Abstract
Background
Recent evidence indicates a potential therapeutic role of fluvoxamine for COVID-19. In the TOGETHER trial for acutely symptomatic patients with COVID-19, we aimed to assess the efficacy of fluvoxamine versus placebo in preventing hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to a tertiary hospital due to COVID-19.
Methods
This placebo-controlled, randomised, adaptive platform trial done among high-risk symptomatic Brazilian adults confirmed positive for SARS-CoV-2 included eligible patients from 11 clinical sites in Brazil with a known risk factor for progression to severe disease. Patients were randomly assigned (1:1) to either fluvoxamine (100 mg twice daily for 10 days) or placebo (or other treatment groups not reported here). The trial team, site staff, and patients were masked to treatment allocation. Our primary outcome was a composite endpoint of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 up to 28 days post-random assignment on the basis of intention to treat. Modified intention to treat explored patients receiving at least 24 h of treatment before a primary outcome event and per-protocol analysis explored patients with a high level adherence (>80%). We used a Bayesian analytic framework to establish the effects along with probability of success of intervention compared with placebo. The trial is registered at ClinicalTrials.gov (NCT04727424) and is ongoing.
Findings
The study team screened 9803 potential participants for this trial. The trial was initiated on June 2, 2020, with the current protocol reporting randomisation to fluvoxamine from Jan 20 to Aug 5, 2021, when the trial arms were stopped for superiority. 741 patients were allocated to fluvoxamine and 756 to placebo. The average age of participants was 50 years (range 18–102 years); 58% were female. The proportion of patients observed in a COVID-19 emergency setting for more than 6 h or transferred to a teritary hospital due to COVID-19 was lower for the fluvoxamine group compared with placebo (79 [11%] of 741 vs 119 [16%] of 756); relative risk [RR] 0·68; 95% Bayesian credible interval [95% BCI]: 0·52–0·88), with a probability of superiority of 99·8% surpassing the prespecified superiority threshold of 97·6% (risk difference 5·0%). Of the composite primary outcome events, 87% were hospitalisations. Findings for the primary outcome were similar for the modified intention-to-treat analysis (RR 0·69, 95% BCI 0·53–0·90) and larger in the per-protocol analysis (RR 0·34, 95% BCI, 0·21–0·54). There were 17 deaths in the fluvoxamine group and 25 deaths in the placebo group in the primary intention-to-treat analysis (odds ratio [OR] 0·68, 95% CI: 0·36–1·27). There was one death in the fluvoxamine group and 12 in the placebo group for the per-protocol population (OR 0·09; 95% CI 0·01–0·47). We found no significant differences in number of treatment emergent adverse events among patients in the fluvoxamine and placebo groups.
Interpretation
Treatment with fluvoxamine (100 mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19 reduced the need for hospitalisation defined as retention in a COVID-19 emergency setting or transfer to a tertiary hospital.
Funding
FastGrants and The Rainwater Charitable Foundation.
Translation
For the Portuguese translation of the abstract see Supplementary Materials section.
Introduction
Although safe and effective vaccines for COVID-19 have been developed and distributed, there remain, particularly in low resource settings, major challenges regarding their production, allocation, and affordability.1 Identifying inexpensive, widely available, and effective therapies against COVID-19 is, therefore, of great importance. In particular, repurposing existing medicines that are widely available and with well understood safety profiles, has particular appeal.2
Research in context.
Evidence before this study
A search of PubMed on Sept 10, 2021 by means of the following search terms “(randomized OR trial) AND (fluvoxamine OR antidepressants OR selective serotonin reuptake inhibitors OR SSRIs) AND (COVID* OR SARS-CoV-2 OR SARS-CoV)”, with no date or language restrictions identified one observational study that reported a significant association between antidepressant use and reduced risk of intubation or death (hazard ratio 0·56; 95% CI 0·43–0·73, p<0·001) and one randomised clinical trial that reported that adult outpatients with symptomatic COVID-19, treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days. In this preliminary randomised trial, 152 participants were randomly assigned to receive 100 mg of fluvoxamine (n=80) or placebo (n=72) three times daily for 15 days; the primary endpoint was clinical deterioration within 15 days of randomisation defined by meeting criteria of shortness of breath, hospitalisation for shortness of breath, pneumonia and oxygen saturation less than 92%, or need for supplemental oxygen to achieve oxygen saturation of 92% or greater.
Added value of this study
TOGETHER is the largest randomised trial to assess the effectiveness of fluvoxamine for patients with COVID-19 in the community. Compared with placebo, patients randomly assigned to fluvoxamine had a lower risk of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to a tertiary hospital due to COVID-19.
Implications of all the available evidence
There are few effective therapies for patients with COVID-19 in the community. Results provide compelling evidence of fluvoxamine's benefit in reducing acute morbidity from COVID-19 illness.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) and a σ-1 receptor (S1R) agonist.3 There are several potential mechanisms for fluvoxamine in treatment of COVID-19 illness, including anti-inflammatory and possible antiviral effects.4 A small placebo-controlled, randomised trial has raised the possibility that fluvoxamine might reduce the risk of clinical deterioration in outpatients with COVID-19, suggesting the need for larger randomised, placebo-controlled studies.5, 6
To evaluate the efficacy of fluvoxamine to prevent progression of COVID-19 and hospitalisation among outpatients with laboratory-documented SARS-CoV-2, we did a randomised, placebo-controlled, adaptive platform trial in Minas Gerais, Brazil. This flexible platform trial design allows for additional agents to be added and tested with standardised operating procedures outlined in a single overarching master protocol.7, 8 Among eight different interventions evaluated in this platform trial, we report here on the clinical evaluation of fluvoxamine by means of a concurrent placebo control group.
