Abstract
Background:
Few studies assess use of parathyroidectomy among older adults with symptomatic primary hyperparathyroidism (PHPT). Our objective was to determine national usage and disparities in parathyroidectomy for symptomatic PHPT among insured older adults.
Methods
We identified older adult patients with symptomatic PHPT using Medicare claims (2006–2017). Primary study variables were race/ethnicity, rurality, and zip-code socioeconomic status (SES). We calculated cumulative incidence of parathyroidectomy and used multivariable Cox proportional hazards regression models to assess the adjusted association of our study variables with parathyroidectomy.
Results:
We included 94,803 patients. The median age at PHPT diagnosis was 76 years old (IQR 71–82). The majority of patients were female(72%), non-Hispanic White(82%), from metropolitan areas(82%) and had a Charlson Comorbidity score ≥3(62%). 9% of patients (n=8,251) underwent parathyroidectomy during follow-up. After adjustment, non-Hispanic Black patients, compared to non-Hispanic White(HR 0.80 95%CI 0.74,0.87), and living in a low SES neighborhood(low SES vs highest SES HR 0.89 95%CI 0.83,0.95) were both associated with lower incidences of parathyroidectomy. Patients from non-metropolitan areas were more likely to undergo parathyroidectomy.
Conclusions:
Parathyroidectomy is underutilized for symptomatic primary hyperparathyroidism in older adults. Quality improvement efforts, rooted in equitable care, should be undertaken to increase access to parathyroidectomy for this disease.
Summary:
In this retrospective cohort study of 94,803 insured older adults with symptomatic primary hyperparathyroidism, only 9% of patients underwent parathyroidectomy with disparities by race/ethnicity, socioeconomic status and rurality. These findings show that national quality improvement efforts are needed to improve access to parathyroidectomy.
Introduction:
Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterized by unregulated overproduction of parathyroid hormone leading to hypercalcemia. PHPT affects roughly 100,000 people annually in the United States.1 PHPT can lead to physical manifestations such as nephrolithiasis, osteoporosis, and fragility fractures, but may also lead to more subtle cognitive, neuropsychiatric and gastrointestinal symptoms.2 In patients with PHPT, parathyroidectomy is curative, cost-effective and improves quality of life.3,4 Parathyroidectomy is indicated in patients younger than 50 and in all symptomatic patients unless they have a prohibitive operative risk.2 Asymptomatic patients over age 50 who have disease manifestations as defined by consensus criteria also should be recommended to undergo parathyroidectomy.5
Despite parathyroidectomy being the most definitive treatment for PHPT, prior research has noted underuse of the procedure for patients with PHPT, particularly among older adults.6,7 Additionally, there are notable disparities in receipt and time to parathyroidectomy among patients with indications for surgery. One recent study found that Black patients had a significantly longer time to parathyroidectomy and were less likely to undergo parathyroidectomy within one year following diagnosis compared to White patients with PHPT.7 In patients who do undergo surgery, Black and Hispanic patients have also been found to have more complications, higher costs, and are less likely to reach a high-volume surgeon.8–10
Prior research assessing use has primarily focused on institutional data from higher volume centers.7,11,12 While these data are able to capture a more detailed clinical picture, a major limitation is that cases diagnosed in the community who are never referred to or never seek care at that center would be missed, overestimating the use of parathyroidectomy in the general population. Additionally, few studies focus on patients with symptomatic disease in whom parathyroidectomy is clearly indicated. Thus, the objectives of this study were to describe use and disparities in parathyroidectomy for symptomatic PHPT among older adults in a national, multi-institutional data source. We hypothesized that use would be low despite patients having universal insurance, with disparities in use by race/ethnicity, socioeconomic status, and rural-urban residence.
Methods:
Study Design and Inclusion/Exclusion Criteria
We performed a retrospective cohort study using Medicare claims from 2006–2017. The Medicare claims data come from a 20% representative sample of all Medicare patients 65 years and older with fee-for-service coverage of Medicare parts A, B and D. Beneficiaries are included in the database if they have simultaneous coverage of parts A, B and D for at least one calendar month from 2006–2017. Once these criteria are met, all subsequent fee-for-service claims are included until the most recent year available (2017), disenrollment, or death.
All older adults (≥67 years old) diagnosed with PHPT by International Classification of Disease Clinical Modification (9th edition: ICD-9-CM; 10th edition: ICD-10-CM) coding were eligible for inclusion (ICD-9-CM: 252.00, 252.01; ICD-10-CM: E21.0, E21.3). All adults were required to have at least two years of Medicare Parts A and B coverage prior to their first PHPT diagnosis (disease-free interval) (Figure 1). Adults with a diagnosis of secondary hyperparathyroidism due to renal origin (ICD-9-CM: 588.81; ICD-10-CM: N25.81) were also excluded.
Figure 1: Study design outlining cohort ascertainment and follow-up for patients with symptomatic primary hyperparathyroidism.
