Abstract
A pericellular basket is a presynaptic configuration of numerous axonal boutons outlining a target neuron soma and its proximal dendrites. Recent studies show neurochemical diversity of pericellular baskets and suggest that neurotransmitter usage together with the dense, soma-proximal boutons may permit strong input effects on different time scales. Here we review the development, distribution, neurochemical phenotypes, and possible functions of pericellular baskets. As an example, we highlight pericellular baskets formed by projections of certain Pet1/Fev neurons of the serotonergic raphe nuclei. We propose that pericellular baskets represent convergence sites of competition or facilitation between neurotransmitter systems on downstream circuitry, especially in limbic brain regions, where pericellular baskets are widespread. Study of these baskets may enhance our understanding of monoamine regulation of memory, social behavior, and brain oscillations.
Keywords: Serotonin, Pet1/Fev, co-transmission, VGLUT3, pericellular basket, memory
Presynaptic pericellular baskets – what and where are they?
The strength and temporal dynamics of neuronal input are modulated by multiple factors. Among them is the spatial arrangement of presynaptic axonal boutons on the postsynaptic cell, i.e., the boutons’ locations and densities. One particularly striking bouton arrangement comprises what is called a pericellular basket, a presynaptic organization of boutons from one or multiple axons that surround the postsynaptic cell body and proximal dendrites. This innervation is typically dense, such that the shape of the postsynaptic soma can be discerned from the basket itself, like climbing vines around the trunk and limbs of a tree [1,2]. Historically, this configuration has also been referred to as a pericellular nest or pericellular array [3–5]. Pericellular baskets are thought to confer privileged control over the targeted cell via the high number of boutons and their location proximal to the soma and axon hillock, potentially overriding effects of more distal inputs [6].
Pericellular baskets in the cerebellum, hippocampus, and cortex were originally described by Ramon y Cajal, and later determined to be GABAergic [7]. Since this foundational work, diverse neuron types have been reported to configure pericellular baskets at their axon termini. These include certain monoaminergic neuron subtypes [2,8], some neuropeptide-releasing neurons [4,9], some glutamatergic projection neurons [10], and cells themselves called basket cells found in the cerebellum [11], cerebral cortex, and hippocampus [12,13]. These basket-extending neurons have cell soma residing in regions such as the median raphe (MR) nucleus [1,8,10,14], cerebral cortex [15], hypothalamus [16], hippocampus [13], and cerebellum [11]. While basket cell interneurons project locally to excitatory principal cells [15], other basket-extending monoaminergic neurons send long-range projections to target primarily GABAergic cells [10,14,17]. The presence of pericellular baskets is phylogenetically widespread, being found in reptiles, songbirds, rodents, non-human primates, and humans [2,3,18–21]. Even with their prevalence across organisms and brain regions and their likely gate-keeper role in controlling target neuron activity, little is known about the development, electrophysiology, and specific functions of pericellular baskets.
In this article, we review pericellular baskets, focusing on those formed by projection neurons of the serotonergic brainstem raphe nuclei. We discuss pericellular baskets as sites of convergence of neurotransmitter systems, suggesting that their privileged control over postsynaptic neuron excitability is complex and may span different time scales if the different neurotransmitters signal ionotropically (e.g., “fast” glutamatergic signaling) versus metabotropically (e.g., “slow” serotonergic signaling). We consider functional roles for pericellular baskets, for example, in the regulation of target neuron activity in the hippocampus and septum, possibly shaping brain theta rhythm and memory formation. We close with a set of questions, intending to stimulate future advances in this exciting area.
Neurochemical and structural diversity of pericellular baskets
A diverse set of neurotransmitters have been detected singly or co-expressed in boutons comprising pericellular baskets. These include serotonin (5-hydroxytryptamine, 5-HT), dopamine, noradrenaline, acetylcholine, glutamate, gamma-amino-butyric acid (GABA), enkephalin (Met- and Leu-), substance P, somatostatin, neuropeptide Y (NPY), and cocaine- and amphetamine-regulated transcript (CART) peptide [2,4,14,22–25]. Precedent for co-transmission deploying glutamate has been reported in monoaminergic, cholinergic, and GABAergic neurons [26], perhaps applying to pericellular basket terminals as well.
Architectural features of pericellular baskets
Pericellular baskets, even of different neurotransmitter phenotypes, share certain cytoarchitectural features. Boutons are characteristically large, and typically 20–30 of them decorate the target soma [12,14]. The contained synapses are typically symmetric, as revealed in electron micrographs of septal and hippocampal pericellular baskets [27–30]. In these cases, the immunodetected neurotransmitters have included GABA, 5-HT, and/or Met-Enkephalin. Collectively, these features predict inhibitory postsynaptic effects, albeit still to be discerned electrophysiologically in most cases. Smaller boutons in baskets have been found to deploy CART peptide and to harbor asymmetric synapses [22], suggesting postsynaptic excitation. Excitatory control also seems possible by some pericellular baskets using glutamate and/or 5-HT. These neurotransmitters may trigger excitatory postsynaptic receptors such as ionotropic and metabotropic glutamate receptors [26,31] or the excitatory ionotropic 5-HT receptor 3A (5-HT3aR) [32] and the metabotropic 5-HT receptors 2A [33,34] and 2C [35], as examples. Additionally, cultured 5-HT neurons have been found to release glutamate at asymmetric synapses [36], suggesting asymmetric synapses may be more common in cases of co-transmission of glutamate and serotonin.
