Abstract
Age-related cognitive decline has been extensively studied in humans, but the majority of research designs are cross-sectional and compare across younger and older adults. Longitudinal studies are necessary to capture variability in cognitive aging trajectories, but are difficult to carry out in humans and long-lived nonhuman primates. Marmosets are an ideal primate model for neurocognitive aging as their naturally short lifespan facilitates longitudinal designs. In a longitudinal study of marmosets tested on reversal learning starting in middle-age, we found that, on average, the group of marmosets declined in cognitive performance around 8 years of age. However, we found highly variable patterns of cognitive aging trajectories across individuals. Preliminary analyses of brain tissues from this cohort also shows highly variable degrees of neuropathology. Future work will tie together behavioral trajectories with brain pathology and provide a window into the factors that predict age-related cognitive decline.
Keywords: Marmoset, aging, longitudinal study, reversal learning, neuropathology
Graphical Abstract
Introduction
Age-related cognitive decline is well documented in humans; however, the bulk of the literature is based on cross-sectional studies that compare the performance of old adults and younger adults in the same tasks. These studies have highlighted differences between young and older participants in a range of cognitive abilities sometimes referred to as “fluid abilities”, such as episodic memory, speed of processing and working memory (Hedden & Gabrieli, 2004; Park & Schwarz, 2000; Salthouse, 2010). However, cross-sectional studies are subject to cohort effects and often over-estimate age effects (Schaie, 2009 but see Salthouse, 2010). Although longitudinal studies possess their own challenges, due to nonrandom attrition and practice effects (e.g. Lindenberger et al., 2002; Rabbitt et al., 2004; Salthouse, 1996), they are necessary to capture true age-related change and examine whether between-individual differences in age-related change are correlated across different aspects of brain and behavior (Lindenberger, 2014). In addition, longitudinal studies can determine normative and non-normative influences on lifespan cognitive trajectories (Steinerman, 2010). Longitudinal studies in humans have highlighted the heterogeneity of cognitive and brain trajectories, with some people showing little or no change, while others display marked changes with advancing age (Nyberg et al., 2020). This individual variability likely results from differences in brain maintenance, brain compensation and cognitive reserve (Cabeza et al., 2018; Stern et al., 2020), under the influence of various biological (e.g., genetic) and environmental (e.g., lifestyle) factors (Nyberg et al., 2020).
Due to the complexity of these interactions in humans (different lifestyles, diet, medications, exercise, etc.), it is crucial to study cognitive and brain aging in animal models for which these sources of variation can be better controlled (McQuail et al., 2021). Nonhuman primates (NHP) are arguably the best animal models of human cognitive aging due to their phylogenetic proximity and overlap with humans in many aspects of brain function and behavior (Baxter, 2001; Emery Thompson et al., 2020; Hara et al., 2012; Herndon et al., 1997; Lacreuse & Herndon, 2009; Voytko & Tinkler, 2004). To date, several NHP species have been used as models for human cognitive aging (Shively et al., this issue), but longitudinal studies remain remarkably rare (Hopkins et al., 2020; Lacreuse et al., 2014; Pifferi & Aujard, 2019; Suomi et al., 1996). A major challenge to longitudinal approaches in NHPs is their long lifespan; our closest great ape relatives, the chimpanzees, have a life expectancy in captivity of 28.3 years at birth, that increases to 34.6 for animals who reach one year of age, with an estimated maximum lifespan of 74 years (Havercamp et al., 2019). Likewise, the most common NHP used in biomedical research, rhesus macaques, have an average life expectancy of approximately 26 years, with a maximum lifespan of 40 years (Colman, 2018).
