Abstract
Introduction
In 2020, the US and New York City experienced unprecedented deaths due to the COVID-19 pandemic and drug overdoses. Policy changes reduced burdensome regulations for medication treatment for opioid use disorder (OUD). Despite these policy changes, few studies examined buprenorphine treatment outcomes during the pandemic. We compared treatment outcomes among Bronx patients referred to office-based buprenorphine treatment before versus during the pandemic.
Methods
In a retrospective cohort study, we compared patients referred to buprenorphine treatment in a Bronx community clinic before (March–August 2019) versus during (March–August 2020) the pandemic. We describe changes to buprenorphine treatment during the pandemic, including telehealth and prioritizing harm reduction. Using data from medical records and program logs, main outcomes included steps of the OUD treatment cascade of care—initial visit scheduled and completed, treatment initiated, and retained in treatment at 90 days. Using chi square and t-tests, we examined differences in patient characteristics and OUD treatment cascade steps before versus during the pandemic.
Results
Before and during the pandemic, 72 and 35 patients were referred to buprenorphine treatment, respectively. Patients' mean age was 46 years, most were male (67.3%) or Hispanic (52.3%), and few had private insurance (19.6%). Patients referred during (vs. before) the pandemic were more likely to have private insurance (31.4% vs. 13.9%, p < 0.05) and be referred from acute care settings (37.1% vs. 19.4%, p < 0.05). No significant differences in OUD cascade of care outcomes existed between those referred during versus before the pandemic. However, among patients who initiated buprenorphine treatment, those referred during (vs. before) the pandemic were more likely to be retained in treatment at 90 days (68.0% vs. 42.9%, p < 0.05).
Conclusions
Despite the COVID-19 pandemic's unprecedented devastation to the Bronx, along with worsening drug overdose deaths, OUD cascade of care outcomes were similar among patients referred to buprenorphine treatment before versus during the pandemic. Among patients who initiated buprenorphine treatment, treatment retention was better during (versus before) the pandemic. During a public health emergency, incorporating telehealth and prioritizing harm reduction are key strategies to maintain optimal OUD treatment outcomes.
Keywords: Opioid use disorder, Buprenorphine, COVID-19 pandemic, Cascade of care
1. Background
Opioid overdose deaths remain a growing and devasting problem. In 2020 during the COVID-19 pandemic, the provisional number of opioid overdose deaths is the highest ever recorded in the US and New York City, and particularly the Bronx (Centers for Disease Control and Prevention [CDC] Health Alert Network, 2020; CDC National Center for Health Statistics; New York City Department of Health and Mental Hygiene [NYC DOHMH], 2021). From March to May 2020, NYC, and particularly the Bronx, was the epicenter of the pandemic. During this time, more than 15,000 people died from COVID-19 in NYC, and more than 3300 died from COVID-19 in the Bronx (New York City Department of Health and Mental Hygiene, 2020a, New York City Department of Health and Mental Hygiene, 2020b). The colliding drug overdose epidemic and COVID-19 pandemic led to rapid and unprecedented changes in health care delivery.
On March 7, 2020, a statewide public health emergency was declared in New York, and NYC was the first to enact shelter-in-place policies that required non-essential workers to stay at home and non-essential businesses to close. As part of these policies, numerous ambulatory clinics, substance use disorder treatment programs, and harm reduction organizations dramatically scaled back services, and many of them closed. As a result, the intersection of the pandemic with the opioid and overdose epidemics became particularly challenging for people with opioid use disorder (OUD). They faced the threat of reduced access to life-saving medication to treat OUD and multiple factors that likely increased their risk of overdose. These factors included reduced access to effective medication treatment (including buprenorphine, methadone, and naltrexone), reduced access to naloxone for opioid overdose reversal, social isolation which likely led to using opioids alone, and decreased opioid tolerance due to shifts in the drug market (CDC Health Alert Network, 2020; Wainwright et al., 2020). Because of these challenges, access to medication treatment for OUD during the pandemic has been more critical than ever.
