AGT (Angiotensinogen) is the unique substrate of the RAS (renin-angiotensin system). While many cells synthesize AGT, studies in mice have demonstrated that plasma AGT is predominantly liver-derived.1 Indeed, either pharmacological inhibition or genetic deficiency of hepatocyte-derived AGT reduces blood pressure and atherosclerosis in mice.2 Locally synthesized AGT in the kidney may contribute to renal Ang (angiotensin) II generation, possibly in a blood pressure-independent manner, although the literature is inconsistent.3, 4 Based on this concept, AGT measurements in urine or renal biopsies are used often as an independent marker of the renal RAS activation in humans. However, it remains unclear whether renal Ang II generation in humans depends on kidney-derived AGT.
AGT is cleaved by renin into two products: Ang I, which consists of 10 amino acids, and des(Ang I)AGT, which has 443 amino acids in mice and 442 amino acids in humans and NHP (nonhuman primates). Although sequences of AGT vary substantially between mouse and human, this protein is highly conserved in humans and NHP. Plasma total AGT concentrations were 3-4 μg/mL in mice, 15-41 μg/mL in humans, and 11-20 μg/mL in cynomolgus monkeys. Plasma AGT was predominantly present as des(AngI)AGT in mice (~92%) in contrast to both humans and cynomolgus monkeys (< 40% of des(AngI)AGT). Despite differences in plasma AGT, the distribution of AGT protein accumulation within the kidney was comparable among the 3 species. AGT protein accumulation was most abundant in the renal proximal convoluted tubules (S1 and S2 segments), modest in the proximal straight tubules (S3 segment), and not detected in glomeruli and other tubules of the kidneys (Figure A).
Figure. Contributions of liver-derived AGT on renal RAS in cynomolgus monkeys.
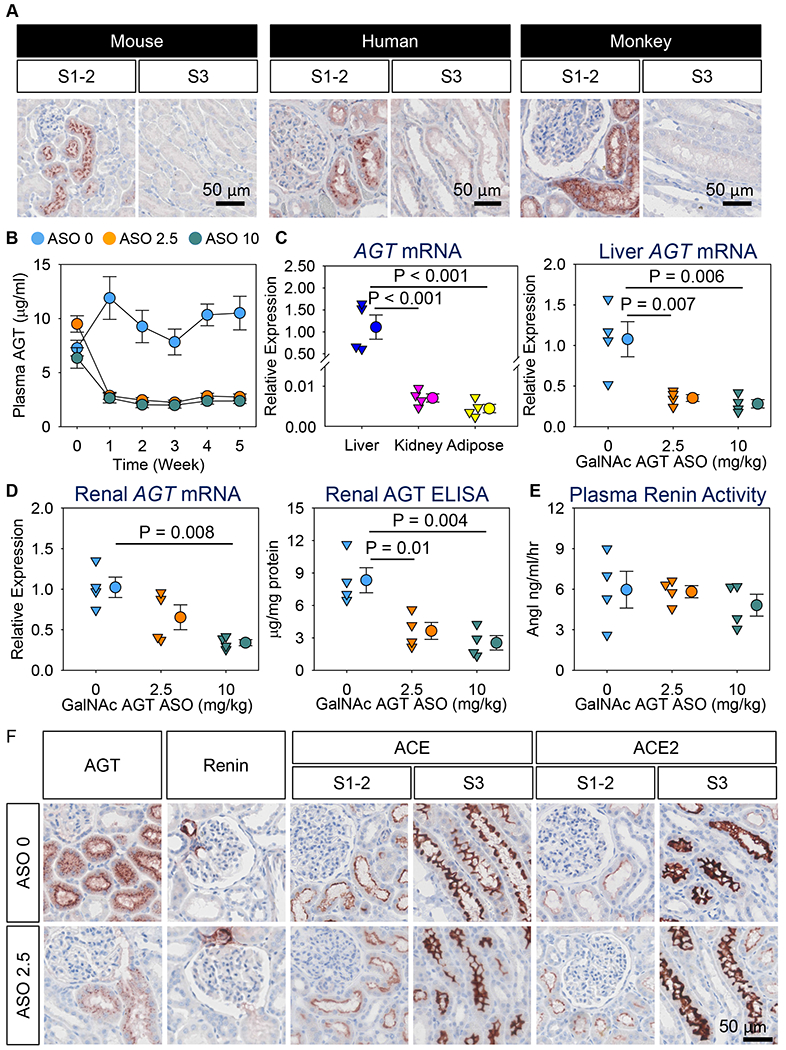
(A) Immunostaining of AGT in kidney sections from mice, humans, and cynomolgus monkeys using a rabbit anti-mouse AGT antibody for mice (IBL America 28101; 0.3 μg/ml) and a mouse anti-human AGT antibody for both humans and monkeys (IBL America 10417; 1 μg/ml). (B-F) Female cynomolgus monkeys were injected subcutaneously with either saline (indicated as “ASO 0” in the figure) or GalNAc AGT ASO (2.5 or 10 mg/kg; indicated as “ASO 2.5” and “ASO 10”, respectively) for 5 weeks (N = 4/group). (B) Plasma total AGT