Abstract
Euryhaline fishes are capable of maintaining osmotic homeostasis in a wide range of environmental salinities. Several pleiotropic hormones, including prolactin, growth hormone, and thyroid hormones (THs) are mediators of salinity acclimation. It is unclear, however, the extent to which THs and the pituitary-thyroid axis promote the adaptive responses of key osmoregulatory organs to freshwater (FW) environments. In the current study, we characterized circulating thyroxine (T4) and 3-5-3’-triiodothyronine (T3) levels in parallel with the outer ring deiodination (ORD) activities of deiodinases (dios) and mRNA expression of dio1, dio2, and dio3 in gill during the acclimation of Mozambique tilapia (Oreochromis mossambicus) to FW. Tilapia transferred from seawater (SW) to FW exhibited reduced plasma T4 and T3 levels at 6 h. These reductions coincided with an increase in branchial dio2-like activity and decreased branchial dio1 gene expression. To assess whether dios respond to osmotic conditions and/or systemic signals, gill filaments were exposed to osmolalities ranging from 280 to 450 mOsm/kg in an in vitro incubation system. Gene expression of branchial dio1, dio2, and dio3 was not directly affected by extracellular osmotic conditions. Lastly, we observed that dio1 and dio2 expression was stimulated by thyroid-stimulating hormone in hypophysectomized tilapia, suggesting that branchial TH metabolism is regulated by systemic signals. Our collective findings suggest that THs are involved in the FW acclimation of Mozambique tilapia through their interactions with branchial deiodinases that modulate their activities in a key osmoregulatory organ.
Keywords: deiodinase, thyroid hormones, T3, T4, TSH, fish, freshwater, salinity, osmoregulation, gill
1. Introduction
Euryhaline fishes are capable of tolerating marked changes in environmental salinity such as those that occur during the tidal cycles inherent to coastal areas. To successfully transition between salinities, euryhaline fishes must efficiently control varied physiological processes to maintain osmotic homeostasis. A number of pleiotropic hormones, including prolactin (PRL), growth hormone (GH), cortisol, and thyroid hormones (THs), direct processes central to salinity acclimation (McCormick, 1996; Pickford and Phillips, 1959; Specker et al., 1984). Thyroxine (T4) is the major thyroid hormone secreted by thyroid follicles, with its synthesis primarily regulated by thyroid-stimulating hormone (TSH) released from the pituitary. T4 is further processed by the removal of a single iodine from the outer ring to become 3-5-3’-triiodothyronine (T3), the most biologically active form of TH. Iodine removal is achieved through the activities of iodothyronine deiodinases (dios), and three dio isoforms, denoted dio1, dio2, and dio3, operate in vertebrates (Bianco et al., 2002; Marsili et al., 2011). Outer ring deiodination (ORD) of T4 by dio1 and dio2 results in T3, which upon interacting with nuclear TH receptors elicits an array of biological effects through the regulation of gene transcription. On the other hand, inner ring deiodination (IRD) by either dio1 or dio3 results in reverse T3 (rT3), a mostly inactive end-product of TH metabolism (Brent, 2012). By balancing the deiodination activities of three distinct dios, peripheral tissues fine-tune their own responsiveness to TH signaling. Indeed, the capacity for THs to regulate a range of functions, including growth, development, metabolism, and reproduction relies upon the activities of dios (Brent, 2012; Larsen and Zavacki, 2012; Marsili et al., 2011).
Previous studies that investigated the osmoregulatory roles of THs in fish suggested that treatment with T3 or T4 promotes the seawater (SW) tolerance of salmonids (Refstie, 1982; Saunders et al., 1985) and flounder (Paralichthys dentatus) (Schreiber and Specker, 1999). In the euryhaline Mozambique tilapia (Oreochromis mossambicus), both T3 and T4 increased branchial Na+/K+-ATPase (Nka) activity and the size of ionocytes (Peter et al., 2000). Residing in the gill and integument, ionocytes play a central role in systemic osmoregulation by mediating either active ion uptake or extrusion, depending upon the environmental salinity (Kaneko et al., 2008). Accordingly, the exposure of mummichogs (Fundulus heteroclitus), coho salmon (Oncorhynchus kisutch), and rainbow trout (Oncorhynchus mykiss) to SW increased plasma T4 levels (Knoeppel et al., 1982; Specker and Kobuke, 1987). The SW-induced increase in T4 coincided with decreased renal dio1 and dio2 activities, as shown in rainbow trout (Orozco et al., 2002). Interestingly, plasma T4 levels may also increase in response to a reduction in environmental salinity. For instance, in gilthead sea bream (Sparus aurata), T4 levels rose, and branchial dio1 activity dropped, following a change in salinity from 35 to 1 ppt (Klaren et al., 2007). On the other hand, hyposmotic conditions induced hepatic dio2 activity in mummichogs (Lopez-Bojorquez et al., 2007). In striped parrotfish (Scarus iseri), hepatic expression of dio2, but not dio1, was elevated following experimentally-induced hyperthyroidism (Johnson and Lema, 2011). By contrast, dio1 is the primary regulator of circulating T4/T3 levels in mammals (Maia et al., 2011). When considered collectively, these findings suggest that species-specific patterns of thyroid axis regulation are associated with salinity acclimation in fishes. It is also evident that patterns of circulating THs and deiodinase activity have been understudied in paradigms employing hyposmotic challenges (e.g., the transfer of fish from SW to fresh water (FW)).
Because of their capacity to acclimate to a wide range of environmental conditions, tilapiine cichlids continue to be suitable models from which to characterize how various components of the thyroid system operate to support hydromineral balance in fishes. The gene expression and activity of deiodinases show tissue-specific patterns of distribution in tilapia (Mol et al., 1993; Mol et al., 1997; Seale et al., 2014). In Nile tilapia (O. niloticus), both dio1 gene expression and dio1 activity were highest in the kidney (Sanders et al., 1997), while dio2 activity was highest in the liver (Mol et al., 1993). Experimentally-induced reductions of circulating TH levels in Nile tilapia and blackchin tilapia (Sarotherodon melanotheron) stimulated hepatic dio1 and dio2 gene expression and the activities of their encoded proteins (Van der Geyten et al., 2001). On the other hand, dio3 activity was highest in the brain of Nile tilapia (Sanders et al., 1999), with IRD activity (typical of both dio1 and dio3) also reported in the brain and gill of blue tilapia (O. aureus) (Mol et al., 1997). In Mozambique tilapia, dio1 and dio3 expression was highest in the gill, while dio2 was prevalent in both the brain and gill. The branchial expression of all three dios was elevated in FW-acclimated fish compared with SW-acclimated fish (Seale et al., 2014); however, these patterns were observed in fish permanently acclimated to steady-state conditions and may not reflect dio expression patterns when fish are experiencing changes in salinity. The mummichog dio2 gene contains two functional osmotic response elements in its promoter (Lopez-Bojorquez et al., 2007), further implicating dios in mediating adaptive responses to changes in salinity.
Although deiodinases have been identified as key players in the tissue-level regulation of TH availability during salinity challenges (Orozco and Valverde, 2005), the role of branchial deiodinases, especially during a hyposmotic challenge, remains unclear. Given that hyperosmotic challenges (e.g., FW to SW transfer) generally increase plasma T4 levels in euryhaline teleosts (Dickhoff et al., 1978; Orozco et al., 2002; Peyghan et al., 2013; Specker and Kobuke, 1987), we hypothesized that a hyposmotic challenge would evoke the opposite response in Mozambique tilapia. Moreover, because TH metabolism is fine-tuned by dios in peripheral tissues, we sought to further resolve how branchial dio activity and expression may support adaptive responses to salinity transitions. In the current study, we transferred SW-acclimated tilapia to FW and characterized attendant patterns of plasma T3 and T4, branchial dio activity, and branchial dio mRNA levels. We also employed a gill filament incubation system to probe the local regulation of dio expression by extracellular osmotic conditions, and hypophysectomy with TSH replacement to investigate the systemic regulation of branchial dio expression.
