Abstract
The mechanistic target of rapamycin (mTOR) pathway integrates metabolic cues into cell fate decisions. A particularly fateful event during the adaptive immune response is the engagement of a T cell receptor by its cognate antigen presented by an antigen-presenting cell (APC). Here, the induction of adequate T cell activation and lineage specification is critical to mount protective immunity; at the same time, inadequate activation, which could lead to autoimmunity, must be avoided. mTOR forms highly conserved protein complexes 1 and 2 that shape lineage specification by integrating signals originating from TCR engagement, co-stimulatory or co-inhibitory receptors and cytokines and availability of nutrients. If one considers autoimmunity as the result of aberrant lineage specification in response to such signals, the importance of this pathway becomes evident; this provides the conceptual basis for mTOR inhibition in the treatment of systemic autoimmunity, such as systemic lupus erythematosus (SLE). Clinical trials in SLE patients have provided preliminary evidence that mTOR blockade by sirolimus (rapamycin) can reverse pro-inflammatory lineage skewing, including the expansion of Th17 and double-negative T cells and plasma cells and the contraction of regulatory T cells. Moreover, sirolimus has shown promising efficacy in the treatment of refractory idiopathic multicentric Castleman disease, newly characterized by systemic autoimmunity due to mTOR overactivation. Alternatively, mTOR blockade enhances responsiveness to vaccination and reduces infections by influenza virus in healthy elderly subjects. Such seemingly contradictory findings highlight the importance to further evaluate the clinical effects of mTOR manipulation, including its potential role in treatment of COVID-19 infection. mTOR blockade may extend healthy lifespan by abrogating inflammation induced by viral infections and autoimmunity.
This review provides a mechanistic assessment of mTOR pathway activation in lineage specification within the adaptive and innate immune systems and its role in health and autoimmunity. We then discuss some of the recent experimental and clinical discoveries implicating mTOR in viral pathogensis and aging.
Keywords: Mechanistic target of rapamycin, mTOR, Sirolimus, Immune cell lineage specification, Autoimmunity, Systemic lupus erythematosus, Antiviral immunity, Lifespan extension;
Abbreviations: 4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; 4E-BP2, eukaryotic translation initiation factor 4E-binding protein 2; AMPK, AMP-activated protein kinase; ANA, antinuclear antibodies; APC, antigen-presenting cell; CCR7, CC-chemokine receptor 7; COVID-19, coronavirus disease 2019; DAMP, damage-associated molecular pattern; DC, dendritic cell; DEPTOR, DEP domain-containing mTOR-interacting protein; EAE, experimental autoimmune encephalomyelitis; EBV, Epstein-Barr virus; eiF4E, Elongation initiation factor 4E; ETV7, transcription factor ETV7; FAO, fatty acid oxidation; FKBP8, FKBP prolyl isomerase 8; G6PD, glucose-6-phosphate-dehydrogenase; HHV-8, human herpesvirus-8; IKK-β, inhibitor of nuclear factor kappa-B kinase subunit beta; iMCD, idiopathic multicentric Castleman disease; IFN-γ, interferon gamma; KSHV, Kaposi's sarcoma-associated herpesvirus; Lc3b, microtubule-associated protein 1 light chain 3β; MHC, Major histocompatibility complex; mLST8, mammalian lethal with SEC13 protein 8; MOG, Myelin oligodendrocyte glycoprotein; MSU, monosodium urate; mTOR, mechanistic target of rapamycin; mTORC, mTOR complex; NAC, N-Acetylcysteine; PD-1, programmed cell death protein 1; p70S6K, P70-S6 kinase; PI3K, phosphoinositide 3-kinase; PRAS40, proline-rich Akt substrate of 40 kDa; PRR5, Proline-rich protein 5; RA, rheumatoid arthritis; Rapa, rapamycin; Raptor, regulatory-associated protein of mTOR; Rheb, ras homolog enriched in brain; Rictor, rapamycin-insensitive companion of mTOR; SIN1, Stress-activated map kinase-interacting protein 1; SLE, systemic lupus erythematosus; S1P1, sphingosine-1-phosphate receptor 1; TLR, Toll-like receptor; ULK1, Unc-51 like autophagy activating kinase; TCR, T cell receptor; TH, T helper cell; TNF, tumor necrosis factor; Treg, regulatory T cell; TSC, tuberous sclerosis complex
1. Structure and evolution of the mTOR pathway
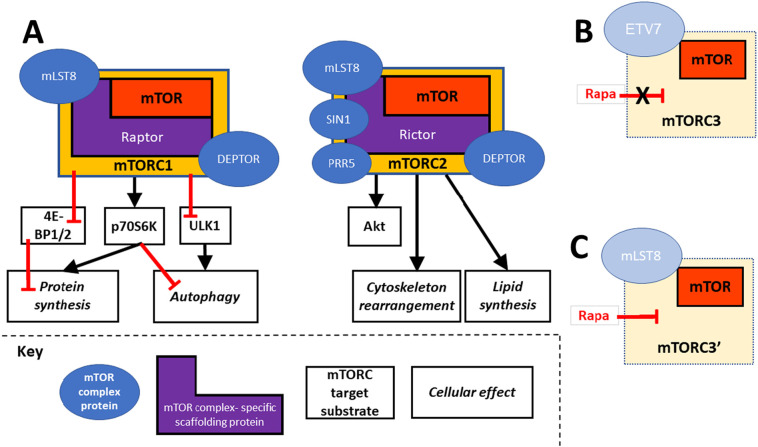
mTOR, a serine/threonine kinase [[1], [2], [3]], executes its cell control functions by forming at least two complexes — mTOR complex 1 and 2 [4,5]. Both complexes control specific functions and share mTOR ‘companion’ proteins but can be distinguished by differential participation of two scaffolding proteins, Raptor and Rictor (see Fig. 1A); additional mTOR complexes (Fig. 1B and C) have been postulated [6,7].
