Class 3: elective neurosurgical conditions with optimal treatment <1–2 months | |
| Revascularization for moyamoya disease for unstable neurological symptoms | |
| Stereotactic EEG lead placement | |
| Hemispherotomy | |
| Resection of seizure focus | |
| Laminectomy for stenosis or spinal fusion in nontraumatic spondylolisthesis with worsening neurological symptoms | |
| Craniosynostosis reconstruction (minimally invasive) | |
| Resection of brain or spinal tumors associated without neurological compromise | |
| Class 4: neurosurgical conditions able to delay treatment >1–2 months | |
| Chiari decompression | |
| Revascularization for asymptomatic or chronic symptoms of moyamoya disease | |
| Laminectomy for tethered cord release | |
| Arachnoid cyst fenestration for nonruptured arachnoid cysts | |
| Cranioplasty | |
| Selective dorsal rhizotomy | |
| Intrathecal baclofen pump | |
| Laminectomy for stenosis or spinal fusion in non-traumatic spondylolisthesis with stable neurological symptoms | |
| Craniosynostosis reconstruction (whole vault) | |
| Nonruptured resection of vascular malformations, clipping/coiling of aneurysms | |
| Benign skull/scalp lesions |
The inpatient and outpatient records, radiologic studies, and operative reports were analyzed. The patient data, including demographic characteristics, clinical presentation and course, laboratory and imaging results, underlying neurosurgical pathology, medications administered, and follow-up details, were abstracted from the electronic medical records. If available, the arterial blood gas carbon dioxide levels, oxygen saturation values, blood pressure results, intracranial pressure values, mechanical ventilation parameters, and medications were reviewed.
Formal N95 fitting and personal protection equipment (PPE) training sessions were administered to ensure the health and safety of the multidisciplinary team. The decision to use a powered air-purifying respirator (PAPR) versus an N95 mask by the attending pediatric neurosurgeon was decided by the results of the N95 fit test and the surgical complexity. The pediatric anesthesia care team used an N95 mask or PAPR during intubation and mechanical ventilation.
Prescreening for COVID-19 risk was conducted by the pediatric anesthesia care team during the preanesthesia evaluation. During admission, all the patients had undergone 24-hour preoperative screening, including symptom history, body temperature, oxygen saturation, and SARS-CoV-2 PCR testing. The SARS-CoV-2 PCR result was obtained within 72 hours before surgery throughout the study period (March 2020 to October 2021).
Results
Demographics
Similar to other pediatric institutions, the neurosurgical activity at our hospital was affected by the COVID-19 pandemic, leading to the postponement and cancellation of elective procedures.18, 19, 20 From March 2020 to October 2021, 1981 surgical procedures were performed. During the first peak of the COVID-19 pandemic in the United States, 10 elective procedures were postponed during March and April of 2020. From May 2020 to October 2021, an additional 7 elective procedures were postponed, and 1 elective procedure was cancelled because their SARS-CoV-2 PCR test results were positive.
Eight children had met the inclusion criteria for requiring an urgent neurosurgical operation with a positive SARS-CoV-2 PCR result and were included in the present study (Table 2 ). All the patients had undergone surgery within 2 days of admission. Of the 8 patients in this cohort, 6 were male (75%), with a mean age of 8.83 years (median, 8.5 years; range, 0.58–18 years).
Table 2.