Pericellular baskets as sites of neurotransmitter convergence
The degree to which neurochemically distinct pericellular baskets target the same soma is largely unknown. In the septum, different neurotransmitter systems form pericellular baskets in broadly similar distributions [1,2,4], raising the possibility that different basket systems interact by projecting to the same downstream target neurons or by axo-axonic synapses onto other baskets. A convergence-organization model suggests that different neurotransmitter systems may compete with each other or facilitate modulation of the targeted cell, either by affecting postsynaptic cellular processes or by inhibiting or exciting other basket terminals. Indeed, multiple neurochemically distinct fibers (serotonergic vs. non-serotonergic) have been observed as making baskets on the same septal cells [8,14], supporting the idea of basket convergence (Figure 1). An alternative possibility is that separate basket systems “tile” innervated regions, targeting largely distinct postsynaptic cells. A distributed, non-overlapping pattern of basket systems would suggest high target specificity for postsynaptic cell types, and possibly even repulsive or non-permissive environments underlying the development of basket stratification. In the septum, glutamatergic pericellular baskets (expressing vesicular glutamate transporter 3 [VGLUT3]) rarely overlap topographically with baskets immunopositive for parvalbumin (PV), tryptophan hydroxylase 2 (TPH2, the rate-limiting enzyme for 5-HT synthesis), calretinin, or choline acetyltransferase [2]. However, they do occasionally target the same somata as do separate, tyrosine hydroxylase+ (presumably dopaminergic) baskets [2] (Figure 1A). The extent of pericellular basket convergence may vary between different basket systems or as a function of region.
Figure 1. Combinations of neurochemically distinct pericellular baskets can target a shared cell soma.
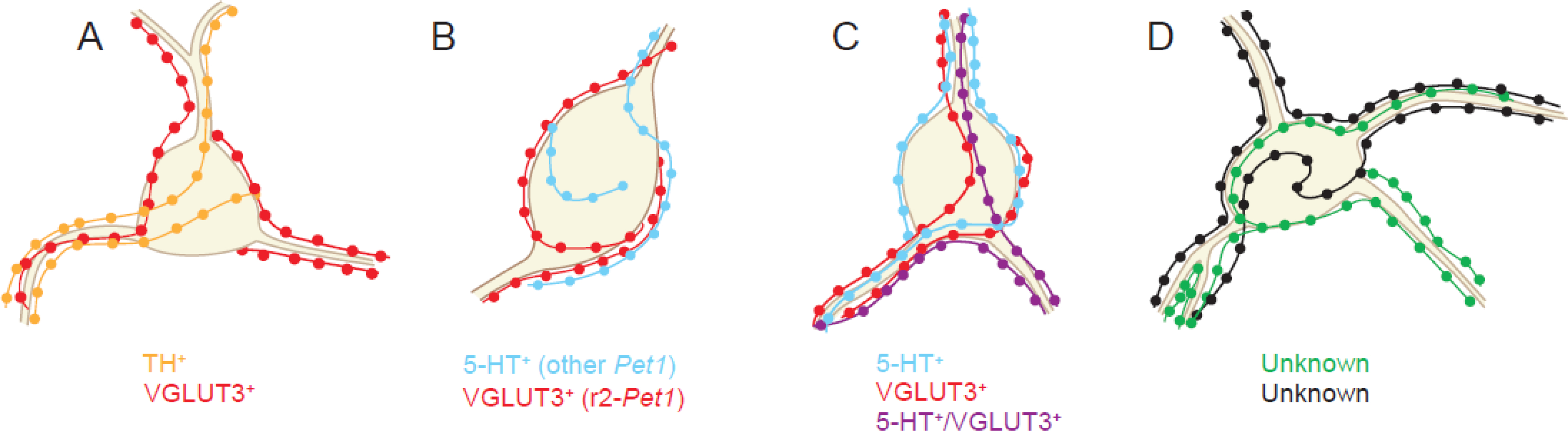
(A) Convergence of TH+ and VGLUT3+ pericellular baskets has been reported in the lateral septum [2]. (B) 5-HT+ and VGLUT3+ baskets from separate Pet1 neuron lineages converge on the same targets in the cortex, hippocampus, and septum [14]. (C) Baskets comprised of 5-HT+, VGLUT3+, and VGLUT3+/5-HT+ fibers are common in the septum [14]. (D) It remains to be tested whether other combinations of axons known to form pericellular baskets converge on their target neurons.
Pet1 neuronal subsystems form serotonergic and glutamatergic baskets
Brainstem raphe neurons defined molecularly by expression of Pet1 (aka Fev) are referred to as Pet1 neurons [37–40] and include a neuron subset that collectively forms pericellular baskets in the septum, hippocampus, and cerebral cortex [14]. Pet1 encodes for a transcription factor master regulator of differentiation of the serotonergic fate [41–43]. Recent findings, however, show that a substantial subset of basket-forming Pet1 neurons express low or undetectable levels of serotonergic pathway genes such as Tph2 and Slc6a4, the latter encoding the serotonin re-uptake transporter. Rather, these Pet1 neurons express high levels of VGLUT3, enabling glutamate packaging into synaptic vesicles [26,44]. This pattern contrasts some other Pet1 neurons, which co-express serotonergic and glutamatergic identity genes, permitting glutamate and serotonin co-transmission [38]. These Pet1-neuron soma that are high in Vglut3 but low in Tph2 transcripts reside in the MR and comprise part of the Pet1 neuronal population that derives developmentally from the hindbrain compartment referred to as rhombomere 2 (r2) [14,37,38]. Pet1 neurons arising from r2 are referred to as ‘r2-Pet1’ neurons [37] and can be accessed genetically by exploiting the overlap (intersection) of expression of two driver transgenics – the r2-specific r2Hoxa2-cre [45] and the Pet1-specific Pet1-Flpe [37]. Pericellular baskets are characteristic of a subgroup within the r2-Pet1 neuron population – the VGLUT3-positive, TPH2-low-or-negative subset of r2-Pet1 neurons referred to as r2-Pet1Vglut3-high [14]. The more classical, serotonergic subgroup of r2-Pet1 neurons, referred to as r2-Pet1Tph2-high, expresses high levels of TPH2, 5-HT, and SLC6A4 and are low-or-negative for VGLUT3. This group does not form pericellular baskets, and projects to brain regions different from the basket-forming r2-Pet1Vglut3-high cells [14].