Fig. 1.
mTOR complexes. A) mTOR complexes 1 and 2 are defined by their scaffolding proteins Raptor and Rictor, respectively. mTORC1 is anabolic, via its substrates 4E-BP 1 and 2, p70S6K and ULK1. mTORC2 activation phosphorylates Akt, which in turn reinforces activation of mTORC 1 and 2. B) A proposed mTORC3 assembles independent of Raptor and Rictor, is resistant to rapamycin and associates with ETV7. C) An alternatively proposed third rapamycin-sensitive complex that contains mLST8, here designated mTORC3’, has also been reported. Rapa: Rapamycin.
1.1. Activated mTORC1 increases protein synthesis and inhibits autophagy
mTORC1 formation requires the participation of the mTORC1 specific scaffolding protein Raptor [8,9]. mTORC1 activates anabolic pathways such as protein synthesis and blocks catabolic pathways such as autophagy [10]. mTORC1 substrates that mediate these effects include the translational repressors 4E-BP1 [11] and 2 [12] and the kinase p70S6K [11]: Phosphorylation of 4E-BP1/2 ‘unleashes’ mRNA translation by releasing its inhibition of eiF4E; the phosphorylation of p70S6K ultimately activates the ribosomal machinery to increase protein synthesis. Phosphorylation of ULK1 mediates the inhibition of autophagy by activated mTORC1 [13].
1.2. mTORC2 can enhance mTORC1 activation by phosphorylating Akt
The assembly of mTORC2 requires the presence of Rictor, an alternative scaffolding protein specific for mTORC2 [14]. mTORC2 phosphorylates several AGC family kinases [15], including Akt, leading to Akt activation [16]. Thus, via Akt, activated mTORC2 indirectly promotes the activation of mTORC1.
Additional effects of mTORC2 activation include cytoskeleton rearrangement via actin polymerization [17], relevant for cellular activation and migration, and increased lipid synthesis, which can promote tumorigenesis [18].
1.3. The TSC complex is a key node that controls mTORC1
Rheb, a small GTPase, can activate mTORC1 (via antagonism of its endogenous inhibitor FKBP8, also known as FKBP38) [19]. Rheb activity is typically suppressed via the TSC complex of TSC1/TSC2 [20] but can be modulated depending on the specific inputs. Important sensors feeding into the TSC complex include AMPK, sensing energy ‘starvation’ as well as inhibitory signals originating from TNF (via IKK-β mediated suppression of TSC1 [21,22]) and Insulin/PI3K signaling via Akt/TSC2 [23,24]).
Thus, the TSC complex serves as a node that integrates growth signals with energy availability and controls mTORC1 by either inhibiting or releasing Rheb based on these upstream signals. In summary, the TSC complex can resolve potentially conflicting growth signals and low energy states to ensure context-appropriate mTORC1 activation.
1.4. Regulating mTORC1 localization via the Ragulator/Rag complex provides additional opportunities to control its activation
The spatial control of mTORC1 and its regulators provides an additional control mechanism of mTORC1. To effectively undergo activation by Rheb (which is anchored to the membranes of lysosome and Golgi apparatus [25]), mTORC1 must translocate to these membranes. Ragulator and Rag GTPases form a complex that senses nutrients [26]; based on nutrient sufficiency the Ragulator/Rag complex either permits or inhibits mTORC1 translocation [27].
In combination, the TSC and Ragulator/Rag complexes help ensure that the anabolic cellular programs resulting from activation of mTORC1 are only executed when both growth signals (via the TSC complex) and sufficient substrate availability (via Ragulator/Rag GTPases) can sustain cellular growth or proliferation.
1.5. The mTOR pathway integrates signals in immune cells yet originated in unicellular life
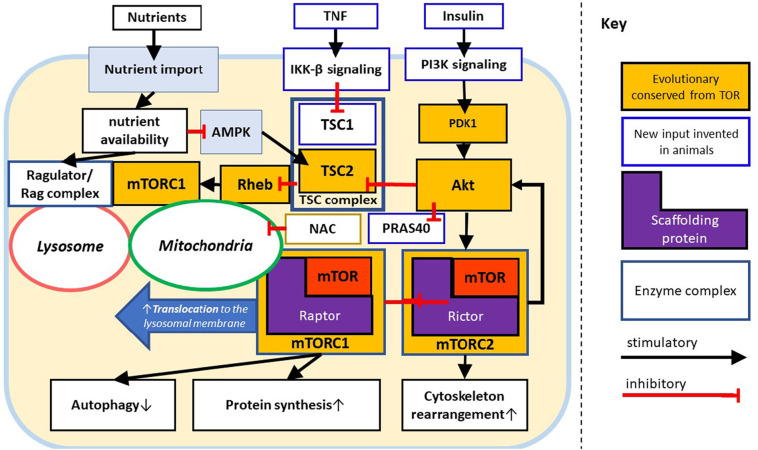
Basic components of mTOR signaling (Fig. 2 , orange components) are preserved almost universally across eukaryotic life [28]. Immune cells can utilize mTOR to integrate cues during the contact with antigens to determine adequate lineage. This function is in part enabled by evolutionary newer inputs into the mTOR pathway such as the TNF and the Insulin signaling (Fig. 2, light blue components) highlighting the flexibility of this ancient pathway to provide integration necessary for cell fate decisions in a variety of contexts [28].
