Abstract
The basolateral amygdala (BLA) is a critical brain region for cocaine-memory reconsolidation. Corticotropin-releasing factor receptor type 1 (CRFR1) is densely expressed in the BLA, and CRFR1 stimulation can activate intra-cellular signaling cascades that mediate memory reconsolidation. Hence, we tested the hypothesis that BLA CRFR1 stimulation is necessary and sufficient for cocaine-memory reconsolidation. Using an instrumental model of drug relapse, male and female Sprague-Dawley rats received cocaine self-administration training in a distinct environmental context over 10 days followed by extinction training in a different context over 7 days. Next, rats were re-exposed to the cocaine-paired context for 15 min to initiate cocaine-memory retrieval and destabilization. Immediately or 6 h after this session, the rats received bilateral vehicle, antalarmin (CRFR1 antagonist; 500 ng/hemisphere), or corticotropin-releasing factor (CRF; 0.2, 30 or 500 ng/hemisphere) infusions into the BLA. Resulting changes in drug context-induced cocaine seeking (index of context-cocaine memory strength) were assessed three days later. Female rats self-administered more cocaine infusions and exhibited more extinction responding than males. Intra-BLA antalarmin treatment immediately after memory retrieval (i.e., when cocaine memories were labile), but not 6 h later (i.e., after memory reconsolidation), attenuated drug context-induced cocaine seeking at test independent of sex, relative to vehicle. Conversely, intra-BLA CRF treatment increased this behavior selectively in females, in a U-shaped dose-dependent fashion. In control experiments, a high (behaviorally ineffective) dose of CRF treatment did not reduce BLA CRFR1 cell-surface expression in females. Thus, BLA CRFR1 signaling is necessary and sufficient, in a sex-dependent manner, for regulating cocaine-memory strength.
Keywords: corticotropin-releasing factor, memory reconsolidation, basolateral amygdala, cocaine self-administration, sex differences
1. Introduction
In the course of chronic drug use, drug-associated environmental stimuli gain powerful control over goal-directed behavior in individuals with substance use disorders (SUDs1). For instance, exposure to cocaine-predictive stimuli leads to the retrieval of context-cocaine memories and evokes drug craving in cocaine users (Ehrman et al. 1992; O’Brien et al. 1992; Foltin and Haney 2000). However, retrieval can also elicit the destabilization of context-cocaine associative memories, and the maintenance of such labile memories requires protein synthesis-dependent reconsolidation into long-term memory stores (Tronson and Taylor 2007; Sorg 2012). Interference with memory reconsolidation reduces conditioned stimulus- and drug context-induced cocaine-seeking behaviors in rats (Lee et al. 2006; Fuchs et al. 2009; Sanchez et al. 2010), and it has been theorized that therapeutic disruption of drug-memory reconsolidation may promote recovery from SUDs (Taylor et al. 2009; Zhao et al. 2011; Sorg 2012; Saladin et al. 2013; Kroes et al. 2016; Paulus et al. 2019; but see Pachas et al. 2015). Accordingly, further research into the pharmacological mechanisms of cocaine-memory reconsolidation is needed to identify suitable therapeutic targets.
The basolateral amygdala (BLA) is a brain region that is critically involved in the reconsolidation of cocaine-associated memories and the subsequent expression of drug context-induced drug-seeking behavior and conditioned place preference (Valjent et al. 2006; Fuchs et al. 2009; Sanchez et al. 2010). Within the BLA, cocaine-memory reconsolidation is dependent on β-adrenergic (Bernardi et al. 2006; Fricks-Gleason and Marshall 2008; Milton et al. 2008b) and NMDA glutamate (Milton et al. 2008a) receptor signaling. Potentially linked to the stimulation of these receptors, cocaine-memory reconsolidation requires the activation of intracellular signaling molecules, including protein kinase A (PKA) (Sanchez et al. 2010; Arguello et al. 2014) and extracellular signal-regulated kinase (ERK) (Valjent et al. 2006; Wells et al. 2013), and the expression of immediate early-genes (IEGs), such as zinc finger 268 (Lee et al. 2005), in the BLA. Similar to β-adrenergic receptor stimulation, corticotropin-releasing factor receptor type 1 (CRFR1) stimulation can initiate PKA (Chen et al. 1993; Kuryshev et al. 1995) and ERK (Kageyama et al. 2007; Van Kolen et al. 2010) activation and IEG expression (Rostkowski et al. 2013), but the contribution of CRFR1 to cocaine-memory reconsolidation has not been investigated.
Corticotropin-releasing factor (CRF) is a neuropeptide that is widely expressed in the rodent brain (Swanson et al. 1983) and involved in memory processing in the amygdala (Liang and Lee 1988; Lee and Sung 1989; Roozendaal et al. 2002; Roozendaal et al. 2008). It is released in response to drug-associated stimuli (Sinha et al. 2003) that trigger drug-memory retrieval and subsequent memory destabilization, as well as in response to other affective stimuli (Merali et al. 1998; Holly et al. 2016). CRF stimulates CRFR1, which is densely expressed in the BLA (Potter et al. 1994). Furthermore, CRFR1 agonist administration into the BLA promotes (Roozendaal et al. 2008), while CRFR1 antagonist administration inhibits (Hubbard et al. 2007), fear-memory consolidation. However, the contribution of BLA CRFR1 signaling to memory reconsolidation, in general, or to cocaine-memory reconsolidation, in particular, has not been evaluated.
The present study investigated the necessity and sufficiency of BLA CRF signaling in cocaine-memory reconsolidation using an instrumental model of drug relapse. To this end, male and female rats were trained to self-administer cocaine infusions in a distinct environmental context to establish context-cocaine associative memories. Responding was then extinguished in a different context. Seven days after the last drug self-administration session, the rats were briefly re-exposed to the previously cocaine-paired context to destabilize cocaine memories. Rats received CRFR1-selective antagonist or exogenous CRF administration into the BLA immediately after cocaine-memory retrieval (i.e., while cocaine memories were labile) or 6 h later (i.e., after memory reconsolidation; control), and the effects of these manipulations on cocaine-memory strength were assessed three days later based on the magnitude of drug-seeking behavior in the cocaine-paired context. Potential effects of intra-BLA CRF administration on CRFR1 cell-surface expression and the protracted effects of intra-BLA CRFR1 antagonist and agonist administration on general locomotor activity were also assessed in follow-up experiments.
2. Methods
2.1. Subjects
Sprague-Dawley male (N = 57; 275–300 g at the start of the experiment) and female (N = 88; 240–280 g at the start of the experiment) rats were housed individually on a reversed light cycle (lights off at 6:00 AM) with ad libitum access to water and 20–25 g of standard rat chow per day. Rats were acclimated to handling prior to experimentation. The housing and care of animals was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and approved by the Washington State University Institutional Animal Care and Use Committee.
2.2. Food training
Rats received a 16-h overnight food-training session to facilitate subsequently the acquisition of lever responding for un-signaled cocaine infusions. During the food-training session, rats were placed into standard operant-conditioning chambers (26 × 27 × 27 cm; Coulbourn Instruments, Allentown, PA) housed within sound-attenuation cubicles (Med Associates, Fairfax, Vermont, USA). Each chamber was equipped with two levers, a water bottle with sipper tube, a food tray, and a food-pellet dispenser controlled by Graphic State Notation software version 4.1.04 (Coulbourn). Water was available ad libitum throughout the session. Responses on a designated active lever resulted in food reinforcement (45-mg grain-based pellets; Bio-Serv, Flemington, NJ) under continuous reinforcement schedule. Responses on a second, inactive lever had no programmed consequences. During the session, rats had no access to the visual, olfactory, tactile, and auditory stimuli or to the chambers that were later paired with cocaine access.
2.3. Surgery
Rats were deeply anesthetized using ketamine and xylazine (100.0 mg/kg and 5.0 mg/kg, i.p., respectively). Intravenous catheters were constructed as described previously (Fuchs et al. 2007). The tip of the catheter was inserted into the right jugular vein. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades.
After the catheter surgery, the rats received bilateral guide cannula implants. Small holes were drilled into the skull, and stainless-steel guide cannulae (P1 Technologies, Roanoke, Virginia, USA) were positioned 2 mm dorsal to the BLA (DV −6.5 mm, AP −2.7 mm, ML +/−4.8 mm relative to bregma). The guide cannulae were affixed to the skull using dental cement and jewelers’ screws. Perioperative analgesic treatment was provided for 24 hours prior to (bacon-flavored placebo tablet, 5 g/tablet; Bio-Serv) and for at least 48 hours after (bacon-flavored Rimadyl® MD tablets, 2 mg carprofen/5-g tablet, Bio-Serv; 5–8 mg/kg) surgery.
Rats received five days for post-surgical recovery, during which time the catheters were flushed daily with an antibiotic solution of cefazolin (1.0 mg/0.1 mL; West-Ward, Eatontown, NJ; dissolved in 70 U/mL heparinized saline, Sagent Pharmaceuticals, Schaumburg, IL) followed by heparinized saline (0.1 mL, 10 U/mL) to help maintain catheter patency. The catheter and cannulae were capped when not in use to prevent clogging (P1 Technologies). Catheter patency was assessed periodically using propofol (1 mg/0.1 mL; Actavis Pharma, Parsippany, NJ), a short-acting anesthetic that produces rapid, temporary loss of muscle tone when administered intravenously.
2.4. Drug self-administration and extinction training
Drug self-administration training was conducted in operant-conditioning chambers configured to form two contexts. Context 1 consisted of a continuous red house light (0.4 fc-brightness), intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine scented air freshener (Car Freshener Corp., Watertown, NY), and wire-mesh flooring (26 cm × 27 cm). Context 2 consisted of an intermittent white stimulus light over the inactive lever (1.2-fc brightness; 2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener (Sopus Products, Moorpark, CA), and a slanted ceramic tile wall that bisected the bar flooring (19 cm × 27 cm). Rats were randomly assigned to context 1 or context 2 for cocaine self-administration training.
Drug self-administration training sessions took place during the rats’ dark cycle. During each session, active-lever responses resulted in the delivery of i.v. cocaine infusions (0.5 mg/kg; 50-μL infusion delivered over 2 s; NIDA Drug Supply Program, Research Triangle Park, NC) under a fixed ratio 1 reinforcement schedule with a 20-s timeout period following each infusion. During the timeout period, active-lever responses had no scheduled consequences. Inactive-lever responses had no programmed consequences at any time. Training continued for a minimum of 10 days or until rats reached the acquisition criterion of ≥10 infusions/session during at least ten 2-h sessions. Rats then received daily 2-h extinction sessions in the alternate context. During the extinction sessions, lever responses were recorded on both levers but had no programmed consequences. The number of extinction sessions was set to seven to control memory age at the time of memory reactivation. Immediately after extinction session 4, the rats were acclimated to the intracranial infusion procedure. Stainless-steel injection cannulae (33-gauge; P1 Technologies) were inserted into the guide cannulae to a depth of 2 mm below the tip of the guide cannulae. Injector cannulae remained in place for 4 min with no infusion of fluids, while the rats were held gently by the experimenter.
2.5. Memory reactivation
On post-cocaine day 8, all rats were placed into the cocaine-paired context for 15 min to elicit the retrieval and destabilization of context-cocaine memories. Session length was selected based on parametric research indicating that it is sufficient to destabilize cocaine memories without producing overt behavioral extinction (Fuchs et al. 2009). During the memory reactivation session, the rats were connected to the infusion lines to allow for similar perception of the context as during cocaine self-administration training. The levers were extended, but lever responses were not reinforced so as to eliminate the acute pharmacological effects of cocaine on CRF in the amygdala (Sarnyai et al. 1993).
2.6. Experiment 1: Effects of post-reactivation CRFR1 antagonism in the BLA on drug context-induced cocaine seeking three days later
Experiment 1 was designed to determine whether CRFR1 stimulation was necessary for cocaine-memory reconsolidation. Rats were assigned to receive bilateral intra-BLA infusions of vehicle (0.5 μL/hemisphere; 100% dimethyl sulfoxide; DMSO) or the CRFR1-selective antagonist, antalarmin (500 ng per 0.5 μL/hemisphere; Sigma-Aldrich), immediately after the memory-reactivation session to disrupt CRFR1 signaling during memory reconsolidation. This antalarmin dose was selected based on its effectiveness to block CRF-induced reinstatement of cocaine seeking when administered into the ventral tegmental area (Blacktop et al. 2011). Treatment-group assignment in experiment 1 and all other experiments was balanced based on average active-lever responding and cocaine intake during the last three drug self-administration training sessions. Intracranial infusions were administered over 2 min. Injection cannulae were inserted into the guide cannulae to a depth of 2 mm below the tip of the guide cannulae. Injector cannulae were left in place for 1 min before and after infusion.
Twenty-four h after the memory reactivation session, daily extinction training sessions resumed in the designated extinction context. Lever responses during the first of these extinction sessions were analyzed to evaluate whether antalarmin had off-target effects on extinction memories, as indicated by a change in lever responding. Daily extinction training continued until the rats reached an extinction criterion of ≤ 25 active-lever responses per session on a minimum of two consecutive days (mean ± SEM = 2.16 ± 0.08 days). Drug-seeking behavior was then assessed in the cocaine-paired context during a 2-h reinstatement test session, approximately three days after memory reactivation and intracranial manipulation. During the extinction training sessions and the reinstatement test, lever responses had no programmed consequences.