Buprenorphine treatment for OUD is effective and reduces overdose deaths by up to 50% (Larochelle et al., 2018; Sordo et al., 2017; Wakeman et al., 2020). However, buprenorphine treatment remains woefully underutilized, with less than 20% of people with OUD receiving treatment (Substance Abuse and Mental Health Services Administration [SAMHSA], 2020). During the pandemic, federal and state policies were changed to reduce burdensome regulations for medication treatment for OUD. Key changes included waiving the requirement to have an in-person evaluation prior to prescribing buprenorphine, waiving the sanctions and penalties for not using HIPAA-compliant telehealth applications to deliver care, and changes to billing/compensation for telehealth visits (Health and Human Services, 2020; New York State Department of Health, 2020; United States Department of Justice, 2020). These policy changes have the potential to improve access to and retention in OUD treatment (Kleinman & Morris, 2021). However, few studies have examined patient outcomes in buprenorphine treatment during the pandemic (Nordeck, Buresh, Krawczyk, Fingerhood, & Agus, 2020; Tofighi et al., 2021). In the context of the worst drug overdose epidemic ever in the US, it is critical to understand how rapid changes in health care policies and health care delivery during the COVID-19 pandemic impacted buprenorphine treatment outcomes. Therefore, we compared treatment outcomes among Bronx patients who were referred to office-based buprenorphine treatment for OUD before and during the pandemic.
2. Methods
We conducted a retrospective cohort study of patients with OUD referred to office-based buprenorphine treatment in the Bronx before and during the COVID-19 pandemic. This project was approved by the affiliated institutional review board.
2.1. Setting
2.1.1. Montefiore medical center
Montefiore Medical Center (Montefiore) is the largest health system in the Bronx and consists of 4 hospitals, 4 emergency rooms, and more than 20 ambulatory community-based clinics. During the pandemic, Montefiore provided care to an unprecedented number of patients with COVID-19 infections (Tomer et al., 2021). During a single day in April 2020, Montefiore hospitalized 1172 patients with COVID-19 infection.
Montefiore's Buprenorphine Treatment Network includes six primary care community-based clinics. Buprenorphine treatment (treatment with any buprenorphine-containing medication approved for OUD treatment, typically the sublingual buprenorphine-naloxone co-formulation) at Montefiore was first established in 2005 in a single clinic, and then expanded to six clinics between 2016 and 2019. Since its establishment, over 50 medical providers have provided buprenorphine treatment to over 1300 patients with OUD. The structure of buprenorphine treatment has been described in detail previously (Cunningham et al., 2008). Briefly, each clinic has a physician champion, a treatment coordinator (nurse, pharmacist, or physician assistant), and several buprenorphine prescribers who are primary care providers board-certified in internal medicine or family medicine. No substance use counselors are available at the clinics, but social workers are available to all clinic patients, including those who receive buprenorphine treatment. Buprenorphine treatment is guided by patient-centered and harm reduction principles; if there is evidence of ongoing illicit opioid use despite buprenorphine treatment or repeated problems with adherence to scheduled visits, treatment is typically intensified through more frequent visits and/or referrals for psychosocial support. Patients are referred for treatment from a variety of sources, including self-referral, referrals from providers within and outside of Montefiore (e.g. clinics, hospitals, substance use disorder treatment programs, jails), and referrals from community-based organizations (e.g. harm reduction organizations).
Buprenorphine Treatment before the COVID-19 pandemic: Prior to the pandemic, all patients with OUD seeking or receiving buprenorphine treatment were required to present to in-person visits to the ambulatory clinics. New patients were typically expected to attend visits every 1–2 weeks during the first month of treatment. Once patients' buprenorphine maintenance dose was established, for the next 6–12 months, they typically had monthly visits. Once stable for 6–12 months, patients could then have visits every 2–3 months. Accordingly, patients typically received prescriptions for buprenorphine-naloxone every 1–2 weeks during their first month of treatment, every month during the subsequent 6–12 months of treatment, and then could receive prescriptions with refills for the subsequent months.
Prior to the pandemic, providers ordered urine toxicology tests for all patients at every visit. This policy was created in part to minimize known racial and ethnic disparities in rates of urine drug testing (Gaither et al., 2018; Hausmann, Gao, Lee, & Kwoh, 2013; Morasco et al., 2016). Finally, naloxone take-home kits were dispensed to all patients at their initial buprenorphine treatment visit and at subsequent visits as needed.
