Summary
Cytoplasmic dynein is activated by the dynactin complex, cargo adapters and LIS1 (Lissencephaly 1). How this process is regulated in vivo remains unclear. The dynein motor ring contains six AAA+ (ATPases Associated with diverse cellular Activities) domains. Here we used the filamentous fungus Aspergillus nidulans to examine whether ATP hydrolysis at AAA3 regulates dynein activation in the context of other regulators. In fungal hyphae, early endosomes undergo dynein-mediated movement away from the microtubule plus ends near the hyphal tip. Dynein normally accumulates at the microtubule plus ends. The early endosomal adapter Hook protein, together with dynactin, drives dynein activation to cause its relocation to the microtubule minus ends. This activation process depends on LIS1, but LIS1 tends to dissociate from dynein after its activation. In this study, we found that dynein containing a mutation blocking ATP hydrolysis at AAA3 can undergo LIS1-independent activation, consistent with our genetic data that the same mutation suppresses the growth defect of the A. nidulans LIS1-deletion mutant. Our data also suggest that blocking AAA3 ATP hydrolysis allows dynein activation by dynactin without the early endosomal adapter. As a consequence, dynein accumulates at microtubule minus ends while early endosomes stay near the plus ends. Dynein containing a mutation blocking ATP binding at AAA3 largely depends on LIS1 for activation, but this mutation abnormally prevents LIS1 dissociation upon dynein activation. Together, our data suggest that the AAA3 ATPase cycle regulates the coordination between dynein activation and cargo binding as well as the dynamic dynein-LIS1 interaction.
Keywords: Dynein, dynactin, LIS1, cargo adapter, early endosomes, filamentous fungi, Aspergillus nidulans, AAA3 ATPase cycle, microtubule plus ends, microtubule minus ends
eTOC blurb
Qiu et al. link the ATPase cycle at the AAA3 domain of cytoplasmic dynein to the spatial regulation of dynein in fungal cells. Specifically, the AAA3 ATPase cycle allows dynein to accumulate at the microtubule plus ends before being activated by cargo adapters, dynactin and LIS1; It also allows LIS1 to dissociate from dynein after its activation.
Introduction
Cytoplasmic dynein-1 (called “dynein” hereafter for simplicity) is a minus-end-directed microtubule motor that transports a variety of cellular cargoes [1, 2]. Dynein is a multi-protein complex, and the two dynein heavy chains form a homodimer [1, 2]. Each heavy chain contains a motor ring with six AAA+ (ATPases Associated with diverse cellular Activities) domains, a stalk connecting the microtubule-binding domain, and a buttress that interacts with the stalk [3]. At the N-terminus of AAA1 is the linker domain that generates motion and force, in part by controlling the buttress-mediated sliding of the stalk helices to shift between strong and weak microtubule-binding states [4]. At the N-terminus of the linker is the dynein tail that binds other dynein subunits and the multi-component dynactin complex [1, 2, 5, 6]. The dynactin complex and specific cargo adapters allow dynein to associate with cargoes such as early endosomes [1, 2, 7-10]. Importantly, several coiled-coils-containing cargo adapters, including the BicD family and the Hook family of proteins, activate the processive movement of mammalian dynein in the presence of dynactin [1, 2, 11, 12]. Dynein activation is caused by a series of conformational changes [13]. The dynein dimer initially forms an autoinhibited “phi” conformation, which can be switched to an open conformation with the motor domains being separated from each other [13]. Binding of dynactin and a cargo adapter to the dynein tails turns the dynein dimer to a parallel configuration, allowing it to walk along a microtubule [13]. Cargo adapter-mediated dynein activation is a mechanism that allows dynein to be activated only after it binds its cargo. Function of the cargo adapter has been implicated in enhancing the interaction between purified mammalian dynein and dynactin [11, 12, 14, 15]. However, since dynein and dynactin interact before cargo binding in some in vivo systems [16, 17], it is likely that additional regulatory mechanisms exist in live cells to ensure that dynein is not activated by dynactin alone before cargo binding.
Dynein stepping requires multiple elements of the dynein heavy chain [3, 4, 18, 19]. ATP binding at AAA1 drives the conformational change of the AAA ring, and it also causes the linker to change from an extended conformation with its N-terminus docked at AAA5 to a bent conformation with its N-terminus moved to AAA2/3 [20, 21]; these changes are transmitted to the buttress and stalk, causing the stalk to shift its coiled-coil registry, which switches the microtubule-binding domain to a low-affinity state [3, 4, 18, 20]. ATP hydrolysis at AAA3 and ATP binding at AAA4 regulate the AAA1-dependent release of dynein from the microtubule [19, 21-27]. Most relevant to our current study, structural studies combined with biophysical analyses in vitro on purified yeast dynein motor domain have provided significant insights into the function of the AAA3 ATPase cycle [21, 25, 26]. Specifically, when the AAA3 ATP hydrolysis is blocked, the linker fails to bend in response to ATP binding at AAA1 and dynein fails to release from the microtubule [21, 25, 26]. Indeed, mutations affecting AAA3 ATP-hydrolysis cause a significant reduction in dynein velocity and force generation in vitro [19, 23, 24, 28]. However, the significance of the AAA3 ATPase cycle has never been examined in the context of cargo adapter and dynactin. Another critical dynein regulator is LIS1, which is required for dynein function in many organisms and cell types [16]. LIS1 binds directly to the dynein motor ring around AAA3/AAA4/AAA5 and to a site on the stalk [28-31]. A study on purified yeast dynein motor domain suggested that different AAA3 nucleotide states may cause LIS1 to bind dynein differently [30]. Given the recent knowledge on LIS1’s role in dynein activation in the context of dynactin and cargo adapters [16, 31-37], it is important to examine the relationship between LIS1 and the AAA3 ATPase cycle in the context of all these regulators.
We have been studying the in vivo regulation of dynein using the filamentous fungus Aspergillus nidulans, a genetically amenable model organism. In filamentous fungi, dynein is required for nuclear distribution and also drives early endosome transport away from the hyphal tip [38, 39]. Dynein, dynactin and LIS1 all accumulate at the microtubule plus ends near the hyphal tip as comet-like structures, and the plus-end accumulation of dynein needs dynactin and kinesin-1 [40-46]. As first discovered in Ustilago maydis, early endosomes in filamentous fungi undergo bidirectional movements driven by kinesin-3 and dynein [38, 42, 47], and the kinesin-1-mediated plus-end dynein targeting is important for dynein-mediated early endosome transport [42]. The dynein-early endosome interaction needs the dynactin complex, and genetic approaches have identified fungal hook proteins as early endosome dynein adapters [7-10]. Upon overexpression of ΔC-HookA, the A. nidulans Hook protein HookA lacking the C-terminal cargo-binding domain [10], dynein and dynactin relocate from the microtubule plus ends to the minus ends at septa or the spindle-pole bodies (SPBs) [35, 48], both of which contain active microtubule-organizing centers [49, 50]. This relocation is consistent with cargo-adapter-mediated dynein activation observed in vitro as well as the cortical adapter Num1-mediated dynein activation in yeasts [11, 12, 32, 34, 51, 52]. By using this relocation as a readout for dynein activation in vivo, we found that dynein activation requires NudF/LIS1 in A. nidulans [35]. In addition, the phi mutation preventing the formation of the phi structure [13] allows the requirement for NudF/LIS1 to be partially bypassed, suggesting that LIS1 promotes the open state of dynein [35], a conclusion largely consistent with recent studies in vitro and in budding yeast [31, 36, 37].
In this study, we first found that a previously identified NudF/LIS1-bypass suppressor mutation in the AAA4 arginine finger [28, 53, 54] allows cargo adapter-mediated dynein activation to occur without LIS1. Since the AAA4 arginine finger is predicted to be involved in AAA3 ATP hydrolysis [18, 21], we further tested the roles of the nucleotide states of AAA3 in dynein activation.
Results:
The AAA4 arginine-finger (RF-AAA4) mutation allows dynein to bypass the requirement of NudF/LIS1 for ΔC-HookA-driven activation
As previously published, GFP-tagged dynein heavy chain (called GFP-dynein for simplicity) carrying the AAA4 arginine finger mutation (RF-AAA4 or nudAR3086C) localizes along microtubules with a clear plus-end enrichment (Figure S1A) [53]. Septal (minus-end) accumulation of dynein was also readily noticed in some hyphae (Figures S1A and S1B). Upon overexpression of ΔC-HookA (gpdA-ΔC-hookA-S), the RF-AAA4 dynein accumulates strongly at the septal minus ends (Figures S1A and S1B), similar to that exhibited by the wild-type dynein [35], suggesting that dynein with the RF-AAA4 mutation is activated by the cargo adapter. We then introduced the temperature-sensitive (ts) loss-of-function mutation of nudF/lis1, nudF6 [55] (or nudFL304S [35]), to test if NudF/LIS1 is needed for this activation. In sharp contrast to the plus-end accumulation of wild-type dynein in the nudF6, gpdA-ΔC-hookA-S background [35], a strong septal accumulation of the RF-AAA4 dynein was observed in the nudF6, gpdA-ΔC-hookA-S background (Figures S1A and S1B). Thus, the RF-AAA4 mutation allows the requirement of NudF/LIS1 for dynein activation to be bypassed to a significant extent.