2.7. Experiment 2: Effects of delayed CRFR1 antagonism in the BLA on drug context-induced cocaine seeking three days later
Experiment 2 was designed to examine the potential memory reactivation-independent effects of CRFR1 antagonism in the BLA. The procedures were identical to those in experiment 1 except that rats received intra-BLA infusions of vehicle or antalarmin 6 h after the memory-reactivation session to disrupt CRFR1 signaling outside of the 2- to 4-h time window of memory reconsolidation (Tronson and Taylor, 2007). The rats then received additional daily extinction training sessions (mean ± SEM = 2.12 ± 0.07 days) followed by a reinstatement test.
2.8. Experiment 3: Effects of post-reactivation CRF administration in the BLA on drug context-induced cocaine seeking three days later
Experiment 3 was designed to evaluate whether CRF signaling was sufficient to increase cocaine-memory strength during reconsolidation. Rats were assigned to receive bilateral intra-BLA infusions of vehicle (20% ascorbic acid in ddH2O, Sigma-Aldrich) or exogenous CRF (0.2, 30, or 500 ng per 0.5 μL/hemisphere; Sigma-Aldrich) immediately after the memory reactivation to stimulate CRFR1 during memory reconsolidation. These CRF doses were selected based on their effectiveness to activate BLA principal neurons (Rostkowski et al. 2013), facilitate inhibitory avoidance learning when administered into the amygdala (Liang et al. 1988), or elicit reinstatement of cocaine seeking when administered into the ventral tegmental area (Blacktop et al. 2011). The rats then received additional daily extinction-training sessions (mean ± SEM = 2.14 ± 0.05 days) followed by a reinstatement test.
2.9. Experiment 4: Effects of delayed CRF administration in the BLA on drug context-induced cocaine seeking three days later
Experiment 4 was designed to examine the potential memory reactivation-independent effects of CRF in the BLA on cocaine seeking. Only female rats were included in experiment 4 based on the results of experiment 3. Rats received intra-BLA infusions of vehicle or CRF (0.2 or 30 ng/hemisphere) 6 h after the memory reactivation session to stimulate CRFR1 after, but not during, memory reconsolidation. The rats then received additional daily extinction training sessions (mean ± SEM = 2.04 ± 0.04 days) followed by a reinstatement test.
2.10. Test of general motor activity
Changes in general activity can alter lever-pressing performance. To evaluate whether the intra-BLA treatments altered general activity three days post-treatment, subsets of rats from experiments 1, 3, and 4 received bilateral intra-BLA infusions of antalarmin (500 ng/hemisphere), CRF (0.2, 30, or 500ng), or corresponding vehicles at least three days after the reinstatement test. Locomotor activity was assessed in novel Plexiglas chambers (42 × 20 × 20 cm) three days post treatment to maintain the treatment-to-testing interval implemented in the reinstatement experiments. The chambers were equipped with eight infrared light sources and photodetectors. Photobeam breaks were measured for 2 h by a computerized system (San Diego Instruments, San Diego, CA).
2.11. Brain histology
In experiments 1–4, rats were overdosed with ketamine hydrochloride and xylazine (100 and 5 mg/kg, i.v., or 300 and 15 mg/kg, i.p., respectively, depending on catheter patency). The brains were extracted, rapidly frozen in methyl butane, and stored at −80 °C. Brains were sectioned in the coronal plane at 50 μm on a cryostat (Leica Biosystems, Buffalo Grove, Illinois, USA). Sections were mounted on glass slides and stained with cresyl violet (Fisher Scientific, Waltham, Massachusetts, USA). The most ventral portion of each cannula tract was identified under a light microscope. The data of rats with cannula placements outside of the BLA were excluded from all statistical analyses.
2.12. Cell-surface CRFR1 biotinylation
Samples from a subset of male and female rats in experiment 4 were used to assess the effects of a high (behaviorally ineffective) dose of CRF on CRFR1 cell-surface expression in the BLA based on previous reports indicating agonist-induced effects on membrane CRFR1 trafficking (Bangasser et al. 2010). At least two weeks after completing behavioral testing, the rats received bilateral infusions of CRF (30 ng/hemisphere) or vehicle into the BLA. Tissue collection took place 30 min after intracranial treatment based on evidence that agonist-induced CRFR1 internalization peaks by this time point (Reyes et al. 2006; Holmes et al. 2006).
Brains were removed, cooled in an ice slurry of artificial cerebrospinal fluid (ACSF). The brains were sectioned (300-μm, frequency = 60, speed = 10) on a Leica VT1000S Vibratome (Leica Biosystems, Buffalo Grove, Illinois, USA) while submerged in ice-cold, oxygenated ACSF. Cannula placements were recorded during sectioning. BLA tissue was dissected out from the sections and recovered in oxygenated ACSF at 32 °C for at least 40 min. BLA tissue samples were then washed with cold ACSF (3 × 3 min) to remove proteins released from broken cells. The samples were incubated with 1-mg/ml Sulfo-NHS-SS-Biotin solution (sulfosuccinimidyl-20(biotinamido)ethyl-1,3-dithiopropionate in cold ACSF; ApexBio, Houston, TX) on ice for 30–45 min with shaking and washed again with ACSF (3 × 1 min plus 10 min). Excess Sulfo-NHS-SS-Biotin was inactivated by shaking in ice-cold quenching buffer (100mM glycine in ACSF, pH 8.0–8.3) for 30 min. After additional ACSF washes (3 × 5 min), the samples were centrifuged at 200 g for 1 min 4 °C. The buffer was aspirated, and 200 μL of immunoprecipitation lysis buffer (IPLB; 25mM Tris-HCl, 150mM NaCl, 1mM EDTA, 1% Triton X-100, and 5% glycerol) containing 1X protease inhibitor cocktail (P8340, Sigma) and 1X phosphatase inhibitor cocktail 1 (P0044, Sigma) (IPLB-Plus) was added to each tissue sample. Samples were stored at −80 °C until further processing.
Biotinylated tissue samples were thawed, disrupted by pipetting, and lysed for 1h at 4 °C. Homogenized tissue samples were centrifuged for 15 min at 14,000g at 4 °C, and the supernatant (total protein fraction) was collected. To pull down biotinylated proteins, 200 μg of total protein from the supernatant in 150 μl of IPLB-Plus was combined with 60 μl of pre-washed Pierce High Capacity NeutrAvidin Agarose beads (PI29202, Fisher Scientific) and shaken at 4 °C overnight. The agarose beads were then washed 4 times with 1 ml of IPLB, with vortexing, centrifuging at RT, and aspirating. To extract biotinylated proteins from the beads, 40 μL of 1X sample buffer (ProteinSimple, San Jose, California, USA) containing 50mM Dithiothreitol (DTT; Sigma-Aldrich) was added to the nearly dry beads and incubated at 4 °C for 30 min with shaking. After centrifugation, 40 μl of supernatant (biotinylated fraction) was collected and stored at −20 °C. Due to low yield, biotinylated fractions were concentrated by acetone precipitation as previously described (Nickerson and Doucette, 2020). A sample of the biotinylated fraction (35 μL) was combined with 2M NaCl followed by acetone to a final concentration of 100mM NaCl and 80% acetone. The samples were kept at RT for 1 h then centrifuged. The supernatant was removed and the tubes were air-dried. The precipitate was dissolved in 5 μL of 1X sample buffer (ProteinSimple) by gentle pipetting and sonication.
2.13. Capillary electrophoresis immunoassay (Simple Western)
All samples were denatured in the presence of 1X Fluorescent Master Mix (PS-FL01–8, ProteinSimple) for 5 min at 95 °C. Total protein fractions (500 μg/ml) and acetone-precipitated biotinylated fractions (at 1:1.25 dilution with 5X Fluorescent Master Mix) were assayed on a ProteinSimple Wes automated capillary-based electrophoresis instrument with Wes Separation Module protocol (ProteinSimple) using the 12–230 kDa Separation Module 8 × 25 capillary cartridges (SM-W004, ProteinSimple) and the Anti-Rabbit Detection Module (DM-001, ProteinSimple). Anti-goat HRP-labeled secondary antibody (043–522-2, ProteinSimple) was also used. Proteins were identified using goat anti-CRFR1 (1:10, NBP1–00175, Novus Biologicals, Centennial, Colorado), rabbit anti-MAPK1/2 (1:4000, ABS44, EMD Millipore, Darmstadt, Germany), and rabbit anti-ATPase (1:400, 3010S, Cell Signaling Technology, Beverly, MA) primary antibodies. Cytoplasmic MAPK1/2 was used as an indicator of BLA cell integrity, and ATPase as a loading control. Results (area under the curve, AUC) were analyzed using Compass for SW software v4.0.0 (see representative electropherograms in Fig. S5). Total and biotinylated CRFR1 values were normalized to total and biotinylated loading control values, respectively.
2.14. Data analysis
The data of rats that failed to reach the acquisition or extinction criteria (n = 14) and the data of vehicle control rats whose responding varied from the group mean by more than two standard deviations at test (n = 6) were excluded from all statistical analyses. Potential pre-existing group differences in drug intake and/or lever responses during drug self-administration training, extinction training, and memory reactivation were assessed using mixed-factorial analyses of variance (ANOVA) with subsequent treatment (vehicle, antalarmin or CRF dose) and sex as between-subjects factors and time (session) as the within-subject factor or t-tests, where appropriate. The effects of intracranial treatments on lever responses at test were assessed using mixed-factorial ANOVAs with treatment (vehicle, antalarmin or CRF dose) and sex as between-subjects factors and context (extinction, cocaine-paired) and time (20-min bins) as within-subject factors, where appropriate. Locomotor activity was analyzed using mixed-factorial ANOVAs with treatment and sex as between-subjects factors, where appropriate, and time (20-min bins) as the within-subjects factor. Protein levels were analyzed using between-subjects ANOVAs with treatment (vehicle, CRF) and sex as factors. Significant interaction and time main effects were further probed using Sidak’s or Tukey’s HSD post hoc tests, where appropriate. Alpha was set at 0.05 for all analyses.
3. Results
3.1. Female rats exhibit more cocaine-reinforced responding than males
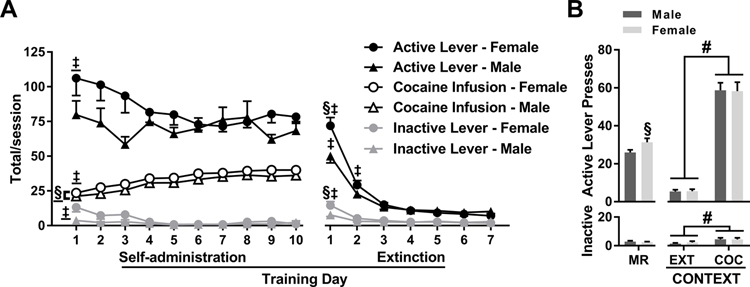
In experiments 1–4, active-lever responding decreased after the first cocaine self-administration training session independent of sex (Fig. 1A; 2 × 10 ANOVA, time main effect, F(9,1269) = 1.94, p = 0.04, Tukey’s tests, day 1 > days 5–10, ps < 0.05; sex main and interaction effects, Fs ≤ 1.79, ps ≥ 0.07). However, cocaine intake varied by sex and time (Fig. 1A; 2 × 10 ANOVA, sex main effect, F(1, 141) = 7.31, p = 0.01; time main effect, F(9, 1269) = 75.36, p < 0.001; sex x time interaction, F(9, 1269) = 0.36, p = 0.95). Specifically, females obtained more cocaine infusions than males by making fewer timeout responses. Furthermore, the number of cocaine infusions obtained by males and females increased over time (Tukey’s tests, day 1 < days 3–10, ps < 0.05), independent of sex. Inactive-lever responding remained low and decreased after the first training session, independent of sex (Fig. 1A; 2 × 10 ANOVA, time main effect, F(9, 1269) = 3.00, p = 0.002, Tukey’s tests, day 1 > days 4–10, ps < 0.05; sex main and interaction effects, Fs ≤ 3.39, ps ≥ 0.07).
Figure 1. Sex differences in cocaine intake and lever responding.
(A) Active- and inactive-lever responses and cocaine infusions (mean ± SEM) obtained by male (n = 57) and female (n = 88) rats in experiments 1–4 during daily 2-h cocaine-self-administration and extinction training sessions. (B) Active- and inactive-lever responses during the 15-min cocaine-memory reactivation (MR) session (before intra-BLA treatment in males, n = 57, and females, n = 88) and upon first re-exposure to the extinction (EXT) and cocaine-paired (COC) contexts (after intra-BLA vehicle treatment in males (n = 24) and females (n = 36). Symbols: Double daggers indicate difference from subsequent training sessions, ‡ ANOVA time main effect collapsed across sex, ‡time simple-main effect in a sex, Tukey’s test. Silcrows indicate difference from males, §ANOVA sex main effect collapsed across time, §sex simple-main effect during a session, Tukey’s test, or sex effect during the MR session, t-test. Pound sign indicates difference from the extinction context, #ANOVA context main effect collapsed across sex. All ps < 0.05.