The common marmoset (Callithrix jacchus) is gaining attention as an ideal model for translational neuroscience research (Abbott et al., 2003), including for aging research (Ross, 2019; Tardif et al., 2011). First, marmosets are ideally suited for longitudinal investigations because they have a relatively short life expectancy; captive laboratories report average lifespan between 5 and 9 years for females and 5 and 13 years for males (Nishijima et al., 2012; Ross, 2019). Maximum lifespan was previously reported to be about 16 years (Tardif et al., 2011) , but increased to 21 years in a Japanese colony (Nishijima et al., 2012). Extended maximum lifespans may be found in marmosets colonies with reduced exposure to potential pathogens (see Ross, 2019). Second, this small-bodied species (300-500 g) is easier to handle than larger primates and easier to group-house in captivity. Third, they have a rich behavioral repertoire, are highly social (Miller, 2017; Miller et al., 2016), are able to perform a range of cognitive tasks (Nakamura et al., 2018; Nummela et al., 2019; Spinelli et al., 2004) and have a brain organization typical of anthropoid primates (Fukushima et al., 2018; Liu et al., 2019; Miller et al., 2016). Aging studies in the marmoset are only in their infancy, but have documented age-related changes in a number of biological systems between ages 5 and 8 (Ross et al., 2012), including weight loss (Tardif et al., 2011), hearing loss (Harada et al., 1999) and cartilage aging (Berkovitz & Pacy, 2000). With regards to the brain, some age-related changes are similar to those observed in humans and include age-related deposition of amyloid-β protein in cortical areas, which has been found in marmosets as early as ages 8 (Geula et al., 2002, Freire Cobo et al., this issue) to 10 (Maclean et al., 2000; Rodriguez-Callejas et al., 2016), reduced neurogenesis in the dentate gyrus (Leuner et al., 2007), accumulation of dystrophic and activated microglia in specific regions (Rodriguez-Callejas et al., 2016; Freire Cobo et al., this issue) and age-related alterations of the corpus callosum (Phillips et al., 2019).
In contrast, studies of age-related cognitive decline in the marmoset remain extremely sparse. Three cross-sectional studies have recently documented age differences in executive function (Munger et al., 2017; Sadoun et al., 2019) working memory (Sadoun et al., 2019) and detour reaching (Phillips et al., 2019) in this species, suggesting that aging influences cognitive domains that are also sensitive to aging in other primates (see Baxter, 2001; Lacreuse & Herndon, 2009). Yet, one of the main advantages of the marmoset over traditional primate models of human aging such as macaque monkeys, is their much shorter lifespan of about 10 years, particularly well-suited to longitudinal designs. In the first study of this type (Rothwell et al., 2021), our group tested marmosets on reversal learning, a task of executive function, starting in middle-age (about 5 years old) until the marmosets were about 9 years old. In order to examine associations between cellular and pathological changes in the brain and the observed behavioral changes, brain tissues were collected post-behavioral assessments for the analysis of prefrontal cortex (PFC) and hippocampal neuropathology.
Variability in Marmoset Cognitive Aging Trajectories
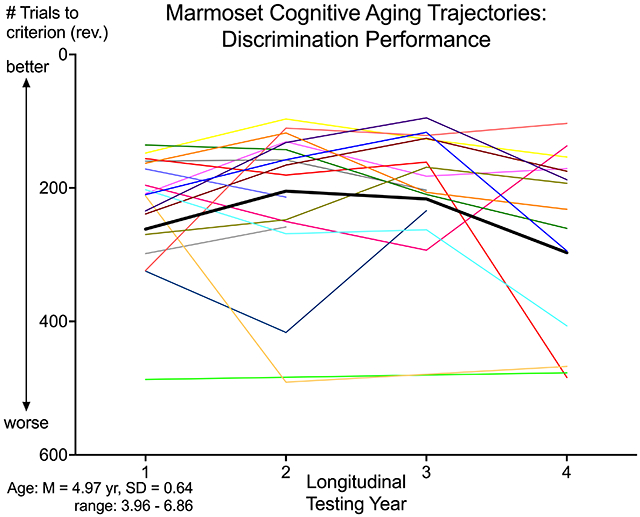
Age-related decline in cognitive performance appeared around age 8, consistent with Sadoun et al.’s cross-sectional study of marmosets (2019). In addition, sex differences were observed, with females exhibiting an earlier and steeper decline than males. Importantly, highly variable patterns of cognitive aging trajectories were seen in both sexes (Figure 1). The existence of substantial individual variability in cognitive aging patterns has been recognized for a long time, both in humans (Hedden & Gabrieli, 2004) and NHP (Rapp & Amaral, 1992). Cognitive variability not only includes differences across individuals – not all individuals will exhibit robust cognitive deficits with age – but also within-individual differences across time (MacDonald et al., 2009) and across tasks (Roalf et al., 2016). The consideration of such indices are critical for assessing cognitive decline (Hultsch et al., 2008; MacDonald et al., 2006), in particular for identifying individuals at risk for dementia (Roalf et al., 2016) or those exhibiting brain pathology (Ferreira et al., 2017).