Dynein function in vivo is affected by AAA3 ATP-binding or hydrolysis mutation; the AAA3 ATP-hydrolysis mutation suppresses the ΔnudF/lis1 colony phenotype
Next, we sought to directly analyze the role of the AAA3 ATPase cycle in dynein activation in vivo mediated by dynactin, LIS1 and cargo adapter. Based on the similarity of dynein heavy chain sequences (Figure S2A), we constructed the AAA3 ATP-binding (Walker A or wA-AAA3, nudAK2599A) and hydrolyzing (Walker B or wB-AAA3, nudAE2663Q) mutants. In comparison to the RF-AAA4 mutant, which formed a colony with asexual spores (note that the green color of the colony is from the asexual spores), both AAA3 mutants formed smaller colonies with a defect in asexual spore production (indicated by the lack of colony color) (Figures 1A and S2B), consistent with a defect in nuclear migration [55]. Thus, although the AAA4 arginine finger is implicated in AAA3 ATP hydrolysis, it is not essential for this process in A. nidulans. Nevertheless, the colonies formed by the wA-AAA3 and wB-AAA3 mutants are bigger than those of the ΔnudA (dynein heavy chain-deletion mutant) [56] and the ΔnudF (NudF/LIS1-deletion mutant) [54] strains (Figures 1A and S2B). Thus, the wA-AAA3 or wB-AAA3 mutant dynein is partially functional in vivo. Consistently, both the wA-AAA3 and wB-AAA3 mutants exhibited a partial defect in nuclear distribution, which is significantly less severe than that exhibited by nudA1, a typical temperature-sensitive nud (nuclear distribution) mutant in which dynein is unstable at a higher temperature [39, 56] (Figures 1B and 1C) (Note that nuclei are labeled by NLS-DsRed [57]). The partial functionality of the wA-AAA3 or wB-AAA3 dynein is also consistent with that of an AAA3 sensor II (involved in ATP binding) mutant dynein in A. nidulans [58]. Importantly, the wB-AAA3 mutation suppressed the colony growth defect of the ΔnudF mutant, suggesting that the wB-AAA3 mutation allows the function of NudF/LIS1 to be bypassed (Figures 1D and S2C). The wA-AAA3 mutation also made the ΔnudF colony slightly bigger at 32°C but the suppression is not as significant as that caused by the wB-AAA3 mutation (Figures 1D and S2C). Surprisingly, adding the nudF6 temperature-sensitive mutation to the wA-AAA3 mutant makes the mutant colony bigger at nudF6’s semi-permissive temperature of 32°C (Figures 1D and S2C). Because the nudF6 mutation makes NudF/LIS1 protein less stable [55], this result suggests that a decrease in the NudF/LIS1 protein level alleviates the defect caused by the wA-AAA3 mutation.
Figure 1. Both the wA-AAA3 and wB-AAA3 mutations affect dynein function but partially suppress the ΔnudF growth defect.
(A) Colony phenotypes of the wA-AAA3 and wB-AAA3 mutants in comparison with the wild type, RF-AAA4 (the AAA4 arginine-finger mutant), ΔnudA and ΔnudF strains. See also Figure S1 for the effect of the RF-AAA4 mutation on dynein activation. See also Figure S2A for a sequence alignment showing positions of the wA-AAA3 and wB-AAA3 mutations. The plate was incubated at 32°C for 3 days. See also Figure S2B for a quantitative analysis of colony diameters. (B) Images of nuclei labeled with NLS-DsRed in wild type, the wA-AAA3 mutant, the wB-AAA3 mutant and the temperature-sensitive nudA1 mutant. The spore head is indicated by a brown arrow. Bar, 5 μm. See also Figure S2D for the colony phenotype of the nudA1 mutant, which is similar to that of the ΔnudA mutant at 37°C. (C) A quantitative analysis on the percentage of germ tubes containing 0, 1, 2, 3, 4, 5, or >5 nuclei in the spore head. Column bar graphs with mean and S.D. values were generated from three experiments (for each experiment, at least 28 germ tubes from each strain were counted). The number of nuclei in the spore head of the wA-AAA3 mutant (n=94) or the wB-AAA3 mutant (n=87) is significantly different from that of wild type (n=135) or the nudA1 mutant (n=93) (p<0.0001 in all cases, Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired). However, the number of nuclei in the spore head of the wA-AAA3 mutant is not significantly different from that in the wB-AAA3 mutant (p>0.99; Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired). (D) Colony phenotypes of various single and double mutant strains. The plate was incubated at 32°C for 3 days. See also Figure S2C for a quantitative analysis of colony diameters.
The minus-end localization of dynein at septa is enhanced mildly by the wA-AAA3 mutation and more dramatically by the wB-AAA3 mutation.
We next used live-cell imaging to observe the localization of GFP-dynein [53], carrying either the wA-AAA3 or wB-AAA3 mutation. We also observed early endosomes in the same hyphae labeled by mCherry-RabA [44, 59, 60]. In wild-type hyphae, dynein mainly accumulates at the microtubule plus ends near the hyphal tip as comet-like structures [40, 53], although localization of dynein at the septa (minus ends) is also seen [50, 61]. The wA-AAA3 mutant dynein signals are seen near the hyphal tip, although in some hyphae the signals do not look like typical comets (microtubule plus-end accumulation) and seem to partially overlap with early endosomes abnormally accumulated at the hyphal tip (Figures 2A). In addition, the wA-AAA3 dynein signals also form a faint microtubule decoration with enrichment at septa (Figures 2A and 2C). In comparison, the wB-AAA3 mutant dynein shows no hyphal tip-enriched signals but a more prominent accumulation at septa and microtubule decoration (Figures 2A and 2C). In some hyphae, the wB-AAA3 dynein is also accumulated as bright dots, which correspond to the SPB signals near the individual nuclei (Figure S3). In the case of the wild-type dynein, such a strong septal and SPB accumulation was only observed when ΔC-HookA was overexpressed to drive dynein activation [35]. As the SPB signals are cell cycle-specific [48], we focus only on the septal accumulation that is more consistently observed. Previously, we showed that the phi mutant dynein (open dynein) also accumulates at the septa without the overexpression of ΔC-HookA, and it is co-localized with early endosomes at the septa [35]. However, early endosomes in either the wB-AAA3 mutant or the wA-AAA3 mutant exhibited a clear hyphal-tip accumulation (100% hyphae, n>30) instead of any obvious septal accumulation (Figure 2A). These observations suggest that most mutant dynein molecules go to the microtubule minus ends without its early endosome cargo.
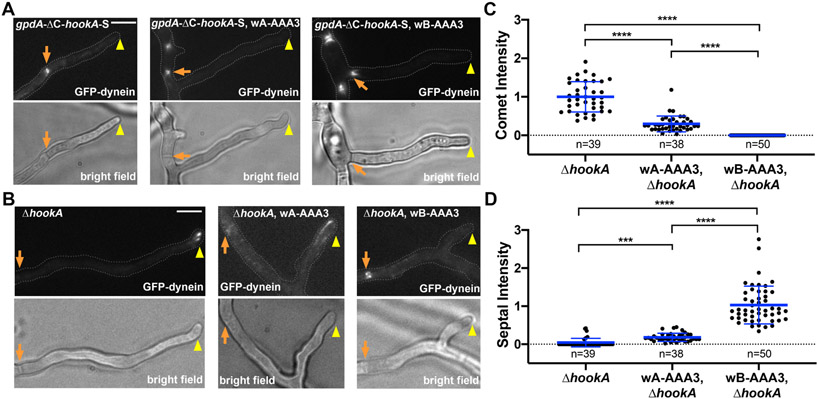
Figure 2. The wA-AAA3 or wB-AAA3 dynein partially accumulates at the microtubule minus ends at septa, and this accumulation requires dynein functionality.
(A) Localization of GFP-dynein and early endosomes (mCherry-RabA) in wild type, the wA-AAA3 mutant and the wB-AAA3 mutant. Bright-field images are shown below to indicate the hyphal shape and position of the septum. The hyphal tip is indicated by a yellow arrowhead and septum by a light brown arrow. Note that 100% of wild-type hyphae (n=39) show comet-like structures near the hyphal tip (representing microtubule plus-end accumulation), 100% of wA-AAA3 hyphae (n=39) show hyphal-tip signals (although some appear to overlap with those of early endosomes) and also obvious septal signals, and 100% of the wB-AAA3 hyphae (n=30) show strong septal signals but no microtubule plus-end accumulation. Bar, 5 μm. See also Figure S3 for the spindle-pole-body (SPB) localization of the wB-AAA3 dynein. See also Figure S4 for images of dynactin (p150-GFP) in the AAA3 mutants and quantitative analyses of the septal signals of dynein and dynactin in these mutants. See also Figure S5 for the motility of wB-AAA3 dynein in vitro. See also Figure S6 for the effect of benomyl on the localization of wB-AAA3 dynein in vivo. (B) The dynein tail mutation nudAF208V (F208V) diminishes the septal accumulation of the wA-AAA3 or wB-AAA3 mutant dynein. Bar, 5 μm. (C) A quantitative analysis on septal dynein intensity in wild type (n=39), wA-AAA3 (n=39), wB-AAA3 (n=30) strains and in the F208V strain (n=36), the wA-AAA3, F208V (n=32) strain and the wB-AAA3, F208V (n=31) strain. The average value for the wB-AAA3 strain is set as 1. Scatter plots with mean and S.D. values were generated by Prism 8. ****p<0.0001; ***p<0.001; ns, non-significant or p>0.05 (Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired).
Next, we examined the localization of dynactin in the wA-AAA3 and wB-AAA3 mutants by introducing the p150-GFP fusion into strains containing the mutations. In both AAA3 mutants, the localization pattern of dynactin is very similar to that of dynein (Figure S4A, S4B). However, faint p150-GFP comets (indicative of microtubule-plus-end accumulation) were seen in some (9 out of 33) wB-AAA3 hyphal tips while wB-AAA3 dynein comets were never observed (0 out of 30). These observations suggest that dynactin is largely associated with dynein in these mutants, although a low level of individual dynactin complexes may localize at the microtubule plus ends in the wB-AAA3 background.
Although the wB-AAA3 dynein decorates microtubules besides forming the minus-end accumulation, the microtubule-bound dynein signals are mainly non-motile. Thus, the minus-end accumulation must have been established during many hours of hyphal growth. To address whether the full-length wB-AAA3 dynein is motile, we prepared cell extract from a strain containing GFP-labeled wB-AAA3 dynein for an in vitro motility assay. A strain containing GFP-labeled wild-type dynein was used as a positive control. In this control strain, the gpdA-ΔC-hookA-S allele was present to ensure that dynein mainly moves towards the microtubule minus end. We found that wild-type dynein moved robustly in the motility assay with a speed of 342.0±114.1 nm/s (mean±S.D., n=26). The full-length wB-AAA3 dynein also moved processively along the microtubule (when 3 mM ATP was used instead of 1 mM ATP), albeit with a very low speed (5.0±2.9 nm/s, mean±S.D., n=25) (Figures S5A, S5B and S5C). The mean velocity of the wB-AAA3 dynein (5.0 nm/s) is similar to that of the wB-AAA3 yeast dynein mutant motor domain (4.8 nm/s or 4.6 nm/s measured in two independent in vitro studies) [19, 24]. This very slow motility was also observed in vivo for the septum-directed movement of p150-GFP (6.5±2.6 nm/s, mean±S.D., n=10), which was detected very occasionally (Figure S5D), and it is also consistent with the notion that the wB-AAA3 dynein is partially functional in vivo (Figure 1).