The majority of baskets from r2-Pet1Vglut3-high cells are immunoreactive for VGLUT3 but not 5-HT, perhaps unsurprisingly given their soma transcriptome just mentioned [14,38]. Interestingly, some of the r2-Pet1Vglut3-high-targeted postsynaptic cells are ensheathed by additional baskets that are serotonergic and derive from non-r2 Pet1 neurons (Figure 1B). These baskets are likely formed by other MR serotonergic neuron subgroups referred to as r1En1-Pet1 or r3Egr2-Pet1 neurons [37,46]. Thus, in some cases, axons from developmentally distinct subsets of Pet1 neurons (derived from different hindbrain rhombomeres) converge and ensheath the same target cell with one deploying glutamate and the other 5-HT. Such ‘composite’ pericellular baskets are prevalent in the septum [14; Figure 1C] and likely explain at least a portion of the baskets formed by serotonergic and non-serotonergic fibers reported decades ago [8].
Proposed models of action of composite baskets
We propose various models for composite baskets based on existing data on serotonin and glutamate postsynaptic effects. A cooperative model describes fast glutamatergic input as prepotentiating the target cell, setting up enhanced responsiveness to subsequent excitatory serotonergic receptor signaling [47]. Also possible is that neuromodulatory input from 5-HT+ pericellular basket terminals may prime synapses to be more or less inducible to plasticity, as has been reported with cholinergic, dopaminergic, and adrenergic fibers [48,49]. An alternative oppositional model involves postsynaptic inhibitory 5-HT receptors, such that serotonergic signaling would oppose that of excitatory glutamate [50]. Relevant to both cooperative and oppositional models, serotonergic and glutamatergic signaling typically operate at different timescales. Glutamate typically elicits fast synaptic responses through ionotropic receptors while serotonin typically operates with slower dynamics usually through metabotropic receptors [47,51]. Resolving the functional impact of these basket complexities is an exciting next step.
Some individual r2-Pet1 neuron baskets co-localize VGLUT3 and 5-HT to the same boutons, raising the possibility for co-transmission [26,44,52,53]. This is especially prevalent for r2-Pet1 basket boutons in the septum, as compared to non-basket Pet1 boutons [14]. Thus, septal pericellular baskets from Pet1 neurons may be centers for co-transmission that offer concurrent yet kinetically different control of the postsynaptic neuron and its embedded network via glutamate versus 5-HT signaling. It is possible that glutamate versus 5-HT may require different thresholds of excitation for release. Indeed, certain serotonergic fibers in the amygdala, though not comprising baskets, showed differential neurotransmitter release depending on stimulation frequency: low frequency stimulation was sufficient to evoke glutamate release, higher frequencies were needed to elicit 5-HT release [54]. This suggests that 5-HT deployment is reserved for specific environmental or physiological circumstances. It also suggests that 5-HT and glutamate are packaged into separate synaptic vesicles. Pericellular baskets have yet to be probed for such graded transmission. An alternative possibility worth exploring is co-release of 5-HT and glutamate from the same synaptic vesicle. Both options suggest the possibility for sophisticated and complex modes of target cell modulation and septal network control by r2-Pet1 neuron baskets.
Developmental elaboration of basket structure parallels target neuron maturation
In rodents, septal pericellular baskets typically form in the early postnatal period. For example, it is during the first postnatal week of life that dopaminergic (TH+) baskets and met-enkephalin+ baskets are first detectable developmentally, increasing in abundance and complexity by week two [55]. In addition to this temporal axis of septal basket development, there is also a significant spatial axis, with baskets elaborating first in the medial septum, and later in the lateral septum. Notably, this pattern matches that of septal neuron maturation including dendritic arborization [56]. Similar temporal dynamics describe the formation of pericellular baskets in other brain regions, such as the cerebellum [57], cortex and hippocampus [58–60]. It may be a common feature for pericellular baskets to form as the cytoarchitectonics of a region and its resident cells mature. The development of Pet1 neuron pericellular baskets remains to be mapped, though in rat, 5-HT+ fibers form baskets in the septum starting after postnatal day (P) 7 [28]. These baskets increase in number and complexity throughout the early postnatal period. Serotonin axon arborization and morphology reach an adult-like pattern shortly after weaning, ~P28 [61,62]. Also unknown is whether early life experiences, such as stress or sensory experiences, affect the formation of pericellular baskets. Indeed, a different pericellular structure comprised of secreted glycoproteins, called the perineuronal net (described in Box 1), is affected in its development by postnatal stressors [63].
Box 1. Pericellular baskets and perineuronal nets: the first, a presynaptic elaboration; the second, an extracellular matrix network; both regulate target cell function.
Pericellular baskets and perineuronal nets (PNNs) are sometimes mistaken for each other conceptually, and while they do share features related to ensheathing neuron soma, they are quite different structures. Pericellular baskets are a presynaptic neuronal specialization comprised of axonal boutons decorating the soma and proximal dendrites of the postsynaptic target cell. They are formed by multiple neuron types, including basket cell interneurons and monoaminergic neurons, among others [4,15]. By contrast, PNNS are extracellular structures composed of secreted chondroitin sulfate proteoglycans that ensheath neuronal soma [63]. The extracellular matrix components that form PNNs are expressed by both neurons and glia [63]. Pericellular baskets have been observed targeting excitatory and inhibitory neurons [8,14,15,17,21]. Similarly, PNNs most commonly target GABAergic interneurons, typically fast-spiking parvalbumin neurons, but have also been observed to surround excitatory neurons [66,67]. In mice, pericellular baskets and PNNs form during the early postnatal period when neurons in many brain regions are maturing and establishing synaptic connectivity, suggesting roles in circuit maturation. PNN formation is dependent on experience and sensory input during critical periods, as PNNs fail to form in visual cortex without exposure to light [68]. Whether early life experience shapes the formation of pericellular baskets remains an open question. Also unclear is whether or how often pericellular baskets and perineuronal nets overlap in cellular target. The gaps in perineuronal ‘netting’ are typically occupied by synaptic boutons [63], suggesting the PNN acts as a scaffold for highly specific synapse formation that also limits further plasticity [66]. A study in mice found that loss of PNNs around parvalbumin interneurons reduced the perisomatic innervation targeting them, suggesting the PNN scaffolding may be necessary to stabilize perisomatic innervation [69]. The same study proposed that a threshold of perisomatic innervation may be necessary for PNNs to form and stabilize this connectivity. These results suggest a potential interplay between pericellular baskets and PNNs, though there are relatively few reports examining whether PNNs and pericellular baskets target the same neurons. In the lateral septum for example, PNNs and glutamatergic (VGLUT3+) pericellular baskets are reported to rarely overlap [2]. These observations offer perhaps a limited view, though, as only one type of PNN has been well characterized – that which binds the lectin Wisteria floribunda agglutinin (WFA) – whereas other PNNs exist as well, for instance those labeled by antibodies to aggrecan [63,70]. Additionally, a subset of serotonergic neurons forms pericellular baskets around parvalbumin interneurons [8], which as a population are common targets of PNNs [63]. As another line of evidence suggesting potential interactions between the serotonergic system and PNNs, selective serotonin reuptake inhibitors (SSRIs) administered during the early postnatal period affect PNN formation, reducing their number in the hippocampus [71]. Determining whether different pericellular basket systems target the same somata as PNNs, and whether the formation of each structure affects the other are compelling direction for future research.