Methods
Study design
The TOGETHER trial is a randomised, adaptive platform trial to investigate the efficacy of repurposed treatments for COVID-19 disease among high-risk adult outpatients.9 The trial was designed and done in partnership with local public health authorities from 11 participating cities in Brazil to simultaneously test potential treatments for early disease by means of a master protocol. A master protocol defines prospective decision criteria for discontinuing interventions for futility, stopping because of superiority against placebo, or adding new interventions. Interventions evaluated in the TOGETHER trial, thus far, include, hydroxychloroquine (protocol 1), lopinavir–ritonavir (protocol 1),10 metformin, ivermectin, fluvoxamine, doxasozin, and pegylated interferon lambda versus matching placebos (protocol 2). The TOGETHER trial is centrally coordinated by Platform Life Sciences (Vancouver, Canada) with local implementation provided by Cardresearch (Belo Horizonte, Brazil). Statistical analyses were done by Cytel (Waltham, MA, USA).
The trial complies with the International Conference of Harmonization—Good Clinical Practices as well as local regulatory requirements. It was approved for research ethics by local and national ethics boards in Brazil (CONEP CAAE: 41174620.0.1001.5120, approval letter 5.501.284) and the Hamilton Integrated Research Ethics Board (approval letter 13390) in Canada. The full protocol and statistical analysis plan have previously been published,9 and additional details are in appendix 2 (p 2). The adaptive designs Consolidated Standards of Reporting Trials extension statement guided this trial report.10, 11 An independent data safety monitoring committee (DSMC) provided trial oversight.
Participants
The cities and investigators of the 11 clinical sites in Brazil who participated in the trial are listed in appendix 2 (p 3). Local investigators, in partnership with local public health authorities, recruited participants at community health facilities (emergency settings, influenza-symptom referral centres, or primary care community centres). We used several community outreach strategies including physical and social media as per local public health authorities, in order to create awareness of the trial.
On presentation to one of the trial outpatient care clinics, local investigators screened potential participants to identify those who met the eligibility criteria. The key inclusion criteria were patients older than 18 years, presenting to an outpatient care setting with an acute clinical condition consistent with COVID-19 and symptoms beginning within 7 days of the screening date, or positive rapid test for SARS-CoV-2 antigen done at the time of screening or patient with positive SARS-CoV-2 diagnostic test within 7 days of symptom onset. Eligible patients also had at least one additional criterion for high risk: diabetes; systemic arterial hypertension requiring at least one oral medication for treatment; known cardiovascular disease (heart failure, congenital heart disease, valve disease, coronary artery disease, cardiomyopathies being treated, clinically manifested heart disease and with clinical repercussion); symptomatic lung disease or treatment for such (emphysema, fibrosing diseases); symptomatic asthma requiring chronic use of agents to control symptoms; smoking; obesity, defined as body-mass index greater than 30 kg/m2 (weight and height information provided by the patient); having had a transplant; stage IV chronic kidney disease or on dialysis; immunosuppression or use of corticosteroid therapy (equivalent to at least 10 mg of prednisone per day) or immunosuppressive therapy; history of cancer in the last 0·5 years or undergoing current cancer treatment or aged 50 years or older; and unvaccinated status.
Patients who met any of the following key criteria were excluded from the trial: diagnostic examination for SARS-CoV-2 negative associated with acute flu-like symptoms (patients with negative test taken early and becoming positive a few days later were eligible, if they were less than 7 days after the onset of flu-like symptoms); acute respiratory condition compatible with COVID-19 treated in primary care and previously requiring hospitalisation; acute respiratory condition owing to other causes; received vaccination for SARS-CoV-2; dyspnoea secondary to other acute and chronic respiratory causes or infections (eg, decompensated chronic obstructive pulmonary disease, acute bronchitis, pneumonia, primary pulmonary arterial hypertension); current use of SSRIs (use of other serotonin reuptake inhibitors were not excluded); uncontrolled psychiatric disorders or suicidal ideation; inability or unwillingness to follow research guidelines and procedures. A full list of exclusion criteria is provided in the trial protocol.
If a patient met the aforementioned eligibility criteria, study personnel obtained written informed consent. After obtaining informed consent a rapid antigen test for COVID-19 (Panbio, Abbott Laboratories Jena, Jena, Germany) and a pregnancy test for women of childbearing age were done. If the COVID-19 test was negative or if the pregnancy test was positive, the participant was not included in the trial. After informed consent, study personnel collected the following data before randomisation: demographics, medical history, concomitant medications, comorbidities, exposure to index case information, WHO clinical worsening scale, and the patient-reported outcomes measurement information (PROMIS) Global Health Scale.
Randomisation and masking
Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol-related procedures and contracted specifically for this process. Sites requested randomisation by text message to the pharmacist at the coordinating centre. This maintained concealment of allocation. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years). The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group. Only the third-party pharmacist responsible for releasing the randomisation was aware of which letter was associated with which drug or placebo. As this is a multiarm trial and all active interventions have a matching inert placebo, the matching placebo represents the proportion of the control group for the number of arms in the trial at any given time.
Procedures
All participants received usual standard care for COVID-19 provided by health-care professionals at public health facilities. Patients were randomly assigned to fluvoxamine (Luvox, Abbott) at a dose of 100 mg twice a day for 10 days or corresponding placebo starting directly after randomisation (day 1). Research personnel provided participants with a welcome video, which gave information on the trial, study drug, adverse events, and follow-up procedures. Clinicians providing usual care in public health facilities typically focus on the management of symptoms and provide antipyretics or recommend antibiotics only if they suspect bacterial pneumonia.
Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video-teleconferencing. We collected outcome data irrespective of whether participants took study medication. In case of adverse events, unscheduled visits (during the treatment period) outside of clinical care could occur at any time.
Considering the transmissible characteristics of SARS-CoV-2 and the isolation recommendations of positive individuals, we collected few vital sign data. Cardiac safety was assessed by means of a six-lead electrocardiogram (Kardiamobile, Mountain View, CA, USA) at the baseline visit. The digital recordings were deidentified and transferred to a central facility (Cardresearch, Belo Horizonte, Brazil) for reading. Oxygen status was assessed by means of a pulse oximeter for non-invasive arterial oxygen saturation and pulse (Jumper Medical Equipment, Shenzhen, China), and temperature by means of a standard digital oral thermometer administered by research personnel. Mid-turbinate nasal swab kits and sterile recipient storage were provided for collection of nasopharyngeal swab or sputum–saliva. Nasal swabs for PCR testing was completed on the first quarter of participants enrolled in the trial on days 3 and 7. Viral clearance was assessed to establish whether active drugs showed any antiviral effects.
All serious and non-serious adverse events were reported to study personnel as per local regulatory requirements. Reportable adverse events included serious adverse events, adverse events resulting in study medication discontinuation, and adverse events assessed as possibly related to study medication.
Outcomes
Our primary outcome was a composite endpoint of medical admission to a hospital setting due to COVID-19-related illness defined as COVID-19 emergency setting visits with participants remaining under observation for more than 6 h or referral to further hospitalisation due to the progression of COVID-19 within 28 days of randomisation. Because many patients who would ordinarily have been hospitalised were prevented from admission due to hospital over-capacity during peak waves, the composite endpoint addresses both hospitalisation and a proxy for hospitalisation, retention in a COVID-19 emergency hospital setting. This region of Brazil implemented hospital-like services in the emergency settings with 50–80 bed units providing services including multiday stays, oxygenation, and mechanical ventilation. The 6 h threshold referred only to periods of time recommended for observation by a clinician and does not include waiting times. Key secondary outcomes include viral clearance, time to clinical improvement, number of days with respiratory symptoms, time to hospitalisation for any cause or due to COVID-19 progression, all-cause mortality and time to death from any causes, WHO clinical worsening scale score, days in hospital and on ventilator and adverse events, adverse reactions to the study medications, and the proportion of participants who are non-adherent with the study drugs. All secondary outcomes were assessed up to 28 days following randomisation.
Statistical analysis
The Adaptive Design Protocol and the Master Statistical Analysis Plan provide details of sample size calculation and statistical analysis.9 This trial is adaptive and applies sample size reassessment approaches. To plan for each arm, we assumed a minimum clinical utility of 37·5% (relative risk reduction) to achieve 80% power with 0·05 two-sided type 1 error for a pairwise comparison against the placebo assuming a control event rate of 15%. This resulted in an initial plan to recruit 681 participants per arm. The statistical team did planned interim analyses. Stopping thresholds for futility were established if the posterior probability of superiority was less than 40% at interim analysis. An arm could be stopped for superiority if the posterior probability of superiority met the threshold of 97·6%.
Baseline characteristics are reported as count (%) or median and IQR for continuous variables. We applied a Bayesian framework for our primary outcome analysis and a frequentist approach for all sensitivity analyses and secondary outcomes. Bayesian analysis allows us to report the posterior probability of treatment efficacy at the end of the trial, independently of the decisions made along the way. Posterior efficacy of fluvoxamine for the primary outcome is calculated by means of the beta-binomial model for event rates, as detailed in the appendix of the statistical analysis plan,12 assuming informed priors on the basis of observational data for both placebo and fluvoxamine, for both intention-to-treat and per-protocol analyses (defined as taking >80% of possible doses). Modified intention to treat (mITT) was defined as receiving treatment for at least 24 h before a primary outcome. We accounted for any temporal changes in event rates by means of only the concurrent randomised population. We assessed subgroup effects according to the preplanned statistical analysis plan. We calculated the number needed to treat.
Secondary outcomes were assessed by means of a prespecified frequentist approach. For viral clearance we fitted a longitudinal, mixed-effect logistic regression model with a treatment and time interaction term for binary patient outcomes (COVID-19 positive–negative) reported on day 3 and 7 from randomisation, with subject random effect. We assessed time-to-event outcomes using Cox proportional hazard models and binary outcomes using logistic regression. Model assumptions were evaluated by testing for proportionality. We did a subgroup analysis and reported p values for the interactions. Per-protocol analyses were considered sensitivity analyses to assess the robustness of the results. We followed the statistical analysis plan and provided a post-hoc analysis where requested by reviewers. All analyses were done by means of R version 4.0.3. Full details of the statistical analysis plan can be found in the Open Science Framework under Data Section.
A data and safety monitoring committee provided independent oversight for this trial. We planned a fourth and final interim analysis of the fluvoxamine group based on data up to Aug 2, 2021. Herein, we present follow-up of all patients up to Sept 9, 2021. The trial is registered at ClinicalTrials.gov (NCT04727424).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit for publication. The executive committee take responsibility for the integrity of the data and the accuracy of the data analysis. The trial executive committee oversaw all aspects of trial conduct, completeness, data accuracy and adherence of trial conduct to the protocol and the committee vouch for the accuracy and completeness of the data and for fidelity to the protocol.