In addition, we performed in vivo experiments to show that microtubules are important for the septal accumulation of the wB-AAA3 dynein. First, we treated the wB-AAA3 mutant with benomyl, a microtubule-depolymerizing drug. This treatment caused a significant decrease in the septal accumulation and a significant increase in the hyphal-tip accumulation of the wB-AAA3 dynein (Figure S6A-D). The signals of hyphal-tip dynein partially overlap with those of abnormally accumulated early endosomes (Figure S6B), suggesting that the cytosolic wB-AAA3 dynein molecules can associate with early endosomes. We then replaced the benomyl-containing medium with a regular medium to let the cells recover. The recovery caused a significant increase in the septal accumulation but a significant decrease in the hyphal-tip accumulation of the wB-AAA3 dynein compared to cells treated with benomyl. During the recovery, we were able to capture some transient dynein movements away from the hyphal tip (plus ends) (Figure S6E), further supporting the idea that the wB-AAA3 dynein can move towards the minus ends in live cells.
Next, we sought to use a molecular genetic approach to confirm that the septal (minus-end) accumulation of the AAA3 mutant dynein needs dynein functionality. To do that, we constructed GFP-tagged dynein heavy chain containing an AAA3 mutation and the nudAF208V (F208V) dynein tail mutation that significantly impairs dynein activity but does not impair the dynein-dynactin interaction or dynein-early endosome interaction [35, 62]. Dynein carrying the wA-AAA3 and F208V double mutations showed hyphal-tip signals (Figure 2B). Dynein carrying the wB-AAA3 and nudAF208V (F208V) mutations showed a microtubule decoration but ~64% of the hyphae (n=30) also showed a clear microtubule plus-end enrichment, which was not observed in the wB-AAA3 single mutant (n=30) (Figure 2B). Importantly, the dynein F208V mutation significantly diminished or abolished the minus-end accumulation of the AAA3 mutant dynein at septa (Figure 2C). This result confirmed that the minus-end accumulation in both the wA-AAA3 or wB-AAA3 single mutants requires dynein functionality.
Activation of wA-AAA3 dynein depends largely on HookA, but activation of wB-AAA3 dynein occurs without HookA, although it still needs dynactin.
In A. nidulans, the early endosomal adapter HookA activates dynein, and overexpression of the cytosolic ΔC-HookA drives dynein relocation from the microtubule plus ends to the minus ends [35], similar to yeast dynein activation caused by the cortical adapter Num1 [51, 52]. We suspected that activation of the AAA3 mutant dynein could be HookA-independent because the HookA-bound early endosomes did not accumulate with dynein at the septa. However, it would be hard to exclude the possibility that the mutant dynein is activated by the HookA-bound early endosome and then dissociates from the early endosome. To directly test whether HookA is needed for activating the wA-AAA3 and wB-AAA3 dynein, we introduced the gpdA-ΔC-hookA-S allele (causing overexpression of ΔC-HookA) [35] or the ΔhookA allele [10] into a strain background with GFP-dynein harboring the wA-AAA3 or wB-AAA3 mutation. We found that activation of the wA-AAA3 dynein is largely but not absolutely HookA-dependent. Overexpression of ΔC-HookA (gpdA-ΔC-hookA-S) drives the septal accumulation of the wA-AAA3 dynein, just like wild-type dynein (Figure 3A). In the ΔhookA mutant, wild-type dynein forms strong comets (indicative of microtubule-plus-end accumulation) near the hyphal tip, and the wA-AAA3 dynein forms plus-end comets as well but also a microtubule decoration and a septal enrichment (Figure 3B). As a consequence, the wA-AAA3 dynein’s plus-end signal intensity is lower than that of wild-type dynein in the ΔhookA background (Figure 3C). These results suggest that wA-AAA3 dynein needs HookA for activation just like wild-type dynein, but a small portion of the wA-AAA3 dynein molecules may be abnormally activated in a HookA-independent fashion. On the other hand, the wB-AAA3 dynein shows a strong septal accumulation not only upon overexpression of ΔC-HookA (gpdA-ΔC-hookA-S) but also in the ΔhookA background (Figure 3A, 3B). In the ΔhookA background, the wB-AAA3 dynein shows a significantly higher septal signal intensity than the wA-AAA3 dynein, suggesting a higher degree of HookA-independent activation (Figure 3D). However, the microtubule decoration by the wB-AAA3 dynein was obvious in the ΔhookA background (100%, n=30) while overexpression of ΔC-HookA (gpdA-ΔC-hookA-S) drives all the wB-AAA3 mutant dynein to septa (100%, n=30). Thus, HookA still can facilitate the activation of wB-AAA3, despite the tendency of the mutant dynein to undergo HookA-independent activation.
Figure 3. Different effects of the cargo adapter HookA on the localization of wild-type dynein, wA-AAA3 dynein and wB-AAA3 dynein.
(A) Wild-type dynein, wA-AAA3 dynein and wB-AAA3 dynein in the gpdA-ΔC-hookA-S (overexpression of ΔC-HookA) background. Bright-field images are shown below to indicate the hyphal shape and position of the septum. The hyphal tip is indicated by a yellow arrowhead and septum by a light brown arrow. Bar, 5 μm. (B) Wild-type dynein, wA-AAA3 dynein and wB-AAA3 dynein in the ΔhookA mutant background. Bar, 5 μm. (C)(D) Quantitative analyses on comet intensity and septal intensity of wild type (n=39), wA-AAA3 (n=38), and wB-AAA3 (n=50) dynein in the ΔhookA background. Scatter plots with mean and S.D. values were generated by Prism 8. ****p<0.0001, ***p<0.001 (Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired). In (C), the average value for wild-type dynein in the ΔhookA strain is set as 1. In (D), the average value for wB-AAA3, ΔhookA strain is set as 1.
The dynactin complex is a critical component in dynein activation, as the Arp1 mini-filament provides the dynein tail binding sites [5, 6, 12, 13, 63, 64]. However, this function of dynactin has not been directly addressed in A. nidulans due to its additional role in the microtubule plus-end accumulation of wild-type dynein [41, 43, 45, 46]. To determine whether dynactin is required for activating the wA-AAA3 or wB-AAA3 dynein, we introduced the Arp1 conditional-null (alcA-nudKArp1) allele [43] into the strain background with GFP-dynein harboring the wA-AAA3 or wB-AAA3 mutation. In the background of wild-type dynein, shutting down Arp1 expression using this alcA-nudKArp1 allele causes a loss of microtubule plus-end accumulation of dynein [43]. Since the alcA promoter is repressed by glucose but de-repressed by glycerol [65], we used both glycerol- and glucose-containing media to culture the cells containing wA-AAA3, alcA-nudKArp1 and wB-AAA3, alcA-nudKArp1 double mutants. In the single AAA3 mutants without the alcA-nudKArp1 allele, the same localization patterns were observed under these two culture conditions (Figure 4A). In a glycerol medium that allows the expression of Arp1 from the alcA promoter, the double mutants containing the alcA-nudKArp1 allele showed localization patterns similar to those in the single mutants (Figure 4A). However, the double mutants showed drastically different localization patterns when Arp1 expression is shut off by glucose. Specifically, loss of Arp1 causes the wA-AAA3 dynein to become diffused in the cytoplasm, consistent with the previously identified role of dynactin in dynein’s plus-end accumulation [41-43, 45, 46]. Loss of Arp1 significantly diminishes the septal (minus-end) accumulation of the wB-AAA3 dynein while allows it to decorate along microtubules (Figures 4A and 4B), indicating that dynactin is needed for activating the wB-AAA3 dynein.
Figure 4. Dynactin is required for the septal accumulation of the wA-AAA3 or wB-AAA3 dynein.
(A) Images of GFP-labeled wA-AAA3 dynein and wB-AAA3 dynein in the wild type or the Arp1 conditional-null (alcA-nudKArp1) background. The alcA promoter is active on glycerol but repressed by glucose, and thus, the expression of Arp1 is shut off in strains containing the alcA-nudKArp1 allele grown on glucose. The strains without the alcA-nudKArp1 allele grown on glycerol or glucose were shown as controls. Bright-field images are shown below to indicate the hyphal shape and position of the septum. The hyphal tip is indicated by a yellow arrowhead and septum by a light brown arrow. Bar, 5 μm. (B) A quantitative analysis on septal intensity of the wA-AAA3 strain (n=30), the wA-AAA3, alcA-nudKArp1 strain (n=30) , the wB-AAA3 strain (n=31) and the wB-AAA3, alcA-nudKArp1 strain (n=35) grown on glucose. The average value for the wB-AAA3 strain is set as 1. Scatter plots with mean and SD values were generated by Prism 8. ****p<0.0001 (Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired).
Activation of wB-AAA3 dynein is NudF/LIS1-independent; activation of wA-AAA3 dynein requires NudF/LIS1, although this requirement is partially bypassed by ΔC-HookA overexpression.
Next, we observed GFP-dynein harboring the wA-AAA3 or wB-AAA3 mutation in the ΔnudF mutant background. The wA-AAA3 dynein is totally accumulated at the microtubule plus ends as represented by bright comets in the absence of NudF/LIS1 (Figure 5A), suggesting that even the residual HookA-independent septal accumulation of the wA-AAA3 dynein is still dependent on NudF/LIS1. In contrast, the wB-AAA3 dynein accumulated strongly at septa regardless of whether NudF/LIS1 is present or not (Figure 5A). This result indicates that the requirement of NudF/LIS1 can be bypassed by the wB-AAA3 mutation, consistent with the strong suppression of the ΔnudF colony defect by the wB-AAA3 mutation (Figures 1D and S2C).
Figure 5. NudF/LIS1 is dispensable for the septal accumulation of the wB-AAA3 dynein but not the wA-AAA3 dynein, but overexpression of ΔC-HookA allows the wA-AAA3 mutation to partially bypass the requirement of NudF/LIS1.