Buprenorphine Treatment during the COVID-19 pandemic: During the pandemic, buprenorphine treatment changed dramatically. These changes were driven by several factors—relaxation of national treatment regulations, deployment of buprenorphine prescribers to care for hospitalized patients with COVID-19 infection, deployment of buprenorphine treatment coordinators to address other needs related to the pandemic, dramatic reduction in public transportation available to patients, and a marked reduction in all ambulatory visits at all Montefiore clinics. In addition, while the Montefiore Buprenorphine Treatment Network has been guided by harm reduction principles since its inception, the focus on harm reduction principles during the pandemic intensified. The program's goals were simply to help patients survive by reducing the risk of overdose and the risk of COVID-19 infection.
During the pandemic, from March 7, 2020 to June 15, 2020, all in-person visits were suspended and visits occurred exclusively via telehealth. Phased re-opening with limited in-person visit availability occurred through August 31, 2020. While telehealth visits with video were prioritized, because many patients had limited access to the necessary technology or internet, many telehealth visits occurred via telephone only without a video component. For new patients, telehealth visits occurred every 2–4 weeks, and quickly advanced to monthly visits. Once patients were stable with their treatment, telehealth visits occurred every 1–2 months. Accordingly, most new patients received prescriptions for a 1-month supply of buprenorphine-naloxone, and stable patients often received prescriptions for a 1-month supply plus refills for subsequent months. Providers worked closely with local pharmacies to arrange home delivery of medications or utilized mail order pharmacies when available. All urine toxicology tests were halted; providers focused on self-report of adherence to buprenorphine-naloxone and self-report of substance use. Finally, providers prescribed naloxone to local pharmacies for patients to pick up or mailed naloxone take-home kits directly to patients if needed.
2.1.2. Sample and exposure to the COVID-19 pandemic
We included all patients who were referred for buprenorphine treatment at Montefiore's Buprenorphine Treatment Network during two time periods based on exposure to the pandemic. Patients who were referred for treatment between March 1, 2019 and August 31, 2019 were considered referred before the pandemic. Patients who were referred for treatment between March 7, 2020 and August 31, 2020 were considered referred during the pandemic. We included all patients referred to treatment, including those from within and outside of Montefiore Medical Center, and those who were initiating or transferring buprenorphine treatment. We chose our exposure time frames for several reasons: a) a public health emergency was declared in New York on March 7, 2020; b) to reduce the risk of bias related to potential differences in outcomes due to seasonal variation, we selected the same time of year before and during the pandemic (March–August); and c) accounting for a potential 30-day lag between time of referral and time of treatment initiation, those who were referred to treatment through August 31, 2019 had the opportunity to reach 90-day treatment retention (a standard treatment outcome) prior to the beginning of the pandemic.
2.1.3. Data collection
We extracted data from March 1, 2019 through December 31, 2019, and from March 7, 2020 through December 31, 2020 for patients referred before and during the pandemic, respectively. Data were extracted electronically from Montefiore's electronic medical record (EMR) system and manually from the Montefiore Buprenorphine Treatment Network program log. Sociodemographic data from the EMR included: age; sex (male, female); race and ethnicity (Hispanic, non-Hispanic Black, non-Hispanic white and other); and private insurance status at the time of treatment referral (yes, no). Clinical data from the EMR included buprenorphine prescription data (date, dose, quantity, refills) and in-person and telehealth visits scheduled and completed. Clinical data from the program log included: history of injection drug use (yes, no); heroin use at the time of referral (yes, no); medication for OUD treatment at the time of referral (methadone, buprenorphine, none); referral source from an acute care setting (e.g., hospital or emergency department; yes, no), referral date.
2.1.4. Main outcomes
Our main outcomes included the steps of the OUD treatment cascade of care – referred for treatment, initial visit scheduled, initial visit completed, treatment initiated, and retained in treatment at 90 days. Referred for treatment was defined as having a referral date in the program log. Initial visit scheduled was defined as having an initial appointment scheduled in the Montefiore EMR. Initial visit completed was defined as a patient having completed an initial visit in the Montefiore EMR. Treatment initiated was defined as having a buprenorphine prescription in the Montefiore EMR. Finally, retained in treatment at 90 days was defined as having an active buprenorphine prescription at least 90 days after treatment initiation.
2.1.5. Analyses
We first conducted descriptive analyses to describe the patients referred to buprenorphine treatment. We then examined whether differences in sociodemographic and clinical characteristics existed between patients referred before versus during the pandemic. Finally, we examined whether completion of each step of the OUD treatment cascade differed between patients referred before versus during the pandemic. To determine whether there were significant differences, we used chi square or Fisher exact tests for categorical variables and t-tests for continuous variables.
