Highlights
-
•
In patients with T2DM and hypercholesterolemia untreated with lipid-lowering drugs:
-
•
BA + EZE FDC lowered LDL-C levels by 38.8% at week 12
-
•
BA + EZE FDC significantly reduced LDL-C and non-HDL-C vs ezetimibe or placebo
-
•
BA + EZE FDC significantly reduced hsCRP vs ezetimibe or placebo
-
•
BA + EZE FDC was safe and generally well tolerated
Keywords: Bempedoic acid, Diabetes mellitus type 2, Ezetimibe, Hypercholesterolemia
Abstract
Objective
Statins are sometimes associated with worsened glycemic control. Patients with type 2 diabetes mellitus (T2DM) may require non-statin therapies to achieve low-density lipoprotein cholesterol (LDL-C) lowering goals. This study evaluated the efficacy and safety of bempedoic acid 180 mg plus ezetimibe 10 mg fixed-dose combination (BA + EZE FDC) in patients with T2DM and hypercholesterolemia who were not receiving background statins or other lipid-lowering therapy.
Methods
Patients with T2DM and elevated LDL-C levels were enrolled into this phase 2, double-blind study (NCT03531905). Patients received placebo during a 5-week washout period where background lipid-lowering therapies (including statins) were discontinued. Eligible patients were then randomized 1:1:1 to receive either BA + EZE FDC, ezetimibe 10 mg, or placebo once daily for 12 weeks. Assessments included the percent change from baseline to week 12 in LDL-C, other lipid parameters, and high-sensitivity C-reactive protein (hsCRP); and the monitoring of safety and tolerability.
Results
Among 179 randomized patients, baseline characteristics following the washout period were similar across treatment groups, with mean LDL-C levels of 142.6 mg/dL and mean glycated hemoglobin of 8.0%. At week 12, BA + EZE FDC therapy lowered mean LDL-C levels by 38.8%, significantly more than ezetimibe alone (19.2%; difference, 19.5% [95% confidence interval (CI), 13.4%–25.7%]; p < 0.001) or placebo (increase of 0.9%; difference, 39.6% [95% CI, 33.4%–45.8%]; p < 0.001). BA + EZE FDC significantly reduced hsCRP levels from baseline vs ezetimibe (29.2%; p = 0.005) and vs placebo (36.7%; p < 0.001). Incidence of treatment-emergent adverse events was low in all treatment groups, with no indication of worsened glycemic control.
Conclusion
In patients with T2DM and hypercholesterolemia who were not receiving statins or other lipid-lowering drugs, BA + EZE FDC significantly lowered LDL-C levels and was generally well tolerated.
1. Introduction
Patients with type 2 diabetes mellitus (T2DM) and hypercholesterolemia are at high risk for atherosclerotic cardiovascular disease (ASCVD) [1,2]. Guidelines recommend statins as first-line therapy to treat elevated low-density lipoprotein cholesterol (LDL-C) levels in these patients, adding non-statins as needed to achieve risk-based LDL-C lowering goals [2,3]. Statin therapy, however, is associated with increased rates of new-onset diabetes and worsening of glycemic control in patients with established T2DM [4], [5], [6], [7]. Further, many patients with hypercholesterolemia (including patients with T2DM) are unable or unwilling to take statins, or they may respond inadequately to them, and thus may not achieve their risk-based LDL-C lowering goals with statins alone [8]. Bempedoic acid (NEXLETOLⓇ, Esperion Therapeutics, Inc.), a first-in-class oral ATP-citrate lyase inhibitor, and a bempedoic acid plus ezetimibe fixed-dose combination (BA + EZE FDC; NEXLIZETⓇ) are approved in the United States as adjuncts to diet and maximally tolerated statin therapy for patients with ASCVD and/or heterozygous familial hypercholesterolemia who require additional LDL-C lowering [9], [10], [11], [12], [13], [14]. In Europe, bempedoic acid and BA + EZE FDC are approved as NILEMDOⓇ and NUSTENDIⓇ with different prescribing information and indications. Limited data are available regarding efficacy and tolerability of BA + EZE FDC in patients with T2DM. This short report describes findings from a phase 2 study evaluating the effects of BA + EZE FDC compared with ezetimibe monotherapy or placebo on LDL-C and other atherogenic lipoproteins and lipids, high-sensitivity C-reactive protein (hsCRP), and safety in patients with T2DM who were not receiving background statins or other lipid-modifying therapies.