3.2. Female rats exhibit more robust extinction responding than male rats
After the removal of cocaine reinforcement, active-lever responding varied by sex depending on time across the seven extinction sessions (Fig. 1A; 2 × 7 ANOVA, sex x time interaction, F(6, 858) = 6.64, p < 0.001; time main effect, F(6, 858) = 127.12, p < 0.001; sex main effect, F(1, 143) = 2.37, p = 0.13). Females exhibited more active-lever responding than males during the first extinction training session (Tukey’s test, p < 0.05), and active-lever responding in males and females declined after the first extinction session (Tukey’s tests: day 1 > days 2–7, p < 0.05). Inactive-lever responding during extinction training varied by sex depending on time (Fig. 1A; 2 × 7 ANOVA, sex x time interaction, F(6, 858) = 3.57, p = 0.002; time main effect, F(6, 858) = 19.79, p < 0.001; sex main effect, F(1, 143) = 2.01, p = 0.16). Females exhibited more inactive-lever responding than males during the first extinction training session (Tukey’s test, p < 0.05), and inactive-lever responding declined in both groups after that (Tukey’s tests, male and female day 1 > days 2–7, p < 0.05).
3.3. Female rats exhibit more responding than males during the memory-reactivation session, but not at test
Females exhibited more active-lever responding (t(143) = −2.16, p = 0.03), but not inactive-lever responding (t(143) = 0.66, p = 0.51), compared to males in the previously cocaine-paired context during the 15-min memory reactivation session (Fig. 1B). Furthermore, time-course analysis indicated that active-lever responding decreased during the last 3-min interval of the memory reactivation session, independent of sex (data not shown; 2 × 5 ANOVA, sex main effect, F(1,66) = 6.58, p = 0.01; time main effect, F(4,264) = 6.31, p < 0.001, bins 1–4 > bin 5, Tukey’s tests, p < 0.05; sex x time interaction effect, F(4,264) = 0.33, p = 0.86). Active-lever responding at test varied by testing context, but not sex (Fig. 1B upper panel; 2 × 2 ANOVA, context main effect, F(1,57) = 244.91, p < 0.001; sex main and interaction effects, Fs ≤ 0.12, ps ≥ 0.73). Male and female control groups (after post-reactivation vehicle treatment) exhibited more active-lever responding upon re-exposure to the cocaine-paired context than upon re-exposure to the extinction context, independent of sex. Similarly, these groups exhibited more inactive-lever responding in the cocaine-paired context at test than in the extinction context, independent of sex (Fig. 1B lower panel; 2 × 2 ANOVA, context main effect, F(1,62) = 12.89, p < 0.001; sex main and interaction effects, Fs ≤ 0.34, ps ≥ 0.57).
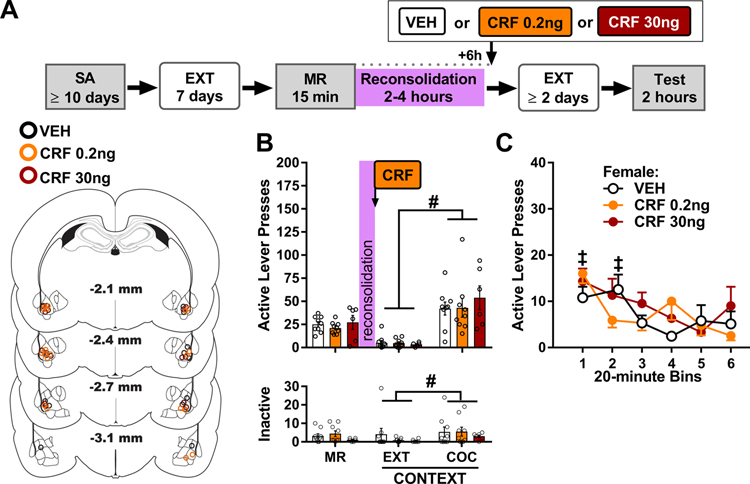
3.4. Experiment 1: BLA CRFR1 antagonism immediately after memory reactivation attenuates subsequent drug context-induced cocaine seeking
Experiment 1 evaluated whether BLA CRFR1 stimulation is necessary for cocaine-memory reconsolidation (Fig. 2A). Bilateral intra-BLA infusions of antalarmin (500 ng/hemisphere) or vehicle were administered immediately after the 15-min cocaine-memory reactivation session. The effects of these manipulations on drug context-induced cocaine-seeking behavior were assessed three days after treatment. Only data from rats with bilateral injector placement in the lateral, basolateral, or accessory basal nucleus of the amygdala (i.e., BLA) were included in data analyses in each experiment. The resulting sample sizes/group (male, female) in experiment 1 were: vehicle (n = 6, 6), antalarmin (n = 5, 7).
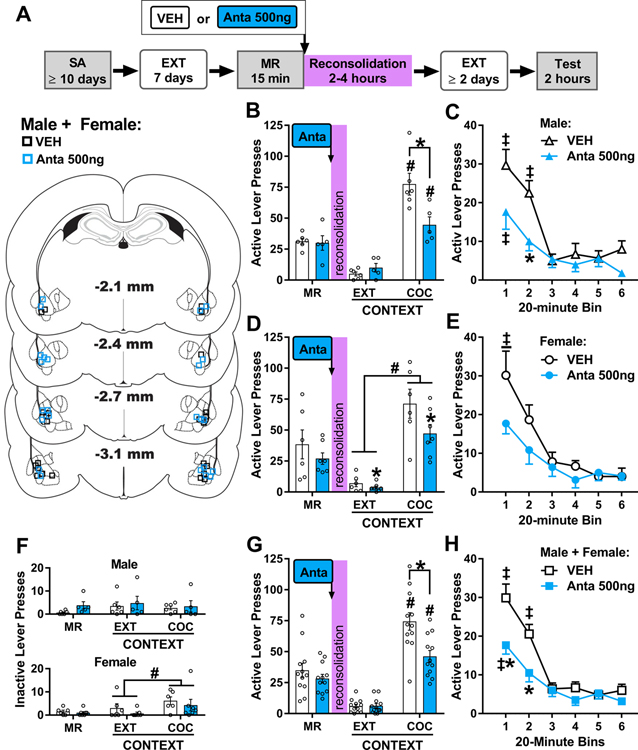
Figure 2. BLA CRFR1 antagonism immediately after memory reactivation attenuates drug context-induced cocaine seeking three days later.
(A) Experimental timeline. Rats were trained to self-administer cocaine (SA) in a distinct context, and their lever responding was then extinguished (EXT) in a different context. On post-cocaine day 8, rats were re-exposed to the cocaine-paired context for 15 min to elicit memory reactivation (MR). They then received bilateral intra-BLA vehicle (VEH) or antalarmin (Anta, 500 ng/0.5 μL per hemisphere) infusions. Cocaine-seeking behavior was assessed in the cocaine-paired context (Test) three days later, after at least two additional daily extinction training sessions in the extinction context. The schematic illustrates cannula placements in experiment 1. (B) Active-lever responses (mean ± SEM) in males (n = 5–6/group) in the cocaine-paired (COC) context during the MR session (before treatment) and upon first re-exposure to the extinction (EXT) and COC contexts at test (after treatment). (C) Time-course of active-lever responses (mean ± SEM) in males in the COC context at test. (D) Active-lever responses (mean ± SEM) in females (n = 6–7/group) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (E) Time-course of active-lever responses (mean ± SEM) in females in the COC context at test. (F) Inactive-lever responses (mean ± SEM) in males (upper panel) and females (lower panel) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (G) Active-lever responses (mean ± SEM; collapsed across sex based on the lack of sex effects; n = 12/group) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (H) Time-course of active-lever responses (mean ± SEM; collapsed across sex based on the lack of sex effects) in the COC context at test. Symbols: Asterisks indicate difference from vehicle treatment, *ANOVA treatment simple main effect or main effect collapsed across testing context (Panel D). Pound signs indicate difference from the extinction context, #ANOVA context main effect collapsed across treatment. Double daggers indicate difference from subsequent training sessions, ‡ ANOVA time main effect collapsed across treatment, ‡time simple-main effect in a treatment group, Tukey’s test. All ps < 0.05.
There were no differences between the subsequent treatment groups in cocaine intake, lever responding during drug self-administration and extinction training (Fig. S1), and active-lever responding during the memory-reactivation session (MR in Fig. 2B, 2D, and 2G; 2 × 2 ANOVA; all treatment and sex main and interaction effects, Fs ≤ 0.83, ps ≥ 0.37). Inactive-lever responding during the memory-reactivation session varied by sex and subsequent treatment (MR in Fig. 2F; 2 × 2 ANOVA, sex x treatment interaction effect, F(1,20) = 5.03, p = 0.04; sex and treatment main effects, F(1,9) < 2.64, p > 0.12); however, post-hoc comparisons failed to detect significant group differences (Tukey’s tests, p > 0.05).
In male rats, active-lever responding at test varied by antalarmin treatment administered immediately after memory reactivation depending on the testing context (Fig. 2B; 2 × 2 ANOVA, treatment x context interaction, F(1,9) = 9.72, p = 0.01; context main effect, F(1, 9) = 77.63, p < 0.001; treatment main effect, F(1, 9) = 5.67, p = 0.04). The cocaine-paired context elicited more active-lever responding than the extinction context (Sidak’s tests, p < 0.05). However, intra-BLA antalarmin treatment immediately after cocaine-memory reactivation reduced active-lever responding in the cocaine-paired context (Sidak’s test, p < 0.05), but not in the extinction context, relative to vehicle treatment. Time-course analysis of active-lever responding in the cocaine-paired context indicated that responding varied by treatment depending on time (Fig. 2C; 2 × 6 ANOVA, treatment x time interaction, F(5, 45) = 2.45, p = 0.05; treatment main effect, F(1, 11) = 8.59, p = 0.02; time main effect, F(5, 45) = 18.70, p < 0.001). Antalarmin treatment immediately after memory reactivation reduced active-lever responding during the second 20-minute interval of the test session, relative to vehicle treatment (Tukey’s test, p < 0.05). Furthermore, antalarmin treatment reduced the persistence of active-lever responding (Tukey’s tests, bin 1 > bins 2–6, ps < 0.05) compared to vehicle treatment (Tukey’s tests, bin 1–2 > bins 3–6, p < 0.05). Inactive-lever responding remained low and did not vary as a function of testing context or treatment (Fig 2F upper panel, treatment and context main and interaction effects, Fs ≤ 0.30, ps ≥ 0.59).
In female rats, active-lever responding at test varied by antalarmin treatment administered immediately after memory reactivation and testing context (Fig. 2D; 2 × 2 ANOVA, context main effect, F(1,11) = 53.19, p < 0.001; treatment main effect, F(1,11) = 4.96, p = 0.05; context x treatment interaction, F(1, 11) = 1.87, p = 0.20). The cocaine-paired context elicited more active-lever responding than the extinction context, independent of treatment. Furthermore, intra-BLA antalarmin treatment immediately after memory reactivation reduced active-lever responding relative to vehicle treatment, independent of context. Time-course analysis of active-lever responding in the cocaine-paired context revealed that responding decreased after the first 20-minute interval of the test session, independent of treatment (Fig. 2E; 2 × 6 ANOVA, time main effect, F(5, 55) = 18.22, p < 0.001, Tukey’s tests, bin 1 > bin 2 > bins 4–6, ps < 0.05; treatment main and interaction effects, Fs ≤ 3.21, p = 0.10). Re-exposure to the cocaine-paired context increased inactive-lever responding compared to re-exposure to the extinction context, independent of treatment (Fig. 2F lower panel; 2 × 2 ANOVA, context main effect, F(1,11) = 6.82, p = 0.02; group main and interaction effects, Fs ≤ 0.86, ps ≥ 0.37).
The omnibus ANOVA of active-lever responding at test revealed that the effects of antalarmin treatment administered immediately after memory reactivation depended on testing context (Fig. 2G, 2 × 2 × 2 ANOVA, context x treatment interaction, F(1,20) = 8.75, p = 0.008; context main effect, F(1,20) = 119.70, p < 0.001; treatment main effect, F(1,20) = 10.30, p = 0.004), but not sex (sex main and all sex interaction effects, Fs(1,20) ≤ 0.80, ps ≥ 0.38). The cocaine-paired context elicited more active-lever responding than the extinction context, independent of sex (Tukey’s tests, p < 0.05). Furthermore, intra-BLA antalarmin treatment immediately after cocaine-memory reactivation reduced active-lever responding in the cocaine-paired context (Tukey’s test, p < 0.05), but not in the extinction context, relative to vehicle treatment, independent of sex. Time-course analysis of active-lever responding in the cocaine-paired context indicated that the effects of antalarmin treatment varied by time (Fig. 2H; 2 × 2 × 6 ANOVA, treatment x time interaction, F(5,100) = 3.92, p = 0.003; time main effect, F(5,100) = 36.01, p < 0.001; treatment main effect, F(1,20) = 10.14, p = 0.005), but not sex (sex main and sex two- or three-way interaction effects, Fs(1,20) ≤ 0.25, ps ≥ 0.62). Antalarmin treatment immediately after memory reactivation reduced active-lever responding during the first and second 20-minute intervals of the test session relative to vehicle treatment (Tukey’s tests, p < 0.05). Furthermore, antalarmin treatment immediately after memory reactivation reduced the persistence of active-lever responding (Tukey’s tests, bin 1 > bin 2–6, ps < 0.05) relative to vehicle treatment (Tukey’s tests, bin 1 > bin 2 > bins 3–6, p < 0.05). Inactive-lever responding remained low but varied by testing context depending on sex (Fig. 2F; 2 × 2 × 2 ANOVA, sex x context interaction, F(1,20) = 7.16, p = 0.02; context, sex, and treatment main and all other two- and three-way interaction effects, Fs(1,20) ≤ 1.81, ps ≥ 0.19). Females (Tukey’s test, p < 0.05), but not males, exhibited more inactive-lever responses in the cocaine-paired context than in the extinction context, independent of treatment.