Results
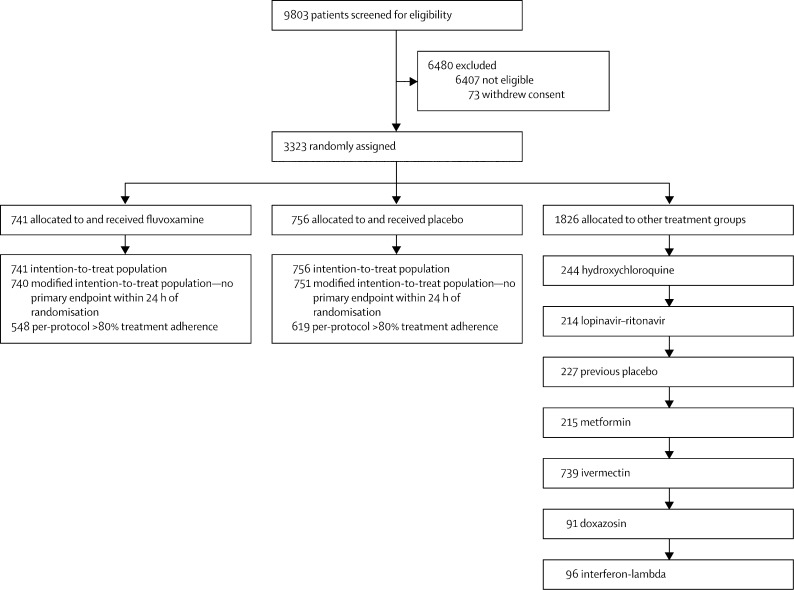
We have screened 9803 potential participants for inclusion in this trial to date. The TOGETHER trial enrolled its first participant on June 2, 2020 and enrolment into the fluvoxamine group began on Jan 20, 2021. As the trial is ongoing, herein we provide descriptive summaries of only those randomly assigned to fluvoxamine and its concurrent control. By Aug 5, 2021, 1497 recruited participants were randomly assigned to fluvoxamine (n=741) or placebo (n=756), and 1826 were randomly assigned to other treatment groups (figure 1 ). Herein, we present data on all patients completing 28 days of follow-up as of Sept 9, 2021. The median age was 50 years (range 18–102) and 862 (58%) were women (table 1 ). Most participants self-identified as mixed race 1428 (95%), 12 (1%) as white, 10 (1%) as black or African heritage, the rest self-identified as unknown 47 (3%). With respect to covariates of age, body-mass index, and comorbidities, the groups were generally well balanced (table 1). The mean number of days with symptoms before randomisation was 3·8 days (SD 1·87).
Figure 1.
Trial profile
Table 1.
Patient characteristics by treatment allocation in the TOGETHER trial
| Fluvoxamine (n=741) | Placebo (n=756) | |
|---|---|---|
| Sex | ||
| Female | 409 (55%) | 453 (60%) |
| Male | 332 (45%) | 303 (40%) |
| Race | ||
| Mixed race* | 709 (96%) | 719 (95%) |
| White | 6 (1%) | 6 (1%) |
| Black or African American | 5 (1%) | 5 (1%) |
| Unknown | 21 (3%) | 26 (3%) |
| Age, years | ||
| <50 | 379 (51%) | 368 (49%) |
| ≥50 | 327 (44%) | 328 (43%) |
| Unspecified | 46 (6%) | 49 (6%) |
| Age descriptive statistics | ||
| Median (IQR) | 50 (39–56) | 49 (38–56) |
| Body-mass index | ||
| <30 kg/m2 | 355 (48%) | 373 (49%) |
| ≥30 kg/m2 | 376 (51%) | 375 (50%) |
| Unspecified | 10 (1%) | 8 (1%) |
| Time since onset of symptoms, days | ||
| 0–3 | 328 (44%) | 310 (41%) |
| 4–7 | 239 (32%) | 267 (35%) |
| Unspecified | 174 (23%) | 179 (24%) |
| Risk factors | ||
| Chronic cardiac disease | 9 (1%) | 7 (1%) |
| Uncontrolled hypertension | 106 (14%) | 88 (12%) |
| Chronic pulmonary disease | 6 (1%) | 3 (<1%) |
| Asthma | 12 (2%) | 16 (2%) |
| Chronic kidney disease | 2 (<1%) | 2 (<1%) |
| Rheumatological disorder | 1 (<1%) | 0 |
| Chronic neurological disorder | 8 (1%) | 6 (1%) |
| Type 1 diabetes | 25 (3%) | 22 (3%) |
| Type 2 diabetes | 104 (14%) | 92 (12%) |
| Autoimmune disease | 0 | 2 (<1%) |
| Any other risk factor(s) or comorbidities | 25 (3%) | 24 (3%) |
Data are n (%).
Self-identified as someone with mixed ancestry.
All patients accessed care via a COVID-19 emergency setting. There were a total of 180 patients in the fluvoxamine group and 251 patients in the placebo group who had any interaction with a COVID-19 emergency setting. The relative risk (RR) for ever visiting a COVID-19 emergency setting was 0·73 (95% CI 0·62–0·88).