concentrations were determined by an ELISA kit (IBL America 27412). Data are represented as mean ± SEM. Piecewise linear mixed model with a split point at week 1 was used to compare plasma AGT concentration changes over time among the three groups. P < 0.001 between week 0 and week 1 for both ASO 2.5 and ASO 10, compared to ASO 0, and the differences remained during the study. (C) mRNA abundance of AGT was quantified by qPCR. Data were calculated using the ΔΔCt method and normalized to the mean of two reference genes: GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) and ZFP91 (Zinc finger protein 91). Data were log10 transformed for normality and equal variance tests. (D) mRNA abundance of renal AGT was quantified by qPCR. Renal AGT protein concentrations were determined using an ELISA kit (IBL America 27412) and normalized by total protein concentrations. (E) Plasma renin activity was determined using an angiotensin I ELISA kit (IBL America IB57101). Since all data passed normality and equal variance test, one-way analysis of variance with the Holm-Sidak method was performed for Figures C-E. (F) Immunostaining of AGT, renin, ACE, and ACE2 in kidney sections. Antibody information: mouse anti-human AGT (IBL America 10417; 1 μg/ml), rabbit anti-human renin (Sigma HPA005131; 1 μg/ml), rabbit anti-human ACE (Sigma HPA029298; 2 μg/ml), and rabbit anti ACE2 (Abcam ab108252; 0.3 μg/ml). Mouse anti-human AGT antibody (IBL America 10417) recognizes the C-terminus of AGT, it detects both intact AGT and its cleaved form, des(AngI) AGT.
Given the similarity between humans and cynomolgus monkeys, findings in the latter likely have greater translational significance, compared to rodent models, in defining the origin of kidney AGT in humans. To determine whether liver-derived AGT contributes to AGT protein accumulated in the kidney of NHP, female cynomolgus monkeys (3-4 years of age) were injected subcutaneously with either saline or ASO (antisense oligonucleotides) targeting liver-derived human AGT (Ionis, GalNAc AGT ASO: conjugated with N-acetyl galactosamine; 2.5 or 10 mg/kg). This human GalNAc AGT ASO has an identical sequence match to AGT mRNA in cynomolgus monkeys. Saline or ASO was injected on day 1 and day 4, and then once weekly for a subsequent 4 weeks. Neither dose of ASO affected body weight or liver and kidney functions.
