Abstract
Several organs, such as the heart, breasts, intestine, testes, and ovaries, have been reported to be target tissues of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. To date, no studies have demonstrated SARS-CoV-2 infection in the female reproductive system. In the present study, we investigated the effects of SARS-CoV-2 infection on ovarian function by comparing follicular fluid (FF) from control and recovered coronavirus disease 2019 (COVID-19) patients and by evaluating the influence of these FF on human endothelial and non-luteinized granulosa cell cultures. Our results showed that most FFs (91.3%) from screened post COVID-19 patients were positive for IgG antibodies against SARS-CoV-2. Additionally, patients with higher levels of IgG against SARS-CoV-2 had lower numbers of retrieved oocytes. While VEGF and IL-1β were significantly lower in post COVID-19 FF, IL-10 did not differ from that in control FF. Moreover, in COV434 cells stimulated with FF from post COVID-19 patients, steroidogenic acute regulatory protein (StAR), estrogen-receptor β (Erβ), and vascular endothelial growth factor (VEGF) expression were significantly decreased, whereas estrogen-receptor α (ERα) and 3β-hydroxysteroid dehydrogenase (3β-HSD) did not change. In endothelial cells stimulated with post COVID-19 FF, we observed a decrease in cell migration without changes in protein expression of certain angiogenic factors. Both cell types showed a significantly higher γH2AX expression when exposed to post COVID-19 FF. In conclusion, our results describe for the first time that the SARS-CoV-2 infection adversely affects the follicular microenvironment, thus dysregulating ovarian function.
Keywords: COVID-19, SARS-CoV-2 IgG antibodies, Follicular fluid, Retrieved oocytes, Angiogenesis
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread throughout the world. As of June 14, 2021, more than 175 million cases and 3.7 million deaths are attributed to this virus worldwide. In Argentina, around 3.48 million cases were confirmed with 73,391 reported deaths [1], [2].
SARS-CoV-2 invades the target cell by binding to angiotensin-converting enzyme 2 (ACE-2). The viral entry is further processed by the transmembrane serine protease 2 (TMPRSS2), thus allowing the fusion of the cell membranes of virus and host cell (3). It is public knowledge that SARS-CoV-2 can cause severe damage, particularly in the respiratory system (4). The most frequently observed symptoms in COVID-19 patients include fever, cough, and pneumonia. However, other symptoms such as thrombosis, pulmonary embolism, and high blood pressure have been reported as well, suggesting that the virus targets the endothelium [5], [6]. It is well known that ACE-2 is also expressed in endothelial cells [7], [8]. Additionally, dysregulated immune responses, as those observed in COVID-19, are a major culprit in endothelial dysfunction, since they alter microvascular permeability and induce vascular inflammation (6). Nonetheless, other organs such as the heart, breasts, intestine, testes, and ovaries have also been reported to be target tissues of this viral infection [9], [10] . To date, no studies have presented evidence of SARS-CoV-2 infecting the female reproductive system.
ACE-2 is expressed in the uterus, vagina, placenta, and ovary [11], [12]. In particular, ACE-2 mRNA transcripts have been detected in ovaries from reproductive-age and postmenopausal women. Both stromal and granulosa cells have been found to be positive for ACE-2 in the human ovary (13). Furthermore, ACE-2 expression in rat and bovine granulosa cells is regulated by gonadotropic hormones [14], [15]. Whether this virus binds to ACE-2 receptors in the ovary and which effects, if any, this infection would have on ovarian function and oocyte quality remains unclear. Nevertheless, to the best of our knowledge, no reports have addressed the consequences of COVID-19 on ovarian function.
During the final stages of folliculogenesis, the oocyte is localized in an antral follicle in the ovary. The female gamete is exposed to a microenvironment that includes follicular fluid (FF) and somatic cells (namely granulosa and theca cells) within the follicle. The composition of FF differs from that of serum—it is a complex mixture of hormones, cytokines, metabolites, and other proteins secreted mainly by granulosa cells [16], [17]. FF composition reflects the stage of oocyte development and oocyte quality [18], [19]. Therefore, an altered FF composition is associated with a reduced reproductive function.
Based on these considerations, we hypothesized that the SARS-CoV-2 infection can potentially affect ovarian function, disturbing the follicular microenvironment and thus affecting oocyte quality in recovered women. Hence, we evaluated the presence of SARS-CoV-2 IgG antibodies and antigens, interleukin-1β (IL-1β), interleukin-10 (IL-10), and vascular endothelial growth factor (VEGF) levels in FF from healthy and recovered SARS-CoV-2 women undergoing assisted reproductive technology (ART) procedures. We also examined the effect of FFs obtained from the above-mentioned patients on: a) the proliferation, migration, angiopoietins 1 and 2 (ANGPT-1/2), and VEGF expression of a human endothelial cell culture; and b) the proliferation and protein expression of estrogen-receptor α (ERα) and β (ERβ), steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (3β-HSD), and VEGF in human non-luteinized granulosa cells. Additionally, we analyzed the effect of FFs on nuclear DNA damage in both cell types.
2. Materials and methods
2.1. Ethical approval
This study was approved by the ethics committee of the Instituto de Biología y Medicina Experimental (IBYME-CONICET; Study No. 2850). Written informed consent was given by all patients before recruitment.
2.2. Study population and FF collection
For this study, we enrolled a total of 80 women (21–41 years old) undergoing assisted reproductive technology procedures between November 2020 and April 2021 at PREGNA Medicina Reproductiva (Buenos Aires, Argentina), IVI Buenos Aires (Buenos Aires, Argentina), Fertilis (Buenos Aires, Argentina) and InVitro (Buenos Aires, Argentina). Patients with pathologies such as uterine fibroids, endometriosis, pelvic inflammatory disease, premature ovarian failure, and PCOS were excluded from the study. Additionally, we excluded patients with poor ovarian response (less than three antral follicles). The patients were classified into two groups: control patients (n = 34), who had never tested positive for COVID-19 or experienced any COVID-related symptoms, and post COVID-19 patients (n = 46), who had at least one positive PCR test for COVID-19 but were given medical clearance before starting the fertility treatment. The patients in this group were asymptomatic or presented mild symptoms such as anosmia, dysgeusia, and flu-like symptoms (fever, sore throat, and cough) [20], [21], [22] .
The time interval between the infection of the patients with SARS-CoV-2 and the retrieval of FF varied between 2 and 9 months, the average being 4.5 months. None of the patients were vaccinated against COVID-19 prior to the study. A protocol for ovarian stimulation was assigned to patients according to their ovarian reserve and following the standard protocol of each clinic. In all cases, it consisted of a gonadotropin protocol (recombinant FSH, highly purified human menopausal gonadotropin, or a combination of both) for an average of 10 days (range, 9–12 days). Ovulation was induced by subcutaneous administration of a GnRH agonist or hCG. All patients were included in the statistical analysis, since there were no differences between the parameters studied in either group receiving GnRHa or hCG trigger for ovulation.