Patient Demographics and Clinical Course
| Pt. No.; Age; Sex | Presenting Signs or Symptoms | Imaging Findings | Neurosurgery | Intubation Duration (Days) | Hospital length of stay (Days) | Symptoms Related to COVID-19 or Intervention |
|---|---|---|---|---|---|---|
| 1; 7 months; male | CSF leakage from ETV incision | Head CT: right subdural hygroma, right frontal pseudomeningocele from ETV incision | ETV wound revision for CSF leak; EVD; VPS placement; EVD and VPS removal and wound revision for CSF leak | During surgery only | 30 | None |
| 2; 10 months; female | Seizures, lethargy | Head CT: large right parietotemporal hemorrhage with bilateral intraventricular hemorrhage | EVD × 2; coiling of posterior cerebral artery intranidal aneurysm | 14 | 58 | None |
| 3; 15 months; male | Obtundation; vomiting; bradycardia; hypertension | Head CT: increased dilation of lateral, third, and fourth ventricles from baseline | VA shunt removal and EVD placement; new VA shunt placement and EVD removal | During surgery only | 5 | Tachypnea; chest radiograph for aspiration pneumonitis associated with tracheal intubation; readmitted for fever |
| 4; 6 years; male | Neck pain; headache; vomiting; lethargy | Head CT: left frontal intraparenchymal hemorrhage with 2–3-mm midline shift; cerebral angiogram: AVM of terminal MCA branch with intranidal aneurysm | Onyx embolization of intranidal aneurysm and AVM nidus; left frontal craniotomy for intranidal aneurysm resection and hemorrhage evacuation | During surgery only | 8 | None |
| 5; 11 years; female | CSF leakage from incision | Brain MRI: increased edema around ETV tract with increased ventricular size | Wound washout and revision; CSF sampling | During surgery only | 6 | None |
| 6; 16 years; male | Swelling on right neck over shunt tubing | Radiograph: disconnection of shunt tubing at neck | Distal shunt revision | During surgery only | 1 | None |
| 7; 17 years; male | Headache; blurry vision; leg weakness; vomiting | Brain MRI: large hemorrhagic cystic vermian mass | Craniotomy for tumor resection | During surgery only | 5 | None |
| 8; 18 years; male | Headache; blurry vision; gait instability; URI symptoms | Head CT: enlarged subdural hygroma collection | Proximal shunt revision and valve exchange | During surgery only | 3 | URI symptoms; headaches; chest radiograph for postoperative hypoxia |
Pt. No., patient number; COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; ETV, endoscopic third ventriculostomy; CT, computed tomography; VPS, ventriculoperitoneal shunt; EVD, external ventricular drain; VA, ventriculoatrial; AVM, arteriovenous malformation; MCA, middle cerebral artery; MRI, magnetic resonance imaging; URI, upper respiratory infection.
Admission, Anesthesia, and Intraoperative Course
All the patients had been admitted to a specialized isolation unit with negative-pressure rooms and dedicated staff in our pediatric intensive care unit (PICU). Negative-pressure operating rooms were also used to minimize exposure through an isolated and restricted area by trained personnel wearing PPE. Consistent protocolized handwashing before and after donning or doffing the PPE was used. The pediatric anesthesiologist wore a disposable skull cap, goggles, a face shield, and an N95 mask or PAPR or controlled air-purifying respirator, scrubs or a surgical gown, a disposable biological-proof protective suit in the outer layer, disposable gloves, and disposable shoe covers. All patients aged >9 months (with special considerations for certain patients aged <9 months) were given the option of a premedication with an anxiolytic medication (midazolam) before transport to the operating room in accordance with the protocol to minimize fear and anxiety. In the present case series, all the patients had received midazolam without any clinical deterioration. The use of midazolam also has the potential benefit of minimizing crying or coughing by the SARS-CoV-2–infected child to reduce the risk of aerosolization of SARS-CoV-2 viral particles. Endotracheal intubation was performed using a video laryngoscope by the most experienced anesthesiology personnel to maximize the distance from the child's face and success at the first attempt. A clear, transparent plastic drape between the anesthesiologist and patient was also used. Only essential personnel were in the room during anesthesia induction, which excluded the neurosurgical and surgical nursing team. A cuffed orotracheal tube was placed to minimize air leakage, and complete muscle relaxation with intravenous neuromuscular blockade was used to prevent coughing and decrease the risk of virus particle aerosolization. Our specialized pediatric anesthetic protocol was developed with multi-institutional collaboration and was consistent with other pediatric anesthesia protocols.13 , 14
After intubation and mechanical ventilator support, the pediatric neurosurgical team and surgical nursing team entered the operating room with their proper PPE in place and the attending pediatric neurosurgeon present for the entire duration of the critical portion of the case to minimize the operative time. To minimize airborne transmission, the entrance of the surgical team was staggered until after intubation. In addition to the attending pediatric neurosurgeon and anesthesiologist, the other individuals present in the operating room included a neurosurgery resident or fellow, an anesthesiology resident or fellow or a certified registered nurse anesthetist, a nurse, and a scrub technician.