3.5. Experiment 2: BLA CRFR1 antagonism 6 h after cocaine-memory reactivation does not alter subsequent drug context-induced cocaine seeking
Experiment 2 evaluated whether the effects of intra-BLA CRFR1 antagonism observed in experiment 1 were independent of memory destabilization (Fig. 3A) because bona fide deficits in memory reconsolidation are expected to be dependent on memory destabilization (Tronson and Taylor, 2007). Bilateral intra-BLA infusions of antalarmin (500 ng/hemisphere; ns = 7 males, 6 females) or vehicle (ns = 7 males, 5 females) were administered 6 h after the memory reactivation session, when memories were no longer expected to be labile. The effects of these manipulations on drug context-induced cocaine seeking were assessed three days later. There were no differences between the subsequent treatment groups in cocaine intake, lever responding during drug self-administration and extinction training (Fig. S2), or lever responding during the memory-reactivation session (MR in Fig. 3B, 3D; 2 × 2 ANOVAs; all treatment and sex main and interaction effects, Fs ≤ 2.42, ps ≥ 0.14).
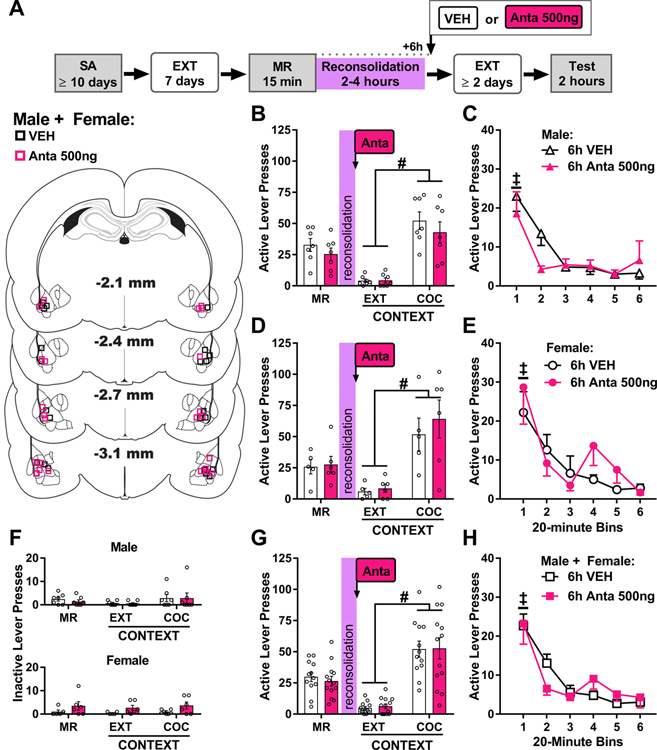
Figure 3. BLA CRFR1 antagonism 6 h after cocaine-memory reactivation does not alter drug context-induced cocaine seeking three days later.
(A) Experimental timeline. Rats were trained to self-administer cocaine (SA) in a distinct context, and their lever responding was then extinguished (EXT) in a different context. On post-cocaine day 8, rats were re-exposed to the previously cocaine-paired context for 15 min to elicit memory reactivation (MR). Six h after MR, they received bilateral intra-BLA vehicle (VEH) or antalarmin (Anta, 500 ng/0.5 μL per hemisphere) infusions. Cocaine-seeking behavior was assessed in the cocaine-paired context (Test) three days later, after at least two additional daily extinction training sessions in the extinction context. The schematic illustrates cannula placements in experiment 2. (B) Active-lever responses (mean ± SEM) in males (n = 7/group) in the cocaine-paired (COC) context during the MR session (before treatment) and upon first re-exposure to the extinction (EXT) and COC contexts at test (after treatment). (C) Time-course of active-lever responses (mean ± SEM) in males in the COC context at test. (D) Active-lever responses (mean ± SEM) in females (n = 5–6/group) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (E) Time-course of active-lever responses (mean ± SEM) in females in the COC context at test. (F) Inactive-lever responses (mean ± SEM) in males (upper panel) and females (lower panel) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (G) Active-lever responses (mean ± SEM; collapsed across sex based on the lack of sex effects; n = 12–13/group) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (H) Time-course of active-lever responses (mean ± SEM; collapsed across sex based on the lack of sex effects) in the COC context at test. Symbols: Pound signs indicate difference from the extinction context, #ANOVA context main effect collapsed across treatment. Double daggers indicate difference from subsequent training sessions, ‡ ANOVA time main effect collapsed across treatment. All ps < 0.05.
In male rats, active-lever responding varied by testing context, but not antalarmin treatment administered 6 h after cocaine-memory reactivation (Fig. 3B; 2 × 2 ANOVA, context main effect, F(1,12) = 51.58, p < 0.001; treatment main and interaction effects, Fs ≤ 0.74, ps ≥ 0.44). The cocaine-paired context elicited more active-lever responding than the extinction context, and delayed antalarmin treatment did not alter active-lever responding in either context relative to vehicle treatment. Time-course analysis of active-lever responding in the cocaine-paired context at test indicated that responding declined after the first 20-min interval of the test session independent of delayed antalarmin treatment (Fig. 3C, 2 × 6 ANOVA, time main effect, F(5, 60) = 11.81, p < 0.001, Tukey’s tests, bin 1 > bins 2–6, ps < 0.05; all treatment main and interaction effects, Fs ≤ 1.37, ps ≥ 0.25). Inactive-lever responding remained low and did not vary as a function of testing context or delayed antalarmin treatment (Fig. 3F upper panel, all Fs ≤ 2.67, ps ≥ 0.13).
In female rats, active-lever responding varied by testing context, but not delayed antalarmin treatment (Fig. 3D; 2 × 2 ANOVA, context main effect, F(1,9) = 22.23, p = 0.001; all treatment main and interaction effects, Fs ≤ 0.53, ps ≥ 0.49). The cocaine-paired context elicited more active-lever responding than the extinction context, and delayed antalarmin treatment did not alter active-lever responding in either context compared to vehicle treatment. The time-course analysis of active-lever responding in the cocaine-paired context indicated that responding declined after the first 20-min interval of the test session independent of delayed antalarmin treatment (Fig. 3E; 2 × 6 ANOVA, time main effect, F(5, 45) = 9.05, p < 0.001, Tukey’s tests, bin 1 > bins 2–6, ps < 0.05; all treatment main and interaction effects, Fs ≤ 0.90, ps ≥ 0.49). Inactive-lever responding remained low and did not vary as a function of testing context or delayed antalarmin treatment (Fig. 3F lower panel, all Fs ≤ 3.49, ps ≥ 0.09).
The omnibus ANOVA of active-lever responding in males and females at test indicated that responding varied by testing context, but not sex or delayed antalarmin treatment (Fig 3G; 2 × 2 × 2 ANOVA, context main effect, F(1,21) = 65.18, p < 0.001; sex and treatment main and all interaction effects, Fs ≤ 1.23, ps ≥ 0.27). The cocaine-paired context elicited more active-lever responding than the extinction context, independent of sex or delayed treatment. The time-course analysis of active-lever responding in the cocaine-paired context indicated that responding decreased after the first 20-min interval of the test session, independent of sex or delayed treatment (Fig. 3H; 2 × 2 × 6 ANOVA, time main effect, F(5,105) = 20.49, p < 0.001; Tukey’s tests, bin 1 > bins 2–6, ps < 0.05; all sex and treatment main and two-way and three-way interaction effects, Fs ≤ 1.32, ps ≥ 0.26). Inactive-lever responding remained low and did not vary as a function of sex, testing context, or delayed treatment (Fig. 3F; 2 × 2 × 2 ANOVA, all context, sex, and treatment main and two- and three-way interaction effects, Fs ≤ 3.59, ps ≥ 0.07).
3.6. Experiment 3: Intra-BLA CRF treatment immediately after memory reactivation increases subsequent drug context-induced cocaine seeking in a sex- and dose-dependent manner
Experiment 3 evaluated whether CRF in the BLA was sufficient to increase cocaine-memory strength during memory reconsolidation (Fig. 4A). Bilateral intra-BLA infusions of CRF (0.2, 30, or 500 ng/hemisphere; n = 6–8 males/dose, 8 females/dose) or vehicle (n = 11 males, 14 females) were administered immediately after the memory reactivation session. The effects of these manipulations on drug context-induced cocaine-seeking behavior were assessed three days later. There were no differences between the subsequent treatment groups in cocaine intake and in lever responding during drug self-administration and extinction training (Fig. S3). Females exhibited more active-lever responding than males during the 15-min memory reactivation session independent of subsequent treatment group (Fig. 4B, 4D upper panels; 2 × 4 ANOVA, sex main effect, F(1,62) = 8.11, p = 0.01; treatment main and interaction effects, Fs ≤ 1.9, ps ≥ 0.13), and inactive-lever responding varied by sex depending on subsequent treatment group (Fig. 4B lower panel; 2 × 4 ANOVA, sex x treatment interaction effect, F(1,62) = 4.04, p = 0.01; sex and treatment main effects, Fs ≤ 1.78, ps ≥ 0.18). Specifically, the 30ng dose of CRF administered after memory reactivation reduced inactive lever responding in females compared to males, and compared to the 0.2ng dose of CRF in females (Tukey’s tests, ps < 0.05).
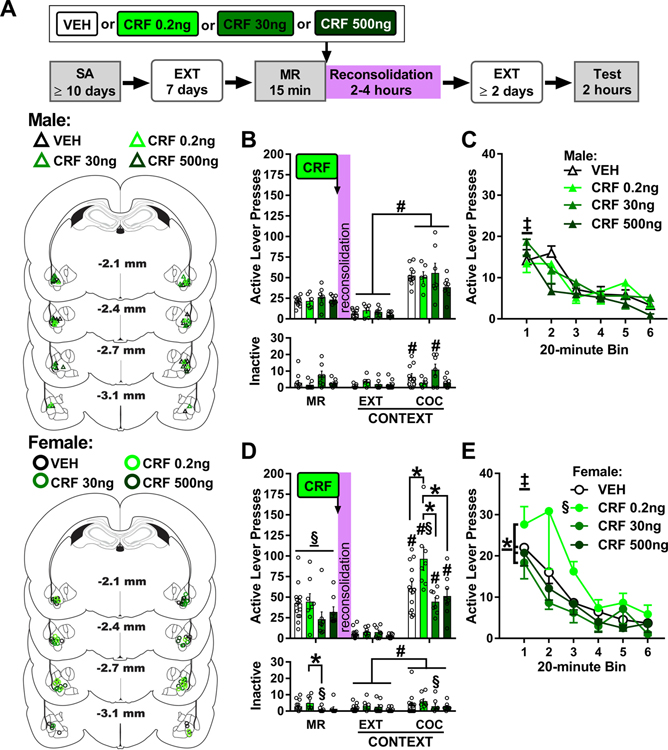
Figure 4. Intra-BLA CRF treatment immediately after memory reactivation dose-dependently increases drug context-induced cocaine seeking in female, but not male, rats three days later.
A) Experimental timeline. Rats were trained to self-administer cocaine (SA) in a distinct context, and their lever responding was then extinguished (EXT) in a different context. On post-cocaine day 8, rats were re-exposed to the cocaine-paired context for 15 min to elicit memory reactivation (MR). They then received bilateral intra-BLA vehicle (VEH) or corticotropin-releasing factor (CRF; 0.2, 30, or 500 ng/0.5 μL per hemisphere) infusions. Cocaine-seeking behavior was assessed in the cocaine-paired context (Test) three days later, after at least two additional daily extinction training sessions in the extinction context. The schematics illustrate cannula placements in males (upper) and females (lower) in experiment 3. (B) Active and inactive-lever responses (mean ± SEM) in males (n = 6–11/group) in the cocaine-paired (COC) context during the MR session (before treatment) and upon first re-exposure to the extinction (EXT) and COC contexts at test (after treatment). (C) Time-course of active-lever responses (mean ± SEM) in males in the COC context at test. (D) Active- and inactive lever responses (mean ± SEM) in females (n = 8–14/group) in the COC context during the MR session (before treatment) and upon first re-exposure to the EXT and COC contexts at test (after treatment). (E) Time-course of active-lever responses (mean ± SEM) in females in the COC context at test. Symbols: Pound signs indicate difference from the extinction context, #ANOVA context main effect collapsed across treatment or time, or context simple-main effect in a treatment group, Tukey’s tests. Double daggers indicate difference from subsequent training sessions, ‡ANOVA time main effect independent of treatment. Silcrows indicate difference from males, §ANOVA sex main effect collapsed across treatment, §sex simple-main effect for a treatment condition, Tukey’s test. All ps < 0.05.