Fig. 2.
mTOR signaling. The evolutionary conserved core of mTOR complexes 1 and 2 activated via PDK1, Akt, TSC2, and Rheb. In humans and other mammals, nutrients, insulin and TNF signaling pathways (light blue) provide additional upstream inputs. The TNF pathway is integrated with the starvation sensor AMPK via the TSC complex (bold dark blue); insulin via the PI3K/PDK1/Akt axis. The Ragulator/Rag complex provides spatial control over mTORC1 that is dependent on mitochondrial oxidative stress, nutrient sufficiency and traffic to the lysosome. Additional innovations in animals include PRAS40 [7,23] through which Akt provides a disinhibitory signal to Raptor, thus favoring assembly of mTORC1, which provides an additional regulatory axis for mTORC1 activation.
2. A model of the role of mTOR during immune responses
In the context of the immune response, the mTOR pathway integrates cues that arise during the contact with antigens to determine T cell lineage. The relative activity of mTORC1 and 2 specifies resulting lineages of CD4+ [29] and CD8+ naïve T cells [30]. Highlighting the importance for adequate balance between the mTOR complexes is that overactivation of mTORC1 can lead to autoimmunity, including the systemic autoimmune disease SLE (reviewed in [31]).
2.1. mTOR pathway and TLR integrate cues surrounding an antigen
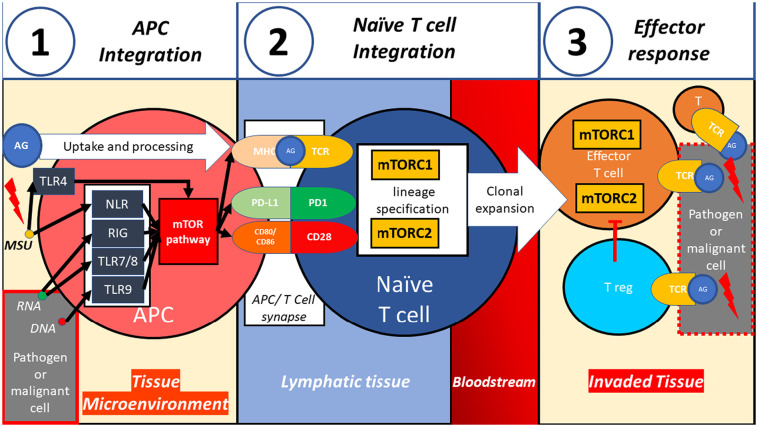
Upon contact between an antigen-presenting cell (APC) and an antigen, the antigenic signal is contextualized (Fig. 3 .1). Local signals provide cues to categorize the antigen in terms of its threat potential. Damage and pathogen-associated molecular patterns serve as cues, which are sensed via pattern recognition receptors such as TLR [32]. TLR can directly activate autophagy in APC [33,34] which overall enhances antigen uptake and presentation [[35], [36], [37]]. Alternatively, TLR can induce mTORC1 in APC [38], which attenuates autophagy [10].
Fig. 3.
A possible model how the mTOR pathway can integrate signals to achieve adequate lineage specification during immune responses. 1) Concomitantly with antigen uptake and processing, the mTOR pathway integrates cues received by pattern recognition receptors, such as DNA, RNA and monosodium urate to contextualize the antigenic signal. 2) The antigenic signal, along with co-stimulatory and inhibitory signals, is transduced and integrated via relative activity of mTORC1 and 2.3) Effector T cells, resulting from clonal expansion of adequate T cell lineages are guided by mTORC1 and 2 activation, execute the appropriate immune response to neutralize the pathogen or malignant cell.(Many additional important immune cell populations, not depicted here, participate in immune responses) AG: antigen, TCR: T cell receptor, MSU: monosodium urate.
Interestingly, mTOR/mTORC1 blockade leads to increased expression of CCR7 in human monocytes and dendritic cells [39] which can facilitate migration to lymphatic tissues to provide antigenic stimulation to T cells. Thus, at least in some contexts, mTOR blockade, via enhanced antigen presentation to T cells could potentially confer enhanced protection against viral and other infections.
2.2. T cells integrate antigen-specific and metabolic cues via mTOR
Following successful migration to lymphatic tissues, the APC can form a synapse with a naïve T cell [40,41]. Here, the antigenic signal is transduced along with co-stimulatory and co-inhibitory signals (reviewed in [42]) and crosstalk with the JAK-STAT signaling pathways [43]. Appropriate APC signals can switch naïve T cell metabolism from a catabolic (reviewed in [44]) to an anabolic profile, characterized by mTOR/mTORC1 activation [45].
The totality of signals transduced by the APC can lead to differential activation of mTORC1 and 2 [29] within T cells to induce lineage defining master transcription factors (such as T-bet for TH1 [46]), thus shaping T cell differentiation (Fig. 3.2).
2.3. mTOR complexes exert control over T effector functions beyond antigen presentation and lineage specification
mTOR complex activity continues to influence the function of differentiated effector cells. (Fig. 3.3) For example, while low levels of mTORC1 and mTORC2 activity favor T reg differentiation [29,47], mTOR deficient T cells show lineage instability, defined as a loss of the transcription factor FoxP3 [48].