The elaboration of septal pericellular baskets during the early postnatal period parallels the development of certain septum-dependent social behaviors. One example is kinship recognition. At about two weeks postnatally, rat pups switch preference from siblings to non-siblings, which is blocked by lesioning the lateral septum [64]. Moreover, lateral septal neurons responsive to sibling versus non-sibling cues differentially localize across the intermediate lateral septum, a subregion rich in pericellular baskets, including Pet1 neuron baskets [2,8,14]. These baskets elaborate and mature along a similar time course to behavioral preference switching, suggesting they may mediate or reflect this behavioral shift. Consistent with this notion, albeit in the adult rodent, optogenetic stimulation of the MR reduced aggression to a novel (non-sibling) intruder mouse [65].
Pet1 axon-derived pericellular baskets in the septohippocampal circuit may modulate memory
Based on functional anatomy, Pet1-derived pericellular baskets seem to be well positioned to influence memory and reinforcement of learned behaviors through modulating theta rhythm generation in the septohippocampal circuit. Theta rhythm describes sinusoidal (4–12 Hz) electroencephalographic oscillations related to activity in the hippocampus, neocortex, and amygdala during attentive wake and REM sleep [72,73]. Hippocampal theta oscillations are important in memory encoding [72–75] and abnormalities in theta rhythm are associated with attention and cognitive disorders such as schizophrenia [76] and attention deficit/hyperactivity disorder [77]. Hippocampal theta rhythms are generated within a broader limbic septohippocampal system in which projections from the medial septum drive theta in the hippocampus. The hippocampus in turn sends reciprocal regulatory connections back to the septum including both medial and lateral subdivisions [78]. This circuit is modulated by the MR, generally in a desynchronizing fashion [79–81] driven by serotonergic (Pet1) neurons residing therein [82,83]. Serotonergic neurons show diversity in their activity during theta [84], suggesting some subpopulations are better positioned to interact with theta-generating circuits. Subsets of Pet1 neurons form pericellular baskets in the hippocampus, medial septum, and lateral septum [14], positioning them to modulate hippocampal theta and memory at several key nodes.
Perhaps the most direct route whereby Pet1 neuron pericellular baskets may modulate theta rhythms is via hippocampal GABAergic neurons, which they preferentially innervate relative to excitatory principal cells [17]. Theta oscillations can be generated by interactions between pyramidal neurons and specific classes of interneurons, including those referred to as basket cells. Basket cells send highly collateralized axons to form pericellular baskets on many pyramidal neurons, coordinating their activity [15,85]. Basket cell interneurons are divided into two classes: the fast-spiking parvalbumin-expressing (PV) basket cells [86,87] and the regular-spiking cholecystokinin-expressing (CCK) basket cells [29]. The latter express 5-HT3aR and are heavily innervated by 5-HT+ and VGLUT3+ MR fiber pericellular baskets, suggesting excitatory, fast responsiveness to 5-HT and glutamate [14,15,29,88,89]. 5-HT3aR antagonists promote theta [90], suggesting 5-HT signaling to CCK basket cells may desynchronize theta. It is possible that glutamate vs. 5-HT release from Pet1 neuron pericellular baskets has differential effects on CCK basket cell firing and thus theta synchrony. Glutamate acting on CCK basket cells may promote inhibitory tone, theta, and thus memory formation [72,75]. Conversely, 5-HT inputs may desynchronize CCK basket cells, suppress theta [83], and promote extinction of memories through 5-HT3 receptors [91]. CCK basket cells have a slow membrane time constant and high input resistance [85,92]. Thus, it is possible they are especially desynchronized by coincident glutamatergic and serotonergic basket input, which may force adaptation (see Box 2).
Box 2: Pet1 neuronal pericellular baskets are poised to modulate memory.
Hippocampal CCK basket cells deliver strong perisomatic inhibition to pyramidal cells. This inhibition is thought to gate circuit transmission such that only the strongest signals persist, creating sparse, precise encoding of memories with minimal overlap in circuit representation [12,87,104]. The most highly recruited pyramidal cells are able to thwart CCK basket cell inhibition by depolarization-induced suppression of inhibition (DSI). DSI can involve, for instance, activity-dependent retrograde release of cannabinoids, which activate presynaptic CB1 receptors on the CCK basket cell terminals, thereby preventing additional release of GABA [85]. r2-Pet1 pericellular baskets might act to excite these CCK basket cells via glutamate release, and thereby promote a sparser neural code. When the pericellular basket is active presynaptically, the postsynaptic CCK basket cell is also likely excited. Subsequent stronger downstream inhibition of pyramidal cells would raise the threshold of circuit recruitment for a pyramidal cell to remain active. Alternatively, coincident glutamate and 5-HT release may greatly depolarize the CCK basket cell, causing adaptation that reduces CCK basket cell firing and acts to ‘reset’ this gain control and increase circuit plasticity.