The surgical team also wore disposable skull caps, eye protection, and an N95 mask or a PAPR or controlled air-purifying respirator, scrubs, surgical gowns, disposable gloves, and disposable shoe covers. The pediatric neurosurgical team did not leave the room until the case was completed. All procedures were performed in negative-pressure operating rooms. To prevent aerosol generation, the use of aerosol-generating instruments, including electrocautery, powered drills, insufflators, and lasers, was limited, if possible. Nonpower tools were used to create burr holes. A smoke evacuator was used in conjunction with Bovie electrocautery.
All equipment and instruments normally kept in the operating rooms were removed, and only the equipment and instruments required for the ongoing case were stocked (including sutures). A staff of nurses waited outside the operating room and used walkie-talkies to decrease door opening and closing, with 1 nurse designated to obtain new equipment or instruments when needed. Our pediatric neurosurgical protocol was consistent with specialized protocols used in Wuhan City, China, and other institutions.16 , 21 , 22
Postoperative PICU Course
On exiting the operating room, all outer clothing of the operating room staff was properly discarded, and the room, including the anesthesia machine, underwent a thorough disinfection and sterilization. To minimize contamination, the multidisciplinary team changed into new scrubs after each COVID-19 case. The COVID-19–infected pediatric patient was transported back to the negative pressure specialized COVID-19 unit in our PICU through an isolated and restricted area by anesthesia and neurosurgical personnel wearing PPE. All PICU personnel specially trained for COVID-19 and wearing proper PPE cared for these children. In addition to the PICU and neurosurgery personnel, the pediatric infectious disease and infection control personnel were closely involved to help monitor the patient and minimize exposure risk. The visitor policies limited patient visitors to only 2 parents, with no siblings allowed. None of the healthcare personnel involved had become infected with COVID-19.
Clinical Presentations and Outcomes
During the study period, 16 neurosurgical operations or procedures were performed in 8 patients. The most common procedure was CSF diversion (external ventricular drain placement for 5 and shunt revision or placement for 4). The next most common surgery was revision of a wound because of CSF leakage (n = 3; Table 2). All the patients, except for 1, had been immediately extubated in the operating room and remained at their neurological baseline. One patient had required prolonged intubation and mechanical ventilation owing to a poor neurological status secondary to intracranial hemorrhage and was extubated without difficulty once her mental status had improved.
Case Report
Patient 1
A 7-month-old male infant with a history of occipital encephalocele resection and hydrocephalus after endoscopic third ventriculostomy (ETV) had presented with a persistent pseudomeningocele at the ETV site with CSF leakage. Head computed tomography (CT) showed a hygroma and persistently enlarged ventricles. He had tested positive for SARS-CoV-2 at the routine admission screening but was asymptomatic. He underwent wound revision of the ETV incision. Four days later, he required placement of an externalized ventriculostomy drain (EVD) because of repeat CSF leakage from the ETV incision. He subsequently underwent placement of a ventriculoperitoneal shunt (VPS). However, CSF cultures obtained during this operation were positive for Streptococcus agalactiae. Consequently, he was returned to the operating room for VPS removal, EVD placement, and a second wound revision of the ETV incision. Overall, he had required intubation and extubation 6 times (including anesthesia for imaging studies) and had not experienced any viral symptoms or neurological sequelae during his month-long hospitalization.