Oocyte retrieval was conducted under vaginal ultrasound guidance 34–36 h after ovulation induction. Human FF was extracted from all 16- to 20-mm follicles of each patient. No flush was used after the aspiration of all accessible ovarian follicles. Only macroscopically clear fluids, indicating lack of contamination and blood, were considered in the study. Immediately after oocyte removal, the FF was centrifuged for 10 min at 2000g to remove cellular components and debris. Once transferred to sterile polypropylene tubes, the supernatant was stored at −20 °C until assayed. For in vitro experiments we selected randomly 20 patients per group, and each patient's FF was used individually. The biochemical analyses were performed in the Laboratory for Studies of the Physiopathology of the Ovary at IBYME-CONICET (www.ibyme.org.ar/laboratorios/51/estudios-de-la-fisiopatologia-del-ovario).
Serum samples for estradiol determination were obtained on the day of the ovulation trigger. Basal hormone levels prior to ovarian stimulation (estradiol, progesterone, and prolactin) were obtained from the patients' clinic history, when available. Various parameters were used to evaluate the efficacy of ovarian stimulation, including the numbers of retrieved cumulus–oocyte complexes and of mature oocytes that reached metaphase II (MII). In addition, each group of patients (control and post COVID-19) was divided into two subsets according to age as follows: control; ≤35 (n = 19) and > 35 (n = 15) and post COVID-19 ≤ 35(n = 22) and > 35 (n = 24), respectively. The analyses of ovarian stimulation outcomes were duplicated for each subset.
2.3. Immunoassays
The levels of SARS-COV-2 IgG in FF samples were measured using an enzyme-linked immunosorbent assay (ELISA) designed specifically to measure immunoreactive IgG against SARS-COV-2 in human fluids (COVIDAR IgG, Argentina) (23). This kit, which was generously donated by Dr. Andrea Gamarnik (Fundación Instituto Leloir-CONICET, Buenos Aires, Argentina), uses two viral proteins as antigens—a trimer stabilized spike protein and the receptor binding domain (RBD). The presumed presence or absence of specific IgG antibodies against the SARS-CoV-2 virus was analyzed taking into account the cut-off value, which was defined as the mean optical density (OD) of the negative control +0.2, according to the manufacturer's instructions. We classified the level of immunoreactivity in each patients' FF based on their absorbance values: low (between 0.22 and 0.5), medium (between 0.5 and 1), and high (greater than 1).
The presence of SARS-CoV-2 viral antigens in FF was determined using the Panbio™ COVID-19 Ag Rapid Test Device (Abbott Diagnostics, Jena, Germany) following manufacturer's instructions.
VEGF concentrations in FF were measured with a commercial ELISA kit (Catalog# 900-TM10; Peprotech, NJ, United States), according to the manufacturer's instructions. IL-1β and IL-10 concentrations in FF were measured using commercials kits (IL-1β Catalog# 557953; IL-10 Catalog# 555157; BD Biosciences, CA, United States), as previously described by Gori et al. (24).
2.4. Granulosa and endothelial cells culture
Human granulosa cell lines are useful, well-known models to study the physiopathological mechanisms that govern follicular development and oocyte maturation in vitro. Therefore, we utilized the immortalized human granulosa cell line COV434 (25), which was donated by Dr. M Begoña Ruiz-Larrea (University of the Basque Country UPV/EHU, Leioa, Spain). COV434 cells were maintained in Dulbecco's Medium (DMEM, Invitrogen, NY, USA) with 10% fetal bovine serum (FBS) and 200 mM l-glutamine (Gibco, WI, USA), in the presence of 100 U/ml penicillin G and 100 mg/ ml streptomycin sulfate at 37 °C with 5% CO2.
As for EA.hy926, this is a continuous, cloneable human cell line that displays numerous features of vascular endothelial cells (26) and is a useful in vitro model for studying angiogenic processes in the ovary [27], [28], [29], [30]. EA.hy926 cells were donated by Dr. Gareth Owen (Pontifical Catholic University of Chile, Santiago, Chile). EA.hy926 cells were maintained in Iscove's Modified Dulbecco's Medium (IMDM, Invitrogen, NY, USA) with 10% FBS in the presence of 100 U/ml penicillin G and 100 mg/ ml streptomycin sulfate at 37 °C with 5% CO2. The number of passages used in both cell lines has not exceeded the 20th.
2.5. Western blot
For protein analysis, COV434 and EA.hy926 cells were seeded into 24-well cell culture plates at a density of 0.5*106 cells/well, allowed to adhere to the surface, and grown to confluence. Then, cells were incubated with FF (25% FF in media) from either control or post COVID-19 patients for 24 h at 37 °C. After treatment with FF, EA.hy926 or COV434 cells were lysed in lysis buffer (20 mM Tris–HCl pH 8, 137 mM NaCl, 1% Nonidet P-40 and 10% glycerol) supplemented with protease inhibitors (0.5 mM PMSF, 0.025 mMN-CBZ-l-phenylalanine chloromethyl ketone, 0.025 mMN-p-tosyl-lysine chloromethyl ketone and 0.025 mM l-1-tosylamide-2-phenyl–ethylchloromethyl ketone). The cell lysates were centrifuged at 10,000g for 10 min at 4 °C. Protein concentration was measured using the Bradford assay. After boiling for 5 min, 20 μg of protein was applied to a SDS–polyacrylamide gel, and electrophoresis was performed at 25 mA for 1.5 h. The resolved proteins were transferred for 2 h onto nitrocellulose membranes. The blot was preincubated in blocking buffer (5% nonfat milk, 0.05% Tween 20 in 20 mM TBS pH 8.0) for 1 h at room temperature and incubated overnight in blocking buffer at 4 °C with diluted primary antibodies as follows: β-actin 1:3000 (sc-1616), 3β-HSD 1:1000 (sc-30,820), ERα 1:100 (sc-787), ERβ 1:500 (sc-390,243), StAR 1:1000 (sc-25,806), purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, USA); VEGF 1:1000 (ab46154), γH2AX 1:1000 (ab26350), ANGPT-1 1:1000 (ab133425), ANGPT-2 1:1000 (ab180820) purchased from Abcam (Cambridge, USA); and GAPDH 1/8000 (#2118) from Cell Signaling Technology, Inc. (Danvers, MA, USA). The immunoblots were then incubated with HRP-conjugated secondary antibodies, namely anti-rabbit 1:1000 (A4914) (Sigma Aldrich), anti-mouse 1:1000 (HAF007) from R&D Systems (MN, USA) or anti-goat 1:2000 (#1721034), as required. Signal was detected by chemiluminescence. Protein levels were analyzed by densitometry using Scion Image for Windows (Scion Corporation, Worman's Mill, CT, USA). OD data are expressed as arbitrary units ± SEM. All blots shown were representative of at least three independent experiments.
2.6. Proliferation assay
EA.hy926 and COV434 cells were exposed for 24 h to control FF and post COVID-19 FF at 37 °C with 5% CO2, after which proliferation was determined using WST-1 reagent (4-[3-(4-Iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate; Roche Diagnostics, Mannheim, Germany), following the manufacturer's instructions. Briefly, after stimulation with FF, 10 μL of WST-1 was added to each well and cells were incubated for an additional 2 h. Absorbance was measured using a microplate reader at 450 nm and 620 nm. Experiments were conducted in triplicate.