2. Materials and Methods
2.1. Chemicals
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified.
2.2. Animals
Mature Mozambique tilapia of both sexes were obtained from a population maintained at the Hawai’i Institute of Marine Biology, University of Hawai’i. Fish were reared in outdoor tanks (700 L) with a continuous flow of FW (municipal water) or SW (Kaneohe Bay, Hawai’i, USA) under natural photoperiod. SW-acclimated tilapia employed in this experiment were spawned, and continuously reared in SW, having never been previously exposed to FW. Animals were fed ~5% of their body weight per day with Trout Chow (Skretting, Tooele, UT). All experiments were conducted in accordance with the principles and procedures approved by the Institutional Animal Care and Use Committee, University of Hawai’i.
2.3. Salinity transfer experiments
Two separate salinity-transfer experiments were performed with fish of both sexes. In both experiments, at the time of transfer, tanks were transitioned from SW (34 ppt) to FW (0 ppt) with minimal disturbance by opening an incoming FW-valve and closing a SW-valve. FW conditions were reached after 60 min. Time-matched control tanks, which contained fish from the original pool raised in SW, were held at constant salinity throughout the duration of the experiment. Fish were fed for the duration of the experiment and water temperature was maintained between 24 and 26 °C. In the first experiment, fish weighing 41.4 ± 1.4 g (mean ± S.E.M.) were sampled (n = 6–10) at 0 h (immediately prior to opening the FW-valve), 6 h, 48 h, and 10 d after transfer and gill filaments were collected for deiodinase activity assays. In the second experiment, fish weighing 362.7 ± 23.1 g were sampled (n = 8) at 0 h, 6 h, 24 h, 48 h, and 7 d after transfer, and blood plasma samples were used for the assessment of plasma osmolality and THs. Gill filaments were also collected for dio gene expression analyses. Fish were fasted for 24 h prior to sample collection. At the time of sampling, fish were netted and anesthetized with 2-phenoxyethenol (2-PE; 0.3 mL/L). Blood from the caudal vasculature was collected using a syringe coated with heparin ammonium salt (200 U/mL). Anesthetized fish were euthanized by rapid decapitation prior to the collection of gill filaments. All tissue samples were stored at −80 °C prior to their analysis.
2.4. Plasma osmolality and thyroid hormones
Plasma was collected following the centrifugation of blood samples (10,000 rpm for 10 min). Plasma osmolality was measured using a vapor pressure osmometer (Wescor 5100C, Logan, UT, USA). Two 25 μL volumes of plasma were used to assay THs using the AccuDiag™ ELISA – T4 kit (Diagnostic Automation; Woodland Hills, CA) and the T3 (Total) ELISA kit (Abnova; Taipei, Taiwan) according to the manufacturers’ instructions. Both assays were validated using stripped tilapia plasma following overnight incubation with 20 mg/mL of dextran-coated charcoal at 4 °C. Stripped tilapia plasma was spiked with known concentrations of T4 or T3. Stock solutions of THs were made in 0.01 N sodium hydroxide and diluted in phosphate buffer. The standard curves obtained with stripped tilapia plasma showed parallelism with those generated by commercial kits (Supplementary Fig. 1). Intra-assay coefficients of variation were 6.5% and 5.6% for T4 and T3, respectively, while inter-assay coefficients of variation were 9.6% and 1.8% for T4 and T3, respectively.
2.5. Deiodinase activity assays
Deiodinase assays were performed as previously described (Mol et al., 1997; Steinsapir et al., 1998; Zavacki et al., 2005). Briefly, gill filaments were sonicated in chilled 0.1 M potassium phosphate buffer (pH 6.9) containing 1 mM EDTA (PE buffer), 0.25 M sucrose, 10 mM dithiothreitol (DTT), and a protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentration was determined using the colorimetric Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA) with a bovine serum albumin (BSA) standard curve. Freshly Sephadex-purified outer ring labeled [I125]-T4 averaged ~109,790 counts per min (cpm; PerkinElmer, Waltham, MA). For each sample, 100 μg of protein was incubated overnight at 37 °C in PE buffer containing 20 mM DTT, ~80,000 cpm of outer ring labeled [I125]-T4, and either 0.5 nM or 100 nM of cold T4, in a total reaction volume of 110 μl. BSA (100 μg) was used as background (~2,623 cpm) under the same reaction conditions as above, and subtracted from experimental sample counts.
Deiodination was stopped by precipitation with 67 μl of horse serum (Gibco® Thermo Fisher Scientific, Waltham, MA) and 33 μl 50% trichloroacetic acid, followed by vortexing and centrifugation (12,000 rpm for 3 min). 120 μl of supernatant was counted with a Packard Cobra gamma-counter (PerkinElmer). The activity from purified human Dio2 (hDio2) was used to represent total activity, for subsequent calculations, based on its obligate outer ring deiodination activity (Bianco et al., 2002). hDio2 was immunopurified after transient transfection of N-terminal peptide FLAG-tagged plasmid construct of hDio2 cDNA (NM_013989.5) overexpressed in a human embryonic kidney (HEK-293) cell line. Transient transfection was carried out by the calcium phosphate method for 48h (Brent et al.,1989). Total deiodination was calculated based on the cpm of samples when incubated with 0.5 nM cold T4. High Km T4 ORD activity was calculated by subtracting blank counts from the counts obtained with 100 nM cold T4. Low Km T4 ORD activity was calculated by subtracting counts obtained from samples incubated with 0.5 nM cold T4 from counts obtained with samples incubated with 100 nM cold T4. For these calculations, we used the approach and nomenclature suggested previously (Mol et al., 1997) regarding the kinetic characteristics of the ORD enzymes. Specifically, considering that some dio1 activity cannot be excluded from counts at 0.5 nM T4, and some dio2 activity cannot be excluded from counts at 100 nM T4, the high and low Km T4 ORD activities are referred to as dio1-like and dio2-like, respectively. All results were calculated by expressing the cpm values as a percentage of the counts obtained with the hDio2 sample.
2.6. Gill filament incubation
To assess whether extracellular osmolality can directly affect branchial dio transcript levels, we incubated filaments from the second and third gill arches of male FW-acclimated tilapia (~100 g) following a previously described protocol (Watanabe et al., 2016) and experimental design (Inokuchi et al., 2015). Briefly, excised gill arches were first washed in sterilized balanced salt solution (BSS: NaCl 120 mM; KCl 4.0 mM; MgSO4 0.8 mM; MgCl2 1.0 mM; NaHCO3 2.0 mM; CaCl2 1.5 mM; KH2PO4 0.4 mM; Na2HPO4 1.3 mM; CaCl2 2.1 mM; Hepes 10 mM; pH 7.4) and then incubated in 0.025% KMnO4 for 1 min. After a second wash in BSS, individual gill filaments were cut from the arches, cut sagittally under a dissecting microscope, and then placed (3 filaments/well) in 24-well plates (Becton-Dickinson, Franklin Lakes, NJ) containing sterilized isosmotic (330 mOsm/kg) Leibovitz’s L-15 culture medium (Gibco® - Thermo Fisher Scientific) supplemented with 5.99 mg/L penicillin and 100 mg/L streptomycin. Filaments were incubated in 500 μL of incubation medium adjusted to four different osmolalities, 280, 330, 380, and 450 mOsm/kg (n = 8), which reflect the range of plasma osmolalities encountered by Mozambique tilapia following abrupt salinity changes (Seale et al., 2012a). The hyposmotic medium (280 mOsm/kg) was produced by diluting incubation medium with distilled water. Isosmotic (330 mOsm/kg) and hyperosmotic (380 and 450 mOsm/kg) media were produced by adding 5 mol/l NaCl solution to the hyposmotic medium. Osmolalities of incubation media were verified using a vapor pressure osmometer (Wescor 5520, Logan, UT). After incubation for 3 and 6 h at 26 °C, gill filaments were frozen in liquid nitrogen and stored at −80°C prior to RNA extraction and gene expression analyses.
