Abstract
While humans exhibit a significant degree of neuropathological changes associated with deficits in cognitive and memory functions during aging, nonhuman primates present with more variable expressions of pathological alterations among individuals and species. As such, nonhuman primates with long life expectancy in captivity offer an opportunity to study brain senescence in the absence of the typical cellular pathology caused by age-related neurodegenerative illnesses commonly seen in humans. Age-related changes at the neuronal population, single cell, and synaptic levels have been well documented in macaques and marmosets, while age-related and Alzheimer’s disease-like neuropathology has been characterized in additional species including lemurs as well as great apes. We present a comparative overview of existing neuropathologic observations across the primate order, including classic age-related changes such as cell loss, amyloid deposition, amyloid angiopathy, and tau accumulation. We also review existing cellular and ultrastructural data on neuronal changes, such as dendritic attrition and spine alterations, synaptic loss and pathology, and axonal and myelin pathology, and discuss their repercussions on cellular and systems function and cognition.
Keywords: Brain senescence, nonhuman primates, neuron morphology, proteinopathy, glia
1. INTRODUCTION
Humans exhibit a significant degree of neuropathological changes associated with the cognitive and memory deficits during aging. Some of these cellular and pathological alterations vary among individuals and species of nonhuman primates (NHP) (Hof, Gilissen, et al., 2002; Rapp & Amaral, 1992). NHP experience some cognitive dysfunction with increasing age and, similar to aging humans, they have deficits in executive function, working memory and recognition memory (Moss et al., 1988; Rapp & Amaral, 1989, 1992; Voytko, 1998). Along with the fact that many NHP species have long life expectancy in captivity, primates offer a unique opportunity to study brain senescence.
Here we summarize evidence from lemurs, ceboids, cercopithecids and hominids. For some of the genera included in this work, the data reviewed come from only a limited number of species that are most accessible to laboratory investigation. These include lemurs (mouse lemurs, Microcebus murinus) and ceboids (mainly common marmosets, Callithrix jacchus; also squirrel monkeys, Saimiri spp., and cotton-top tamarins, Saguinus oedipus). In cercopithecids, age-related and disease-like alterations are well documented in macaques, particularly the rhesus macaque (Macaca mulatta) and to a lesser degree from the long-tailed macaque (Macaca fascicularis). In this group, we also include studies of Patas monkeys (Erythrocebus patas), baboons (Papio spp.), vervets (Chlorocebus spp.), and guenons (Cercopithecus campbelli). The review concludes with studies in hominids, which comprise chimpanzees (Pan troglodytes), bonobos (Pan paniscus), gorillas (Gorilla gorilla and Gorilla beringei), and orangutans (Pongo pygmaeus and Pongo abelii), as well as studies using postmortem human brain with the aim of highlighting certain parallels with the NHP.
For the purpose of this review, we summarize cell type-specific and age-related changes to neuronal populations, dendritic arborization, spine morphology, synaptic loss, and axonal, myelin and glial pathology at the structural and ultrastructural level in NHP species. Here, we focus on Alzheimer’s disease (AD)-like pathologies in NHP that may contribute to cognitive decline during aging. AD is a progressive, irreversible brain disorder characterized pathologically by amyloid β protein (Aβ) plaques and angiopathy, neurofibrillary tangles (NFT), extensive neocortical and hippocampal neuronal loss, and neuroinflammation, accompanied by severe cognitive impairment (Serrano-Pozo et al., 2011). We present a comparative overview of existing neuropathologic observations across primate species that resemble some of the pathological hallmarks of AD such as cellular loss, amyloid deposition, amyloid angiopathy, and tau accumulation. For both structural and pathological age-related changes, we mainly reviewed data derived from the dorsolateral prefrontal cortex (dlPFC, Brodmann areas 8–10 and 46), entorhinal cortex, and hippocampal complex (CA subfields 1–4, dentate gyrus, and subiculum) for rhesus macaques and humans, but also include relevant information found in other brain areas for those NHP species where less information is available.
2. LEMURS
2.1. Structural changes and altered cell morphology in the aging brain
Grey mouse lemurs
Significant degeneration of axonal terminals in neocortical layers III and IV and of pyramidal neurons in neocortical layer III has been observed in the brain of old grey mouse lemurs (8 to 11 years of age, n = 5 out of 10), coinciding with general atrophy of the brain compared to young animals (2–3 years, n = 15). Affected pyramidal neurons and apical dendrites showed argyrophilic filamentous inclusions, as well as dendritic degeneration, resulting in neuritic debris (Bons et al., 1992). Unfortunately, population-level analysis of neurons or quantitative single-cell analysis of changes to dendrites, spines or synapses are unavailable in this species.
Astrogliosis was also assessed in grey mouse lemurs (> 7 years old, n = 4) showing brain atrophy-associated increase in the intensity of glial fibrillary acidic protein (GFAP)-expressing cells in the cerebral cortex, corpus callosum, and hippocampus (Kraska et al., 2011).
2.2. Neuropathological changes in the aging brain
Grey mouse lemurs
Neuropathological examination of brains from grey mouse lemurs by silver staining revealed few amyloid-like deposits in neocortical vasculature but more frequent neuritic amyloid-like plaques, mostly in layer IV of the parietal cortex and occipital cortex of old individuals (8–11 years old, n = 2 of 10) compared to younger animals (2–3 years of age) (Bons et al., 1992). Amyloid antibody immunocytochemistry confirmed Aβ40 peptide deposits in old animals (8–12 years of age, n = 8), with amyloid accumulations found mostly in the cerebral vasculature and cortical neuropil (Bons et al., 1994; Kraska et al., 2011). Extracellular diffuse Aβ plaques, but few mature and neuritic plaques, were present in the neocortex and even less frequently in the hippocampus (Giannakopoulos, Silhol, et al., 1997). Intracellular Aβ has also been detected in the neocortex and hippocampus of grey mouse lemurs past 7 years of age (Kraska et al., 2011). In a different study, cell bodies and proximal dendrites of pyramidal neurons in layer III neocortex and axons in the stratum radiatum of CA3/4 of the hippocampus, as well as glial cells and microvessel walls in the brains of grey mouse lemurs over 8 years of age, were strongly immunoreactive using antibodies against the amyloid precursor protein (APP) (Silhol et al., 1996). Ex vivo magnetic resonance microscopy revealed dark spots that increased with age in areas of the cortex and hippocampus between young (1–4 years) and old (6–10 years) grey mouse lemurs, suggesting the presence of Aβ plaques or microhemorrhages (Bertrand et al., 2013).
Normal tau (detected by antibody MV4S4 raised against normal human tau) was present throughout the cortex at most ages (1–11 years of age) in the grey mouse lemur (Bons et al., 1995). However, using antibodies to detect normal and aggregated tau (anti-S400/T429 tau) and phosphorylated tau in paired helical filaments (PHF), aggregated and phosphorylated tau were present in 1–4 year old grey mouse lemurs but more prevalent in those older than 5 years; tau increased as they aged to 13 years and occurred mainly in the soma and dendrites of pyramidal neurons and neurites in frontal cortex, entorhinal cortex, subiculum and amygdala, but not in the hippocampus CA subfields (Bons et al., 1995; Giannakopoulos, Hof, et al., 1997). Of note, the earlier age of onset of tau deposits, consistent prevalence and high density in layers IV, V and VI of the frontal, parietal and temporal cortices, and in layers III, IV and V of the occipital cortex differed from amyloid deposits seen in this species (Giannakopoulos, Hof, et al., 1997). However, a qualitative study using a different tau antibody (against p-S202) reported neuronal accumulation of phosphorylated tau in the hippocampus but not in neocortical regions in old grey mouse lemurs (> 6 years old, n = 3 of 5) (Kraska et al., 2011). Further assessment would be required to confirm whether age-related phosphorylation of tau differs depending on tau epitope or brain area in the grey mouse lemur.
3. CEBOIDS
3.1. Structural changes and altered cell morphology in the aging brain
Common marmosets
No quantitative studies exist of age-related neuronal population changes in the neocortex and hippocampus in the common marmoset, except for altered hippocampal neurogenesis. Neurogenesis in the hippocampus declined from young (1.5–3 years old, n = 8) to middle-aged (3.5–7 years old, n = 9) marmosets, with fewer new cells in the subgranular zone and the subventricular zone, but not in the hilar region of the dentate gyrus (DG) of the middle-aged group (Leuner et al., 2007). Moreover, the decline of neurogenesis in the subgranular zone continued in old common marmosets (8–15 years old, n = 3) and was associated with a decrease in neural progenitor cells (Bunk et al., 2011).
Age-related studies of neuronal morphology in the common marmoset have mainly focused on axonal changes. Pyramidal neurons of layers II and IIIa in areas 10 and 14C showed a shortening of the axon initial segment, the site of action potential initiation, in 12–14 year old marmosets (n = 4) compared to 2–3 year old animals (n = 7). Degeneration of white matter structures was also observed. Myelin thickness and density were reduced in the corpus callosum of old (15–20 years old, n = 4) marmosets compared to young ones (4 years old, n = 2). Moreover, lower myelin fraction of axonal fibers was correlated with the variability in performance of the older animals in a detour reaching task (Phillips et al., 2019). There is currently no evidence describing changes to dendrites, dendritic spines or synapses during aging in marmosets. Some efforts have been directed towards the study of dendrite growth, spinogenesis and normal development of synapses and spines in young and adult marmosets (Ichinohe, 2015; Sasaki et al., 2015). Profiles of dendrite and spine formation and pruning differ across areas of the PFC in NHP. In this regard, basal dendrites of layer III pyramidal neurons in areas 8b/9 and 14r increased in length from birth to 2 months (n = 1 each) and then decreased to a stable length by 6 months of age (n = 2), whereas neurons in area 24 reached a stable length by 2 months of age which was maintained to 2.5 years of age (n = 1). The frontal cortex (area 8b/9) showed the greatest spine loss (43%) from a peak at 2–3 months of age compared to areas 24 (33%) and 14r (29% spine loss) (Sasaki et al., 2015). Preliminary results from our ongoing longitudinal study of old male and female marmosets (7–9 years) show differences between cognitively impaired and non-impaired animals in dendritic spine vulnerability during aging. For example, behaviorally impaired marmosets displayed a decrease in total spine density in layer III of dlPFC (area 8b/9) similar to that seen in macaques (Dumitriu et al., 2010; Luebke et al., 2010; Motley et al., 2018). These preliminary studies form the foundation for more detailed quantitative examination of neuronal populations, dendritic morphology and spine density in larger cohorts of marmosets across age and cognitive status.
3.2. Neuropathological changes in the aging brain
Common marmosets
In the common marmoset, Aβ plaques have been reported in some animals past middle-age (>7 years of age) and more consistently in the very old (>15 years of age). These animals displayed different amounts of Aβ42 deposits in neocortical regions, including the sensorimotor, association, and paralimbic cortices, but not in the hippocampal formation (Geula et al., 2002; Rodriguez-Callejas et al., 2016). While accumulation of Aβ42 was detected in the form of diffuse and compact plaques, Aβ40 diffuse aggregates were found mainly associated with blood vessels (Geula et al., 2002; Rodriguez-Callejas et al., 2016). Aβ deposition was most frequently found in the association cortex, and particularly in occipital cortex. Amyloid angiopathy was seen in cortical and meningeal vessels, particularly in abundance within the calcarine sulcus. Moreover, Aβ deposits showed acetylcholinesterase or butyrylcholinesterase activities, apolipoprotein E (ApoE) and α1-antichymotrypsin immunoreactivity and APP staining in a pattern resembling neuritic plaques (Geula et al., 2002). Our preliminary results in old marmosets (7–9 years of age) also show different degrees of Aβ deposition in plaques and in cerebral vasculature in layer III of dlPFC (Fig.1) and hippocampal CA1 (Rothwell et al., this issue). Marmosets with greater amyloid burden seem to show higher dendritic spine loss in the dlPFC and CA1, suggesting an accelerated aging process accompanied by neurodegenerative changes. Whether the degree of amyloid burden associates with the variable trajectories of cognitive decline in old marmosets requires further study.
Figure 1. Amyloid burden in the marmoset brain.
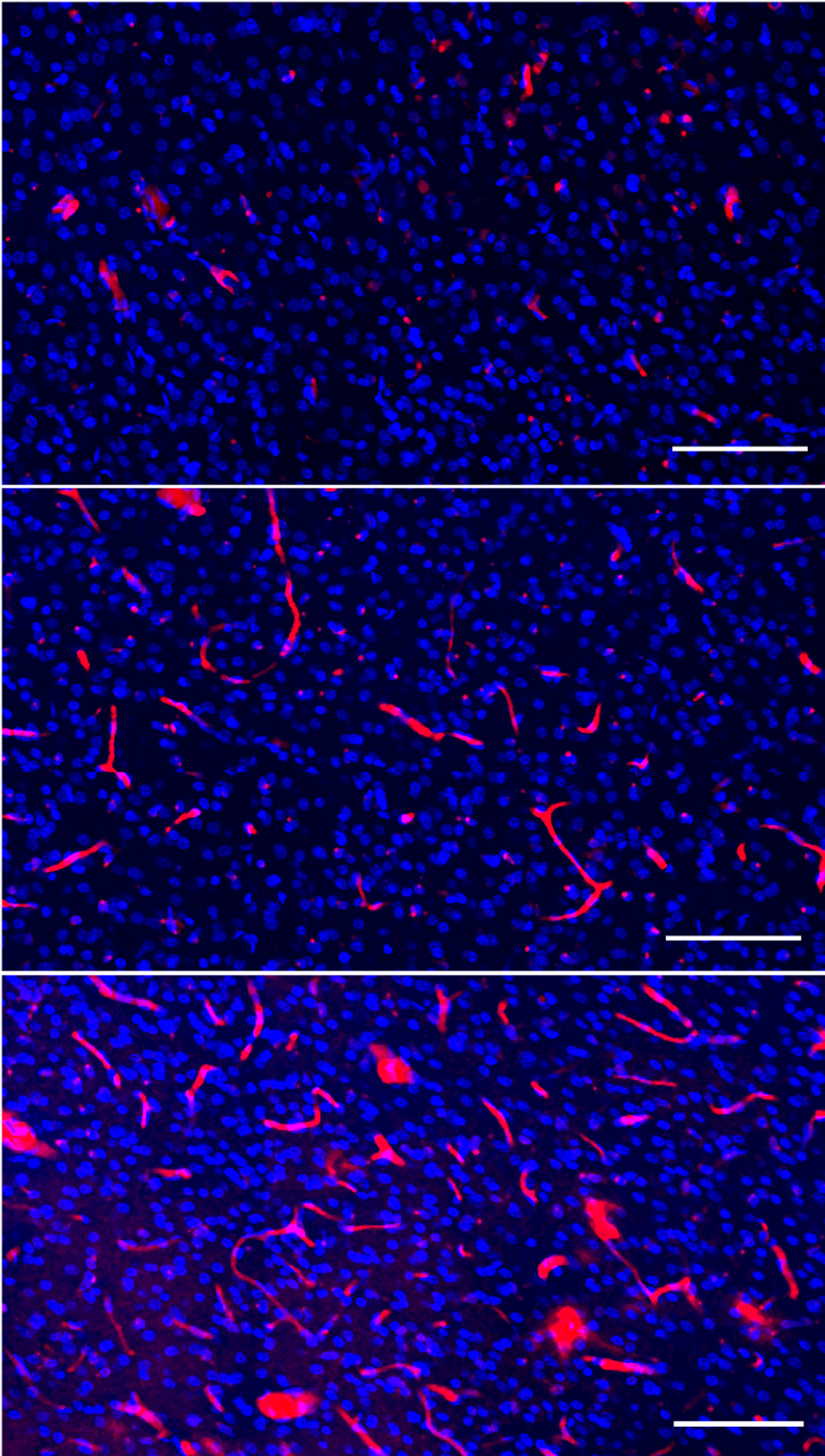
Confocal image stacks showing amyloid β (red) and 4′,6-diamidino-2-phenylindole (DAPI, blue) a nuclear marker. Different degrees of amyloid deposits are observed in 7–9 year old marmosets in dorsolateral prefrontal cortex (dlPFC, area 8b/9, layer III). Both amyloid deposited in diffuse plaques and vascular deposits are detected in behaviorally characterized marmosets, suggesting different neurodegenerative trajectories between individuals. Scale bars = 100 μm.
To investigate the mechanisms involved in the development of cerebral amyloidosis in marmosets, intracerebral injection of brain homogenates from AD patients or Aβ peptides were placed into the caudate nucleus, nucleus accumbens, hippocampus, amygdala and parietal cortex (Maclean et al., 2000; Philippens et al., 2017; Ridley et al., 2006). The experimentally induced amyloidosis was similar to naturally occurring Aβ deposition. Small and large vessels with cerebral amyloid angiopathy (CAA) and some plaques were present throughout the neocortex of injected marmosets, 1–8 years following injection (7–13 years old, n = 7), with no evidence of astrocytic hyperplasia or inflammation. Aβ40 deposits were found in large vessels, especially in the calcarine sulcus, the lateral sulcus and on the surface of the cingulate cortex. Small vessel CAA was found in the superficial layers of the neocortex (Maclean et al., 2000). Diffuse and mature, neuritic plaques, mainly containing Aβ42, were found in the cingulate cortex, the parietal and temporal banks of the lateral sulcus, the temporal cortex and occasionally in the hippocampus, but absent in subcortical areas and the cerebellum when the marmosets were examined from <1 year to >9 years after the injections (6–16 years, n = 27) (Ridley et al., 2006). Injection of fibrillar Aβ triggered tau phosphorylation (p-S262 and p-S396/S404) in neocortical neurons of old (8–10 years, n = 5) but not young marmosets (2–3 years, n = 5) 11–12 days post-injection (Geula et al., 1998). Triggering neuroinflammation along with Aβ seeding resulted in both diffuse and senile plaques in middle-aged (5–8 year old, n = 4) and old (13–14 year old, n = 2) marmosets injected with human Aβ together with lipopolysaccharide in the frontal, sensorimotor and parietal cortices (Philippens et al., 2017). However, findings derived from intracerebral cortical injections need to be interpreted with caution, given the variability in the incubation periods of the amyloid injections and the overlap between the ages at which amyloid deposits occur as a natural consequence of aging in marmosets.
Geula and colleagues did not find abnormally phosphorylated tau (p-S396/S404 tau and p-S262 tau) deposits in old marmosets (> 7 years old, n = 15) where immunoreactivity was detected for Aβ (Geula et al., 2002). However, another study detected low levels of abnormally hyperphosphorylated tau (p-T231 tau and p-T212/S214 tau) and aggregated tau (using antibody Alz-50) in the cytoplasm of neurons starting in adolescence and young adulthood (1.6–5 years, n = 4). These tau markers increased in intensity and abundance in the medial temporal region (hippocampal CA3 field, entorhinal, and inferior temporal cortex) and parietal cortex, in animals past reproductive senescence (> 8 years old, n = 7). The oldest animals (mean age 18 years) showed phosphorylated tau in soma, dendrites and as nuclear inclusions in neurons and aggregated tau as fibrillary inclusions in both neurons and glia. In the DG, glial tau was localized to dystrophic microglia, but not active microglia (Rodriguez-Callejas et al., 2016). More recently, a biochemical study addressed possible reasons for the variability in tau detection, by investigating the expression of different tau isoforms and their phosphorylation status in the marmoset brain (Sharma et al., 2019). These authors reported that while marmoset tau conserves the phosphorylation sites seen in humans, it lacks 10 amino acids in the N-terminal motif unique to primates. Interestingly, adult marmosets (23 months) express only three-repeat tau and 0N or 2N isoforms similar to adult mice and rats, compared to humans where adults express both three- and four-repeat tau and 1N isoform. Western blotting using anti- phospho tau AD epitopes generated by double phosphorylation at S202 and T205 (AT8), at T231 and S235 (AT180), and at S396 and S404 (PHF-1) were used to examine whether both of these sites were phosphorylated in newborn or adult marmoset brain (Sharma et al., 2019). In this study, all three double phosphorylated epitopes were found in newborn marmosets (postnatal day 0, n = 3), with p-S396/p-S404 tau levels being the most robust. In adult marmosets (1.9–5.8 years old, n = 3), only p-S396/p-S404 tau was weakly detected. When adult brains were probed with antibodies to detect single phosphorylation epitopes, p-T205 site was not detected, but strong levels of p-S202 and p-S404 sites were revealed as were lower levels of p-T231, p-S235 and p-S396 (Sharma et al., 2019). However, it remains to be established whether tau phosphorylation patterns change with further aging in marmosets resulting in pathological tau aggregation.
