Abstract
Objectives:
To utilize a Luminex platform to examine multiple cytokines simultaneously as well as clinical laboratory testing in order to identify markers that predict acute pancreatitis (AP) severity in the pediatric population on admission.
Study design:
Patients (<19 years) prospectively enrolled over a 4-year period in a single institution AP database were included in separate derivation and validation cohorts. Plasma samples were obtained within 48 hours of admission and stored for analysis. Samples from mild AP and SAP (moderately severe and severe combined) were analyzed using Luminex panels and C-Reactive Protein (CRP) testing.
Results:
The derivation cohort examined 62 cytokines in 66 subject samples (20 control, 36 mild AP, 10 SAP) and identified interleukin 6 (IL-6) [P = .02] and monocyte chemotactic protein-1 (MCP-1) [p=0.02] as cytokines that were differentially expressed between mild and SAP. Our validation cohort analyzed 76 cytokines between 10 controls, 19 mild AP and 6 SAP subjects. IL-6 (p=0.02) and MCP-1 (p=0.007) were again found to differentiate mild AP from SAP. CRP values were obtained from 53 of the subjects, revealing a strong association between elevated CRP values and progression to severe disease (P<0.0001).
Conclusion:
This study identified and validated IL-6 and MCP-1 as predictors of SAP using 2 distinct cohorts, and showed that CRP elevation is a marker of progression to SAP. These biomarkers have not been extensively studied in the pediatric AP population. Our data allows for risk-stratification of AP patients, and represent novel insight into the immunologic response in SAP.
Keywords: Severe acute pancreatitis, pediatrics, IL-6, MCP-1, CRP
Acute pancreatitis (AP) represents a significant disease burden in the pediatric population, with an estimated incidence that has been increasing over the past two decades now stabilized to greater than 1 in 10,000.(1, 2) Although there have been emerging research efforts dedicated to pediatric AP, there remain limited data regarding optimal or risk-stratified management, and many of the existing guidelines are extrapolated from adult literature and do not apply to children given the different etiologies and disease presentation.(3) The North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) published the first pediatric AP guidelines in 2018.(4) This guideline noted potential predictive models of severity of pediatric AP had been previously published, with varying specificity and sensitivity upon validation, and recommended further investigation to identify predictive markers of severity on admission.(4) Unfortunately, previous efforts to establish predictive markers were limited by the fact that a common definition of severity in pediatric AP was lacking, so standardization across sites and published models were challenging. In 2017, the NASPGHAN Pancreas Committee published severity classification guidelines for pediatric AP, providing a consensus definition of mild, moderately severe, and severe AP in pediatrics for the first time. (5) Mild AP is defined as a largely self-limited phenomenon, whereas moderately severe AP involves local pancreatic complications or transient (<48 hr) organ dysfunction, and severe AP involves persistent organ dysfunction lasting longer than 48 hours.
The identification of patients at highest risk of progression to severe disease can be crucial for a variety of reasons. Because mild AP is most often a self-limited condition, these patients can be cared for by the hospital pediatrics team at most medical centers, or allowed to stay at community locations without the need to transfer to tertiary care centers. If a simple scoring system that accurately identifies patients at lowest (or highest) risk of progression to severe disease exists, resources can be allocated appropriately. Predictive models can help risk stratify patients for directed clinical management or for the design of clinical trials investigating therapies to prevent progression to severe disease. The majority of patients presenting with AP do not have the severity findings on admission, so identifying who will progress to severe becomes very important. The identification of patients at highest risk of progression to severe disease is not as useful without identifying additional therapies that could be applied to severe disease. In order to develop novel therapies, we will need to elucidate more of the underlying immunologic and pathophysiologic responses in these patients.
Adding novel biomarkers can help optimize our previously generated clinical model to predict severity. From clinically obtained data, our previous efforts have identified and validated Blood Urea Nitrogen (BUN) as an early predictive marker for the development of severe disease, but more effort will be required to develop this into an easily distributed model and/or clinical tool that could be deployed in a community-based emergency department or pediatric ward. (6, 7) Under this premise, the goal of our study was to examine the role of new cytokines not previously investigated in AP and the role of C-reactive protein to evaluate combinations of cytokine and chemokine signals early in the hospital course in patients who will ultimately develop SAP.
Methods:
Patient Enrollment
Patients were identified prospectively as having their first episode of pediatric AP at CCHMC, and enrolled in an institutional prospective registry (CCHMC IRB 2012-4050), which prospectively monitors patients’ progression. Patients under 19 years of age with AP were eligible. The diagnosis of AP was based on the INSPPIRE criteria as noted in the NASPGHAN guidelines; all patients had at least two of the following three findings: 1. characteristic abdominal pain of pancreatic origin, 2. amylase and/or lipase at least three times upper limit of normal, and 3. imaging (CT, Ultrasound, MRCP) findings consistent with pancreatic inflammation. (5, 8) The initial blood samples were obtained within 48 hours of admission, with most obtained within 24 hours (>75%). Plasma was extracted, aliquoted and stored at −20°C for future use. The derivation cohort consisted of patients who presented with AP between January 2016 and June 2018. The validation cohort consisted of patients who presented with AP between August 2018 and September of 2019. Controls were recruited from patients having same day surgery without a history of pancreatic disease or other gastroenterological inflammatory conditions who were presenting for other procedures. The majority of these patients were having sedated dental or otolaryngological procedures.