2.7. Hypophysectomy and TSH replacement
Hypophysectomy was performed by the transorbital technique described by (Nishioka, 1994). Prior to surgery, male FW-acclimated fish (118.4 ± 6.9 g) were anesthetized in buffered tricaine methanesulfonate (100 mg/L, Argent Chemical Laboratories, Redmond, WA) and 2-PE (0.3 mL/L) in FW. After the procedure, fish recovered in experimental aquaria containing recirculating brackish water (12 ppt; 24–26 °C) where they were maintained for 3 days. Fish were treated with kanamycin sulfate (National Fish Pharmaceuticals, Tucson, AZ) and not fed following surgery. Three days after hypophysectomy, fish (n = 6–9) were anesthetized with 2-PE (0.3 mL/L) and administered bovine TSH (bTSH; 5 μg/g body weight or 0.01 IU/g body weight) or saline vehicle (0.9% NaCl) by a single intraperitoneal injection (1.0 μl/g body weight). All animals were treated in the same fashion prior to injections. To our knowledge, this is the first study that injected hypophysectomized tilapia with bTSH; the concentration of bTSH administered was based on previous hypophysectomy and hormone replacement studies in this species employing heterologous hormones such as ovine PRL and ovine GH (Breves et al., 2014; Breves et al., 2010b). After injection, fish were returned to the experimental aquaria and sampled after 12 h. At the time of sampling, fish were euthanized and plasma and gill filaments (from the second left arch) were collected. Samples were snap frozen in liquid nitrogen and stored at −80 °C.
2.8. Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using TRI Reagent according to the manufacturer’s protocols (MRC, Cincinnati, OH). The concentration and purity of extracted RNA were assessed using a NanoDrop (NanoDrop One, Thermo Fisher Scientific, Waltham, MA). Total RNA (400 ng) was reverse-transcribed using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The mRNA levels of reference and target genes were determined by the relative quantification method using a StepOnePlus real-time PCR system (Thermo Fisher Scientific). The qPCR reaction mix (15 μl) contained Power SYBR Green PCR Master Mix (Thermo Fisher Scientific), 200 nM of forward and reverse primers, and 1 μl cDNA. PCR cycling parameters were: 2 min at 50 °C, 10 min at 95 °C followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All gene specific primer sequences are provided in Table 1. After verification that elongation factor 1α (ef1a) mRNA levels did not exhibit significant main effects (salinity: P=0.73; time: P=0.11), ef1a levels were used to normalize dio gene expression levels. Reference and target genes were calculated by the relative quantification method with PCR efficiency correction (Pfaffl, 2001). Standard curves were prepared from serial dilutions of gill cDNA and included on each plate to calculate the PCR efficiencies for target and normalization gene assays. Relative gene expression ratios between groups are reported as a fold-change from controls.
Table 1.
Specific primer sequences (5′–3′) for quantitative real-time PCR.
| Gene | Forward | Reverse | Reference |
|---|---|---|---|
|
| |||
| dio1 | AACTATGAGGATTGGGGTCT | TGAGTCTGGAGCTTCTCCT | (Seale et al., 2014) |
| dio2 | CTTCTGTTTGTGCGTTTACA | TTCCAAACACTTTTCTCGTT | (Seale et al., 2014) |
| dio3 | AGAAACTGGCTGGAACAATA | ATGGGTGAACATCTGATAGC | (Seale et al., 2014) |
| ef1a | AGCAAGTACTACGTGACCATCATTG | AGTCAGCCTGGGAGGTACCA | (Breves et al., 2010a) |
2.9. Statistical analyses
Data from salinity transfer experiments were analyzed by two-way analysis of variance (ANOVA) with salinity and time as main effects. Significant (P<0.05) main and interaction effects (marked with asterisks) were followed up by Fisher’s protected LSD test. In gill filament incubation experiments, data are expressed as percent change from isosmotic (330 mOsm/kg) controls and slope analyses were carried out by linear regression. The effects of hypophysectomy and hormone replacement on branchial dios were analyzed by one-way ANOVA followed by Fisher’s protected LSD test. Statistical calculations were performed using Prism v. 9 (GraphPad Software, La Jolla, CA).
3. Results
3.1. Effects of salinity on plasma osmolality and circulating thyroid hormones
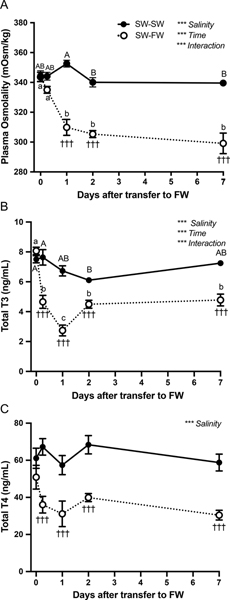
It is well-established that plasma osmolality drops upon transfer of tilapia from SW to FW (Seale et al., 2002). In the transfer paradigm used in the current study, we found a significant reduction in plasma osmolality, from ~340 to ~300 mOsm/kg, at 24 h after transfer to FW; this reduction was sustained until the end of the experiment on day 7. In SW-SW controls, plasma osmolality was maintained at ~340 mOsm/kg throughout the experiment (Fig. 1A). Transfer from SW to FW led to rapid decreases in both plasma T4 and T3 (Fig. 1B, C) by 6 h. T4 levels fell from 50–60 ng/mL to 30–40 ng/mL and remained at the lower levels for the duration of the experiment. T3 levels, on the other hand, fell from ~8 ng/mL to ~5 ng/mL by 6 h, dipped further to ~3 ng/mL by 24 h, and then leveled at ~4.5 ng/mL for the remainder of the experiment. SW-SW controls maintained circulating levels of T4 and T3 at ~60–70 ng/mL and ~6–8 ng/mL, respectively.
Figure 1:
Changes in plasma osmolality (A), T4 (B), and T3 (C) after transfer of Mozambique tilapia from SW to FW (open circles). Symbols represent mean ± S.E.M (n = 7–8). Control fish were maintained in SW (solid circles) and sampled on the same time course as fish subjected to a FW challenge. Differences among groups were evaluated by two-way ANOVA. Main and interaction effects are marked with *** representing P<0.001. Significant (P<0.05) main and interaction effects (marked with asterisks) were followed up by Fisher’s protected LSD test. †, ††, ††† denote significant differences from corresponding SW–SW controls at P<0.05, 0.01, and 0.001, respectively, and different letters correspond to significant differences in means across time within SW-FW (lower case) and SW-SW (upper case) groups.