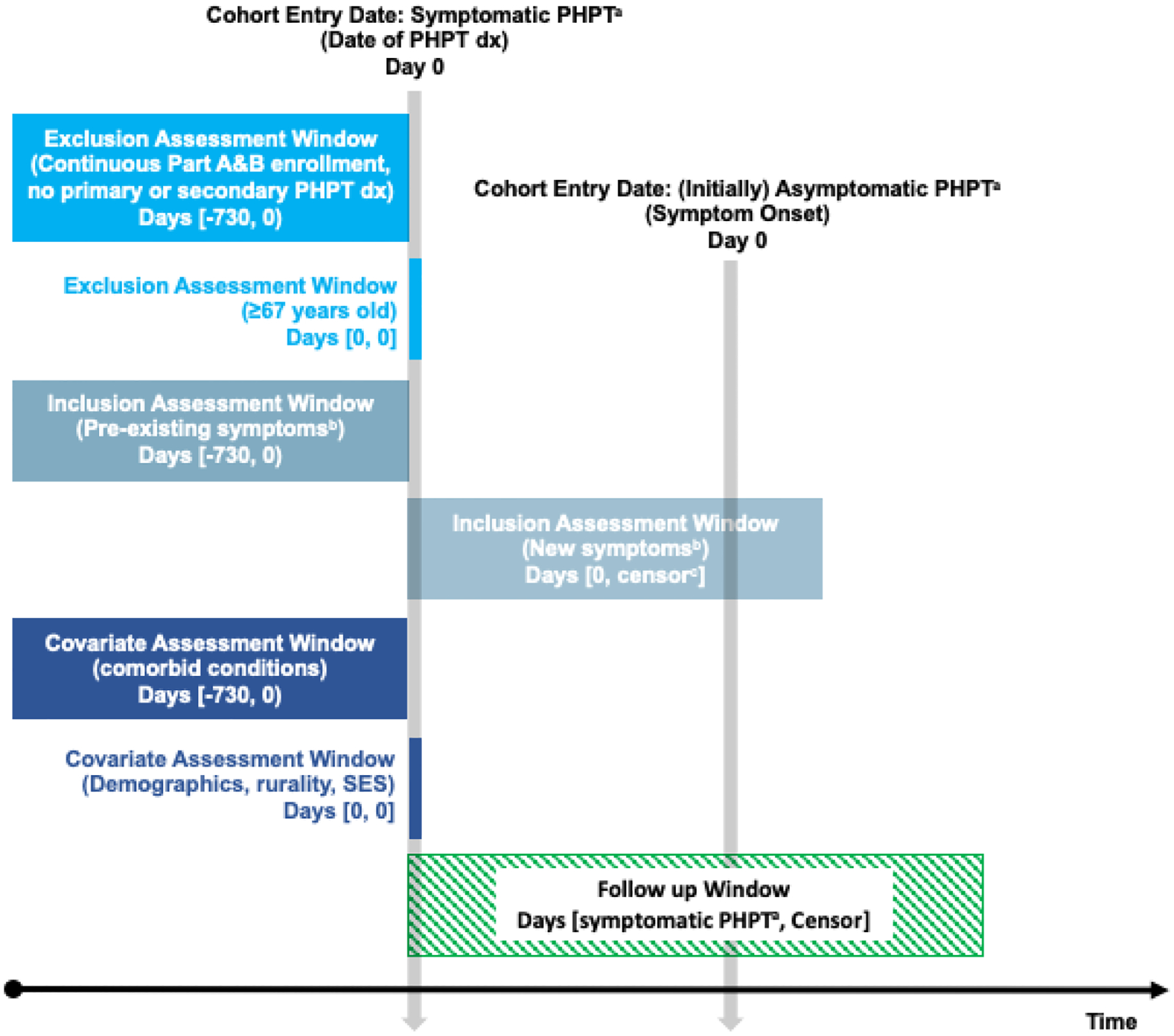
A) Follow-up began at date of PHPT among patients with evidence of pre-existing symptoms. Follow-up among (initially) asymptomatic patients began on the date of their first documented symptom after PHPT diagnosis; patients who were censored prior to symptom development were excluded. B) Symptoms include: nephrolithiasis, hypercalciuria, nephrocalcinosis, decreased glomerular filtration rate (GFR <60), osteopenia, osteoporosis, history of pathologic fractures, neuropsychiatric symptoms, and gastrointestinal manifestations. C) Censored at earliest of: outcome (parathyroidectomy), death, disenrollment, or end of the study period (December 31, 2017). Abbreviations: PHPT, primary hyperparathyroidism; SES, socioeconomic status
Symptomatic disease was defined by claims records of any of the following symptoms or disease manifestations before or after diagnosis of PHPT: nephrolithiasis, hypercalciuria, nephrocalcinosis, decreased glomerular filtration rate (<60 mL/min), osteopenia, osteoporosis with and without fracture, history of pathologic fracture, neuropsychiatric manifestations such as depression or fatigue, or gastrointestinal manifestations (Supplemental Table 1). Codes were identified through online searches, review of prior studies, and by forward and backward mapping with Centers for Medicaid & Medicare Services (CMS) General Equivalence Mappings (GEM). The GEMs are guides from CMS that allow for the conversion of ICD-9-CM codes to ICD-10 codes (forward mapping) and from ICD-10 codes to ICD-9-CM codes (backward mapping).13
Follow-up began on the first date a patient had both received a diagnosis of PHPT and a recorded diagnosis for at least one symptom. Specifically, patients with symptoms reported during the 2-year disease-free interval had follow-up begin on the date of their first PHPT diagnosis. Patients without symptoms during the disease-free interval were assumed to be asymptomatic (e.g., incidental finding of PHPT) and follow-up began on the date the first symptom was documented. All adults were then followed until parathyroidectomy (outcome of interest), death, insurance coverage change or disenrollment, or end of study follow-up on December 31, 2017, whichever came first. Receipt of parathyroidectomy was defined by the Current Procedural Terminology® code 60500.