There are additional pathways through which Pet1 pericellular baskets may modulate theta and memory. r2-Pet1 pericellular baskets also target hippocampal neurons expressing calbindin [14], a population that sends inhibitory projections to the medial septum [93], a major generator of theta [94]. Pet1 neuron pericellular baskets are also prevalent within the medial and lateral septum, where they innervate GABAergic neurons (possibly interneurons), some of which express the excitatory 5-HT2C receptor [8,14]. If Pet1 pericellular basket input (glutamate, serotonin, or both) excites these cell types as predicted by the cognate receptor function, their subsequent release of GABA would increase inhibitory tone in the septum. We predict this suppression of activity would reduce theta [95] and disrupt memory formation [96].
Based on the predicted effects of basket neurotransmission and identity of cellular targets, we propose Pet1 neuron basket activation generally reduces memory durability and increases circuit plasticity. Consistent with this idea, chemogenetic inhibition of r2-Pet1 neurons during the encoding of a cocaine conditioned place preference increased resistance to extinction of that behavioral preference in the cocaine-free state [97]. This suggests that diminished r2-Pet1 neuron activity strengthens the durability of cocaine memory. Conversely, this predicts that r2-Pet1 neuron activity normally functions to limit this durability, allowing for plasticity or flexibility in learning and memory [97]. 5-HT3aRs, expressed by hippocampal neurons targeted by r2-Pet1 pericellular baskets, seem particularly important in erasing stored memories. 5-HT3aR knockout mice are less able to extinguish fear memories [98] and express high levels of anxiety-related behaviors [99]. In line with these findings, systemic administration of 5-HT3aR antagonists improved baseline memory in rodents and primates and counteracted memory deficits induced by scopolamine [100] and pentylenetetrazole-kindling in a rodent model of epilepsy [101]. Exciting next steps will involve 5HT3aR manipulations specific to septohippocampal circuitry.
Approaches to manipulate memory durability may have translational implications. Malleable memories are essential to behavioral flexibility. A foraging animal, for example, must update its internal map to reflect when a food source is depleted, or it risks returning again and again to diminishing returns and enhanced predation risk. In some circumstances, unfruitful perseveration of memories can be highly detrimental and even life threatening. This is the case for instance in certain neuropsychiatric conditions, including post-traumatic stress disorder (PTSD) where prolonged and intrusive stressful memories are highly debilitating. Serotonin system abnormalities are thought to elevate risk for PTSD, and can be treated with serotonin-modulating drugs [102,103]. One may speculate that studies of Pet1 pericellular baskets could offer previously underappreciated circuit nodes and molecular pathways for conceptualizing new therapeutic strategies for PTSD and other psychiatric disorders.
Concluding Remarks
Pericellular baskets are complex structures formed by one or several axons densely innervating the soma and proximal dendrites of a downstream target cell. This spatial organization of boutons may enable strong and even multi-modal control over target cell activity. Formed by many neuron types in different brain regions, the functions and physiology of pericellular baskets remain under-described. In this article, we argue that pericellular baskets may represent a neurochemically and functionally complex interaction point between different neuronal and neurotransmitter systems with potential functional roles regulating emotion processing, brain oscillations, and memory. Understanding the extent of convergence of pericellular baskets across different neurotransmitter systems, as well as addressing central questions about their development and neurotransmission (see Outstanding Questions) would be essential to understanding their circuit function and impact on broader brain function and behavior.
Outstanding Questions.
How frequently do different pericellular baskets target the same neuron, forming a ‘composite basket’? Does the prevalence of composite baskets vary with target region?
Do individual monoamine neurons, which often have highly collateralized axons, make baskets in multiple regions? For neurons forming multiple pericellular baskets, are the baskets of identical or heterogeneous neurochemical phenotypes?
For monoamine neurons, which commonly co-transmit multiple neurotransmitters, are baskets sites of co-transmission and if so, is co-transmission fixed or variable as a function of presynaptic excitation?
For composite baskets, how does the activation of one basket affect the target cell response to subsequent input from the other basket(s)? Do baskets formed by different neurons ever signal to each other via axo-axonic appositions?
How does modulating neurotransmitter release from Pet1 pericellular baskets in target regions such as the septum and hippocampus affect memory formation? Given their often-different neurochemical profiles, do different developmental lineages of basket-forming Pet1 neurons have differential effects on memory durability?
How do pericellular baskets form around targeted cells? Are cell-cell adhesion proteins involved in guiding axons to specific downstream targets?
Pet1 neuron pericellular baskets are formed in the early postnatal period and are commonplace in brain regions that exhibit neuroplasticity in response to early life stress, such as the hippocampus and septum. Is the formation of pericellular baskets also plastic in response to early life stress, and does this plasticity have functional consequences on later expression of stress coping behavior or memory?
Does disrupting postsynaptic cell maturation also disrupt basket formation? Conversely, does disrupting basket formation disrupt neuronal maturation?
Highlights.
A pericellular basket is a presynaptic array of boutons from single or multiple axons that encase the target, postsynaptic cell body and proximal dendrites.
Pericellular baskets ensheathing cerebellar and cortical neurons were described by Ramón y Cajal in the late 1800s and early 1900s.
Pericellular baskets typically form postnatally after initial innervation of a brain region, suggesting possible induction by maturing target neurons.
Pericellular baskets often deploy multiple neurotransmitters either co-expressed in individual axons or separately deployed by different axons, suggesting complex target cell regulation.
The proximity to the postsynaptic soma of boutons configuring a basket suggests temporally precise inhibition or excitation of the encased cell.
Pet1 neurons of the raphe nuclei offer an example of pericellular basket projections. Pet1 neuron pericellular baskets frequently target distant inhibitory GABAergic interneurons, and thus may exert privileged influence on target region networks and their excitability.
Cell types targeted by Pet1 neuronal pericellular baskets suggest possible roles in regulating memory durability.