(A) Images of GFP-labeled wild-type dynein, wA-AAA3 dynein and wB-AAA3 dynein in the ΔnudF background. Bright-field images are shown below to indicate the hyphal shape and position of the septum. The hyphal tip is indicated by a yellow arrowhead and septum by a light brown arrow. Note that 100% of the ΔnudF (n=30) and wA-AAA3, ΔnudF (n=31) hyphae show comet-like structures representing microtubule plus-end accumulation. In contrast, 100% of the wB-AAA3, ΔnudF hyphae (n=31) show strong septal dynein accumulation (with only less than 7% of hyphal tips show possible plus-end comets with weak signals). Bar, 5 μm. (B) Images of GFP-labeled wA-AAA3 dynein or wild-type dynein in the gpdA-ΔC-hookA-S or the nudF6, gpdA-ΔC-hookA-S background. Bar, 5 μm. (C) (D) Quantitative analysis on comet intensity (C) and septal intensity (D) of the wA-AAA3 dynein in the gpdA-ΔC-hookA-S (n=31) or the nudF6, gpdA-ΔC-hookA-S background (n=40) and that of wild-type dynein in the nudF6, gpdA-ΔC-hookA-S background (n=42). Scatter plots with mean and SD values were generated by Prism 8. ****p<0.0001, **p<0.01; ns, non-significant or p>0.05 (unpaired, Mann-Whitney test, Prism 8).
Since the septal localization of wA-AAA3 dynein depends on NudF/LIS1, we further examined if overexpression of ΔC-HookA would help overcome this dependency. In this experiment, we used the temperature-sensitive nudF6 allele instead of the ΔnudF deletion allele to facilitate strain growth on a solid medium before the live-cell imaging experiments. Previously, we found that while ΔC-HookA overexpression (gpdA-ΔC-hookA-S) causes dynein to relocate from the microtubule plus ends to the minus ends, the nudF6 mutation prevents this relocation and keeps dynein at the plus ends [35]. Consistent with a role of NudF/LIS1 in activation of the wA-AAA3 dynein, the nudF6 mutation also kept the wA-AAA3 dynein as plus-end comets upon overexpression of ΔC-HookA (gpdA-ΔC-hookA-S) (Figure 5B, 5C). However, a septal accumulation of the wA-AAA3 dynein was also obvious in the nudF6, gpdA-ΔC-hookA-S background, which was significantly stronger than wild-type dynein in the same background (Figure 5B, 5D). Thus, the wA-AAA3 mutation bypasses NudF/LIS1 function to a low but detectable degree when ΔC-HookA is overexpressed. This may explain why the wA-AAA3 mutation mildly suppresses the colony growth defect of the ΔnudF mutant (Figure 1D, S2C).
The wA-AAA3 mutation alters the LIS1-dynein interaction
Previously, we found that during the ΔC-HookA-mediated activation of wild-type dynein, NudF/LIS1 accumulates at the microtubule plus ends instead of going to the minus ends with dynein despite its importance for dynein activation [35]. This is consistent with LIS1’s dissociation from the activated dynein complex during minus-end-directed movement [31, 34, 36, 42, 45, 52]. In the wB-AAA3 dynein mutant, although dynein strongly accumulates at the septal minus ends, NudF/LIS1-GFP forms plus-end comets instead of co-localizing with dynein at the septa, which is similar to the behavior of NudF/LIS1-GFP during the ΔC-HookA-driven activation of wild-type dynein (Figure 6A). Surprisingly, in the wA-AAA3 mutant, NudF/LIS1-GFP localizes to both the hyphal tip and septa just like wA-AAA3 dynein. Next, we introduced the gpdA-ΔC-hookA-S allele into the NudF/LIS1-GFP, wA-AAA3 strain and the NudF/LIS1-GFP, wB-AAA3 strain to examine NudF/LIS1-GFP localization upon overexpression of ΔC-HookA (Figure 6B). In the wA-AAA3, gpdA-ΔC-hookA-S background, where the wA-AAA3 dynein accumulates at the septa (Figure 3A), NudF/LIS1-GFP also accumulates at the septa (Figures 6B and 6C), although plus-end comets were still observed. In contrast, NudF/LIS1-GFP does not go to the septa in the wB-AAA3, gpdA-ΔC-hookA-S strain. These results suggest that the wA-AAA3 mutation allows NudF/LIS1 to stay with dynein and co-localize with dynein at the microtubule minus ends, while the wB-AAA3 dynein tends to dissociate from NudF/LIS1 just like wild-type dynein after its cargo adapter-mediated activation.
Figure 6. Images showing that NudF/LIS1 accumulates at the septa in the wA-AAA3 mutant.
(A) Images of NudF/LIS1-GFP localization in wild-type, the wA-AAA3 mutant and the wB-AAA3 mutant. Bright-field images are shown below to indicate the hyphal shape and position of the septum. The hyphal tip is indicated by a yellow arrowhead and septum by a light brown arrow. Bar, 5 μm. (B) Images of NudF/LIS1-GFP localization in wild-type, the wA-AAA3 mutant and the wB-AAA3 mutant in the gpdA-ΔC-hookA-S background. Bar, 5 μm. (C). A quantitative analysis on septal intensity of NudF/LIS1-GFP in the backgrounds of gpdA-ΔC-hookA-S (n=35), wA-AAA3, gpdA-ΔC-hookA-S (n=25), wA-AAA3 (n=30), wB-AAA3 (n=31), and wB-AAA3, gpdA-ΔC-hookA-S (n=31). The average value of the LIS1-GFP septal intensity in the wA-AAA3, gpdA-ΔC-hookA-S strain is set as 1. Scatter plots with mean and S.D. values were generated by Prism 8. ****p<0.0001, ***p<0.001, **p<0.01, ns, non-significant or p>0.05 (Kruskal-Wallis with Dunn’s multiple comparisons test, unpaired).
Discussion:
In this study, we found that the wB-AAA3 mutation allows dynein to bypass the requirement of NudF/LIS1 for its activation. As recent data link the function of LIS1 to promoting the open state of dynein [31, 35-37], our current result suggests that the wB-AAA3 dynein is also open. In the wB-AAA3 mutant, the linker is locked at one position during the AAA1 ATPase cycle, with its N-terminus docked at AAA5 [21]. This is in contrast to wild-type dynein in which the linker bends upon ATP binding to AAA1, with its N-terminus close to AAA2/AAA3 and/or other positions [21]. The phi conformation of dynein is maintained by multiple interactions, and linkers in the phi dynein (PDB code: 5NUG) are bent [13]. Thus, if the linker is docked at AAA5, the phi structure may not be able to form, which implies that the wB-AAA3 dynein is unable to form the phi structure. However, the open conformation of the wB-AAA3 dynein must be different from that of the phi mutant dynein, which is open due to the weakened AAA4-linker ionic interaction between two dynein heavy chains within the dimer [13]. The phi mutant dynein also strongly accumulates at the microtubule minus ends at centrosomes in mammalian cells and the septa in A. nidulans [13, 35]. However, a key difference between the phi mutant and the wB-AAA3 mutant is that early endosomes strongly colocalize with the phi mutant (open) dynein [35] but not with the wB-AAA3 dynein at the septa. Although it is hard to completely exclude the possibility that other cargo adapters may be responsible for this minus-end localization, our data are most easily explained by the possibility that blocking AAA3 ATP hydrolysis allows the requirement of cargo adapter for dynein activation to be bypassed. The function of the cargo adapter has been implicated in enhancing the dynein-dynactin interaction [11, 12, 14, 15]. In fungi, however, the dynein-dynactin interaction clearly occurs before cargo-adapter-mediated dynein activation [16, 17], especially since dynactin is absolutely required for dynein’s plus-end accumulation in filamentous fungi [41-43, 45, 46] and dynein is required for the plus end-accumulation of dynactin in the budding yeast [66]. Thus, the function of the cargo adapter must be needed for changing the binding configuration of the two complexes so that the tails and motor domains of the dynein dimer can assume a parallel configuration [13]. Given the special linker position of the wB-AAA3 dynein [21], could the dynactin complex be sufficient for making the wB-AAA3 dynein parallel? In this context, we would also point out that in some previous in vitro experiments, dynactin alone can indeed cause or enhance dynein processivity in a moderate way [67-70]. Alternatively, could there be an in vivo regulator that reinforces the inactive state of dynein-dynactin before cargo binding and the wB-AAA3 mutation prevents dynein-dynactin from interacting with this regulator? Future work will clearly be needed to address these interesting questions.
The wB-AAA3 dynein does not accumulate at the microtubule plus end. This is likely due to an unscheduled activation of the wB-AAA3 dynein, as its plus-end accumulation can be partially restored if dynein activity is compromised by a dynein tail mutation. The unscheduled activation may also weaken the kinesin-1-dynein interaction if kinesin-1 preferentially transports dynein in the autoinhibited phi conformation [17]; or, the mutant dynein may still bind kinesin-1 but overpowers it [71]. These possibilities will need to be tested in the future. In filamentous fungi, LIS1 is not as important as dynactin for the plus-end accumulation of dynein [41-43, 45, 46]. However, Pac1/LIS1 but not dynactin is critically required for the plus-end accumulation of dynein in the budding yeast [72, 73]. The yeast motor domain with the wA-AAA3 or wB-AAA3 mutation and the full-length wA-AAA3 dynein all accumulate normally at the microtubule plus ends, indicating that they can all bind LIS1 [74]. Here we should emphasize that the mechanism of LIS1 in promoting the plus-end dynein accumulation is unlikely related to strengthening a direct dynein-microtubule binding, because the microtubule-binding domain of yeast dynein is dispensable for its plus-end accumulation [52]. Instead, it is more closely related to the direct interaction between LIS1 and the plus-end-tracking protein Bik1/CLIP170, which recruits dynein via LIS1 [16, 73]. In filamentous fungi, the inessentiality of LIS1 in the plus-end dynein accumulation makes the activating function of LIS1 to be more easily observed in A. nidulans upon cargo-adapter-mediated dynein activation (compared to Num1-mediated activation in yeast [52]), as the lack of NudF/LIS1 prevents dynein from leaving the plus end [35]. In addition, while the A. nidulans AAA4 arginine-finger mutation or the wB-AAA3 mutation suppresses the growth defect of the ΔnudF/lis1 mutant, the yeast AAA4 arginine-finger mutation does not suppress the Δpac1/lis1 mutant phenotype [28], because the additional function of Pac1/LIS1 in plus-end dynein accumulation cannot be bypassed [28, 72, 73]. In mammalian cells or if mammalian proteins are used in vitro, although the plus-end dynein accumulation absolutely needs dynactin, LIS1 has also been shown to be important [14, 32, 34]. Thus, it may be easier to test if the mammalian wB-AAA3 dynein bypasses the requirement of LIS1 in an experimental setting where plus-end dynein accumulation is not a complicating factor.