Study Variables
The exposures of interest were race/ethnicity and neighborhood characteristics, specifically rurality and socioeconomic status. Race/ethnicity in the claims data is based on patient self-report and was categorized as non-Hispanic (NH) White, NH Black, NH Asian/Pacific Islander (PI), Hispanic and other. Patients with unknown race/ethnicity were excluded from these analyses (n=382). Rurality was defined using the United States Department of Agriculture’s 2013 Rural Urban Continuum Codes (RUCC), a classification scheme used to distinguish metropolitan counties by the population size of the metro area and nonmetropolitan counties by degree of urbanization and adjacency to a metro area. Non-metropolitan counties are generally considered rural, but the non-metropolitan RUCCs can also be subdivided by their proximity to a larger metropolitan area.14 Thus, RUCCs were categorized into three groups (1–3: Metropolitan; 4,6,8: Non-metro adjacent; 5,7,9: Non-metro non-adjacent).14,15 The American Community Survey’s social deprivation index (SDI) was used as an area measure of socioeconomic status (SES). The SDI is a composite measure of area level deprivation determined by seven demographic characteristics used to quantify socioeconomic variation in health outcomes (range 1–100),16 with a lower score indicating higher SES. The SDI was split into quartiles and categorized as highest (1–22), high (23–45), medium (46–71), and low (≥72) SES. Patient billing ZIP code was captured at the time of their Medicare enrollment. Patients that were missing a ZIP code (n=293), were living in a United States territory (e.g. Puerto Rico, n=627), or were living in a ZIP code with no SDI score (n=3,251) were excluded from analyses.
Other covariates of interest included age at diagnosis, sex (male/female), comorbidities, and symptoms (as previously described). Comorbidities were captured in the two years prior to diagnosis of PHPT using the Charlson Comorbidity Index (CCI) score. The CCI score was calculated using the methodology described in Deyo et al (1992).17
Statistical Analyses
Differences in patient demographics and comorbidities were assessed using descriptive statistics which were stratified by socioeconomic status (SDI quartiles). Crude and adjusted associations between the primary study variables (race/ethnicity, socioeconomic status, and rurality) and parathyroidectomy were estimated using separate Cox proportional hazard regression models. Death was treated as a competing risk. Each model was adjusted for age at diagnosis (treated as a restricted quadratic spline), sex, CCI score (treated as a restricted quadratic spline) and symptoms (treated as time-varying).
We also performed several sensitivity analyses. Neuropsychiatric symptoms, gastrointestinal manifestations, and osteopenia are common and may be due to causes and conditions other than PHPT. In addition, osteopenia might not be considered an indication for parathyroidectomy. Therefore, we also performed a sensitivity analysis where these conditions were removed from the symptom list. For this analysis, the start of follow-up for each patient was reclassified using the timing of their PHPT diagnosis and first onset of other symptoms. Adjusted Cox proportional hazards models were then re-run. We also performed a sensitivity analysis where we only included patients diagnosed with at least two diagnoses of PHPT. This was done to exclude patients with PHPT diagnoses listed as a rule out diagnosis in patients undergoing parathyroid hormone level testing.
All analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC). This study was classified as exempt by the University of North Carolina at Chapel Hill Institutional Review Board (IRB# 19–2857). Due to the data use agreement, the data are not shared.
Results:
Overall, 94,803 adults were included. 83,570 adults (88%) were symptomatic prior to their PHPT diagnosis and 11,233 adults (12%) became symptomatic after their PHPT diagnosis. Among patients who became symptomatic after their PHPT diagnosis, the median time between PHPT diagnosis and diagnosis of at least one symptom was 58 days (IQR 0–309, range 0–3,176). Overall, 71,190 patients (75%) had at least 1 year of follow-up after being diagnosed with symptomatic PHPT (median follow-up time 915 days, range 0–3,651).
Baseline characteristics are presented in total and by SDI category in Table 1. Characteristics were also stratified by race/ethnicity and rurality (Supplemental Tables 2 and 3). Overall, the median age at diagnosis was 76 years old (IQR 71–82). The majority of patients were female (72%), NH White (82%), from metropolitan areas (82%) and had a CCI score ≥3 (62%). Notable differences by SES included race/ethnicity (94% NH White and 12% NH Black in the highest SES neighborhoods compared to 60% and 30%, respectively, in the lowest SES neighborhoods) and CCI score (70% ≥3 in the lowest SES compared to 55% ≥3 in the highest SES neighborhood).
Table 1.