3.2. Effects of salinity on branchial deiodination activity in vivo
Fish transferred from SW to FW exhibited a doubling of total branchial T4 ORD activity at 6 h. This increase, however, was transient, with activity returning to baseline levels by 48 h (Fig. 2A). Despite a significant main effect of salinity on a high Km T4 ORD activity, no differences between groups at any individual time points were detected by post hoc analyses (Fig. 2B). Resembling the pattern of total branchial T4 ORD activity, low Km T4 ORD activity was markedly increased in the SW-FW treatment compared with SW-SW controls at 6 h (Fig. 2C).
Figure 2:
Branchial T4 ORD (A), dio1-like (B), and dio2-like (C) activities after transfer of Mozambique tilapia from SW to FW (open circles). Dio1-like and dio2-like correspond to high and low Km activities, respectively. Symbols represent mean ± S.E.M (n = 5). Control fish were maintained in SW (solid circles) and sampled on the same time course as fish subjected to a FW challenge. Differences among groups were evaluated by two-way ANOVA. Main and interaction effects are marked with *, **, *** representing P<0.05, 0.01, and 0.001, respectively. Significant effects between means were followed up by Fisher’s protected LSD test. †, ††, ††† denote significant differences from corresponding SW–SW controls at P<0.05, 0.01, and 0.001, respectively, and different letters correspond to significant differences in means across time within SW-FW (lower case) and SW-SW (upper case) groups.
3.3. Effects of salinity on deiodinase gene expression in vivo
We then investigated the branchial gene expression of dios following the transfer of tilapia from SW to FW. There was a significant main effect of time on dio1 expression (Fig. 3A). dio1 expression was transiently reduced at 6 h after transfer from SW to FW and then gradually rose to initial levels by 7 d. There was a significant interaction effect, but no main effects, detected for dio2 expression. A transient increase in dio2 occurred by 6 h in the SW-SW control group (Fig. 3B). There were no significant main effects or an interaction for branchial dio3 expression (Fig. 3C).
Figure 3:
Gene expression of dio1 (A), dio2 (B), and dio3 (C) after transfer of Mozambique tilapia from SW to FW (open circles). Symbols represent mean ± S.E.M (dio1, n = 5–8; dio2, n = 5–8; dio3, n = 6–8; ef1a, n = 6–8). Control fish were maintained in SW (solid circles) and sampled on the same time course as fish subjected to a FW challenge. Differences among groups were evaluated by two-way ANOVA. Main and interaction effects are marked with *, ** representing P<0.05 and 0.01, respectively. Significant effects between means were followed up by Fisher’s protected LSD test. † denote significant differences from corresponding SW–SW controls at P<0.05, and different letters correspond to significant differences in means across time within SW-FW (lower case) and SW-SW (upper case) groups.
3.4. Effects of extracellular osmolality on deiodinase gene expression in vitro
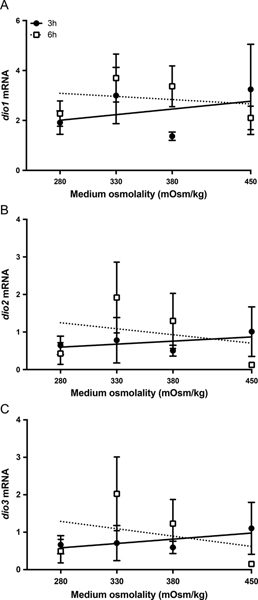
Upon detecting changes in dio expression following transfer to FW in vivo, we investigated whether dio expression directly responds to changes in extracellular osmolality by incubating gill filaments in isosmotic (330 mOsm/kg), hyposmotic (280 mOsm/kg) or hyperosmotic (380 and 450 mOsm/kg) media for 3 and 6 h. The gene expression of all three dios was insensitive to the range of tested extracellular osmotic conditions (Fig. 4A–C).
Figure 4:
Gene expression of dio1 (A), dio2 (B), and dio3 (C) in gill filaments exposed to a range of medium osmolalities for 3 h (solid circles) and 6 h (open squares). Symbols represent mean ± S.E.M (n = 7–8). Differences among groups were evaluated by linear regression analysis.
3.5. Effects of hypophysectomy and TSH replacement on plasma thyroid hormones and branchial deiodinase gene expression
Since dio expression did not directly respond to extracellular osmotic conditions, we hypothesized that dio transcripts may be primarily regulated by systemic signals. To address whether branchial dios are regulated by the pituitary-thyroid axis, we injected hypophysectomized tilapia with bTSH. We measured plasma levels of T3 and T4 to validate the effectiveness of hypophysectomy and bTSH treatment. Plasma T3 was reduced in hypophysectomized tilapia; replacement with bTSH restored plasma T3 to sham-operated levels (Fig. 5A). A similar pattern was observed for plasma T4, with hypophysectomy lowering plasma T4 and treatment with bTSH restoring levels to those of sham-operated fish (Fig. 5B). When compared with sham-operated animals, hypophysectomized animals exhibited reduced dio1 and dio2 expression (Fig. 5C, D). dio3 expression was not affected by hypophysectomy (Fig. 5E). Branchial dio1 and dio2 were both stimulated by bTSH injection (Fig. 5C, D). dio2 returned to levels in the sham-operated group while dio1 increased 3-fold compared with sham-operated animals. bTSH did not elicit a response on branchial dio3 expression (Fig. 5D).
Figure 5:
Plasma levels of T3 (A), T4 (B), and branchial gene expression of dio1 (C), dio2 (D), and dio3 (E) after hypophysectomy of Mozambique tilapia and TSH replacement. Bars represent mean ± S.E.M (n = 4–8). Hypophysectomized fish were sampled 12 h after receiving a single injection of oTSH (shaded bars). Sham-operated (solid bars) and hypophysectomized (open bars) fish receiving saline injections served as controls. Differences among groups were evaluated by one-way ANOVA followed up by Fisher’s protected LSD test. Significant differences between means are represented by different letters and correspond to P<0.05.
4. Discussion
In fishes, the activity of the thyroid axis at the level of its individual components has been largely examined in the context of development, reproduction, and major life-history transitions (Blanton and Specker, 2007; Irachi et al., 2021; Miwa et al., 1988; Specker, 1988). Connections between the thyroid axis and osmoregulatory processes in fishes, however, are less clear. Most studies that investigated thyroid function within the context of salinity acclimation reported that THs are elevated in response to hyperosmotic conditions (Grau, 1988; Knoeppel et al., 1982; McNabb and Pickford, 1970; Parker and Specker, 1990; Prunet et al., 1989; Specker, 1988; Specker and Kobuke, 1987; Young et al., 1989). Given that THs promote specific processes suited to SW conditions (Refstie, 1982; Saunders et al., 1985; Shrimpton and McCormick, 1999), we reasoned that THs may be attenuated when euryhaline species acclimate to FW conditions. This systemic pattern of regulation, however, may be subject to local modulation; TH metabolism may provide support, through branchial dio activation, to ionocyte remodeling during a transition to FW. We transferred tilapia from SW to FW and observed sharp and sustained declines in plasma T3 and T4, while in the gill dio2-like activity was transiently increased by 6 h after exposure to FW. By contrast, branchial dio1-like activity did not change. To our knowledge, this is the first documentation of systemic and local regulation of TH signaling in a euryhaline fish during the initial stages of FW acclimation.