Patient 2
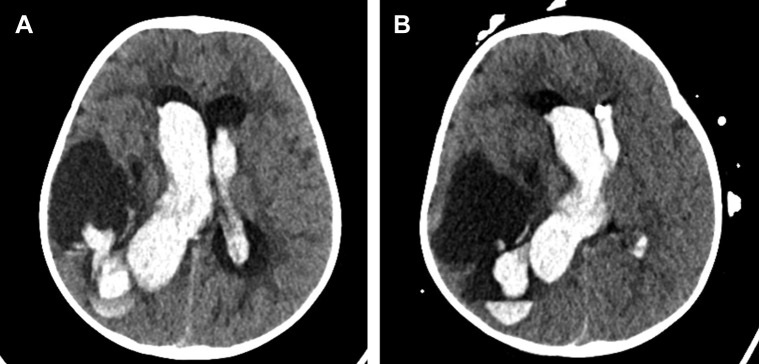
A 10-month-old female infant with a history of a Spetzler-Martin grade 5 thalamic arteriovenous malformation (AVM) after rupture and coiling of an associated anterior choroidal intranidal aneurysm 3 months previously had experienced acute onset of emesis, lethargy, and seizure without any preceding fever or other symptoms of a viral illness. Head CT revealed a large right parietotemporal intraparenchymal hemorrhage, midline shift, and bilateral intraventricular hemorrhage (Figure 1A ). She had tested positive for SARS-CoV-2 incidentally at admission. The patient underwent emergent left frontal EVD placement and endovascular coiling of an intranidal posterior cerebral artery aneurysm. Two days later, she had required placement of a ventricular drain into an enlarging cystic encephalomalacia cavity that had resulted from her first intraparenchymal hemorrhage (Figure 1B). She continued to require intubation and mechanical ventilation for 14 days after her admission owing to her poor neurological status. She was extubated successfully after her mental status had improved. These ongoing neurological symptoms were not attributed to the SAR-CoV-2 infection. On hospital day 20, she underwent exchange of the EVDs and placement of a new EVD. On hospital day 30, she underwent bilateral VPS placement because of EVD weaning failure and a lack of communication between the right temporal cyst and lateral ventricles. During the last 2 operations, she had been successfully extubated in the operating room. She had not experienced any viral symptoms during her hospitalization and was discharged to inpatient rehabilitation 2 months after admission.
Figure 1.
Patient 2. (A) Axial noncontrast-enhanced head computed tomography scan showing a large right parietotemporal intraparenchymal hemorrhage and bilateral intraventricular hemorrhage. (B) Axial noncontrast-enhanced computed tomography scan after left frontal external ventricular drain placement showing an increasing right temporal cystic cavity causing an increased midline shift, which required additional drain placement.
Patient 3
A 15-month-old boy with a history of ventriculoatrial (VA) shunt placement for posthemorrhagic hydrocephalus, prematurity at birth, and prior jejunal perforation with ileostomy had presented with symptoms of vomiting and obtundation. He had had a fever for 2 days before admission with recent exposure to a COVID-19–positive contact and had tested positive for SARS-CoV-2 on the day of admission. Head CT revealed a ventricular size that was increased from baseline, and owing to the bradycardia and obtundation, he emergently underwent VA shunt removal (for distal malfunction) and EVD placement. Owing to aspiration pneumonitis from intubation and respiratory symptoms from COVID-19, he had developed tachypnea and a desaturation episode to 72% on pulse oximetry after extubation and required noninvasive bilevel positive airway pressure ventilation in the PICU. A chest radiograph demonstrated atelectasis superimposed on chronic lung disease of prematurity. Two days later, a new VA shunt was placed. He was discharged on postoperative day 2 after VA shunt placement but had presented again with fever on postoperative day 8. All workup, including CSF, was negative, and he was eventually discharged home without further neurological or respiratory sequelae.
Patient 4
A 6-year-old boy with no significant medical history had presented with acute neck pain, headache, vomiting, and lethargy. Head CT revealed a left frontal intraparenchymal hemorrhage concerning for AVM rupture. He had tested positive for SARS-CoV-2 incidentally at admission. Cerebral angiogram showed an AVM of the terminal middle cerebral artery branch with an intranidal aneurysm, and he emergently underwent onyx embolization of the aneurysm and AVM nidus. The next day, left frontal craniotomy was performed for AVM and intranidal aneurysm resection and hematoma evacuation. The patient was discharged on postoperative day 5 with no respiratory symptoms, fever, or neurological sequelae.