LIS1 tends to dissociate from activated dynein in vivo and in vitro. In U. maydis and A. nidulans, LIS1 but not dynactin dissociates from early endosomes undergoing dynein-mediated transport [42, 45]. In budding yeast, LIS1 dissociates from Num1-activated dynein [52]. Interestingly, an engineered dynein mutation implicated in overcoming dynein autoinhibition allows LIS1 to co-localize with dynein cortically anchored at the Num1 sites [37, 75]. In different in vitro assays, LIS1 can either co-migrate with the dynein-dynactin-cargo adapter complex [32, 33] or dissociate from the activated dynein complex [31, 34, 36]. In A. nidulans, the notion of LIS1-dynein dissociation after dynein activation explains the observation that NudF/LIS1 does not co-localize with activated dynein at the septal minus ends [35]. However, if AAA3 ATP binding is blocked, NudF/LIS1 strongly accumulates at the minus ends just like activated dynein, suggesting that the AAA3 ATPase cycle regulates the dynamic dynein-LIS1 interaction in vivo. The abnormally increased dynein-LIS1 affinity may contribute to the functional defect of the wA-AAA3 dynein since reducing the NudF/LIS1 level in A. nidulans by using a nudF6 mutant grown at a semi-permissive temperature improves the growth of the wA-AAA3 mutant (Figures 1D and S2C). Based on this current study and previous studies from several labs, we propose that the AAA3 ATPase cycle is closely linked to the spatial regulation of dynein activation in vivo in the following ways: First, during kinesin-1-mediated transport to the microtubule plus end, dynein is in the autoinhibited phi conformation with an ADP-bound AAA3 (Figure 7). Second, LIS1 may preferentially bind dynein with an apo-AAA3 (note that LIS1 binding is not compatible with the phi dynein conformation, and the open dynein conformation enhances the affinity of the LIS-dynein interaction [31, 37]). Third, ATP binding to AAA3 occurs after cargo binding, as otherwise dynein may be activated by dynactin alone without the cargo. Finally, after cargo-mediated dynein activation, LIS1 may dissociate at any step except when AAA3 is in the apo state. We should point out that our current study is done in the context of not only cargo adapter and dynactin, but also NudE, another dynein regulator that binds both LIS1 and dynein [16]. Previous work on purified mammalian dynein showed that while LIS1 only binds dynein when ATP and vanadate are added, the inclusion of NudE allows LIS1-dynein binding in the absence of nucleotide [76]. Although the wA-AAA3 mutation clearly alters the dynamic LIS1-dynein interaction in A. nidulans, our hypothesis that LIS1 preferentially binds dynein with an apo-AAA3 before cargo binding will need to be further examined in the presence and absence of NudE.
Figure 7. A hypothetic model of dynein activation involving the AAA3 ATPase cycle.
Note that we only focus on a series of events at the vicinity of the microtubule plus end. During kinesin-1-mediated transport of dynein-dynactin to the microtubule plus end, dynein is most likely in the autoinhibited phi conformation. In the phi dynein, both AAA1 and AAA3 are occupied by ADP, and the linker is in a bent conformation (PDB code: 5NUG) [13]. Two different states (either apo or ATP at both AAA1 and AAA3) with the linkers docked on AAA5 [21, 77] are shown at the plus end. LIS1 may preferentially bind dynein with apo-AAA3, which is followed by cargo binding to cause dynein activation. ATP binding to AAA3 most likely happens after cargo-mediated dynein activation, because otherwise dynein would be activated by dynactin without the cargo. During the minus-end-directed movement of the dynein-dynactin-cargo adapter complex, AAA3 is ADP-bound most of the time, and the linker is flexible and its position would depend on the AAA1 nucleotide state [21, 25], which in turn regulates dynein stepping [4, 19]. Finally, LIS1 can dissociate from the activated dynein-dynactin-cargo adapter complex at any step as long as the AAA3 is not in the apo state.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xin Xiang (Xin.xiang@usuhs.edu).
Materials availability
Aspergillus nidulans strains generated in this study are available upon request to the lead contact.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
The model organism we used for this study is Aspergillus nidulans, a filamentous fungus. A. nidulans strains grow on solid rich medium made of either YAG (0.5% yeast extract and 2% glucose with 2% agar) or YAG+UU (YAG plus 0.12% uridine and 0.11% uracil). Solid minimal medium containing 1% glucose was used for selecting progeny from a genetic cross. Note that the minimal medium (pH 6.5) also contains 0.6% NaNO3, 0.052% KCl, 0.0152% KH2PO4 and 0.051% MgSO4. Asexual spores of the strains can be stored in 70% glycerol at −80°C for several years. For most live-cell imaging experiments, cells were cultured overnight at 32°C in a liquid minimal medium containing 1% glycerol. For the live-cell imaging analysis on nuclear distribution, cells were cultured in minimal glucose medium for 7-8 hours at 37°C. All live-cell imaging experiments involving the nudF6 mutation were done at 37°C. Note that the nudF6 mutant as well as the nudA1 mutant are temperature-sensitive; they form tiny colony lacking asexual spores at a higher temperature (typical of a nud or nuclear-distribution mutant), but some asexual spores are produced at the semi-permissive temperature of 32°C. Thus, for experiments involving these mutations, we harvested spores at 32°C and cultured them at 37°C for imaging analysis. For experiments involving the alcA-nudKArp1 (Arp1 conditional-null) allele, we harvested spores from the solid minimal medium containing 1% glycerol (non-repressive for the alcA promoter) and cultured them in liquid minimal medium containing 1% glucose (repressive for the alcA-promoter [65]) for imaging analysis. In experiments using benomyl, the final concentration of benomyl is 2.4 μg/mL, and the solvent for benomyl (95% ethanol) was used as a control. For in vitro motility assays, cells were grown overnight at 32°C in a liquid YG rich medium (0.5% yeast extract and 2% glucose) before being harvested.
METHOD DETAILS
Live-cell imaging and analyses
All images were captured using an Olympus IX73 inverted fluorescence microscope linked to a PCO/Cooke Corporation Sensicam QE cooled CCD camera. A UPlanSApo 100x objective lens (oil) with a 1.40 numerical aperture was used. A filter wheel system with GFP/mCherry-ET Sputtered series with high transmission (Biovision Technologies) was used. For all images, cells were grown in the LabTek Chambered #1.0 borosilicate coverglass system (Nalge Nunc International, Rochester, NY). Images were taken at room temperature immediately after the cells were taken out of the incubators. The IPLab software (Scanalytics, Inc.) was used for image acquisition and analysis. Image labeling was done using Microsoft PowerPoint and/or Adobe Photoshop. For quantitation of signal intensity, a region of interest (ROI) was selected and the Max/Min tool of the IPLab software was used to measure the maximal intensity within the ROI. The ROI box was then dragged to a nearby area inside the cell to take the background value, which was then subtracted from the intensity value. For measuring the signal intensity of a microtubule plus-end comet formed by GFP-dynein or NudF/LIS1-GFP proteins, only the comet closest to the hyphal tip was measured. For measuring GFP-dynein signal intensity at septa, usually only the septum most proximal to the hyphal tip was measured. For the benomyl-treatment experiment (Figure S6C), because the treatment obviously decreased the signal-containing area, the sum of septal intensity was measured. For quantitative analysis, hyphae were chosen randomly from images acquired under the same experimental conditions. All the observations were made in at least three different experiments done under the same conditions, and the same trend was observed in at least three experiments.
Constructing the wA-AAA3 (nudAK2599A) and wB-AAA3 (nudAE2663Q) mutants and other strains containing the mutant alleles
For making the AAA3 Walker A (wA-AAA3) mutant nudAK2599A, we used fusion PCR to make a DNA fragment with the following primers: WAF (5'-TTCTGGTGCAACCATGACACTGTTTGCCG-3'), WAR (5'-GTGTCATGGTTGC ACCAGAACCGGGAGGAC-3'), NudA58 [53] and NdA310 [53]. For making the AAA3 Walker B (wB-AAA3) mutant nudAE2663Q, we used fusion PCR to make a DNA fragment with the following primers: WBF (5'-ATCTTCTGCGATCAAATCAACCTGCCGGCTC-3'), WBR (5'-CAGGTTGATTTGATCGCAGAAGATAACCAGCCAA-3'), NudA58 [53] and NdA310 [53]. Each fragment was co-transformed with a selective marker pyrG fragment into a pyrG89 strain RQ2 [62] containing GFP-dynein HC (NudA), mCherry-RabA and the Ku70 deletion allele ΔnkuA-argB, which facilitates the selection of transformants in which transformed DNA fragments underwent homologous integration into the genome [78]. Several transformants with growth defects were selected and the mutations confirmed by sequencing analysis of the genomic DNA fragments. For making the strains containing p150-GFP and the wA-AAA3 or the wB-AAA3 allele, the fusion PCR product containing the wA-AAA3 or the wB-AAA3 mutation was co-transformed with the p150-GFP-AfpyrG fragment [7] into a pyrG89 strain XY42 [79] containing mCherry-RabA and ΔnkuA-argB. Note that the p150-GFP-AfpyrG fragment was amplified from the genomic DNA isolated from the XY13 [7] strain by using the following two primers: ORF-F2 (5’-GTCCTTTCAAGCAAGCAGAGAATG-3’) and UTR-R2 (5’-GATGCTGAGCTTGCTGCCTG-3’). Transformants with growth defect were selected and the mutations confirmed by sequencing analysis. For making the strains containing both the dynein heavy chain tail mutation nudAF208V and the wA-AAA3 or the wB-AAA3 allele, the fusion PCR product containing the wA-AAA3 or the wB-AAA3 mutation was co-transformed with the A. nidulans pyrG fragment into a pyrG89 strain (RQ338) containing GFP-nudAF208V, mCherry-RabA and ΔnkuA-argB. Transformants with growth defects were selected and the mutations confirmed by sequencing analysis.
Strains containing multiple mutant alleles were constructed by genetic crosses. Genetic crosses were done by standard methods, and progeny with desired genotypes were selected based on colony phenotype, imaging analysis, western analysis, diagnostic PCR, and/or sequencing of specific regions of the genomic DNA. For genomic DNA isolation, about 0.25 g of hyphal mass was harvested from overnight culture for each sample and pulverized with liquid nitrogen by using mortars and pestles. The DNeasy Plant Mini Kit (QIAGEN) was used for genomic DNA isolation. The QIAquick Gel Extraction Kit (QIAGEN) was used for purifying PCR products from agarose gels. Sequencing was done using the service of Quintarabio (https://www.quintarabio.com/service/ngs_services).