In summary, mTOR and associated proteins have a profound impact along the key events from antigen sensing to fine-tuning effector functions to ensure appropriate inflammatory responses, explaining how dysregulation of mTOR can lead to the inappropriate immune responses that characterize SLE and other systemic autoimmune diseases.
3. mTOR-dependent lineage differentiation controls inflammation
This segment highlights results during the past four years concerning the immunological outcome of mTOR blockade in humans followed by some important advances on the understanding how mTOR and related proteins can modulate immune cell function.
3.1. New insights into clinical effects of mTOR inhibition
3.1.1. mTOR blockade can normalize T cell memory populations in SLE
The benefit of mTOR inhibition for the treatment of the prototypical autoimmune disease SLE [49] occurs primarily by a normalizing effect on lymphocyte subpopulations [49], resulting in a net anti-inflammatory and tolerogenic effect that is mediated at least in part by inhibition of apoptosis of regulatory T cells and decreases in T and B cell activation in SLE patients [49]. Memory T cell subsets on the other hand, expanded in SLE patients following mTOR blockade with sirolimus (rapamycin) which suggests that in SLE patients, sirolimus treatment may confer enhanced T cell mediated immunity to mTOR/mTORC1 blockade treated individuals providing another potential axis through which mTOR blockade may enhance immune function.
3.1.2. mTOR blockade is an effective treatment for idiopathic multicentric Castleman disease
New data supports mTOR/mTORC1 inhibition as an effective treatment for the autoimmune disease idiopathic multicentric Castleman disease (iMCD), a lymphoproliferative disease with autoimmune features. iMCD is associated with increased mTORC1 activation as measured in both the serum proteome as well as the lymphatic tissue and, more importantly, iMCD patients refractory to the IL-6 antagonist tocilizumab, responded to the mTOR inhibitor sirolimus (rapamycin) [50,51] thus highlighting the efficacy of inhibiting this pathway in autoimmune diseases including in patients otherwise refractory to IL-6 inhibition.
3.1.3. Short term mTORC1 inhibition can be immunostimulatory
Interestingly, although mTOR inhibition is generally regarded to be immunosuppressive, short term (6 week) low-dose mTORC1 inhibition using a combination of the allosteric and catalytic mTOR inhibitors daclotisib and everolimus reduced infection and enhanced antiviral vaccination responses in healthy elderly individuals [52]. Following mTORC1 inhibition, an upregulation of interferon related genes, as well as a decrease of CD4 and CD8 positive T cell subsets expressing the co-inhibitory receptor PD-1 occurred, along with an increase of the interferon signature which may be underlying the protective effect of low-dose mTORC1 blockade. [52].
3.1.4. Discussion of clinical findings
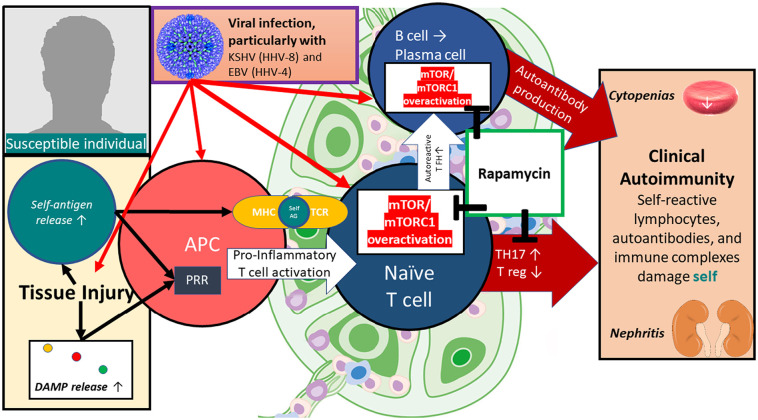
MCD and SLE share many clinical and pathophysiologic features and are unified by profound mTOR overactivation in lymphocytes. Following viral infection with KSHV (HHV-8), some individuals develop MCD, characterized by extensive polyclonal lymphoproliferation and systemic autoimmunity. 50% of MCD cases are negative for HHV-8 — referred to as idiopathic MCD — and additional viral triggers including other human herpesviridae are hypothesized to drive iMCD at least in a subset of cases. Because the majority of herpesvirus-infected individuals do not develop iMCD or SLE, other co-existing environmental or genetic hits must occur, either directly on lymphocytes or mediated via aberrant antigen presentation. Importantly, sirolimus (rapamycin) can prevent pathologic lymphocyte differentiation ‘downstream’ of these pathways which may in part explain its efficacy even in refractory cases. (Fig. 4 ).
Fig. 4.
A possible model how viral and other environmental triggers may promote autoimmunity in predisposed individuals, and the responsiveness to mTOR inhibition using rapamycin (sirolimus). mTOR hyperactivation promotes lymphoproliferation and increased generation of TH17 cells while decreasing tolerogenic T reg formation. Acute and chronic infections, particularly by herpesviridae infecting lymphocytes, antigen-presenting cells, and other tissues are implicated as risk factors in the pathogenesis of lymphoproliferative and autoimmune diseases. Rapamycin, by reversing mTOR overactivation within lymphocytes, can ameliorate the hallmarks of autoimmunity. EM of Herpesvirus by Bernard Heymann, pH.D., NIAMS Laboratory of Structural Biology Research (CC BY NC 2.0).