Patient 5
An 11-year-old girl with a history of midbrain and pontine pilocytic astrocytoma after craniotomy for tumor debulking and ETV had presented with CSF leakage from her ETV incision. She had had no symptoms of SARS-CoV-2 infection on admission but tested positive by PCR. She was not receiving chemotherapy. She was admitted to the PICU for neurological monitoring and subsequently developed a fever on hospital day 0. A lumbar puncture was performed, which demonstrated an elevated white blood cell count, consistent with possible meningitis. Staphylococcus epidermidis was identified on culture, which was ultimately determined to be a contaminant. Broad-spectrum antibiotics were administered, and she was taken to the operating room for wound washout and closure of the leaking incision. The patient was discharged on postoperative day 6 with no respiratory symptoms, ongoing fever, or neurological sequelae.
Patient 6
A 16-year-old boy with a history of a VPS placed at age 2 for congenital hydrocephalus with the last revision at age 11 had developed swelling and pain in his right neck along the course of the VPS. Radiographic imaging revealed a 1.7-cm disconnection of the VPS in the lower neck. He had no symptoms of SARS-CoV-2 infection on admission but tested positive by PCR. The patient subsequently underwent distal shunt revision and was discharged to home on postoperative day 1 with no respiratory symptoms, fever, or neurologic sequelae.
Patient 7
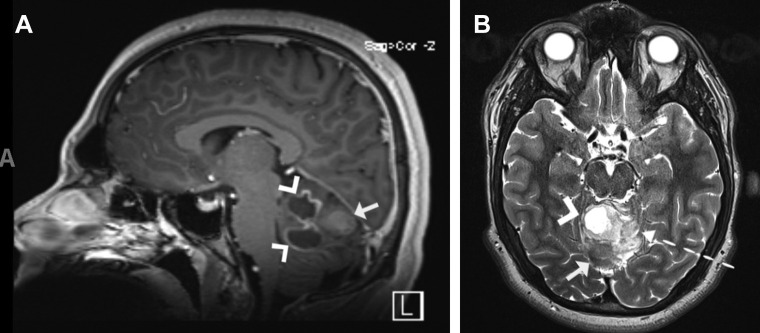
A 17-year-old boy with no significant medical history had presented with headache, blurry vision, vomiting, and intermittent left leg weakness. Admission magnetic resonance imaging revealed a large vermian brain tumor with sites of intratumoral hemorrhage (Figure 2 ) without hydrocephalus. He had no symptoms of SARS-CoV-2 infection on admission but tested positive by PCR. The patient underwent suboccipital craniotomy for tumor resection. Postoperatively, the patient remained neurologically stable and developed no respiratory symptoms or fever. He was discharged home on postoperative day 3 with improvement in his neurologic symptoms.
Figure 2.
Patient 7. (A) Sagittal T1-weighted contrast-enhanced brain magnetic resonance imaging scan showing a large vermian mass with heterogeneous contrast enhancement (regular arrow) and cystic components (arrowhead) without hydrocephalus. (B) Axial T2-weighted brain magnetic resonance imaging scan showing hyperintense components (dotted arrow), cystic components (arrowhead), and an isointense signal (regular arrow) consistent with hemorrhage.