In male rats, active-lever responding at test varied by testing context, but not CRF treatment administered immediately after memory reactivation (Fig. 4B upper panel; 2 × 4 ANOVA, context main effect, F(1,28) = 154.02, p < 0.001, treatment main and interaction effects, Fs ≤ 1.68, ps ≥ 0.19). The cocaine-paired context elicited more active-lever responding than the extinction context independent of CRF treatment (Tukey’s tests, p < 0.05). Time-course analysis of active-lever responding in the cocaine-paired context at test indicated that responding declined after the first 20-min interval of the test session independent of treatment (Fig. 4C; 4 × 6 ANOVA, time main effect, F(5,140) = 20.74, p < 0.001, Tukey’s tests, bin 1 > bins 3–6, ps < 0.05; treatment main and interaction effects, Fs ≤ 1.34, ps ≥ 0.28). Inactive-lever responding remained low at test, but it varied by CRF treatment depending on testing context (Fig. 4B lower panel; 2 × 4 ANOVA, treatment x context interaction, F(3,28) = 4.95, p = 0.01; context main effect, F(1,28) = 15.76, p < 0.001; treatment main effect, F(3,33) = 1.53, p = 0.23). The cocaine-paired context elicited more inactive-lever responding than the extinction context after vehicle or 30 ng of CRF treatment (Sidak tests, ps < 0.05), but not after 0.2 or 500 ng of CRF treatment.
In female rats, active-lever responding varied by CRF treatment immediately after memory reactivation depending on testing context (Fig. 4D upper panel; 2 × 4 ANOVA, treatment x context interaction, F(3,34) = 4.66, p = 0.01; context main effect, F(1,34) = 137.65, p < 0.001; treatment main effect, F(3,34) = 6.00, p = 0.002). The cocaine-paired context elicited more active-lever responding than the extinction context (Tukey’s tests, p < 0.05). The 0.2 ng dose of CRF administered immediately after memory reactivation increased active-lever responding in the cocaine-paired context, but not in the extinction context, relative to vehicle or higher (30 or 500 ng) doses of CRF (Tukey’s tests, p < 0.05). Time-course analysis of active-lever responding in the cocaine-paired context revealed that responding varied by CRF treatment and time (Fig. 4E; 4 × 6 ANOVA, treatment main effect, F(3,34) = 5.43, p = 0.004; time main effect, F(5,170) = 17.29, p < 0.001, Tukey’s tests, bin 1 > bins 3–6, ps < 0.05; treatment x time interaction, F(15,170) = 0.80, p = 0.68). The 0.2 ng dose of CRF administered immediately after memory reactivation elicited more active-lever pressing at test than vehicle or the 30- and 500-ng doses of CRF, independent of time (Sidak’s tests, ps < 0.05). Additionally, active-lever responding declined after the first 20-min interval of the test session, independent of treatment (Tukey’s tests, bin 1 > bins 2–6, ps < 0.05). Inactive-lever responding increased in the cocaine-paired context relative to the extinction context, independent of CRF treatment (Fig. 4D lower panel; 2 × 4 ANOVA, context main effect, F(1, 34) = 4.35, p = 0.05; treatment main and interaction effects, Fs ≤ 1.18, ps ≥ 0.33).
The omnibus ANOVA indicated that active-lever responding varied by CRF treatment depending on sex and testing context (Fig. 4B and 4D upper panels; 2 × 4 × 2 ANOVA, sex x treatment x context interaction, F(3,62) = 3.41, p = 0.02; sex x treatment interaction, F(3,62) = 3.05, p = 0.04; sex x context interaction, F(1,62) = 6.46, p = 0.01; treatment x context, F(1,62) = 2.79, p = 0.05; sex main effect, F(1,62) = 4.10, p = 0.05; treatment main effect, F(3,62) = 4.93, p = 0.004; context main effect, F(1,62) = 258.46, p < 0.001). The cocaine-paired context elicited more active-lever responding than the extinction context (Tukey’s tests, p < 0.05). In the extinction context, neither sex nor CRF treatment altered active-lever responding. In the cocaine-paired context, responding did not vary between males and females following vehicle treatment immediately after memory reactivation. The 0.2-ng dose of CRF administered after memory reactivation increased responding in females compared to males, and compared to vehicle or higher doses (30 or 500ng) of CRF in females (Turkey’s tests, p < 0.05). The time course analysis of active-lever responding in the cocaine-paired context confirmed the same sex and CRF treatment effects and also indicated that responding decreased over time, independent of sex or CRF treatment (Fig 4C and 4E; 2 × 4 × 6 ANOVA, sex x treatment interaction, F(3,62) = 3.37, p = 0.02; sex main effect, F(1,62) = 5.57, p = 0.02; treatment main effect, F(3,62) = 3.90, p = 0.01; time main effect, F(5,310) = 30.85, p < 0.001, Tukey’s tests, bin 1 > bins 3–6, ps < 0.05; all other interaction effects, Fs ≤ 1.91, ps ≥ 0.09). Inactive-lever responding at test remained low and varied by CRF treatment depending on sex and testing context (Fig 4C and 4E lower panels; 2 × 4 × 2 ANOVA, sex x treatment x context interaction, F(3,62) = 3.96, p = 0.01; context main effect, F(1,62) = 19.29, p < 0.001; sex and treatment main and all other interaction effects, Fs ≤ 2.71, ps ≥ 0.11). In females, the cocaine-paired context elicited more inactive-lever responding than the extinction context, independent of CRF treatment (Tukey’s tests, ps < 0.05), whereas the same was only observed in the males following treatment with vehicle or 30 ng of CRF immediately after memory reactivation (Tukey’s tests, ps < 0.05). In addition, inactive-lever responding was lower in females than in males after administration of 30 ng of CRF (Fig. 4D lower panel; Tukey’s test, ps < 0.05).
3.7. Experiment 4: Intra-BLA CRF treatment 6 h after memory reactivation did not alter drug context-induced cocaine-seeking behavior in female rats
Experiment 4 evaluated whether the effects of intra-BLA CRF administration in female rats in experiment 3 were independent of memory reactivation (Fig. 5A). To this end, CRF (0.2 or 30 ng/hemisphere; n = 9, 6 respectively) or vehicle (n = 9) was administered into the BLA of female rats 6 h after the memory reactivation session, when memories were no longer expected to be labile. The effects on drug context-induced cocaine-seeking behavior were assessed three days later. There were no differences between the subsequent treatment groups in cocaine intake and in lever responding during drug self-administration and extinction training (Fig. S4) or during the memory-reactivation session (Fig. 5B; one-way ANOVAs, Fs ≤ 0.59, ps ≥ 0.57).
Figure 5. Intra-BLA CRF treatment in females 6 h after cocaine-memory reactivation does not alter drug context-induced cocaine seeking three days later.
(A) Experimental timeline. Female rats were trained to self-administer cocaine (SA) in a distinct context, and their lever responding was then extinguished (EXT) in a different context. On post-cocaine day 8, rats were re-exposed to the cocaine-paired context for 15 min to elicit memory reactivation (MR). Six hours after MR, they received bilateral intra-BLA vehicle (VEH) or corticotropin-releasing factor (CRF, 0.2 or 30 ng/0.5 μL per hemisphere) infusions. Cocaine-seeking behavior was assessed in the cocaine-paired context (Test) three days later, after at least two additional daily extinction training sessions in the extinction context. The schematic illustrates cannula placements in experiment 4. (B) Active- and inactive-lever responses (mean ± SEM) in females (n = 6–9/group) in the cocaine-paired (COC) context during the MR session (before treatment) and upon first re-exposure to the extinction (EXT) and COC contexts at test (after treatment). (C) Time-course of active-lever responses (mean ± SEM) in females in the COC context at test. Symbols: Pound signs indicate difference from the extinction context, #ANOVA context main effect collapsed across treatment. Double daggers indicate difference from subsequent training sessions, ‡ANOVA time simple-main effect in a treatment group. All ps < 0.05.
Active-lever responding varied as a function of testing context, but not delayed CRF treatment (Fig. 5B upper panel; 3 × 2 ANOVA, context main effect, F(1,21) = 51.52, p = 0.001; treatment main and interaction effects, Fs ≤ 0.48, ps ≥ 0.63). The cocaine-paired context elicited more active-lever responding than the extinction context, independent of delayed CRF treatment. The time-course analysis revealed that active-lever responding in the cocaine-paired context varied by delayed treatment depending on time (Fig. 5C; 3 × 6 ANOVA, treatment x time interaction, F(1,105) = 1.94, p = 0.05; time main effect, F(5,105) = 6.80, p < 0.001; treatment main effect, F(2,21) = 0.36, p = 0.70) due to a decrease in active-lever responding after the first 20-min interval of the test session following delayed treatment with 0.2 ng of CRF (Bin 1 > bins 2, 3, 5, 6, Tukey’s tests, p < 0.05), but not after delayed treatment with vehicle or 30 ng of CRF. There was a similar decrease in responding during the second 20-min interval following delayed vehicle treatment (Bin 1–2 > bin 3, Tukey’s tests, p < 0.05). Inactive-lever responding remained low but increased upon re-exposure to the cocaine-paired context relative to re-exposure to the extinction context, independent of delayed CRF treatment (Fig. 5B lower panel, 2 × 2 ANOVA, context main effect, F(1,21) = 8.62, p = 0.01; all treatment main and interaction effects, Fs ≤ 1.29, ps ≥ 0.30).
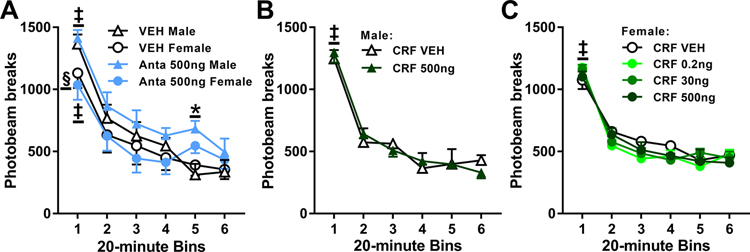
3.8. Intra-BLA antalarmin, but not CRF, treatment transiently alters general activity
To evaluate the potential effects of our intracranial manipulations on motor performance at test, a subset of rats received bilateral infusions of intra-BLA antalarmin (500 ng/0.5 μL per hemisphere, (n = 6 males, 6 females), CRF (500 ng/0.5 μL per hemisphere, n = 4 males; 0.2, 30, or 500 ng/0.5 μL per hemisphere, ns = 5–7 females/dose), or vehicle (n = 10 males, 13 females) at least two days after the last test of cocaine-seeking behavior. Locomotor activity was assessed three days post treatment to match the treatment-to-testing interval employed in the earlier experiments.
Locomotor activity varied by sex, antalarmin pre-treatment, and time (Fig. 6A; 2 × 2 × 6 ANOVA, sex x time interaction, F(5,95) = 2.73, p = 0.02; treatment x time interaction, F(5,95) = 2.73, p = 0.03, time main effect, F(5,95) = 87.84, p < 0.001; sex and treatment main and all other interaction effects, Fs ≤ 3.40, ps ≥ 0.08). Females exhibited less locomotor activity than males during the first 20-min interval of session, independent of antalarmin pre-treatment (Tukey’s test, p < 0.05). Furthermore, intra-BLA antalarmin pre-treatment increased locomotor activity during the fifth 20-min interval relative to vehicle pre-treatment, independent of sex (Tukey’s test, p < 0.05).
Figure 6. Intra-BLA antalarmin or CRF pre-treatment does not alter locomotor activity in a novel context three days later.
Rats received intra-BLA administration of antalarmin (Anta; 500 ng/0.5 μL per hemisphere), corticotropin-releasing factor (CRF; 0.2, 30, or 500 ng/0.5 μL per hemisphere), or their respective vehicles (VEH) three days prior to a 2-h locomotor activity test in a novel chamber. (A) Total number of photobeam breaks (mean/2 h ± SEM) elicited by males and females that received intra-BLA VEH (n = 6 males, 5 females) or Anta pre-treatment (n = 6 males, 6 females). (B) Total number of photobeam breaks (mean/2 h ± SEM) elicited by males that received intra-BLA vehicle or CRF (500 ng/hemisphere) pre-treatment (n = 4/group). (C) Total number of photobeam breaks (mean/2 h ± SEM) elicited by females that received intra-BLA vehicle or CRF (0.2, 30, or 500 ng/hemisphere) pre-treatment (n = 5–8/group). Symbols: Silcrow indicates difference from males during the first time bin, §ANOVA sex simple-main effect collapsed across treatment, Tukey’s test. Asterisk indicates difference from vehicle treatment at a time bin, *ANOVA treatment simple-main effect collapsed across sex. Double daggers indicate difference from subsequent time bins, ‡ANOVA time simple-main effect collapsed across treatment, but not sex (Panel A) or time main effect collapse across treatment (Panels B, C).