The modulation of neuroinflammation by glial cells may also contribute to aging in the marmoset brain. The number of microglia was comparable across ages in marmosets (mean 1.6 to 18 years) (Rodriguez-Callejas et al., 2016). However, the state of microglial activation has been related to their ferritin content in old marmosets. Ferritin, a protein with ferroxidase activity, is capable of storing iron that accumulates in brain during aging. The number of ferritin-containing activated and dystrophic microglia in CA1 and CA2 was higher in middle-aged (mean age 5 years, n = 2) and old marmosets (mean age 11 years, n = 5) compared to adolescents (mean age 2 years, n = 2). However, very old marmosets (mean age 18 years, n = 2) had fewer ferritin-containing activated microglia in the hippocampus and entorhinal cortex than in the old (mean age 11 years), suggesting that protection against oxidative stress damage conferred by ferritin in microglia of adult and old marmosets may be lost in the very old. Conversely, the decrease of ferritin in very old marmosets led to the accumulation of iron in brain tissue, and this may be related to the increase of oxidized RNA in neurons and astrocytes. The same study also showed increased number of and larger astrocytes in the hippocampus of old and very old marmosets compared to adolescent and adult marmosets (Rodríguez-Callejas et al., 2019). We also observed variable levels of microglial activation and altered morphology in old marmosets (7–9 years; Rothwell et al., current issue) and whether these quantitatively correspond with the variability in cognitive decline observed in these animals is under investigation.
Cotton-top tamarins
An immunohistochemical analysis showed that Aβ deposition and associated pathogenesis occur in old (12.4–20.9 years old, n = 20) but not young (6.3 to 11.3 years old, n = 16) cotton-top tamarins. Diffuse and compact plaques were observed in frontal, temporal and occipital cortices in cotton-top tamarins from 12 years of age, but hippocampal plaques were only found over 20 years of age. Both Aβ plaque and vascular deposits occurred initially in frontal and temporal cortices. Vascular Aβ was much more abundant in occipital cortex. In all these brain areas, Aβ42 preceded Aβ40 deposition. Aβ42 was present in both plaques and in the vasculature but Aβ40 was mostly vascular. Moreover, the dense-core plaques were associated with reactive astrocytes, activated microglia, ApoE, and ubiquitin-positive dystrophic neurites, but APP- and tau-immunopositive neuritic plaques were absent (Lemere et al., 2008). Tau hyperphosphorylation (p-S202/205) was absent in tamarins and neither neuritic nor NFT tau was observed in cotton-top tamarins (Lemere et al., 2008).
Squirrel monkeys
A few reports describe the development of significant amyloidosis in the natural course of aging in squirrel monkeys. Amyloid deposits were mostly cerebrovascular and meningeal with fewer parenchymal Aβ deposits consisting of dense-cored and diffuse Aβ in the neocortex, and rarely in the hippocampus, in animals between 23 to 27 years of age (n = 6) but not in 8 year olds (n = 3). Some of the dense plaques were associated with neurites and astrocytes (Walker et al., 1990). Although Aβ-laden vessels were more prevalent in squirrel monkeys older than 15 years (n = 14), certain brain regions, such as the neostriatum showed a relatively higher proportion of plaque-like deposits. A systematic sampling of the entire rostro-caudal extent of the brain confirmed that CAA lesions were denser rostrally than caudally, present more in capillaries, and in severe cases, CAA was associated with other vasculopathies, including microhemorrhages, fibrinoid extravasation, and focal gliosis, more astrocytic than microglial (Elfenbein et al., 2007). Analysis of the composition of the Aβ protein species in CAA identified not only Aβ40 as but also Aβ42 as the main component in amyloid deposits (Sawamura et al., 1997). These two species coexist in most microvascular and parenchymal lesions, but larger arterioles have more Aβ40 (Elfenbein et al., 2007). Squirrel monkeys are homozygous for ApoE ε4 but due to an amino acid substitution, the protein functions like human ApoE ε3 (Morelli et al., 1996). ApoE was detected in amyloid plaques in squirrel monkeys (Ndung’u et al., 2012).
In contrast to Aβ deposition, neurons and plaque-associated neurites with tau hyperphosphorylation (p-S202 tau, p-S396/40 tau and p-S202/205 tau) or aggregated tau (Alz50) were rarely found in the neocortex and NFTs were absent in old squirrel monkeys (14–23 years, n = 11) (Elfenbein et al., 2007; Rosen et al., 2016).
4. CERCOPITHECIDS
4.1. Structural changes and altered cell morphology in the aging brain
Rhesus macaques
Studies of rhesus macaques across the lifespan have demonstrated that within the dlPFC, area 46 is especially vulnerable to age-related cellular changes associated with cognitive decline. This vulnerability did not involve cell loss, as neuron counts and sizes in the PFC were comparable among young (5–6 years, n = 3), middle-aged (12 years, n = 1) and old macaques (25–32 years, n = 5) (Peters et al., 1994). Stable neuron counts in area 46 have been confirmed in much older macaques (22–44 years, n = 18) (Stonebarger et al., 2020). Like in the PFC, neurons in the hippocampus were also preserved when young (0–4 years, n = 8) and old macaques (18–31 years, n = 5) were compared (Keuker et al., 2003). However, a recent study reported a modest increase of granule neurons and volume specifically in the DG, attributed to neurogenesis in this area, when including more subjects over a wider age range (6.1–31.5 years, n = 18) (Ngwenya et al., 2015). Aging in rhesus monkeys was not associated with loss of neurons in entorhinal cortex layers II, III, and V/VI; neuronal area and volume were also conserved in old (20.4–30 years, n = 10) compared to young macaques (8–15.3 years, n = 12) (Gazzaley et al., 1997; Merrill et al., 2000). Although total neuron counts are preserved, fewer reelin-expressing neurons were found in layer II of the entorhinal cortex in old macaques with memory deficits (27–38 years, n = 7) compared to cognitively intact old (27–38 years, n = 7) or to young adults (8–10 years, n = 7), suggesting that specific neuronal classes may be selectively vulnerable to cognitive aging (Long et al., 2020). Moreover, though learning performance correlated with the level of neurogenesis in the hippocampal DG, not all age-related impairment in cognitive performance could be explained by an age-related decline in adult neurogenesis (5.3 ± 0.8 to 29.6 years, n = 18) (Aizawa et al., 2009; Ngwenya et al., 2015).
Despite no neuron loss observed in aging, irregularities in dendritic arborization, altered distribution, number or morphology of spines, loss of synapses or axons may explain the electrophysiological changes in the macaque underlying the detrimental effects of aging on cognitive function. Swollen layer I dendrites, regression of dendritic trees, loss of dendritic spines and synapses (Fig. 2), and alterations in neurotransmitters and receptors in pyramidal neurons in area 46 have been described in old macaques (Luebke et al., 2010; Peters et al., 1994). Old macaques (24–25 years, n = 5) showed a reduction in length and complexity of both apical and basal dendrites of temporal cortical neurons projecting to area 46, compared to young adults (10–12 years, including both rhesus and long-tailed macaques, n = 6) (Duan et al., 2003). DG neurons of old rhesus macaques (24–30.2 years, n = 9) had comparable total length and complexity but showed decreased vertical extent, corresponding to decreased width of the DG molecular layer, compared to young macaques (5.1–10.8 years, n = 11) (Luebke & Rosene, 2003). Length and complexity were increased in the proximal one-third and decreased in the distal one-third of DG dendritic tree, suggesting alterations in specific inputs to the molecular layer with age.
Figure 2. Dendritic spine density and morphology in rhesus macaques.
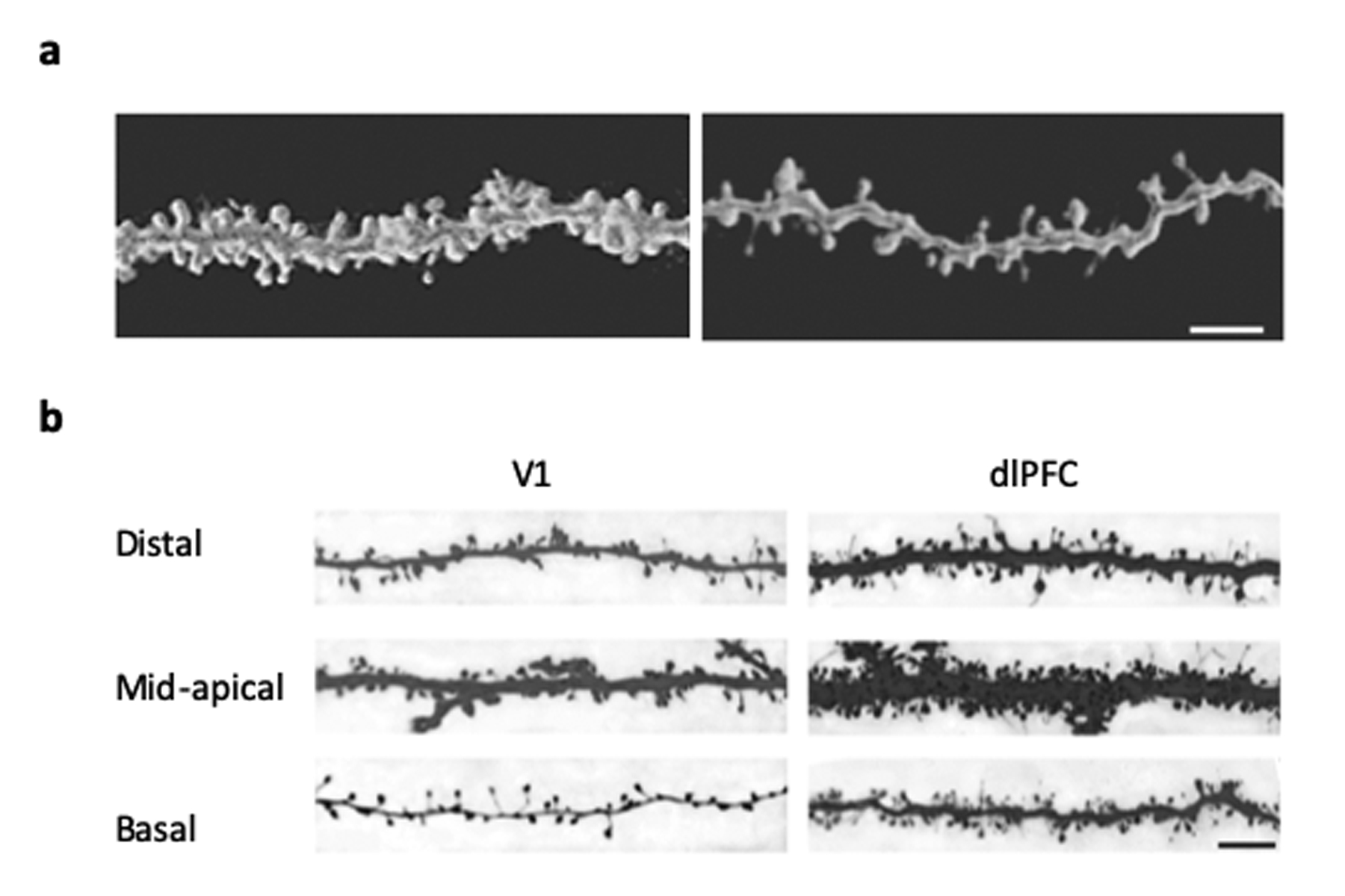
(A) Confocal image stacks from young (8 years old) (left) vs old (24 years old) (right) macaques show the spine loss associated with aging in dorsolateral prefrontal cortex (dlPFC). Scale bar = 5 μm. (Luebke et al., 2010). (B) Confocal image stacks of distal apical, mid-apical and basal dendritic branches (b/w inverted) showing the fewer dendritic spines and lower proportion of thin spines in visual cortex (V1) when compared to dorsolateral prefrontal cortex (dlPFC) neurons. Scale bar = 5 μm. (Amatrudo et al., 2012)
Furthermore, age-related spine loss (estimated at 43%), occurs mainly on proximal branches of apical dendrites, whereas spine loss in basal dendrites (estimated at 27%) occurs primarily on distal branches (Duan et al., 2003). More recent findings have described a selective decrease with age in spine density and increase of head volume of thin spines but not mushroom spines in area 46 (young 9–14 years, n = 12; old 22–35 years, n = 14) (Dumitriu et al., 2010; Young et al., 2014) and in area 7a of the parietal cortex (young 6–13.5 years, n = 6; old 21–34.7 years, n = 12), which is also active during working memory tasks in humans and NHP (Motley et al., 2018).
Complementary electron microscopy (EM) studies have confirmed that dlPFC neurons undergo a marked loss of synapses, during aging (5–35 years, n = 32), mostly in layers II, III and layer V of area 46 (Dumitriu et al., 2010; Peters et al., 2008). Not all synapses are equally vulnerable, with glutamatergic axospinous synapses being the most affected by aging. The synapse loss in layer III correlates with the degree of cognitive impairment with aging. Selective loss of axospinous synapses has also been reported in the DG of macaques with aging (4–35 years, n = 10) (Tigges et al., 1995).
In addition to synapse loss, an age-related disconnection of dlPFC from other cortical regions is supported by extensive loss of myelinated fibers and increase of degenerating myelin sheaths in area 46 (5–32 years, n = 9) (Fig.3) and related white matter tracts (4–32 years, n = 21) of macaques with aging (Bowley et al., 2010; Peters, 2009; Peters et al., 1994). In the hippocampus, old macaques (28.2 ± 1.0 years, n = 8) had shorter cholinergic fibers compared to young adults (8.8 ± 0.5 years, n = 6) (Calhoun et al., 2004).
Figure 3. White matter atrophy in rhesus macaques.
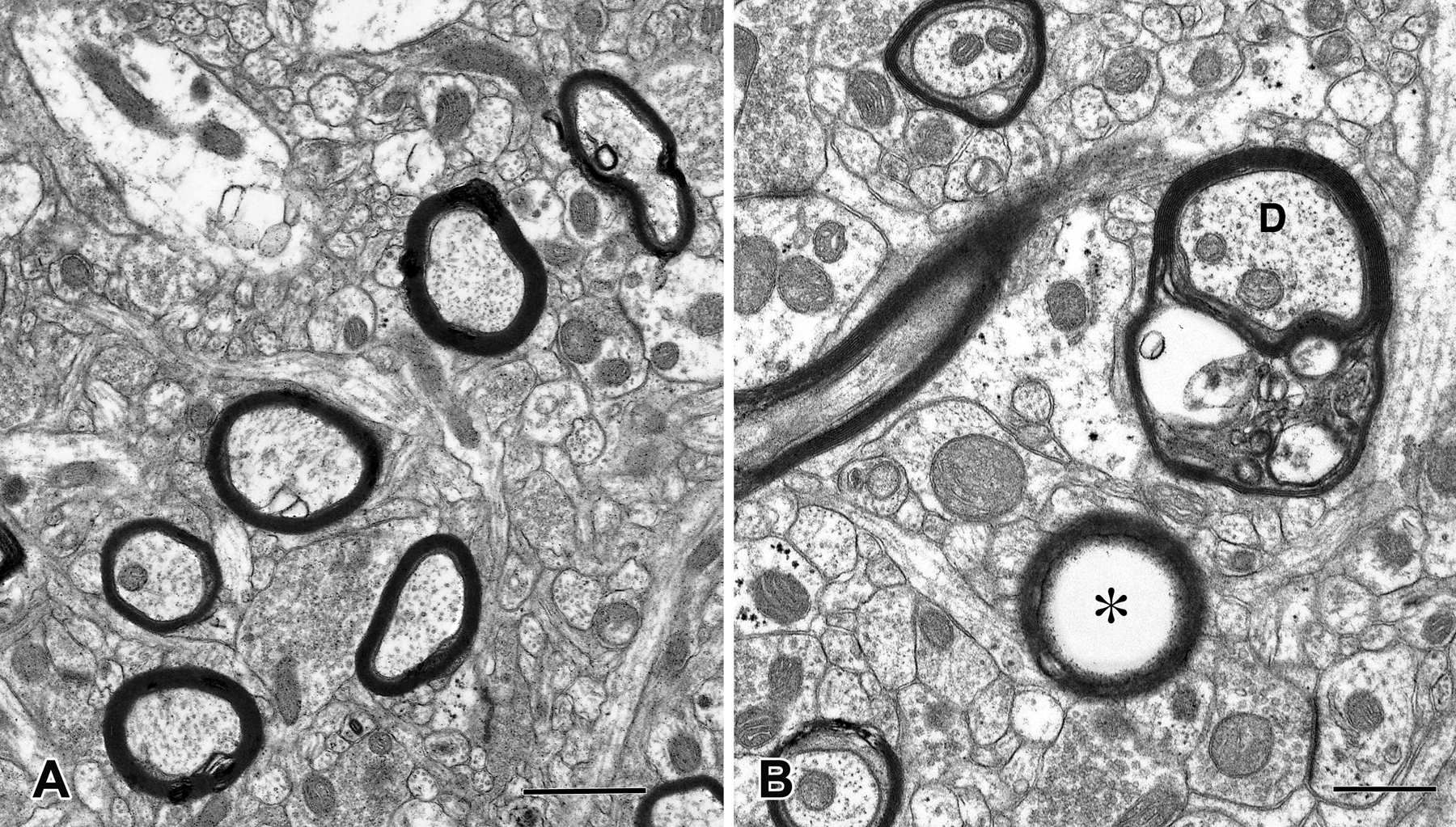
Electron micrographs showing myelinated nerve fibers in area 46. (A) In a young macaque (10 years of age) myelin sheaths around axons are compact, whereas (B) in an old individual (27 years of age) many of the myelin sheaths (labelled with a D in the picture) have begun to degenerate and show dense inclusions within their lamellae. Other sheaths (labelled with an asterisk in the picture) are empty because the axon has degenerated. Scale bar = 1 μm. (Luebke et al., 2010)
The key role of structural alterations of area 46 in cognitive aging is evidenced by whole-cell patch-clamp recordings and high-resolution 3D morphometric analyses of layer III of dlPFC compared to visual cortex (V1). The results demonstrate that dlPFC but not V1 neurons show action potential hyperexcitability that is associated with cognitive decline as well as reduced excitatory and increased inhibitory synaptic activity (Amatrudo et al., 2012; Kabaso et al., 2009; Luebke et al., 2015; Peters et al., 2001). Computational models have considered these unique features of pyramidal neurons and other key determinants of area-specific network behavior in NHP to make predictions about the consequences of these empirically observed age-related changes at both the single neuron and circuit level (Coskren et al., 2015; Ibañez et al., 2019; Rumbell et al., 2016).
Morphological alterations in dendrites and synapses are accompanied by changes in postsynaptic expression and distribution patterns of neurotransmitter receptors in aging macaques. The number of neurons expressing certain ionotropic glutamate receptors (iGluR) and N-methyl-D-aspartate receptor (NMDAR) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunits is significantly reduced during aging. GluR2 and NMDAR1 expression decrease primarily in the long corticocortical projections from the superior temporal cortex to area 46 in old macaques compared to young adults (10–25 years, n = 9 including both rhesus and long-tailed macaques) (Hof, Duan, et al., 2002). Additionally, in situ hybridization experiments confirmed a significant decrease in the mRNA expression of the NR2B subunit of the NMDAR in the PFC and in the caudate nucleus, but not in the hippocampus, in old monkeys (24–26 years, n = 4) compared to young adults (6–8 years, n = 4) (Bai et al., 2004). Age-related neurochemical and electrophysiological changes also take place in the hippocampus of old rhesus monkeys. Decreased glutamatergic neurotransmission with no changes in cholinergic or GABAergic systems has been reported in the neocortex of old (29–34 years, n = 5) compared to young macaques (4–9 years, n = 7) (Wenk et al., 1991). DG cells of behaviorally characterized old rhesus macaques (24–30.2 years, n = 9) showed increased input resistance and reduced perisomatic inhibitory signaling compared to young macaques (5.1–10.8 years, n = 11) (Luebke & Rosene, 2003). Further, the hippocampal DG showed lower density of synaptic GluR2 subunit of the AMPAR on spines expressing both GluR2 and the atypical protein kinase C ζ isoform (PKMζ) coupled with memory deficits in old (29.52 ± 1.26 years, n = 12) compared to young macaques (9.72 ± 0.20 years, n = 5) (Hara, Punsoni, et al., 2012).
It is important to highlight that age- and menopause-related cognitive impairment occurs in NHP species with female endocrine senescence comparable to that of humans. Sex-related effects in cognitive aging have been examined in female macaques. The abundance of estrogen receptor α (ERα) within excitatory synapses in dlPFC is correlated with cognitive function performance in young (9 years ± 7 months, n = 12) and old (22 years ± 7 months, n = 14) ovariectomized female macaques and ERα expression is stable across age, with and without estrogen treatment (Wang et al., 2010). Conversely, G protein-coupled estrogen receptor 1 (GPER1) distribution within layer III dlPFC synapses is reduced with age (young: 10.37 ± 0.65 years, n = 13 vs. old 24.99 ± 0.70, n = 13) and partially restored with estrogen treatment (Crimins et al., 2017).