2.7. Endothelial cell migration
A wound healing assay was performed using the EA.hy926 endothelial cell line to study the effect of FF on endothelial cell migration as previously described by Scotti et al. (2013, 2014, 2016) [27], [28], [30]. Briefly, EA.hy926 cells were detached by trypsinization, resuspended in IMDM, plated at a density of 3*105 cells per well in 24-well plates, and grown to confluence. Cell monolayers were wounded by a 1000 μl micropipette tip in one direction. After the injury, the cells were washed with PBS to remove cellular debris. The wounded cells were then incubated with FF (25%) either from control (n = 20) or post COVID-19 patients (n = 20). Serum-free DMEM/F12 was used as a negative control (n = 16). Cells were then incubated for 15 h at 37 °C. Cell migration was monitored at initial wounding (t 0 h) and at 12 h (t 12 h) under a phase-contrast microscope and pictures were acquired at the same magnification and location every time. The resulting cell migration was calculated as cell-free area at t 0 h – cell-free area at t 12 h and was expressed as a percentage of the mean migration of negative control wells (without FF). Endothelial cell migration in negative control wells (media without FF) is arbitrarily presented as 100%. We quantified the cell-free wounded areas using ImageJ software (National Institutes of Health, Bethesda, MD). Experiments were conducted in duplicate.
2.8. Statistical analysis
Statistical analyses were performed using the statistical software Prism v8.0 (GraphPad Software, San Diego, CA, US). Data are expressed as the mean ± SEM. Differences between groups were tested for significance using the independent samples Student's t-test for parametric variables. For endothelial cell migration, normally distributed data were analyzed using one-way ANOVA followed by Tukey's test for statistical comparison of the groups. Statistical significance was defined as p < 0.05.
3. Results
3.1. Characteristics of the study population and fertility parameters
The characteristics of the study population are shown in Table 1 . No significant differences were found in overall patient age (range, 21–41), which were 33.09 and 33.43 in control and post COVID-19 groups, respectively. Before starting the IVF procedure, patients underwent a general clinical examination. We registered multiple indicators, including BMI, antral follicle count (AFC), basal serum AMH, estradiol, progesterone, and prolactin, as well as estradiol levels on the day of ovulation trigger. There were no significant differences in these parameters when comparing post COVID-19 and control patients. It is worth mentioning that the time interval between the infection of the patients with SARS-CoV-2 and the retrieval of FF varied between 2 and 9 months, being the average 4.5 months. Subsequently, the patients in each group were subdivided into two groups according to their age (≤ 35 years and > 35 years) and we evaluated their response to hormonal stimulation. The results showed that a lower number of oocytes was retrieved from post COVID-19 patients over 35 years old than from age-matched control patients, whereas the number of oocytes retrieved in patients ≤35 years old did not differ between both groups. Oocyte maturation was also evaluated, but no significant differences were observed in the number or percentage of MII oocytes between both groups.
Table 1.
Clinical information of control patients and post COVID-19 patients.
| Baseline characteristics of patients |
Control patients (n = 34) |
Recovered COVID-19 patients (n = 46) |
P value |
||||
|---|---|---|---|---|---|---|---|
| Mean | Min–Max | SEM | Mean | Min–Max | SEM | ||
| Age (years) | 33.09 | 23–38 | 0.60 | 33.43 | 21–44 | 1.02 | n.s. |
| Number of oocytes retrieved in patients ≤ 35 years | 11.84 | 8–23 | 0.85 | 13.80 | 0–30 | 2.21 | n.s. |
| Number of oocytes retrieved in patients > 35 years | 11.11 | 6–16 | 0.95 | 6.95 | 0–15 | 0.95 | 0.0187 |
| MII oocytes (n, %) | 9.03 (79.84%) | 6–16 | 0.61 | 11.98 (82.23%) | 0–30 | 1.41 | n.s. |
| Basal serum estradiol (pg/ml) | 33.00 | 19–46 | 7.81 | 42.70 | 25–56 | 3.45 | n.s. |
| Serum estradiol on trigger day (pg/ml) | 2710 | 400–5772 | 576.9 | 1424 | 325–3728 | 1152 | n.s. |
| Basal serum progesterone (ng/ml) | 1.09 | 0.52–1.86 | 0.18 | 1.37 | 0.30–4.38 | 0.58 | n.s. |
| Basal serum prolactin (ng/ml) | 20.37 | 6.20–48 | 3.01 | 15.74 | 1–36.20 | 1.83 | n.s. |
| AMH (ng/ml) | 2.067 | 0.5–4.4 | 0.32 | 2.917 | 0.31–5.7 | 0.48 | n.s |
| Antral follicles count (AFC) | 12.64 | 7–20 | 0.77 | 12.50 | 4–22 | 0.99 | n.s |
| BMI | 23.43 | 18.70–31 | 0.98 | 23.01 | 18–29.36 | 0.55 | n.s |
| Time from COVID-19 infection (months) | – | – | – | 4.5 | 2–9 | 0.37 | – |
Data are expressed as the mean ± standard error of the mean. Student's t-test was used for comparisons between groups. Statistical significance was defined as <0.05.
3.2. Detection of IgG antibodies against SARS-COV-2 in FF from recovered patients
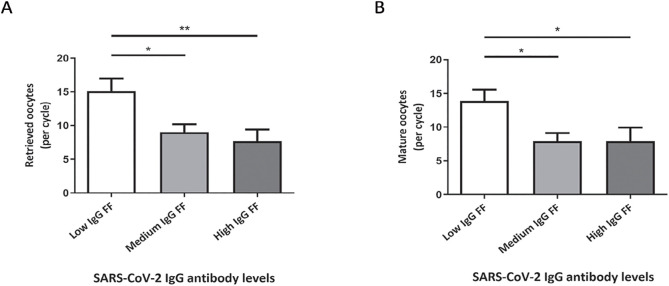
The presence of SARS-CoV-2 IgG antibodies was determined in FF from post COVID-19 and control patients (Table 2 ). The results revealed that 91.3% (42/46) of the FF from post-COVID-19 patients were positive for IgG against SARS-CoV-2, whereas antibodies were not detected in any of the FF from control patients, as expected. Within the post COVID-19 group, the ELISA assay yielded different colorimetric intensities, indicating varying levels of SARS-CoV-2 IgG, which we classified as high (38.1%; 16/42 patients), medium (38.1%; 16/42 patients) and low (23.8%; 10/42 patients). We found no correlation between SARS-CoV-2 IgG levels and the time from infection. We then evaluated the number of retrieved oocytes in each group and found that this parameter significantly decreased with higher titers of SARS-CoV-2 IgG antibodies (low vs. medium, p < 0.05; low vs. high, p < 0.01). Similar results were obtained for the number of mature oocytes (those that reached MII stage) from each patient (low vs. medium and high, p < 0.05). These findings are shown in Fig. 1 A-B. Additionally, we assessed the presence of SARS-CoV-2 viral antigens in FF from patients, but none of the samples presented positive results (data not shown).
Table 2.
Detection of IgG antibodies against SARS-CoV-2 by ELISA in control and recovered COVID-19 patients.
| Immunoreactivity | Negative (%) | Positive (%) |
||
|---|---|---|---|---|
| Low-IgG (%) | Medium-IgG (%) | High-IgG (%) |
||
| Control FF (n = 34) |
34/34 (100%) |
– | – | – |
| Post COVID-19 FF (n = 46) |
4/46 (8.7%) |
42/46 (91.3%) | ||
| 10/42 (23.8%) | 16/42 (38.1%) |
16/42 (38.1%) | ||
Of the 46 post COVID-19 patients, 91.30% tested positive for IgG antibodies against SARS-CoV-2 in FF. The titer of SARS-CoV-2 IgG antibodies in FF were classified according to their absorbance values as high (greater than 1), medium (between 0.5 and 1), and low (between 0.22 and 0.5).