The rapid decreases in plasma T4 and T3 (Fig. 1) suggest strong hyposmotically-induced suppression of TH production and deiodination. These changes in circulating TH levels may impact multiple physiological systems. For example, methimazole-induced hypothyroidism impaired the growth of Nile tilapia (Van der Geyten et al., 2001). In light of the plasma T3 and T4 patterns reported here, the findings of Van der Geyten et al. (2001) are consistent with the reduced growth rates of Mozambique tilapia reared in FW versus SW (Sparks et al., 2003; Zikos et al., 2014). The range of processes controlled by THs reflect the spatiotemporal regulation of dio genes and the enzymatic activities of their encoded proteins. The conversion of circulating T4 into active T3 is attributed to the ORD activities of dio1 and dio2 (Peeters and Visser, 2017). In mammals, hepatic dio1 is associated with the deiodination of T4 to maintain T3 levels in circulation, while dio2 predominantly controls the tissue-specific, localized conversion of T4 into T3 (Marsili et al., 2011). In Atlantic salmon (Salmo salar) undergoing parr-smolt transformation (preparatory phase that precedes the transition from FW to SW environments), T4 ORD was elevated in several tissues; hepatic ORD activity was highly correlated with plasma T3 levels and therefore seemingly determined plasma T3 levels (Morin et al., 1993). In mummichogs hepatic dio2 mRNA and dio2 activity increased in liver explants in response to a hyposmotic stimulus, an effect mediated, at least in part, by an osmotic response element in the dio2 promoter (Lopez-Bojorquez et al., 2007). Additional studies in euryhaline teleosts, including salmonids, mummichogs, and various tilapias, revealed distinct patterns of dio activity that varied across tissues and environmental salinities (Mol et al., 1993; Mol et al., 1998; Mol et al., 1997; Orozco et al., 2000; Orozco et al., 2002). With respect to their sensitivies to THs, however, dio activities across species were generally similar (Mol et al., 1998). It is also possible that upon binding to dio2, T4 enhances ubiquitination and subsequent proteasomal degradation of tilapia dio2, as described for mamalian dio2 in tissues that are highly dependent on its activity (Steinsapir et al., 2000; Zavacki et al., 2009). Such substrate-dependent degradation could explain the subsequent drop in dio2-like activity observed after 6 h of transferring fish from SW to FW. Hence, tissue-specific deiodination activities seemingly play an important role in directing environmental responses that require the targeted action of T3.
Since the gill plays a central role in osmoregulation, salinity acclimation entails the remodelling of branchial ionocytes to favor either ion extrusion or absorption when animals are exposed to SW or FW environments, respectively (Kaneko et al., 2008). During acclimation to hyposmotic conditions, the rapid (within 6 h) induction of gene transcripts that encode mediators of ion uptake, such as Na+/Cl− cotransporter 2, Clc family Cl− channel 2c, and Nka alpha 1a, and the suppression of genes encoding effectors of ion extrusion, such as Na+/K+/2Cl− cotransporter 1a and cystic fibrosis transmembrane conductance regulator 1, follows an abrupt drop in environmental salinity (Breves et al., 2017; Inokuchi et al., 2015; Moorman et al., 2015; Tipsmark et al., 2011). We detected a transient increase in total and dio2-like activity (to promote local T3 production) in the gill that temporally overlaped with the timing of branchial remodeling via the differentiation of FW-type ionocytes (Hiroi et al., 2005) (Fig. 2A, C). Inasmuch as THs stimulate cell differentiation in various vertebrate organs (Brown, 1997), it now remains to be determined whether T3 promotes the differentiation of FW-type ionocytes. Ionocytes are strong candidates for regulation by THs in tilapia given that T3 stimulated branchial Nka activity (Peter et al., 2000). THs may also enhance the sensitivity of the gill to other hormones, especially factors known to regulate osmoregulatory activities such as cortisol (McCormick, 2001). For example, T4 potentiated a cortisol-induced increase in branchial Nka activity (Dangé, 1986). The early and transient induction of dio2 activity, therefore, may further contribute to branchial reconfiguration during FW-acclimation through TH’s interactions with other hormones.
The regulation of dio genes in vertebrates is notably complex and reflects the essential roles they play in a range of biological functions (de Jesus et al., 2001; García-Domingo et al., 1999; Gereben and Salvatore, 2005; Larsen and Zavacki, 2012; Milesi et al., 2017; Williams and Bassett, 2011). Unlike dio activity, the effects of FW acclimation on branchial dio gene expression were variable over the experimental time course (Fig. 3). A salinity effect was only observed for dio1, where expression was transiently decreased at 6 h followed by a gradual rise to pre-transfer levels by 7 d (Fig. 3A). Interestingly, a transient surge in dio2 at 6 h was observed in SW-SW controls (Fig. 3B), a response that could have been triggered by other environmental factors, such as time of day. We previously reported that branchial expression of all three dios was higher in steady-state FW-versus SW-acclimated tilapia (Seale et al., 2014). Moreover, dio3, which did not show a consistent pattern of expression in the current experiment, was transiently upregulated and downregulated following previous FW- and SW-challenge experiments, respectively (Seale et al., 2014). At first, the transient downregulation of dio1 in FW appears consistent with the observed patterns of plasma TH, where concerted suppression of ORD (via dio1) would result in the lowering of active T3. Nonetheless, while the current pattern of branchial dio1 expression stands out for matching the time course of changes in total circulating TH and branchial dio enzymatic activity, the nature of the dio1 and dio2 responses to a hyposmotic environment were different than those of dio1- and dio2-like activities. To some extent, such discrepancies may be influenced by the use of low and high Km activities to distinguish between the predominant ORD, where some dio1 and dio2 activities may still occur at a low and high Km, respectively. The variability in the expression of dios may also be linked to more than one environmental cue. In Atlantic salmon, for example, branchial dio2a was induced following transfer to SW and associated with the metabolic response to osmotic stress (Lorgen et al., 2015). By contrast, its paralog, dio2b, was induced by increasing day length in both the gill and brain, consistent with the anticipatory nature of TH metabolism that is involved in seasonal life history transitions such as smoltification (Lorgen et al., 2015). Moreover, IRD activity of dio1 or dio3 may also respond to environmental cues that modulate local TH metabolism, though activity levels of dio3 in tilapia were found to be several-fold higher in brain than in gill (Sanders et al., 1999). These differences in regulation illustrate the level of complexity regarding the transcriptional control of dios as determined by either an immediate response to environmental salinity or one, cued by other environmental factors such as day light (Wambiji et al., 2011), that prepares the animal for developmental changes.
To discern whether the effects of salinity transfer on the expression of dios in vivo occurred in direct response to a change in extracellular osmolality per se, or were influenced by systemic cues, we examined the osmotic responsiveness of dio genes in vitro. Gill filament incubations have previously allowed us to detect the osmotic responsiveness of a series of genes (Inokuchi et al., 2015). In the current experiment, however, the expression of dios was unresponsive to physiologically relevant extracellular osmolalities ranging between 280 and 450 mOsm/kg (Fig. 4). It is worth noting that while osmotic response elements motifs were identified in the promoter region of mummichog dio2 (Lopez-Bojorquez et al., 2007), the osmotic concentration of hyposmotic media employed to induce responses in hepatic mRNA levels and activity in that study was far below the lowest plasma osmotic concentration experienced by Mozambique tilapia facing a direct transfer from SW to FW. These findings suggest that changes in dio expression following transfer to FW are regulated by systemic factors.