The effects of estrogen treatment reported include changes in dendritic spines and the expression of essential synaptic components. Estrogen-treated groups of ovariectomized young macaque females (6–8 years, n = 10) showed increase of total spinophilin-immunoreactive spines in layer I of area 46 in dlPFC but not in layer I of area V1o (Tang et al., 2004). In layer III pyramidal neurons in dlPFC area 46, estrogen increased spine density, with a shift toward the more plastic thin spines, in old ovariectomized female macaques (22 years ± 7 months, n = 14) (Hao et al., 2006). The cognitive benefits of estrogen replacement in old macaques (24.70 ± 0.66 years, n = 13) compared to young adults (10.01 ± 0.55 years, n −13) may be a result of the decreased levels of synaptic phospho-Tyr1472-GluN2B (pGluN2B) NMDAR subunit in the dlPFC (Hara et al., 2018). Estrogen treatment also improved presynaptic mitochondrial number and morphology (Hara et al., 2014) and restored multisynaptic boutons (MSB) in dlPFC, both of which correlated with better working memory performance in old, ovariectomized macaques (22 years ± 7 months, n = 8) (Hara et al., 2014; Y. Hara et al., 2016).
Long-tailed macaques
Cell counts per unit area in the aging long-tailed macaque showed a trend towards fewer neurons, significantly fewer astrocytes and more microglia in the hippocampus of middle-aged (18–19 years, n = 12) compared to young (3–5 years, n = 12) female macaques (Kodama et al., 2010). Stereological estimates may provide more robust data, as in another study showing fewer total and dividing neural progenitor cells, but not astrocyte progenitors, and fewer immature neurons in the DG of old (20–24 years, n = 4) compared to young macaques (4–7 years, n=3) (Aizawa et al., 2011).
Age-related ultrastructural changes in the brain included decreased number of synapses per neuron in the temporal, but not frontal cortex of 17 year old (n = 2) compared to 10 year old macaques (n = 3), though synapse densities were comparable between the two age groups (Bertoni-Freddari et al., 2006). This reduction in synapses per neuron in the temporal cortex did not persist in older macaques (20 years old, n = 4), but synapse density was decreased at that age and accompanied by an increase in synapse size in both the temporal and frontal cortex (Bertoni-Freddari et al., 2007). These results would require further confirmation using larger sample sizes, random sampling and more rigorous quantification methods.
Patas monkeys
In Patas monkeys, retrograde tract-tracing studies in area 46 of the dlPFC revealed changes in dendritic spines on projection neurons of the temporal lobe. In young (10–12 years, n = 3) and old subjects (21–25 years, n = 3), the total length of basal and apical dendrites and the number of dendritic branches did not significantly change. There was, however, a significant reduction in dendritic spine numbers and densities along the dendritic branches of the old animals compared to the young (Page et al., 2002).
The decrease in spine density on corticocortical projection neurons connecting the temporal to the prefrontal cortex suggests age-related deficits in excitatory inputs. Immunoreactivity against GluR2 and NMDAR subunit 1 was assessed in area 46, inferior parietal area 7a and superior temporal sulcus in young and old Patas monkeys (10–25 years, n = 6). In the old animals, the proportions of projection neurons expressing NMDAR1 were significantly decreased in the temporal cortex and area 7, and those expressing GluR2 were decreased in all three areas compared to the young monkeys. These projection neurons also had lower levels of GluR2 compared to NMDAR1, suggesting a potential vulnerability to excitotoxicity caused by the imbalanced levels of these receptors (Hof, Duan, et al., 2002).
4.2. Neuropathological changes in the aging brain
Rhesus monkeys
Aβ plaques, including diffuse and compact neuritic plaques, were observed in the neocortex and hippocampus of old (20–35 years, n = 6) but not young macaques (15 years, n = 3) (Poduri et al., 1994). Plaque load and amyloid angiopathy increased with age in macaques past 20 years (5–39, n = 109) (Cork et al., 1990; Sloane et al., 1997; Uno et al., 1996; Uno & Walker, 1993). Some senile plaques and cortical and meningeal blood vessels showed immunoreactivity for ApoE (Mufson et al., 1994; Poduri et al., 1994). Neuritic plaques showed acetylcholinesterase activity and immunoreactivity for APP, glutamic acid decarboxylase, tyrosine hydroxylase, somatostatin, neuropeptide Y and choline acetyltransferase in old macaques (20–37 years, n = 11) (Kitt et al., 1984; Kitt et al., 1985; Martin et al., 1991; Struble et al., 1984; Walker et al., 1988; Walker et al., 1985). Aβ deposited in compact plaques showed fibrillar conformation (Shah et al., 2010). Aβ40, Aβ42 and pyroglutamyl-Aβ were present in plaques and vascular deposits in macaques (21–31 years, n = 13) (Gearing et al., 1996; Härtig et al., 2010). Aβ deposits containing oligomers with between 3- to 12-mers, but not dimers, were detected in the brains of rhesus macaques (Zhang et al., 2019).
Infusion of soluble oligomers of Aβ peptide (AβOs) or fibrillar Aβ into the brain of rhesus macaques has been used to create NHP models of AD. Rhesus macaques (11–19 years, n = 4) injected repeatedly with AβOs for 24 days in the lateral ventricle showed dendritic spine loss, similar to that observed in normal aging in macaques, in DG of the hippocampus and layer III of the dlPFC area 46 when examined 6 days after the last injection. However, amyloid plaques or tau pathology were not detected (Beckman et al., 2019). In contrast to soluble Aβ, injection of fibrillar Aβ triggered tau phosphorylation (p-S262 and p-S396/S404) in NFTs, neuropil threads and neurites, as well as microglial clustering and neuronal loss around plaques in the neocortex of old (25–28 years, n = 4) but not young macaques (5 years, n = 2) 11 to 12 days post-injection (Geula et al., 1998).
Previous EM studies showed degenerating neurites and paired filaments in the frontal and temporal cortex of old macaques (16–23 years, n = 4) (Wisniewski et al., 1973). Although p-S396/404 tau NFT pathology was not detected in the neocortex of old macaques (5–31 years, n = 16) (Zhang et al., 2019), clusters of phosphorylated tau-positive (p-S396/404) swollen neurites, a significant loss of neurons and a small reduction in the density of cholinergic axons were observed in the vicinity of compact plaques in the neocortex of old macaques (25–31 years, n = 11) (Shah et al., 2010). At later ages (30–33 years), a few NFTs stained for p-S396/404 were detected and apical and basal dendrites containing p-S396/404 and p-T217 tau were observed in deep layer III dlPFC of macaques. Tau NFTs containing p-S396/404 were found in the entorhinal cortex of old macaques, starting in layer II between 24–26 years and progressing to the deeper layers in 33 to 38 year old animals. Neurons containing p-S214 tau in their soma, axons, dendrites and spines were observed in layer II entorhinal cortex in young macaques (7–9 years) and in the dlPFC at later ages (31–34 years) (7–38 years, n = 10) (Datta et al., 2021; Paspalas et al., 2018). Total levels of p-S214 tau increase with age in the dlPFC. At the ultrastructural level, young macaques (9–11 years, n = 3) showed higher incidence of p-S214 tau in axons and proximal dendrites, whereas in old macaques (24–31 years, n = 5), it was predominant within dendritic spines, suggesting age-related trafficking of phosphorylated tau (Carlyle et al., 2014). Moreover, p-S214 tau within axon terminals and dendritic spines in female rhesus macaques was reduced with age (young 9.9 ± 0.7 years, n = 8 vs. old 25.2 ± 0.9 years, n = 8) and correlated with synapse density but not cognitive status (Crimins et al., 2019).
Growing evidence suggests that astrocytes and microglia become activated during aging in macaques. Whereas glial counts were unchanged in area 46 of old macaques compared to young (5–35 years, n = 21), ultrastructural analysis revealed more cytoplasmic inclusions in astrocytes and microglia of old macaques (Peters et al., 1994; Peters & Sethares, 2002). Some inclusion bodies within astrocytes contained myelin, possibly a result of demyelination with aging (Peters & Sethares, 2003). The area adjacent to compact, but not diffuse, Aβ plaques showed activated microglia (Shah et al., 2010), abnormal microglial morphology, and astrocytosis (Härtig et al., 1997; Zhang et al., 2019). In the frontal cortex of old macaques (19–26 years, n = 4) compared to young adults (7–12 years, n = 5), astrocyte processes were less complex in the gray and white matter (Robillard et al., 2016). In frontal white matter, microglia express a phagocytic phenotype with enlarged hypertrophic morphology during aging (6–31 years, n = 44) (Shobin et al., 2017).
Long-tailed macaques
In long-tailed macaques (n = 34, 6–36 years old), diffuse and compact Aβ plaques were detected in the temporal and parietal cortex and the hippocampus starting around 19 years of age (Kodama et al., 2010; Oikawa et al., 2010; Podlisny et al., 1991; Uchihara et al., 2016). Aβ42 was predominant in diffuse plaques (Darusman et al., 2014; Oikawa et al., 2010) though both Aβ42 and Aβ40 was detected in mature plaques (Nakamura et al., 1996; Nakamura, Nakayama, et al., 1995; Nakamura, Tamaoka, et al., 1995). Neurites associated with mature plaques contained ApoE, APP and ubiquitin but not tau (Kimura et al., 2003). Amyloid deposits were also present as CAA in small blood vessels of the frontal, temporal, and occipital cortex and in hippocampus (Darusman et al., 2014; Kodama et al., 2010; Nakamura et al., 1996; Podlisny et al., 1991).
Tau phosphorylated at S202/T205 was detected as dystrophic neurites or within oligodendrocytes in the frontal and temporal neocortex and basal ganglia of long-tailed macaques over 30 years of age. Rare diffuse, granular deposits of p-S202/T205 tau were also detected in hippocampal pyramidal neurons or in perivascular astrocytes in the oldest (36-year old) macaques (Uchihara et al., 2016). The tau deposits in these macaques were different from those observed in AD, in that they contained only 4-repeat tau and were present in oligodendrocytes of the basal ganglia, pons, medulla and white matter of hippocampus and temporal lobe (Kiatipattanasakul et al., 2000; Oikawa et al., 2010; Uchihara et al., 2016). Rare tau deposits of pT231 were also observed in neurons of the temporal and occipital lobes of a 29-year-old long-tailed macaque (Darusman et al., 2014). Gliosis was present in the frontal, temporal, parietal and occipital cortices of old macaques (over 29 years) (Darusman et al., 2014). Astrocytic processes stained with GFAP were detected proximal to compact Aβ plaques (Nakamura et al., 1996).
Baboons
A recent study reported that old baboons (P. anubis, 20 ± 3 years of age, n = 3) show a decline in attention, learning, and memory compared to young adults (13 ± 3 years of age, n = 3) (Lizarraga et al., 2020). Interestingly, age-related pathology in baboons also appears around the age at which cognitive deficits are apparent. Aβ pathology is rare in the neocortex of baboons before 20 years of age; however, diffuse Aβ plaques and vascular amyloid range from mild to moderate severity in older P. hamadryas and P. anubis (Schultz, Dehghani, et al., 2000; Schultz, Hubbard, et al., 2000). This grading of Aβ pathology has been confirmed (P. hamadryas, P. cynocephalus, P. anubis) in the frontal (area 9 or 10), temporal (area 22), parietal (area 40), and occipital cortices (area 17–18), but not hippocampus and amygdala (1–30 year old, n = 50). However, another study using different antibodies detected Aβ plaques in hippocampus as well as in layers III to V of the neocortex (18–28 years, n = 6). Here, Aβ42 was the prevalent peptide in the diffuse parenchymal plaques, while both Aβ40 and Aβ42 were present in vascular deposits and AβO was present in both plaques and amyloid-laden vessels (Ndung’u et al., 2012).
Abnormally phosphorylated or misfolded tau has also been studied in the brains of P. hamadryas and P. anubis (Schultz, Dehghani, et al., 2000; Schultz, Hubbard, et al., 2000). Similar to the studies in amyloid deposits, grading of abnormal tau (p-S202/T205 tau) showed no tau pathology in young baboons (1–10 years, n = 9), rare tau inclusions in adults after 19 years of age (11–20 years, n = 12) but more frequent and more severe tau pathology with increasing age thereafter (21–30 years, n = 28). In general, the distribution of tau pathology showed no correlation to the location of Aβ deposits. In the hippocampus, abnormal tau-positive inclusions were detected in cell bodies and dendrites of CA1 and CA3 pyramidal neurons, with the highest density of neuronal tau deposits in the deeper granule cell layers. Moreover, in P. anubis layer II neurons of the entorhinal cortex also showed accumulation of abnormal tau (Schultz, Hubbard, et al., 2000). In the few studies available for brain areas outside the hippocampus and entorhinal cortex, abnormal phosphorylated tau (p-S202/T205 tau) was found in rare neurons within cortical layer VI and in glia-like cells in the frontal cortex of P. hamadryas (26 to 28 years of age, n = 2) (Härtig et al., 2000) and in a few neurons or as diffuse extracellular reactivity in the temporal cortex of P. cynocephalus (20 to 24 years of age) (Ndung’u et al., 2012).
Age-related changes in glial cells of baboons included abnormal tau (p-S202/205, p-S396/404, TG-3 to detect phosphorylation and conformation changes) in the cell bodies and processes of perivascular astrocytes in the hippocampus from 19 years of age (Schultz, Hubbard, et al., 2000), as well as in oligodendrocytes in the perforant path of the oldest subjects (26–30 years, n = 2) (Schultz, Dehghani, et al., 2000).
Vervets
In vervets, the number of Aβ plaques has been shown to increase with age in specific brain regions. Density of Aβ42 and Aβ40 plaques increased with age in the cingulate gyrus (areas 6/32 and 23c), and entorhinal cortex, frontal cortex, secondary somatosensory cortex, insular cortex, and auditory cortex when comparing vervets of different ages (6–32 years, n = 15) (Cramer et al., 2018). Interestingly, Aβ plaque distribution showed regional variation in temporal and parietal cortices, with both diffuse and neuritic plaques as well as vascular Aβ deposits observed in old vervets (Latimer et al., 2019). Soluble Aβ42 and Aβ40 levels did not show age-dependency. However, guanidine-extracted insoluble Aβ42 and Aβ40 levels in temporal and parietal cortices of old vervets (19.5–23.4 year old, n = 9) were significantly higher than those of middle-aged vervets (8.2–13.5 year old, n = 9) (Latimer et al., 2019). Also, older animals (> 15 years of age) had higher amyloid deposition of both phosphorylated (phosphorylated serine 8) and non-phosphorylated Aβ variants in the frontal and temporal cortex (Kumar et al., 2018). Generally, no significant age-related changes were observed in the hippocampus or the visual cortex (Cramer et al., 2018), basal ganglia, brainstem, or cerebellum (Latimer et al., 2019). Only a few studies have found extracellular Aβ plaques in the hippocampal region in older vervets. Phosphorylated Aβ was also found in meningeal and parenchymal blood vessels (Kumar et al., 2018).
Cellular phosphorylated tau (p-S202/T205 tau) appeared in granular cytoplasmic deposits in parietal, temporal, and occipital lobes, as well as basal ganglia, brainstem, and cerebellum of most vervets over 8 years of age (n = 18) (Latimer et al., 2019). However, intraneuronal tau tangles (p-S202/T205) were observed in the entorhinal cortex and in stratum radiatum of hippocampal CA1-4 fields of two vervets over 20 years of age (Cramer et al., 2018). Moreover, higher levels of soluble PHF tau were found in the temporal cortex of old vervets, whereas guanidine-extracted insoluble PHF-tau levels were comparable in parietal cortex for both old and middle-aged groups (Latimer et al., 2019). Interestingly, whereas data from CSF showed no significant changes in Aβ levels, an increase in total tau and phospho-tau levels were observed in old vervets (Cramer et al., 2018).
In addition to Aβ and tau pathology, altered distribution of glial cells was observed in old vervets. Increased GFAP expression in astrocytes of the temporal and parietal cortex and hippocampus with aging in vervets (10–24 years old, n = 10) was accompanied by clustering of astrocytes and Iba1-immunoreactive microglia around Aβ plaques (Cramer et al., 2018; Kalinin et al., 2013).
Guenons
A single study of Campbell’s mona monkeys (30 and 27 years of age), showed sparsely scattered phosphorylated tau (p-S202/T205 tau) deposition in the cerebral cortex. Tau-immunoreactive cells were primarily found in the cingulate cortex (Härtig et al., 2000).
5. HOMINIDS
5.1. Structural changes and altered cell morphology in the aging brain
Great apes
Data on neuron density varies in great apes. In a small sample of chimpanzees (n = 6, 13–45 years), total neuron numbers in the hippocampal subfields CA1 and CA3 did not change with age (Perl et al., 2000). However, a larger study of chimpanzees (n = 20, 37–62 years) found moderate age-associated decline in neuron density (~12–19%) in the CA1 and CA3 (Edler et al., 2020), but did not assess total neuron numbers as reported in another study (n = 6, 29–43 years) (Rogers Flattery et al., 2020). The decrease in neuron density with aging in chimpanzees might reflect loss of neuropil space due to atrophy of dendrites and synapses, which would be consistent with what has been observed in humans and other NHP. This possibility remains to be fully examined.
Humans
Age-related atrophy of the prefrontal cortex, hippocampus and entorhinal cortex in the human brain was initially thought to be due to loss of neurons (Anderson et al., 1983; Brody, 1955; Mani et al., 1986). However, quantification using unbiased stereological tools in the frontal cortex showed that total neuronal counts are preserved in subjects aged 65–105 years (n = 23) (Fabricius et al., 2013; Freeman et al., 2008). Reports of lower counts of large neurons in the mid-frontal cortex and area 9 of old subjects (>70 years old, n = 3–25) compared to young (16–49 years old, n = 3–12) (Soreq et al., 2017; Terry et al., 1987) require confirmation using rigorous stereological techniques and larger sample sizes. Similar to observations in the frontal cortex, most studies agree that neurons are generally preserved in aging human subjects (56–105 years old, n = 105) in the entorhinal cortex and most hippocampal subfields (Freeman et al., 2008; Gómez-Isla et al., 1996; Kobayashi et al., 2018; Simić et al., 1997; West, 1993; West et al., 1994; West & Gundersen, 1990). However, a few studies comparing subjects including a younger age range (13–101 years of age) have reported age-related loss of neurons in CA1 and hilar fields of the hippocampus and subiculum (Simić et al., 1997; West, 1993; West et al., 1994; West & Gundersen, 1990). Although some groups showed a decline in hippocampal neurogenesis with age (0–100 years, n =31) (Knoth et al., 2010; Mathews et al., 2017), a stereological estimate using a combination of cell markers showed that hippocampal neurogenesis is maintained in aging (14–79 years, n = 28) (Boldrini et al., 2018). The density of inhibitory neurons in area 9, entorhinal cortex, and hippocampus are also generally maintained in aging (26–93 years of age, n = 11) (Bu et al., 2003).
Another contributor to altered brain volumes in aging could be changes in neuron morphology at the level of axons, dendritic trees or dendritic spines. Total length of myelinated fibers is shorter in 62–90 year old subjects (n = 5) compared to younger subjects (8–57 year old, n = 5), with a preferential loss of thinner axonal fibers (Tang et al., 1997). Lower density of cholinergic axonal fibers has been reported in the entorhinal cortex of old subjects (69–91 year old, n = 3) compared to young (22–43 years old, n = 3) (Geula & Mesulam, 1989). Morphometric analyses of dendrites in old (>50 years, n = 16) versus young (<50 years, n = 10) subjects revealed slightly lower basal dendritic length and complexity in layer III pyramidal neurons of prefrontal area 10 (Jacobs et al., 1997). In areas 9 and 46, basal dendritic length of layer V, but not layer IIIc, pyramidal neurons was inversely correlated with age (49–90 years, n = 8) (de Brabander et al., 1998). In the hippocampus, dendritic length and complexity was preserved in pyramidal neurons of the CA1, CA2-3 and layer III of the subiculum in normal aging (43–95 years, n = 10–12) (Flood, 1991; Flood et al., 1987; Hanks & Flood, 1991). In granule cells of the DG, apical dendritic length increased from middle-age (43–60 years, n = 7) to old age (68–79 years, n = 5) and then decreased after the 8th decade of life (n = 5) (Flood et al., 1985). Length and complexity of apical dendrites on pyramidal neurons in layer II of the parahippocampal gyrus was greater in old subjects (68–92 years, n = 5) compared to middle-aged subjects (44–55 years, n = 5) (Buell & Coleman, 1979). In cognitively intact subjects from 14–106 years of age, spine density was found to decline with age on basal dendrites of pyramidal neurons in layer II and III of area 46, and in layer III of area 10 (Boros et al., 2019; Jacobs et al., 1997). In summary, aging affects dendrites and spines in the frontal cortex more than those in the hippocampus.