In vitro dynein motility assay using A. nidulans extract
About 0.25 g of hyphal mass was harvested from overnight culture for each sample and pulverized with liquid nitrogen by using mortars and pestles. Cell extract was prepared using 150 μL of lysis buffer (30 mM HEPES pH 7.3, 50 mM potassium acetate, 2 mM magnesium acetate, 1 mM EGTA, 10% glycerol, 1 mM DTT, and 0.2 mM ATP). Cell extracts were then centrifuged at 20,000 g for 30min at 4°C to remove cell debris, and the supernatant was kept on ice for motility assays, or snap-frozen with liquid nitrogen and stored at −80°C if motility assays cannot be performed on the same day. Immediately prior to imaging, the cell lysates were warmed to room temperature and diluted 10 times in the lysis buffer containing 1 mM ATP, 1 mM Trolox, 20 μM Taxol, 10 mM DTT, 1 mg/mL BSA, 1 mg/mL β-casein, 15 μg/mL catalase, 40 μM glucose, and 40 μg/mL glucose oxidase.
To make X-rhodamine-labeled microtubule, un-labeled tubulin (Cytoskeleton, Inc) and X-rhodamine-labeled tubulin (Cytoskeleton, Inc) are mixed with a ratio of ~12:1 in BRB80 buffer (80 mM PIPES, pH 7.0, 1 mM EGTA, and 1 mM MgCl2) with 2 mM GTP. After incubating for 12 min at 35°C, the polymerized microtubule was stabilized by adding 20 μM taxol and stored at room temperature.
To prepare flow chambers, 75 mm x 25 mm glass microscope slides and 18 mm x 18 mm coverslips with #1.5 thickness were treated as described [80]. In summary, both the microscope slides and coverslips were pre-cleaned with alkaline in bath sonicator followed by washing with ultrapure water and treating in plasma clear with RF coil set on "high" for 5 min. The pre-cleaned coverslips were further aminosilanized with 2% APTES (Sigma-Aldrich). To assemble the slide chamber, a set of parafilm strips were inserted between the aminosilane surface-coated coverslip and pre-cleaned slide to generate an approximate volume of 15-20 μL and the chamber was then formed after being heat sealed. The aminosilanized surface of the slide chamber was further activated with 8% glutaraldehyde (Sigma-Aldrich) to enhance microtubule binding before an experiment.
To image dynein motility on microtubule, 20 μL of ~0.1 mg/mL microtubule was flushed into the glutaraldehyde treated chamber from one side and incubate at room temperature for 90 seconds, 20 μL BRB80 with 20 μM taxol was then added from the same side and the chamber was incubated at room temperature for 20 min to allow microtubule binding. The flow chamber was then blocked with 2 mg/mL β-casein and 20 μM Taxol for 5 min, and the cell lysates were added in the presence of either 1 mM or 3 mM ATP. Time-lapse movies were acquired immediately with either an Olympus IX73 inverted fluorescence microscope linked to a PCO/Cooke Corporation Sensicam QE cooled CCD camera or an Olympus IX83 microscope equipped with a one-line motorized IX3 cellTIRF illuminator and quad laser TIRF set, outfitted with an ORCA flash 4.0 v3 sCMOS camera (Hamamatsu), a 100x 1.50-NA objective, and CellSens software. A 488-nm laser and a 561-nm laser were used for acquiring images of GFP-labeled dynein and X-Rhodamine-labeled microtubules respectively. Several 1.5 to 2 min movies were acquired at room temperature per chamber with 1-second intervals of each frame. Velocities were measured by using kymographs generated by the Fiji/ImageJ software (the National Institutes of Health).
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analyses were done using GraphPad Prism 8 for Mac (version 8.0.0, 2018). The D’Agostino & Pearson normality test was performed on all data sets. In all the figures of quantitative analyses, non-parametric tests were used without assuming Gaussian distribution, because not all data sets passed the normality test. Specifically, the Kruskal-Wallis (unpaired) with Dunn’s multiple comparisons test was used for analyzing multiple data sets at once (Figures 2C, 3C, 3D, 4B, 6C, S1B, S4B, S6C and S6D). The Mann-Whitney test (unpaired, two-tailed) was used for comparing two data sets at a time (Figures 5C, 5D, S2B, S2C and S5B). In all the figures presenting quantitative analyses of signal intensity, n is the number of hyphae. For the colony-diameter measurement described in S2D legend, ordinary one-way ANOVA with multiple comparisons test was used. Due to the fact that the difference and similarity among the colony sizes are very obvious, we only measured a small number of colonies (n=3), and in this case, ordinary one-way ANOVA is a more powerful method than the non-parametric tests to reveal the difference. For all quantitative analyses, the mean and S.D. values are presented. Note that adjusted p values were generated from the Kruskal-Wallis with Dunn’s multiple comparisons test. In all figures, **** indicates p<0.0001, *** indicates p<0.001, ** indicates p<0.01, * indicates p<0.05 *, and “ns” indicates p>0.05.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and virus strains | ||
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Uridine | Sigma-Aldrich | Cat# U3750 |
| Uracil | Sigma-Aldrich | Cat# U0750 |
| Benomyl | Sigma-Aldrich | Cat# 45339 |
| Trolox | Sigma-Aldrich | Cat# 238813 |
| Paclitaxel (Taxol) | Cytoskeleton, Inc | Cat# TXD01 |
| β-casein | Sigma-Aldrich | Cat# C6905 |
| Catalase | Sigma-Aldrich | Cat# C9322 |
| Glucose oxidase | Sigma-Aldrich | Cat# G2133 |
| Tubulin | Cytoskeleton, Inc | Cat# T238P |
| X-rhodamine-labeled tubulin | Cytoskeleton, Inc | Cat# TL620M |
| (3-Aminopropyl)triethoxysilane (APTES) | Sigma-Aldrich | Cat# A3648 |
| Glutaraldehyde | Sigma-Aldrich | Cat# G6257 |
| D-(+)-Glucose | Sigma-Aldrich | Cat# G7528 |
| GTP | Cytoskeleton, Inc | Cat# BST06 |
| ATP | Cytoskeleton, Inc | Cat# BSA04 |
| Critical commercial assays | ||
| DNeasy Plant Mini Kit | QIAGEN | Cat# 69104 |
| QIAquick Gel Extraction Kit | QIAGEN | Cat# 28706 |
| Deposited data | ||
| Experimental models: Cell lines | ||
| Experimental models: Organisms/strains | ||
| A. nidulans: JZ383: ΔnudF::pyr4; GFP-nudAHC; argB2::[argB*-alcAp::mCherry-RabA] | J. Zhang (Uniformed Services University) [35] | N/A |
| A. nidulans: LZ36: GFP-nudAR3086C; ΔnkuA::argB; pyroA4 | L. Zhuang (Uniformed Services University) [53] | N/A |
| A. nidulans: RQ2: GFP-nudAHC; argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyrG89; pyroA4; yA2 | R. Qiu (Uniformed Services University) [62] | N/A |
| A. nidulans: RQ8: GFP-nudAF208V; argB2::[argB*-alcAp::mCherry-RabA]; pyrG89; wA2 | R. Qiu (Uniformed Services University) [62] | N/A |
| A. nidulans: RQ165: nudF-GFP-AfpyrG; argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyroA4; pyrG89; wA2 | R. Qiu (Uniformed Services University) [35] | N/A |
| A. nidulans: RQ275: nudF6; gpdA-ΔC-hookA-S-AfpyrG; GFP-nudAHC; pyrG89; yA2; possibly ΔnkuA::argB | R. Qiu (Uniformed Services University) [35] | N/A |
| A. nidulans: RQ287: GFP-nudAHC; gpdA-ΔC-hookA-S-AfpyrG; yA2 | R. Qiu (Uniformed Services University) [35] | N/A |
| A. nidulans: XX60: ΔnudA::pyrG; pyrG89 | X. Xiang (Uniformed Services University) [56] | N/A |
| A. nidulans: XX222: GFP-nudAHC; argB2::[argB*-alcAp::mCherry-RabA]; yA2; pantoB100 | X. Xiang (Uniformed Services University) [7] | N/A |
| A. nidulans: XX514: GFP-nudAHC; ΔyA::NLS-DsRed; possibly ΔnkuA::argB | X. Xiang (Uniformed Services University) [35] | N/A |
| A. nidulans: XX566: nudF-GFP-AfpyrG; gpdA-ΔC-hookA-S-AfpyrG; argB2::[argB*-alcAp::mCherry-RabA]; wA2 | X. Xiang (Uniformed Services University) [35] | N/A |
| A. nidulans: XX634: ΔnudF::pyr4; GFP-nudAR1602E, K1645E; argB2::[argB*-alcAp::mCherry-RabA]; possibly ΔnkuA::argB | X. Xiang (Uniformed Services University) [35] | N/A |
| A. nidulans: XY13: p150-GFP-AfpyrG; ΔnkuA::argB; pyrG89; pyroA4 | X. Yao (Uniformed Services University) [7] | N/A |
| A. nidulans: XY42: argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyrG89; yA2; pantoB100 | X. Yao (Uniformed Services University) [79] | N/A |
| A. nidulans: JZ504: GFP-nudAHC; ΔhookA-AfpyrG; argB2::[argB*-alcAp::mCherry-RabA]; pyroA4 | This paper | N/A |
| A. nidulans: RQ102: GFP-nudAHC; ΔhookA-AfpyrG; argB2::[argB*-alcAp::mCherry-RabA]; pantoB100; yA2 | This paper | N/A |
| A. nidulans: RQ293: nudF6; gpdA-ΔC-hookA-S-AfpyrG; GFP-nudAR3086C | This paper | N/A |
| A. nidulans: RQ295: gpdA-ΔC-hookA-S-AfpyrG; GFP-nudAR3086C | This paper | N/A |
| A. nidulans: RQ300: GFP-nudAK2599A(wA-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyroA4; yA2 | This paper | N/A |
| A. nidulans: RQ301: GFP-nudAE2663Q(wB-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyroA4; yA2 | This paper | N/A |
| A. nidulans: RQ306: ΔnudF::pyr4; GFP-nudAK2599A(wA-AAA3); argB2::[argB*-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: RQ307: ΔnudF::pyr4; GFP-nudAE2663Q(wB-AAA3); argB2::[argB*-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: RQ309: GFP-nudAK2599A(wA-AAA3); gpdA-ΔC-hookA-S-AfpyrG | This paper | N/A |
| A. nidulans: RQ311: GFP-nudAK2599A(wA-AAA3); ΔyA::NLS-DsRed; possibly ΔnkuA::argB | This paper | N/A |
| A. nidulans: RQ312: GFP-nudAE2663Q(wB-AAA3); gpdA-ΔC-hookA-S-AfpyrG | This paper | N/A |
| A. nidulans: RQ314: GFP-nudAE2663Q(wB-AAA3); ΔyA::NLS-DsRed; possibly ΔnkuA::argB | This paper | N/A |
| A. nidulans: RQ317: GFP-nudAK2599A(wA-AAA3); gpdA-ΔC-hookA-S-AfpyrG; argB2::[argB*-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: RQ318: GFP-nudAK2599A(wA-AAA3); gpdA-ΔC-hookA-S-AfpyrG; nudF6 | This paper | N/A |
| A. nidulans: RQ323: nudAK2599A(wA-AAA3); p150-GFP-AfpyrG; ΔnkuA::argB; argB2::[argB*-alcAp::mCherry-RabA]; pantoB100; yA2 | This paper | N/A |
| A. nidulans: RQ324: nudAE2663Q(wB-AAA3); p150-GFP-AfpyrG; ΔnkuA::argB; argB2::[argB*-alcAp::mCherry-RabA]; pantoB100; yA2 | This paper | N/A |
| A. nidulans: RQ328: GFP-nudAE2663Q(wB-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; ΔhookA-AfpyrG | This paper | N/A |
| A. nidulans: RQ329: GFP-nudAK2599A(wA-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; ΔhookA-AfpyrG | This paper | N/A |
| A. nidulans: RQ330: GFP-nudAK2599A(wA-AAA3); argB2::[argB*-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: RQ331: GFP-nudAE2663Q(wB-AAA3); argB2::[argB*-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: RQ332: GFP-nudAK2599A(wA-AAA3); alcAp-nudKArp1-pyr4; possibly argB2::[argB*-alcAp::mCherry-RabA]; possibly p25-S | This paper | N/A |