Baseline patient characteristics, at time of hyperparathyroidism diagnosis, stratified by social deprivation index (SDI) score.
| Total | Highest SES N=23,240 | Medium | |||
|---|---|---|---|---|---|
| High SES N=33,302 | SES N=23,958 | Low SES N=24,303 | |||
| Age, median (IQR) | 76 (71–82) | 76 (71–82) | 77 (71–83) | 76 (71–82) | 76 (71–82) |
| Male, No.(%) | 27,012 (28) | 6,959 (30) | 6,740 (29) | 6,569 (27) | 6,744 (28) |
| Race/ethnicity, No.(%) | |||||
| Non-Hispanic White | 77,688 (82) | 21,799 (94) | 21,099 (91) | 20,220 (85) | 14,570 (60) |
| Non-Hispanic Black | 11,419 (12) | 644 (3) | 1,214 (5) | 2,472 (10) | 7,089 (29) |
| Non-Hispanic Asian/PI | 1,615 (2) | 292 (1) | 356 (2) | 355 (1) | 612 (3) |
| Hispanic | 2,017 (2) | 86 (0.4) | 196 (1) | 420 (2) | 1,315 (5) |
| Non-Hispanic other | 1,682 (2) | 291 (1) | 349 (2) | 415 (2) | 627 (3) |
| Rurality, No.(%) | |||||
| Metropolitan | 77,852 (82) | 21,160 (91) | 18,972 (82) | 17,176 (72) | 20,544 (85) |
| Non-Metro Adjacent | 10,833 (11) | 1,243 (5) | 2,590 (11) | 4,417 (18) | 2,583 (11) |
| Non-Metro Non-Adjacent | 6,059 (6) | 825 (4) | 1,717 (7) | 2,354 (10) | 1,163 (5) |
| CCI score, No.(%) | |||||
| 0 | 12,377 (13) | 3,902 (17) | 3,346 (14) | 2,958 (12) | 2,171 (9) |
| 1 | 10,542 (11) | 2,958 (13) | 2,744 (12) | 2,661 (11) | 2,179 (9) |
| 2 | 12,979 (14) | 3,552 (15) | 3,376 (14) | 3,218 (13) | 2,833 (12) |
| ≥3 | 58,905 (62) | 12,828 (55) | 13,836 (59) | 15,121 (63) | 17,120 (70) |
| Pre-PHPT symptoms * , No.(%) | |||||
| Nephrolithiasis | 8,306 (9) | 2,015 (9) | 2,049 (9) | 2,152 (9) | 2,090 (9) |
| Hypercalciuria | 2,558 (3) | 615 (3) | 640 (3) | 604 (3) | 699 (3) |
| Nephrocalcinosis | 1,713 (2) | 415 (2) | 409 (2) | 424 (2) | 465 (2) |
| Decreased GFR <60 | 32,094 (34) | 7,537 (32) | 8,495 (35) | 6,969 (30) | 9,093 (37) |
| Osteopenia | 21,826 (23) | 5,557 (24) | 5,364 (22) | 6,060 (26) | 4,845 (20) |
| Osteoporosis without fracture | 31,191 (33) | 7,810 (34) | 7,683 (32) | 7,973 (34) | 7,725 (32) |
| Osteoporosis with fracture | 3,326 (4) | 893 (4) | 828 (3) | 850 (4) | 755 (3) |
| History of pathologic fracture | 267 (0.3) | 82 (0.4) | 51 (0.2) | 87 (0.4) | 47 (0.2) |
| MDD, single episode | 6,466 (7) | 1,554 (7) | 1,694 (7) | 1,497 (6) | 1,721 (7) |
| MDD, recurrent episode | 5,086 (5) | 1,186 (5) | 1,242 (5) | 1,139 (5) | 1,519 (6) |
| MDD, unspecified | 16,728 (18) | 3,995 (17) | 4,512 (19) | 3,614 (16) | 4,607 (19) |
| Adjustment disorder | 3,863 (4) | 963 (4) | 937 (4) | 1,006 (4) | 957 (4) |
| Malaise and fatigue | 45,736 (48) | 10,957 (47) | 11,808 (49) | 10,715 (46) | 12,256 (50) |
| GI manifestations | 33,075 (35) | 7,656 (33) | 8,616 (36) | 7,118 (31) | 9,685 (40) |
Abbreviations: SES, socioeconomic status; IQR, interquartile range; CCI, Charlson comorbidity index; PHPT, primary hyperparathyroidism; GFR, Glomerular Filtration Rate; MDD, major depressive disorder; GI, gastrointestinal; PI, Pacific Islander
Individuals were able to have multiple symptoms, and column percentages may not sum to 100; pre-diagnosis symptoms were captured during the 2-year disease-free interval.
The overall prevalence of parathyroidectomy after diagnosis and onset of symptoms was 9% (N=8,251). Prevalence of parathyroidectomy by race/ethnicity was as follows: 10% for NH White, 6% for NH Black and 1% for Hispanic. With respect to rurality, 11% of rural patients underwent parathyroidectomy compared to 8% of patients living in metropolitan areas. And, 9% of patients living in the highest SES areas underwent parathyroidectomy compared to 7% in the lowest SES areas (Table 2). Among those who underwent surgery, median time from diagnosis and symptom onset to surgery was 1,411 days (IQR 770–2,279, range 9–3,651). Cumulative incidences of parathyroidectomy are plotted by race/ethnicity, rurality and SDI quartile in Figure 2. Prevalence of surgery decreased with patient age, with 14% of patients 67–69 years old undergoing surgery to 1% of those 90 years of age and older (Table 3).
Table 2.