Altered spine density with aging points towards changes occurring at the synapse. Quantification from EM studies showed reduced synaptic density with aging (26–90 years, n = 24) in the frontal cortex and DG (Bertoni-Freddari et al., 1992; Gibson, 1983). However, after controlling for postmortem interval and neurological diagnosis, no significant change in synaptic density was found in layer III of the middle frontal gyrus or in area 9 with aging (15–89 years, n = 53) (Cragg, 1975; Huttenlocher, 1979; Scheff et al., 2001). Spinophilin, a postsynaptic marker mainly located in dendritic spines, is shown to decrease in area 9 and hippocampus CA1 in relation to cognitive decline in old individuals (74–97 years, n = 18) (Akram et al., 2008). Other groups have used synaptophysin levels as an estimate of presynaptic terminals. Whereas synaptophysin decreased in the frontal cortex with aging (16–98 years, n = 25) (Masliah et al., 1993), it was unchanged in the hippocampus, although there is an age-related decline in the parahippocampal gyrus of subjects over 68 years of age (n = 6) (Eastwood et al., 2006).
Specific neurotransmitter systems at the pre- or postsynaptic terminals may also be affected in aging. Activity of choline acetyltransferase, a presynaptic marker for cholinergic neurons, decreased with age in the hippocampus and entorhinal cortex, but was unchanged in the frontal cortex (32–96 years) (Court et al., 1993). Lower binding of dopamine agonists to D1 receptors in area 9 (de Keyser et al., 1990), or ligands to D1 (Iyo & Yamasaki, 1993; Suhara et al., 1991) and D2/3 receptors in the frontal cortex and hippocampus (Inoue et al., 2001; Kaasinen et al., 2000; Seaman et al., 2019) has been reported with aging (19–83 years, n = 183). Dopamine synthesis in the dlPFC declines with aging (20–67 years, n = 21) (Ota et al., 2006), whereas levels of the dopamine catabolic enzyme monoamine oxidase B in the PFC (21–75 years, n = 22) and hippocampus (14–93 years, n = 27) increase with aging (Galva et al., 1995; Saura et al., 1997). In area 9, lower antagonist binding to NMDAR was observed with age (11–100 years, n = 34) (Piggott et al., 1992). However, in the hippocampus, lower binding activity to AMPAR (32–96 years, n = 43) (Court et al., 1993), but not NMDA or kainate receptors (Johnson et al., 1996) was seen with aging (20–95 years, n = 30). Serotonin 5-HT receptor binding activity in the frontal cortex was reduced with aging (19–85 years, n = 86), with both 5HT1 and 5HT2A reduced in the PFC and hippocampus (Allen et al., 1983; Iyo & Yamasaki, 1993; J. Marcusson et al., 1984; J. O. Marcusson et al., 1984; Wang et al., 1995; Wong et al., 1984). However, one group has reported increase of 5HT2 receptor binding in the PFC and hippocampus past 50 years, though the overall pattern of age-dependent decrease was similar to the other studies (17–81 years, n = 12) (Gross-Isseroff et al., 1990).
5.2. Neuropathological changes in the aging brain
Great apes
In addition to age-related neurological changes, AD-like pathologies have been identified in the great ape brain. Notably, great apes exhibit high sequence homology for amyloid precursor protein (APP) and as all NHP assessed to date, chimpanzees have a fixed Apolipoprotein allele ε4 (ApoE4) profile (McIntosh et al., 2012). Yet studies of AD lesions in the great ape brain are few and typically include only a handful of animals. Extracellular diffuse plaques and vascular amyloid have been detected (Fig. 4) in the brains of old orangutans, gorillas, and chimpanzees (Edler et al., 2017; Gearing et al., 1994; Gearing et al., 1996, 1997; Kimura et al., 2001; Perez et al., 2013; Perez et al., 2016; Rosen et al., 2008). Two previous studies identified diffuse Aβ plaques and occasional amyloid-positive blood vessels in the neocortex of orangutans (n = 5, 10–46 years) (Gearing et al., 1997; Selkoe et al., 1987). Unlike humans, however, plaques in orangutans primarily consisted of Aβ40 and were not associated with gliosis. Diffuse plaques characterized in gorillas (n = 20, 13–55 years) contained APP, oligomeric Aβ, Aβ40, and Aβ42, and were weakly positive for thioflavin S and negative for Congo Red (Kimura et al., 2001; Perez et al., 2013; Perez et al., 2016). Vascular Aβ was noted in meningeal arteries, arterioles, and capillaries, and as perivascular accumulations in the neocortex of western lowland gorillas, mountain gorillas, and chimpanzees (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016; Rogers Flattery et al., 2020). Differing from plaques, Aβ vessels in these apes had robust thioflavin S fluorescence, indicating a fibrillar nature. Moreover, aging was associated with greater APP and Aβ levels in gorillas and chimpanzees, and CAA was present in both species, unlike orangutans (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016). The regional distribution of plaques in chimpanzees aligned with staging patterns typically observed in AD, with the greatest deposition occurring in the neocortex, and Aβ42 appeared to be the primary peptide length in plaques and vessels in the chimpanzee brain (Edler et al., 2017). In contrast to humans, great apes appear to lack dystrophic neurites associated with neuritic plaques (Edler et al., 2017; Gearing et al., 1996; Perez et al., 2016).
Figure 4. Alzheimer’s disease pathology in great apes.
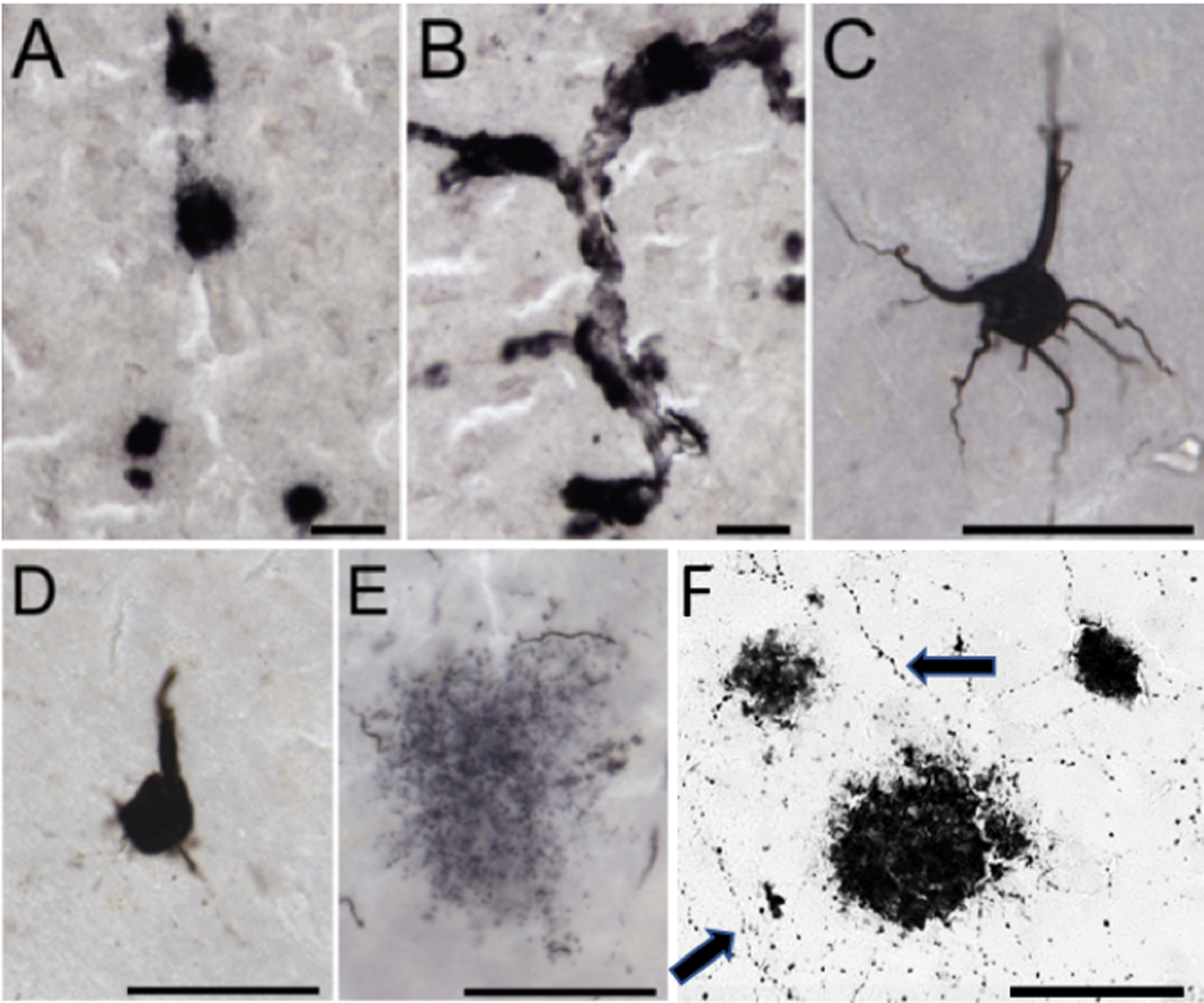
Photomicrographs of immunohistochemical staining in a 33-year-old female chimpanzee brain. Amyloid-β deposition occurs in plaques (A) and vessels (B), and tau deposition is present in pretangles (C), NFT (D), and neuritic clusters of dystrophic neurites (E), which appears unique to chimpanzees and gorillas. (F) Frontal cortex dual stained for choline acetyltransferase (fine fibers; arrows) and APP/Aβ amyloid plaques in a 55-year-old female Western lowland gorilla. Scale bars = 25 μm.
Distinct from Aβ pathologies, tauopathy is rarely observed in great apes (Fig. 4). Tau-positive pretangle neurons and glia have been identified in gorillas and chimpanzees (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016; Rogers Flattery et al., 2020). Classic NFT have been detected in old chimpanzees (37–62 years) (Rosen et al., 2008) but were absent in lowland gorillas, mountain gorillas, and orangutans (Edler et al., 2017; Mufson et al., 2013; Perez et al., 2016; Selkoe et al., 1987). This species variation may be due, in part, to sequence differences in the microtubule-associated protein tau gene, as homology at the nucleotide level is 99.5% in chimpanzees, 96.8% in gorillas, and 96.4% in orangutans (NCBI BLAST Nucleotide, 1988). In addition, neuritic clusters containing tau-immunopositive dystrophic neurites, were identified only in old chimpanzees and gorillas (Edler et al., 2017; Mufson et al., 2013; Perez et al., 2013; Perez et al., 2016; Rogers Flattery et al., 2020; Rosen et al., 2008; Selkoe et al., 1987). Tau lesions have been noted in proximity to Aβ deposition in gorillas and chimpanzees, and moderate and severe CAA was associated with increased tauopathy in old chimpanzees (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016). Significant neocortical and hippocampal neuron loss occurs in the human AD brain, and only a single investigation has examined the association of AD lesions with neuron loss in great apes. A study of 20 chimpanzees, ages 37 to 62 years, found a trend of non-significant moderate change in neuron density (~20–24%) correlated with moderate/severe CAA, but not plaque or tau lesions, in the middle temporal gyrus (Edler et al., 2020). Therefore, based on current evidence, apes appear to lack the severe neuronal loss typically observed in AD.
Cerebrovascular dysfunction and neuroinflammation are common characteristics in human aging, but their study in great apes remains scarce (Kettenmann et al., 2011; Sofroniew & Vinters, 2010; Sonntag et al., 2007). Two case reports documented thickening and calcification of small arteries in the striatum of an old gorilla brain and severe arteriolosclerosis and edema in a 54-year-old female orangutan brain with a history of hemiparesis (Lowenstine et al., 2016; Márquez et al., 2008). A few studies have analyzed glial responses to aging in apes. Gliosis was seen throughout the neocortex of a 40-year-old albino gorilla (Lowenstine et al., 2016; Márquez et al., 2008). Conversely, microglia, astrocyte, and total glia densities in the neocortex and hippocampus of chimpanzees did not vary with age, although morphologic changes indicative of activation were noted (Edler et al., 2020; Edler et al., 2018; Munger et al., 2019). A couple of analyses have examined the neuroinflammatory response to AD pathologies in chimpanzees. Tau lesions and Aβ plaques and vessels were correlated with greater glial activation in the neocortex and hippocampus of old chimpanzees (Edler et al., 2020; Edler et al., 2018; Munger et al., 2019). Greater volumes of Aβ42 plaque and vessel deposition were associated with higher microglial densities and morphologic changes in the hippocampus of old chimpanzees (Edler et al., 2017). Additionally, astrocyte density increased with Aβ plaque deposition in the hippocampus and with tau neuritic clusters in layer I of the PFC (Munger et al., 2019). Astrocyte soma volume was increased with tau neuritic cluster density in CA1. In contrast to AD, microglial activation was not correlated with tau lesions.
Humans
AD neuropathological features, including diffuse and compact plaques containing Aβ peptides, vascular Aβ deposits as well as neurites and NFTs containing hyperphosphorylated tau occur in the brains of old, non-demented humans (ages > 65). Aβ plaques are present in the frontal cortex, area 9, entorhinal cortex, CA1, CA2-3, hilus, and subiculum of non-demented older subjects (Bouras et al., 1994; Bussière et al., 2002; Bussière, Giannakopoulos, et al., 2003; Davis et al., 1999; Giannakopoulos, Hof, Giannakopoulos, et al., 1994; Giannakopoulos et al., 1995; Giannakopoulos et al., 1996; Giannakopoulos, Hof, et al., 1997; Giannakopoulos et al., 1993; Tanprasertsuk et al., 2019; Tsartsalis et al., 2018). CAA is also observed in cognitively normal older subjects, with Aβ-laden vessels present in the frontal cortex and hippocampus, among other areas (Arvanitakis et al., 2011; Kövari et al., 2013; Thal et al., 2003; Vinters & Gilbert, 1983). Low levels of post-translationally modified Aβ, specifically the isoaspartate and pyroglutamylated forms, have been observed in plaques and vascular deposits in aging (Mandler et al., 2014; Moro et al., 2018; Tekirian et al., 1998).
Neurites and NFT consisting of hyperphosphorylated tau appear early and are widely observed in layers II and V of the entorhinal cortex, but are variable in the CA1, CA2-3, hilus, and subiculum of non-demented older subjects (Arriagada et al., 1992; Bouras et al., 1994; Bouras et al., 1993; Davis et al., 1999; Giannakopoulos, Hof, Giannakopoulos, et al., 1994; Giannakopoulos et al., 1995; Giannakopoulos et al., 1996; Giannakopoulos, Hof, et al., 1997; Giannakopoulos, Hof, Mottier, et al., 1994; Giannakopoulos et al., 1993; Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) 2001; Price et al., 1991; Price & Morris, 1999; Tanprasertsuk et al., 2019; Tsartsalis et al., 2018). NFTs are rarely seen in the superior frontal cortex or in area 9 (Bouras et al., 1994; Bussière, Giannakopoulos, et al., 2003; Bussière, Gold, et al., 2003; Giannakopoulos et al., 1995; Giannakopoulos, Hof, et al., 1997; Giannakopoulos, Hof, Mottier, et al., 1994; Giannakopoulos et al., 1993).
Although neurons in the frontal cortex and hippocampus are relatively spared, aging may affect glial cells in these brain regions, either in their total counts or in their phenotypes as revealed by morphological or biochemical changes. The number of astrocytes and microglia in the neocortex (Pelvig et al., 2008), astrocyte counts in layers I and II of the entorhinal cortex (Kobayashi et al., 2018) and astrocyte density in the white matter of the entorhinal cortex and hippocampus (Gefen et al., 2019) were unchanged in healthy aging. Increased prevalence of rod-shaped microglia has been reported in the hippocampus of old non-demented subjects compared to young controls (Bachstetter et al., 2017). Age-related increase of dystrophic microglia (Streit et al., 2004) showing accumulation of ferritin, an iron-storage protein, is observed in the frontal cortex (Lopes et al., 2008) as well as in the entorhinal cortex and hippocampus (DiPatre & Gelman, 1997). Increased ferritin in astrocytes of the hippocampus has also been reported in aging (Connor et al., 1990). These findings on altered microglial and astrocytic phenotypes need replication with more rigorous quantification. Fewer oligodendrocytes have been reported in the frontal lobe with increasing age (Pelvig et al., 2008) and in area 9 of a small sample of older subjects compared to young controls (Soreq et al., 2017).
6. DISCUSSION
6.1. Structural changes and altered cell morphology in the aging brain
In most of the NHP species reviewed showing a loss of grey matter, it was initially thought to result from neuron loss during aging. Evidence suggests, however, that atrophy in the aging brain is generally attributed to the decreased arborization of dendritic trees and loss of dendritic spines (Duan et al., 2002; Duan et al., 2003; Page et al., 2002). In human studies it has been shown that total neuronal counts within the neocortex and hippocampus seem to be preserved with age (Fabricius et al., 2013; Simić et al., 1997). Relatedly, in chimpanzees there is a moderate age-associated reduction in density of neurons in the CA1 and CA3 of the hippocampus, which could be related to deterioration of dendrites and spines in the neuropil (Edler et al., 2020). Cognitively intact human subjects show lower basal dendritic length and complexity. In addition, spine density declines with increasing age on basal dendrites of pyramidal neurons in layer II and III of area 46 and in layer III of area 10 (Akram et al., 2008; Boros et al., 2019; Jacobs et al., 1997). Dendritic spine loss and irregularities in dendritic arborization and spine distribution, number or morphology and synapses in pyramidal neurons in dlPFC area 46 (Fig.2), which has an important role in cognition during aging (Luebke et al., 2010), have been described in macaques (Duan et al., 2003; Dumitriu et al., 2010; Motley et al., 2018; Page et al., 2002) and patas monkeys (Page et al., 2002) among cercopithecids. Preliminary evidence from old marmoset monkeys also shows differences between cognitive impaired and non-impaired animals in dendritic spine vulnerability during aging in dlPFC layer III of area 8b/9.
As a consequence of spine loss, especially in dlPFC, there is a marked excitatory synapse loss during aging and synapses undergo remodeling which has been reported to widely affect neurons receiving excitatory inputs (Luebke & Amatrudo, 2012; Luebke & Rosene, 2003). A decrease in postsynaptic density components like excitatory neurotransmitter receptors has been reported in macaques and patas monkeys during aging in PFC and hippocampus (Bai et al., 2004; Hara, Punsoni, et al., 2012; Hof, Duan, et al., 2002; Wenk et al., 1991). This is in agreement with human studies where lower antagonist binding to NMDAR was observed with age in PFC area 9, as well as lower binding activity to GluR in the hippocampus (Court et al., 1993; Piggott et al., 1992). Overall, synapse loss causes important changes in the excitation-inhibition balance in the cortex, which is associated with age-related cognitive decline as demonstrated in electrophysiological studies in macaque and marmoset monkeys (Atapour & Rosa, 2017; Luebke & Amatrudo, 2012; Peters & Kemper, 2012).
In addition, among the factors involved in spine dynamics and synaptic plasticity changes during aging, it is worth mentioning the importance of the fluctuation of sex hormones as it has been pointed out in several studies in macaques (Hao et al., 2007; Tang et al., 2004). In particular, the involvement of estrogen receptors in synaptic remodeling during senescence may, in part, explain the detrimental effects on cognition (Hao et al., 2006; Hara et al., 2011). These findings also emphasize the need to study sex differences, which is largely overlooked in NHP studies.
Several studies in humans show damage of tissue structure in frontal white matter (Salat et al., 2005). This is often manifested in the shortening of myelinated fibers with loss of thinner axonal fibers (Salat et al., 2005; Tang et al., 1997). The vast majority of NHP reports on white matter changes come from marmosets and macaques. In the former, a decrease in myelin thickness and density in the corpus callosum has been associated with the cognitive performance in old animals (Phillips et al., 2019). In macaques, the loss of myelinated fibers and abnormalities in myelin in dlPFC area 46 (Fig.3) have their direct consequence in the age-related disconnection of dlPFC from other cortical regions (Bowley et al., 2010; Peters, 2009).