Fig. 1.
Retrieved and mature oocytes from patients with low-, medium- and high-level SARS-CoV-2 IgG antibodies in FF. (A) The number of retrieved oocytes was significantly lower in the post COVID-19 subgroups as levels of SARS-CoV-2 IgG were higher (low vs. medium, *p < 0.05; low vs. high, **p < 0.01). (B) Similar results were obtained for the number of mature oocytes (low vs. medium and high, *p < 0.05).
3.3. VEGF, IL-1β, and IL-10 concentration in FF from control and post COVID-19 patients
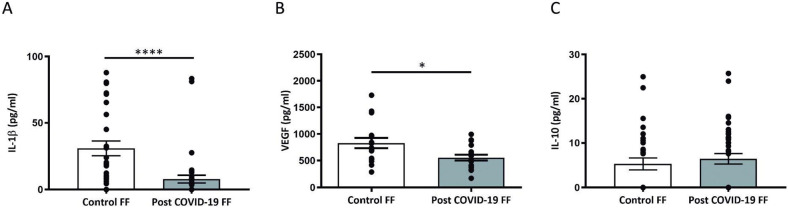
As shown in Fig. 2 A-B, IL-1β and VEGF concentrations were significantly lower in FF from post COVID-19 patients than those in FF from control patients (IL-1β: p < 0.0001,VEGF: p < 0.05). In contrast, the levels of IL-10 from post COVID-19 FF did not differ significantly from those in control FF (Fig. 2 C). Furthermore, no association was found between the IL-1β and VEGF levels in FF from post-COVID-19 patients and the time elapsed from the viral infection to the day of oocyte retrieval (data not shown).
Fig. 2.
VEGF, IL-1β and IL-10 concentration in control and post COVID-19 FF determined by ELISA. IL-1β (A) and VEGF(B) concentrations were decreased in FF from post COVID-19 compared with that in FF from control patients (VEGF: *p < 0.05, IL-1β: ****p < 0.0001). No differences were found between groups in terms of IL-10 levels (C) (p = 0.4).
3.4. Effects of FF from control and post COVID-19 patients on granulosa cell culture
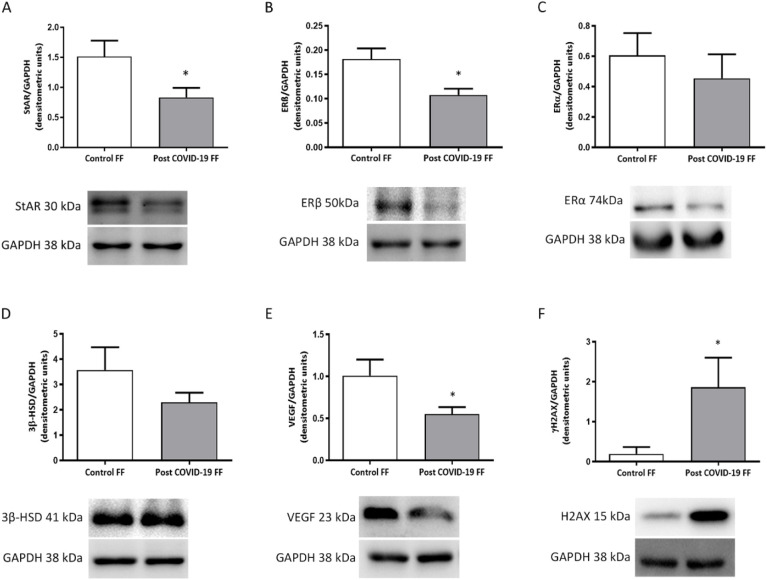
To evaluate whether granulosa cells could be affected by FF from recovered COVID-19 patients, we stimulated COV434 cells with control or post COVID-19 FF. As illustrated in Fig. 3 A-B, the analysis of endocrine-related proteins showed a significant decrease of StAR and ERβ in granulosa cells stimulated with post COVID-19 FF compared with those stimulated with control FF (p < 0.05). Protein expression of ERα and 3β-HSD remained unchanged between both groups (Fig. 3 C—D).
Fig. 3.
Effect of FF from post COVID-19 patients on protein expression in granulosa cells. The following proteins were measured by Western Blot: StAR (A); ERβ (B); ERα (C); 3β-HSD (D); VEGF (E); γH2AX (F). Densitometric quantification showed decreased levels of StAR (A; p < 0.05) and ERβ (B; p < 0.05) in cells stimulated with post COVID-19 FF, whereas protein levels of ERα (C) and 3β-HSD (D) remained unchanged between both groups. VEGF levels were significantly lower (p < 0.05) in COV434 cells incubated with FF from post COVID-19 patients compared with those incubated with control FF (E). Ovarian cells stimulated with FF from post COVID-19 patients expressed higher levels of γH2AX than cells stimulated with control FF (F; p < 0.05). In all cases, representative immunoblots are shown in the lower panels. Data are expressed as means ± SEM normalized to GAPDH. Results were obtained from three independent experiments. * p < 0.05.
Since VEGF is one of the most important angiogenic factors in the ovary, we measured VEGF protein expression in COV434 by Western blot. The densitometric analysis showed that granulosa cells stimulated with FF from post COVID-19 patients expressed lower levels of VEGF than those stimulated with FF from control patients (p < 0.05) (Fig. 3 E).
To study the impact of stimulation with FF from recovered COVID-19 patients on granulosa cells, we evaluated the protein expression of γH2AX, a molecular marker for DNA damage. The results showed that γH2AX expression in COV434 cells incubated with post COVID-19 FF was significantly higher than that in cells incubated with control FF (p < 0.05) (Fig. 3 F).
Finally, in order to determine whether FF from recovered COVID-19 patients altered granulosa cell proliferation, we quantified this parameter using WST-1. No differences were found in COV434 proliferation rates between the groups (data not shown).