Figure 1. Cognitive aging trajectories.
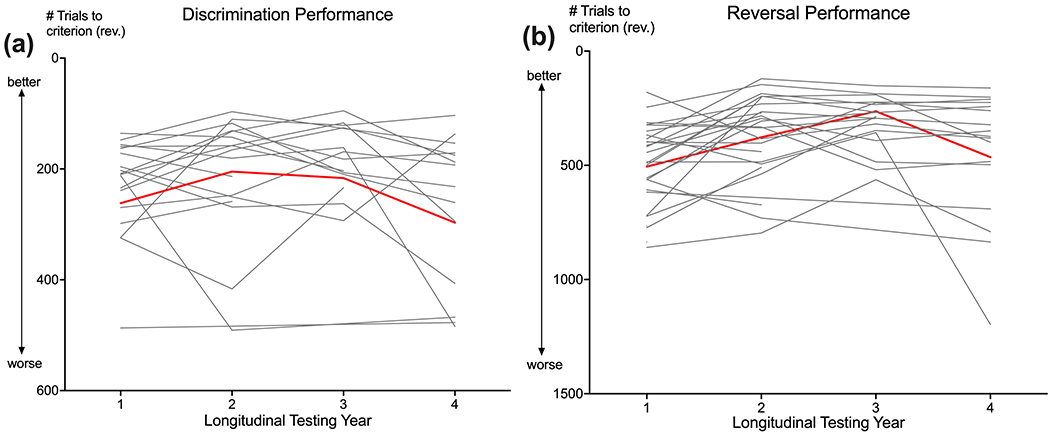
Trajectories of cognitive performance on trials to criterion for (a) discrimination and (b) reversal testing across a 4-year longitudinal study spanning middle to old age. Performance is measured by the number of trials to criterion, with fewer trials representing better performance. Red lines represent the average aging trajectories for all marmosets (N=27) and gray lines represent individual marmosets. Average age at Testing Year 1 was 4.97 years old (SD = 0.64; 3.96 to 6.86 years).
Variability in Marmoset Neuropathology
The analysis of brain tissues from the marmosets at the end of the longitudinal study indicates that the extent of neuropathology is highly variable across individuals. Our preliminary investigations based on 12 marmoset brains revealed large individual differences in loss of dendritic spine densities in dorsolateral prefrontal cortex (dlPFC) and hippocampus and degrees of amyloid-β deposition in dlPFC layer III of area 8b/9 (Freire Cobo et al., this issue) and hippocampus CA1 (Figure 2). Along with the amyloid-β burden, we also observed individual differences in microglial activation (Figure 3) and morphology (Figure 4). Some marmosets showed an increased activation state of microglial cells, in both dlPFC layer III of area 8b/9 and hippocampus CA1 with an increase of the atrophic microglial phenotype (Garaschuk & Verkhratsky, 2019). As our sample sizes increase, we will be able to determine whether neuropathology is more severe in marmosets characterized as cognitively impaired, as well as in females, as would be predicted from sex differences in cognitive trajectories (Rothwell et al., 2021).
Figure 2. Amyloid deposits in hippocampus.