6.2. Neuropathological changes in the aging brain
Most old NHP species present different degrees of amyloidosis in areas important for cognition, like the neocortex and hippocampus (Table 1). Extracellular plaques and vascular amyloid deposits have been detected in the brains of old orangutans, gorillas, and chimpanzees (Edler et al., 2017; Gearing et al., 1997; Kimura et al., 2001; Perez et al., 2013; Rosen et al., 2016) and also in cercopithecids like macaques (Geula et al., 2002; Kodama et al., 2010; Oikawa et al., 2010; Podlisny et al., 1991; Rosen et al., 2016; Uchihara et al., 2016), baboons (Ndung’u et al., 2012; Schultz, Hubbard, et al., 2000), and vervets (Kumar et al., 2018; Latimer et al., 2019). In ceboids, amyloidosis is described for marmosets (Geula et al., 2002; Rodriguez-Callejas et al., 2016), squirrel monkeys (Elfenbein et al., 2007; Walker et al., 1990), and cotton-top tamarins (Lemere et al., 2008). In lemurs, amyloid deposition has been reported in grey mouse lemurs (Bons et al., 1994; Kraska et al., 2011). In the majority of cases, Aβ42 was detected in the form of diffuse and sometimes compact plaques, and Aβ40 was found mainly when CAA was present, in proximity to blood vessels as diffuse aggregates.
Table 1.
Summary of age-related and AD-like neuropathology in NHP and humans
| Species | Life Span (maximum) | Aβ deposits and vascular amyloid | Tau pathology | Other | |
|---|---|---|---|---|---|
| Lemurs | Grey mouse lemurs | 14–16 years | Diffuse Aβ plaques and cerebrovascular Aβ (neocortex, amygdala and hippocampus). Few mature or neuritic plaques. Intra-neuronal, glial and vascular APP (hippocampus) | Aggregated (anti-PHF) and p-S400/T429 tau in neurons and neurites (neocortex, amygdala), p-S202 tau (hippocampus) | Astrogliosis (corpus callosum, hippocampus and neocortex) |
| Ceboids | Common marmosets | 10–17 years | Diffuse and compact Aβ plaques, neuritic plaques with APP and ApoE, amyloid angiopathy (neocortex and hippocampus) | p-S396/S404 and p-S262 tau not detected. Cytoplasmic p-T231 and p-T212/214 tau and aggregated tau (Alz-50) in neurons and dystrophic microglia (medial temporal and parietal cortex, hippocampus) | Dystrophic, ferritin-expressing microglia, larger astrocytes (hippocampus) |
| Cotton top tamarins | 23 years | Diffuse and compact Aβ plaques and vascular deposits (neocortex, hippocampus). No APP neuritic plaques | p-S202/T205 tau not detected. No tau neurites or NFTs | Reactive astrocytes and activated microglia proximal to Aβ plaques (neocortex) | |
| Squirrel monkeys | 15–27 years | Diffuse and dense-cored Aβ plaques and cerebral amyloid angiopathy (neocortex and amygdala) | P-S202, p-S396/40, p-S202/205 tau neurites rarely detected (neocortex). NFTs absent | Focal gliosis detected with severe cerebral amyloid angiopathy (neocortex and amygdala) | |
| Cercopithecids | Rhesus macaques | 23–44 years | Diffuse and compact Aβ plaques and amyloid angiopathy, neurites with acetylcholinesterase, APP, glutamic acid decarboxylase, tyrosine hydroxylase, somatostatin, neuropeptide Y, choline acetyltransferase (neocortex and hippocampus) | p-S396/404 tau in neurites and rare NFTs, p-T217 and p-S214 tau in dendrites and spines (neocortex). p-S396/404 tau NFTs and p-S214 tau in neurons (entorhinal cortex) | Dystrophic microglia. Astrocyte and microglia activation around compact plaques (neocortex and hippocampus) |
| Long-tailed macaque | 25–38 years | Diffuse and compact Aβ plaques, neurites with APP and ApoE, cerebral amyloid angiopathy (neocortex and hippocampus) | p-S202/205 tau in dystrophic neurites and oligodendrocytes (neocortex). Rare intraneuronal p-T231 tau | Gliosis (neocortex) and astrocytes surrounding Aβ plaques | |
| Patas monkeys | 21–24 years | N/A | N/A | N/A | |
| Baboons | 35–45 years | Age-related increase in diffuse Aβ plaques and vascular Aβ (neocortex and hippocampus ) | p-S202/T205 tau increase with age (entorhinal cortex and hippocampus, rare in neocortex). p-S202/205 and p-S396/404 tau in perivascular astrocytes and in oligodendrocytes (hippocampus, entorhinal cortex) | N/A | |
| Vervets | 23–32 years | Increased Aβ plaques with age (neocortex, entorhinal cortex and rare in hippocampus ). Diffuse and neuritic plaques, vascular Aβ | p-S202/T205 tau as cytoplasmic deposits (neocortex, cerebellum, striatum, brainstem) and rare tangles (entorhinal cortex, hippocampus) | Increased astrocytosis with age, Aβ plaque associated astrocytes and microglia (neocortex and hippocampus) | |
| Campbell’s guenons | 20–33 years | N/A | Rare p-S202/T205 tau (neocortex) | N/A | |
| Hominids | Apes | 35–60 years | Orangutans, gorillas, chimpanzees: diffuse and compact Aβ plaques and vascular amyloid (neocortex, hippocampus). No neuritic plaques | Gorillas and chimpanzees: p-S202/T205 and p-S396/404 tau pretangles in neurons and glia. Neuritic clusters. Chimpanzees: NFT (PFC, hippocampus, entorhinal cortex) | Gorillas: gliosis (neocortex). Chimpanzees: Aβ - associated gliosis and microglial dystrophy (hippocampus) and neuritic tau – associated astrogliosis (PFC) |
| Humans | 100–123 years | Diffuse and compact Aβ plaques and cerebral amyloid angiopathy present (PFC, subiculum, entorhinal cortex, hippocampal CA1) | p-tau rare in PFC. High prevalence of p-tau as neurites, NFTs in entorhinal cortex, occasionally in hippocampus CA1-3 and dentate gyrus | Dystrophic, ferritin-expressing microglia (PFC, hippocampus) . Oligodendrocytes- decreased (PFC) | |
Aβ: amyloid beta protein; ApoE: apolipoprotein E; APP: amyloid beta precursor protein; CA: Cornu Ammonis; NFT: neurofibrillary tangles; PFC: prefrontal cortex; p-tau: phosphorylated tau. All references included in text.
There is a growing interest in research with NHP models, particularly for elucidation of the earliest phase of AD pathogenesis in comparison with humans. A study conducted in macaques suggested that the Aβ dimer, absent in macaques, plays an important role in AD pathogenesis (Zhang et al., 2019). In macaques, the injection of Aβ oligomers led to dendritic spine loss in the PFC and increased neuroinflammation, but without detection of amyloid plaques or tau pathology at 6 days post-injection (Beckman et al., 2019). However, at 11–12 days after injection of fibrillar Aβ in macaques, amyloid plaques and tau NFTs were detectable in old macaques (Geula et al., 1998). In marmosets, the intracerebral injection of brain homogenates induced amyloidosis, similar to the natural occurring deposition of Aβ in aging, with large vessel angiopathy and some plaques throughout the cortex (Geula et al., 1998; Maclean et al., 2000; Philippens et al., 2017; Ridley et al., 2006). These studies need to be interpreted with caution, as the time following injection was variable and the age at which some of the pathologies were seen post-injection coincide with their appearance during normal aging.
Although phosphorylated tau in NFT is rarely observed in NHP models, neurites and neuritic plaques do occur, with variability among species in the same suborder that might be a consequence of sequence differences in the tau gene. Also, the lack of uniformity reported for different phosphorylated forms of the tau protein detected may reflect the lack of consistency in tissue processing and specificity of the antibodies used in the immunoassays. Moreover, tau isoform expression pattern and phosphorylation sites associated with AD pathology in humans may change with age and differ across species of NHP (Table 1). In non-demented older human subjects (Braak et al., 2006), NFT consist of hyperphosphorylated tau and were widely observed in entorhinal cortex, although it is variable in hippocampus and rarely seen in the superior frontal cortex or in area 9 (Tanprasertsuk et al., 2019; Tsartsalis et al., 2018). In NHP, the location of tau deposition varies, and generally occurs exclusively in the oldest animals across species. Generally, the distribution of tau pathology is not correlated to amyloid deposits, with the exception of gorillas and chimpanzees, where tau is observed in proximity to Aβ plaques (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016). Moreover, in contrast to humans, great apes appear to lack dystrophic neurites associated with plaques. In old chimpanzees, baboons, rhesus macaques, and vervet monkeys, classic NFT have been detected (see Fig. 4), however they were absent in lowland gorillas, mountain gorillas, and orangutans (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016; Rosen et al., 2008). In addition, neuritic clusters were identified in old chimpanzees and gorillas (Edler et al., 2017; Perez et al., 2013; Perez et al., 2016; Rosen et al., 2008). In other species, grey mouse lemurs showed hyperphosphorylated tau accumulation in neurons and neurites in the neocortex and hippocampus (Bons et al., 1994; Kraska et al., 2011). Among ceboids, marmosets showed hyperphosphorylated tau deposits in hippocampal CA3, entorhinal, temporal and parietal cortex (Rodriguez-Callejas et al., 2016), squirrel monkeys showed rare neuritic tau in the neocortex and no tau was detected in cotton-top tamarins (Lemere et al., 2008; Rosen et al., 2016). In the group of cercopithecids, baboons showed abnormal phosphorylated tau deposition in hippocampus and entorhinal cortex (Härtig et al., 2000; Schultz, Dehghani, et al., 2000), and in macaques, vervets and guenons, neuritic or cytoplasmic tau was sparsely scattered in the cerebral cortex (Cramer et al., 2018; Härtig et al., 2000; Latimer et al., 2019; Shah et al., 2010). Old macaques also showed NFT in the entorhinal cortex (Datta et al., 2021; Paspalas et al., 2018). In long-tailed macaques, hyperphosphorylated tau aggregates were more prevalent in oligodendrocytes than in neurons, reminiscent of the pathology seen in progressive supranuclear palsy and corticobasal degeneration (Kiatipattanasakul et al., 2000; Oikawa et al., 2010; Uchihara et al., 2016).
A neuroinflammatory response associated to AD-like pathologies has been described in many primate models in the form of abnormal changes in glia and microglia (Table 1). Generally, in the NHP species reviewed, except in cases where data is scarce, like in baboons, patas monkeys, guenons and squirrel monkeys, astrocytosis was widely described in the area adjacent to compact amyloid deposits. Along with changes in astrocyte volume and/or density in great apes, macaque and marmoset also demonstrate higher microglial densities and morphologic changes (Darusman et al., 2014; Edler et al., 2018; Munger et al., 2019; Nakamura et al., 1996; Rodríguez-Callejas et al., 2019; Rodriguez-Callejas et al., 2016; Shobin et al., 2017). Age-related changes have been described in microglial cells, where a shortening of their processes and a hypertrophic soma are related to the increase of their phagocytic activity. Preliminary results in old marmosets revealed comparable changes in microglia associated with impaired behavioral performance (Rothwell et al, this issue). Interestingly, in old marmosets, the state of microglia activation has also been related to the ferritin content in the microglial cell that parallels the observed in human studies (Lopes et al., 2008; Rodríguez-Callejas et al., 2019). The number of ferritin-containing microglia decrease in old individuals, suggesting that ferritin confers protection to oxidative stress damage of microglia during aging.
Finally, the differences between Aβ and tau pathology or neuroinflammatory changes in the neocortex and hippocampus of aging NHP and those seen in AD may only partly explain the absence of neurodegenerative disease such as AD, Parkinson’s disease or frontotemporal dementia in NHP. Other age-related changes, such as TDP-43 inclusions or accumulation of α-synuclein in Lewy bodies, are also relevant to neurodegeneration in these age-related diseases.
7. CONCLUSION AND FUTURE DIRECTIONS
Primates share many aspects of brain biology and life history in common due to evolutionary ancestry. However, different species of primates display a wide variation in brain size, neuroanatomical structure, development, and lifespan. As an example, with respect to lifespan, the estimates we provide in Table 1 are of maximum lifespan and have been revised to reflect recent reports of extreme aging in some NHP (Finch & Sapolsky, 1999; Hakeem et al., 1996; Stonebarger et al., 2020). Consequently, there are important differences among primate taxa in aging and neurodegeneration. Overall, the use of NHP as models in aging and neurodegeneration research provide a unique and highly valuable source with direct translation to humans.
One important example is the beneficial effect that hormone replacement therapy (HRT) regimen has upon cognition, as shown in morphometric analyses in female macaques (Hao et al., 2003; Hara et al., 2018). The expression of estrogen receptors shows high sensitivity to aging. Estrogen therapy enhanced cognition and induced spinogenesis in neuronal circuits in macaques. In a similar manner, HRT benefits on cognitive aging in this species were associated with structural plasticity and seem, in part, to contribute to the maintenance of complex presynaptic organizations in the PFC (Hao et al., 2007; Hao et al., 2006; Hara et al., 2018; Hara et al., 2011; Hara, Park, et al., 2012; Y. Hara et al., 2016). Interestingly, androgens may also affect the male brain in primates (Leranth et al., 2004) but little data are available on this subject. Overall, such findings emphasize the critical need to incorporate both sexes in NHP aging research, especially in light of sex differences characterizing cognitive aging trajectories and AD in humans (McCarrey et al., 2016; Mielke, 2020).
Regarding the investigation of the AD-like neuropathology, the apparent resistance of most primates to overt NFT pathology and AD-like neurodegeneration could offer insight into the predisposition of humans to develop AD. Moreover, the presence of Aβ pathology and CAA in most aging NHP and tau pathology in some NHP offers opportunity for testing anti-Aβ therapies or p-tau reducing therapies for their effects on cognition. Aβ proteins are the targets of immunotherapy treatments and vaccinations for AD. In old vervets, subcutaneous injections of Aβ40 and Aβ42 was administered for antibody generation in blood and CSF. The immunized animals showed a decrease in amyloid plaques along with a decrease in gliosis and neuritic dystrophy (Lemere et al., 2004). Similarly, recombinant adeno-associated virus (rAAV) vector expressing Aβ1–43 was used to vaccinate African green monkeys. Vaccinated animals showed fewer plaques measured as total area and Aβ-positive neurons compared to the control group (H. Hara et al., 2016). Anti-Aβ therapies, both immunization-based and antibody-based, have also been tested in rhesus macaques and the latter were found to reduce Aβ aggregation (Boado et al., 2007; Evans et al., 2014; Gandy, DeMattos, Lemere, Heppner, Leverone, Aguzzi, Ershler, Dai, Fraser, Hyslop, et al., 2004; Gandy, DeMattos, Lemere, Heppner, Leverone, Aguzzi, Ershler, Dai, Fraser, St George Hyslop, et al., 2004; Lambracht-Washington et al., 2017). Other Aβ reduction therapies tested in macaques include γ-secretase inhibition (Cook et al., 2010). Among therapies targeting tau, pharmaceutical modulation of calcium-mediated regulators of tau phosphorylation in old macaques improved cognition (Datta et al., 2021).
Moreover, recent efforts have been directed towards the development of new therapeutics modulating neuroinflammatory responses in the aging brain. Extracellular vesicles (EVs) from mesenchymal stem cells (MSCs) are lipid-bound, nanoscale vesicles that mediate cell-to-cell inflammatory and trophic signaling. Old rhesus macaques treated with MSC-EVs after cortical injury showed a recovery in motor function, possibly mediated by an increase in density of ramified, homeostatic microglia, along with reduced pro-inflammatory microglial markers (Go et al., 2020). This modulation of neuroinflammatory pathways affects neural plasticity by reducing injury-related physiological and morphologic changes in perilesional layer III pyramidal neurons. These neurons showed a greater apical dendritic branching complexity and spine density (Medalla et al., 2020).
New lines of investigation in NHP, that parallel human studies, include the study of age-related changes in DNA methylation levels. DNA methylation is an epigenetic mark involving the additional of methyl chemical groups to the DNA base cytosine. It functions as a gene regulatory mechanism that is essential for normal development and the maintenance of cell identity. In humans, altered DNA methylation profiles have been associated with cognitive decline and neuropathology, including AD in both peripheral blood and brain tissue (Levine et al., 2015; Marioni et al., 2015; McCartney et al., 2018; Wei et al., 2020). Similar studies in chimpanzees show an accelerated rate of epigenetic aging in blood compared with humans, potentially reflecting their accelerated life pacing and shorter lifespans (Guevara et al., 2020). Intriguingly, humans appear to show faster epigenetic aging in the PFC compared to other tissues, which may reflect greater age-related change in this brain structure (Marioni et al., 2015) despite overall slower organismal aging in humans compared with chimpanzees (Guevara et al., in press). Loci showing age-related change in methylation in both species, human and chimpanzees, include genes implicated in neuroinflammation and AD, like somatostatin (SST) (Saito et al., 2005). Whereas genes found to show significant differential methylation between humans and other primates in the brain include dipeptidyl peptidase 10 (DPP10) in the PFC, which plays a role in synaptic transmission via potassium channel function and shows increased expression at Alzheimer’s disease-related pathologies, and ankyrin repeat and sterile alpha motif domain containing 1B (ANKS1B) in the cerebellum, which is a postsynaptic scaffold that binds APP (Chen et al., 2014; Guevara et al., 2021; Mendizabal et al., 2016). These differences may reflect human-specific aspects of pathways involved in brain aging (Guevara et al., 2021).
Despite all of the ongoing research in NHP, there is still a paucity of approaches including both postmortem neuropathologic analyses and antemortem cognitive data. The lack of these structure-function investigations is attributable to ethical reasons, slower breeding rates, high care costs, requirement of large spaces with enriched environments and in some cases the restricted numbers of certain species available. Emerging models like the marmoset have been proposed as complementary to larger primate models, due to their ease of handling, quick reproduction, availability of neuroanatomy atlases, and brain organization comparable to larger NHP and humans. Thus, marmosets may offer a suitable bridge between NHP and rodents for neuroscience and aging research (Miller, 2017; Tardif et al., 2011). In addition, the National Chimpanzee Brain Resource (www.chimpanzeebrain.org) is an integrated biobanking resource that curates MRI data, postmortem brain tissue, health records, rearing histories, pedigrees, and performance on cognitive and behavioral tasks. Further investigation incorporating suitable NHP models and advanced therapeutic interventions would be pivotal to understand the clinical manifestation of AD and address the progressive decline in cognition seen during normal aging and in AD.
ACKNOWLEDGEMENTS
Funding:
AG014449 (Mufson, Perez), AG014308 (Erwin), AG059028 and AG049870 (Hof, Luebke), AG046266 (Lacreuse, Hof), AG067419 (Raghanti, Edler, Guevara, Sherwood), AG005138 and AG066514 (Hof).
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interests.