3.5. Effect of FF from control and post COVID-19 patients on endothelial cell culture
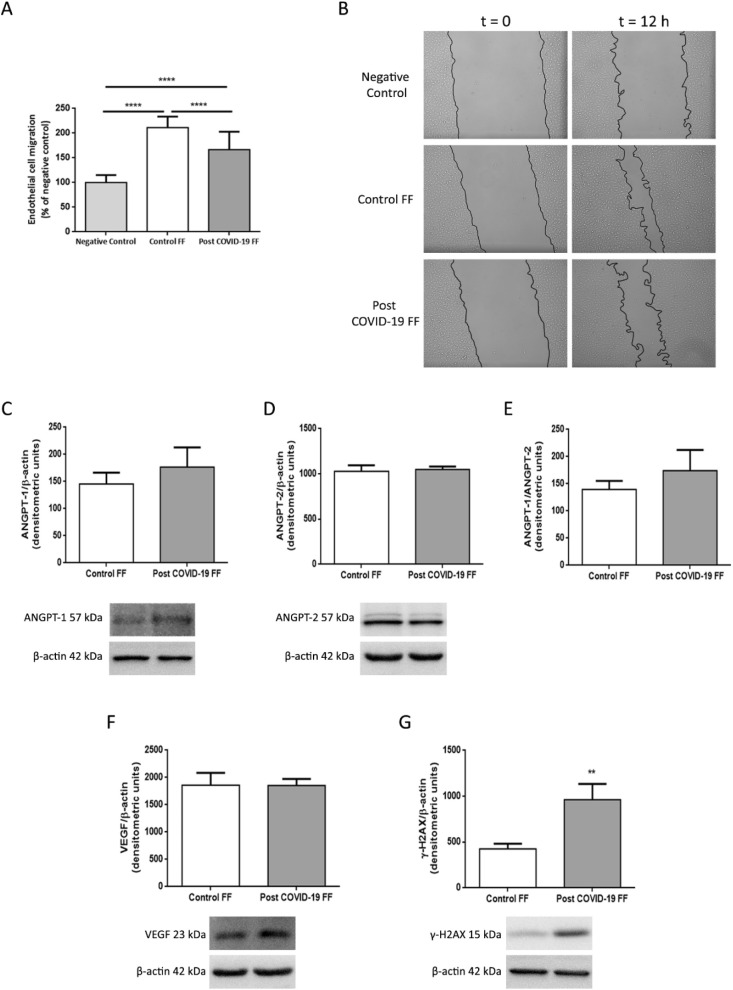
To analyze the specific effect of COVID-19 infection on ovarian angiogenesis, migration was quantified in EA.hy926 cells stimulated with FF from control and post COVID-19 patients using a wound healing assay. After 12 h, results showed that FF from post COVID-19 patients significantly decreased endothelial cell migration compared with FF from control patients (p < 0.0001) (Fig. 4 A-B). In addition, we analyzed ANGPT-1 and ANGPT-2 protein expression in endothelial cells and no changes were found between groups (Fig. 4 C-E), as well as in VEGF protein levels when cells were stimulated with either control or post COVID-19 FF (Fig. 4 F).
Fig. 4.
Effects of FF from recovered COVID-19 patients on endothelial cells
Endothelial migration of EA.hy926 cells stimulated with control or post COVID-19 FF.
(A) Quantification of the wound-healing assay. The columns show the percentage of endothelial cell migration normalized to the negative control, which is presented as 100%. Data are expressed as means ± SEM. (B) Representative images taken immediately after wound scratching (t 0) and after 12 h (t 12). Black lines represent the migration fronts. Effects of stimulation with control or post COVID-19 FF on the expression of ANGPT-1 (C); ANGPT-2 (D); ANGPT-1/ANGPT-2 (E); VEGF (F) and γH2AX (G) in EA.hy926 cells. The graphs show the densitometric analysis of protein levels. The density of each band was normalized to the density of the β-actin bands. Lower panels show representative blots for each protein analyzed. * p < 0.05.
Furthermore, we studied the effect of FF from recovered COVID-19 patients on DNA damage, as determined by endothelial cell expression of γH2AX. Protein levels of γH2AX in EA.hy926 cells incubated with FF from post COVID-19 patients were significantly higher than in those with FF from control patients (p < 0.01) (Fig. 4 G). Lastly, we evaluated whether endothelial cell proliferation was affected by the presence of FF from recovered COVID-19 patients, but no differences were found between both groups (data not shown).
4. Discussion
The data presented in this study demonstrate for the first time the presence of IgG antibodies against SARS-CoV-2 in FF from recovered COVID-19 patients undergoing ART treatments. Additionally, we demonstrated that VEGF and IL-1β levels in FF from post COVID-19 patients were decreased compared with those in the control group (patients that were never infected with SARS-CoV-2). FF contributes to maintaining the controlled microenvironment required to support female gamete development and is composed of a complex mixture of proteins, metabolites, and cytokines. Based on these considerations, several biochemical characteristics of the FF may determine oocyte quality and thus influence the potential reproductive performance.
In our study, in addition to detecting antibodies against SARS-CoV-2, we found that the numbers of retrieved oocytes as well as the mature oocytes were lower in the subgroups with higher SARS-CoV-2 IgG levels. Recently, Peghin et al. (2021) have indicated that the persistent high titers of the serological response against SARS-CoV-2 might play a crucial role as an independent risk factor for severe post COVID-19 symptoms, in addition to gender and the number of symptoms at onset and ICU admission (31).
A number of studies have reported that the presence of certain pathogens can affect the success of IVF treatments. Cortiñas et al. (2004) and Pacchiarotti et al. (2009) have demonstrated that high levels of anti-Chlamydia trachomatis IgG and IgA, both in serum and FF, harm implantation rate in women undergoing IVF [32], [33]. Therefore, it is reasonable to think that the high titers of SARS-COV-2 IgG antibodies could alter oocyte number and/or quality. Nonetheless, further research is necessary to elucidate whether the presence of immunoglobulins for SARS-CoV-2 adversely affects the reproductive outcome of women. In particular, the impact of these antibodies in FF on oocyte quality should be further explored and its assessment is beyond the objective of the present work.
Several of the growth factors and interleukins in FF are known to be associated with ovarian response and fertilization rates [17], [34], [35]. The follicles and corpora lutea are able to produce many of these, including numerous angiogenic factors. This is especially relevant since a functional ovarian microvasculature is crucial to guarantee the supply of cytokines, hormones, and oxygen that make follicular growth and corpus luteum formation possible.
VEGF, one of the central angiogenic factors, plays a key role in the regulation of normal and abnormal angiogenesis in the ovary (36). Inhibition of VEGF expression results in reduced follicle angiogenesis and lack of antral follicle development. In addition, it is well known that VEGF is involved in the ovulatory process. Accordingly, increased expression of VEGF after administration of an ovulatory dose of gonadotropins is correlated with prostaglandin concentration (37). In our study, VEGF levels in FF from recovered COVID-19 patients undergoing in vitro fertility treatments were lower than those in the control group. Decreased VEGF levels could lead to an abnormal development of the ovarian vasculature and, consequently, fail to provide nutrients and hormones to the growing follicles, affecting the oocyte quality.
In the ovary, cytokines and macrophages are intimately involved in follicular development, ovulation, and luteal function. Our study evidenced that IL-1β levels were decreased in FF from recovered COVID-19 patients compared with those in control FF. In particular, it has been reported that IL-1β promotes several processes associated with ovulation, as well as regulates folliculogenesis and atresia [38], [39]. Intra-ovarian macrophages, which represent from 5 to 15% of the total cellular population in FF (40), are responsible for the production of IL-1β (41), a cytokine that is also secreted by oocytes, granulosa, theca, and cumulus cells in human ovaries [42], [43], [44]. Additionally, IL-1β is hormonally regulated and its levels increase during the peri-ovulatory period [45], [46]. IL-1β, IL-6, and TNFα have been detected in FF of women undergoing in vitro fertilization [47], [48], and serum levels of IL-1β positively correlate with estradiol levels on the day of hCG injection (49). Furthermore, Mendoza et al. (2019) demonstrated that increased levels of IL-1β in FF were associated with enhanced fertilization rates (35). Although we did not find any correlation between IL-1β levels and the reproductive outcome in terms of retrieved or mature oocyte numbers, this IL-1β deficiency observed in FF from post COVID-19 patients could have implications for oocyte quality and for prospective reproductive outcomes.