Crude and adjusted associations between primary study variables and parathyroidectomy.
| Parathyroidectomy, No. (%) | HR (95% CI) | aHR (95% CI)* | |
|---|---|---|---|
| Total | 8,251 (8.7) | - | - |
| Race/Ethnicity | |||
| Non-Hispanic White | 7,348 (9.5) | 1.0 (ref) | 1.0 (ref) |
| Non-Hispanic Black | 647 (5.7) | 0.624 (0.576, 0.676) | 0.803 (0.742, 0.868) |
| Non-Hispanic Asian/PI | 53 (3.3) | 0.337 (0.258, 0.442) | 0.413 (0.318, 0.536) |
| Hispanic | 66 (0.8) | 0.345 (0.271, 0.439) | 0.497 (0.392, 0.630) |
| Non-Hispanic Other | 98 (1.2) | 0.615 (0.504, 0.750) | 0.654 (0.431, 0.992) |
| Rurality | |||
| Metropolitan | 6,457 (8.3) | 1.0 (ref) | 1.0 (ref) |
| Non-Metro Adjacent | 1,134 (10.5) | 1.298 (1.219, 1.383) | 1.341 (1.260, 1.427) |
| Non-Metro Non-Adjacent | 654 (10.8) | 1.436 (1.237, 1.453) | 1.363 (1.259, 1.475) |
| Social Deprivation Index | |||
| Highest SES | 2,183 (9.4) | 1.0 (ref) | 1.0 (ref) |
| High SES | 2,183 (9.1) | 0.954 (0.899, 1.012) | 1.044 (0.985 1.106) |
| Medium SES | 2,287 (9.8) | 0.944 (0.890, 1.001) | 1.085 (1.024, 1.150) |
| Low SES | 1,598 (6.6) | 0.677 (0.635, 0.722) | 0.887 (0.831, 0.948) |
Abbreviations: HR, Hazard Ratio; aHR, adjusted Hazard Ratio; CI, Confidence Interval; SES: Socioeconomic Status; PI, Pacific Islander
Adjusted for year of diagnosis, age (treated as a restricted quadratic spline), sex, Charlson comorbidity index, and symptoms; symptoms were treated as time-varying. Among adults symptomatic prior to diagnosis of primary hyperparathyroidism, follow-up time begins at time of initial diagnosis; in patients who become symptomatic after diagnosis, follow-up time begins at the time first symptom is recorded.
Figure 2: Cumulative incidence curves displaying time to parathyroidectomy by a) socioeconomic status; b) race/ethnicity; and c) rural-urban status.
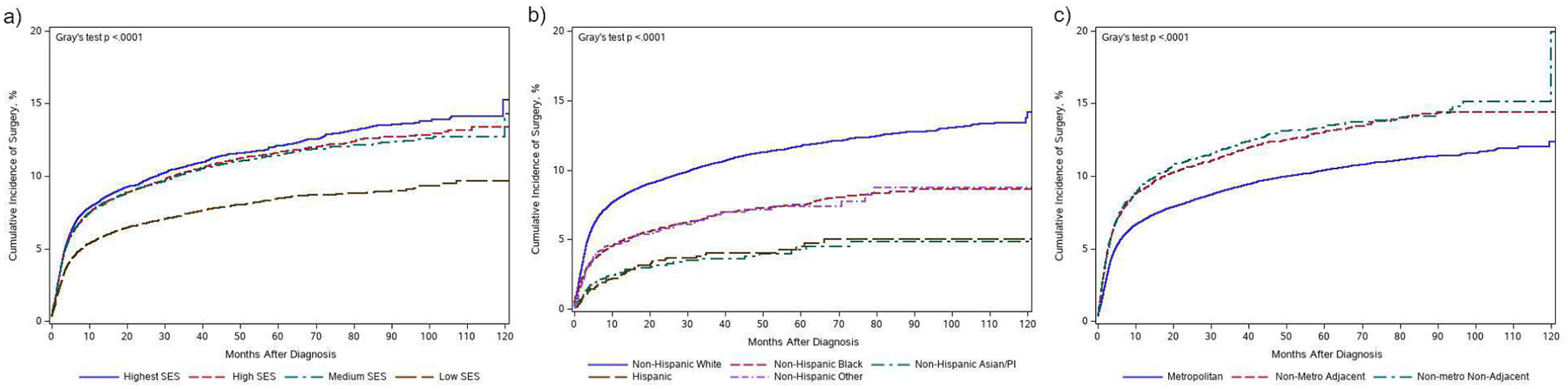
Cumulative incidence curves for receipt of parathyroidectomy by primary study variables. A significant Gray’s test indicates overall significant difference among curves.