To address the systemic control of branchial dio expression, we targeted the pituitary gland, the major site of TSH synthesis and secretion. Hypophysectomy decreased plasma THs and branchial expression of dio1 and dio2, but not dio3. Hormone replacement with TSH restored circulating levels of both T4 and T3 and stimulated dio1 and dio2 expression to levels above those of sham-operated fish (Fig. 5A, B), indicating that these two dios are regulated by TSH and/or any number of TSH-responsive factors. It is unclear, however, if O. mossambicus expresses a receptor for TSH in the gills, and whether signaling through the TSH receptor affects dio expression. In mummichog, plasma T4 was reduced by hypophysectomy and shown to increase following injection with oTSH (Brown and Stetson, 1983; Grau and Stetson, 1977b). Thus, TSH-induced TH release is a plausible link between the pituitary and branchial dios. The observed pituitary-dependent responses in branchial dio transcription are also consistent with the reduction of branchial dio1 that mirrored the concomitant reductions in circulating THs following the transfer of fish from SW to FW. At the systemic level, however, a range of complex interactions may occur between factors within the thyroid axis and other hormones linked with salinity acclimation. For instance, PRL is released from the pituitary of euryhaline fishes in reponse to reductions in extracellular osmolality that occur following exposure to FW (Seale et al., 2012b), and studies in mummichog showed that injections of oPRL prevented the oTSH-induced rise in circulating T4 through suppression of thyroid function rather than through changes in peripheral TH activity (Brown and Stetson, 1983; Grau and Stetson, 1977a). Given that PRL rises within 6 h after tilapia are transferred from SW to FW (Seale et al., 2006; Seale et al., 2002), PRL may inhibit the release of T4 from the thyroid and thereby affect plasma T4 levels. The extent to which hormonal interactions may be at play, and whether the branchial expression of dios is directly and/or differentially regulated by THs and TSH in Mozambique tilapia, however, remain to be investigated.
In conclusion, our results suggest that TH signaling is locally modulated in the gill during the FW acclimation of Mozambique tilapia. This modulation is achieved through the regulation of branchial deiodinase gene expression and activity. Furthermore, with the concomitant reductions in circulating THs, it is apparent that systemic regulation of TH metabolism is also associated with a hyposmotic challenge. We propose that acute changes in branchial dio activity, and thus tissue T3 levels, support the functional remodelling of the gill during FW acclimation. The next challenge is to identify specific branchial processes that respond to TH signaling to improve the collective understanding of how discrete hormones underlie euryhalinity in fishes.
Supplementary Material
Highlights:
Transfer of Mozambique tilapia from seawater to fresh water reduces circulating thyroid hormones
T4 outer ring deiodination activity is transiently increased upon transfer to fresh water
Branchial dio1 is transiently downregulated upon transfer to fresh water
Branchial dios are insensitive to extracellular osmolality in vitro
Branchial dio1 and dio2 are reduced following pituitary removal and stimulated by TSH replacement
Acknowledgments
We are grateful to Profs. E. Gordon Grau and Marla J. Berry for their discussions and encouragement during the course of this study, and to Mr. John W. Harney for his outstanding technical support. This work was funded in part by the Hawai’i Community Foundation [20ADVC-102166 to L.A.S.], start-up funds to L.A.S., a Travel Award from the Endocrine Society to C.L.G., the National Science Foundation [IOS-1755016 and -1755131 to A.P.S. and J.P.B.], the National Oceanic and Atmospheric Administration [NA18OAR4170347 to A.P.S.], the National Institute of Diabetes and Digestive and Kidney Diseases [R21DK111775 to A.P.S., R01DK128390 to L.A.S. and R01DK044128 to A.M.Z. and P.R.L.], and the National Institute of Food and Agriculture [Hatch no. HAW02051-H to A.P.S.].
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as hindering the impartiality of the research reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR, 2002. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev 23, 38–89. [DOI] [PubMed] [Google Scholar]
- Blanton ML, Specker JL, 2007. The Hypothalamic-Pituitary-Thyroid (HPT) Axis in Fish and Its Role in Fish Development and Reproduction. Crit. Rev.Toxicol. 37, 97–115. [DOI] [PubMed] [Google Scholar]
- Brent GA, 2012. Mechanisms of thyroid hormone action. J. Clin. Invest. 122, 3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA, Larsen PR, Harney JW, Koenig RJ, Moore DD, 1989. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J. Biol. Chem. 264, 178–82. [PubMed] [Google Scholar]
- Breves JP, Hirano T, Grau EG, 2010a. Ionoregulatory and endocrine responses to disturbed salt and water balance in Mozambique tilapia exposed to confinement and handling stress. Comp. Biochem. Physiol. 155, 294–300. [DOI] [PubMed] [Google Scholar]
- Breves JP, Watanabe S, Kaneko T, Hirano T, Grau EG, 2010b. Prolactin restores branchial mitochondrion-rich cells expressing Na+/Cl− cotransporter in hypophysectomized Mozambique tilapia. Am. J. Physiol. 299, R702–710. [DOI] [PubMed] [Google Scholar]
- Breves JP, Keith PLK, Hunt BL, Pavlosky KK, Inokuchi M, Yamaguchi Y, Lerner DT, Seale AP, Grau EG, 2017. clc-2c is regulated by salinity, prolactin and extracellular osmolality in tilapia gill. J. Mol. Endocrinol. 59, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breves JP, Tipsmark CK, Stough BA, Seale AP, Flack BR, Moorman BP, Lerner DT, Grau EG, 2014. Nutritional status and growth hormone regulate insulin-like growth factor binding protein (igfbp) transcripts in Mozambique tilapia. Gen. Comp. Endocrinol. 207, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Stetson MH, 1983. Prolactin--thyroid interaction in Fundulus heteroclitus. Gen. Comp. Endocrinol. 50, 167–171. [DOI] [PubMed] [Google Scholar]
- Brown DD, 1997. The role of thyroid hormone in zebrafish and axolotl development. Proc. Natl. Acad. Sci. U.S.A. 94, 13011–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangé AD, 1986. Branchial Na+ - K+ - ATPase activity in freshwater or seawater acclimated tilapia, Oreochromis (Sarotherodon) mossambicus: effects of cortisol and thyroxine. Gen. Comp. Endocrinol. 62, 341–343. [DOI] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC, 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J. Clin. Invest. 108, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickhoff WW, Folmar LC, Gorbman A, 1978. Changes in plasma thyroxine during smoltification of coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 36, 229–232. [DOI] [PubMed] [Google Scholar]