2. Methods
2.1. Patients
Eligible patients included men and women aged 18–75 years with reported ≥6 months history of T2DM and elevated LDL-C levels (>70 mg/dL at screening and 100–220 mg/dL after lipid-lowering therapy [LLT] washout) who were receiving stable diabetes treatment for ≥3 months and had a glycated hemoglobin (HbA1c) level of 7.0% to ≤10.0% at screening. Patients were ineligible if the investigator chose not to discontinue all LDL-C–lowering drugs and lipid-altering nutritional supplements for 17 weeks, or if patients had a body mass index >40 kg/m2, had documented cardiovascular disease, had a fasting triglyceride (TG) level >400 mg/dL after LLT washout, had a history of type 1 diabetes, or had uncontrolled hypothyroidism, significant hepatic or renal dysfunction, gastrointestinal or hematologic disorders, active malignancy, unexplained elevated levels of serum creatine kinase (>3 × ULN), or recent history of drug or alcohol abuse. Patients were encouraged to follow a healthful diet and exercise regularly throughout the study.
2.2. Study design and treatment
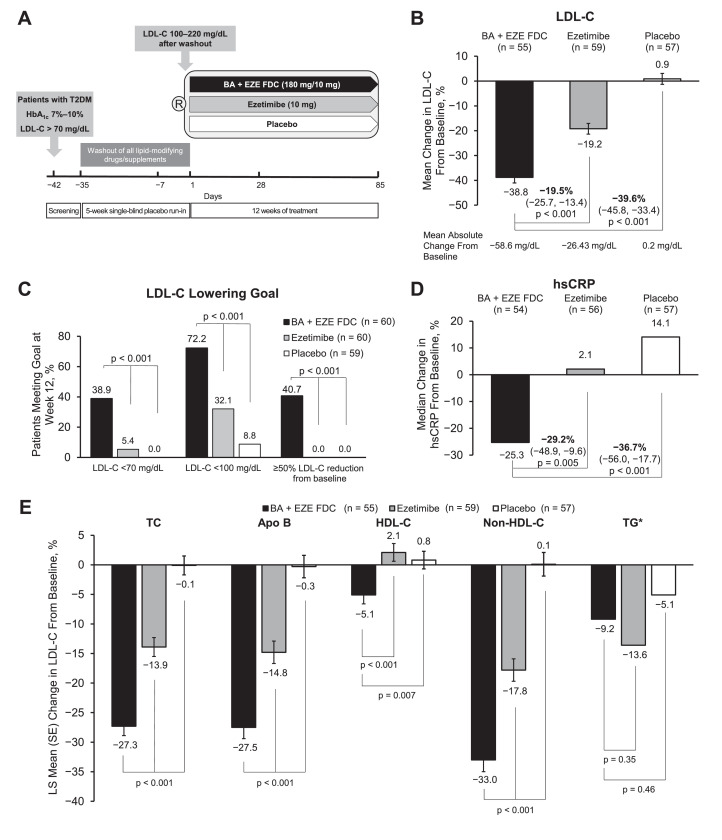
This was a double-blind, parallel-group, placebo-controlled, multicenter, phase 2 trial (NCT03531905) conducted between May 9, 2018, and June 18, 2019, at 28 US clinical research sites. Patients were randomized 1:1:1 to receive once daily BA + EZE FDC (180 mg BA + 10 mg EZE), ezetimibe alone (10 mg), or placebo (Fig. 1A). The study consisted of a 5-week LLT washout period during which patients received single-blind, once-daily placebo treatment, followed by 12 weeks of study drug. The study was approved by individual institutional review boards, all patients provided written informed consent prior to the study onset, and the study was conducted under the guidance of the Good Clinical Practice Guideline principles as defined by the Declaration of Helsinki.