Table 3:
Frequency of parathyroidectomy among patients with symptomatic primary hyperparathyroidism in Medicare claims by age, 2006–2017.
| Age, years | Total Diagnosed N, (column %) | Parathyroidectomy N, (row %) |
|---|---|---|
| 67–69 | 15,245 (16.1) | 2098 (13.8) |
| 70–74 | 24,036 (25.4) | 2865 (11.9) |
| 75–79 | 21,303 (22.5) | 1963 (9.2) |
| 80–84 | 17,303 (18.3) | 974 (5.6) |
| 85–89 | 11,222 (11.8) | 310 (2.8) |
| 90+ | 5,694 (6.0) | 41 (0.7) |
| All | 94803 (100) | 8251 (8.7) |
After adjustment, compared to NH White patients, patients from all other measured racial/ethnic backgrounds were significantly less likely to undergo parathyroidectomy, Table 2. Patients from non-metro non-adjacent and non-metro adjacent areas had a higher likelihood of parathyroidectomy compared to patients from metropolitan areas (non-metro non-adjacent aHR 1.50 95% CI 1.32; 1.70 and non-metro adjacent aHR 1.33 95% CI 1.26, 1.40). Compared to patients living in the highest SES neighborhoods, patients living in the lowest SES neighborhoods had a lower likelihood of parathyroidectomy (aHR 0.89 95% CI 0.83, 0.95).
In a post-hoc analysis, we evaluated whether receipt of parathyroidectomy differed by sex. Among 27,012 male patients, 6% (n=1,605) underwent parathyroidectomy. Among 67,791 female patients, 10% (n=6,646) underwent parathyroidectomy. After adjusting for SES, age at diagnosis, CCI score, and symptoms, female sex was still associated with an increased likelihood of parathyroidectomy (aHR 1.18 95% CI 1.11, 1.25).
The sensitivity analysis, where gastrointestinal, neuropsychiatric, and osteopenia diagnoses were excluded from the definition of symptomatic PHPT, did not change our results (Supplemental Table 4). Within 1 year of their first PHPT diagnosis, 59% (n=56,181) of the original cohort had at least one additional PHPT diagnosis. Among these patients, 7,930 (14%) underwent a parathyroidectomy during follow-up. When we restricted the analysis to patients with multiple claims with a primary diagnosis of PHPT, similar disparities were still seen (Supplemental Table 5).
Discussion
Our objective was to characterize use and potential disparities in parathyroidectomy among a nationally representative cohort of universally insured older adults with symptomatic PHPT. We found an overall low rate of parathyroidectomy use, and that patients who are racial and ethnic minorities or were from lower SES areas were less likely to undergo parathyroidectomy. In contrast to our hypothesis, we found that rural patients were more likely to undergo parathyroidectomy.
Although underuse of parathyroidectomy has been previously reported, our work adds to the body of literature by characterizing this in a large, nationally representative cohort of insured older patients who are symptomatic.6,7,12,18 Two recent studies by Seib and colleagues using national claims data, one from a large private insurance claims data and another using Medicare claims, also found a underuse of parathyroidectomy. Their study using Medicare claims, published after initial submission of this manuscript, used very similar methods to ours and found use of parathyroidectomy to be approximately 30%.19,20 Our study found the prevalence was lower, and ranged from 8–14% depending on inclusion criteria. Though our methods were similar, there are some differences that could account for a difference in findings. We used a 20% Medicare sample while Seib et al used 100% of Medicare claims. We included 94,803 subjects (7,900 per year of inclusion). In contrast, Seib et al included 210,206 subjects (19,110 per year of inclusion). Therefore, though Seib et al.’s starting population was 5 times larger, their included subject population was only 2.4 times larger. Therefore, our methods resulted in broader inclusion, and therefore a proportionally larger denominator, thus explaining the lower proportion receiving parathyroidectomy in our study. When we restricted to patients with multiple records with a PHPT diagnosis, the overall estimated prevalence of parathyroidectomy did increase, suggesting some of the cohort may have only been tested for the diagnosis. However, we feel that despite some methodological differences, our findings are similar to Seib and others, and indicate that parathyroidectomy is strikingly underused in this patient population.
The use we found was surprisingly low estimate among a cohort of patients who were symptomatic and had insurance coverage. It is possible that our necessary reliance on diagnostic coding may be capturing patients who have concurrent diseases (e.g. gastrointestinal manifestations) that are not truly related to hyperparathyroidism, thus falsely inflating our denominator.5 Some complications of hyperparathyroidism (e.g. neuropsychiatric manifestations) may not be accurately attributed to hyperparathyroidism and therefore patients are not being offered definitive treatment. Our results were robust to several sensitivity analyses removing less specific diagnosis codes in our definition of symptomatic. It is also likely that using claims to assess symptoms underestimates their prevalence. For example, fatigue is a common symptom of hyperparathyroidism, but it is likely not to be coded as a diagnosis, especially among older adults. These symptomatic patients would therefore not be captured in our analysis unless they had other symptoms documented. Therefore, the true denominator of symptomatic patients could be larger, and the use of parathyroidectomy is even lower than we have found.
Prior work has studied reasons for underuse of parathyroidectomy in patients with PHPT. This disorder is both underdiagnosed and undertreated.11,21,22 Low rates of parathyroidectomy among patients already diagnosed are primarily due to a lack of surgical referral. Some have estimated that only 20% of patients diagnosed with PHPT receive a surgical referral.11,21 This may be due to primary care providers and other non-surgeons underestimating the need for curative treatment and overestimating the risks of parathyroidectomy in elderly patients and patients with comorbidities.23
Among a background of global underuse in our study, there were notable disparities in the use of parathyroidectomy by individual characteristics including race/ethnicity and sex. Prior studies have identified racial disparities in complications, costs, and access to high-volume surgeons, but fewer studies have evaluated racial or sex disparities in access to parathyroidectomy at all.7–10 Our results confirm recent work.7,19,20 And while our findings are not unique, as racial disparities in particular are nearly ubiquitous in healthcare, we add to this literature by characterizing this among other racial/ethnic groups where data are more scarce, by sex, and in a nationally representative cohort.19,20,24,25 As efforts to improve access to parathyroidectomy are undertaken, interventions that incorporate automated decision support tools, standardized care pathways and culturally appropriate patient navigation may improve these disparities.26–28
Both low SES and living in a rural area have previously been shown to affect surgical access and outcomes, and may do so through due to fewer available health resources in these areas as well as limited access to those resources (eg. longer travel distances).29,30 Thus, we believe our results highlight how community level poverty can impact access to treatment for PHPT and that multi-level interventions may be needed to improve access to parathyroidectomy.