Given that follicles developing healthy oocytes produce high levels of IL-1β and TNFα (35), it is therefore likely that both cytokines are involved in oocyte quality. IL-1β also induces the secretion of TNFα and directly increases vascular permeability (50) and, in turn, TNFα enhances new blood vessel growth during inflammatory processes (51). Based on these data from the literature, it could conceivably be hypothesized that lower IL-1β levels in post COVID-19 patients decrease TNFα production, which can lead to impaired blood vessel formation in the ovary. This explanation would be consistent with the decrease in VEGF levels observed in post COVID-19 FF. More studies are needed to elucidate the effect of these cytokines on ovarian angiogenesis in recovered COVID-19 patients.
To study the potentially detrimental consequences of altered FF composition in post COVID-19 women, we evaluated the effects of these FF on two pivotal cell types in the ovary—granulosa and endothelial cells. To this purpose, we stimulated a granulosa cell line (COV434) and an endothelial cell line (EA.hy926) with FF from either control or post COVID-19 patients and analyzed endocrine-related proteins, angiogenic markers, and nuclear DNA damage in these cells.
Endocrine-related proteins, such as steroidogenic enzymes and hormone receptors, are essential for ovarian function since follicular development depends on steroid hormone production. Despite being different, these hormones are all synthesized from a common precursor substrate: cholesterol. Since the rate-limiting step in follicular steroidogenesis is the transport of cholesterol to the site of steroid biosynthesis, this makes steroidogenic acute regulatory protein (StAR) a key player. Indeed, the StAR protein predominantly modulates steroid biosynthesis during the folliculogenesis. Furthermore, estrogens and their receptors, α and β, play a crucial role in the pathogenesis of gynecological disorders and/or cancers, i.e., endometriosis as well as breast, endometrial, and ovarian cancers [52], [53]. In the present study, we showed that FF from post COVID-19 patients decreased StAR and ERβ expression in human non-luteinized granulosa cells compared with those stimulated with control FF. Dang et al. (2017) have previously shown that cytokines such as IL-1β induce the expression of StAR and stimulate steroid synthesis in human granulosa-lutein cells (54). Taken together, these data suggest that low IL-1β levels detected in FF from post COVID-19 patients could be partially responsible for the decreased StAR expression and alter the steroid synthesis in granulosa cells, thus affecting oocyte development and maturation.
As described above, VEGF is the main angiogenic factor involved in the formation of microvasculature within ovarian follicles (55). Other angiogenic factors, such as angiopoietins, are required for the maturation of newly formed blood vessels. Previously, we showed in a rat model that inhibition of VEGF and ANGPT-1 causes an imbalance in the ratio of antiapoptotic: proapoptotic proteins that leads more follicles to atresia [56], [57]. In the present study, stimulation with FF from post COVID-19 patients resulted in a significant decrease in endothelial cell migration compared with that of control FF. This finding is consistent with the decrease in VEGF concentration that we observed in post COVID-19 FF. Indeed, altered endothelial migration could be a direct consequence of low VEGF concentrations since this affects new blood vessel formation.
Conversely, several studies, including ours, have demonstrated the cytoprotective effect of VEGF in the bovine and rat ovary [56], [58]. Here, we showed that FF from post COVID-19 patients significantly decreased the expression of VEGF in non-luteinized human granulosa cells. Even though the presence of post-COVID-19 FF did not seem to influence granulosa cell proliferation compared with that of control FF, our results indicate that decreased VEGF levels might affect follicular cell function and, consequently, damage oocytes.
Double-strand breaks (DSBs) affect the stability of the genome and represent one of the most critical lesions for cell survival (59). γH2AX is a well-known marker for the detection of chromatin modifications linked to DNA damage and is used to assess various cellular processes such as aging, cancer, and inflammation [60], [61], [62]. In particular, γH2AX is utilized to predict chronic inflammatory conditions that precede cancer as well as cardiovascular and nervous system disorders. Moreover, the influx of viral antigens can cause an inflammatory response, making γH2AX a potential marker of viral infection. For instance, Nichols et al. (2009) observed that increased γH2AX levels were induced by replicating viral proteins during adenovirus infection (63). In our study, we demonstrated that FF from recovered COVID-19 patients increased γH2AX levels compared with FF from control patients in both endothelial and granulosa cells. Therefore, a possible explanation for this is that systemic and/or local infection of SARS-CoV-2 may promote the entry of lymphocytes and macrophages to the ovary. This would affect the synthesis of pro- and anti-inflammatory cytokines that regulate the release of reactive oxygen species, possibly leading to disruption of the DNA integrity of follicular and endothelial cells.
In conclusion, the results described for the first time in this study evidence that infection with SARS-CoV-2 could damage ovarian function, alter the follicular microenvironment and potentially affect reproductive outcomes. Our results indicate that this viral infection leads to the presence of IgG antibodies against SARS-CoV-2 in FF, in addition to decreased VEGF and IL-1β levels in FF. We also found a negative relationship between SARS-CoV-2 IgG levels in FF and the numbers of retrieved and mature oocytes from the same patients, further corroborating that COVID-19 might jeopardize reproductive outcomes. Additionally, post COVID-19 FF alters steroidogenic parameters and VEGF expression in granulosa cells as well as impair migration in endothelial cells. Moreover, these FF severely damage DNA stability and integrity in both granulosa and endothelial cells. Further research on SARS-CoV-2 infection and its impact on ovarian microvasculature and folliculogenesis is of the essence. In particular, elucidating which FF components in the ovarian microenvironment have a negative impact on oocytes should be examined more closely.
One of the limitations of our study is that the enrolled patients were analyzed 3 to 9 months after SARS-CoV-2 infection. Therefore, more studies are needed to evaluate whether these ovarian alterations can be reverted after longer periods of time, which would allow physicians to design an optimal fertility protocol for patients recovered from COVID-19 and to prevent potential complications during the ART treatments due to a recent SARS-CoV-2 infection. Studies with a larger population size could provide more definite evidence on the reproductive performance of recovered COVID-19 female patients.
5. Conclusion
Greater efforts are needed to ensure that COVID-19 is taken into account as a relevant factor influencing female reproduction and to use this information to further improve clinical interventions and public health policies. Finally, our study provides a solid groundwork for future research to continue evaluating the potential effects of SARS-CoV-2 infection on ovarian function and its implications on women's fertility.
Funding information
This study was supported by Ferring Covid-19 Investigational Grant (2020); National Agency for Scientific and Technological Promotion (ANPCyT) (PICT 1117-2015 and PICT 1603-2017); and the Baron and Williams Foundations, Argentina.
CRediT authorship contribution statement
Yamila Herrero: Investigation, Conceptualization, Formal analysis, Data curation, Validation, Visualization, Writing – review & editing. Natalia Pascuali: Investigation, Data curation, Writing – review & editing. Candela Velázquez: Investigation, Data curation. Gonzalo Oubiña: Investigation, Data curation. Vanesa Hauk: Investigation, Data curation.
Ignacio de Zúñiga: Collection of clinical samples. Mariana Gómez Peña: Collection of clinical samples. Gustavo Martínez: Collection of clinical samples. Mariano Lavolpe: Collection of clinical samples. Florencia Veiga: Collection of clinical samples. Fernando Neuspiller: Collection of clinical samples. Dalhia Abramovich: Writing – review & editing. Leopoldina Scotti: Investigation, Data curation, Writing – review & editing. Fernanda Parborell: Conceptualization, Supervision, Formal analysis, Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration.
All authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr. Andrea Gamarnik (Fundación Instituto Leloir-CONICET, Buenos Aires, Argentina) for donating the tests to evaluate IgG antibody responses to SARS-CoV-2.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) dashboard. 2021 May 22. https://covid19.who.int/ Available from:
- 2.John Hopkins University Center for Systems Science and Engineering Coronavirus Resource Center 2021 May 22. https://coronavirus.jhu.edu/map.html Available from:
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss S.A., Seo C., Carrier M., Jetty P. From cellular function to global impact: the vascular perspective on COVID-19. Can. J. Surg. 2021;64(3):E289–E297. doi: 10.1503/cjs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teuwen L.-A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albini A., Di Guardo G., Noonan D.M., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor-and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Phys. Heart Circ. Phys. 2008;295(4):H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 9.Sharma I., Kumari P., Sharma A., Saha S.C. SARS-CoV-2 and the reproductive system: known and the unknown.!! Middle East Fertil. Soc. J. 2021;26(1):1. doi: 10.1186/s43043-020-00046-z. 2021/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020:1–8. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz-Silva J., Carneiro M., Ferreira M., Pinheiro S., Silva D., Silva A., et al. The vasoactive peptide angiotensin-(1–7), its receptor mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod. Sci. 2009;16(3):247–256. doi: 10.1177/1933719108327593. [DOI] [PubMed] [Google Scholar]
- 12.Valdes G., Neves L., Anton L., Corthorn J., Chacon C., Germain A., et al. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27(2–3):200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Reis F.M., Bouissou D.R., Pereira V.M., Camargos A.F., dos Reis A.M., Santos R.A. Angiotensin-(1–7), its receptor mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil. Steril. 2011;95(1):176–181. doi: 10.1016/j.fertnstert.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 14.Barreta M.H., Gasperin B.G., Ferreira R., Rovani M., Pereira G.R., Bohrer R.C., et al. The components of the angiotensin-(1–7) system are differentially expressed during follicular wave in cattle. J. Renin-Angiotensin-Aldosterone Syst. 2015;16(2):275–283. doi: 10.1177/1470320313491996. [DOI] [PubMed] [Google Scholar]
- 15.Pereira V.M., Reis F.M., Santos R.A., Cassali G.D., Santos S.H., Honorato-Sampaio K., et al. Gonadotropin stimulation increases the expression of angiotensin-(1–7) and MAS receptor in the rat ovary. Reprod. Sci. 2009;16(12):1165–1174. doi: 10.1177/1933719109343309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi L., Gagliardi A., Landi C., Focarelli R., De Leo V., Luddi A., et al. Protein pathways working in human follicular fluid: the future for tailored IVF? Expert Rev. Mol. Med. 2016;18 doi: 10.1017/erm.2016.4. [DOI] [PubMed] [Google Scholar]
- 17.Chen F., Spiessens C., D’Hooghe T., Peeraer K., Carpentier S. Follicular fluid biomarkers for human in vitro fertilization outcome: proof of principle. Proteome Sci. 2016;14(1):1–11. doi: 10.1186/s12953-016-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y.-T., Wu Y., Zhang J.-Y., Hou N.-N., Liu A.-X., Pan J.-X., et al. Preliminary proteomic analysis on the alterations in follicular fluid proteins from women undergoing natural cycles or controlled ovarian hyperstimulation. J. Assist. Reprod. Genet. 2015;32(3):417–427. doi: 10.1007/s10815-014-0419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Revelli A., Delle Piane L., Casano S., Molinari E., Massobrio M., Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009;7(1):1–13. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagheri S.H., Asghari A., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K., et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med. J. Islam Repub. Iran. 2020;34:62. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye R., Chang C.D., Kazahaya K., Brereton J., Denneny J.C., III COVID-19 anosmia reporting tool: initial findings. Otolaryngol. Head Neck Surg. 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 22.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojeda D.S., Gonzalez Lopez Ledesma M.M., Pallarés H.M., Costa Navarro G.S., Sanchez L., Perazzi B. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gori S., Soczewski E., Fernández L., Grasso E., Gallino L., Merech F., et al. Decidualization process induces maternal monocytes to tolerogenic IL-10-producing dendritic cells (DC-10) Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Vollmer M., De Geyter M., Litzistorf Y., Ladewig A., Dürrenberger M., et al. Characterization of an immortalized human granulosa cell line (COV434) Mol Human Reprod. 2000;6(2):146–153. doi: 10.1093/molehr/6.2.146. [DOI] [PubMed] [Google Scholar]
- 26.Edgell C.J., McDonald C.C., Graham J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl Acad. Sci. USA. 1983;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. 6/1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scotti L., Abramovich D., Pascuali N., Oubina A., Kopcow L., de Z I. Involvement of the ANGPTs/Tie-2 system in ovarian hyperstimulation syndrome (OHSS) Mol. Cell. Endocrinol. 2013;365(2):223–230. doi: 10.1016/j.mce.2012.10.022. 1/30/2013. [DOI] [PubMed] [Google Scholar]
- 28.Scotti L., Parborell F., Irusta G., Bisioli C., Pettorossi H., de Z I. Platelet-derived growth factor BB and DD and angiopoietin1 are altered in follicular fluid from polycystic ovary syndrome patients. Mol. Reprod. Dev. 2014;81(8):748–756. doi: 10.1002/mrd.22343. 8/2014. [DOI] [PubMed] [Google Scholar]
- 29.Scotti L., Abramovich D., Pascuali N., Durand L.H., Irusta G., de Zúñiga I., et al. Inhibition of angiopoietin-1 (ANGPT1) affects vascular integrity in ovarian hyperstimulation syndrome (OHSS) Reprod. Fertil. Dev. 2016;28(6):690–699. doi: 10.1071/RD13356. [DOI] [PubMed] [Google Scholar]
- 30.Scotti L., Di Pietro M., Pascuali N., Irusta G., Zuniga Id., Gomez Pena M., et al. Sphingosine-1-phosphate restores endothelial barrier integrity in ovarian hyperstimulation syndrome. Mol. Hum. Reprod. 2016 Dec;22(12):852–866. doi: 10.1093/molehr/gaw065. [DOI] [PubMed] [Google Scholar]