Fig. 1.
Study design and efficacy of BA + EZE FDC in patients with T2DM. (A) Schematic of the study design. (B) Percent change from baseline in LDL-C levels at week 12. (C) Proportion of patients meeting LDL-C lowering goals at week 12. (D) Percent change from baseline in hsCRP levels at week 12. (E) Percent change from baseline in TC, Apo B, HDL-C, non-HDL-C, and TG levels at week 12. *Data for TG are presented as median; interquartile range is 47.4 mg/dL for BA + EZE FDC (n = 54), 29.9 mg/dL for ezetimibe (n = 56), and 32.0 mg/dL for placebo (n = 57). Bars represent the standard error (SE) of the least squares mean. Abbreviations: Apo B, apolipoprotein B; BA + EZE FDC, bempedoic acid plus ezetimibe fixed-dose combination; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; R, randomization; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
2.3. Assessments
The primary endpoint was percent change from baseline to week 12 in LDL-C levels for BA + EZE FDC vs ezetimibe alone or placebo. The secondary endpoints were percent change from baseline to week 12 in hsCRP, non–high-density lipoprotein cholesterol (non-HDL-C), total cholesterol (TC), apolipoprotein B (Apo B), TG, and high-density lipoprotein cholesterol (HDL-C) levels, and the proportion of patients achieving LDL-C <70 mg/dL and/or a ≥ 50% reduction from baseline. The proportion of patients achieving LDL-C levels <100 mg/dL was determined post hoc.
Safety and tolerability were assessed by treatment-emergent adverse events (TEAEs), vital signs, physical examinations, and laboratory tests. Adverse events of special interest (AESIs) were prespecified based on potential or theoretical risks of bempedoic acid or other lipid-lowering therapies and included categories of new-onset or worsening diabetes mellitus (including blood glucose increased), hepatic enzyme elevations, hypoglycemia, muscular disorders, renal disorders, and neurocognitive events.
2.4. Statistical analysis
Fifty-six patients per treatment group were estimated to provide ≥95% power to detect an estimated 15% treatment effect (±20% standard deviation [SD]) in the primary endpoint. A total of 659 patients were screened and 242 patients were randomized to receive BA + EZE FDC (n = 81), ezetimibe alone (n = 81), or placebo (n = 80). Before unblinding of the data, concerns arose regarding data integrity at three sites (as in another study of BA + EZE FDC [15]) and data from these three sites were excluded from the analyses. A total of 179 patients who were randomized and received at least one dose of study medication were included in the final efficacy and safety analyses (BA + EZE FDC [n = 60], ezetimibe 10 mg alone [n = 60], or placebo [n = 59]). The percent change from baseline to week 12 in LDL-C, Apo B, TC, HDL-C, and non-HDL-C were analyzed using the analysis of covariance model, which included treatment as a factor and baseline value as a covariate. The percent change in hsCRP and TG levels from baseline to week 12 was analyzed using a non-parametric (Wilcoxon rank-sum test) analysis with Hodges-Lehmann estimates because of non-normal distribution.