- García-Domingo D, Leonardo E, Grandien A, Martínez P, Albar JP, Izpisúa-Belmonte JC, Martínez-A C, 1999. DIO-1 is a gene involved in onset of apoptosis in vitro, whose misexpression disrupts limb development. Proc. Natl. Acad. Sci. U.S.A. 96, 7992–7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Salvatore D, 2005. Pretranslational regulation of type 2 deiodinase. Thyroid 15, 855–864. [DOI] [PubMed] [Google Scholar]
- Grau EG, 1988. Environmental Influences on Thyroid Function in Teleost Fish. Integr. Comp. Biol. 28, 329–335. [Google Scholar]
- Grau EG, Stetson MH, 1977a. The effects of prolactin and TSH on thyroid function in Fundulus heteroclitus. Gen. Comp. Endocrinol. 33, 329–335. [DOI] [PubMed] [Google Scholar]
- Grau EG, Stetson MH, 1977b. Pituitary autotransplants in Fundulus heteroclitus: effect onf thyroid function. Gen. Comp. Endocrinol. 32, 427–431. [DOI] [PubMed] [Google Scholar]
- Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T, 2005. Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+-ATPase, Na+/K+/2Cl− cotransporter and CFTR anion channel. J. Exp. Biol. 208, 2023–2036. [DOI] [PubMed] [Google Scholar]
- Inokuchi M, Breves JP, Moriyama S, Watanabe S, Kaneko T, Lerner DT, Grau EG, Seale AP, 2015. Prolactin 177, prolactin 188, and extracellular osmolality independently regulate the gene expression of ion transport effectors in gill of Mozambique tilapia. Am. J. Physiol. 309, R1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irachi S, Hall DJ, Fleming MS, Maugars G, Björnsson BT, Dufour S, Uchida K, McCormick SD, 2021. Photoperiodic regulation of pituitary thyroid-stimulating hormone and brain deiodinase in Atlantic salmon. Mol. Cell. Endocrinol. 519, 111056. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Lema SC, 2011. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri). Gen. Comp. Endocrinol. 172, 505–517. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Watanabe S, Lee KM, 2008. Functional Morphology of Mitochondrion-Rich Cells in Euryhaline and Stenohaline Teleosts. Aqua-BioSci. Monogr. 1, 1–68. [Google Scholar]
- Klaren PHM, Guzmán JM, Reutelingsperger SJ, Mancera JM, Flik G, 2007. Low salinity acclimation and thyroid hormone metabolizing enzymes in gilthead seabream (Sparus auratus). Gen. Comp. Endocrinol. 152, 215–222. [DOI] [PubMed] [Google Scholar]
- Knoeppel SJ, Atkins DL, Packer RK, 1982. The role of the thyroid gland in osmotic and ionic regulation in Fundulus heteroclitus acclimated to freshwater and seawater. Comp. Biochem. Physiol. 73, 25–29. [Google Scholar]
- Larsen PR, Zavacki AM, 2012. The role of the iodothyronine deiodinases in the physiology and pathophysiology of thyroid hormone action. Eur. Thyroid J. 1, 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bojorquez L, Villalobos P, Garcia GC, Orozco A, Valverde RC, 2007. Functional identification of an osmotic response element (ORE) in the promoter region of the killifish deiodinase 2 gene (FhDio2). J. Exp. Biol. 210, 3126–3132. [DOI] [PubMed] [Google Scholar]
- Lorgen M, Casadei E, Król E, Douglas A, Birnie MJ, Ebbesson LO, Nilsen TO, Jordan WC, Jørgensen EH, Dardente H, Hazlerigg DG, Martin SA, 2015. Functional divergence of type 2 deiodinase paralogs in the Atlantic salmon. Curr. Biol. 25, 936–941. [DOI] [PubMed] [Google Scholar]
- Maia AL, Goemann IM, Meyer EL, Wajner SM, 2011. Deiodinases: the balance of thyroid hormone: type 1 iodothyronine deiodinase in human physiology and disease. J. Endocrinol. 209, 283–297. [DOI] [PubMed] [Google Scholar]
- Marsili A, Zavacki AM, Harney JW, Larsen PR, 2011. Physiological role and regulation of iodothyronine deiodinases: a 2011 update. J. Endocrinol. Invest. 34, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SD, 1996. Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar): interaction with cortisol. Gen. Comp. Endocrinol. 101, 3–11. [DOI] [PubMed] [Google Scholar]
- McCormick SD, 2001. Endocrine control of osmoregulation in teleost fish. Am. Zool. 41, 781–794. [Google Scholar]
- McNabb RA, Pickford GE, 1970. Thyroid function in male killifish, Fundulus heteroclitus, adapted to high and low temperatures and to fresh water and sea water. Comp. Biochem. Physiol. 33, 783–792. [DOI] [PubMed] [Google Scholar]
- Milesi S, Simonneaux V, Klosen P, 2017. Downregulation of Deiodinase 3 is the earliest event in photoperiodic and photorefractory activation of the gonadotropic axis in seasonal hamsters. Sci. Rep. 7, 17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S, Tagawa M, Inui Y, Hirano T, 1988. Thyroxine surge in metamorphosing flounder larvae. Gen. Comp. Endocrinol. 70, 158–163. [DOI] [PubMed] [Google Scholar]
- Mol K, Kaptein E, Darras VM, de Greef WJ, Kuhn ER, Visser TJ, 1993. Different thyroid hormone-deiodinating enzymes in tilapia (Oreochromis niloticus) liver and kidney. FEBS Lett. 321, 140–144. [DOI] [PubMed] [Google Scholar]
- Mol KA, Van der Geyten S, Burel C, Kühn ER, Boujard T, Darras VM, 1998. Comparative study of iodothyronine outer ring and inner ring deiodinase activities in five teleostean fishes. Fish Physiol. Biochem. 18, 253–266. [Google Scholar]
- Mol KA, Van Der Geyten S, Darras VM, Visser TJ, Kuhn ER, 1997. Characterization of iodothyronine outer ring and inner ring deiodinase activities in the blue tilapia, Oreochromis aureus. Endocrinol. 138, 1787–1793. [DOI] [PubMed] [Google Scholar]
- Moorman BP, Lerner DT, Grau EG, Seale AP, 2015. The effects of acute salinity challenges on osmoregulation in Mozambique tilapia reared in a tidally changing salinity. J. Exp. Biol. 218, 731–739. [DOI] [PubMed] [Google Scholar]
- Morin PP, Hara TJ, Eales JG, 1993. Thyroid hormone deiodination in brain, liver, gill, heart and muscle of Atlantic salmon (Salmo salar) during photoperiodically-induced parr-smolt transformation. I. Outer- and inner-ring thyroxine deiodination. Gen. Comp. Endocrinol. 90, 142–156. [DOI] [PubMed] [Google Scholar]
- Nishioka RS, 1994. Hypophysectomy of fish, in: Hochachka PW, Mommsen TP (Eds.), Biochemistry and Molecular Biology of Fishes: Analytical Techniques. Elsevier, New York, pp. 49–58. [Google Scholar]
- Orozco A, Linser P, Valverde C, 2000. Kinetic characterization of outer-ring deiodinase activity (ORD) in the liver, gill and retina of the killifish Fundulus heteroclitus. Comp. Biochem. Physiol. 126, 283–290. [DOI] [PubMed] [Google Scholar]
- Orozco A, Valverde RC, 2005. Thyroid hormone deiodination in fish. Thyroid 15, 799–813. [DOI] [PubMed] [Google Scholar]
- Orozco A, Villalobos P, Valverde RC, 2002. Environmental salinity selectively modifies the outer-ring deiodinating activity of liver, kidney and gill in the rainbow trout. Comp. Biochem. Physiol. 131, 387–395. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Specker JL, 1990. Salinity and temperature effects on whole-animal thyroid hormone levels in larval and juvenile striped bass, Morone saxatilis. Fish Physiol. Biochem. 8, 507–514. [DOI] [PubMed] [Google Scholar]
- Peeters RP, Visser TJ, 2017. Metabolism of Thyroid Hormone MDText.com, Inc; 2000, South Dartmouth (MA). [Google Scholar]