Acknowledgements
This work was supported by funding from the National Institutes of Health grants F31 NS108406 (RS), R21 DA036056 (SD), R01 DA034022 (SD), and R01 MH116223 (SD). We would also like to thank Ms. Mallory Rice for her work on the figure and Drs. Benjamin Okaty and Yasmin Escobedo-Lozoya for discussions and feedback on the manuscript.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Köhler C et al. (1982) The distribution and origin of serotonin-containing fibers in the septal area: a combined immunohistochemical and fluorescent retrograde tracing study in the rat. J. Comp. Neurol 209, 91–111 [DOI] [PubMed] [Google Scholar]
- 2.Riedel A et al. (2008) Vesicular glutamate transporter 3-immunoreactive pericellular baskets ensheath a distinct population of neurons in the lateral septum. J. Chem. Neuroanat 36, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin-Padilla M (1974) Three-dimensional reconstruction of the pericellular nests (baskets) of the motor (area 4) and visual (area 17) areas of the human cerebral cortex: A Golgi study. Z. Für Anat. Entwicklungsgeschichte 144, 123–135 [DOI] [PubMed] [Google Scholar]
- 4.Gall C and Moore RY (1984) Distribution of enkephalin, substance P, tyrosine hydroxylase, and 5-hydroxytryptamine immunoreactivity in the septal region of the rat. J. Comp. Neurol 225, 212–227 [DOI] [PubMed] [Google Scholar]
- 5.Hornung JP et al. (1990) Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J. Comp. Neurol 297, 165–181 [DOI] [PubMed] [Google Scholar]
- 6.Strüber M et al. (2015) Strength and duration of perisomatic GABAergic inhibition depend on distance between synaptically connected cells. Proc. Natl. Acad. Sci 112, 1220–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis DR et al. (1970) GABA and hippocampal inhibition. Br. J. Pharmacol 40, 881–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aznar S et al. (2004) Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience 124, 573–581 [DOI] [PubMed] [Google Scholar]
- 9.Olucha-Bordonau FE et al. (2012) Distribution and targets of the relaxin-3 innervation of the septal area in the rat. J. Comp. Neurol 520, 1903–1939 [DOI] [PubMed] [Google Scholar]
- 10.Szőnyi A et al. (2019) Median raphe controls acquisition of negative experience in the mouse. Science 366, [DOI] [PubMed] [Google Scholar]
- 11.Zhou J et al. (2020) Purkinje cell neurotransmission patterns cerebellar basket cells into zonal modules defined by distinct pinceau sizes. eLife 9, e55569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acsády L et al. (2000) Unusual Target Selectivity of Perisomatic Inhibitory Cells in the Hilar Region of the Rat Hippocampus. J. Neurosci 20, 6907–6919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelkey KA et al. (2017) Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev 97, 1619–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senft RA et al. (2021) Neurochemically and hodologically distinct ascending VGLUT3 versus serotonin subsystems comprise the r2-Pet1 median raphe. J. Neurosci DOI: 10.1523/JNEUROSCI.1667-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong C and Soltesz I (2012) Basket cell dichotomy in microcircuit function. J. Physiol 590, 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szeidemann Z et al. (1995) Hypothalamic Leu-enkephalin-immunoreactive fibers terminate on calbindin-containing somatospiny cells in the lateral septal area of the rat. J. Comp. Neurol 358, 573–583 [DOI] [PubMed] [Google Scholar]
- 17.Hornung JP and Celio MR (1992) The selective innervation by serotoninergic axons of calbindin-containing interneurons in the neocortex and hippocampus of the marmoset. J. Comp. Neurol 320, 457–467 [DOI] [PubMed] [Google Scholar]
- 18.Font C et al. (1997) Septal complex of the telencephalon of the lizard Podarcis hispanica. II. Afferent connections. J. Comp. Neurol 383, 489–511 [PubMed] [Google Scholar]
- 19.Goodson JL et al. (2004) Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J. Comp. Neurol 473, 293–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghanti MA et al. (2008) Differences in Cortical Serotonergic Innervation among Humans, Chimpanzees, and Macaque Monkeys: A Comparative Study. Cereb. Cortex 18, 584–597 [DOI] [PubMed] [Google Scholar]
- 21.Marin-Padilla M (1969) Origin of the pericellular baskets of the pyramidal cells of the human motor cortex: A golgi study. Brain Res. 14, 633–646 [DOI] [PubMed] [Google Scholar]
- 22.Janzsó G et al. (2010) Cocaine- and amphetamine-regulated transcript (CART) peptide-immunopositive neuronal elements in the lateral septum: Rostrocaudal distribution in the male rat. Brain Res. 1362, 40–47 [DOI] [PubMed] [Google Scholar]
- 23.Pickavance LC et al. (1992) Distributions and colocalization of neuropeptide Y and somatostatin in the goldfish brain. J. Chem. Neuroanat 5, 221–233 [DOI] [PubMed] [Google Scholar]
- 24.Wähle P et al. (1986) Localization of NPY-immunoreactivity in the cat’s visual cortex. Exp. Brain Res 61, 364–374 [DOI] [PubMed] [Google Scholar]
- 25.Paspalas CD and Papadopoulos GC (1999) Noradrenergic Innervation of Peptidergic Interneurons in the Rat Visual Cortex. Cereb. Cortex 9, 844–853 [DOI] [PubMed] [Google Scholar]
- 26.Trudeau L-E and El Mestikawy S (2018) Glutamate Cotransmission in Cholinergic, GABAergic and Monoamine Systems: Contrasts and Commonalities. Front. Neural Circuits 12, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beauvillain JC et al. (1991) GABA and enkephalin in the lateral septum of the guinea pig: Light and electron microscopic evidence for interrelations. J. Comp. Neurol 308, 103–114 [DOI] [PubMed] [Google Scholar]
- 28.Dinopoulos A et al. (1993) Serotonergic innervation of the mature and developing lateral septum of the rat: A light and electron microscopic immunocytochemical analysis. Neuroscience 55, 209–222 [DOI] [PubMed] [Google Scholar]
- 29.Fasano C et al. (2017) Regulation of the Hippocampal Network by VGLUT3-Positive CCK-GABAergic Basket Cells. Front. Cell. Neurosci 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hioki H et al. (2004) Chemically Specific Circuit Composed of Vesicular Glutamate Transporter 3- and Preprotachykinin B-producing Interneurons in the Rat Neocortex. Cereb. Cortex 14, 1266–1275 [DOI] [PubMed] [Google Scholar]