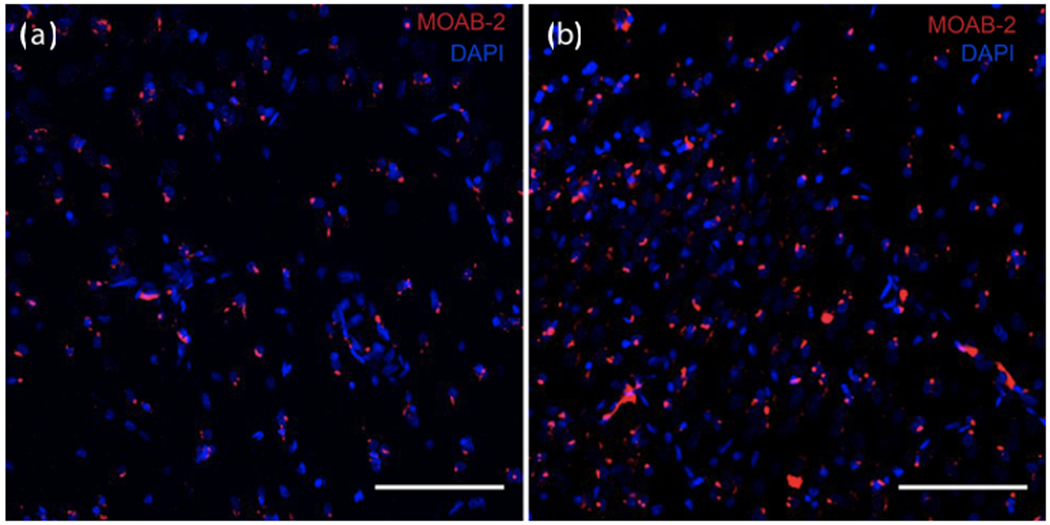
Varying degrees of amyloid deposits in CA1 of old marmosets. Amyloid-β peptide detected by MOAB-2 antibody (red); cell nuclei stained with DAPI (blue). Scale bar = 100 μm. Amyloid burden in two male marmosets: (a) low burden, 9 years old and (b) high burden 8 years old.
Figure 3. Microglia activation in hippocampus.
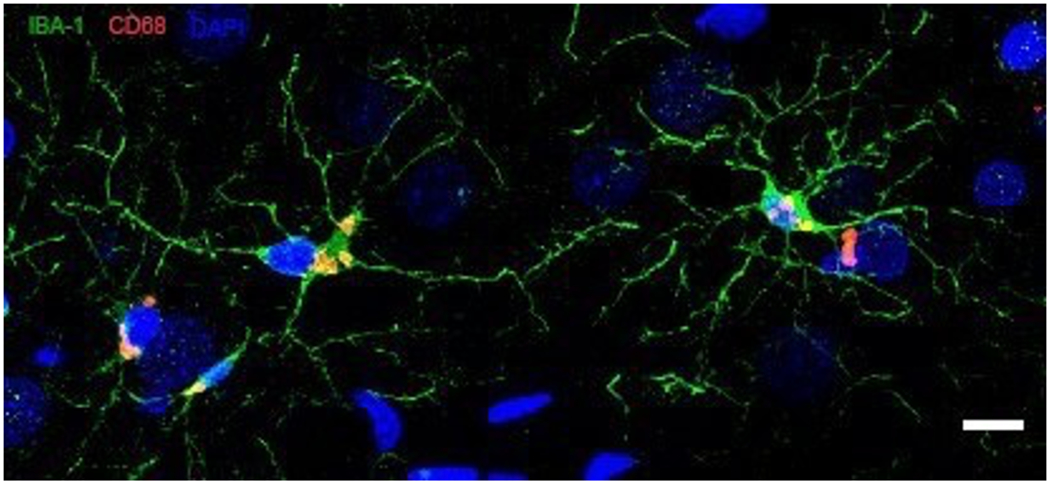
Activated microglia in CA1 of an old marmoset. Confocal images of microglia expressing Iba-1 (green) and CD68 (red). Nuclei are stained with DAPI (blue). Scale bar = 10 μm.
Figure 4. Microglia morphology in dlPFC.