Luminex Assay
Luminex assays were performed by the Human Immune Monitoring Center (HIMC) at Stanford University (http://iti.stanford.edu.stanford.idm.oclc.org/himc/protocols.html). For the exploratory study, Human 62-plex Procarta kits were purchased from eBiosciences/Affymetrix/Thermo Fisher (Santa Clara, California, USA) and used according to the manufacturer’s recommendations with modifications as described. In brief, beads were added to a 96 well plate and washed in a Biotek ELx405 washer. Samples were then added to the plate containing the mixed antibody-linked beads and incubated at room temperature (RT) for 1 hour followed by overnight incubation at 4°C with shaking. All incubation steps were performed on an orbital shaker at 500-600 rpm. Plates were then washed in a Biotek ELx405 washer and biotinylated detection antibody was added for 75 minutes at RT with shaking. The plate was washed as above and streptavidin-PE added. After incubation for 30 minutes at RT wash was performed as above and reading buffer was added to the wells. Each sample was measured in duplicate. Plates were read using a Luminex 200 or a FM3D FlexMap instrument with a lower bound of 50 beads per sample per cytokine. Custom Assay Chex control beads were purchased from Radix Biosolutions (Georgetown, Texas) and added to all wells.
Validation cohort kits were purchased from EMD Millipore Corporation (Burlington, MA) and used according to the manufacturer’s recommendations with modifications described as follows: H76 kits include 3 panels which are combined to make a 76 plex. Panel 1 is Milliplex HCYTMAG60PMX41BK with IL-18 and IL-22 added to generate a 43 plex. Panel 2 is Milliplex HCP2MAG62KPX23BK with MIG/CXCL9 added to generate a 24 plex. Panel 3 includes the Milliplex HSP1MAG-63K with Resistin, Leptin and HGF add to generate a 9 plex. The setup of assay was performed as recommended. In brief, samples were mixed with antibody-linked magnetic beads on a 96-well plate and incubated overnight at 4°C with shaking. Incubation steps were performed on an orbital shaker at 500-600 rpm. Plates were washed twice with wash buffer in a Biotek ELx405 washer. Following a one-hour incubation at RT with biotinylated detection antibody, streptavidin-PE was added for 30 minutes with shaking. Plates were washed as above and PBS added to wells for reading in the Luminex FlexMap3D Instrument with a lower bound of 50 beads per sample per cytokine. Each sample was measured in duplicate. Custom Assay Chex control beads were purchased from Radix Biosolutions (Georgetown, Texas) and added to all wells. For both the 62- and 76-plex assays, median fluorescence intensity (MFI), an estimate of analyte concentration, was used to compare expression in each sample. The cytokines/ chemokines / adhesion molecules measured by each Luminex assay can be found in Table 1 (available at www.jpeds.com).
Table 1:
Cytokines investigated.
| H62 | H76 |
|---|---|
| BDNF | CD40Ligand |
| NGF | EGF |
| EGF | EOTAXIN |
| ENA78 | FGFb |
| EOTAXIN | FIt-3L |
| FGFB | Fractalkine |
| GCSF | G-CSF |
| GMCSF | GM-CSF |
| GROA | GRO |
| HGF | IFN-a2 |
| IFNA | IFN-G |
| IFNB | IL-10 |
| IFNG | IL-12-P70 |
| IL10 | IL-12P40 |
| IL12P40 | IL-13 |
| IL12P70 | IL-15 |
| IL13 | IL-17 |
| IL15 | IL-18 |
| IL17A | IL-1a |
| IL17F | IL-1B |
| IL18 | IL-1RA |
| IL1A | IL-2 |
| IL1B | IL-22 |
| IL1RA | IL-3 |
| IL2 | IL-4 |
| IL21 | IL-5 |
| IL22 | IL-6 |
| IL23 | IL-7 |
| IL27 | IL-8 |
| IL31 | IL-9 |
| IL4 | IP-10 |
| IL5 | MCP-1 |
| IL6 | MCP-3 |
| IL7 | MDC |
| IL8 | MIP-1B |
| IL9 | MIP1a |
| IP10 | PDGF-AA |
| LEPTIN | PDGF-BB/AB |
| LIF | RANTES |
| MCSF | TGF-a |
| MCP1 | TNF-A |
| MCP3 | TNF-B |
| MIG | VEGF |
| MIP1A | Eotaxin-2 |
| MIP1B | MCP-2 |
| PAI1 | BCA-1 |
| PDGFBB | MCP-4 |
| RANTES | I-309 |
| RESISTIN | IL-16 |
| CD40L | TARC |
| SDF1A | Eotaxin-3 |
| FASL | LIF |
| ICAM1 | TPO |
| VCAM1 | SCF |
| TGFA | TSLP |
| TGFB | IL-33 |
| TNFA | CXCL9/MIG |
| TNFB | IL-20 |
| TRAIL | IL-21 |
| VEGF | IL-23 |
| VEGFD | Trail |
| CTACK | |
| SDF-1a+b | |
| ENA-78 | |
| MIP-1d | |
| IL-28A | |
| HGF | |
| Leptin | |
| MIF | |
| PAI-1 | |
| Resistin | |
| sFAS | |
| sFASL | |
| sICAM1 | |
| sVCAM-1 |
C Reactive Protein (CRP) Assay:
CRP assay was performed on the Siemens Atellica Analyzer. This utilized the CH C-Reactive Protein_2 (CRP_2) latex reagent, which is a suspension of uniform polystyrene latex particles with anti-CRP antibody. When the sample containing the CRP is agglutinated with the reagent, there is an increase in the turbidity, which is then measured at 571 nm. Ultimately, the CRP concentration is then determined from a generated calibration curve.