Patient 8
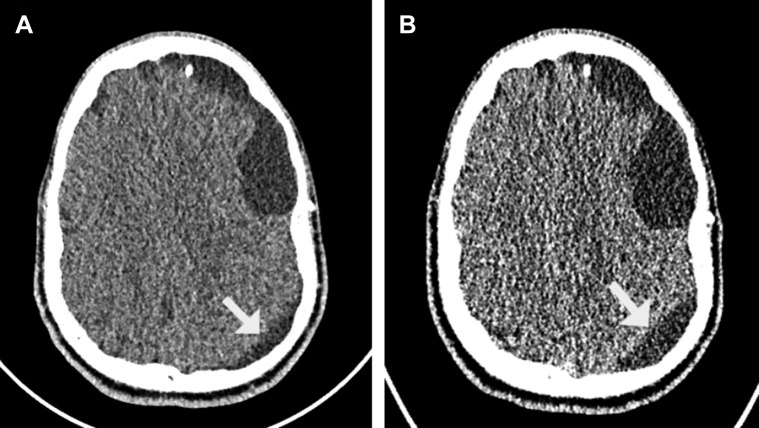
An 18-year-old man with a history of a subdural–peritoneal shunt placed 1 year prior for a subdural hygroma due to a ruptured arachnoid cyst had presented with an acute onset of headaches, blurry vision, and gait instability. He also had mild upper respiratory infection symptoms, including rhinorrhea and cough. Admission head CT revealed enlargement of the shunted subdural hygroma compared with a baseline head CT (Figure 3 ). He underwent proximal shunt revision and valve exchange because of a proximal catheter and valve malfunction. The patient remained neurologically unchanged throughout the postoperative course. On postoperative day 0, he had experienced headaches and several episodes of hypoxia with oxygen saturation percentages as low as the 40s within the first 12 hours after surgery, all of which had self-resolved without intervention. The findings from a chest radiograph were reassuring, and these episodes did not recur after the first postoperative day. However, he continued to experience headaches through postoperative day 3. A repeat head CT showed further reduction of the subdural hygroma, suggesting that the headaches might have been related to the viral syndrome instead of shunt malfunction, highlighting the complexity of postoperative management of SARS-CoV-2–infected patients. He was discharged on postoperative day 3 with follow-up resolution of the headache.
Figure 3.
Patient 8. (A) Axial head computed tomography scan showing baseline findings of left subdural hygroma collection (regular arrow) and associated arachnoid cyst after subdural peritoneal shunt placement without shunt malfunction. (B) Preoperative axial head computed tomography scan after shunt malfunction showing larger left subdural hygroma (regular arrow) collection.
Discussion
Worldwide, >125 million worldwide cases of COVID-19 have occurred, and our knowledge of the disease has continuously evolved.23 However, since originally discovered, most reports have been focused on symptomatic adults. Much uncertainty still remains regarding the anesthetic and inflammatory response in infected children who might require emergent or urgent surgical procedures. These concerns are even more important because, as of October 2021, young children are not vaccine candidates and new variant strains of SARS-CoV-2 have begun to emerge. To the best of our knowledge, we have reported the largest known series of pediatric patients with known SARS-CoV-2 infection who have undergone anesthesia and urgent neurosurgical interventions. All of the pediatric patients tolerated the anesthesia and neurosurgery operation well without worsening sequelae, and no patient experienced significant worsening of COVID-19–related viral symptoms requiring COVID-19–directed therapies, aside from supportive care.
One recent study demonstrated that pediatric patients with nonsevere COVID-19 had higher rates of perianesthetic respiratory complications such as laryngospasm, bronchospasm, hypoxemia, or postoperative supplemental oxygen requirements compared with uninfected patients but not an increased risk of nonrespiratory complications or mortality.24 Although none of the patients in our series had developed laryngospasm or bronchospasm, 1 patient had had transient postoperative hypoxia attributable to SARS-CoV-2, 1 patient had experienced acute respiratory failure requiring noninvasive ventilation attributable to SARS-CoV-2 infection superimposed on a history of bronchopulmonary dysplasia, and 1 patient had remained intubated and mechanically ventilated for a prolonged period owing to neurologic concerns unrelated to SARS-CoV-2 infection. None of the patients in the present case series died.
A recent report of 4 pediatric patients with MIS-C described meningismus symptoms and intracranial hypertension based on an elevated opening pressure by lumbar puncture. None of the 4 patients had required neurosurgical intervention, and all were successfully treated with pharmacological methods.12 None of our cohort had met the criteria for MIS-C. This population warrants specific study, given that they will experience a more severe clinical spectrum, including a higher mortality rate, a greater prevalence of cardiovascular dysfunction (including shock and myocardial dysfunction) and respiratory symptoms such as hypoxemia and pneumonia, and, of relevance to pediatric neurosurgeons, a greater prevalence of neurologic sequelae.11 , 25 , 26 It is clear that further clinical observations of pediatric anesthesia and urgent neurosurgical interventions for children across the spectrum of clinical manifestations of SARS-CoV-2, including acute symptomatic or asymptomatic infection and MIS-C, will be of paramount importance.