3.2. New insights into mTOR complexes in lymphocytes
3.2.1. mTOR complexes continue to define the function of differentiated T cell subsets after T cell differentiation
While it has been known that mTORC1 and 2 suppression is required to induce Tregs, it remains less clear whether their activity is required for the functioning of differentiated Tregs. Treg function, especially migration to non-lymphoid tissues and their stability, are dependent on mTOR as Tregs deficient in mTOR showed lineage instability. mTOR-deficient Treg show migration defects (increased levels of CD62L and CCR7, S1P1; decreased expression of CD69; retention in lymphoid organs) and increasingly become “Ex-Treg” (Tregs that have lost the transcription factor Foxp3) suggesting that mTOR may be required for Treg effector function. [48]
Regarding the effects of mTOR within conventional T cell (T conv) effectors, the presence of mTORC1 in CD4+ TH17 cells is required for their inflammatory effector function: Within TH17 cells, mTORC1 is critical for TH17 plasticity as mTORC1 deficient TH17 cells are unable to transdifferentiate into TH1-like (IFN-γ producing) TH17 cells after antigen stimulation; mice with mTORC1 (Raptor) deficient TH17 cells are protected from MOG-induced EAE. Phenotypically, the mTORC1 positive TH17 subset can be distinguished by a lack of CD27 expression [53] whereas CD27 positive TH17 cells are associated with a memory-like transcriptome, express low levels of mTORC1 and are capable to proliferate and turn into CD27 negative TH17 cells. Overall, this supports a continued role of mTORC1 in inflammatory TH17 responses and may provide a rationale for mTORC1 blockade in TH17-mediated neuroinflammation.
3.3. New insights into mTOR complexes in antigen-presenting cells (APC)
As an important integrator of cell biology, mTOR can be expected to play a role within APC activation, but the precise effects have been a matter of debate. It is likely that individual APC subsets respond differently to mTOR induction or inhibition. For example, mTOR inhibition negatively regulates the activation of IL-4/GM-CSF-differentiated monocyte-derived dendritic cells while it augments maturation of conventional DC from human peripheral blood [54]. This section focuses on a few findings in recent years that helped delineate the role of mTOR in these cells; for an in depth discussion on these complexities we refer to a recent excellent review [55].
3.3.1. mTOR in APC shapes the character of inflammation via metabolic adaptation
mTORC1 deficiency within APC can skew T cell differentiation towards TH17 lineages by affecting cellular metabolism: Whereas mTORΔAPC mice (mice with a CD11c+-specific mTOR deletion) globally develop normally they show tissue-specific APC alterations with metabolic reprogramming, most pronounced within the lung. Here, mTOR deficiency in APC shifts the APC composition: expansions of CD11c+ CD11b+ towards macrophage/monocytic lineages; CD11c+ MHC II+ SIRPalpha+ Heat stable antigen (HSA)+ cells were reduced; the same was observed in mice deficient in mTORC1 (Raptor) but not in mTORC2 (Rictor) deficient mice. In a murine asthma model, these “inflammatory” mTOR-deficient APC skewed inflammation from eosinophilic T helper cell 2 (TH2) to neutrophilic TH17 polarity. Interestingly, targeting the two downstream mTORC1 substrates S6K and 4E-BP1 does not affect the APC composition suggesting that this effect is translation-independent. Rather, FAO inhibition using etomoxir abrogates the lineage shift thus showing that mTOR in APC influences the character of inflammation on the metabolic rather than on the translational level. [56]
3.3.2. mTOR (mTORC1) activation in APC by regulatory T cells can ameliorate immune responses by inhibiting autophagy
Demonstrating an anti-inflammatory role for mTORC1 in APC, autophagy inhibition of APC, induced by Tregs via mTORC1 ameliorates immune responses. Tregs, via CTLA-4, can activate the PI3K/AKT/mTOR axis, leading to inhibition of autophagy, as determined by a decreased synthesis of the autophagy component Lc3b. In vitro pharmacologic treatment with CTLA4-Ig led to decreased autophagosome formation; DC from RA patients treated with CTLA4-Ig showed decreased transcripts of Lc3b as compared to patients treated with alternative TNF inhibition [57] suggesting that the clinical benefit of CTLA4-Ig in RA may in part be mediated via its effect on decreased autophagy and antigen presentation.
3.4. The effect of mTOR blockade on lifespan is context dependent
Mice with growth hormone receptor knockout show a paradoxical effect. While rapamycin promotes longevity in mice and other model animals, mice with growth hormone receptor knockout showed the opposite effect — a shortening of life span — presumably related to increased levels of inflammation, as measured by circulating IL-6, and decreased IL-2 levels in growth hormone receptor knockout mice that was induced by rapamycin treatment [58]. Contrary to what was observed in healthy humans [52], rapamycin blockade in growth-hormone knockout mice led to increased levels of circulating PD1 positive CD4 and CD8 T cells, suggesting that mTOR blockade — although generally a promotor of lifespan [59,60] — may impair longevity under such growth inhibitory conditions.