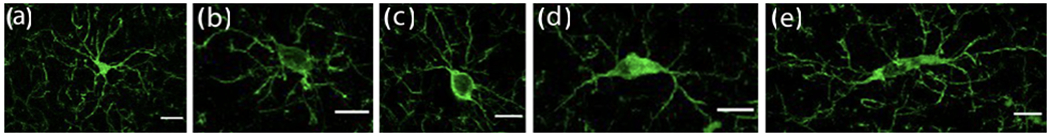
Microglia detected by Iba-1 expression (green). (a) Highly ramified morphology with small cell soma and fine processes; (b-c) intermediate morphology with enlarged cell soma and thick, short processes; (d-e) ameboid and dystrophic microglia, with extensive loss of processes. Scale bars = 10 μm.
Discussion
Few studies to date have leveraged the advantages of small, short-lived NHP (Fischer & Austad, 2011) for longitudinal investigations of neurocognitive aging (Pifferi & Aujard, 2019; Rothwell et al., 2021). Longitudinal assessments of cognitive, behavioral and biological parameters, combined with multiple neuroimaging assessments (Laclair et al., 2019; Nephew et al., 2020) and post-mortem neuropathology in the aging marmoset will provide invaluable insight into healthy and pathological aging, including Alzheimer’s disease (AD). While transgenic rodent models have advanced our understanding of AD, clinical trials have routinely failed for almost two decades (King, 2018). A need exists to study animal models that naturally undergo cerebral and behavioral changes more similar to humans to understand the development of AD-like neuropathology better. Furthermore, individual variability in susceptibility or resistance to AD-like neuropathology can only be understood with longitudinal studies. In this context it is therefore crucial to ensure that marmosets are made readily available for aging research (Miller & Lee, 2019; Servick, 2018; Shively, this issue), as we expect that the marmoset will become a major NHP model in which to address these fundamental questions.
Acknowledgements
Supported by NIH grants R01 AG046266 to AL and F32 AG064925 to ER. This research adhered to the guidelines for the Ethical Treatment of Non-Human Primates provided by the American Society of Primatologists. This research was approved by the University of Massachusetts-Amherst Institutional Animal Care and Use Committee. Research data used for this manuscript are not shared at this time. The authors report no conflicts of interest.
References
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, & Schultz-Darken NJ (2003). Aspects of Common Marmoset Basic Biology and Life History Important for Biomedical Research. Comparative Medicine, 53(4), 339–350. [PubMed] [Google Scholar]
- Baxter MG (2001). Cognitive Aging in Nonhuman Primates. In Functional Neurobiology of Aging (pp. 407–419). Elsevier. 10.1016/b978-012351830-9/50028-7 [DOI] [Google Scholar]
- Berkovitz BKB, & Pacy J (2000). Age changes in the cells of the intra-articular disc of the temporomandibular joints of rats and marmosets. Archives of Oral Biology, 45(11), 987–995. 10.1016/S0003-9969(00)00067-4 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, & Rajah MN (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. In Nature Reviews Neuroscience (Vol. 19, Issue 11, pp. 701–710). Nature Publishing Group. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ (2018). Non-human primates as a model for aging. In Biochimica et Biophysica Acta-Molecular Basis of Disease (Vol. 1864, Issue 9, pp. 2733–2741). Elsevier B.V. 10.1016/j.bbadis.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Rosati AG, & Snyder-Mackler N (2020). Insights from evolutionarily relevant models for human ageing. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1811), 20190605. 10.1098/rstb.2019.0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira D, Machado A, Molina Y, Nieto A, Correia R, Westman E, & Barroso J (2017). Cognitive Variability during Middle-Age: Possible Association with Neurodegeneration and Cognitive Reserve. Frontiers in Aging Neuroscience, 9(JUN), 188. 10.3389/fnagi.2017.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire-Cobo C, Edler M, Munger E, Laffey J, Raia S, In S, Varghese M, Wicinsky B, Medalla M, Perez S, Mufson EJ, Erwin JM, Guevara E, Sherwood C,Luebke JI, Lacreuse A, Raghanti MA, & Hof PR (this issue). Comparative primate neuropathology: a perspective. American Journal of Primatology Special Issue on Aging, Cognitive Decline, and Neuropathology in Nonhuman Primates. [Google Scholar]