ETHICAL STATEMENT
The research included in this article adhered to the guidelines for the Ethical Treatment of Non-Human Primates provided by the American Society of Primatology. All research conducted was approved by the University Institutional Animal Care and Use Committee.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aizawa K, Ageyama N, Terao K, & Hisatsune T (2011, January). Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging, 32(1), 140–150. 10.1016/j.neurobiolaging.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Yokoyama C, & Hisatsune T (2009, July). Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim, 58(4), 403–407. 10.1538/expanim.58.403 [DOI] [PubMed] [Google Scholar]
- Akram A, Christoffel D, Rocher AB, Bouras C, Kovari E, Perl DP, Morrison JH, Herrmann FR, Haroutunian V, Giannakopoulos P, & Hof PR (2008, September). Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiol Aging, 29(9), 1296–1307. 10.1016/j.neurobiolaging.2007.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Benton JS, Goodhardt MJ, Haan EA, Sims NR, Smith CC, Spillane JA, Bowen DM, & Davison AN (1983, July). Biochemical evidence of selective nerve cell changes in the normal ageing human and rat brain. J Neurochem, 41(1), 256–265. 10.1111/j.1471-4159.1983.tb11837.x [DOI] [PubMed] [Google Scholar]
- Amatrudo JM, Weaver CM, Crimins JL, Hof PR, Rosene DL, & Luebke JI (2012, October 3). Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci, 32(40), 13644–13660. 10.1523/jneurosci.2581-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Hubbard BM, Coghill GR, & Slidders W (1983, February). The effect of advanced old age on the neurone content of the cerebral cortex. Observations with an automatic image analyser point counting method. J Neurol Sci, 58(2), 235–246. 10.1016/0022-510x(83)90220-4 [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, & Hyman BT (1992, September). Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology, 42(9), 1681–1688. 10.1212/wnl.42.9.1681 [DOI] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, & Schneider JA (2011, February). Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol, 69(2), 320–327. 10.1002/ana.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atapour N, & Rosa MGP (2017, September). Age-related plasticity of the axon initial segment of cortical pyramidal cells in marmoset monkeys. Neurobiol Aging, 57, 95–103. 10.1016/j.neurobiolaging.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Ighodaro ET, Hassoun Y, Aldeiri D, Neltner JH, Patel E, Abner EL, & Nelson PT (2017, April). Rod-shaped microglia morphology is associated with aging in 2 human autopsy series. Neurobiol Aging, 52, 98–105. 10.1016/j.neurobiolaging.2016.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Hof PR, Standaert DG, Xing Y, Nelson SE, Young AB, & Magnusson KR (2004, February). Changes in the expression of the NR2B subunit during aging in macaque monkeys. Neurobiol Aging, 25(2), 201–208. 10.1016/s0197-4580(03)00091-5 [DOI] [PubMed] [Google Scholar]
- Beckman D, Ott S, Donis-Cox K, Janssen WG, Bliss-Moreau E, Rudebeck PH, Baxter MG, & Morrison JH (2019, December 23). Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc Natl Acad Sci U S A, 116(52), 26239–26246. 10.1073/pnas.1902301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Giorgetti B, Grossi Y, Balietti M, Casoli T, Di Stefano G, & Perretta G (2006, Spring). Synaptic pathology in the brain cortex of old monkeys as an early alteration in senile plaque formation. Rejuvenation Res, 9(1), 85–88. 10.1089/rej.2006.9.85 [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Giorgetti B, Grossi Y, Balietti M, Casoli T, Di Stefano G, & Perretta G (2007, January). Alterations of synaptic turnover rate in aging may trigger senile plaque formation and neurodegeneration. Ann N Y Acad Sci, 1096, 128–137. 10.1196/annals.1397.078 [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Pieroni M, Meier-Ruge W, & Ulrich J (1992, June). Enlargement of synaptic size as a compensative reaction in aging and dementia. Pathol Res Pract, 188(4–5), 612–615. 10.1016/s0344-0338(11)80066-x [DOI] [PubMed] [Google Scholar]
- Bertrand A, Pasquier A, Petiet A, Wiggins C, Kraska A, Joseph-Mathurin N, Aujard F, Mestre-Francés N, & Dhenain M (2013). Micro-MRI study of cerebral aging: ex vivo detection of hippocampal subfield reorganization, microhemorrhages and amyloid plaques in mouse lemur primates. PLoS One, 8(2), e56593. 10.1371/journal.pone.0056593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Xia CF, & Pardridge WM (2007, Mar-Apr). Fusion antibody for Alzheimer’s disease with bidirectional transport across the blood-brain barrier and abeta fibril disaggregation. Bioconjug Chem, 18(2), 447–455. 10.1021/bc060349x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, & Mann JJ (2018, April 5). Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell, 22(4), 589–599.e585. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N, Jallageas V, Silhol S, Mestre-Frances N, Petter A, & Delacourte A (1995, July). Immunocytochemical characterization of Tau proteins during cerebral aging of the lemurian primate Microcebus murinus. C R Acad Sci III, 318(7), 741–747. https://www.ncbi.nlm.nih.gov/pubmed/7583762 [PubMed] [Google Scholar]
- Bons N, Mestre N, & Petter A (1992, Jan-Feb). Senile plaques and neurofibrillary changes in the brain of an aged lemurian primate, Microcebus murinus. Neurobiol Aging, 13(1), 99–105. 10.1016/0197-4580(92)90016-q [DOI] [PubMed] [Google Scholar]
- Bons N, Mestre N, Ritchie K, Petter A, Podlisny M, & Selkoe D (1994, Mar-Apr). Identification of amyloid beta protein in the brain of the small, short-lived lemurian primate Microcebus murinus. Neurobiol Aging, 15(2), 215–220. 10.1016/0197-4580(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Boros BD, Greathouse KM, Gearing M, & Herskowitz JH (2019, January). Dendritic spine remodeling accompanies Alzheimer’s disease pathology and genetic susceptibility in cognitively normal aging. Neurobiol Aging, 73, 92–103. 10.1016/j.neurobiolaging.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Hof PR, Giannakopoulos P, Michel JP, & Morrison JH (1994, Mar-Apr). Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex, 4(2), 138–150. 10.1093/cercor/4.2.138 [DOI] [PubMed] [Google Scholar]
- Bouras C, Hof PR, & Morrison JH (1993, April 30). Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett, 153(2), 131–135. 10.1016/0304-3940(93)90305-5 [DOI] [PubMed] [Google Scholar]
- Bowley MP, Cabral H, Rosene DL, & Peters A (2010, August 1). Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol, 518(15), 3046–3064. 10.1002/cne.22379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, & Del Tredici K (2006, October). Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol, 112(4), 389–404. 10.1007/s00401-006-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody H (1955, April). Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol, 102(2), 511–516. 10.1002/cne.901020206 [DOI] [PubMed] [Google Scholar]
- Bu J, Sathyendra V, Nagykery N, & Geula C (2003, July). Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp Neurol, 182(1), 220–231. 10.1016/s0014-4886(03)00094-3 [DOI] [PubMed] [Google Scholar]
- Buell SJ, & Coleman PD (1979, November 16). Dendritic growth in the aged human brain and failure of growth in senile dementia. Science, 206(4420), 854–856. 10.1126/science.493989 [DOI] [PubMed] [Google Scholar]
- Bunk EC, Stelzer S, Hermann S, Schäfers M, Schlatt S, & Schwamborn JC (2011, February). Cellular organization of adult neurogenesis in the Common Marmoset. Aging Cell, 10(1), 28–38. 10.1111/j.1474-9726.2010.00639.x [DOI] [PubMed] [Google Scholar]
- Bussière T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Giannakopoulos P, Robakis NK, Morrison JH, Perl DP, & Hof PR (2002). Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience, 112(1), 75–91. 10.1016/s0306-4522(02)00056-8 [DOI] [PubMed] [Google Scholar]
- Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, & Hof PR (2003, August 25). Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol, 463(3), 281–302. 10.1002/cne.10760 [DOI] [PubMed] [Google Scholar]
- Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, & Hof PR (2003). Stereologic analysis of neurofibrillary tangle formation in prefrontal cortex area 9 in aging and Alzheimer’s disease. Neuroscience, 117(3), 577–592. 10.1016/s0306-4522(02)00942-9 [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Mao Y, Roberts JA, & Rapp PR (2004, July 19). Reduction in hippocampal cholinergic innervation is unrelated to recognition memory impairment in aged rhesus monkeys. J Comp Neurol, 475(2), 238–246. 10.1002/cne.20181 [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, Simen AA, Ramos BP, Bordner KA, Craft GE, Davies P, Pletikos M, Sestan N, Arnsten AF, & Paspalas CD (2014, April 1). cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A, 111(13), 5036–5041. 10.1073/pnas.1322360111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Gai WP, & Abbott CA (2014). Dipeptidyl peptidase 10 (DPP10(789)): a voltage gated potassium channel associated protein is abnormally expressed in Alzheimer’s and other neurodegenerative diseases. Biomed Res Int, 2014, 209398. 10.1155/2014/209398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Menzies SL, St Martin SM, & Mufson EJ (1990, December). Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res, 27(4), 595–611. 10.1002/jnr.490270421 [DOI] [PubMed] [Google Scholar]
- Cook JJ, Wildsmith KR, Gilberto DB, Holahan MA, Kinney GG, Mathers PD, Michener MS, Price EA, Shearman MS, Simon AJ, Wang JX, Wu G, Yarasheski KE, & Bateman RJ (2010, May 12). Acute gamma-secretase inhibition of nonhuman primate CNS shifts amyloid precursor protein (APP) metabolism from amyloid-beta production to alternative APP fragments without amyloid-beta rebound. J Neurosci, 30(19), 6743–6750. 10.1523/JNEUROSCI.1381-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork LC, Masters C, Beyreuther K, & Price DL (1990, December). Development of senile plaques. Relationships of neuronal abnormalities and amyloid deposits. Am J Pathol, 137(6), 1383–1392. https://www.ncbi.nlm.nih.gov/pubmed/1701963 [PMC free article] [PubMed] [Google Scholar]
- Coskren PJ, Luebke JI, Kabaso D, Wearne SL, Yadav A, Rumbell T, Hof PR, & Weaver CM (2015, April). Functional consequences of age-related morphologic changes to pyramidal neurons of the rhesus monkey prefrontal cortex. J Comput Neurosci, 38(2), 263–283. 10.1007/s10827-014-0541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court JA, Perry EK, Johnson M, Piggott MA, Kerwin JA, Perry RH, & Ince PG (1993, July 16). Regional patterns of cholinergic and glutamate activity in the developing and aging human brain. Brain Res Dev Brain Res, 74(1), 73–82. 10.1016/0165-3806(93)90085-o [DOI] [PubMed] [Google Scholar]
- Cragg BG (1975, March). The density of synapses and neurons in normal, mentally defective ageing human brains. Brain, 98(1), 81–90. 10.1093/brain/98.1.81 [DOI] [PubMed] [Google Scholar]
- Cramer PE, Gentzel RC, Tanis KQ, Vardigan J, Wang Y, Connolly B, Manfre P, Lodge K, Renger JJ, Zerbinatti C, & Uslaner JM (2018, April). Aging African green monkeys manifest transcriptional, pathological, and cognitive hallmarks of human Alzheimer’s disease. Neurobiol Aging, 64, 92–106. 10.1016/j.neurobiolaging.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Crimins JL, Puri R, Calakos KC, Yuk F, Janssen WGM, Hara Y, Rapp PR, & Morrison JH (2019, March 1). Synaptic distributions of pS214-tau in rhesus monkey prefrontal cortex are associated with spine density, but not with cognitive decline. J Comp Neurol, 527(4), 856–873. 10.1002/cne.24576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimins JL, Wang AC, Yuk F, Puri R, Janssen WGM, Hara Y, Rapp PR, & Morrison JH (2017, March 1). Diverse Synaptic Distributions of G Protein-coupled Estrogen Receptor 1 in Monkey Prefrontal Cortex with Aging and Menopause. Cereb Cortex, 27(3), 2022–2033. 10.1093/cercor/bhw050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darusman HS, Gjedde A, Sajuthi D, Schapiro SJ, Kalliokoski O, Kristianingrum YP, Handaryani E, & Hau J (2014). Amyloid Beta1–42 and the Phoshorylated Tau Threonine 231 in Brains of Aged Cynomolgus Monkeys (Macaca fascicularis). Front Aging Neurosci, 6, 313. 10.3389/fnagi.2014.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Leslie SN, Wang M, Morozov YM, Yang S, Mentone S, Zeiss C, Duque A, Rakic P, Horvath TL, van Dyck CH, Nairn AC, & Arnsten AFT (2021, April 7). Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimers Dement 10.1002/alz.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DG, Schmitt FA, Wekstein DR, & Markesbery WR (1999, April). Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol, 58(4), 376–388. 10.1097/00005072-199904000-00008 [DOI] [PubMed] [Google Scholar]
- de Brabander JM, Kramers RJ, & Uylings HB (1998, April). Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci, 10(4), 1261–1269. 10.1046/j.1460-9568.1998.00137.x [DOI] [PubMed] [Google Scholar]
- de Keyser J, De Backer JP, Vauquelin G, & Ebinger G (1990, October 1). The effect of aging on the D1 dopamine receptors in human frontal cortex. Brain Res, 528(2), 308–310. 10.1016/0006-8993(90)91672-4 [DOI] [PubMed] [Google Scholar]
- DiPatre PL, & Gelman BB (1997, February). Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J Neuropathol Exp Neurol, 56(2), 143–149. 10.1097/00005072-199702000-00004 [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Morrison JH, & Hof PR (2002). Quantitative analysis of the dendritic morphology of corticocortical projection neurons in the macaque monkey association cortex. Neuroscience, 114(2), 349–359. 10.1016/s0306-4522(02)00305-6 [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, & Hof PR (2003, September). Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex, 13(9), 950–961. 10.1093/cercor/13.9.950 [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, & Morrison JH (2010, June 2). Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci, 30(22), 7507–7515. 10.1523/jneurosci.6410-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Weickert CS, Webster MJ, Herman MM, Kleinman JE, & Harrison PJ (2006). Synaptophysin protein and mRNA expression in the human hippocampal formation from birth to old age. Hippocampus, 16(8), 645–654. 10.1002/hipo.20194 [DOI] [PubMed] [Google Scholar]
- Edler MK, Munger EL, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, Sherwood CC, & Raghanti MA (2020, November 9). Neuron loss associated with age but not Alzheimer’s disease pathology in the chimpanzee brain. Philos Trans R Soc Lond B Biol Sci, 375(1811), 20190619. 10.1098/rstb.2019.0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Hopkins WD, Ely JJ, Erwin JM, Mufson EJ, Hof PR, & Raghanti MA (2017, November). Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol Aging, 59, 107–120. 10.1016/j.neurobiolaging.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edler MK, Sherwood CC, Meindl RS, Munger EL, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, & Raghanti MA (2018). Microglia changes associated to Alzheimer’s disease pathology in aged chimpanzees. J Comp Neurol, 526, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein HA, Rosen RF, Stephens SL, Switzer RC, Smith Y, Pare J, Mehta PD, Warzok R, & Walker LC (2007, February). Cerebral beta-amyloid angiopathy in aged squirrel monkeys. Histol Histopathol, 22(2), 155–167. 10.14670/hh-22.155 [DOI] [PubMed] [Google Scholar]
- Evans CF, Davtyan H, Petrushina I, Hovakimyan A, Davtyan A, Hannaman D, Cribbs DH, Agadjanyan MG, & Ghochikyan A (2014, May). Epitope-based DNA vaccine for Alzheimer’s disease: translational study in macaques. Alzheimers Dement, 10(3), 284–295. 10.1016/j.jalz.2013.04.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Jacobsen JS, & Pakkenberg B (2013, January). Effect of age on neocortical brain cells in 90+ year old human females--a cell counting study. Neurobiol Aging, 34(1), 91–99. 10.1016/j.neurobiolaging.2012.06.009 [DOI] [PubMed] [Google Scholar]
- Finch CE, & Sapolsky RM (1999, Jul-Aug). The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging, 20(4), 407–428. 10.1016/s0197-4580(99)00053-6 [DOI] [PubMed] [Google Scholar]
- Flood DG (1991, February 1). Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. II. Subiculum. Brain Res, 540(1–2), 83–95. 10.1016/0006-8993(91)90494-g [DOI] [PubMed] [Google Scholar]
- Flood DG, Buell SJ, Defiore CH, Horwitz GJ, & Coleman PD (1985, October 21). Age-related dendritic growth in dentate gyrus of human brain is followed by regression in the ‘oldest old’. Brain Res, 345(2), 366–368. 10.1016/0006-8993(85)91018-2 [DOI] [PubMed] [Google Scholar]
- Flood DG, Buell SJ, Horwitz GJ, & Coleman PD (1987, February 3). Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res, 402(2), 205–216. 10.1016/0006-8993(87)90027-8 [DOI] [PubMed] [Google Scholar]
- Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP, Hedley-Whyte ET, Locascio JJ, Lipsitz LA, & Hyman BT (2008, December). Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol, 67(12), 1205–1212. 10.1097/NEN.0b013e31818fc72f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galva MD, Bondiolotti GP, Olasmaa M, & Picotti GB (1995). Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect, 101(1–3), 83–94. 10.1007/bf01271547 [DOI] [PubMed] [Google Scholar]
- Gandy S, DeMattos RB, Lemere CA, Heppner FL, Leverone J, Aguzzi A, Ershler WB, Dai J, Fraser P, Hyslop PS, Holtzman DM, Walker LC, & Keller ET (2004, Jan-Mar). Alzheimer A beta vaccination of rhesus monkeys (Macaca mulatta). Alzheimer Dis Assoc Disord, 18(1), 44–46. 10.1097/00002093-200401000-00009 [DOI] [PubMed] [Google Scholar]
- Gandy S, DeMattos RB, Lemere CA, Heppner FL, Leverone J, Aguzzi A, Ershler WB, Dai J, Fraser P, St George Hyslop P, Holtzman DM, Walker LC, & Keller ET (2004, February). Alzheimer’s Abeta vaccination of rhesus monkeys (Macaca mulatta). Mech Ageing Dev, 125(2), 149–151. 10.1016/j.mad.2003.12.002 [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, & Morrison JH (1997, Sep-Oct). Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging, 18(5), 549–553. 10.1016/s0197-4580(97)00112-7 [DOI] [PubMed] [Google Scholar]
- Gearing M, Rebeck GW, Hyman BT, Tigges J, & Mirra SS (1994). Neuropathology and apolipoprotein E profile of aged chimpanzees: Implications for Alzheimer disease. Proc Natl Acad Sci USA, 91, 9382–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, & Mirra SS (1996). Aβ40 is a major form of β-amyloid in nonhuman primates. Neurobiol Aging, 17, 903–908. 10.1016/S0197-4580(96)00164-9 [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, & Mirra SS (1997). Beta-amyloid (A-beta) deposition in the brains of aged orangutans. Neurobiol Aging, 18, 139–146. [DOI] [PubMed] [Google Scholar]
- Gefen T, Kim G, Bolbolan K, Geoly A, Ohm D, Oboudiyat C, Shahidehpour R, Rademaker A, Weintraub S, Bigio EH, Mesulam MM, Rogalski E, & Geula C (2019). Activated Microglia in Cortical White Matter Across Cognitive Aging Trajectories. Front Aging Neurosci, 11, 94. 10.3389/fnagi.2019.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, & Mesulam MM (1989). Cortical cholinergic fibers in aging and Alzheimer’s disease: a morphometric study. Neuroscience, 33(3), 469–481. 10.1016/0306-4522(89)90399-0 [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, & Wu CK (2002, January). Amyloid-beta deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol, 103(1), 48–58. 10.1007/s004010100429 [DOI] [PubMed] [Google Scholar]
- Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, & Yankner BA (1998, July). Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med, 4(7), 827–831. 10.1038/nm0798-827 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Giannakopoulos AS, Buée-Scherrer V, Surini M, Delacourte A, & Bouras C (1994, Nov-Dec). Dementia in the oldest-old: quantitative analysis of 12 cases from a psychiatric hospital. Dementia, 5(6), 348–356. 10.1159/000106745 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Giannakopoulos AS, Herrmann FR, Michel JP, & Bouras C (1995, December). Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol, 52(12), 1150–1159. 10.1001/archneur.1995.00540360028012 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Kövari E, Vallet PG, Herrmann FR, & Bouras C (1996, December). Distinct patterns of neuronal loss and Alzheimer’s disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol, 55(12), 1210–1220. 10.1097/00005072-199612000-00004 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Michel JP, Guimon J, & Bouras C (1997, October). Cerebral cortex pathology in aging and Alzheimer’s disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res Brain Res Rev, 25(2), 217–245. 10.1016/s0165-0173(97)00023-4 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Mottier S, Michel JP, & Bouras C (1994). Neuropathological changes in the cerebral cortex of 1258 cases from a geriatric hospital: retrospective clinicopathological evaluation of a 10-year autopsy population. Acta Neuropathol, 87(5), 456–468. 10.1007/bf00294172 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Surini M, Michel JP, & Bouras C (1993). Quantitative immunohistochemical analysis of the distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of nonagenarians and centenarians. Acta Neuropathol, 85(6), 602–610. 10.1007/bf00334669 [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Silhol S, Jallageas V, Mallet J, Bons N, Bouras C, & Delaère P (1997, August). Quantitative analysis of tau protein-immunoreactive accumulations and beta amyloid protein deposits in the cerebral cortex of the mouse lemur, Microcebus murinus. Acta Neuropathol, 94(2), 131–139. 10.1007/s004010050684 [DOI] [PubMed] [Google Scholar]