- 31.Crupi R et al. (2019) Role of Metabotropic Glutamate Receptors in Neurological Disorders. Front. Mol. Neurosci 12, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyama Y et al. (2017) Building a 5-HT3A Receptor Expression Map in the Mouse Brain. Sci. Rep 7, 42884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L-B et al. (2015) Activation of serotonin2A receptors in the medial septum-diagonal band of Broca complex enhanced working memory in the hemiparkinsonian rats. Neuropharmacology 91, 23–33 [DOI] [PubMed] [Google Scholar]
- 34.Zhang G and Stackman RW (2015) The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios JM et al. (2017) A short history of the 5-HT2C receptor: from the choroid plexus to depression, obesity and addiction treatment. Psychopharmacology (Berl.) 234, 1395–1418 [DOI] [PubMed] [Google Scholar]
- 36.Johnson MD (1994) Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron 12, 433–442 [DOI] [PubMed] [Google Scholar]
- 37.Jensen P et al. (2008) Redefining the serotonergic system by genetic lineage. Nat. Neurosci 11, 417–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okaty BW et al. (2015) Multi-Scale Molecular Deconstruction of the Serotonin Neuron System. Neuron 88, 774–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okaty BW et al. (2020) A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. eLife 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okaty BW et al. (2019) Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci 20, 397–424 [DOI] [PubMed] [Google Scholar]
- 41.Deneris E and Gaspar P (2018) Serotonin neuron development: shaping molecular and structural identities. Wiley Interdiscip. Rev. Dev. Biol 7, e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu C et al. (2010) Pet-1 is required across different stages of life to regulate serotonergic function. Nat. Neurosci 13, 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyler SC et al. (2016) Pet-1 Switches Transcriptional Targets Postnatally to Regulate Maturation of Serotonin Neuron Excitability. J. Neurosci 36, 1758–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amilhon B et al. (2010) VGLUT3 (Vesicular Glutamate Transporter Type 3) Contribution to the Regulation of Serotonergic Transmission and Anxiety. J. Neurosci 30, 2198–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awatramani R et al. (2003) Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet 35, 70–75 [DOI] [PubMed] [Google Scholar]
- 46.Bang SJ et al. (2012) Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci 35, 85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson J et al. (2009) Nonserotonergic projection neurons in the midbrain raphe nuclei contain the vesicular glutamate transporter VGLUT3. Synapse 63, 31–41 [DOI] [PubMed] [Google Scholar]
- 48.Brzosko Z et al. (2019) Neuromodulation of Spike-Timing-Dependent Plasticity: Past, Present, and Future. Neuron 103, 563–581 [DOI] [PubMed] [Google Scholar]
- 49.Ruan H et al. (2014) Dopamine-enabled anti-Hebbian timing-dependent plasticity in prefrontal circuitry. Front. Neural Circuits 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hannon J and Hoyer D (2008) Molecular biology of 5-HT receptors. Behav. Brain Res 195, 198–213 [DOI] [PubMed] [Google Scholar]
- 51.Vaaga CE et al. (2014) Dual-transmitter neurons: Functional implications of co-release and co-transmission. Curr. Opin. Neurobiol 0, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belmer A et al. (2019) Axonal Non-segregation of the Vesicular Glutamate Transporter VGLUT3 Within Serotonergic Projections in the Mouse Forebrain. Front. Cell. Neurosci 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varga V et al. (2009) Fast Synaptic Subcortical Control of Hippocampal Circuits. Science 326, 449–453 [DOI] [PubMed] [Google Scholar]
- 54.Sengupta A et al. (2017) Control of Amygdala Circuits by 5-HT Neurons via 5-HT and Glutamate Cotransmission. J. Neurosci 37, 1785–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verney C et al. (1987) Postnatal sequential development of dopaminergic and enkephalinergic perineuronal formations in the lateral septal nucleus of the rat correlated with local neuronal maturation. Anat. Embryol. (Berl.) 176, 463–475 [DOI] [PubMed] [Google Scholar]
- 56.Iyer A and Tole S (2020) Neuronal diversity and reciprocal connectivity between the vertebrate hippocampus and septum. WIREs Dev. Biol 9, e370. [DOI] [PubMed] [Google Scholar]
- 57.Ichikawa R et al. (2011) Developmental Switching of Perisomatic Innervation from Climbing Fibers to Basket Cell Fibers in Cerebellar Purkinje Cells. J. Neurosci 31, 16916–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pelkey KA et al. 13-February-(2020), Paradoxical network excitation by glutamate release from VGluT3+ GABAergic interneurons., eLife. [Online]. Available: https://elifesciences.org/articles/51996/figures. [Accessed: 01-Mar-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chattopadhyaya B et al. (2004) Experience and Activity-Dependent Maturation of Perisomatic GABAergic Innervation in Primary Visual Cortex during a Postnatal Critical Period. J. Neurosci 24, 9598–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doischer D et al. (2008) Postnatal Differentiation of Basket Cells from Slow to Fast Signaling Devices. J. Neurosci 28, 12956–12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lidov HG and Molliver ME (1982) Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res. Bull 9, 559–604 [DOI] [PubMed] [Google Scholar]
- 62.Maddaloni G et al. (2017) Development of Serotonergic Fibers in the Post-Natal Mouse Brain. Front. Cell. Neurosci 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fawcett JW et al. (2019) The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci 20, 451–465 [DOI] [PubMed] [Google Scholar]
- 64.Clemens AM et al. (2020) The lateral septum mediates kinship behavior in the rat. Nat. Commun 11, 3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balázsfi D et al. (2018) Differential Roles of the Two Raphe Nuclei in Amiable Social Behavior and Aggression – An Optogenetic Study. Front. Behav. Neurosci 12, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sorg BA et al. (2016) Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci 36, 11459–11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morikawa S et al. (2017) Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci. Rep 7, 46024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kind PC et al. (2013) The development and activity-dependent expression of aggrecan in the cat visual cortex. Cereb. Cortex N. Y. N 1991 23, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carceller H et al. (2020) Perineuronal Nets Regulate the Inhibitory Perisomatic Input onto Parvalbumin Interneurons and γ Activity in the Prefrontal Cortex. J. Neurosci 40, 5008–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthews RT et al. (2002) Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J. Neurosci. Off. J. Soc. Neurosci 22, 7536–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukhopadhyay S et al. (2021) Postnatal Fluoxetine Treatment Alters Perineuronal Net Formation and Maintenance in the Hippocampus. eNeuro 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buzsáki G and Moser EI (2013) Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci 16, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasselmo ME (2005) What is the function of hippocampal theta rhythm?--Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15, 936–949 [DOI] [PubMed] [Google Scholar]