- Fischer KE, & Austad SN (2011). The Development of Small Primate Models for Aging Research. ILAR Journal, 52(1), 78–88. 10.1093/ilar.52.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Ichinohe N, & Okano H (2018). Neuroanatomy of the marmoset. In The Common Marmoset in Captivity and Biomedical Research (pp. 43–62). Elsevier. 10.1016/B978-0-12-811829-0.00003-0 [DOI] [Google Scholar]
- Garaschuk O, & Verkhratsky A (2019). Physiology of Microglia. In Methods in Molecular Biology (Vol. 2034, pp. 27–40). Humana Press Inc. 10.1007/978-1-4939-9658-2_3 [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, & Wu CK (2002). Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): Incidence and chemical composition. Acta Neuropathologica, 103(1), 48–58. 10.1007/s004010100429 [DOI] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, & Morrison JH (2012). Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. In Age (Vol. 34, Issue 5, pp. 1051–1073). 10.1007/s11357-011-9278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Tokuriki M, & Tanioka Y (1999). Age-related changes in the brainstem auditory evoked potentials of the marmoset. Hearing Research, 128(1–2), 119–124. 10.1016/S0378-5955(98)00201-9 [DOI] [PubMed] [Google Scholar]
- Havercamp K, Watanuki K, Tomonaga M, Matsuzawa T, & Hirata S (2019). Longevity and mortality of captive chimpanzees in Japan from 1921 to 2018. Primates, 60(6), 525–535. 10.1007/s10329-019-00755-8 [DOI] [PubMed] [Google Scholar]
- Hedden T, & Gabrieli JDE (2004). Insights into the ageing mind: A view from cognitive neuroscience. In Nature Reviews Neuroscience (Vol. 5, Issue 2, pp. 87–96). European Association for Cardio-Thoracic Surgery. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, & Killiany RJ (1997). Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research, 87(1), 25–34. 10.1016/S0166-4328(96)02256-5 [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Mareno MC, Neal Webb SJ, Schapiro SJ, Raghanti MA, & Sherwood CC (2020). Age-related changes in chimpanzee ( Pan troglodytes ) cognition: Cross-sectional and longitudinal analyses. American Journal of Primatology. 10.1002/ajp.23214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, & MacDonald SWS (2008). Intraindividual variability, cognition, and aging. (Craik FIM & Salthouse TA (Eds.)). https://psycnet.apa.org/record/2007-10440-010 [Google Scholar]
- King A (2018). The search for better animal models of Alzheimer’s disease. Nature, 559(7715), S13–S13. https://go.gale.com/ps/i.do?p=HRCA&sw=w&issn=00280836&v=2.1&it=r&id=GALE%7CA572728089&sid=googleScholar&linkaccess=fulltext [DOI] [PubMed] [Google Scholar]
- Laclair M, Febo M, Nephew B, Gervais NJ, Poirier G, Workman K, Chumachenko S, Payne L, Moore MC, King JA, & Lacreuse A (2019). Sex differences in cognitive flexibility and resting brain networks in middle-aged marmosets. ENeuro, 6(4). 10.1523/ENEURO.0154-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Chang J, Metevier CM, LaClair M, Meyer JS, & Ferris CM (2014). Oestradiol Modulation of Cognition in Adult Female Marmosets ( Callithrix jacchus ). Journal of Neuroendocrinology, 26(5), 296–309. 10.1111/jne.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, & Herndon JG (2009). Nonhuman Primate Models of Cognitive Aging. In Animal Models of Human Cognitive Aging (pp. 1–30). Humana Press. 10.1007/978-1-59745-422-3_2 [DOI] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, & Gould E (2007). Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America, 104(43), 17169–17173. 10.1073/pnas.0708228104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Singer T, & Baltes PB (2002). Longitudinal Selectivity in Aging Populations: Separating Mortality-Associated Versus Experimental Components in the Berlin Aging Study (BASE). The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57(6), P474–P482. 10.1093/geronb/57.6.P474 [DOI] [PubMed] [Google Scholar]
- Lindenberger Ulman. (2014). Human cognitive aging: Corriger lafortune? In Science (Vol. 346, Issue 6209, pp. 572–578). American Association for the Advancement of Science. 10.1126/science.1254403 [DOI] [PubMed] [Google Scholar]