Surprisingly, patients from rural areas had a higher likelihood of parathyroidectomy. This is in contrast to the broader rural health literature generally showing worse access to care.31–33 However, selection bias may explain this unexpected finding. If patients from rural areas presented later and with more symptomatic disease, they may be more likely to be referred and undergo parathyroidectomy. Additionally, rural patients with limited access to any health care may be less likely to ever be diagnosed and would thus not be included in our study, thus underestimating the denominator. Rural patients included in our study by definition saw a provider and were diagnosed with PHPT. These rural patients may be more likely to utilize healthcare at baseline. However, these questions deserve further study outside of Medicare claims data.
Our study has several limitations. The main limitation is that by using claims data we do not have access to more granular details such as laboratory data, functional status, and patient preferences, that are more readily available through institutional studies. Additionally, we cannot account for the potential subjectivity of symptomatic PHPT when using claims data. This was a trade-off when broadening the scope of the evaluated population. It is also possible that some symptomatic patients disenrolled from Medicare prior to surgery or had surgery after our follow-up ended in 2017, thus underestimating the number undergoing parathyroidectomy. Finally, we examined an older adult population with universal health insurance and this may limit generalizability to the broader population with PHPT.
Despite prior reports of underuse of parathyroidectomy in PHPT in older adults, our nationally representative study found this may be more severe than previously reported. Additionally, we found significant disparities by race/ethnicity and SES among an insured population. Our study highlights the continued need for quality improvement efforts, rooted in equitable care, to improve access to parathyroidectomy.
Supplementary Material
Acknowledgments:
Funding/Support:
Dr. Herb is supported by a National Research Service Award Pre-Doctoral/Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Grant No. 5T32 HS000032. Dr. Paula Strassle is supported by the Division of Intramural Research, National Institute of Minority Health and Health Disparities, National Institutes of Health. Brooke Staley is supported by a Genetic Epidemiology of Heart, Lung, and Blood (HLB) Traits Training Grant (GenHLB, T32HL129982). The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
The authors have no conflicts of interest to report.
References:
- 1.Lal G, Clark O. Thyroid, Parathyroid, and Adrenal. In: Schwartz’s Principles of Surgery. 11th ed. New York: McGraw-Hill; 2019:1663–1665. [Google Scholar]
- 2.Wilhelm SM, Wang TS, Ruan DT, et al. The American association of endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310 [DOI] [PubMed] [Google Scholar]
- 3.Zanocco KA, Wu JX, Yeh MW. Parathyroidectomy for asymptomatic primary hyperparathyroidism: A revised cost-effectiveness analysis incorporating fracture risk reduction. Surg (United States). 2017;161(1):16–24. doi: 10.1016/j.surg.2016.06.062 [DOI] [PubMed] [Google Scholar]
- 4.Pasieka JL, Parsons L, Jones J. The long-term benefit of parathyroidectomy in primary hyperparathyroidism: A 10-year prospective surgical outcome study. Surgery. 2009;146(6):1006–1013. doi: 10.1016/j.surg.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 5.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: Summary statement from the fourth international workshop. J Clin Endocrinol Metab. 2014;99(10):3561–3569. doi: 10.1210/jc.2014-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu B, Haigh PI, Hwang R, et al. Underutilization of parathyroidectomy in elderly patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2010;95(9):4324–4330. doi: 10.1210/jc.2009-2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallick R, Xie R, Kirklin JK, Chen H, Balentine CJ. Race and Gender Disparities in Access to Parathyroidectomy: A Need to Change Processes for Diagnosis and Referral to Surgeons. Ann Surg Oncol. 2020. doi: 10.1245/s10434-020-08707-z [DOI] [PubMed] [Google Scholar]
- 8.Al-Qurayshi Z, Hauch A, Srivastav S, Kandil E. Ethnic and economic disparities effect on management of hyperparathyroidism. Am J Surg. 2017;213(6):1134–1142. doi: 10.1016/j.amjsurg.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Jang S, Mandabach M, Aburjania Z, Balentine CJ, Chen H. Racial disparities in the cost of surgical care for parathyroidectomy. J Surg Res. 2018;221:216–221. doi: 10.1016/j.jss.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 10.Noureldine SI, Abbas A, Tufano RP, et al. The impact of surgical volume on racial disparity in thyroid and parathyroid surgery. Ann Surg Oncol. 2014;21(8):2733–2739. doi: 10.1245/s10434-014-3610-0 [DOI] [PubMed] [Google Scholar]
- 11.Asban A, Dombrowsky A, Mallick R, et al. Failure to Diagnose and Treat Hyperparathyroidism Among Patients with Hypercalcemia: Opportunities for Intervention at the Patient and Physician Level to Increase Surgical Referral. Oncologist. 2019;24(9):828–834. doi: 10.1634/theoncologist.2018-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh MW, Wiseman JE, Ituarte PHG, et al. Surgery for primary hyperparathyroidism: Are the Consensus Guidelines Being Followed? Parathyr Anatomy, Funct Disord. 2012;255(6):125–149. doi: 10.1097/sla.0b013e31824dad7d [DOI] [PubMed] [Google Scholar]
- 13.General Equivalence Mappings: ICD-9-CM to and from ICD-10-CM and ICD-10-PCS. Centers for Medicare & Medicaid Services Medicare Learning Network. https://www.cms.gov/Medicare/Coding/ICD10/downloads/ICD-10_GEM_fact_sheet.pdf. Published 2009. Accessed May 2, 2021.