4. Activation and therapeutic blockade of mTOR in COVID-19
Viruses hijack the ribosomal machinery of their host cells to enforce viral replication, which implicates mTOR/mTORC1 over-activation in viral disease pathogenesis and offers a potential antiviral treatment target. Indeed, rapamycin (sirolimus) is predicted to have direct antiviral efficacy, via its interference with mRNA translation [61,62]. mTOR inhibitors have demonstrated in vitro antiviral properties against SARS-CoV-2 [63] and the related MERS-CoV virus. [64]
Beyond such expected direct antiviral properties, mTOR blockade has the potential to ameliorate excessive systemic and pulmonary inflammation, characteristic for severe Covid-19 [65], via its modulating effects on the host immune system. As reviewed in the previous section, mTOR blockade can enhance primary immune responses [52], promote expansions of the T cell memory compartment [49], which could promote antiviral memory and recall responses, and shift differentiation of T cell lineages away from excessively inflammatory phenotypes [49].
SARS-CoV-2 exploits the ACE2 receptor to invade host cells [66]. Interestingly, mitochondrial oxidative stress retains ACE2 in its functional reduced form [67]. Consistent with its role in SLE [68], mTOR can sense mitochondrial oxidative stress in COVID-19 [69]. N-Acetylcysteine (NAC) can protect against oxidative stress and can ameliorate mTOR overactivation in T cells [70].
Thus, as supported by early clinical observations [71], NAC treatment may have therapeutic efficacy in COVID-19, via at least two mechanisms; by relieving the oxidative stress that facilitates viral entry through ACE2 and by blocking the virally-induced overactivation of the mTOR pathway that — similar to the situation in SLE and MCD — may drive the COVID-19 features of systemic autoimmunity.
Ongoing clinical trials aimed to evaluate the safety and efficacy of mTOR blockade for treatment or prevention of Covid-19 are summarized in Table 1 .
Table 1.
Overview of active clinical trials of mTOR blockade for the treatment or prevention of Covid-19 (www.clinicaltrials.gov) and a pilot study for the use of NAC. RTB101: a proprietary ATP-competitive mTORC1 inhibitor.
| Trial Name | Population | mTOR inhibitor and dose | Control | Primary Outcome |
Status (July 2021) |
|---|---|---|---|---|---|
| Efficacy and Safety of Sirolimus in COVID-19 Infection (NCT04461340) | Adults ≥18 years with COVID-19 pneumonia | Rapamycin (sirolimus) 6 mg × 1 (day 1) 2 mg daily (days 2–10) |
Standard of care | Time to clinical recovery | Recruiting |
| Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia (NCT04341675) | Adults ≥18 years with Covid-19 pneumonia, hypoxia, and poor prognostic biomarkers | Rapamycin (sirolimus) 6 mg × 1 (day 1) 2 mg daily (days 2–14; or until hospital discharge) |
Placebo | Survival free from advanced respiratory support at day 28 | Recruiting |
| A Phase 2 Study of RTB101 as COVID-19 Post-Exposure Prophylaxis in Older Adults (NCT04584710) | Asymptomatic Adults ≥65 years with SARS-CoV-2 on a surveillance swab OR who live in the same building as someone who has COVID-19 | RTB101 10 mg daily for 14 days | Placebo | Time to first positive SARS-CoV-2 test | Active, not recruiting |
| Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine (https://pubmed.ncbi.nlm.nih.gov/32707089/) | Ten patients with ventilator-dependent Covid-19 pneumonia, one of which had G6PD deficiency | Intravenous N-acetylcysteine varying doses; eight patients received 600 mg twice daily for 2–9 days | N/A | N/A (clinical data, C-reactive protein and ferritin were assessed) | Completed |
5. mTOR blockade in the context of longevity
The enhanced antiviral immunity conferred by mTOR (mTORC1) blockade to healthy elderly individuals is in interesting contrast to the immunosuppressive effects traditionally ascribed to mTOR (mTORC1) antagonists. At least in SLE, mTOR blockade relieves the proliferative pressure of T lymphocytes towards inflammatory effector lineages which appears to permit expansion of T memory cell subsets.
While healthy aged individuals would not be expected to show the same degree of T cell subset shifts as lupus patients, the immune system changes dramatically with age, leading to declines in T cell memory and susceptibility to infections and neoplasms.
Thus, promoting the expansion of the T cell memory compartment by blocking mTOR/mTORC1 provides an important intervention to enhance T cellular immunity in elderly individuals, which could — by reducing mortality from neoplastic and infectious, particularly viral, threats — provide an important axis to promote human longevity.
6. Concluding remarks
Thirty years after the initial identification of TOR [1], these recent findings add new nuances to the understanding how an ancient signaling pathway can be highly conserved across eukaryotic cells and yet form a flexible network that is capable to maintain immune homeostasis under dynamic conditions and, how aberrant mTOR pathway activity can lead to autoimmune disease when this network fails.
The findings in the treatment of SLE [49] and iMCD [51] further validate the concept of targeting mTOR to control aberrant inflammation as a treatment strategy for systemic autoimmunity. Additionally, there have been promising early reports in the treatment of vasculitis [72] and RA [73].
For the coming years, we anticipate that the integration of single cell technologies, high-dimensional flow cytometry and RNA sequencing, with traditional proteomics and metabolomics will further unravel how mTOR controls inflammation in health and disease. These studies could enable the development of new therapeutic options that extend healthy lifespan by protecting from autoimmunity while enhancing beneficial immune responses, such as those directed against viral pathogens.
Funding
This work was supported by grants AI141304, AI072648, AI122176, and AR076092 from the National Institutes of Health and the Department of Medicine at the SUNY Upstate Medical University.