3. Results
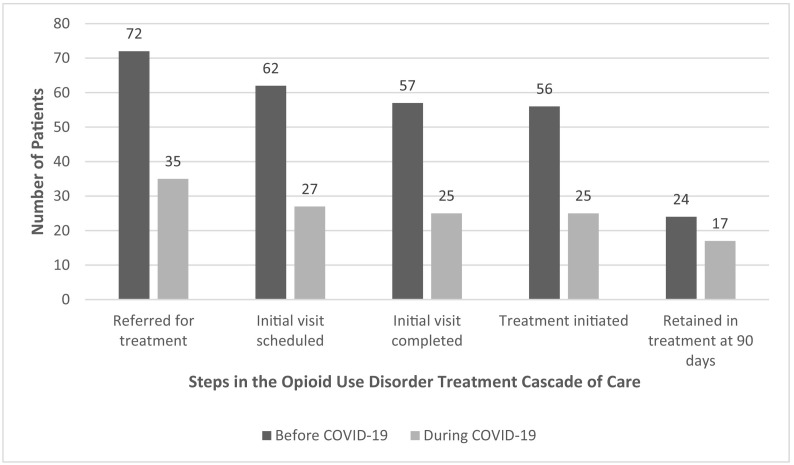
From March–August 2019, before the COVID-19 pandemic, 72 patients were referred for office-based buprenorphine treatment. From March–August 2020, during the pandemic, 35 patients were referred. Patients' mean age was 46 years, most were male (67.3%) or Hispanic (52.3%), and few had private insurance (19.6%) (see Table 1 ). At the time of referral, most patients used heroin (58.9%), nearly half had previously been prescribed buprenorphine for OUD treatment (46.7%), and a quarter (25.9%) were referred from an acute care setting. Among all 107 patients, 89 (83.2%) scheduled an initial visit, 82 (76.6%) completed an initial visit, 81 (75.7%) initiated buprenorphine treatment, and 41 were retained in treatment at 90 days (38.3% of all referred patients and 50.6% of patients who initiated buprenorphine treatment).
Table 1.
Characteristics of patients referred to office-based buprenorphine treatment before and during the COVID-19 pandemic.
| Patients' characteristic | Total |
Time of referral |
|
|---|---|---|---|
|
N = 107 n (%) |
Before the COVID-19 pandemic N = 72 n (%) |
During the COVID-19 pandemic N = 35 n (%) |
|
| Mean age in years (±SD) | 45.9 ± 14.1 | 45.4 ± 14.1 | 46.9 ± 14.1 |
| Female sex | 35 (32.7) | 23 (31.9) | 12 (34.3) |
| Race/ethnicity | |||
| Hispanic | 56 (52.3) | 36 (50.0) | 20 (57.1) |
| Non-Hispanic Black | 21 (19.6) | 15 (20.8) | 6 (17.1) |
| Non-Hispanic white | 19 (17.8) | 14 (19.4) | 5 (14.3) |
| Non-Hispanic other or unknown | 11 (10.3) | 7 (9.7) | 4 (11.4) |
| Private insurance | 21 (19.6) | 10 (13.9) | 11 (31.4)⁎ |
| Heroin use at time of referral | 63 (58.9) | 38(52.8) | 25 (71.4) |
| History of injection drug use | 22 (20.6) | 15(20.8) | 7 (20.0) |
| Medication for OUD at time of referral | |||
| None | 50 (46.7) | 33 (45.8) | 17 (48.6) |
| Buprenorphine | 50 (46.7) | 35 (48.6) | 15 (42.9) |
| Methadone | 7 (6.5) | 4 (5.6) | 3 (8.6) |
| Referred from an acute care setting | 27 (25.2) | 14 (19.4) | 13 (37.1)⁎ |
| Opioid use disorder cascade of care | |||
| Initial visit scheduled | 89 (83.2) | 62 (86.1) | 27 (77.1) |
| Initial visit completion | 82 (76.6) | 57 (79.2) | 25 (71.4) |
| Treatment initiation | 81 (75.7) | 56 (77.8) | 25 (71.4) |
| 90-day treatment retention | 41 (38.3) | 24 (33.3) | 17 (48.6) |
p < 0.05.