The potential effects of CRF on locomotor activity were analyzed separately for males and females. In males, locomotor activity decreased after the first 20-minute interval of the test session, and it was not altered by CRF pretreatment (500 ng) relative to vehicle (Fig. 6B; 2 × 6 ANOVA, time main effect, F(5,30) = 72.18, Tukey’s tests, bin 1 > bins 2–6, ps < 0.05; treatment main and interaction effects, Fs ≤ 0.71, ps ≥ 0.62). Similarly, in females, locomotor activity decreased after the first 20-minute interval of the test session, and it was not altered by CRF pretreatment (Fig. 6C; 4 × 6 ANOVA, time main effect, F(5,115) = 148.75, p < 0.001, Tukey’s tests, bin 1 > bins 2–6, ps < 0.05; treatment main and interaction effects, Fs ≤ 1.33, ps ≥ 0.20).
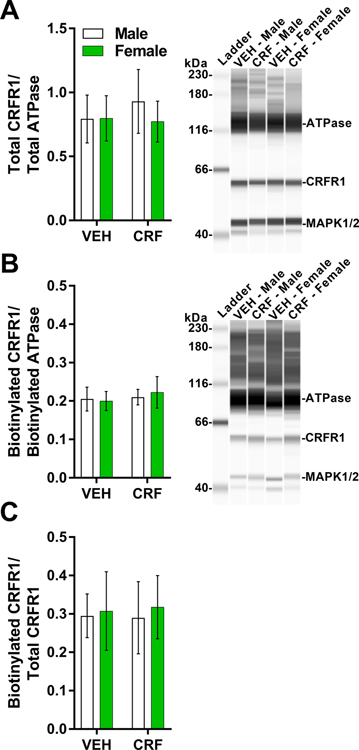
3.9. Intra-BLA CRF treatment does not alter CRFR1 cell-surface expression
To examine whether the non-significant effects of higher doses of CRF on memory strength in experiment 3 were due to compensatory changes in CRFR1 levels or cell-surface CRFR1 levels in the BLA, male and female rats received intra-BLA infusions of CRF (30 ng; lowest dose ineffective in both sexes; n = 4 males, 4 females) or vehicle (n = 4 males, 4 females). Tissue was collected 30 min later. Tissue quality was consistent across groups as indicated by no effect of sex or treatment on cytosolic protein MAPK levels in the biotinylated fraction (data not shown; 2 × 2 ANOVA, all treatment and sex main and interaction effects, Fs ≤ 0.18, ps ≥ 0.68). There were no effects of sex or CRF treatment on total CRFR1 expression normalized to ATPase loading control, (Fig. 7A; 2 × 2 ANOVA, all treatment and sex main and interaction effects, Fs ≤ 0.17, ps ≥ 0.69), on biotinylated CRFR1 expression normalized to biotinylated ATPase loading control (Fig. 7B, 2 × 2 ANOVA, all treatment and sex main and interaction effects, Fs ≤ 0.18, ps ≥ 0.68), or on the ratio of biotinylated to total CRFR1, normalized to the appropriate loading controls (Fig. 7C; 2 × 2 ANOVA, all treatment and sex main and interaction effects, Fs ≤ 0.06, ps ≥ 0.82).
Figure 7. Intra-BLA CRF treatment did not alter total or biotinylated (cell-surface) CRFR1 expression in the BLA.
Male and female rats received bilateral infusions of CRF (30 ng/hemisphere) or vehicle (VEH; DMSO) into the BLA. BLA tissue was collected 30 min later for cell-surface protein biotinylation and analysis total and biotinylated protein levels using an automated capillary electrophoresis immunoassay on the Wes ProteinSimple instrument. (A) Total of CRFR1 area under the curve (AUC) values normalized to total ATPase (loading control) AUC values (mean ratio of AUCs ± SEM). Representative digital western blot image generated by Compass for SW software v4.0.0 (ProteinSimple). The bands corresponding to protein peaks observed for ATPase (99 or 129 kDa), CRFR1 (61 kDa), and MAPK1/2 (41 and 45 kDa) in the total and biotinylated (acetone precipitated) fractions, respectively. See Fig. S5 for representative electropherograms used to measure AUC. MAP1/2 was measured as an indicator of BLA cell integrity. (B) Biotinylated (cell-surface) CRFR1 normalized to biotinylated ATPase (loading control) (mean ratio of AUCs ± SEM). Representative digital western blot image generated by Compass for SW software (ProteinSimple, v4.0.0). A separate, high-contrast image of the ladder was taken and superimposed on the original to increase image quality. (C) Ratio of normalized biotinylated to normalized total CRFR1 AUC values (mean ± SEM).
4. Discussion
In the present study, we investigated the role of CRF signaling in the BLA in cocaine-memory reconsolidation. We observed that CRFR1 antagonist treatment in the BLA immediately after cocaine-memory reactivation (i.e., when cocaine memories were labile) attenuated the reinstatement of extinguished drug-seeking behavior in the previously cocaine-paired context three days later, without altering responding in the extinction context, relative to vehicle treatment (Fig. 2). Conversely, exogenous CRF treatment in the BLA after cocaine-memory reactivation increased drug-seeking behavior selectively in the previously cocaine-paired context compared to vehicle treatment, albeit in a sex- and dose-dependent manner (Fig. 4). These effects on drug-seeking behavior reflected bona fide changes in context-cocaine memory strength as opposed to protracted alterations in motivation or motor performance. In support of this, neither CRFR1 antagonist (Fig. 3) nor CRF (Fig. 5) administration altered cocaine seeking at test when these manipulations were administered 6 h after memory reactivation, by which time labile cocaine memories were expected to be reconsolidated into long-term memories and invulnerable to manipulation (Tronson and Taylor, 2007; Fuchs et al. 2009). The CRFR1 antagonist, antalarmin, elicited mild hyperactivity selectively during the fifth 20-min interval of the locomotor test (Fig. 6A), but that subtle change in motor activity is unlikely to have interfered with drug-seeking behavior (Fig. 2F). Furthermore, intra-BLA CRF administration did not alter locomotor activity (Fig. 6B).
Notably, the observed changes in memory strength may signify changes in cocaine-memory reconsolidation or extinction memory consolidation. Modest within-session extinction learning during the non-reinforced memory reactivation session, as indicated by a slight decrease in lever responding during the last 3-minute interval of the session (data not show, see result in Section 3.3), could evoke extinction-memory consolidation. Furthermore, CRFR1 might modulate this phenomenon based on a report that intra-BLA administration of a CRFR2 antagonist/CRFR1 partial agonist facilitates, whereas intra-BLA administration of a CRF binding protein (CRF-BP) inhibitor that augments CRF bioavailability impairs, fear extinction-memory consolidation (Abiri et al. 2014). Accordingly, BLA CRFR1 antagonist treatment in our study could reduce cocaine-memory strength either through interference with context-cocaine memory reconsolidation or through enhancement of context-no-cocaine extinction memory consolidation; even though, reconsolidation was likely the dominant process (Eisenberg et al. 2003). Intra-BLA CRF treatment could increase context-cocaine memory strength during, or the efficacy of, reconsolidation or interfere with extinction memory consolidation. Through these mechanisms, CRFR1 signaling in the BLA can regulate both the mnemonic and/or motivational effects of cocaine-paired contextual stimuli.
CRF may regulate cocaine-memory strength through multiple intracellular signaling pathways, given the complexity of effector systems connected with CRFR1. CRFR1 can be coupled with Gs and Gq G-proteins, but it can also signal through β-arrestin (for review, see Hauger et al. 2006). Thus far, evidence from the cocaine-memory reconsolidation literature is consistent with the idea that modulation of Gs- or β-arrestin-mediated signaling pathways in the BLA may mediate the effects of CRFR1 manipulations on cocaine-memory reconsolidation in the present study. Stimulation of Gs-coupled CRFR1 results in cAMP formation and PKA activation (Aguilara et al. 1983), which drives synaptic plasticity associated with learning and memory (Esteban et al. 2003). PKA activation is also required for the reconsolidation of both aversive (Koh and Bernstein 2003; Tronson et al. 2006) and appetitive associative memories (Kemenes et al. 2006). In particular, inhibition of PKA in the BLA impairs drug context-induced (Arguello et al. 2014) and explicit conditioned stimulus-induced (Sanchez et al. 2010) reinstatement of cocaine-seeking behaviors in a memory-reactivation dependent manner. These findings confirm that PKA activation in the BLA is necessary for cue-cocaine memory reconsolidation, but future research will need to ascertain whether PKA activation downstream from CRFR1 stimulation per se is requisite for this phenomenon.
PKA-dependent, as well as PKA-independent (e.g., β-arrestin-mediated), ERK activation may be another critical mediator of CRFR1-dependent effects on memory strength (Wan and Huang 1998; Lefkowitz and Sudha 2005; Kageyama et al. 2007). Similar to PKA activation, ERK activation is key to memory storage, including Pavlovian fear-memory consolidation (Schafe et al. 2000) and object recognition memory consolidation and reconsolidation (Kelly et al. 2003). Importantly, ERK activation in the BLA is necessary for cocaine-memory reconsolidation, as ERK inhibitor administration into the BLA immediately after cocaine-memory reactivation attenuates subsequent drug context-induced cocaine-seeking behavior (Wells et al. 2013). Since CRFR1 in the BLA may mediate cocaine-memory reconsolidation through a number of distinct intracellular signaling pathways, additional systematic research will be needed to identify the most critical signaling molecules involved.
Intra-BLA administration of the 0.2-ng dose of CRF enhanced cocaine-memory strength selectively in female rats (Fig. 4D–E), whereas higher doses (30 or 500 ng) failed to alter memory strength in either female or male rats (Fig. 4B–C). Below we consider the dose-dependent and sex-dependent aspects of these responses.
The inverted U-shaped dose-effect relationship observed in females suggests that CRF concentration must remain within an optimal range to augment memory strength. This may be related to the effects of CRF on glutamate neurotransmission in the BLA, based on observations that intra-BLA CRF administration elicits c-Fos-mediated neuronal activation in CaMKII-immunoreactive BLA principal neurons in an inverted U-shaped dose-dependent manner (Rostkowski et al. 2013) and glutamatergic signaling in the BLA is critical for cocaine-memory reconsolidation (Milton et al. 2008; Higginbotham et al. 2021b) and extinction memory consolidation (Feltenstein and See, 2007). There are several possible mechanistic explanations for the null effects of high-dose CRF treatment. First, CRFR1 stimulation enhances BLA excitability in part by augmenting hyperpolarization-activated inwardly rectifying (Ih) current (Giesbrecht et al. 2010). However, high concentrations of CRF might result in over-recruitment of Ih, leading to a depolarization block (Bianchi et al. 2012), disruption in pyramidal neuronal output, and impairment in memory processing. Second, CRF has ~40-fold greater affinity for CRFR1 than for CRF receptor type 2 (CRFR2) (Dautzenberg et al. 2001), a receptor subtype that is expressed in low abundance within the BLA (Chalmers et al. 1995; Pett et al. 2000). Nonetheless, high-dose CRF treatment might significantly increase the stimulation of CRFR2, which contributes differently to physiological responses to stress compared to CRFR1 (Janssen and Kozicz 2013) and may exert opposite effects on cocaine-memory strength. Third, CRF at high concentrations might lead to CRFR1 desensitization or β-arrestin-mediated CRFR1 internalization in the BLA (Reisine and Hoffman 1983; Perry et al. 2005; Ferguson 2001; Rasmussen et al. 2004; Teli et al. 2005; Dunn et al. 2013). To our knowledge, BLA CRFR1 internalization has not been studied. Therefore, we examined whether intra-BLA CRF administration (30 ng) reduced CRFR1 cell-surface expression 30-minutes post-treatment based on the in vivo kinetics of agonist-induced CRFR1 internalization in the locus coeruleus (LC; Reyes et al. 2006). We found no evidence of agonist-induced receptor internalization in the BLA (Fig. 6). Thus, the null effects of high-dose CRF treatment on memory strength may be related to the additive effects of exogenous CRF treatment and memory reactivation-associated endogenous CRF release (DeVries et al. 1998; Sinha et al. 2003; Stringfield et al. 2017; Higginbotham et al. 2021a), which we did not investigate. Alternatively, these may involve CRFR1 desensitization, including receptor phosphorylation and G-protein uncoupling (Teli et al. 2005; Bangasser et al. 2010; Howerton et al. 2014).