References
- 1.Heitman J., Movva N., Hall M. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 2.Loewith R., Hall M.N. Target of Rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas G., Hall M.N. TOR signalling and control of cell growth. Curr Opin Cell Biol. 1997;9:782–787. doi: 10.1016/s0955-0674(97)80078-6. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harwood F.C., Geltink R.I.K., O’Hara B.P., Cardone M., Janke L., Finkelstein D., et al. ETV7 is an essential component of a rapamycin-insensitive mTOR complex in cancer. Sci Adv. 2018;4 doi: 10.1126/sciadv.aar3938. eaar3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y., Liu L., Wu Y., Singh K., Su B., Zhang N., et al. Rapamycin inhibits mSin1 phosphorylation independently of mTORC1 and mTORC2. Oncotarget. 2015;6:4286–4298. doi: 10.18632/oncotarget.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.C., Guan K.-L. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choo A.Y., Yoon S.-O., Kim S.G., Roux P.P., Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banko J.L., Poulin F., Hou L., DeMaria C.T., Sonenberg N., Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the Hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C., Wang H., Zhang D., Luo W., Liu R., Xu D., et al. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat Commun. 2018;9:3492. doi: 10.1038/s41467-018-05449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarbassov D.D., Ali S.M., Kim D.-H., Guertin D.A., Latek R.R., Erdjument-Bromage H., et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Mossmann D., Park S., Hall M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–757. doi: 10.1038/s41568-018-0074-8. [DOI] [PubMed] [Google Scholar]
- 16.Guertin D.A., Stevens D.M., Thoreen C.C., Burds A.A., Kalaany N.Y., Moffat J., et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Huang W., Zhu P.J., Zhang S., Zhou H., Stoica L., Galiano M., et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci. 2013;16:441–448. doi: 10.1038/nn.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guri Y., Colombi M., Dazert E., Hindupur S.K., Roszik J., Moes S., et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32:807–823. doi: 10.1016/j.ccell.2017.11.011. e12. [DOI] [PubMed] [Google Scholar]
- 19.Bai X., Ma D., Liu A., Shen X., Wang Q.J., Liu Y., et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Gao X., Saucedo L.J., Ru B., Edgar B.A., Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 21.Lee D.-F., Kuo H.-P., Chen C.-T., Hsu J.-M., Chou C.-K., Wei Y., et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 22.Lee D.-F., Kuo H.-P., Chen C.-T., Wei Y., Chou C.-K., Hung J.-Y., et al. IKKβ suppression of TSC1 function links the mTOR pathway with insulin resistance. Int J Mol Med. 2008;22:633. doi: 10.3892/ijmm_00000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H.H., Huang J., Düvel K., Boback B., Wu S., Squillace R.M., et al. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon S., Dibble C.C., Talbott G., Hoxhaj G., Valvezan A.J., Takahashi H., et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao F., Kondo K., Itoh T., Ikari S., Nada S., Okada M., et al. Rheb localized on the Golgi membrane activates lysosome-localized mTORC1 at the Golgi-lysosome contact site. J Cell Sci. 2018;131 doi: 10.1242/jcs.208017. jcs208017. [DOI] [PubMed] [Google Scholar]
- 26.Bar-Peled L., Schweitzer L.D., Zoncu R., Sabatini D.M. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancak Y., Bar-Peled L., Zoncu R., Markhard A.L., Nada S., Sabatini D.M. Ragulator-rag complex targets mTORC1 to the Lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dam T.J.P., Zwartkruis F.J.T., Bos J.L., Snel B. Evolution of the TOR pathway. J Mol Evol. 2011;73:209–220. doi: 10.1007/s00239-011-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollizzi K.N., Patel C.H., Sun I.-H., Oh M.-H., Waickman A.T., Wen J., et al. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J Clin Invest. 2015;125:2090–2108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oaks Z., Winans T., Huang N., Banki K., Perl A. Activation of the mechanistic target of Rapamycin in SLE: explosion of evidence in the last five years. Curr Rheumatol Rep. 2016;18:73. doi: 10.1007/s11926-016-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanjuan M.A., Dillon C.P., Tait S.W.G., Moshiach S., Dorsey F., Connell S., et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 34.Delgado M.A., Deretic V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009;16:976–983. doi: 10.1038/cdd.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dengjel J., Schoor O., Fischer R., Reich M., Kraus M., Müller M., et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimmerjahn F., Milosevic S., Behrends U., Jaffee E.M., Pardoll D.M., Bornkamm G.W., et al. Major histocompatibility complex class II-restricted presentation of a cytosolic antigen by autophagy. Eur J Immunol. 2003;33:1250–1259. doi: 10.1002/eji.200323730. [DOI] [PubMed] [Google Scholar]
- 37.Dörfel D., Appel S., Grünebach F., Weck M.M., Müller M.R., Heine A., et al. Processing and presentation of HLA class I and II epitopes by dendritic cells after transfection with in vitro-transcribed MUC1 RNA. Blood. 2005;105:3199–3205. doi: 10.1182/blood-2004-09-3556. [DOI] [PubMed] [Google Scholar]
- 38.Weichhart T., Hengstschläger M., Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sordi V., Bianchi G., Buracchi C., Mercalli A., Marchesi F., D’Amico G., et al. Differential effects of immunosuppressive drugs on chemokine receptor CCR7 in human monocyte-derived dendritic cells: selective upregulation by rapamycin. Transplantation. 2006;82:826–834. doi: 10.1097/01.tp.0000235433.03554.4f. [DOI] [PubMed] [Google Scholar]
- 40.Reichert P., Reinhardt R.L., Ingulli E., Jenkins M.K. Cutting edge: in vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J Immunol. 2001;166:4278–4281. doi: 10.4049/jimmunol.166.7.4278. [DOI] [PubMed] [Google Scholar]