- Liu C, Yen CCC, Szczupak D, Ye FQ, Leopold DA, & Silva AC (2019). Anatomical and functional investigation of the marmoset default mode network. Nature Communications, 10(1), 1–8. 10.1038/s41467-019-09813-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SWS, Li SC, & Bäckman L (2009). Neural Underpinnings of Within-Person Variability in Cognitive Functioning. Psychology and Aging, 24(4), 792–808. 10.1037/a0017798 [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Nyberg L, & Bäckman L (2006). Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. In Trends in Neurosciences (Vol. 29, Issue 8, pp. 474–480). Elsevier Current Trends. 10.1016/j.tins.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, & Mori H (2000). Naturally occurring and experimentally induced β-amyloid deposits in the brains of marmosets (Callithrix jacchus). Journal of Neural Transmission, 107(7), 799–814. 10.1007/s007020070060 [DOI] [PubMed] [Google Scholar]
- McQuail JA, Dunn AR, Stern Y, Barnes CA, Kempermann G, Rapp PR, Kaczorowski CC, & Foster TC (2021). Cognitive Reserve in Model Systems for Mechanistic Discovery: The Importance of Longitudinal Studies. Frontiers in Aging Neuroscience, 12, 532. 10.3389/fnagi.2020.607685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT (2017). Why Marmosets? Developmental Neurobiology, 77(3), 237–243. 10.1002/dneu.22483 [DOI] [PubMed] [Google Scholar]
- Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, & Wang X (2016). Marmosets: A Neuroscientific Model of Human Social Behavior. Neuron, 90(2), 219–233. 10.1016/J.NEURON.2016.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, & Lee K-F (2019). 2019 Marmoset Community White Paper. [Google Scholar]
- Munger EL, Takemoto A, Raghanti MA, & Nakamura K (2017). Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neuroscience Research, 124, 57–62. 10.1016/j.neures.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Koba R, Miwa M, Yamaguchi C, Suzuki H, & Takemoto A (2018). A Method to Train Marmosets in Visual Working Memory Task and Their Performance. Frontiers in Behavioral Neuroscience, 12, 46. 10.3389/fnbeh.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Febo M, Cali R, Workman KP, Payne L, Moore CM, King JA, & Lacreuse A (2020). Robustness of sex-differences in functional connectivity over time in middle-aged marmosets. Scientific Reports, 10(1), 16647. 10.1038/s41598-020-73811-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, & Kitajima S (2012). Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology, 13, 439–443. 10.1007/s10522-012-9388-1 [DOI] [PubMed] [Google Scholar]
- Nummela SU, Jutras MJ, Wixted JT, Buffalo EA, & Miller CT (2019). Recognition Memory in Marmoset and Macaque Monkeys: A Comparison of Active Vision. Journal of Cognitive Neuroscience, 31(9), 1318–1328. 10.1162/jocn_a_01361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Boraxbekk CJ, Sörman DE, Hansson P, Herlitz A, Kauppi K, Ljungberg JK, Lövheim H, Lundquist A, Adolfsson AN, Oudin A, Pudas S, Rönnlund M, Stiernstedt M, Sundström A, & Adolfsson R (2020). Biological and environmental predictors of heterogeneity in neurocognitive ageing: Evidence from Betula and other longitudinal studies. In Ageing Research Reviews (Vol. 64, p. 101184). Elsevier Ireland Ltd. 10.1016/j.arr.2020.101184 [DOI] [PubMed] [Google Scholar]
- Park DC, & Schwarz N (Eds.). (2000). Cognitive aging: A primer. Psychology Press. https://psycnet.apa.org/record/2000-07430-000 [Google Scholar]