Few studies have reported on pediatric COVID-19 patients needing neurosurgical intervention. One case has been reported of a pediatric COVID-19–positive patient who had undergone shunt revision.17 An 8-month-old male infant in Italy with complex hydrocephalus had presented with a mild temperature, dry cough, and occipital CSF collection.17 A head CT scan indicated shunt disconnection, and the nasopharyngeal swab was positive for SARS-CoV-2. He underwent shunt revision under general anesthesia without respiratory complications and was promptly extubated, and the neurosurgical course was routine. That report has highlighted the feasibility and safety of performing urgent neurosurgical procedures in pediatric patients with minimal or no COVID-19–related symptoms.17 Our case series of 8 children who had undergone general anesthesia and urgent neurosurgical interventions builds on their case report and has demonstrated that despite SARS-CoV-2 infection, the care of patients in the PICU and operating room, the timing of anesthetic and operative intervention, and postoperative PICU care were able to proceed without delay or adverse sequelae to the children. None of the healthcare personnel involved had become infected with COVID-19.
One of the limitations of our study, in addition to the small number of patients, was that most patients were asymptomatic with respect to COVID-19 infection. Thus, it is unclear whether a greater prevalence of respiratory or other clinical complications would have been observed if the cohort had been larger or had had more severe symptoms of COVID-19. However, because many children with SARS-CoV-2 will be asymptomatic, our cohort reflects the real-world practice likely to be encountered by pediatric anesthesiologists and neurosurgeons. Although further studies are clearly needed, our experience supports that urgent pediatric neurosurgical operations should not be delayed because our growing familiarity with PPE and other safe practices has facilitated the care of these patients, even during urgent operations.
Conclusions
The relative successes of our minimally symptomatic infected pediatric patients in the present small case series support the relative resiliency to severe manifestations of COVID-19 in children, despite the stresses of general anesthesia and major neurosurgery. As our knowledge and understanding of the clinical manifestations of COVID-19 in the pediatric population expand, we hope to be able to determine more accurately the risk factors for poor outcomes among pediatric neurosurgery patients and the optimal protocols for perioperative management. We hope that our report will help guide clinical care and help determine the optimal anesthetic and perioperative guidelines for COVID-19–infected pediatric patients who require urgent neurosurgical treatment.
CRediT authorship contribution statement
Shih-Shan Lang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision. Avi A. Gajjar: Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Alexander M. Tucker: Validation, Writing – review & editing. Phillip B. Storm: Validation, Writing – review & editing. Raphia K. Rahman: Validation, Writing – review & editing. Peter J. Madsen: Validation, Writing – review & editing. Aidan O'Brien: Methodology, Validation, Formal analysis, Investigation, Writing – original draft. Kathleen Chiotos: Validation, Writing – review & editing. Todd J. Kilbaugh: Validation, Writing – review & editing. Jimmy W. Huh: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Shih-Shan Lang and Avi A. Gajjar contributed equally to the present report.
References
- 1.Berlin D.A., Gulick R.M., Martinez F.J. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 2.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Demographic Trends of COVID-19 Cases and Deaths in the US Reported to CDC. https://covid.cdc.gov/covid-data-tracker Available at: Accessed October 19, 2021.
- 4.Alnajjar A.A., Dohain A.M., Abdelmohsen G.A., Alahmadi T.S., Zaher Z.F., Abdelgalil A.A. Clinical characteristics and outcomes of children with COVID-19 in Saudi Arabia. Saudi Med J. 2021;42:391–398. doi: 10.15537/smj.2021.42.4.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 6.Leidman E., Duca L.M., Omura J.D., Proia K., Stephens J.W., Sauber-Schatz E.K. COVID-19 trends among persons Aged 0-24 years—United States, March 1-December 12, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:88–94. doi: 10.15585/mmwr.mm7003e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics Children and COVID-19: State-Level Data Report. http://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ Available at: Accessed October 19, 2021.