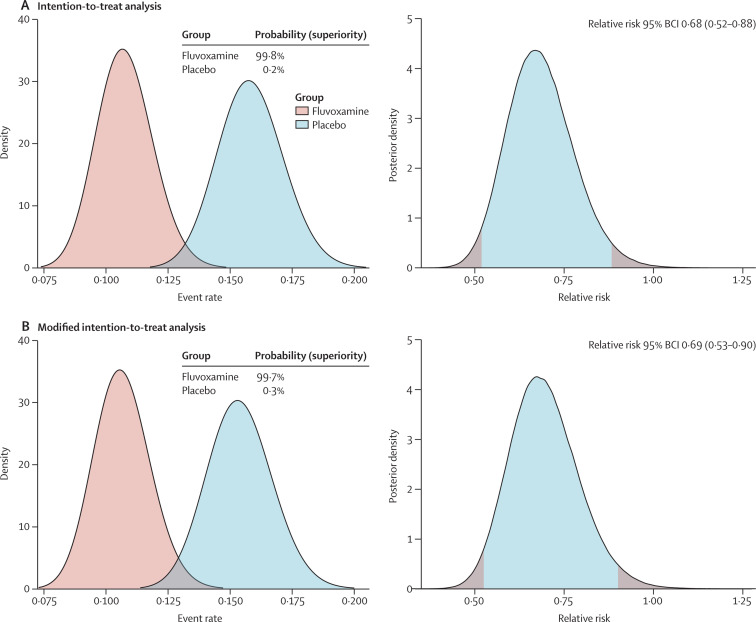
In the fluvoxamine group 79 (11%) participants had a primary outcome event compared with 119 (16%) in the placebo group (table 2 ). Most events (87%) were hospitalisations. On the basis of the Bayesian beta-binomial model, there was evidence of a benefit of fluvoxamine reducing the composite primary endpoint of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 (RR 0·68; 95% Bayesian credible interval [BCI] 0·52–0·88) in the ITT population (figure 2A ) and RR 0·69; 95% BCI 0·53–0·90 in a modified ITT population (figure 2B). The number needed to treat was 20. Per-protocol analysis showed a larger treatment effect (0·34, 95% BCI 0·21–0·54). The probability that the event rate was lower in the fluvoxamine group compared with placebo was 99·8% for the ITT population and 99·7% for the mITT population (figure 2A, B). When the DSMC met on Aug 5, 2021, it recommended that the TOGETHER trial stop randomly assigning patients to the fluvoxamine group, as this comparison had met the prespecified superiority criterion for the primary endpoint (prespecified superiority threshold 97·6%).
Table 2.
Proportion of primary outcome events and relative risk of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 for patients allocated fluvoxamine versus placebo
|
Intention-to-treat analysis |
Modified intention-to-treat analysis |
|||||
|---|---|---|---|---|---|---|
| N | n (%) | Relative risk (95% BCI) | N | n (%) | Relative risk (95% BCI) | |
| Fluvoxamine | 741 | 79 (11%) | 0·68 (0·52–0·88) | 740 | 78 (11%) | 0·69 (0·53–0·90) |
| Placebo | 756 | 119 (16%) | 1 (ref) | 752 | 115 (15%) | 1 (ref) |
BCI=Bayesian credible interval.
Figure 2.
Probability of efficacy and Bayesian relative risk of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19 for fluvoxamine versus placebo
BCI=Bayesian credible interval.
Table 3 presents findings from secondary outcome analyses. There were no significant differences between fluvoxamine and placebo for viral clearance at day 7 (p=0·090) and hospitalisations due to COVID (p=0·10), all-cause hospitalisations (p=0·09), time to hospitalisation (p=0·11), number of days in hospital (p=0·06), mortality (p=0·24), time to death (p=0·49), number of days on mechanical ventilation (p=0·90), time to recovery (p=0·79) or the PROMIS Global Physical (p=0·55) or Mental Scale (p=0·32; appendix 2 p 8).
Table 3.
Secondary outcomes of fluvoxamine versus placebo in the TOGETHER trial
| Fluvoxamine | Placebo | Estimated treatment effect (95% CI) | p value | ||
|---|---|---|---|---|---|
| Viral clearance (day 7) | 40/207 (19%) | 58/221 (26%) | 0·67 (0·42–1·06)* | 0·090 | |
| Hospitalised for COVID | 75/741 (10%) | 97/756 (13%) | 0·77 (0·55–1·05)* | 0·10 | |
| All-cause hospitalisation | 76/741 (10%) | 99/756 (13%) | 0·76 (0·58–1·04)* | 0·088 | |
| Time to hospitalisation, days | 5 (3–7) | 5 (3–7·5) | 0·79 (0·58–1·06)† | 0·11 | |
| Period of hospitalisation, days | 8 (5–13) | 6 (3–10·75) | 1·23 (0·99–1·53)‡ | 0·059 | |
| Emergency setting visit for at least 6 h | 7/741 (1%) | 36/756 (5%) | 0·19 (0·08–0·41)* | 0·0001 | |
| Time to the emergency visit for at least 6 h, days | 4 (3–7) | 5 (3–8·25) | 0·20 (0·09–0·44)† | 0·002 | |
| Death, intention to treat | 17/741 (2%) | 25/756 (3%) | 0·69 (0·36–1·27)* | 0·24 | |
| Time to death, days | 17 (9–21) | 14 (8–20) | 0·80 (0·43–1·51)† | 0·49 | |
| Mechanical ventilation | 26 | 34 | 0·77 (0·45–1·30) | 0·33 | |
| Time on mechanical ventilator, days | 5·5 (3–12·75) | 6·5 (2·25–12) | 1·03 (0·64–1·67)‡ | 0·90 | |
| Adherence | 548/741 (74%) | 618/738 (82%) | 0·62 (0·48–0·77)* | 0·0003 | |
| Death, per protocol | 1/548 (<1%) | 12/618 (2%) | 0·09 (0·01–0·47) | 0·022 | |
| Treatment emergent adverse event | |||||
| Grade 1 | 20/741 (3%) | 11/756 (1%) | 1·88 (0·91–4·09)* | 0·096 | |
| Grade 2 | 72/741 (10%) | 81/756 (11%) | 0·91 (0·64–1·25)* | 0·52 | |
| Grade 3 | 38/741 (5%) | 50/756 (7%) | 0·76 (0·49–1·18)* | 0·22 | |
| Grade 4 | 21/741 (3%) | 20/756 (3%) | 1·07 (0·58–2·01)* | 0·82 | |
| Grade 5 | 18/741 (2%) | 26/756 (3%) | 0·70 (0·37–1·28)* | 0·25 | |
Data are n/N (%) or median (IQR) unless otherwise stated.