- Phillips KA, Watson CM, Bearman A, Knippenberg AR, Adams J, Ross C, & Tardif SD (2019). Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. American Journal of Primatology, 81(2), e22949. 10.1002/ajp.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi F, & Aujard F (2019). Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. In Progress in Neuro-Psychopharmacology and Biological Psychiatry (Vol. 95, p. 109702). Elsevier Inc. 10.1016/j.pnpbp.2019.109702 [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Holland F, & McInnes L (2004). Practice and Drop-Out Effects During a 17-Year Longitudinal Study of Cognitive Aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 59(2), P84–P97. 10.1093/geronb/59.2.P84 [DOI] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1992). Individual differences in the cognitive and neurobiological consequences of normal aging. In Trends in Neurosciences (Vol. 15, Issue 9, pp. 340–345). Elsevier Current Trends. 10.1016/0166-2236(92)90051-9 [DOI] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Mechanic-Hamilton D, Wolk DA, Arnold SE, & Moberg PJ (2016). Within-individual variability: An index for subtle change in neurocognition in mild cognitive impairment. Journal of Alzheimer’s Disease, 54(1), 325–335. 10.3233/JAD-160259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Callejas JD, Fuchs E, & Perez-Cruz C (2016). Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset. Frontiers in Aging Neuroscience, 8, 315. 10.3389/fnagi.2016.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN (2019). Marmosets in Aging Research. The Common Marmoset in Captivity and Biomedical Research, 355–376. 10.1016/B978-0-12-811829-0.00021-2 [DOI] [Google Scholar]
- Ross CN, Davis K, Dobek G, & Tardif SD (2012). Aging phenotypes of common marmosets (Callithrix jacchus). Journal of Aging Research, 2012. 10.1155/2012/567143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoun A, Rosito M, Fonta C, & Girard P (2019). Key periods of cognitive decline in a nonhuman primate model of cognitive aging, the common marmoset (Callithrix jacchus). Neurobiology of Aging, 74, 1–14. 10.1016/J.NEUROBIOLAGING.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (1996). Constraints on theories of cognitive aging. Psychonomic Bulletin and Review, 3(3), 287–299. 10.3758/BF03210753 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. In Journal of the International Neuropsychological Society (Vol. 16, Issue 5, pp. 754–760). Cambridge University Press. 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2014). Selective review of cognitive aging. 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW (2009). “When does age-related cognitive decline begin?” Salthouse again reifies the “cross-sectional fallacy.” In Neurobiology of Aging (Vol. 30, Issue 4, pp. 528–529). Elsevier Inc. 10.1016/j.neurobiolaging.2008.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servick K (2018). U.S. Labs clamor for marmosets: Shortage develops as new transgenic models for neurological diseases stoke interest. In Science (Vol. 362, Issue 6413, pp. 383–384). American Association for the Advancement of Science. 10.1126/science.362.6413.383 [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, & Pryce CR (2004). Performance of the marmoset monkey on computerized tasks of attention and working memory. Cognitive Brain Research, 19(2), 123–137. 10.1016/j.cogbrainres.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Steinerman JR (2010). Minding the aging brain: Technology-enabled cognitive training for healthy elders. In Current Neurology and Neuroscience Reports (Vol. 10, Issue 5, pp. 374–380). Current Medicine Group LLC. 10.1007/s11910-010-0124-4 [DOI] [PubMed] [Google Scholar]
- Stern Y, Arenaza-Urquijo EM, Bartrés-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, & Vuoksimaa E (2020). Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s & Dementia, 16(9), 1305–1311. 10.1016/j.jalz.2018.07.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, Novak MA, & Well A (1996). In the public domain Aging in Rhesus Monkeys: Different Windows on Behavioral Continuity and Change. In Developmental Psychology (Vol. 32, Issue 6). [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, & Ziegler TE (2011). The Marmoset as a Model of Aging and Age-Related Diseases. ILAR Journal, 52(1), 54–65. 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, & Tinkler GP (2004). Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer’s disease, and menopause. In Frontiers in Bioscience (Vol. 9, pp. 1899–1914). Frontiers in Bioscience. 10.2741/1370 [DOI] [PubMed] [Google Scholar]