- 8.Alsohime F., Temsah M.-H., Al-Nemri A.M., Somily A.M., Al-Subaie S. COVID-19 infection prevalence in pediatric population: etiology, clinical presentation, and outcome. J Infect Public Health. 2020;13:1791–1796. doi: 10.1016/j.jiph.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beslow L.A., Linds A.B., Fox C.K., et al. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2021;89:657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 10.Boronat S. Neurologic care of COVID-19 in children. Front Neurol. 2020;11:613832. doi: 10.3389/fneur.2020.613832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein L.R., Rose E.B., Horwitz S.M., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker A.E., Chiotos K., McGuire J.L., Bruins B.B., Alcamo A.M. Intracranial hypertension in multisystem inflammatory syndrome in children. J Pediatr. 2021;233:263–267. doi: 10.1016/j.jpeds.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soneru C.N., Nunez K., Petersen T.R., Lock R. Anesthetic concerns for pediatric patients in the era of COVID-19. Pediatr Anesth. 2020;30:737–742. doi: 10.1111/pan.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matava C.T., Kovatsis P.G., Lee J.K., et al. Pediatric airway management in COVID-19 patients: consensus guidelines from the Society for Pediatric Anesthesia’s pediatric difficult intubation collaborative and the Canadian Pediatric Anesthesia Society. Anesth Analg. 2020;131:61–73. doi: 10.1213/ANE.0000000000004872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed L.K., Wen J., Liang B., Wang X., Feng D., Huang J.H. Safely performing neurosurgical procedures during COVID-19 pandemic. Neurol Res. 2020;42:811–817. doi: 10.1080/01616412.2020.1781455. [DOI] [PubMed] [Google Scholar]
- 17.Carrabba G., Tariciotti L., Guez S., Calderini E., Locatelli M. Neurosurgery in an infant with COVID-19. Lancet. 2020;395:e76. doi: 10.1016/S0140-6736(20)30927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballestero M.F.M., Furlanetti L., de Oliveira R.S. Pediatric neurosurgery during the COVID-19 pandemic: update and recommendations from the Brazilian Society of Pediatric Neurosurgery. Neurosurg Focus. 2020;49:E2. doi: 10.3171/2020.9.FOCUS20703. [DOI] [PubMed] [Google Scholar]
- 19.Ceraudo M., Balestrino A., Cama A., Macrina G., Piatelli G., Consales A. Pediatric neurosurgery after the COVID-19 pandemic: management strategies from a single pediatric hospital in Italy. World Neurosurg. 2021;146:e1079–e1082. doi: 10.1016/j.wneu.2020.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos De Oliveira R., Ballestero M.F.M. The COVID-19 outbreak and pediatric neurosurgery guidelines. Arch Pediatr Neurosurg. http://www.archpedneurosurg.com.br/pkp/index.php/sbnped2019/article/view/26 Available at: Accessed October 19, 2021.
- 21.Tan Y.-T., Wang J.-W., Zhao K., et al. Preliminary recommendations for surgical practice of neurosurgery department in the central epidemic area of 2019 coronavirus infection. Curr Med Sci. 2020;40:281–284. doi: 10.1007/s11596-020-2173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iorio-Morin C., Hodaie M., Sarica C., et al. Letter: the risk of COVID-19 infection during neurosurgical procedures: a review of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) modes of transmission and proposed neurosurgery-specific measures for mitigation. Neurosurgery. 2020;87:E178–E185. doi: 10.1093/neuros/nyaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johns Hopkins University and Medicine Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html Available at: Accessed May 1, 2021.
- 24.Saynhalath R., Alex G., Efune P.N., Szmuk P., Zhu H., Sanford E.L. Anesthetic complications associated with SARS-CoV-2 in pediatric patients. Anesth Analg. 2021;133:483–490. doi: 10.1213/ANE.0000000000005606. [DOI] [PubMed] [Google Scholar]
- 25.Pereira M.F.B., Litvinov N., Farhat S.C.L., et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics (Sao Paulo) 2020;75:e2209. doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaRovere K.L., Riggs B.J., Poussaint T.Y., et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]