Compared to patients referred before the pandemic, those referred during the pandemic more likely to have private insurance (13.9% vs. 31.4%, p < 0.05) and be referred from an acute care setting (19.4% vs. 37.1%, p < 0.05). Among all patients, there were no significant differences in each completed step of the OUD cascade of care between those referred before versus during the pandemic (see Fig. 1 ). In an exploratory analysis limited to the 81 patients who initiated buprenorphine treatment, compared to those referred before the pandemic, patients referred during the pandemic were more likely to be retained in treatment at 90 days (42.9% vs. 68.0%, p < 0.05).
Fig. 1.
Steps of the opioid use disorder cascade of care for patients referred to office-based buprenorphine treatment before and during the COVID-19 pandemic.
4. Discussion
Within a well-established buprenorphine treatment network of six primary care community-based clinics in the Bronx that underwent rapid changes to buprenorphine treatment during the COVID-19 pandemic, 50% fewer referrals for buprenorphine treatment occurred during (versus before) the pandemic. Compared to patients referred before the pandemic, those referred during the pandemic were more likely to have private insurance and be referred from acute care settings. Despite rapid changes to the health care system and health care delivery during the pandemic, among all patients referred for buprenorphine treatment, we found no differences in the proportion of patients who completed each step of the OUD cascade of care. However, when we explored patients who initiated buprenorphine treatment, 90-day treatment retention was significantly better during (versus before) the pandemic.
Although published narratives describe changes in OUD treatment delivery during the pandemic (Buchheit, Wheelock, Lee, Brandt, & Gregg, 2021; Clark et al., 2021; Samuels et al., 2020; Wang et al., 2021), to our knowledge, only two manuscripts have reported patient outcomes (Nordeck et al., 2020; Tofighi et al., 2021), and none have compared the full cascade of OUD treatment outcomes before versus during the pandemic. Of the two studies that examined patient outcomes, neither included patients who were referred to buprenorphine treatment but did not initiate care. Tofighi and colleagues examined outcomes of patients who initiated buprenorphine treatment via telehealth during the initial surge of the pandemic in New York City (Tofighi et al., 2021). In that study, 54% of patients were retained in treatment at 8 weeks (and 27% were transitioned to a community treatment program). In the other study, Nordeck and colleagues reported a sharp increase in patients who completed an initial visit and initiated treatment during the pandemic in Baltimore (Nordeck et al., 2020). They found no differences in patient characteristics or treatment outcomes among patients who initiated treatment before versus during the pandemic. In addition, 64% of their patients were retained in treatment at 30 days. While our 90-day treatment retention rate during the pandemic is similar to these studies (68% of patients who initiated treatment during the pandemic were retained at 90 days), we found approximately a 50% reduction in the number of patients who were referred, completed an initial visit, and initiated treatment during the pandemic. In addition, unlike Nordeck and colleagues, we found differences in patient characteristics between those referred before versus during the pandemic. One important difference we found is that those referred to buprenorphine treatment during (versus before) the pandemic were more likely to have private insurance. Therefore, the reduction in referrals could reflect the lack of access to technology needed to access buprenorphine treatment or competing priorities, particularly among those without private insurance. The differences in findings between our study and others' studies may also reflect the severity of the COVID-19 pandemic, the availability of buprenorphine treatment, and the subsequent public health responses in New York City versus Baltimore.
Our findings add to the literature, as we examined treatment outcomes throughout the OUD cascade of care, including referrals to treatment, scheduled and completed initial visits, treatment initiation, and 90-day retention. In addition, our comparison of patient outcomes before versus during the COVID-19 pandemic adds rigor to our study. Despite the devastation in the Bronx and unprecedented changes in health care delivery, treatment outcomes were similar before and during the pandemic. In addition, in exploratory analyses, we found improved treatment retention during the pandemic among patients who initiated treatment. This finding remained when treatment retention was defined using prescription data, visit data, or a combination of both (data not shown). During the pandemic, we believe that our patients were likely to achieve similar or better outcomes to patients prior to the pandemic probably because of two key reasons. First, although not optimal for all patients, telehealth likely increased access to care for some patients, allowing for buprenorphine treatment without increasing risk of COVID-19 infection. Second, focusing on harm reduction as a guiding principle in treatment likely improved access and retention in care. It is also possible that other unmeasured patient factors, like motivation, could have influenced this outcome. While incorporating telehealth and harm reduction into buprenorphine treatment during a public health emergency was critical, extending these strategies into routine buprenorphine treatment may further improve access to OUD treatment and OUD treatment outcomes.