- Gibson PH (1983). EM study of the numbers of cortical synapses in the brains of ageing people and people with Alzheimer-type dementia. Acta Neuropathol, 62(1–2), 127–133. 10.1007/bf00684929 [DOI] [PubMed] [Google Scholar]
- Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, & Moore TL (2020, February). Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience, 42(1), 1–17. 10.1007/s11357-019-00115-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, McKeel DW Jr., Morris JC, Growdon JH, & Hyman BT (1996, July 15). Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci, 16(14), 4491–4500. 10.1523/jneurosci.16-14-04491.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Isseroff R, Salama D, Israeli M, & Biegon A (1990, June 11). Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res, 519(1–2), 223–227. 10.1016/0006-8993(90)90081-l [DOI] [PubMed] [Google Scholar]
- Guevara EE, Hopkins WD, Hof PR, Ely JJ, Bradley BJ, & Sherwood CC (2021). Comparative analysis reveals distinctive epigenetic features of the human cerebellum. PLoS Genet, 17(5), e1009506. 10.1371/journal.pgen.1009506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara EE, Lawler RR, Staes N, White CM, Sherwood CC, Ely JJ, Hopkins WD, & Bradley BJ (2020). Age-associated epigenetic change in chimpanzees and humans. Philos Trans R Soc, B, 375, 20190616. 10.1098/rstb.2019.0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeem A, Sandoval R, Jones M, & Allman J (1996). Brain and life span in primates. In Birren J (Ed.), Handbook of the Psychology of Aging (pp. 78–104). Academic Press, San Diego. [Google Scholar]
- Hanks SD, & Flood DG (1991, February 1). Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res, 540(1–2), 63–82. 10.1016/0006-8993(91)90493-f [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, & Morrison JH (2003, October 27). Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol, 465(4), 540–550. 10.1002/cne.10837 [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, & Morrison JH (2007, July 3). Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A, 104(27), 11465–11470. 10.1073/pnas.0704757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, & Morrison JH (2006, March 1). Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci, 26(9), 2571–2578. 10.1523/jneurosci.3440-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Ono F, Nakamura S, Matsumoto SE, Jin H, Hattori N, & Tabira T (2016, October 4). An Oral Aβ Vaccine Using a Recombinant Adeno-Associated Virus Vector in Aged Monkeys: Reduction in Plaque Amyloid and Increase in Aβ Oligomers. J Alzheimers Dis, 54(3), 1047–1059. 10.3233/jad-160514 [DOI] [PubMed] [Google Scholar]
- Hara Y, Crimins JL, Puri R, Wang ACJ, Motley SE, Yuk F, Ramos TM, Janssen WGM, Rapp PR, & Morrison JH (2018, December 1). Estrogen Alters the Synaptic Distribution of Phospho-GluN2B in the Dorsolateral Prefrontal Cortex While Promoting Working Memory in Aged Rhesus Monkeys. Neuroscience, 394, 303–315. 10.1016/j.neuroscience.2018.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, & Morrison JH (2011, May 25). Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci, 31(21), 7737–7744. 10.1523/jneurosci.0822-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, & Rapp PR (2012, February). Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging, 33(2), 421.e417–428. 10.1016/j.neurobiolaging.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Punsoni M, Yuk F, Park CS, Janssen WG, Rapp PR, & Morrison JH (2012, May 23). Synaptic distributions of GluA2 and PKMζ in the monkey dentate gyrus and their relationships with aging and memory. J Neurosci, 32(21), 7336–7344. 10.1523/jneurosci.0605-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, & Morrison JH (2014, January 7). Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A, 111(1), 486–491. 10.1073/pnas.1311310110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, & Morrison JH (2016, January 20). Estrogen Restores Multisynaptic Boutons in the Dorsolateral Prefrontal Cortex while Promoting Working Memory in Aged Rhesus Monkeys. J Neurosci, 36(3), 901–910. 10.1523/jneurosci.3480-13.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W, Bruckner G, Schmidt C, Brauer K, Bodewitz G, Turner JD, & Bigl V (1997, March 21). Co-localization of beta-amyloid peptides, apolipoprotein E and glial markers in senile plaques in the prefrontal cortex of old rhesus monkeys. Brain Res, 751(2), 315–322. 10.1016/s0006-8993(96)01423-0 [DOI] [PubMed] [Google Scholar]
- Härtig W, Goldhammer S, Bauer U, Wegner F, Wirths O, Bayer TA, & Grosche J (2010, September). Concomitant detection of beta-amyloid peptides with N-terminal truncation and different C-terminal endings in cortical plaques from cases with Alzheimer’s disease, senile monkeys and triple transgenic mice. J Chem Neuroanat, 40(1), 82–92. 10.1016/j.jchemneu.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Härtig W, Klein C, Brauer K, Schüppel KF, Arendt T, Brückner G, & Bigl V (2000, September). Abnormally phosphorylated protein tau in the cortex of aged individuals of various mammalian orders. Acta Neuropathol, 100(3), 305–312. 10.1007/s004010000183 [DOI] [PubMed] [Google Scholar]
- Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, & Morrison JH (2002, February 22). Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res, 928(1–2), 175–186. 10.1016/s0006-8993(01)03345-5 [DOI] [PubMed] [Google Scholar]
- Hof PR, Gilissen EP, Sherwood CC, Duan H, Lee PWH, Delman BN, Naidich TP, Gannon PJ, Perl DP, & Erwin JM (2002). Comparative Neuropathology of Brain Aging in Primates. Interdisciplinary Topics in Gerontology and Geriatrics, 31, 130–154. 10.1159/000061462 [DOI] [Google Scholar]
- Huttenlocher PR (1979, March 16). Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res, 163(2), 195–205. 10.1016/0006-8993(79)90349-4 [DOI] [PubMed] [Google Scholar]
- Ibañez S, Luebke JI, Chang W, Draguljić D, & Weaver CM (2019). Network Models Predict That Pyramidal Neuron Hyperexcitability and Synapse Loss in the dlPFC Lead to Age-Related Spatial Working Memory Impairment in Rhesus Monkeys. Front Comput Neurosci, 13, 89. 10.3389/fncom.2019.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N (2015, April). On-going elucidation of mechanisms of primate specific synaptic spine development using the common marmoset (Callithrix jacchus). Neurosci Res, 93, 176–178. 10.1016/j.neures.2014.10.019 [DOI] [PubMed] [Google Scholar]
- Inoue M, Suhara T, Sudo Y, Okubo Y, Yasuno F, Kishimoto T, Yoshikawa K, & Tanada S (2001, July 20). Age-related reduction of extrastriatal dopamine D2 receptor measured by PET. Life Sci, 69(9), 1079–1084. 10.1016/s0024-3205(01)01205-x [DOI] [PubMed] [Google Scholar]
- Iyo M, & Yamasaki T (1993, May). The detection of age-related decrease of dopamine D1, D2 and serotonin 5-HT2 receptors in living human brain. Prog Neuropsychopharmacol Biol Psychiatry, 17(3), 415–421. 10.1016/0278-5846(93)90075-4 [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, & Schall M (1997, October 6). Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol, 386(4), 661–680. [PubMed] [Google Scholar]
- Johnson M, Perry RH, Piggott MA, Court JA, Spurden D, Lloyd S, Ince PG, & Perry EK (1996, Jul-Aug). Glutamate receptor binding in the human hippocampus and adjacent cortex during development and aging. Neurobiol Aging, 17(4), 639–651. 10.1016/0197-4580(96)00064-4 [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, & Rinne J (2000, Sep-Oct). Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging, 21(5), 683–688. 10.1016/s0197-4580(00)00149-4 [DOI] [PubMed] [Google Scholar]
- Kabaso D, Coskren PJ, Henry BI, Hof PR, & Wearne SL (2009, October). The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex, 19(10), 2248–2268. 10.1093/cercor/bhn242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinin S, Willard SL, Shively CA, Kaplan JR, Register TC, Jorgensen MJ, Polak PE, Rubinstein I, & Feinstein DL (2013, October). Development of amyloid burden in African Green monkeys. Neurobiol Aging, 34(10), 2361–2369. 10.1016/j.neurobiolaging.2013.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, & Verkhratsky A (2011). Physiology of microglia. Physiol Rev, 91, 461–553. [DOI] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, & Fuchs E (2003, Jan-Feb). Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging, 24(1), 157–165. 10.1016/s0197-4580(02)00062-3 [DOI] [PubMed] [Google Scholar]
- Kiatipattanasakul W, Nakayama H, Yongsiri S, Chotiapisitkul S, Nakamura S, Kojima H, & Doi K (2000, November). Abnormal neuronal and glial argyrophilic fibrillary structures in the brain of an aged albino cynomolgus monkey (Macaca fascicularis). Acta Neuropathol, 100(5), 580–586. 10.1007/s004010000215 [DOI] [PubMed] [Google Scholar]
- Kimura N, Nakamura S, Goto N, Narushima E, Hara I, Shichiri S, Saitou K, Nose M, Hayashi T, Kawamura S, & Yoshikawa Y (2001). Senile plaques in an aged western lowland gorilla. Exp Anim, 50, 77–81. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, & Yoshikawa Y (2003, October 17). Age-related changes of Alzheimer’s disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Commun, 310(2), 303–311. 10.1016/j.bbrc.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Kitt CA, Price DL, Struble RG, Cork LC, Wainer BH, Becher MW, & Mobley WC (1984, December 21). Evidence for cholinergic neurites in senile plaques. Science, 226(4681), 1443–1445. 10.1126/science.6505701 [DOI] [PubMed] [Google Scholar]
- Kitt CA, Struble RG, Cork LC, Mobley WC, Walker LC, Joh TH, & Price DL (1985, November). Catecholaminergic neurites in senile plaques in prefrontal cortex of aged nonhuman primates. Neuroscience, 16(3), 691–699. 10.1016/0306-4522(85)90202-7 [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, & Kempermann G (2010, January 29). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One, 5(1), e8809. 10.1371/journal.pone.0008809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Nakano M, Kubota K, Himuro N, Mizoguchi S, Chikenji T, Otani M, Mizue Y, Nagaishi K, & Fujimiya M (2018, January 26). Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep, 8(1), 1712. 10.1038/s41598-018-19442-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama R, Yang X, Saski Y, Iwashige S, Tanigawa Y, Yoshikawa T, Nagaoka T, Kamimura Y, & Maeda H (2010, February). Age-related lesions in the cerebrum in middle-aged female cynomolgus monkeys. Toxicol Pathol, 38(2), 303–311. 10.1177/0192623309358904 [DOI] [PubMed] [Google Scholar]
- Kövari E, Herrmann FR, Hof PR, & Bouras C (2013, August). The relationship between cerebral amyloid angiopathy and cortical microinfarcts in brain ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol, 39(5), 498–509. 10.1111/nan.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraska A, Dorieux O, Picq JL, Petit F, Bourrin E, Chenu E, Volk A, Perret M, Hantraye P, Mestre-Frances N, Aujard F, & Dhenain M (2011, May). Age-associated cerebral atrophy in mouse lemur primates. Neurobiol Aging, 32(5), 894–906. 10.1016/j.neurobiolaging.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Frost JL, Cotman CW, Head E, Palmour R, Lemere CA, & Walter J (2018, May). Deposition of phosphorylated amyloid-β in brains of aged nonhuman primates and canines. Brain Pathol, 28(3), 427–430. 10.1111/bpa.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambracht-Washington D, Fu M, Frost P, & Rosenberg RN (2017, April 26). Evaluation of a DNA Abeta42 vaccine in adult rhesus monkeys (Macaca mulatta): antibody kinetics and immune profile after intradermal immunization with full-length DNA Abeta42 trimer. Alzheimers Res Ther, 9(1), 30. 10.1186/s13195-017-0257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer CS, Shively CA, Keene CD, Jorgensen MJ, Andrews RN, Register TC, Montine TJ, Wilson AM, Neth BJ, Mintz A, Maldjian JA, Whitlow CT, Kaplan JR, & Craft S (2019, January). A nonhuman primate model of early Alzheimer’s disease pathologic change: Implications for disease pathogenesis. Alzheimers Dement, 15(1), 93–105. 10.1016/j.jalz.2018.06.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, & Ervin FR (2004, July). Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol, 165(1), 283–297. 10.1016/s0002-9440(10)63296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Oh J, Stanish HA, Peng Y, Pepivani I, Fagan AM, Yamaguchi H, Westmoreland SV, & Mansfield KG (2008, April). Cerebral amyloid-beta protein accumulation with aging in cotton-top tamarins: a model of early Alzheimer’s disease? Rejuvenation Res, 11(2), 321–332. 10.1089/rej.2008.0677 [DOI] [PubMed] [Google Scholar]
- Leranth C, Prange-Kiel J, Frick KM, & Horvath TL (2004, May). Low CA1 spine synapse density is further reduced by castration in male non-human primates. Cereb Cortex, 14(5), 503–510. 10.1093/cercor/bhh012 [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, & Gould E (2007, October 23). Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A, 104(43), 17169–17173. 10.1073/pnas.0708228104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Bennett DA, & Horvath S (2015, December). Epigenetic age of the prefrontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging (Albany NY), 7(12), 1198–1211. 10.18632/aging.100864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga S, Daadi EW, Roy-Choudhury G, & Daadi MM (2020, May 19). Age-related cognitive decline in baboons: modeling the prodromal phase of Alzheimer’s disease and related dementias. Aging (Albany NY), 12(11), 10099–10116. 10.18632/aging.103272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Perez EJ, Roberts JA, Roberts MT, & Rapp PR (2020, March). Reelin in the Years: decline in the number of reelin immunoreactive neurons in layer II of the entorhinal cortex in aged monkeys with memory impairment. Neurobiol Aging, 87, 132–137. 10.1016/j.neurobiolaging.2019.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes KO, Sparks DL, & Streit WJ (2008, August 1). Microglial dystrophy in the aged and Alzheimer’s disease brain is associated with ferritin immunoreactivity. Glia, 56(10), 1048–1060. 10.1002/glia.20678 [DOI] [PubMed] [Google Scholar]
- Lowenstine LJ, Mcmanamon R, & Terio KA (2016). Comparative pathology of aging great apes: Bonobos, chimpanzees, gorillas, and orangutans. Vet Pathol, 53, 250–276. [DOI] [PubMed] [Google Scholar]
- Luebke J, Barbas H, & Peters A (2010, March). Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev, 62(2), 212–232. 10.1016/j.brainresrev.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, & Amatrudo JM (2012, June). Age-related increase of sI(AHP) in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiol Aging, 33(6), 1085–1095. 10.1016/j.neurobiolaging.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Medalla M, Amatrudo JM, Weaver CM, Crimins JL, Hunt B, Hof PR, & Peters A (2015, June). Age-related changes to layer 3 pyramidal cells in the rhesus monkey visual cortex. Cereb Cortex, 25(6), 1454–1468. 10.1093/cercor/bht336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, & Rosene DL (2003, June 9). Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J Comp Neurol, 460(4), 573–584. 10.1002/cne.10668 [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Baker HF, Ridley RM, & Mori H (2000). Naturally occurring and experimentally induced beta-amyloid deposits in the brains of marmosets (Callithrix jacchus). J Neural Transm (Vienna), 107(7), 799–814. 10.1007/s007020070060 [DOI] [PubMed] [Google Scholar]
- Mandler M, Walker L, Santic R, Hanson P, Upadhaya AR, Colloby SJ, Morris CM, Thal DR, Thomas AJ, Schneeberger A, & Attems J (2014, July). Pyroglutamylated amyloid-β is associated with hyperphosphorylated tau and severity of Alzheimer’s disease. Acta Neuropathol, 128(1), 67–79. 10.1007/s00401-014-1296-9 [DOI] [PubMed] [Google Scholar]
- Mani RB, Lohr JB, & Jeste DV (1986, October). Hippocampal pyramidal cells and aging in the human: a quantitative study of neuronal loss in sectors CA1 to CA4. Exp Neurol, 94(1), 29–40. 10.1016/0014-4886(86)90269-4 [DOI] [PubMed] [Google Scholar]
- Marcusson J, Oreland L, & Winblad B (1984, December). Effect of age on human brain serotonin (S-1) binding sites. J Neurochem, 43(6), 1699–1705. 10.1111/j.1471-4159.1984.tb06098.x [DOI] [PubMed] [Google Scholar]
- Marcusson JO, Morgan DG, Winblad B, & Finch CE (1984, October 8). Serotonin-2 binding sites in human frontal cortex and hippocampus. Selective loss of S-2A sites with age. Brain Res, 311(1), 51–56. 10.1016/0006-8993(84)91397-0 [DOI] [PubMed] [Google Scholar]
- Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, Gibson J, Redmond P, Cox SR, Pattie A, Corley J, Taylor A, Murphy L, Starr JM, Horvath S, Visscher PM, Wray NR, & Deary IJ (2015, August). The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol, 44(4), 1388–1396. 10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez M, Serafin A, FernÁndez-Bellon H, Serrat S, Ferrer-Admetlla A, Bertranpetit J, Ferrer I, & Pumarola M (2008). Neuropathologic findings in an aged albino gorilla. Vet Pathol, 45, 531–537. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Sisodia SS, Koo EH, Cork LC, Dellovade TL, Weidemann A, Beyreuther K, Masters C, & Price DL (1991, February 15). Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci U S A, 88(4), 1461–1465. 10.1073/pnas.88.4.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, & Terry RD (1993, January). Quantitative synaptic alterations in the human neocortex during normal aging. Neurology, 43(1), 192–197. 10.1212/wnl.43.1_part_1.192 [DOI] [PubMed] [Google Scholar]
- Mathews KJ, Allen KM, Boerrigter D, Ball H, Shannon Weickert C, & Double KL (2017, October). Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell, 16(5), 1195–1199. 10.1111/acel.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, & Resnick SM (2016, March). Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging, 31(2), 166–175. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney DL, Stevenson AJ, Walker RM, Gibson J, Morris SW, Campbell A, Murray AD, Whalley HC, Porteous DJ, McIntosh AM, Evans KL, Deary IJ, & Marioni RE (2018). Investigating the relationship between DNA methylation age acceleration and risk factors for Alzheimer’s disease. Alzheimers Dement (Amst), 10, 429–437. 10.1016/j.dadm.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, Fontenot MB, & Bradley BJ (2012). The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (pan troglodytes). PLoS One, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Chang W, Calderazzo SM, Go V, Tsolias A, Goodliffe JW, Pathak D, De Alba D, Pessina M, Rosene DL, Buller B, & Moore TL (2020, April 22). Treatment with Mesenchymal-Derived Extracellular Vesicles Reduces Injury-Related Pathology in Pyramidal Neurons of Monkey Perilesional Ventral Premotor Cortex. J Neurosci, 40(17), 3385–3407. 10.1523/jneurosci.2226-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal I, Shi L, Keller TE, Konopka G, Preuss TM, Hsieh TF, Hu E, Zhang Z, Su B, & Yi SV (2016, November). Comparative Methylome Analyses Identify Epigenetic Regulatory Loci of Human Brain Evolution. Mol Biol Evol, 33(11), 2947–2959. 10.1093/molbev/msw176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, & Tuszynski MH (2000, July 3). Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol, 422(3), 396–401. [DOI] [PubMed] [Google Scholar]
- Mielke MM (2020, January 1). Consideration of Sex Differences in the Measurement and Interpretation of Alzheimer Disease-Related Biofluid-Based Biomarkers. J Appl Lab Med, 5(1), 158–169. 10.1373/jalm.2019.030023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT (2017, March). Why marmosets? Dev Neurobiol, 77(3), 237–243. 10.1002/dneu.22483 [DOI] [PubMed] [Google Scholar]
- Morelli L, Wei L, Amorim A, McDermid J, Abee CR, Frangione B, Walker LC, & Levy E (1996, January 29). Cerebrovascular amyloidosis in squirrel monkeys and rhesus monkeys: apolipoprotein E genotype. FEBS Lett, 379(2), 132–134. 10.1016/0014-5793(95)01491-8 [DOI] [PubMed] [Google Scholar]
- Moro ML, Phillips AS, Gaimster K, Paul C, Mudher A, Nicoll JAR, & Boche D (2018, January 3). Pyroglutamate and Isoaspartate modified Amyloid-Beta in ageing and Alzheimer’s disease. Acta Neuropathol Commun, 6(1), 3. 10.1186/s40478-017-0505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, & Peters A (1988, Sep-Dec). Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging, 9(5–6), 495–502. 10.1016/s0197-4580(88)80103-9 [DOI] [PubMed] [Google Scholar]
- Motley SE, Grossman YS, Janssen WGM, Baxter MG, Rapp PR, Dumitriu D, & Morrison JH (2018, December 5). Selective Loss of Thin Spines in Area 7a of the Primate Intraparietal Sulcus Predicts Age-Related Working Memory Impairment. J Neurosci, 38(49), 10467–10478. 10.1523/jneurosci.1234-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Benzing WC, Cole GM, Wang H, Emerich DF, Sladek JR Jr., Morrison JH, & Kordower JH (1994, Sep-Oct). Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: colocalization with amyloid plaques. Neurobiol Aging, 15(5), 621–627. 10.1016/0197-4580(94)00064-6 [DOI] [PubMed] [Google Scholar]