The sex-dependent nature of intra-BLA CRF treatment effects might be related to differences between male and female rats in the sensitivity of the CRF system at the molecular and behavioral levels. Prior research indicates that female rats exhibit more robust cocaine-seeking behavior upon intracerebroventricular CRF administration (Buffalari et al. 2012). Furthermore, LC neurons in female rats are activated by 10–30-fold lower concentrations of exogenous CRF than in males (Curtis et al. 2006; Bangasser et al., 2010). These sex differences in the sensitivity of the CRF system are produced at least in part by greater CRFR1 Gs coupling within the LC and cortex of unstressed female rats (Bangasser et al. 2010) and by increased β-arrestin-mediated CRFR1 internalization in the LC of stress-exposed males (Bangasser et al. 2010). Conversely, female rats are less sensitive to the memory-impairing effects of CRF in the medial septum than males, and this may be related to more robust expression of CRF-BP, an endogenous inhibitor of CRF and urocortin 1 bioavailability (Potter et al. 1991; Behan et al. 1995), in females than in males (Wiersielis et al. 2019). Since sex differences in the sensitivity of the CRF system are brain region specific, future research will need to evaluate whether the increased memory strength observed in females in the present study reflects a shift of the inverted U-shaped CRF dose-effect curve to the left (i.e., increased sensitivity to a CRF dose that falls on the ascending limb of the dose-effect curve) or to the right (i.e., tolerance to a CRF dose that falls on the descending limb of the dose-effect curve for males).
We have extensively demonstrated that brain regions dorsally adjacent to the BLA are not involved in cocaine-memory reconsolidation (Fuchs et al. 2009; Wells et al. 2013; Arguello et al. 2014; Stringfield et al. 2017; Higginbotham et al. 2021a). Injection cannula placements in the present study were located in the ventral lateral BLA, but it is possible that CRF could diffuse to the central nucleus of the amygdala (CeA), a CRFR1-expressing brain region (Pett et al. 2000; Weera et al. 2021). Previous studies indicate that the CeA is recruited during memory reconsolidation if memory reactivation is elicited using interoceptive cues (e.g., cocaine itself, Zhu et al. 2018; cue signaling post-ingestive nutrient state, Yan et al. 2020) or the labile memory is manipulated using the retrieval-extinction procedure (Olshavsky et al. 2013), possibly based on the CeA’s involvement in valence and salience encoding (Kong and Zweifel 2021). Conversely, the CeA is not recruited during memory reconsolidation if memory reactivation elicited using environmental stimuli (e.g., shock-predictive auditory cue, Si et al. 2012; cocaine-conditioned context, Zhu et al. 2018), suggesting that it is unlikely that CeA CRFR1 mediated the alterations in memory strength in the present study.
Overall, our results indicate that the level of CRFR1 stimulation in the BLA critically regulates cocaine-memory strength; however, the source of the relevant pool of CRF in the BLA has yet to be identified. CRF-expressing neurons are located throughout the neocortex and limbic system (Swanson et al. 1983), and several brain regions may supply CRF to support cocaine-memory reconsolidation through monosynaptic projections to the BLA (for review, Sah et al. 2003). The medial prefrontal cortex is one such candidate brain region based on its neuropeptide chemistry (Swanson et al. 1983; Hupalo et al. 2019), monosynaptic connectivity with the BLA (McDonald et al. 1996), and role in the reconsolidation of Pavlovian and explicit conditioned stimulus-cocaine memories (Otis et al. 2013; Sorg et al. 2015). However, we have been unable to confirm the involvement of the medial prefrontal cortex in context-cocaine memory reconsolidation per se (Ramirez et al. 2009). A second potential source of CRF is the CeA. In the absence of direct CeA inputs, CRF from the CeA may passively diffuse to the BLA. This mechanism of action has been proposed to underlie fear-memory consolidation in rats and is supported by the observation that foot-shock exposure increases CRF-like immunoreactivity in the dorsal-medial BLA, adjacent to the CeA (Roozendaal et al. 2002). Finally, a third possible source of CRF is the oval nucleus of the bed nucleus of the stria terminals based on its monosynaptic CRF projections to the BLA (Dabrowska et al. 2016). Future research will need to systematically map the specific CRF circuits involved in cocaine-memory reconsolidation.
The present study confirmed previously reported patterns of sex differences in cocaine intake and cocaine-motivated goal-directed behaviors in rats. Female rats obtained more cocaine infusions than males by making fewer timeout responses (Fig. 1). Similar results have been reported in rats trained to self-administer the same dose of cocaine coupled with discrete light-tone conditioned stimuli (Fuchs et al. 2005; Zhou et al. 2014). It has also been shown that female rats acquire cocaine-reinforced responding faster and in greater proportions than males (Lynch and Carroll 1999). These preclinical findings parallel results from clinical studies which indicate that female cocaine users self-report more intense cocaine craving upon cocaine-cue exposure and are more severely impacted by cocaine use disorder than male users (Griffin and Mirin 1989; Kosten et al. 1993; Robbins et al. 1999).
Females also exhibited more responding on the previously cocaine-reinforced lever than males during extinction training in the extinction context (Fig. 1A) and during the memory reactivation session, but not the reinstatement test, in the cocaine-paired context (Fig. 1B). On one hand, the absence of this sex difference at test might result from more effective extinction-memory consolidation in females after the memory reactivation test. On the other hand, the literature has been inconsistent regarding sex differences in cue-induced reinstatement with studies reporting either more robust cue-induced cocaine-seeking in females than in males (Zhou et al. 2014) or no sex differences (Fuchs et al. 2005; Feltenstein et al. 2011).
The present study included free-cycling females without estrous cycle monitoring, and this approach did not permit the investigation of the potential impact of estrous state on cocaine-memory strength or reconsolidation efficacy. Previous research suggests that estrous phase at the time of memory reconsolidation does not alter contextual-fear memory strength or the ability of an amnesic agent to disrupt reconsolidation (Franzen et al. 2019). However, our findings indicate that sex differences exist in the efficacy of CRF in the BLA to enhance cocaine-memory strength. The potential contribution of estrous state to these effects will have to be investigated in preparations that permit maximal experimental control and sensitivity to detect the potentially nuanced, circuit and cell-type specific influence of ovarian hormones on cocaine-memory reconsolidation.
5. Conclusion
The results from the present study suggest that interference with CRFR1 signaling may weaken cocaine-related maladaptive memories as part of an anti-relapse treatment strategy. Antalarmin has low oral bioavailability (Habib et al. 2000), which precludes its use as a pharmacotherapeutic agent. However, the efficacy of several other CRFR1 antagonists has been evaluated for the treatment of psychiatric disorders that may involve abnormally strong or intrusive memories, including alcohol use disorder (Kwako et al. 2015; Schwandt et al. 2016), post-traumatic stress disorder (Dunlop et al. 2017), major depression (Binneman et al. 2008), and generalized anxiety disorder (Coric et al. 2010), in clinical trials. Despite encouraging results in animal models (Mansbach et al. 1997; Le et al. 2000; Boyson et al. 2011), CRFR1 antagonist treatments were ineffective, albeit well tolerated (Binneman et al. 2008; Coric et al. 2010; Schwandt et al. 2016; Dunlop et al. 2017), in these clinical samples. The effects of CRFR1 antagonist treatment on memory reconsolidation has not been investigated in human subjects, but our results provide impetus for future research in this direction. Moving forward, understanding the critical effector systems responsible for CRFR1 antagonist effects on memory strength in preclinical models will be key to identify viable therapeutic approaches for drug relapse prevention.
Supplementary Material
Highlights:
Female rats self-administer more cocaine than males
Cocaine memory strength can be altered during memory reconsolidation
BLA CRFR1 antagonism weakens an index of cocaine-memory strength in both sexes
BLA CRF treatment increases this index in females only at low, but not high, doses
High dose CRF treatment did not reduce CRFR1 cell-surface expression
Off-target motor effects did not account for changes in cocaine-memory strength
6. Acknowledgments
The authors are grateful to Dr. Jessica Higginbotham, Jennifer Wang, Jaclyn Roland-McGowan, Taylor Brown, and Erik Glueck for expert technical assistance. The authors also thank Drs. Jim Peters, Ilia Karatsoreos, Beverly Reyes, and William Colmers for helpful discussions. This work was supported by NIH NIDA 2 R01 DA025646 (RAF) and by Washington State Initiative 171 research funds administered through the WSU Alcohol and Drug Abuse Research program (JLR).
Footnotes
Declarations of interest: None.
Abbreviations: ACSF - artificial cerebrospinal fluid, ANOVA – analysis of variance, Anta – antalarmin, ATPase – adenosine triphosphatase, AUC – area under the curve, BLA – basolateral amygdala, COC – cocaine-paired context, CRF – corticotropin-releasing factor, CRF-BP – CRF binding protein, CRFR1 – CRF receptor type 1, CRFR2 – CRF receptor type 2, CeA - central nucleus of the amygdala, DMSO – dimethyl sulfoxide, DTT – dithiothreitol, ERK – extracellular signal-regulated kinase, EXT – extinction, IEG – immediate-early gene, Ih – hyperpolarization-activated inwardly rectifying current, IPBL-Plus – immunoprecipitation lysis buffer containing protease and phosphatase inhibitors, MAPK – mitogen activated protein kinase, MR – memory reactivation, NIDA – National Institute on Drug Abuse, NMDA – N-methyl-D-aspartate, PKA – protein kinase A, SUD – substance use disorder, Sulfo-NHS-SS-Biotin – sulfosuccinimidyl-20(biotinamido)ethyl-1,3-dithiopropionate, VEH – vehicle, VTA – ventral tegmental area.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.1 Works Cited
- Abiri D, Douglas CE, Calakos KC, Barbayannis G, Roberts A, Bauer EP, 2014. Fear extinction learning can be impaired or enhanced by modulation of the CRF system in the basolateral nucleus of the amygdala. Behav Brain Res 271, 234–239. 10.1016/j.bbr.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilara G, Hardwood JP, Wilson JX, Morell W, Brown JH, Catt KJ, 1983. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J. Biol. Chem. 258, 8039–8045. 10.1016/S0021-9258(20)82024-9. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Hodges MA, Wells AM, Lara H 3rd, Xie X, Fuchs RA, 2014. Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology (Berl) 231, 55–65. 10.1007/s00213-013-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ, 2010. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15, 896–904. 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan D, De Souza E, Lowry P, Potter E, Sawchenko P, Vale W, 1995. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol 16, 362–382. 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP, 2006. Postretrieval propranolol disrupts a cocaine conditioned place preference. NeuroReport 17, 1443–1447. 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Bianchi D, Marasco A, Limongiello A, Marchetti C, Marie H, Tirozzi B, Migliore M, 2012. On the mechanisms underlying the depolarization block in the spiking dynamics of CA1 pyramidal neurons. J Comput Neurosci 33, 207–225. 10.1007/s10827-012-0383-y. [DOI] [PubMed] [Google Scholar]
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T, 2008. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am Journal Psychiatry 165, 617–620. 10.1176/appi.ajp.2008.07071199. [DOI] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR, 2011. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci 31, 11396–11403. 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA, 2011. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 218, 257–269. 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE, 2012. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105, 209–214. 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB, 1995. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15, 6340–6350. 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW, 1993. Expression cloning of a human corticotropin-releasing factor receptor. PNAS 90, 8967–8971. 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coric V, Feldman HH, Oren DA, Shekhar A, Pultz J, Dockens RC, Wu X, Gentile KA, Huang SP, Emison E, Delmonte T, D’Souza BB, Zimbroff DL, Grebb JA, Goddard AW, Stock EG, 2010. Multicenter, randomized, double-blind, active comparator and placebo-controlled trial of a corticotropin-releasing factor receptor-1 antagonist in generalized anxiety disorder. Depress Anxiety 27, 417–425. 10.1002/da.20695. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ, 2006. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31, 544–554. 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, Muller JF, Young LJ, Rainnie DG, 2011. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology 36, 1312–1326. 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, Rainnie DG, 2016. Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol 28. 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Py-Lang G, Higelin J, Fischer C, Wright MB, Huber G, 2001. Different binding modes of amphibian and human corticotropin-releasing factor type 1 and type 2 receptors: evidence for evolutionary differences. J Pharmacol Exp Ther 296, 113–120. 11123370. [PubMed] [Google Scholar]
- DeVries A, Taymans SE, Sundstrom JM, Pert A, 1998. Conditioned release of corticosterone by contextual stimuli associated with cocaine is mediated by corticotropin-releasing factor. Brain Res 786, 39–46. 10.1016/s0006-8993(97)01328-0. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Iosifescu D, Mathew SJ, Neylan TC, Pape JC, Carrillo-Roa T, Green C, Kinkead B, Grigoriadis D, Rothbaum BO, Nemeroff CB, Mayberg HS, 2017. Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic stress disorder. Biol Psychiatry 82, 866–874. 10.1016/j.biopsych.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn HA, Walther C, Godin CM, Hall RA, Ferguson SS, 2013. Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling. J Biol Chem 288, 15023–15034. 10.1074/jbc.M113.473660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP, 1992. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107, 523–529. 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Eisenberg M, Kobilo T, Berman DE, Dudai Y, 2003. Stability of retrieved memory: Inverse correlation with trace dominance. Science 301, 1102–1104. DOI: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R, 2003. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6, 136–143. 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE, 2011. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 216, 53–62. 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE, 2007. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem 88, 435–444. 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S, 2001. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacology Review 53, 1–24. . [PubMed] [Google Scholar]