- 14.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Heal. 2006;83(2):162–175. doi: 10.1007/s11524-005-9016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rural-Urban Continuum Codes. United States Department of Agriculture Economic Research Service. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/. Published 2019. Accessed November 24, 2020.
- 16.Social Deprivation Index. Robert Graham Center. https://www.graham-center.org/rgc/maps-data-tools/sdi/social-deprivation-index.html.
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 18.Kuo EJ, Al-Alusi MA, Du L, et al. Surgery for Primary Hyperparathyroidism: Adherence to Consensus Guidelines in an Academic Health System. Ann Surg. 2019;269(1):158–162. doi: 10.1097/SLA.0000000000002474 [DOI] [PubMed] [Google Scholar]
- 19.Seib CD, Meng T, Suh I, et al. Undertreatment of primary hyperparathyroidism in a privately insured US population: Decreasing utilization of parathyroidectomy despite expanding surgical guidelines. Surg (United States). 2021;169(1):87–93. doi: 10.1016/j.surg.2020.04.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seib CD, Suh I, Meng T, et al. Patient Factors Associated with Parathyroidectomy in Older Adults with Primary Hyperparathyroidism. JAMA Surg. 2021;94305:1–8. doi: 10.1001/jamasurg.2020.6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balentine, Xie Courtney J., Rongbing. Kirklin James K. Chen H. Failure to Diagnose Hyperparathyroidism in 10,432 Patients with Hypercalcemia: Opportunities for System-Level Intervention to Increase Surgical Referrals and Cure. Ann Surg. 2017;266(4):632–640. doi: 10.1097/SLA.0000000000002370.Failure [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sippel RS. Under-recognition of the benefits of parathyroidectomy leads to underdiagnosis of the disease. Surg (United States). 2013;154(6):1230–1231. doi: 10.1016/j.surg.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 23.Dombrowsky A, Borg B, Xie R, Kirklin JK, Chen H, Balentine CJ. Why Is Hyperparathyroidism Underdiagnosed and Undertreated in Older Adults? Clin Med Insights Endocrinol Diabetes. 2018;11. doi: 10.1177/1179551418815916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haider A, Scott V, Rehman K, et al. Racial Disparities in Surgical Care and Outcomes in The United States: A Comprehensive Review of Patient, Provider and Systemic Factors. J Am Coll Surg. 2013;216(3):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurie N, Dubowitz T. Health disparities and access to health. JAMA. 2007;297(10):1118–1121. doi: 10.1097/01.ogx.0000269083.12366.d8 [DOI] [PubMed] [Google Scholar]
- 26.Lau BD, Haider AH, Streiff MB, et al. Eliminating Health Care Disparities With Mandatory Clinical Decision Support. Med Care. 2015;53(1):18–24. doi: 10.1097/mlr.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cykert S, Eng E, Walker P, et al. A system-based intervention to reduce Black-White disparities in the treatment of early stage lung cancer: A pragmatic trial at five cancer centers. Cancer Med. 2019;8(3):1095–1102. doi: 10.1002/cam4.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavender MA, Rassi AN, Fonarow GC, et al. Relationship of race/ethnicity with door-to-balloon time and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: Findings from get with the guidelines-coronary artery disease. Clin Cardiol. 2013;36(12):749–756. doi: 10.1002/clc.22213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehaffey JH, Hawkins RB, Charles EJ, et al. Community level socioeconomic status association with surgical outcomes and resource utilisation in a regional cohort : a prospective registry analysis. Br Med J Qual Saf. 2020;29:232–237. doi: 10.1136/bmjqs-2019-009800 [DOI] [PubMed] [Google Scholar]
- 30.Meilleur A, Subramanian S, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural Residence and Cancer Outcomes in the US: Issues and Challenges. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1657–1667. doi: 10.1158/1055-9965.EPI-13-0404.Rural [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolin JN, Bellamy GR, Ferdinand AO, et al. Rural Healthy People 2020: New Decade, Same Challenges. J Rural Heal. 2015;31(3):326–333. doi: 10.1111/jrh.12116 [DOI] [PubMed] [Google Scholar]
- 32.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Heal. 2006;22(2):140–146. doi: 10.1111/j.1748-0361.2006.00022.x [DOI] [PubMed] [Google Scholar]
- 33.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909–918. doi: 10.1002/cncr.23229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