Our study has limitations, including examining buprenorphine treatment occurring in a single health care system in the Bronx, which was extraordinarily impacted by the COVID-19 pandemic. Thus, our findings may not be generalizable to other areas or health care settings which experienced milder effects of the pandemic. However, because the Bronx was the early epicenter of the pandemic in the US, our findings reflect treatment outcomes that occurred in one of the most challenging circumstances of a public health emergency. In addition, our sample size was small. Therefore, we were likely underpowered to detect differences between the groups of patients referred before versus during the pandemic, and we had limited ability to conduct robust multivariable analyses. Our study was limited to patients' characteristics and outcomes that were available in our program logs and EMR. Finally, nearly half of the patients referred to buprenorphine treatment reported they were taking buprenorphine at the time of referral (e.g., prescribed buprenorphine while hospitalized and upon discharge—the time of referral). While this is not unusual, clinical sites that are referred patients without buprenorphine experience could have different outcomes.
In conclusion, despite unprecedented devastation of the COVID-19 pandemic to the Bronx in the spring of 2020, OUD cascade of care outcomes (initial visit scheduled, initial visit completed, treatment initiated, retained in treatment at 90 days) were similar among patients referred to office-based buprenorphine treatment before versus during the COVID-19 pandemic. Among patients who initiated buprenorphine treatment, treatment retention was better during (versus before) the pandemic. During a public health emergency, incorporating telehealth and prioritizing harm reduction are key strategies to maintain optimal OUD treatment outcomes.
CRediT authorship contribution statement
Conceived of the project (COC, TL, LK), drafted manuscript (COC), critically reviewed the manuscript (all), interpreted findings (all), secured funding (COC, TL), supervised research activities (COC, TL), extracted data (LK, YT, ST), managed and cleaned data (YT), conducted data analyses (YT, CZ).
Declaration of competing interest
No authors have conflicts of interests to report.
Acknowledgments
Acknowledgements
We thank the physician champions and treatment coordinators who helped with rapid practice changes for Montefiore's Buprenorphine Treatment Network during the COVID-19 pandemic, in particular Dr. Joel Bumol, Shannon Morrissey, and Tanya Williams.
Funding/support
This work was supported by the National Institutes of Health (K24DA036955, R01DA044171, P30AI124414) and the Health Resources and Services Administration (H80CS00626).
References
- Buchheit B.M., Wheelock H., Lee A., Brandt K., Gregg J. Low-barrier buprenorphine during the COVID-19 pandemic: A rapid transition to on-demand telemedicine with wide-ranging effects. Journal of Substance Abuse Treatment. 2021;131 doi: 10.1016/j.jsat.2021.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, Health Alert Network Increase in fatal drug overdoses across the united states driven by synthetic opioids before and during the COVID-19 pandemic. 2020. https://emergency.cdc.gov/han/2020/han00438.asp Accessed on 8/8/21 at.
- Centers for Disease Control and Prevention, National Center for Health Statistics Vital Statistics Rapid Release, Provisional Drug Overdose Death Counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm Accessed on 8/8/21 at.