- Foltin RW, Haney M, 2000. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology 149, 24–33. 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fanzen JM, Giachero M, Bertoglio LJ, 2019. Dissociating retrieval-dependent contextual aversive memory processes in female rats: Are there cycle-dependent differences? Neuroscience 406, 542–553. 10.1016/j.neuroscience.2019.03.035. [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF, 2008. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem 15, 643–648. 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH, 2007. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26, 487–498. 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI, 2009. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30, 889–900. 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE, 2005. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 179, 662–672. 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Giesbrecht CJ, Mackay JP, Silveira HB, Urban JH, Colmers WF, 2010. Countervailing modulation of Ih by neuropeptide Y and corticotrophin-releasing factor in basolateral amygdala as a possible mechanism for their effects on stress-related behaviors. J Neurosci 30, 16970–16982. 10.1523/jneurosci.2306-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M, Mirin S, 1989. A comparison of male and female cocaine abusers. Archives of General Psychiatry 46, 122–126. 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW, 2000. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proceedings of the National Academy of Sciences of the United States of America 97, 6079–6084. 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger R, Risbrough V, Brauns O, Dautzenberg F, 2006. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets 5, 453–479. 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham JA, Jones NM, Wang R, Christian RJ, Ritchie JL, McLaughlin RJ, Fuchs RA, 2021a. Basolateral amygdala CB1 receptors gate HPA axis activation and context-cocaine memory strength during reconsolidation. Neuropsychopharmacology, 1–11. 10.1038/s41386-020-00919-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham JA, Wang R, Richardson BD, Shiina H, Tan SM, Presker MA, Rossi DJ, Fuchs RA, 2021b. CB1 receptor signaling modulates amygdalar plasticity during context-cocaine memory reconsolidation to promote subsequent cocaine seeking. J Neurosci 41, 613–629. 10.1523/JNEUROSCI.1390-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA, 2016. Episodic social stress-escalated cocaine self-administration: Role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci 36, 4093–4105. 10.1523/JNEUROSCI.2232-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KD, Babwah AV, Dale LB, Poulter MO, Ferguson SS, 2006. Differential regulation of corticotropin releasing factor 1 alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem 96, 934–949. 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- Hubbard DT, Nakashima BR, Lee I, Takahashi LK, 2007. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience 150, 818–828. 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupalo S, Martin AJ, Green RK, Devilbiss DM, Berridge CW, 2019. Prefrontal corticotropin-releasing factor (CRF) neurons act locally to modulate frontostriatal cognition and circuit function. J Neurosci 39, 2080–2090. 10.1523/jneurosci.2701-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D, Kozicz T, 2013. Is it really a matter of simple dualism? Corticotropin-releasing factor receptors in body and mental health. Front Endocrinol (Lausanne) 4, 28. 10.3389/fendo.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Moriyama T, Imaizumi T, Satoh K, Suda T, 2007. Differential regulation of CREB and ERK phosphorylation through corticotropin-releasing factor receptors type 1 and 2 in AtT-20 and A7r5 cells. Mol Cell Endocrinol 263, 90–102. 10.1016/j.mce.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Hanada K, Moriyama T, Nigawara T, Sakihara S, Suda T, 2006. G protein-coupled receptor kinase 2 involvement in desensitization of corticotropin-releasing factor (CRF) receptor type 1 by CRF in murine corticotrophs. Endocrinology 147, 441–450. 10.1210/en.2005-0376. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S, 2003. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci 12, 5354–5360. 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes G, Kemenes I, Michel M, Papp A, Muller U, 2006. Phase-dependent molecular requirements for memory reconsolidation: differential roles for protein synthesis and protein kinase A activity. J Neurosci 26, 6298–6302. 10.1523/JNEUROSCI.0890-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL, 2003. Inhibition of protein kinase A activity during conditioned taste aversion retrieval: interference with extinction or reconsolidation of a memory? NeuroReport 14, 405–407. 10.1097/00001756-200303030-00021. [DOI] [PubMed] [Google Scholar]
- Kong MS, Zweifel LS, 2021. Central amygdala circuits in valence and salience processing. Behav Brain Res 410, 113355. 10.1016/j.bbr.2021.113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin F, Kosten TR, Rounsaville B, 1993. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10, 63–66. 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Schiller D, LeDoux JE, Phelps EA, 2016. Translational approaches targeting reconsolidation. Curr Top Behav Neurosci 28, 197–230. 10.1007/7854_2015_5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev YA, Childs GV, Ritchie AK, 1995. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology 136, 3925–3935. 10.1210/endo.136.9.7649101. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M, 2015. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 40, 1053–1063. 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y, 2000. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 150, 317–324. 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lee E, Sung Y, 1989. Differential influences of corticotropin-releasing factor on memory retention of aversive learning and appetitive learning in rats. Behav Neural Biol, 285–294. 10.1016/s0163-1047(89)90412-3. [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ, 2005. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47, 795–801. 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ, 2006. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26, 5881–5887. 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R, Sudha S, 2005. Transduction of receptor signals by b-arrestins. Science 308, 512–517. 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Liang K, Lee E, 1988. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology, 232–236. 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME, 1999. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144, 77–82. 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Brooks EN, Chen YL, 1997. Antidepressant-like effects of CP-154,526, a selective CRF1 receptor antagonist. Eur J Pharmacol 323, 21–26. 10.1016/s0014-2999(97)00025-3. [DOI] [PubMed] [Google Scholar]
- McDonald A, Mascagni F, Guo L, 1996. Projections of the medial and lateral prefrontal cortices to the amygdala: Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71, 55–75. 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, David M, Anisam H, 1998. Aversive and appetitive events evoke the release of corticotropinreleasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 18, 4758–4766. 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ, 2008a. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28, 8230–8237. 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson JL, Doucette AA, 2020. Rapid and quantitative protein precipitation for proteome analysis by mass spectrometry. J Proteome Res 19, 2035–2042. 10.1021/acs.jproteome.9b00867. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ, 2008b. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem 15, 88–92. 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- O’brien CP, Childress AR, Mclellan AT, Ehrman R, 1992. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 654, 400–415. 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Olshavsky ME, Song BJ, Powell DJ, Jones CE, Monfils MH, Lee HJ, 2013. Updating appetitive memory during reconsolidation window: critical role of cue-directed behavior and amygdala central nucleus. Front Behav Neurosci 7, 186. 10.3389/fnbeh.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D, 2013. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci 33, 1271–1281a. 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachas GN, Gilman J, Orr SP, Hoeppner B, Carlini SV, Grasser EB, Loebl T, Nino J, Pitman RK, Evins AE, 2015. Single dose propranolol does not affect physiologic or emotional reactivity to smoking cues. Psychopharmacology (Berl) 232, 1619–1628. 10.1007/s00213-014-3797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus DJ, Kamboj SK, Das RK, Saladin ME, 2019. Prospects for reconsolidation-focused treatments of substance use and anxiety-related disorders. Curr Opin Psychol 30, 80–86. 10.1016/j.copsyc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SJ, Junger S, Kohout TA, Hoare SR, Struthers RS, Grigoriadis DE, Maki RA, 2005. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem 280, 11560–11568. 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW, 1991. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature 349, 423–426. 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Potter E, S. S, Donaldson C, Chen R, Perrin M, Lewis K, Sawchenko P, Vale W, 1994. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. PNAS 91, 8777–8781. 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA, 2009. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci 30, 901–912. 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TN, Novak I, Nielsen SM, 2004. Internalization of the human CRF receptor 1 is independent of classical phosphorylation sites and of beta-arrestin 1 recruitment. Eur J Biochem 271, 4366–4374. 10.1111/j.1432-1033.2004.04371.x. [DOI] [PubMed] [Google Scholar]
- Reisine T, Hoffman A, 1983. Desensitization of corticotropin-releasing factor receptors. Biochem Biophys Res Commun 111, 919–925. 10.1016/0006-291X(83)91387-6. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ, 2006. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci 23, 2991–2998. 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Robbins S, Ehrman R, Childress A, O’Brien C, 1999. Comparing levels of cocaine cue reactivity in male and female outpatiens. Drug Alcohol Depend 53, 223–230. 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Brunson K, Holloway B, McGaugh J, Baram T, 2002. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. PNAS 99, 13908–13913. 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL, 2008. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the beta-adrenoceptor-cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci 28, 6642–6651. 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostkowski AB, Leitermann RJ, Urban JH, 2013. Differential activation of neuronal cell types in the basolateral amygdala by corticotropin releasing factor. Neuropeptides 47, 273–280. 10.1016/j.npep.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber E, Lopez De Armentia M, Power J, 2003. The amygdaloid complex: Anatomy and physiology. Physiol Rev 83, 803–834. 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD, Baker NL, Hartwell KJ, Brady KT, 2013. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 226, 721–737. 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR, 2010. Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci 30, 4401–4407. 10.1523/JNEUROSCI.3149-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, B.E., Gardi J, Vecsernyés M, Julesz J, Telegdy G, 1993. Alterations of corticotropin-releasing factor-like immunoreactivity in different brain regions after acute cocaine administration in rats. Brain Res 616, 315–319. 10.1016/0006-8993(93)90224-b. [DOI] [PubMed] [Google Scholar]
- Schafe G, Atkins C, Swank M, Bauer E, Sweatt J, LeDoux J, 2000. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci 20, 8177–8187. 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, Grigoriadis DE, Pich EM, Leggio L, Heilig M, 2016. The CRF1 antagonist verucerfont in anxious alcohol-dependent women: Translation of neuroendocrine, but not of anti-craving effects. Neuropsychopharmacology 41, 2818–2829. 10.1038/npp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ, 2003. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 170, 62–72. 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Sorg BA, 2012. Reconsolidation of drug memories. Neurosci Biobehav Rev 36, 1400–1417. 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Todd RP, Slaker M, Churchill L, 2015. Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology 92, 25–33. 10.1016/j.neuropharm.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfield SJ, Higginbotham JA, Wang R, Berger AL, McLaughlin RJ, Fuchs RA, 2017. Role of glucocorticoid receptor-mediated mechanisms in cocaine memory enhancement. Neuropharmacology 123, 349–358. 10.1016/j.neuropharm.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW, 1983. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology 36, 165–186. 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM, 2009. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology 56 Suppl 1, 186–195. 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teli T, Markovic D, Levine MA, Hillhouse EW, Grammatopoulos DK, 2005. Regulation of corticotropin-releasing hormone receptor type 1 alpha signaling: structural determinants for G protein-coupled receptor kinase-mediated phosphorylation and agonist-mediated desensitization. Mol Endocrinol 19, 474–490. 10.1210/me.2004-0275. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR, 2007. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8, 262–275. 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR, 2006. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci 9, 167–169. 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille A, Bertran-Gonzalez J, Herve D, Girault J, 2006. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. PNAS 103, 2932–2937. 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kolen K, Dautzenberg FM, Verstraeten K, Royaux I, De Hoogt R, Gutknecht E, Peeters PJ, 2010. Corticotropin releasing factor-induced ERK phosphorylation in AtT20 cells occurs via a cAMP-dependent mechanism requiring EPAC2. Neuropharmacology 58, 135–144. 10.1016/j.neuropharm.2009.06.022. [DOI] [PubMed] [Google Scholar]
- Van Pett K, C. R, Perrin M, Viau V, Li H, Vale W, Bittencourt J, Arias C, Sawchenko P, 2000. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428, 191–212. . [DOI] [PubMed] [Google Scholar]
- Wan Y, Huang X, 1998. Analysis of the Gs/mitogen-activated protein kinase pathway in mutant S49 cells. J Biol Chem 273, 14533–14537. 10.1074/jbc.273.23.14533. [DOI] [PubMed] [Google Scholar]
- Weera MM, Agoglia AE, Douglass E, Jiang Z, Rajamanikam S, Shackett RS, Justice NJ, Herman MA, Gilpin NW, 2021. Cellular, physiological, and behavioral validation of a CRFR1:Cre - tdTomato transgenic rat for use in basic neuroscience research. BioRxiv, 10.1101/2021.02.23.432551. [DOI] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA, 2013. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology 38, 753–762. 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersielis KR, Ceretti A, Hall A, Famularo ST, Salvatore M, Ellis AS, Jang H, Wimmer ME, Bangasser DA, 2019. Sex differences in corticotropin releasing factor regulation of medial septum-mediated memory formation. Neurobiol Stress 10, 1–7. 10.1016/j.ynstr.2019.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Zhang L, Zhu T, Deng S, Ma B, Lv H, Shan X, Cheng H, Jiang K, Zhang T, Meng B, Mei B, Li W-G, Li F, 2020. Reconsolidation of a post-ingestive nutrient memory requires mTOR in the central amygdala. Molecular Psychiatry. 10.1038/s41380-020-00874-5. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Sun LL, Shi J, Li P, Zhang Y, Lu L, 2011. Effects of beta-adrenergic receptor blockade on drug-related memory reconsolidation in abstinent heroin addicts. Drug Alcohol Depend 118, 224–229. 10.1016/j.drugalcdep.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE, 2014. Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct 219, 1831–1840. 10.1007/s00429-013-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhou Y, Liu Z, Chen X, Li Y, Liu X, Ma L, 2018. Beta1-adrenoceptor in the central amygdala is required for unconditioned stimulus-induced drug memory reconsolidation. Int J Neuropsychopharmacol 21, 267–280. 10.1093/ijnp/pyx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.