- 41.Stoll S., Delon J., Brotz T.M., Germain R.N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 42.Huppa J.B., Davis M.M. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 43.Saleiro D., Platanias L.C. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2015;36:21–29. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Windt G.J.W., Pearce E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waickman A.T., Powell J.D. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenner R.G., Townsend M.J., Jackson I., Sun K., Bouwman R.D., Young R.A., et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delgoffe G.M., Kole T.P., Zheng Y., Zarek P.E., Matthews K.L., Xiao B., et al. mTOR differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vallion R., Divoux J., Glauzy S., Ronin E., Lombardi Y., di Ricco M.L., et al. Regulatory T cell stability and migration are dependent on mTOR. J Immunol. 2020;205:1799–1809. doi: 10.4049/jimmunol.1901480. [DOI] [PubMed] [Google Scholar]
- 49.Lai Z.-W., Kelly R., Winans T., Marchena I., Shadakshari A., Yu J., et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet Lond Engl. 2018;391:1186–1196. doi: 10.1016/S0140-6736(18)30485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arenas D.J., Floess K., Kobrin D., Pai R.-A.L., Srkalovic M.B., Tamakloe M.-A., et al. Increased mTOR activation in idiopathic multicentric Castleman disease. Blood. 2020;135:1673–1684. doi: 10.1182/blood.2019002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fajgenbaum D.C., Langan R.-A., Japp A.S., Partridge H.L., Pierson S.K., Singh A., et al. Identifying and targeting pathogenic PI3K/AKT/mTOR signaling in IL-6-blockade-refractory idiopathic multicentric Castleman disease. J Clin Invest. 2019;129:4451–4463. doi: 10.1172/JCI126091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mannick J.B., Morris M., Hockey H.-U.P., Roma G., Beibel M., Kulmatycki K., et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aaq1564. eaaq1564. [DOI] [PubMed] [Google Scholar]
- 53.Karmaus P.W.F., Chen X., Lim S.A., Herrada A.A., Nguyen T-LM Xu B., et al. Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature. 2019;565:101–105. doi: 10.1038/s41586-018-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haidinger M., Poglitsch M., Geyeregger R., Kasturi S., Zeyda M., Zlabinger G.J., et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol Baltim Md. 2010;1950(185):3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 55.Snyder J.P., Amiel E. Regulation of dendritic cell immune function and metabolism by cellular nutrient sensor mammalian target of Rapamycin (mTOR) Front Immunol. 2018;9:3145. doi: 10.3389/fimmu.2018.03145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinclair C., Bommakanti G., Gardinassi L., Loebbermann J., Johnson M.J., Hakimpour P., et al. mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science. 2017;357:1014–1021. doi: 10.1126/science.aaj2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alissafi T., Banos A., Boon L., Sparwasser T., Ghigo A., Wing K., et al. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J Clin Invest. 2017;127:2789–2804. doi: 10.1172/JCI92079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang Y., Hill C.M., Darcy J., Reyes-Ordoñez A., Arauz E., McFadden S., et al. Effects of rapamycin on growth hormone receptor knockout mice. Proc Natl Acad Sci U S A. 2018;115:E1495–E1503. doi: 10.1073/pnas.1717065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller R.A., Harrison D.E., Astle C.M., Baur J.A., Boyd A.R., de Cabo R., et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66A:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y., et al. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun Rev. 2020;19:102571. doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mullen P.J., Garcia G., Purkayastha A., Matulionis N., Schmid E.W., Momcilovic M., et al. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat Commun. 2021;12:1876. doi: 10.1038/s41467-021-22166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal Kinome analysis. Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Valle D.M., Kim-Schulze S., Huang H.-H., Beckmann N.D., Nirenberg S., Wang B., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol Orlando FLA. 2020;215:108410. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez D.R., Telarico T., Bonilla E., Li Q., Banerjee S., Middleton F.A., et al. Activation of mTOR controls the loss of TCRζ in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol Baltim Md. 2009;1950(182):2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maiese K. The mechanistic target of Rapamycin (mTOR): novel considerations as an antiviral treatment. Curr Neurovasc Res. 2020;17:332–337. doi: 10.2174/1567202617666200425205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai Z.-W., Hanczko R., Bonilla E., Caza T.N., Clair B., Bartos A., et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibrahim H., Perl A., Smith D., Lewis T., Kon Z., Goldenberg R., et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol Orlando FLA. 2020;219:108544. doi: 10.1016/j.clim.2020.108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J., Zhao L., Wang J., Cheng Z., Sun M., Zhao J., et al. Targeting mechanistic target of Rapamycin complex 1 restricts proinflammatory T cell differentiation and ameliorates Takayasu arteritis. Arthritis Rheumatol Hoboken NJ. 2020;72:303–315. doi: 10.1002/art.41084. [DOI] [PubMed] [Google Scholar]
- 73.Wen H.-Y., Wang J., Zhang S.-X., Luo J., Zhao X.-C., Zhang C., et al. Low-dose sirolimus immunoregulation therapy in patients with active rheumatoid arthritis: a 24-week follow-up of the randomized, open-label, parallel-controlled trial. J Immunol Res. 2019;2019 doi: 10.1155/2019/7684352. [DOI] [PMC free article] [PubMed] [Google Scholar]