3. Results
3.1. Patients
The efficacy and safety analysis populations included 179 patients who were randomized to BA + EZE FDC (n = 60), ezetimibe 10 mg alone (n = 60), or placebo (n = 59). Patient characteristics were generally similar across treatment groups, with an average duration of T2DM ≥ 11 years (range, 0.7–39.7 years) and mean body mass index of 31.3 kg/m2 (Table S1). The study population was diverse and generally representative of the US population: 48% of the patients were female, 25% were black, and 21% were Hispanic or Latino. After LLT washout, mean LDL-C levels were 145.1 ± 31.5 mg/dL in the BA + EZE FDC group, 139.2 ± 28.1 mg/dL in the ezetimibe group, and 143.4 ± 26.4 mg/dL in the placebo group. Other baseline parameters were generally comparable, although median baseline hsCRP levels were higher in the placebo group (3.5 mg/L) vs BA + EZE FDC or ezetimibe (2.6 and 2.4 mg/L, respectively).
3.2. Efficacy
At week 12, BA + EZE FDC therapy lowered mean LDL-C by 38.8%, which was significantly greater than with ezetimibe (19.2%; difference, 19.5% [95% confidence interval (CI), 13.4%–25.7%]; p < 0.001) or placebo (increased by 0.9%; difference, 39.6% [95% CI, 33.4%–45.8%]; p < 0.001; Fig. 1B). The mean absolute reductions in LDL-C levels were 58.6 mg/dL with BA + EZE FDC and 26.4 mg/dL with ezetimibe; LDL-C levels were increased by 0.2 mg/dL with placebo. Attainment of LDL-C lowering goals was greater with BA + EZE FDC than with ezetimibe or placebo. Significantly more patients achieved LDL-C levels <70 mg/dL when treated with BA + EZE FDC (38.9%) than did patients taking ezetimibe (5.4%) or placebo (0%; p < 0.001 for all; Fig. 1C). Similarly, the proportion of patients who achieved LDL-C levels <100 mg/dL was 72.2% in the BA + EZE FDC group, 32.1% in the ezetimibe group, and 8.8% in the placebo group (p < 0.001 for BA + EZE FDC vs ezetimibe and vs placebo). Of patients who were treated with BA + EZE FDC, 40.7% achieved a reduction in LDL-C of ≥50% from baseline, while no patients who received ezetimibe or placebo achieved ≥50% reduction in LDL-C levels from baseline. At week 12, BA + EZE FDC therapy reduced median hsCRP by 25.3%, which was significantly greater than with ezetimibe alone (increased by 2.1%; difference, 29.2% [95% CI, 9.6–48.9]; p = 0.005) or with placebo (increased by 14.1%; difference, 36.7% [95% CI, 17.7–56.0]; p < 0.001) as shown in Fig. 1D. Similar to reductions in LDL-C levels, BA + EZE FDC significantly reduced TC, Apo B, and non-HDL-C levels compared with ezetimibe alone or placebo (p < 0.001 for all; Fig. 1E).
3.3. Safety and tolerability
The patient incidence of TEAEs was generally comparable across the treatment groups (Table 1). The most common TEAEs (occurring in ≥3 patients in any treatment group) were blood glucose increased, urinary tract infection, and bronchitis. No patients reported elevated uric acid levels, gout, or tendon rupture. Patients treated with BA + EZE FDC did not experience any serious TEAEs, discontinuations due to TEAEs, or increased frequency of muscle related or hepatic TEAEs. Two serious TEAEs were reported during the study, one in the ezetimibe group (duodenal ulcer) and one in the placebo group (angioedema), both of which were not considered to be related to the study drug. Only one patient (in the ezetimibe group) had a TEAE leading to discontinuation of study drug due to gastrointestinal pain. No deaths were reported in any treatment group.
Table 1.