Unadjusted odds ratio.
Unadjusted hazard ratio.
Exponentiated unadjusted estimates from a log-transformed linear regression.
84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability. Per-protocol findings among patients who reported optimal adherence (greater than 80% for possible days) indicated a significant treatment effect (RR 0·34; 95% BCI 0·21–0·54 for the primary outcome and for mortality (odds ratio 0·09; 95% CI 0·01–0·47). With respect to adverse events, there were no significant differences in number of treatment emergent adverse events among patients in the fluvoxamine and placebo groups.
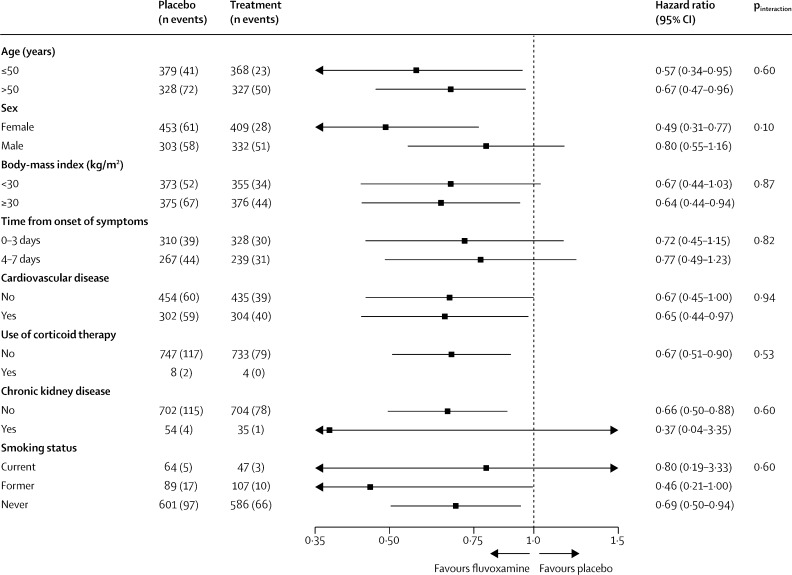
In the prespecified subgroup analysis, we found no evidence of moderation of treatment effect for fluvoxamine compared with placebo, for subgroups of age, sex, days since symptom onset, smoking status, or comorbidities (figure 3 , appendix 2 p 9).
Figure 3.
Subgroup analyses of fluvoxamine versus placebo in the TOGETHER Trial
Discussion
This is, to the best of our knowledge, the first large, randomised controlled trial to test the efficacy of fluvoxamine for acute treatment of COVID-19. We found a clinically important absolute risk reduction of 5·0%, and 32% RR reduction, on the primary outcome of hospitalisation defined as either retention in a COVID-19 emergency setting or transfer to tertiary hospital due to COVID-19, consequent on the administration of fluvoxamine for 10 days. This study is only the second study to show an important treatment benefit for a repurposed drug in the early treatment population.13 Our findings represent the complete analysis of the trial after the DSMC recommended stopping the active fluvoxamine group and all 28-day follow-up of randomly assigned patients. Given fluvoxamine's safety, tolerability, ease of use, low cost, and widespread availability, these findings might influence national and international guidelines on the clinical management of COVID-19.
Our results are consistent with an earlier smaller trial done in the USA (led by EJL and AMR).6 That study used a higher dose of fluvoxamine (100 mg three times a day for 15 days) and included a lower risk group for the primary outcome but found no clinical deterioration among 80 patients receiving fluvoxamine versus six cases among 72 patients receiving placebo. A large observational study from France involved a different population, 7230 hospitalised COVID-19 patients, and reported a reduction in use of intubation or death with use of SSRIs.5
The underlying mechanism of fluvoxamine for COVID-19 disease remains uncertain. Although hypotheses include several potential mechanisms,4 the main reason for the initial study of fluvoxamine as a treatment of COVID-19 was its anti-inflammatory action through activation of the S1R.14 S1R is an endoplasmic reticulum (ER) chaperone membrane protein involved in many cellular functions,15 including regulation of ER stress response–unfolded protein response and regulation of cytokine production in response to inflammatory triggers.16 In the presence of fluvoxamine, S1R might prevent the ER stress sensor inositol-requiring enzyme 1α from splicing and activating the mRNA of X-box protein 1, a key regulator of cytokine production including interleukins IL-6, IL-8, IL-1β, and IL-12. In a 2019 study by Rosen and colleagues, fluvoxamine showed benefit in preclinical models of inflammation and sepsis through this mechanism.16
A second mechanism might be fluvoxamine's antiplatelet activity.17 SSRIs can prevent loading of serotonin into platelets and inhibit platelet activation, which might reduce the risk of thrombosis, and these antiplatelet effects can be cardioprotective. Finally, another potential mechanism of action might be related to the effect of fluvoxamine in increasing plasma levels of melatonin.16 In vitro and animal studies are needed to help clarify the most probable mechanism(s). Biomarker studies included as part of future randomised controlled trials might also help to clarify mechanisms.
Since the start of the COVID-19 pandemic, there have been more than 2800 randomised controlled trials registered on ClinicalTrials.gov. However, fewer than 300 have been reported and most clinical trials have been small and underpowered, with sample sizes less than 100. In many cases, these trials have been unsuccessful at recruiting as the local epidemics occur in waves and sustainable infrastructure to maintain staff or local interest for recruitment is lacking. The trials that provide the clearest medical understanding tend to be the larger platform trials, such as SOLIDARITY,17 RECOVERY,18 PRINCIPLE,11 and REMAP-CAP.19 As a result, we actively collaborate with other investigators running trials with overlapping interventions so that they can be aware of our study decisions and establish whether they should influence their respective trials.