- 31.Peghin M., Palese A., Venturini M., De Martino M., Gerussi V., Graziano E., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021;27(10):1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortinas P., Munoz M.G., Loureiro C.L., Pujol F.H. Follicular fluid antibodies to chlamydia trachomatis and human heat shock protein-60 kDa and infertility in women. Arch. Med. Res. 2004;35(2):121–125. doi: 10.1016/j.arcmed.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Pacchiarotti A., Sbracia M., Mohamed M.A., Frega A., Pacchiarotti A., Espinola S.M., et al. Autoimmune response to chlamydia trachomatis infection and in vitro fertilization outcome. Fertil. Steril. 2009;91(3):946–948. doi: 10.1016/j.fertnstert.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Ocal P., Aydin S., Cepni I., Idil S., Idil M., Uzun H., et al. Follicular fluid concentrations of vascular endothelial growth factor, inhibin a and inhibin B in IVF cycles: are they markers for ovarian response and pregnancy outcome? Eur. J. Obstet. Gynecol. Reprod. Biol. 2004;115(2):194–199. doi: 10.1016/j.ejogrb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza C., Cremades N., Ruiz-Requena E., Martinez F., Ortega E., Bernabeu S., et al. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum. Reprod. 1999;14(3):628–635. doi: 10.1093/humrep/14.3.628. [DOI] [PubMed] [Google Scholar]
- 36.Kaczmarek M.M., Schams D., Ziecik A.J. Role of vascular endothelial growth factor in ovarian physiology-an overview. Reprod. Biol. 2005;5(2):111–136. [PubMed] [Google Scholar]
- 37.Knobil E., Neill J.D. 3 Rev Ed edition. Vol. 2005. 2005. Physiology of Reproduction. [Google Scholar]
- 38.Bonello N., McKie K., Jasper M., Andrew L., Ross N., Braybon E., et al. Inhibition of nitric oxide: effects on interleukin-lβ-enhanced ovulation rate, steroid hormones, and ovarian leukocyte distribution at ovulation in the rat. Biol. Reprod. 1996;54(2):436–445. doi: 10.1095/biolreprod54.2.436. [DOI] [PubMed] [Google Scholar]
- 39.Kaipia A., Hsueh A.J. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997;59(1):349–363. doi: 10.1146/annurev.physiol.59.1.349. [DOI] [PubMed] [Google Scholar]
- 40.Loukides J.A., Loy R.A., Edwards R., Honig J., Visintin I., Polan M.L. Human follicular fluids contain tissue macrophages. J. Clin. Endocrinol. Metab. 1990;71(5):1363–1367. doi: 10.1210/jcem-71-5-1363. [DOI] [PubMed] [Google Scholar]
- 41.Machelon V., Nome F., Durand-Gasselin I., Emilie D. Macrophage and granulosa interleukin-1β mRNA in human ovulatory follicles. Hum. Reprod. 1995;10(8):2198–2203. doi: 10.1093/oxfordjournals.humrep.a136268. [DOI] [PubMed] [Google Scholar]
- 42.Zolti M., Ben-Rafael Z., Meirom R., Shemesh M., Bider D., Mashiach S., et al. Cytokine involvement in oocytes and early embryos. Fertil. Steril. 1991;56(2):265–272. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
- 43.Barak V., Yanai P., Treves A.J., Roisman I., Simon A., Laufer N. Interleukin-1: local production and modulation of human granulosa luteal cells steroidogenesis. Fertil. Steril. 1992;58(4):719–725. doi: 10.1016/s0015-0282(16)55318-7. [DOI] [PubMed] [Google Scholar]
- 44.José de los Santos M., Anderson D.J., Racowsky C., Simón C., Hill J.A. Expression of interleukin-1 system genes in human gametes. Biol. Reprod. 1998;59(6):1419–1424. doi: 10.1095/biolreprod59.6.1419. [DOI] [PubMed] [Google Scholar]
- 45.Machelon V., Emilie D. Production of ovarian cytokines and their role in ovulation in the mammalian ovary. Eur. Cytokine Netw. 1997;8(2):137–143. [PubMed] [Google Scholar]
- 46.Hurwitz A., Loukides J., Ricciarelli E., Botero L., Katz E., McAllister J.M., et al. Human intraovarian interleukin-1 (IL-1) system: highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J. Clin. Invest. 1992;89(6):1746–1754. doi: 10.1172/JCI115777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L., Norman R. Concentrations of immunoreactive interleukin-1 and interleukin-2 in human preovulatory follicular fluid. Hum. Reprod. 1992;7(2):147–150. doi: 10.1093/oxfordjournals.humrep.a137607. [DOI] [PubMed] [Google Scholar]
- 48.Wang L.J., Brännström M., Robertson S.A., Norman R.J. Tumor necrosis factor α in the human ovary: presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil. Steril. 1992;58(5):934–940. doi: 10.1016/s0015-0282(16)55438-7. [DOI] [PubMed] [Google Scholar]
- 49.Wang X.-F., Xing F.-Q., Chen S.-L. Interleukin-1beta expression on ovarian granulosa cells and its clinical implication in women undergoing in vitro fertilization. Di 1 Jun Yi Da Xue Xue Bao. 2002;22(10):934–936. [PubMed] [Google Scholar]
- 50.Dinarello C.A. The interleukin-1 family: 10 years of discovery 1. FASEB J. 1994;8(15):1314–1325. [PubMed] [Google Scholar]
- 51.Leibovich S.J., Polverini P.J., Shepard H.M., Wiseman D.M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 52.Bulun S.E., Lin Z., Imir G., Amin S., Demura M., Yilmaz B., et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol. Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 53.Bulun S.E., Simpson E.R. Aromatase expression in women’s cancers. Innov. Endocrinol. Cancer. 2008:112–132. doi: 10.1007/978-0-387-78818-0_8. [DOI] [PubMed] [Google Scholar]
- 54.Dang X., Zhu Q., He Y., Wang Y., Lu Y., Li X., et al. IL-1 β upregulates StAR and progesterone production through the ERK1/2-and p38-mediated CREB signaling pathways in human granulosa-lutein cells. Endocrinology. 2017;158(10):3281–3291. doi: 10.1210/en.2017-00029. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77(7):527–543. doi: 10.1007/s001099900019. 7/1999. [DOI] [PubMed] [Google Scholar]
- 56.Abramovich D., Parborell F., Tesone M. Effect of a vascular endothelial growth factor (VEGF) inhibitory treatment on the folliculogenesis and ovarian apoptosis in gonadotropin-treated prepubertal rats. Biol. Reprod. 2006;75(3):434–441. doi: 10.1095/biolreprod.106.051052. 9/2006. [DOI] [PubMed] [Google Scholar]
- 57.Parborell F., Abramovich D., Tesone M. Intrabursal administration of the antiangiopoietin 1 antibody produces a delay in rat follicular development associated with an increase in ovarian apoptosis mediated by changes in the expression of BCL2 related genes. Biol. Reprod. 2008;78(3):506–513. doi: 10.1095/biolreprod.107.063610. 3/2008. [DOI] [PubMed] [Google Scholar]
- 58.Greenaway J., Connor K., Pedersen H.G., Coomber B.L., Petrik J., LaMarre J. Vascular endothelial growth factor and its receptor, Flk-1/KDR, are cytoprotective in the extravascular compartment of the ovarian follicle. Endocrinology. 2004;145(6):2896–2905. doi: 10.1210/en.2003-1620. 6/2004. [DOI] [PubMed] [Google Scholar]
- 59.Lieber M.R., Ma Y., Pannicke U., Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat. Rev. Mol. Cell Biol. 2003;4(9):712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 60.Rogakou E.P., Pilch D.R., Orr A.H., Ivanova V.S., Bonner W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 61.Bonner W.M., Redon C.E., Dickey J.S., Nakamura A.J., Sedelnikova O.A., Solier S., et al. γH2AX and cancer. Nat. Rev. Cancer. 2008;8(12):957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedelnikova O.A., Horikawa I., Zimonjic D.B., Popescu N.C., Bonner W.M., Barrett J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004;6(2):168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 63.Nichols G.J., Schaack J., Ornelles D.A. Widespread phosphorylation of histone H2AX by species C adenovirus infection requires viral DNA replication. J. Virol. 2009;83(12):5987–5998. doi: 10.1128/JVI.00091-09. [DOI] [PMC free article] [PubMed] [Google Scholar]