Treatment-emergent adverse events.
| Safety Parameter | Patients, n (%) |
||
|---|---|---|---|
| BA + EZE FDC (n = 60) | Ezetimibe (n = 60) | Placebo (n = 59) | |
| Any TEAEa | 26 (43.3) | 18 (30.0) | 22 (37.3) |
| Treatment-related TEAE | 1 (1.7) | 3 (5.0) | 3 (5.1) |
| Serious TEAE | 0 | 1 (1.7) | 1 (1.7) |
| TEAE leading to discontinuation of study drug | 0 | 1 (1.7) | 0 |
| TEAEs with a fatal outcome | 0 | 0 | 0 |
| AESIsb | 9 (15.0) | 5 (8.3) | 5 (8.5) |
| Hypoglycemia | 0 | 1 (1.7) | 2 (3.4) |
| Blood glucose increased | 3 (5.0) | 0 | 0 |
| Glycosylated hemoglobin increased | 0 | 1 (1.7) | 2 (3.4) |
| Urine ketone body present | 2 (3.3) | 0 | 0 |
| Glucose urine present | 1 (1.7) | 0 | 0 |
| Diabetes mellitusc | 2 (3.3) | 0 | 0 |
| Type 2 diabetes mellitusc | 1 (1.7) | 1 (1.7) | 0 |
| Diabetes mellitus inadequate control | 1 (1.7) | 0 | 0 |
| Hyperglycemia | 0 | 1 (1.7) | 0 |
| Muscle spasms | 1 (1.7) | 1 (1.7) | 0 |
| Myalgia | 0 | 0 | 1 (1.7) |
| Hepatic enzyme increased | 1 (1.7) | 0 | 0 |
Abbreviations: AESI, adverse event of special interest; BA + EZE FDC, bempedoic acid plus ezetimibe fixed-dose combination; TEAE, treatment-emergent adverse event.
TEAEs were defined as adverse events that began or worsened in severity after the first dose of double-blind study drug until 30 days after the last dose. The subject incidence of TEAEs were summarized by treatment group. TEAEs were coded according to Medical Dictionary of Regulatory Activities (MedDRA), version 20.1.
AESIs were prespecified based on potential or theoretical risks of bempedoic acid or other lipid-lowering therapies and included preferred terms related to metabolic acidosis; hepatic, muscular, renal, cardiovascular, and neurocognitive/neurologic events; and new-onset diabetes mellitus or hyperglycemia. AESIs were coded according to Medical Dictionary of Regulatory Activities (MedDRA), version 21.0.
Indicates worsening or uncontrolled diabetes.
Rates of treatment-emergent AESIs were also low in all treatment groups, although higher in the BA + EZE FDC group (15.0% [n = 9]) compared with the ezetimibe (8.3% [n = 5]) or placebo (8.5% [n = 5]) groups. The only AESI preferred term occurring in ≥3 patients in any treatment group was blood glucose increased in three patients taking BA + EZE FDC. None of these reported increases were severe, and only one case was considered by the investigator to possibly be treatment related. There were no treatment-emergent reports of blood glucose increased in the ezetimibe and placebo groups.
4. Discussion
In a diverse population of patients with T2DM not receiving background LLTs, treatment with BA + EZE FDC significantly lowered LDL-C levels compared with ezetimibe alone or placebo, and significantly more patients achieved LDL-C lowering goals while taking BA + EZE FDC than did those taking ezetimibe or placebo. BA + EZE FDC also lowered total cholesterol, non-HDL-C, Apo B, and hsCRP levels significantly more than ezetimibe or placebo. All three study treatments were generally well tolerated.
Although statin use is well known to increase glycemia and new-onset diabetes [4], [5], [6], [7], these events were not associated with bempedoic acid in phase 3 studies [[9], [10], [11], [12],16,17]. In a pooled analysis of four phase 3 studies, the incidence of new-onset diabetes was lower in patients who received bempedoic acid compared with patients who received placebo (4.7 vs 6.4 events per 100 person-years, respectively) [16]. Both statins and bempedoic acid lower LDL-C levels by blocking intrahepatic cholesterol synthesis and increasing LDL-receptor expression, but bempedoic acid inhibits ATP-citrate lyase, an enzyme distinct from 3-hydroxy‑3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibited by statins. Genetic studies suggest lower activity of HMG-CoA reductase may be associated with increased risk of diabetes [18,19]; in contrast, data from a Mendelian randomization study suggest no such connection with genetically lower ATP-citrate lyase activity [19]. Thus, the apparent lack of adverse glycemic effects with bempedoic acid vs their presence with statins may be mechanistically based [19]. Further investigation of the glycemic effects of bempedoic acid are needed to confirm these findings [19], and should be forthcoming from the ongoing CLEAR Outcomes study (NCT02993406) [20]. This cardiovascular outcomes trial will provide roughly 3.5 years of follow-up in over 14,000 patients, approximately 43% of whom had diabetes at baseline [20].