- Clark S.A., Davis C., Wightman R.S., Wunsch C., Keeler L.A.J., Reddy N., Samuels E.A. Using telehealth to improve buprenorphine access during and after COVID-19: A rapid response initiative in Rhode Island. Journal of Substance Abuse Treatment. 2021;124 doi: 10.1016/j.jsat.2021.108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C., Giovanniello A., Sacajiu G., Whitley S., Mund P., Beil R., Sohler N. Buprenorphine treatment in an urban community health center: What to expect. Family Medicine. 2008;40(7):500–506. [PMC free article] [PubMed] [Google Scholar]
- Gaither J.R., Gordon K., Crystal S., Edelman E.J., Kerns R.D., Justice A.C., Becker W.C. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug and Alcohol Dependence. 2018;192:371–376. doi: 10.1016/j.drugalcdep.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann L.R.M., Gao S., Lee E.S., Kwoh K.C. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain. 2013;154(1):46–52. doi: 10.1016/j.pain.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Health and Human Services . 2020. Secretary Azar announces historic expansion of telehealth access to combat COVID-19.https://public3.pagefreezer.com/browse/HHS%20%E2%80%93%C2%A0About%20News/20-01-2021T12:29/https://www.hhs.gov/about/news/2020/03/17/secretary-azar-announces-historic-expansion-of-telehealth-access-to-combat-covid-19.html Accessed on 9/26/21 at. [Google Scholar]
- Kleinman R.A., Morris N.P. Rapid access to medications for opioid use disorder. Journal of General Internal Medicine. 2021;1–2 doi: 10.1007/s11606-021-06850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M.R., Bernson D., Land T., Stopka T.J., Wang N., et al. 2018. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco B.J., Peters D., Krebs E.E., Kovas A.E., Hart K., Dobscha S.K. Predictors of urine drug testing for patients with chronic pain: Results from a national cohort of U.S. veterans. Substance Abuse. 2016;37(1):82–87. doi: 10.1080/08897077.2015.1110742. [DOI] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene . 2020. Coronavirus disease 2019 (COVID-19) daily data summary. May 17, 2020.https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-19-daily-data-summary-05172020-1.pdf Accessed on 8/5/21 at. [Google Scholar]
- New York City Department of Health and Mental Hygiene . 2020. Coronavirus disease 2019 (COVID-19) daily data summary. May 17, 2020.https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-19-daily-data-summary-hospitalizations-05172020-1.pdf Accessed on 8/5//21 at. [Google Scholar]
- New York City Department of Health and Mental Hygiene . April 2021. 2021. Unintential drug poisoning (overdose) deaths quarters 1-3, 2020, New York City.https://www1.nyc.gov/assets/doh/downloads/pdf/basas/provisional-overdose-report-third-quarter-2020.pdf Accessed on 8/5/21 at. [Google Scholar]
- New York State Department of Health New York state medicaid coverage and reimbursement policy for services related to coronavirus disease 2019 (COVID-19) medicaid update. 2020;36(7) https://www.health.ny.gov/health_care/medicaid/program/update/2020/docs/mu_no07_2020-03-27_covid-19_reimbursement.pdf Accessed on 9/26/21 at. [Google Scholar]
- Nordeck C.D., Buresh M., Krawczyk N., Fingerhood M., Agus D. Adapting a low-threshold buprenorphine program for vulnerable populations during the COVID-19 pandemic. Journal of Addiction Medicine. 2020 doi: 10.1097/adm.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E.A., Clark S.A., Wunsch C., Jordison Keeler L.A., Reddy N., Vanjani R., Wightman R.S. Innovation during COVID-19: Improving addiction treatment access. Journal of Addiction Medicine. 2020;14(4):e8–e9. doi: 10.1097/adm.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L., et. a. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357 doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Rockville, MD: 2020. Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55)https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf Accessed on 8/22/21 at. [Google Scholar]
- Tofighi B., McNeely J., Walzer D., Fansiwala K., Demner A., Chaudhury C.S., et al. A telemedicine buprenorphine clinic to serve New York City: Initial evaluation of the NYC public hospital system's initiative to expand treatment access during the COVID-19 pandemic. J Addict Med. 2021 doi: 10.1097/adm.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y., Ng Gong M., Keller M.J., Southern W., Kitsis E.A., Kajita G.R., et. a. Teamwork and leadership under fire at the epicenter of the COVID-19 epidemic in the bronx. Frontiers in Medicine. 2021;8 doi: 10.3389/fmed.2021.610100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Administration D.E., Division D.C., United States Department of Justice . 2020. COVID-19 Information Page.https://www.deadiversion.usdoj.gov/coronavirus.html Accessed on 9/26/21 at. [Google Scholar]
- Wainwright J.J., Mikre M., Whitley P., Dawson E., Huskey A., Lukowiak A., Giroir B.P. Analysis of drug test results before and after the US declaration of a National Emergency Concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674–1677. doi: 10.1001/jama.2020.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman S.E., Larochelle M.R., Ameli O., Chaisson C.E., McPheeters J.T., Crown W.H., et. a. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Network Open. 2020;3(2) doi: 10.1001/jamanetworkopen.2019.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Weiss J., Ryan E.B., Waldman J., Rubin S., Griffin J.L. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. Journal of Substance Abuse Treatment. 2021;124 doi: 10.1016/j.jsat.2020.108272. [DOI] [PMC free article] [PubMed] [Google Scholar]