- Mufson EJ, He B, Nadeem M, Perez SE, Counts SE, Leurgans S, Fritz J, Lah J, Ginsberg D, Wuu J, & Scheff SW (2013). Hippocampal ProNGF Signaling Pathways and β-Amyloid Levels in Mild Cognitive Impairment and Alzheimer Disease. J Neuropathol Exp Neurol, 71, 1018–1029. 10.1097/NEN.0b013e318272caab.Hippocampal [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger EL, Edler MK, Hopkins WD, Ely JJ, Erwin JM, Perl DP, Mufson EJ, Hof PR, Sherwood CC, & Raghanti MA (2019). Astrocytic changes with aging and Alzheimer’s disease-type pathology in chimpanzees. J Comp Neurol, 527, 1179–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Kiatipattanasakul W, Nakayama H, Ono F, Sakakibara I, Yoshikawa Y, Goto N, & Doi K (1996, August). Immunohistochemical characteristics of the constituents of senile plaques and amyloid angiopathy in aged cynomolgus monkeys. J Med Primatol, 25(4), 294–300. 10.1111/j.1600-0684.1996.tb00213.x [DOI] [PubMed] [Google Scholar]
- Nakamura S, Nakayama H, Goto N, Ono-Ochikubo F, Sakakibara I, & Yoshikawa Y (1995, October). [Histopathological studies on senile plaques and cerebral amyloid angiopathy in aged cynomolgus monkeys]. Exp Anim, 43(5), 711–718. 10.1538/expanim1978.43.5_711 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tamaoka A, Sawamura N, Shoji S, Nakayama H, Ono F, Sakakibara I, Yoshikawa Y, Mori H, Goto N, & et al. (1995, December 8). Carboxyl end-specific monoclonal antibodies to amyloid beta protein (A beta) subtypes (A beta 40 and A beta 42(43)) differentiate A beta in senile plaques and amyloid angiopathy in brains of aged cynomolgus monkeys. Neurosci Lett, 201(2), 151–154. 10.1016/0304-3940(95)12160-9 [DOI] [PubMed] [Google Scholar]
- Ndung’u M, Härtig W, Wegner F, Mwenda JM, Low RW, Akinyemi RO, & Kalaria RN (2012, August). Cerebral amyloid β(42) deposits and microvascular pathology in ageing baboons. Neuropathol Appl Neurobiol, 38(5), 487–499. 10.1111/j.1365-2990.2011.01246.x [DOI] [PubMed] [Google Scholar]
- Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, & Rosene DL (2015). Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci, 9, 102. 10.3389/fnsys.2015.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa N, Kimura N, & Yanagisawa K (2010, February 22). Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain Res, 1315, 137–149. 10.1016/j.brainres.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, & Suhara T (2006, July 17). Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[beta-11C]DOPA. Life Sci, 79(8), 730–736. 10.1016/j.lfs.2006.02.017 [DOI] [PubMed] [Google Scholar]
- Page TL, Einstein M, Duan H, He Y, Flores T, Rolshud D, Erwin JM, Wearne SL, Morrison JH, & Hof PR (2002, January 4). Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett, 317(1), 37–41. 10.1016/s0304-3940(01)02428-4 [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Carlyle BC, Leslie S, Preuss TM, Crimins JL, Huttner AJ, van Dyck CH, Rosene DL, Nairn AC, & Arnsten AFT (2018, May). The aged rhesus macaque manifests Braak stage III/IV Alzheimer’s-like pathology. Alzheimers Dement, 14(5), 680–691. 10.1016/j.jalz.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). (2001, January 20). Lancet, 357(9251), 169–175. 10.1016/s0140-6736(00)03589-3 [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, & Pakkenberg B (2008, November). Neocortical glial cell numbers in human brains. Neurobiol Aging, 29(11), 1754–1762. 10.1016/j.neurobiolaging.2007.04.013 [DOI] [PubMed] [Google Scholar]
- Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, & Mufson EJ (2013). Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (gorilla gorilla gorilla). J Comp Neurol, 521, 4318–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Sherwood CC, Cranfield MR, Erwin JM, Mudakikwa A, Hof PR, & Mufson EJ (2016). Early Alzheimer’s disease-type pathology in the frontal cortex of wild mountain gorillas (Gorilla beringei beringei). Neurobiol Aging, 39, 195–201. 10.1016/j.neurobiolaging.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl DP, Good PF, Bussière T, Morrison JH, Erwin JM, & Hof PR (2000). Practical approaches to stereology in the setting of aging- and disease-related brain banks. J Chem Neuroanat, 20, 7–19. [DOI] [PubMed] [Google Scholar]
- Peters A (2009). The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat, 3, 11. 10.3389/neuro.05.011.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, & Kemper T (2012, October). A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging, 33(10), 2357–2372. 10.1016/j.neurobiolaging.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, & McNally KJ (1994, Nov-Dec). The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex, 4(6), 621–635. 10.1093/cercor/4.6.621 [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, & Sethares C (2001, February). The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb Cortex, 11(2), 93–103. 10.1093/cercor/11.2.93 [DOI] [PubMed] [Google Scholar]
- Peters A, & Sethares C (2002, January). The effects of age on the cells in layer 1 of primate cerebral cortex. Cereb Cortex, 12(1), 27–36. 10.1093/cercor/12.1.27 [DOI] [PubMed] [Google Scholar]
- Peters A, & Sethares C (2003, May 26). Is there remyelination during aging of the primate central nervous system? J Comp Neurol, 460(2), 238–254. 10.1002/cne.10639 [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, & Luebke JI (2008, April 9). Synapses are lost during aging in the primate prefrontal cortex. Neuroscience, 152(4), 970–981. 10.1016/j.neuroscience.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippens IH, Ormel PR, Baarends G, Johansson M, Remarque EJ, & Doverskog M (2017). Acceleration of Amyloidosis by Inflammation in the Amyloid-Beta Marmoset Monkey Model of Alzheimer’s Disease. J Alzheimers Dis, 55(1), 101–113. 10.3233/jad-160673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Watson CM, Bearman A, Knippenberg AR, Adams J, Ross C, & Tardif SD (2019, February). Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. Am J Primatol, 81(2), e22949. 10.1002/ajp.22949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott MA, Perry EK, Perry RH, & Court JA (1992, August 21). [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res, 588(2), 277–286. 10.1016/0006-8993(92)91586-4 [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Tolan DR, & Selkoe DJ (1991, June). Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer’s disease. Am J Pathol, 138(6), 1423–1435. https://www.ncbi.nlm.nih.gov/pubmed/1905108 [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Gearing M, Rebeck GW, Mirra SS, Tigges J, & Hyman BT (1994, June). Apolipoprotein E4 and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. Am J Pathol, 144(6), 1183–1187. [PMC free article] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, & White DL (1991, Jul-Aug). The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging, 12(4), 295–312. 10.1016/0197-4580(91)90006-6 [DOI] [PubMed] [Google Scholar]
- Price JL, & Morris JC (1999, March). Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol, 45(3), 358–368. [DOI] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1989, October). Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci, 9(10), 3568–3576. 10.1523/jneurosci.09-10-03568.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, & Amaral DG (1992, September). Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci, 15(9), 340–345. 10.1016/0166-2236(92)90051-9 [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Windle CP, & Cummings RM (2006, September). Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm (Vienna), 113(9), 1243–1251. 10.1007/s00702-005-0385-2 [DOI] [PubMed] [Google Scholar]
- Robillard KN, Lee KM, Chiu KB, & MacLean AG (2016, July). Glial cell morphological and density changes through the lifespan of rhesus macaques. Brain Behav Immun, 55, 60–69. 10.1016/j.bbi.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Callejas JD, Cuervo-Zanatta D, Rosas-Arellano A, Fonta C, Fuchs E, & Perez-Cruz C (2019, February). Loss of ferritin-positive microglia relates to increased iron, RNA oxidation, and dystrophic microglia in the brains of aged male marmosets. Am J Primatol, 81(2), e22956. 10.1002/ajp.22956 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Callejas JD, Fuchs E, & Perez-Cruz C (2016). Evidence of Tau Hyperphosphorylation and Dystrophic Microglia in the Common Marmoset. Front Aging Neurosci, 8, 315. 10.3389/fnagi.2016.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Flattery CN, Rosen RF, Farberg AS, Dooyema JM, Hof PR, Sherwood CC, Walker LC, & Preuss TM (2020, November). Quantification of neurons in the hippocampal formation of chimpanzees: comparison to rhesus monkeys and humans. Brain Struct Funct, 225(8), 2521–2531. 10.1007/s00429-020-02139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare JF, Duong TQ, Hopkins WD, Preuss TM, & Walker LC (2008, July 20). Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol, 509(3), 259–270. 10.1002/cne.21744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Tomidokoro Y, Farberg AS, Dooyema J, Ciliax B, Preuss TM, Neubert TA, Ghiso JA, LeVine H 3rd, & Walker LC (2016, August). Comparative pathobiology of β-amyloid and the unique susceptibility of humans to Alzheimer’s disease. Neurobiol Aging, 44, 185–196. 10.1016/j.neurobiolaging.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbell TH, Draguljić D, Yadav A, Hof PR, Luebke JI, & Weaver CM (2016, August). Automated evolutionary optimization of ion channel conductances and kinetics in models of young and aged rhesus monkey pyramidal neurons. J Comput Neurosci, 41(1), 65–90. 10.1007/s10827-016-0605-9 [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, & Saido TC (2005, April). Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med, 11(4), 434–439. 10.1038/nm1206 [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, & Dale AM (2005, Aug-Sep). Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging, 26(8), 1215–1227. 10.1016/j.neurobiolaging.2004.09.017 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Aoi H, Oga T, Fujita I, & Ichinohe N (2015, November). Postnatal development of dendritic structure of layer III pyramidal neurons in the medial prefrontal cortex of marmoset. Brain Struct Funct, 220(6), 3245–3258. 10.1007/s00429-014-0853-2 [DOI] [PubMed] [Google Scholar]
- Saura J, Andrés N, Andrade C, Ojuel J, Eriksson K, & Mahy N (1997, Sep-Oct). Biphasic and region-specific MAO-B response to aging in normal human brain. Neurobiol Aging, 18(5), 497–507. 10.1016/s0197-4580(97)00113-9 [DOI] [PubMed] [Google Scholar]
- Sawamura N, Tamaoka A, Shoji S, Koo EH, Walker LC, & Mori H (1997, August 1). Characterization of amyloid beta protein species in cerebral amyloid angiopathy of a squirrel monkey by immunocytochemistry and enzyme-linked immunosorbent assay. Brain Res, 764(1–2), 225–229. 10.1016/s0006-8993(97)00624-0 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, & Sparks DL (2001, May-Jun). Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging, 22(3), 355–365. 10.1016/s0197-4580(01)00222-6 [DOI] [PubMed] [Google Scholar]
- Schultz C, Dehghani F, Hubbard GB, Thal DR, Struckhoff G, Braak E, & Braak H (2000, January). Filamentous tau pathology in nerve cells, astrocytes, and oligodendrocytes of aged baboons. J Neuropathol Exp Neurol, 59(1), 39–52. 10.1093/jnen/59.1.39 [DOI] [PubMed] [Google Scholar]
- Schultz C, Hubbard GB, Rüb U, Braak E, & Braak H (2000, Nov-Dec). Age-related progression of tau pathology in brains of baboons. Neurobiol Aging, 21(6), 905–912. 10.1016/s0197-4580(00)00176-7 [DOI] [PubMed] [Google Scholar]
- Seaman KL, Smith CT, Juarez EJ, Dang LC, Castrellon JJ, Burgess LL, San Juan MD, Kundzicz PM, Cowan RL, Zald DH, & Samanez-Larkin GR (2019, July). Differential regional decline in dopamine receptor availability across adulthood: Linear and nonlinear effects of age. Hum Brain Mapp, 40(10), 3125–3138. 10.1002/hbm.24585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Bell DS, Podlisny MB, Price DL, & Cork LC (1987). Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science, 235, 873–877. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, & Hyman BT (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med, 1, a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Lal N, Leung E, Traul DE, Gonzalo-Ruiz A, & Geula C (2010, July). Neuronal and axonal loss are selectively linked to fibrillar amyloid-{beta} within plaques of the aged primate cerebral cortex. Am J Pathol, 177(1), 325–333. 10.2353/ajpath.2010.090937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Huo A, Kimura T, Shiozawa S, Kobayashi R, Sahara N, Ishibashi M, Ishigaki S, Saito T, Ando K, Murayama S, Hasegawa M, Sobue G, Okano H, & Hisanaga SI (2019, July 26). Tau isoform expression and phosphorylation in marmoset brains. J Biol Chem, 294(30), 11433–11444. 10.1074/jbc.RA119.008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobin E, Bowley MP, Estrada LI, Heyworth NC, Orczykowski ME, Eldridge SA, Calderazzo SM, Mortazavi F, Moore TL, & Rosene DL (2017, April). Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. Geroscience, 39(2), 199–220. 10.1007/s11357-017-9965-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol S, Calenda A, Jallageas V, Mestre-Frances N, Bellis M, & Bons N (1996). beta-Amyloid protein precursor in Microcebus murinus: genotyping and brain localization. Neurobiol Dis, 3(3), 169–182. 10.1006/nbdi.1996.0017 [DOI] [PubMed] [Google Scholar]
- Simić G, Kostović I, Winblad B, & Bogdanović N (1997, March 24). Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol, 379(4), 482–494. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, & Abraham CR (1997, November). Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol, 94(5), 471–478. 10.1007/s004010050735 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, & Vinters HV (2010). Astrocytes: Biology and pathology. Acta Neuropathol, 119, 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation of cerebrovascular aging, Brain Aging: Models, Methods, and Mechanisms (CRC Press/Taylor & Francis; 2007). [Google Scholar]
- Soreq L, Rose J, Soreq E, Hardy J, Trabzuni D, Cookson MR, Smith C, Ryten M, Patani R, & Ule J (2017, January 10). Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep, 18(2), 557–570. 10.1016/j.celrep.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonebarger GA, Urbanski HF, Woltjer RL, Vaughan KL, Ingram DK, Schultz PL, Calderazzo SM, Siedeman JA, Mattison JA, Rosene DL, & Kohama SG (2020, December). Amyloidosis increase is not attenuated by long-term calorie restriction or related to neuron density in the prefrontal cortex of extremely aged rhesus macaques. Geroscience, 42(6), 1733–1749. 10.1007/s11357-020-00259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, & Sparks DL (2004, January 15). Dystrophic microglia in the aging human brain. Glia, 45(2), 208–212. 10.1002/glia.10319 [DOI] [PubMed] [Google Scholar]
- Struble RG, Hedreen JC, Cork LC, & Price DL (1984, Fall). Acetylcholinesterase activity in senile plaques of aged macaques. Neurobiol Aging, 5(3), 191–198. 10.1016/0197-4580(84)90062-9 [DOI] [PubMed] [Google Scholar]
- Suhara T, Fukuda H, Inoue O, Itoh T, Suzuki K, Yamasaki T, & Tateno Y (1991). Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl), 103(1), 41–45. 10.1007/bf02244071 [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, & Morrison JH (2004, February). Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex, 14(2), 215–223. 10.1093/cercor/bhg121 [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, & Gundersen HJ (1997, Nov-Dec). Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging, 18(6), 609–615. 10.1016/s0197-4580(97)00155-3 [DOI] [PubMed] [Google Scholar]
- Tanprasertsuk J, Johnson EJ, Johnson MA, Poon LW, Nelson PT, Davey A, Martin P, Barbey AK, Barger K, Wang XD, & Scott TM (2019). Clinico-Neuropathological Findings in the Oldest Old from the Georgia Centenarian Study. J Alzheimers Dis, 70(1), 35–49. 10.3233/jad-181110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, & Ziegler TE (2011). The marmoset as a model of aging and age-related diseases. Ilar j, 52(1), 54–65. 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekirian TL, Saido TC, Markesbery WR, Russell MJ, Wekstein DR, Patel E, & Geddes JW (1998, January). N-terminal heterogeneity of parenchymal and cerebrovascular Abeta deposits. J Neuropathol Exp Neurol, 57(1), 76–94. 10.1097/00005072-199801000-00009 [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, & Hansen LA (1987, June). Neocortical cell counts in normal human adult aging. Ann Neurol, 21(6), 530–539. 10.1002/ana.410210603 [DOI] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Orantes M, & Wiestler OD (2003, December). Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol, 62(12), 1287–1301. 10.1093/jnen/62.12.1287 [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, & Rosene DL (1995). Mild age-related changes in the dentate gyrus of adult rhesus monkeys. Acta Anat (Basel), 153(1), 39–48. 10.1159/000147713 [DOI] [PubMed] [Google Scholar]
- Tsartsalis S, Xekardaki A, Hof PR, Kövari E, & Bouras C (2018, February). Early Alzheimer-type lesions in cognitively normal subjects. Neurobiol Aging, 62, 34–44. 10.1016/j.neurobiolaging.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihara T, Endo K, Kondo H, Okabayashi S, Shimozawa N, Yasutomi Y, Adachi E, & Kimura N (2016, November 14). Tau pathology in aged cynomolgus monkeys is progressive supranuclear palsy/corticobasal degeneration- but not Alzheimer disease-like -Ultrastructural mapping of tau by EDX. Acta Neuropathol Commun, 4(1), 118. 10.1186/s40478-016-0385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Alsum PB, Dong S, Richardson R, Zimbric ML, Thieme CS, & Houser WD (1996, Mar-Apr). Cerebral amyloid angiopathy and plaques, and visceral amyloidosis in aged macaques. Neurobiol Aging, 17(2), 275–281. 10.1016/0197-4580(95)02063-2 [DOI] [PubMed] [Google Scholar]
- Uno H, & Walker LC (1993, September 24). The age of biosenescence and the incidence of cerebral beta-amyloidosis in aged captive rhesus monkeys. Ann N Y Acad Sci, 695, 232–235. 10.1111/j.1749-6632.1993.tb23058.x [DOI] [PubMed] [Google Scholar]
- Vinters HV, & Gilbert JJ (1983, Nov-Dec). Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke, 14(6), 924–928. 10.1161/01.str.14.6.924 [DOI] [PubMed] [Google Scholar]
- Voytko ML (1998, December). Nonhuman primates as models for aging and Alzheimer’s disease. Lab Anim Sci, 48(6), 611–617. [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Cork LC, Struble RG, Dellovade TL, & Price DL (1988, March). Multiple transmitter systems contribute neurites to individual senile plaques. J Neuropathol Exp Neurol, 47(2), 138–144. 10.1097/00005072-198803000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Struble RG, Schmechel DE, Oertel WH, Cork LC, & Price DL (1985, August 30). Glutamic acid decarboxylase-like immunoreactive neurites in senile plaques. Neurosci Lett, 59(2), 165–169. 10.1016/0304-3940(85)90194-6 [DOI] [PubMed] [Google Scholar]
- Walker LC, Masters C, Beyreuther K, & Price DL (1990). Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol, 80(4), 381–387. 10.1007/bf00307691 [DOI] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, & Morrison JH (2010, September 22). Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci, 30(38), 12770–12776. 10.1523/jneurosci.3192-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Fowler JS, Schlyer D, MacGregor RR, Hitzemann RJ, Gur RC, & Wolf AP (1995). Evaluation of age-related changes in serotonin 5-HT2 and dopamine D2 receptor availability in healthy human subjects. Life Sci, 56(14), Pl249–253. 10.1016/0024-3205(95)00066-f [DOI] [PubMed] [Google Scholar]
- Wei X, Zhang L, & Zeng Y (2020, October). DNA methylation in Alzheimer’s disease: In brain and peripheral blood. Mech Ageing Dev, 191, 111319. 10.1016/j.mad.2020.111319 [DOI] [PubMed] [Google Scholar]
- Wenk GL, Walker LC, Price DL, & Cork LC (1991, Mar-Apr). Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging, 12(2), 93–98. 10.1016/0197-4580(91)90047-n [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ (1993, Jul-Aug). Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging, 14(4), 287–293. 10.1016/0197-4580(93)90113-p [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, & Troncoso JC (1994, September 17). Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet, 344(8925), 769–772. 10.1016/s0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- West MJ, & Gundersen HJ (1990, June 1). Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol, 296(1), 1–22. 10.1002/cne.902960102 [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Ghetti B, & Terry RD (1973, October). Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol, 32(4), 566–584. 10.1097/00005072-197310000-00007 [DOI] [PubMed] [Google Scholar]
- Wong DF, Wagner HN Jr., Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, & et al. (1984, December 21). Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science, 226(4681), 1393–1396. 10.1126/science.6334363 [DOI] [PubMed] [Google Scholar]
- Young ME, Ohm DT, Dumitriu D, Rapp PR, & Morrison JH (2014, August 22). Differential effects of aging on dendritic spines in visual cortex and prefrontal cortex of the rhesus monkey. Neuroscience, 274, 33–43. 10.1016/j.neuroscience.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen B, Lu J, Wu Y, Wang S, Yao Z, Zhu L, Qiao Y, Sun Q, Qin W, Zhao Q, Jia J, & Wei C (2019, August). Brains of rhesus monkeys display Aβ deposits and glial pathology while lacking Aβ dimers and other Alzheimer’s pathologies. Aging Cell, 18(4), e12978. 10.1111/acel.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]