Limitations of this study include a relatively small patient population treated for a relatively short duration in a clinical trial setting. Additionally, given that all background LLTs were washed out prior to this trial, these results may differ from a real-world setting where most patients with T2DM would likely receive BA + EZE FDC added to background statin therapy. However, these findings provide useful insights into the efficacy, safety, and tolerability of BA + EZE FDC for clinical use in patients with T2DM.
5. Summary and conclusions
In the absence of background statins or other LLTs, patients with T2DM experienced substantially greater lowering of LDL-C and related atherogenic lipids with BA + EZE FDC compared with ezetimibe alone or placebo. Similarly, the proportion of patients who achieved LDL-C lowering goals was significantly higher in the BA + EZE FDC group vs the ezetimibe or placebo groups. Additionally, BA + EZE FDC significantly reduced levels of the inflammatory biomarker hsCRP. Differences in adverse events were generally not clinically meaningful.
Data sharing statement
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedures.
Author contributions
Harold E. Bays: Investigation, Writing - review & editing. Seth J. Baum: Investigation, Writing - review & editing. Eliot A. Brinton: Investigation, Writing - review & editing. Jeff Plutzky: Conceptualization, Investigation, Writing - review & editing. Jeff C. Hanselman: Conceptualization, Data curation, Investigation, Supervision, Writing - review & editing. Rujun Teng: Data curation, Formal analysis, Investigation, Writing - review & editing. Christie M. Ballantyne: Investigation, Writing - review & editing.
Declaration of Competing Interest
Dr. Bays’ research site has received research grants from 89Bio, Acasti, Akcea, Allergan, Alon Medtech/Epitomee, Amarin, Amgen, AstraZeneca, Axsome, BioHaven, Bionime, Boehringer Ingelheim, Civi, Eli Lilly, Esperion, Evidera, Gan and Lee, Home Access, Janssen, Johnson and Johnson, Lexicon, Matinas, Merck, Metavant, Novartis, Novo Nordisk, Pfizer, Regeneron, Sanofi, Selecta, TIMI, and Urovant. He has served as a consultant/advisor for 89Bio, Amarin, Esperion, Matinas, and Gelesis, and as a speaker for Esperion and Novo Nordisk.
Dr. Brinton has received fees as a speaker from Akcea, Amarin, Amgen, Esperion, Kowa, Medicure, Regeneron, and Sanofi-Aventis, and consulting fees from 89bio, Akcea, Amarin, Amgen, Daiichi-Sankyo, Esperion, Kowa, Medicure, PTS Diagnostics, Regeneron, and Sanofi-Aventis.
Dr. Plutzky has received grants from Boehringer Ingelheim and NIH/NIDDK as well as consultancy fees from Amarin, Amgen, Esperion, Janssen, Merck, Novo Nordisk, Sanofi, and Vivus.
Dr. Baum is a consultant/advisory board member/principal investigator for Merck, Akcea, Amgen, Inc., Regeneron, Sanofi, AstraZeneca, Eli Lilly, Esperion, Boehringer Ingelheim, Gemphire, Madrigal, Novartis, Gerson Lehrman Group, Novo Nordisk, and Guidepoint Global.
Mr. Hanselman and Ms. Teng are current (Hanselman) or former (Teng) employees of Esperion Therapeutics, Inc., and may hold stock or stock options.