Strengths of our trial include the rapid recruitment and enrolment of high-risk patients for the development of severe COVID-19. Our recruitment strategy involves engagement with the local public health system, thus allowing recruitment that frequently exceeds 20 patients per day. We enrolled only participants with diagnosed COVID-19 and less than 7 days of symptom onset using a commercially available COVID-19 rapid antigen test (Panbio, Abbott Rapid Diagnostics Jena, Jena, Germany). The concordance of COVID-19 positive tests with RT-PCR was evaluated on the group of participants with PCR evaluations and a concordance rate of greater than 99% on both tests collected at baseline was found. In this trial we did not enrol participants without positive COVID-19 tests, nor those who were asymptomatic SARS-CoV-2 positive. Our primary outcome is hospitalisation defined as either retention in a COVID-19 emergency setting for more than 6 h or transfer to tertiary hospital due to COVID-19. The event adjudication committee did count patient wait times as contributing to a primary endpoint. Specialised emergency settings were developed to respond to the Brazilian epidemic and we considered prolonged observation and treatment in these settings as equivalent in importance to hospitalisation as many patients who typically would be hospitalised were prevented from doing so owing to hospital over-capacity. In our trial, 87% of all primary outcome events eventually resulted in transfer to a tertiary hospital. Patients observed in both the emergency setting and hospital were counted only once. Our sub-group analyses examined pre-determined population groups and tests for interaction did not detect differing effects for any sub-group. Female sex was identified as a signficant sub-group favouring fluvoxamine while male sex was not, however we did not detect differing effects between the groups.
Our understanding of the epidemiology of COVID-19 as well as its disease progression and outcomes have evolved since beginning this platform trial in June, 2020. Early studies assessed the effects of interventions on viral load and clearance, whereas later studies also evaluate more clinical outcomes. We made adjustments to the trial according to prespecified rules and in communication with the appropriate ethics review committees that allowed us to respond to the epidemic waves while maintaining high rates of recruitment. Unlike many outpatient clinical trials, our study involves direct patient contact through the use of medical students, nurses, and physicians who do at-home visits as well as follow-up via telecommunications. Given the rapid recruitment of patients in combination with the high event rate of COVID-19 emergency setting visits and hospitalisations, we were able to evaluate the effects of interventions when portions of the planned population had been recruited. The period between first recruitment of a patient on fluvoxamine and the final data cut for our trial was 219 days.
Major limitations of our trial are related to the challenges of doing a trial in a disease that is not well characterised. There is no standard of care that exists for early treatment of COVID-19 and various advocacy groups promote different interventions, including some of those evaluated in this and our previous trials.20 Furthermore, there is little understanding of who is at greatest risk of disease progression from this disease as some patients with numerous risk factors do recover quickly whereas some others with less established risk factors might not. Our population had a higher rate of hospitalisation events than observed in most clinical trials,20 thus permitting inferences on treatment effects in this higher-risk population. Although intention-to-treat analysis provides more real-world evidence than per-protocol analysis, we found that patients who reported optimal adherence (greater than 80% for possible days—our per-protocol analysis) had a greater treatment benefit, suggesting that intensifying adherence to treatment might have considerable clinical benefits. However, adherence might be related to tolerability. 84 participants stopped fluvoxamine and 64 participants stopped in the placebo group for this reason. Finally, when the trial began, vaccines were not available in Brazil but became more widely available as the trial progressed. Although we modified inclusion criteria and permitted vaccinated patients during the trial, we believe this had minimal effect on the primary outcome as only 86 (6%) of 1497 reported at least one dose of a COVID-19 vaccine at the end of the trial.
Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population. A 10-day course of fluvoxamine costs approximately US$4 even in well-resourced settings.21 Our study compares favourably with the treatment effects of more expensive treatments including monoclonal antibodies for outpatient treatment.20, 22, 23 The absolute number of serious adverse events associated with fluvoxamine was lower than for placebo and this might reflect the modulatory effect of fluvoxamine on systemic inflammation in these participants. Lower respiratory tract infections were reported less frequently in patients in the fluvoxamine group than those in the placebo group. This is concordant with the reduction of hospital admissions in patients with confirmed COVID-19 treated with fluvoxamine, and the numerically lower number of patients requiring mechanical ventilation.
Fluvoxamine is widely available but is not on the WHO Essential Medicines List,24 whereas a closely related SSRI, fluoxetine, is on the list. It is now crucial to establish whether a class effect exists and whehese drugs can be used interchangeably for COVID-19. The important findings that inhaled budesonide decreased time to recovery11 among a similar population to our trial and had a trend towards decreased hospitalisations suggests that this as an alternative or additional intervention for outpatient care that should be evaluated. The PRINCIPLE trial evaluated time to recovery by means of self-reported recovery up to 28 days after randomisation to budesonide.11 Our trial differed as we evaluated improvement in the WHO categorisation of disease disability up to days 14 and then 28 (appendix 2 p 7). Finally, our study was among primarily unvaccinated patients. Further evidence of treatment benefits are needed to establish the effect of fluvoxamine among vaccinated populations.
Use of interventions, including fluvoxamine, to prevent progression of illness and hospitalisation is critically dependent on identifying higher-risk individuals. Unselected populations will have a lower risk. What absolute reduction in risk of clinical deterioration would motivate patients to choose treatment (probably the approximately 5% that we observed, but perhaps not much lower) remains uncertain. These considerations raise the importance of the development of a validated prediction rule for deterioration in patients in the early stages of COVID-19 infection.