- 74.Hutchison IC and Rathore S (2015) The role of REM sleep theta activity in emotional memory. Front. Psychol 6, 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whissell PD et al. (2019) Selective Activation of Cholecystokinin-Expressing GABA (CCK-GABA) Neurons Enhances Memory and Cognition. eNeuro 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adams RA et al. (2020) Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia. Brain 143, 1261–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo J et al. (2020) Abnormal modulation of theta oscillations in children with attention-deficit/hyperactivity disorder. NeuroImage Clin. 27, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khakpai F et al. (2013) Septo-Hippocampo-Septal Loop and Memory Formation. Basic Clin. Neurosci 4, 5–23 [PMC free article] [PubMed] [Google Scholar]
- 79.Bland BH et al. (2016) Median raphe stimulation-induced motor inhibition concurrent with suppression of type 1 and type 2 hippocampal theta. Hippocampus 26, 289–300 [DOI] [PubMed] [Google Scholar]
- 80.Hsiao Y-T et al. (2013) Disruption of footshock-induced theta rhythms by stimulating median raphe nucleus reduces anxiety in rats. Behav. Brain Res 247, 193–200 [DOI] [PubMed] [Google Scholar]
- 81.Jackson J et al. (2008) Median Raphe Stimulation Disrupts Hippocampal Theta Via Rapid Inhibition and State-Dependent Phase Reset of Theta-Related Neural Circuitry. J. Neurophysiol 99, 3009–3026 [DOI] [PubMed] [Google Scholar]
- 82.Gutiérrez-Guzmán BE et al. (2017) Serotonergic modulation of septo-hippocampal and septo-mammillary theta activity during spatial learning, in the rat. Behav. Brain Res 319, 73–86 [DOI] [PubMed] [Google Scholar]
- 83.Olvera-Cortés ME et al. (2013) Serotonergic modulation of hippocampal theta activity in relation to hippocampal information processing. Exp. Brain Res 230, 407–426 [DOI] [PubMed] [Google Scholar]
- 84.Kocsis B et al. (2006) Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci 103, 1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bartos M and Elgueta C (2012) Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J. Physiol 590, 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amilhon B et al. (2015) Parvalbumin Interneurons of Hippocampus Tune Population Activity at Theta Frequency. Neuron 86, 1277–1289 [DOI] [PubMed] [Google Scholar]
- 87.Klausberger T et al. (2005) Complementary Roles of Cholecystokinin- and Parvalbumin-Expressing GABAergic Neurons in Hippocampal Network Oscillations. J. Neurosci 25, 9782–9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Férézou I et al. (2002) 5-HT3 Receptors Mediate Serotonergic Fast Synaptic Excitation of Neocortical Vasoactive Intestinal Peptide/Cholecystokinin Interneurons. J. Neurosci 22, 7389–7397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee S et al. (2010) The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. Off. J. Soc. Neurosci 30, 16796–16808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Staubli U and Xu FB (1995) Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J. Neurosci 15, 2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buhot M-C et al. (2000) Role of serotonin in memory impairment. Ann. Med 32, 210–221 [DOI] [PubMed] [Google Scholar]
- 92.Lee SY and Soltesz I (2011) Cholecystokinin: A multi-functional molecular switch of neuronal circuits. Dev. Neurobiol 71, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tóth K and Freund TF (1992) Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: Their immunoreactivity for GABA and projection to the medial septum. Neuroscience 49, 793–805 [DOI] [PubMed] [Google Scholar]
- 94.Tsanov M (2018) Differential and complementary roles of medial and lateral septum in the orchestration of limbic oscillations and signal integration. Eur. J. Neurosci 48, 2783–2794 [DOI] [PubMed] [Google Scholar]
- 95.Sörman E et al. (2011) Control of hippocampal theta rhythm by serotonin: Role of 5-HT2c receptors. Neuropharmacology 61, 489–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Calandreau L et al. (2007) Dissociated roles for the lateral and medial septum in elemental and contextual fear conditioning. Learn. Mem 14, 422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baskin BM et al. (2020) Cocaine reward and memory after chemogenetic inhibition of distinct serotonin neuron subtypes in mice. Psychopharmacology (Berl.) DOI: 10.1007/s00213-020-05560-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kondo M et al. (2013) The 5-HT3A receptor is essential for fear extinction. Learn. Mem 21, 740–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelley SP et al. (2003) Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur. J. Pharmacol 461, 19–25 [DOI] [PubMed] [Google Scholar]
- 100.Barnes JM et al. (1990) The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol. Biochem. Behav 35, 955–962 [DOI] [PubMed] [Google Scholar]
- 101.Mishra A and Goel RK (2016) Chronic 5-HT3 receptor antagonism ameliorates seizures and associated memory deficit in pentylenetetrazole-kindled mice. Neuroscience 339, 319–328 [DOI] [PubMed] [Google Scholar]
- 102.Zhao M et al. (2017) Meta-analysis of the interaction between serotonin transporter promoter variant, stress, and posttraumatic stress disorder. Sci. Rep 7, 16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu L et al. (2018) Serotonin transporter 5-HTTLPR genotype is associated with intrusion and avoidance symptoms of DSM-5 posttraumatic stress disorder (PTSD) in Chinese earthquake survivors. Anxiety Stress Coping 31, 318–327 [DOI] [PubMed] [Google Scholar]
- 104.Klausberger T and Somogyi P (2008) Neuronal Diversity and Temporal Dynamics: The Unity of Hippocampal Circuit Operations. Science 321, 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]