- Peter PMC, Lock RA, Wendelaar Bonga SE, 2000. Evidence for an osmoregulatory role of thyroid hormones in the freshwater mozambique tilapia Oreochromis mossambicus. Gen. Comp. Endocrinol. 120, 157–167. [DOI] [PubMed] [Google Scholar]
- Peyghan R, Enayati A, Sabzevarizadeh M, 2013. Effect of salinity level on TSH and thyroid hormones of grass carp, Ctenophayngodon idella. Vet. Res. Forum 4, 175–178. [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford GE, Phillips JG, 1959. Prolactin, a factor in promoting survival of hypophysectomized killifish in fresh water. Science 130, 454–455. [DOI] [PubMed] [Google Scholar]
- Prunet P, Boeuf G, Bolton JP, Young G, 1989. Smoltification and seawater adaptation in Atlantic salmon (Salmo salar): Plasma prolactin, growth hormone, and thyroid hormones. Gen. Comp. Endocrinol. 74, 355–364. [DOI] [PubMed] [Google Scholar]
- Refstie T, 1982. The effect of feeding thyroid hormones on saltwater tolerance and growth rate of Atlantic salmon. Can. J. of Zool. 60, 2706–2712. [Google Scholar]
- Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ, 1997. Characterization of a propylthiouracil-insensitive type I iodothyronine deiodinase. Endocrinol. 138, 5153–5160. [DOI] [PubMed] [Google Scholar]
- Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ, 1999. Cloning and characterization of type III iodothyronine deiodinase from the fish Oreochromis niloticus. Endocrinol. 140, 3666–3673. [DOI] [PubMed] [Google Scholar]
- Saunders RL, McCormick SD, Henderson EB, Eales JG, Johnston CE, 1985. The effect of orally administered 3,5,3′-triiodo-L-thyronine on growth and salinity tolerance of Atlantic salmon (Salmo salar L.). Aquaculture 45, 143–156. [Google Scholar]
- Schreiber AM, Specker JL, 1999. Metamorphosis in the summer flounder, Paralichthys dentatus: thyroidal status influences salinity tolerance. J. Exp. Zool. 284, 414–424. [DOI] [PubMed] [Google Scholar]
- Seale AP, Fiess JC, Hirano T, Cooke IM, Grau EG, 2006. Disparate release of prolactin and growth hormone from the tilapia pituitary in response to osmotic stimulation. Gen. Comp. Endocrinol. 145, 222–231. [DOI] [PubMed] [Google Scholar]
- Seale AP, Moorman BP, Stagg JJ, Breves JP, Lerner D, Grau G, 2012a. Prolactin 177, prolactin 188 and prolactin receptor 2 in the pituitary of the euryhaline tilapia, Oreochromis mossambicus, are differentially osmosensitive. J. Endocrinol. 213, 89–98. [DOI] [PubMed] [Google Scholar]
- Seale AP, Riley LG, Leedom TA, Kajimura S, Dores RM, Hirano T, Grau EG, 2002. Effects of environmental osmolality on release of prolactin, growth hormone and ACTH from the tilapia pituitary. Gen. Comp. Endocrinol. 128, 91–101. [DOI] [PubMed] [Google Scholar]
- Seale AP, Watanabe S, Grau EG, 2012b. Osmoreception: Perspectives on signal transduction and environmental modulation. Gen. Comp. Endocrinol. 176, 354–360. [DOI] [PubMed] [Google Scholar]
- Seale LA, Gilman CL, Moorman BP, Berry MJ, Grau EG, Seale AP, 2014. Effects of acclimation salinity on the expression of selenoproteins in the tilapia, Oreochromis mossambicus. J. Trace Elem. Med. Biol. 28, 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton JM, McCormick SD, 1999. Responsiveness of gill Na+/K+-ATPase to cortisol is related to gill corticosteroid receptor concentration in juvenile rainbow trout. J. Exp. Biol. 202 (Pt 8), 987–995. [DOI] [PubMed] [Google Scholar]
- Sparks RT, Shepherd BS, Ron B, Richman NH, Riley LG, Iwama GK, Hirano T, Grau EG, 2003. Effects of environmental salinity and 17 alpha-methyltestosterone on growth and oxygen consumption in the tilapia, Oreochromis mossambicus. Comp. Biochem. Phys. 136, 657–665. [DOI] [PubMed] [Google Scholar]
- Specker J, 1988. Preadaptive Role of Thyroid Hormones in Larval and Juvenile Salmon: Growth, the Gut and Evolutionary Considerations. Integr. Comp. Biol. 28, 337–349. [Google Scholar]
- Specker JL, DiStefano JJ 3rd, Grau EG, Nishioka RS, Bern HA, 1984. Development-associated changes in thyroxine kinetics in juvenile salmon. Endocrinol. 115, 399–406. [DOI] [PubMed] [Google Scholar]
- Specker JL, Kobuke L, 1987. Seawater-acclimation and the thyroidal response to thyrotropin in juvenile coho salmon (Oncorhynchus kisutch). J. Exp. Zool. 241, 327–332. [DOI] [PubMed] [Google Scholar]
- Steinsapir J, Bianco AC, Buettner C, Harney J, Larsen PR, 2000. Substrate-induced down-regulation of human type 2 deiodinase (hD2) is mediated through proteasomal degradation and requires interaction with the enzyme’s active center. Endocrinology 141, 1127–1135. [DOI] [PubMed] [Google Scholar]
- Steinsapir J, Harney J, Larsen PR, 1998. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J. Clin. Invest. 102, 1895–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipsmark CK, Breves JP, Seale AP, Lerner DT, Hirano T, Grau EG, 2011. Switching of Na+, K+-ATPase isoforms by salinity and prolactin in the gill of a cichlid fish. J. Endocrinol. 209, 237–244. [DOI] [PubMed] [Google Scholar]
- Van der Geyten S, Toguyeni A, Baroiller JF, Fauconneau B, Fostier A, Sanders JP, Visser TJ, Kuhn ER, Darras VM, 2001. Hypothyroidism induces type I iodothyronine deiodinase expression in tilapia liver. Gen. Comp. Endocrinol. 124, 333–342. [DOI] [PubMed] [Google Scholar]
- Wambiji N, Park YJ, Kim SJ, Hur SP, Takeuchi Y, Takemura A, 2011. Expression of type II iodothyronine deiodinase gene in the brain of a tropical spinefoot, Siganus guttatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160:447–52. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Itoh K, Kaneko T, 2016. Prolactin and cortisol mediate the maintenance of hyperosmoregulatory ionocytes in gills of Mozambique tilapia: Exploring with an improved gill incubation system. Gen. Comp. Endocrinol. 232, 151–159. [DOI] [PubMed] [Google Scholar]
- Williams GR, Bassett JH, 2011. Deiodinases: the balance of thyroid hormone: local control of thyroid hormone action: role of type 2 deiodinase. J. Endocrinol. 209, 261–272. [DOI] [PubMed] [Google Scholar]
- Young G, Björnsson BT, Prunet P, Lin RJ, Bern HA, 1989. Smoltification and seawater adaptation in coho salmon (Oncorhynchus kisutch): Plasma prolactin, growth hormone, thyroid hormones, and cortisol. Gen. Comp. Endocrinol. 74, 335–345. [DOI] [PubMed] [Google Scholar]
- Zavacki AM, Arrojo E Drigo R, Freitas BCG, Chung M, Harney JW, Egri P, Wittmann G, Fekete C, Gereben B, Bianco AC, 2009. The E3 ubiquitin ligase TEB4 mediates degradation of type 2 iodothyronine deiodinase. Mol. Cell. Biol. 29, 5339–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavacki AM, Ying H, Christoffolete MA, Aerts G, So E, Harney JW, Cheng S. y., Larsen PR, Bianco AC, 2005. Type 1 Iodothyronine Deiodinase Is a Sensitive Marker of Peripheral Thyroid Status in the Mouse. Endocrinol. 146, 1568–1575. [DOI] [PubMed] [Google Scholar]
- Zikos A, Seale AP, Lerner DT, Grau EG, Korsmeyer KE, 2014. Effects of salinity on metabolic rate and branchial expression of genes involved in ion transport and metabolism in Mozambique tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. 178, 121–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.