This online publication has been corrected. The corrected version first appeared at thelancet.com/lancetgh on February 24, 2022
Data sharing
Data from the TOGETHER trial will be made available following publication of this manuscript to interested investigators through the International COVID-19 Data Alliance after accreditation and approval by the TOGETHER trial principal investigators (EJM and GR). Other study related documents can be found in Vivli.
This online publication has been corrected. The corrected version first appeared at thelancet.com/lancetgh on February 24, 2022 and further corrections were made on August 9, 2022.
Acknowledgments
Acknowledgments
The trial was supported by FastGrants and the Rainwater Foundation. Our research network consists of partnerships between academics and clinicians at McMaster University in Ontario, Canada, and Pontificia Universidade Catolica de Minas Gerais, Claros State University, and University of Ouro Preto in Minas Gerais, Brazil. Other partners include Cytel, Platform Life Sciences, MMS Holdings, WHO Therapeutic Guidelines Committee, and the Society for Clinical Trials. Data safety and monitoring committee members were William Cameron, University of Ottawa (Canada); James Orbinski, York University (Canada); Sonal Singh, University of Massachusetts (USA); Kristian Thorlund, McMaster University (Canada); and Jonas Haggstrom, Cytel (Sweden).
Contributors
EJM and GR decided to publish the paper. EJM, GR, EAdSM-S, DCMS, LT, TSF, CVQdS, VHdSC, AMRN, APFGA, EDC, ADdFN, LCMS, MICS, LBR, RO, OH, JIF, HR, SS, PM, AVG, CRR, EJL, AMR, and GHG contributed to trial design. EJM, GR, JIF, EASdMS, DCMS, LT, TSF, CVQdS, VHdSC, AMRN, APFGA, EDC, ADdFN, LCMS, MICS, LBR, RO, OH, JIF, HR, SS, PM, AVG, CRR, EJL, and AMR helped plan the trial and ongoing recruitment. GR, EJM, EAdSMS, ACM, and DCMS were responsible for acquisition of data. EJM, GR, JIF, OH, HR, PM, SS, RO, CRR, LT, ACM, and GHG drafted the manuscript. OH, HR, and EJM contributed to the statistical analysis. GR and EJM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript. The members of the TOGETHER investigators group and their roles in the conduct of the trial are listed in appendix 2 (pp 2–4). All authors had full access to all the data in the study and take final responsibility for the decision to submit for publication. EJM and GR accessed and verified the underlying data.
Declaration of interests
EJM, AVG, SS, PM, and JIF have been employed by Platform Life Sciences. EJM, JIF, OH, and HR have been employed by Cytel. CRR has been employed by Certara. GR has been employed by Cardresearch. AMR and EJL are co-inventors on a patent application filed by Washington University for methods of treating COVID-19. All other authors declare no competing interests.
Supplementary Materials
References
- 1.Torres I, Artaza O, Profeta B, Alonso C, Kang J. COVID-19 vaccination: returning to WHO's Health For All. Lancet Glob Health. 2020;8:e1355–e1356. doi: 10.1016/S2214-109X(20)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayner CR, Dron L, Park JJH, et al. Accelerating clinical evaluation of repurposed combination therapies for COVID-19. Am J Trop Med Hyg. 2020;103:1364–1366. doi: 10.4269/ajtmh.20-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omi T, Tanimukai H, Kanayama D, et al. Fluvoxamine alleviates ER stress via induction of sigma-1 receptor. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.652688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoertel N, Sánchez-Rico M, Vernet R, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021 doi: 10.1038/s41380-021-01021-4. published online Feb 4. [DOI] [PubMed] [Google Scholar]
- 6.Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JJH, Siden E, Zoratti MJ, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials. 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 9.Reis G, Silva EAdSM, Silva DCM, et al. A multi-center, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and high-risk for complications: the TOGETHER master trial protocol. Gates Open Res. 2021;5:117. [Google Scholar]
- 10.Anderson GM. Fluvoxamine, melatonin and COVID-19. Psychopharmacology (Berl) 2021;238:611. doi: 10.1007/s00213-020-05753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19 - interim WHO Solidarity Trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis G, Moreira Silva EADS, Medeiros Silva DC, et al. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. doi: 10.1016/S0140-6736(21)01744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashaei Y. Drug repurposing of selective serotonin reuptake inhibitors: could these drugs help fight COVID-19 and save lives? J Clin Neurosci. 2021;88:163–172. doi: 10.1016/j.jocn.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol. 2014;727:167–173. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 16.Rosen DA, Seki SM, Fernández-Castañeda A, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlienger RG, Meier CR. Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction? Am J Cardiovasc Drugs. 2003;3:149–162. doi: 10.2165/00129784-200303030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021 https://doi.org/2021.02.11.21249258 published online Feb 11 (preprint). [Google Scholar]
- 19.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Levi J, Ellis L, Hill A. Minimum manufacturing costs, national prices and estimated global availability of new repurposed therapies for COVID-19. medRxiv. 2021 doi: 10.1101/2021.06.01.21258147. published online June 3. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . World Health Organization; Geneva: 2019. World Health Organization model list of essential medicines: 21st list 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the TOGETHER trial will be made available following publication of this manuscript to interested investigators through the International COVID-19 Data Alliance after accreditation and approval by the TOGETHER trial principal investigators (EJM and GR). Other study related documents can be found in Vivli.
This online publication has been corrected. The corrected version first appeared at thelancet.com/lancetgh on February 24, 2022 and further corrections were made on August 9, 2022.