Dr. Ballantyne has received research grant(s)/support from Abbott Diagnostics, Akcea, Amarin, Amgen, Esperion, Ionis, Novartis, Regeneron, Roche Diagnostic, Sanofi-Synthelabo, NIH, AHA, and ADA (all paid to the institution, not to the individual). He has also served as a consultant for Abbott Diagnostics, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esperion, Intercept, Ionis, Matinas BioPharma, Merck, Novartis, Novo Nordisk, Regeneron, Roche Diagnostic, and Sanofi-Synthelabo.
Acknowledgments
Funding
This study was supported by Esperion Therapeutics, Inc., Ann Arbor, MI.
Acknowledgments
All authors had access to the data and participated in the development, review, and approval of the manuscript. Zhan Ye, a former employee of Esperion Therapeutics, contributed to the study design, statistical analyses, and development of the first draft of the manuscript. Medical writing support (funded by Esperion Therapeutics, Inc.) was provided by Callie A. S. Corsa, PhD, and Kelly M. Cameron, PhD, CMPP, of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in implementing author revisions while adhering to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2021.100278.
Appendix. Supplementary materials
References
- 1.Bertoluci M.C., Rocha V.Z. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. doi: 10.1186/s13098-017-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy S.M., Stone N.J., Bailey A.L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;2019(73) e285-e350. [Google Scholar]
- 3.Mach F., Baigent C., Catapano A.L. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;2020(41):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 4.Preiss D., Sattar N. Statins and the risk of new-onset diabetes: a review of recent evidence. Curr Opin Lipidol. 2011;22:460–466. doi: 10.1097/MOL.0b013e32834b4994. [DOI] [PubMed] [Google Scholar]
- 5.Erqou S., Lee C.C., Adler A.I. Statins and glycaemic control in individuals with diabetes: a systematic review and meta-analysis. Diabetologia. 2014;57:2444–2452. doi: 10.1007/s00125-014-3374-x. [DOI] [PubMed] [Google Scholar]
- 6.Newman C.B., Preiss D., Tobert J.A. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–e81. doi: 10.1161/ATV.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N., Preiss D., Murray H.M. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 8.Boekholdt S.M., Hovingh G.K., Mora S. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol. 2014;64:485–494. doi: 10.1016/j.jacc.2014.02.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne C.M., Banach M., Mancini G.B.J. Efficacy and safety of bempedoic acid added to ezetimibe in statin-intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg A.C., Leiter L.A., Stroes E.S.G. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA. 2019;322:1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray K.K., Bays H.E., Catapano A.L. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 12.Laufs U., Banach M., Mancini G.B.J. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nexletol (bempedoic acid) tablets for oral use . Esperion Therapeutics; Ann Arbor, MI: 2020. Prescribing information. [Google Scholar]
- 14.Nexlizet . Esperion Therapeutics; Ann Arbor, MI: 2020. (bempedoic acid and ezetimibe) prescribing information. [Google Scholar]
- 15.Ballantyne C.M., Laufs U., Ray K.K. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27:593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bays H.E., Banach M., Catapano A.L. Bempedoic acid safety analysis: pooled data from four phase 3 clinical trials. J Clin Lipidol. 2020;14:649–659. doi: 10.1016/j.jacl.2020.08.009. e646. [DOI] [PubMed] [Google Scholar]
- 17.Leiter L.A., Banach M., Catapano A.L. Abstract 11417: bempedoic acid and glycemic control: a pooled analysis of 4 phase 3 clinical trials. Circulation. 2019;140:A11417. [Google Scholar]
- 18.Ference B.A., Robinson J.G., Brook R.D. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–2153. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 19.Ference B.A., Ray K.K., Catapano A.L. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019;380:1033–1042. doi: 10.1056/NEJMoa1806747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholls S., Lincoff A.M., Bays H.E. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–112. doi: 10.1016/j.ahj.2020.10.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.