Both doses of GalNAc AGT ASO reduced plasma AGT concentrations within 1 week by up to 80% (Figure B). Liver had approximately 160-fold more AGT mRNA abundance than kidney and visceral adipose tissue (Figure C). Both doses of GalNAc AGT ASO profoundly reduced hepatic mRNA abundance of AGT (Figure C). The low dose (2.5 mg/kg) of GalNAc AGT ASO did not affect renal AGT mRNA abundance, whereas the high dose (10 mg/kg) reduced renal AGT mRNA abundance (Figure D). Of note, both doses of GalNAc AGT ASO produced equivalent reductions in both plasma (Figure B) and liver AGT (Figure C). This illustrates that GalNAc ASO can be detected in kidney and exhibits some activity at sufficiently high doses such as 10 mg/kg in cynomolgus monkeys. Irrespective of renal AGT mRNA, both doses of GalNAc AGT ASO diminished renal AGT protein accumulation to a similar extent (Figure D). Plasma renin activity was not altered by either dose of GalNAc AGT ASO (Figure E), consistent with the recent data evaluating IONIS-AGT-LRX in hypertensive patients.4 These data imply that renin upregulation must have matched AGT downregulation to keep angiotensin generation in the normal range.3
As shown by immunostaining, diminished AGT protein accumulation following dosing with GalNAc AGT ASO was noted in the S1 and S2 segments of renal proximal tubules (Figure F). These findings support the notion that liver supplies the bulk of AGT protein to the kidney in NHP, independent of the presence of renal AGT mRNA. Figure F also shows the distribution of other RAS components. Renin was observed predominantly in juxtaglomerular cells, and ACE (angiotensin-converting enzyme) and ACE2 were present in all 3 segments of the proximal tubules, being most abundant in the S3 portion. GalNAc AGT ASO did not change the renal distribution of these enzymes.
In conclusion, the liver is the major source of AGT in kidneys of cynomolgus monkeys. Hepatic AGT accumulation in the S1 and S2 segments of the proximal tubules coincides with the observation in humans that tubular reabsorption via megalin, an endocytic receptor on the proximal tubules, is the main determinant of urinary AGT.1, 5 Taken together, these data are consistent with renal AGT originating predominantly in the liver. This implies that renal Ang II production in NHP and humans relies on hepatic AGT, and that the concept that AGT in urine or renal biopsies reflects an independent renal RAS needs to be reconsidered.
Acknowledgment
We thank CCTS Biospecimens Core (Supported by UL1TR001998) at the University of Kentucky for providing human samples.
Sources of Funding
This project was supported by NIH grants R01HL139748 and R01HL111932.
Abbreviations:
- AGT
Angiotensinogen
- Ang
Angiotensin
- RAS
Renin angiotensin system
- ASO
Antisense oligonucleotides
- NHP
Nonhuman primates
- ACE
Angiotensin-converting enzyme
Footnotes
Disclosures
Adam Mullick is an employee of Ionis Pharmaceuticals, Inc.
References
- 1.Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A and Ichikawa I. Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol. 2012;23:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H, Wu C, Howatt DA, Balakrishnan A, Moorleghen JJ, Chen X, Zhao M, Graham MJ, Mullick AE, Crooke RM, Feldman DL, Cassis LA, Vander Kooi CW and Daugherty A. Angiotensinogen exerts effects independent of angiotensin II. Arterioscler Thromb Vasc Biol. 2016;36:256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bovée DM, Ren L, Uijl E, Clahsen-van Groningen MC, van Veghel R, Garrelds IM, Domenig O, Poglitsch M, Zlatev I, Kim JB, Huang S, Melton L, Lu X, Hoorn EJ, Foster D and Danser AHJ. Renoprotective effects of small interfering RNA targeting liver angiotensinogen in experimental chronic kidney disease. Hypertension. 2021;77:1600–1612. [DOI] [PubMed] [Google Scholar]
- 4.Morgan ES, Tami Y, Hu K, Brambatti M, Mullick AE, Geary RS, Bakris GL and Tsimikas S. Antisense inhibition of angiotensinogen with IONIS-AGT-L(Rx): Results of phase 1 and phase 2 studies. JACC Basic Transl Sci. 2021;6:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roksnoer LC, Heijnen BF, Nakano D, Peti-Peterdi J, Walsh SB, Garrelds IM, van Gool JM, Zietse R, Struijker-Boudier HA, Hoorn EJ and Danser AH. On the origin of urinary renin: A translational approach. Hypertension. 2016;67:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]