| A. nidulans: RQ333: GFP-nudAE2663Q(wB-AAA3); alcAp-nudKArp1-pyr4; possibly argB2::[argB*-alcAp::mCherry-RabA]; possibly p25-S | This paper | N/A |
| A. nidulans: RQ338: GFP-nudAF208V; argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyrG89; pyroA4 | This paper | N/A |
| A. nidulans: RQ343: GFP-nudAK2599A, F208V; argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyroA4 | This paper | N/A |
| A. nidulans: RQ344: GFP-nudAE2663Q, F208V; argB2::[argB*-alcAp::mCherry-RabA]; ΔnkuA::argB; pyroA4 | This paper | N/A |
| A. nidulans: RQ354: nudF-GFP-AfpyrG; nudAK2599A(wA-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; possibly ΔnkuA::argB; pyrG89 | This paper | N/A |
| A. nidulans: RQ355: nudF-GFP-AfpyrG; nudAE2663Q(wB-AAA3); argB2::[argB*-alcAp::mCherry-RabA]; possibly ΔnkuA::argB; pyrG89 | This paper | N/A |
| A. nidulans: XX632: GFP-nudAR3086C; ΔnudF::pyr4; argB2::[argB*-alcAp::mCherry-RabA]; possibly ΔnkuA::argB; possibly pyrG89 | This paper | N/A |
| A. nidulans: XX644: GFP-nudAK2599A(wA-AAA3); nudF6; argB2::[argB*-alcAp::mCherry-RabA]; possibly ΔnkuA::argB | This paper | N/A |
| A. nidulans: XX684: nudF-GFP-AfpyrG; nudAK2599A(wA-AAA3); gpdA-ΔC-hookA-S; possibly ΔnkuA::argB; possibly pyrG89; possibly argB2::[argB⁄-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: XX689: nudF-GFP-AfpyrG; nudAE2663Q(wB-AAA3); gpdA-ΔC-hookA-S; possibly ΔnkuA::argB; possibly pyrG89; possibly argB2::[argB⁄-alcAp::mCherry-RabA] | This paper | N/A |
| A. nidulans: XX725: nudA1; ΔyA::NLS-DsRed | This paper | N/A |
| Oligonucleotides | ||
| Primer: WAF: TTCTGGTGCAACCATGACACTGTTTGCCG | This paper | N/A |
| Primer: WAR: GTGTCATGGTTGC ACCAGAACCGGGAGGAC |
This paper | N/A |
| Primer: NudA58: AGAAAGATCGTGGACAACCTC |
L. Zhuang (Uniformed Services University) [53] | N/A |
| Primer: NdA310 CACGGTTAACTCGTTCGTATG |
L. Zhuang (Uniformed Services University) [53] | N/A |
| Primer: WBF: ATCTTCTGCGATCAAATCAACCTGCCGGCTC |
This paper | N/A |
| Primer: WBR: CAGGTTGATTTG ATCGCAGAAGATAACCAGCCAA |
This paper | N/A |
| Primer: ORF-F2: GTCCTTTCAAGCAAGCAGAGAATG |
J. Zhang (Uniformed Services University) [7] | N/A |
| Primer: UTR-R2: GATGCTGAGCTTGCTGCCTG |
J. Zhang (Uniformed Services University) [7] | N/A |
| Recombinant DNA | ||
| Software and algorithms | ||
| FIJI/ImageJ | NIH, USA | https://imagej.net/Fiji |
| Kymograph and velocity (FIJI plugin) | EMBL | https://www.embl.de/eamnet/html/kymograph.html |
| IPLab software | Scanalytics, Inc | https://www.bioprocessonline.com/doc/image-processing-software-0001 |
| Prism 8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| 8-well #1 chambered coverglass with cover | Thermo Fisher | Cat# 155411 |
Highlights:
Dynein activation in vivo is regulated by the ATPase cycle at its AAA3 domain.
AAA3 ATP hydrolysis allows coordination between cargo binding and dynein activation.
AAA3 ATP hydrolysis allows the participation of LIS1 in dynein activation.
ATP binding at AAA3 allows LIS1 to dissociate from dynein after its activation.
Acknowledgements
We thank Drs. Berl Oakley, Stephen Osmani and Miguel Peñalva for Aspergillus strains. We thank Dr. Peter Höök (a former postdoctoral fellow in Dr. Richard Vallee’s lab) and Dr. Samara Reck-Peterson for informing us many years ago that nudAR3086 represents an arginine-finger. We also thank Dr. Erika Holzbaur and her lab, especially former members Drs. Jennifer Ross and Mara Olenick, for advice/tips on in vitro motility experiments. This work was funded by the National Institutes of Health RO1 GM121850 (to X. Xiang) and RO1 GM134104 (to J. Rotty). J. Rotty was also supported by Uniformed Services University start-up fund. Disclaimer: The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Footnotes
Declaration of interests
The authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Reck-Peterson SL, Redwine WB, Vale RD, and Carter AP (2018). The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol 19, 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olenick MA, and Holzbaur ELF (2019). Dynein activators and adaptors at a glance. J Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhabha G, Johnson GT, Schroeder CM, and Vale RD (2016). How Dynein Moves Along Microtubules. Trends Biochem Sci 41, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao L, Berger F, Nicholas MP, and Gennerich A (2019). Molecular mechanism of cytoplasmic dynein tension sensing. Nat Commun 10, 3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury S, Ketcham SA, Schroer TA, and Lander GC (2015). Structural organization of the dynein-dynactin complex bound to microtubules. Nat Struct Mol Biol 22, 345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urnavicius L, Zhang K, Diamant AG, Motz C, Schlager MA, Yu M, Patel NA, Robinson CV, and Carter AP (2015). The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Yao X, Fischer L, Abenza JF, Penalva MA, and Xiang X (2011). The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. J Cell Biol 193, 1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh TY, Quintyne NJ, Scipioni BR, Eckley DM, and Schroer TA (2012). Dynactin's pointed-end complex is a cargo-targeting module. Mol Biol Cell 23, 3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielska E, Schuster M, Roger Y, Berepiki A, Soanes DM, Talbot NJ, and Steinberg G (2014). Hook is an adapter that coordinates kinesin-3 and dynein cargo attachment on early endosomes. J Cell Biol 204, 989–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Qiu R, Arst HN Jr., Penalva MA, and Xiang X (2014). HookA is a novel dynein-early endosome linker critical for cargo movement in vivo. J Cell Biol 204, 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, and Vale RD (2014). Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science 345, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlager MA, Hoang HT, Urnavicius L, Bullock SL, and Carter AP (2014). In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J 33, 1855–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K, Foster HE, Rondelet A, Lacey SE, Bahi-Buisson N, Bird AW, and Carter AP (2017). Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Cell 169, 1303–1314.e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, et al. (2012). BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures. Mol Biol Cell 23, 4226–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olenick MA, Tokito M, Boczkowska M, Dominguez R, and Holzbaur EL (2016). Hook Adaptors Induce Unidirectional Processive Motility by Enhancing the Dynein-Dynactin Interaction. J Biol Chem 291, 18239–18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus SM, Marzo MG, and McKenney RJ (2020). New insights into the mechanism of dynein motor regulation by lissencephaly-1. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang X, and Qiu R (2020). Cargo-Mediated Activation of Cytoplasmic Dynein in vivo. Front Cell Dev Biol 8, 598952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt H, and Carter AP (2016). Review: Structure and mechanism of the dynein motor ATPase. Biopolymers 105, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Rao L, and Gennerich A (2020). The regulatory function of the AAA4 ATPase domain of cytoplasmic dynein. Nat Commun 11, 5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt H, Zalyte R, Urnavicius L, and Carter AP (2015). Structure of human cytoplasmic dynein-2 primed for its power stroke. Nature 518, 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhabha G, Cheng HC, Zhang N, Moeller A, Liao M, Speir JA, Cheng Y, and Vale RD (2014). Allosteric communication in the dynein motor domain. Cell 159, 857–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvanovich A, Li MG, Serr M, Mische S, and Hays TS (2003). The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol Biol Cell 14, 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kon T, Nishiura M, Ohkura R, Toyoshima YY, and Sutoh K (2004). Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266–11274. [DOI] [PubMed] [Google Scholar]
- 24.Cho C, Reck-Peterson SL, and Vale RD (2008). Regulatory ATPase sites of cytoplasmic dynein affect processivity and force generation. J Biol Chem 283, 25839–25845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeWitt MA, Cypranowska CA, Cleary FB, Belyy V, and Yildiz A (2015). The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat Struct Mol Biol 22, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholas MP, Berger F, Rao L, Brenner S, Cho C, and Gennerich A (2015). Cytoplasmic dynein regulates its attachment to microtubules via nucleotide state-switched mechanosensing at multiple AAA domains. Proc Natl Acad Sci U S A 112, 6371–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivagurunathan S, Schnittker RR, Razafsky DS, Nandini S, Plamann MD, and King SJ (2012). Analyses of dynein heavy chain mutations reveal complex interactions between dynein motor domains and cellular dynein functions. Genetics 191, 1157–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Roberts AJ, Leschziner AE, and Reck-Peterson SL (2012). Lis1 Acts as a "Clutch" between the ATPase and Microtubule-Binding Domains of the Dynein Motor. Cell 150, 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toropova K, Zou S, Roberts AJ, Redwine WB, Goodman BS, Reck-Peterson SL, and Leschziner AE (2014). Lis1 regulates dynein by sterically blocking its mechanochemical cycle. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeSantis ME, Cianfrocco MA, Htet ZM, Tran PT, Reck-Peterson SL, and Leschziner AE (2017). Lis1 Has Two Opposing Modes of Regulating Cytoplasmic Dynein. Cell 170, 1197–1208.e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Htet ZM, Gillies JP, Baker RW, Leschziner AE, DeSantis ME, and Reck-Peterson SL (2020). LIS1 promotes the formation of activated cytoplasmic dynein-1 complexes. Nat Cell Biol 22, 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumbach J, Murthy A, McClintock MA, Dix CI, Zalyte R, Hoang HT, and Bullock SL (2017). Lissencephaly-1 is a context-dependent regulator of the human dynein complex. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutierrez PA, Ackermann BE, Vershinin M, and McKenney RJ (2017). Differential effects of the dynein-regulatory factor Lissencephaly-1 on processive dynein-dynactin motility. J Biol Chem 292, 12245–12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha R, Roostalu J, Cade NI, Trokter M, and Surrey T (2017). Combinatorial regulation of the balance between dynein microtubule end accumulation and initiation of directed motility. Embo j 36, 3387–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu R, Zhang J, and Xiang X (2019). LIS1 regulates cargo-adapter-mediated activation of dynein by overcoming its autoinhibition in vivo. J Cell Biol 218, 3630–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elshenawy MM, Kusakci E, Volz S, Baumbach J, Bullock SL, and Yildiz A (2020). Lis1 activates dynein motility by modulating its pairing with dynactin. Nat Cell Biol 22, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzo MG, Griswold JM, and Markus SM (2020). Pac1/LIS1 stabilizes an uninhibited conformation of dynein to coordinate its localization and activity. Nat Cell Biol 22, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg G, Peñalva MA, Riquelme M, Wösten HA, and Harris SD (2017). Cell Biology of Hyphal Growth. Microbiol Spectr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang X (2018). Nuclear movement in fungi. Semin Cell Dev Biol 82, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han G, Liu B, Zhang J, Zuo W, Morris NR, and Xiang X (2001). The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics. Curr Biol 11, 719–724. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Li S, Fischer R, and Xiang X (2003). Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent. Mol Biol Cell 14, 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenz JH, Schuchardt I, Straube A, and Steinberg G (2006). A dynein loading zone for retrograde endosome motility at microtubule plus-ends. Embo j 25, 2275–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Wang L, Zhuang L, Huo L, Musa S, Li S, and Xiang X (2008). Arp11 affects dynein-dynactin interaction and is essential for dynein function in Aspergillus nidulans. Traffic 9, 1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Zhuang L, Lee Y, Abenza JF, Penalva MA, and Xiang X (2010). The microtubule plus-end localization of Aspergillus dynein is important for dynein-early-endosome interaction but not for dynein ATPase activation. J Cell Sci 123, 3596–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egan MJ, Tan K, and Reck-Peterson SL (2012). Lis1 is an initiation factor for dynein-driven organelle transport. J Cell Biol 197, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao X, Zhang J, Zhou H, Wang E, and Xiang X (2012). In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function. Traffic 13, 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wedlich-Soldner R, Straube A, Friedrich MW, and Steinberg G (2002). A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J 21, 2946–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bieger BD, Osmani AH, Xiang X, and Egan MJ (2021). The spindle pole-body localization of activated cytoplasmic dynein is cell cycle-dependent in Aspergillus nidulans. Fungal Genet Biol 148, 103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oakley BR, Oakley CE, Yoon Y, and Jung MK (1990). Gamma-tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 61, 1289–1301. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Gao X, Manck R, Schmid M, Osmani AH, Osmani SA, Takeshita N, and Fischer R (2017). Microtubule-organizing centers of Aspergillus nidulans are anchored at septa by a disordered protein. Mol Microbiol 106, 285–303. [DOI] [PubMed] [Google Scholar]
- 51.Ananthanarayanan V, Schattat M, Vogel SK, Krull A, Pavin N, and Tolić-Nørrelykke IM (2013). Dynein motion switches from diffusive to directed upon cortical anchoring. Cell 153, 1526–1536. [DOI] [PubMed] [Google Scholar]
- 52.Lammers LG, and Markus SM (2015). The dynein cortical anchor Num1 activates dynein motility by relieving Pac1/LIS1-mediated inhibition. J Cell Biol 211, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang L, Zhang J, and Xiang X (2007). Point mutations in the stem region and the fourth AAA domain of cytoplasmic dynein heavy chain partially suppress the phenotype of NUDF/LIS1 loss in Aspergillus nidulans. Genetics 175, 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willins DA, Liu B, Xiang X, and Morris NR (1997). Mutations in the heavy chain of cytoplasmic dynein suppress the nudF nuclear migration mutation of Aspergillus nidulans. Mol Gen Genet 255, 194–200. [DOI] [PubMed] [Google Scholar]
- 55.Xiang X, Osmani AH, Osmani SA, Xin M, and Morris NR (1995). NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell 6, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang X, Roghi C, and Morris NR (1995). Characterization and localization of the cytoplasmic dynein heavy chain in Aspergillus nidulans. Proc Natl Acad Sci U S A 92, 9890–9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen KF, and Osmani SA (2013). Regulation of mitosis by the NIMA kinase involves TINA and its newly discovered partner, An-WDR8, at spindle pole bodies. Mol Biol Cell 24, 3842–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan K, Roberts AJ, Chonofsky M, Egan MJ, and Reck-Peterson SL (2014). A microscopy-based screen employing multiplex genome sequencing identifies cargo-specific requirements for dynein velocity. Mol Biol Cell 25, 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abenza JF, Pantazopoulou A, Rodriguez JM, Galindo A, and Penalva MA (2009). Long-distance movement of Aspergillus nidulans early endosomes on microtubule tracks. Traffic 10, 57–75. [DOI] [PubMed] [Google Scholar]
- 60.Abenza JF, Galindo A, Pantazopoulou A, Gil C, de los Ríos V, and Peñalva MA (2010). Aspergillus RabB Rab5 integrates acquisition of degradative identity with the long distance movement of early endosomes. Mol Biol Cell 21, 2756–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu B, Xiang X, and Lee YR (2003). The requirement of the LC8 dynein light chain for nuclear migration and septum positioning is temperature dependent in Aspergillus nidulans. Mol Microbiol 47, 291–301. [DOI] [PubMed] [Google Scholar]
- 62.Qiu R, Zhang J, and Xiang X (2013). Identification of a novel site in the tail of dynein heavy chain important for dynein function in vivo. J Biol Chem 288, 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grotjahn DA, Chowdhury S, Xu Y, McKenney RJ, Schroer TA, and Lander GC (2018). Cryo-electron tomography reveals that dynactin recruits a team of dyneins for processive motility. Nat Struct Mol Biol 25, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urnavicius L, Lau CK, Elshenawy MM, Morales-Rios E, Motz C, Yildiz A, and Carter AP (2018). Cryo-EM shows how dynactin recruits two dyneins for faster movement. Nature 554, 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waring RB, May GS, and Morris NR (1989). Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin-coding genes. Gene 79, 119–130. [DOI] [PubMed] [Google Scholar]
- 66.Moore JK, Li J, and Cooper JA (2008). Dynactin function in mitotic spindle positioning. Traffic 9, 510–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.King SJ, and Schroer TA (2000). Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol 2, 20–24. [DOI] [PubMed] [Google Scholar]
- 68.Ross JL, Wallace K, Shuman H, Goldman YE, and Holzbaur EL (2006). Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol 8, 562–570. [DOI] [PubMed] [Google Scholar]
- 69.Kardon JR, Reck-Peterson SL, and Vale RD (2009). Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. Proc Natl Acad Sci U S A 106, 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tripathy SK, Weil SJ, Chen C, Anand P, Vallee RB, and Gross SP (2014). Autoregulatory mechanism for dynactin control of processive and diffusive dynein transport. Nat Cell Biol 16, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belyy V, Schlager MA, Foster H, Reimer AE, Carter AP, and Yildiz A (2016). The mammalian dynein-dynactin complex is a strong opponent to kinesin in a tug-of-war competition. Nat Cell Biol 18, 1018–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee WL, Oberle JR, and Cooper JA (2003). The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J Cell Biol 160, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheeman B, Carvalho P, Sagot I, Geiser J, Kho D, Hoyt MA, and Pellman D (2003). Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr Biol 13, 364–372. [DOI] [PubMed] [Google Scholar]
- 74.Markus SM, Punch JJ, and Lee WL (2009). Motor- and tail-dependent targeting of dynein to microtubule plus ends and the cell cortex. Curr Biol 19, 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Markus SM, and Lee WL (2011). Regulated offloading of cytoplasmic dynein from microtubule plus ends to the cortex. Dev Cell 20, 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKenney RJ, Vershinin M, Kunwar A, Vallee RB, and Gross SP (2010). LIS1 and NudE induce a persistent dynein force-producing state. Cell 141, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt H, Gleave ES, and Carter AP (2012). Insights into dynein motor domain function from a 3.3-A crystal structure. Nat Struct Mol Biol 19, 492–497, s491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, and Oakley BR (2006). A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172, 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu R, Zhang J, and Xiang X (2018). p25 of the dynactin complex plays a dual role in cargo binding and dynactin regulation. J Biol Chem 293, 15606–15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicholas MP, Rao L, and Gennerich A (2014). Covalent immobilization of microtubules on glass surfaces for molecular motor force measurements and other single-molecule assays. Methods Mol Biol 1136, 137–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.