Statistical Analyses
The primary analysis of this study was to identify candidate biomarkers that were differentially expressed between healthy controls, mild acute pancreatitis (AP) and the non-mild cases, which included all forms of severe AP (including both moderately severe and severe disease). Initial assessment of differentially expressed biomarkers between healthy control and all combined AP patients was performed by Morpheus Heatmap analysis of the Broad Institute. We first looked for significant differences between the samples based on t-tests with FDR correction, between the controls and the AP group, and then between the mild and combined severity (moderately severe and severe) samples. In the secondary analysis, we separated all aspects of severity to look for a trend. Ultimately, due to the small sample size of the validation cohort, the t-test was replaced with Wilcoxon analysis. For continuous data, group comparisons were done using Wilcoxon-Mann-Whitney or Kruskal-Wallis tests as appropriate. For categorical data, Chi-square or Fisher exact tests were used for analysis between groups. MFI was used for all calculations, and since the MFI across plates could not be reliably combined, the derivation and validation cohorts were kept separate across all cytokine analysis. Receiver Operating Characteristic (ROC) curves were derived for each studied variable, and the Akaike’s Information Criterion (AIC) was calculated for the models and evaluated to compare performance, with a lower score suggesting a better model. For the ROC analysis, the derivation and validation cohorts were combined when examining CRP and BUN but kept separate for the cytokine comparisons as stated above. The lower limit of detection for the CRP assay is 0.4 mg/dL, and many of our patients, including all but one of the control samples, had undetectable values of <0.4 mg/dL. In order to characterize these values for graphic purposes, we used a correction factor for undetectable samples as previously described in the literature.(9) By definition, CRP values less than 0.8 mg/dL in this assay is suggestive of the absence of inflammation. For the purposes of the ROC curves, we separated the CRP into 3 groups: <0.4 mg/dL, 0.4 −2.5 mg/dL, and >2.5 mg/dL. In the secondary analysis, data were analyzed using SAS®, version 9.4 (SAS Institute, Cary, NC). A p-value <0.05 was considered statistically significant.
Results:
A total of 50 samples were identified for analysis from our institution. It was found that 3 of the samples were from repeat attacks among patients during the study time period, and one sample was not included due to poor performance on the cytoplex assay based on comparison with internal controls. We limited patients to their known first attack (index AP) within our hospital system. Ultimately 46 unique patient samples were included in the derivation cohort for the analysis, alongside 20 distinct controls. The validation cohort consisted of 25 unique patient samples and 10 distinct controls. For internal validation, we included 10 controls from the derivation cohort during the validation run. Severity was determined using the NASPGHAN criteria at time of discharge.(5) There was no significant difference between the derivation and validation cohorts on presentation based on age, sex, BMI, etiology, index case, or presence of any severity on admission (Table 2). There were no significant differences between the derivation and validation cohorts based on clinically obtained biochemical characteristics when examining initial lipase, amylase, albumin, white blood count, creatinine, hematocrit, hemoglobin, or BUN values (Table 3; available at www.jpeds.com).
Table 2:
Baseline Characteristics of Subjects
| Derivation AP Cohort (n=46) | Derivation Controls (n=20) | Validation AP Cohort (n=25) | Validation Controls (n=10) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 13.7 (9.1-16.2) | 12.8 (5.9-16.4) | 14.2 (11.1-17.3) | 10.4 (6.3-15.9) | 0.45 |
| Sex (male) | 24 (52%) | 11 (55%) | 13 (52%) | 6 (60%) | 0.98 |
| BMI percentile | 73.2 (38.5-97.0) n=39 | 48.6 (40.3-77.3) n=18 | 66.9 (31.8-96.6) | 64.3 (41.4-66.5) n=8 | 0.60 |
| Etiology | NA | NA | |||
| 1. Divisum/obstructive | 1 (2%) | 2 (8%) | 0.28 | ||
| 2. Biliary/gallstone | 6 (13%) | 7 (28%) | 0.20 | ||
| 3. Trauma | 2 (4%) | 0 (0%) | 0.54 | ||
| 4. Genetic† | 8 (17%) | 1 (4%) | NA | ||
| 5. Infectious/systemic disease/metabolic | 12 (26%) | 2 (8%) | 0.12 | ||
| 6. Idiopathic/other | 10 (22%) | 8 (32%) | 0.34 | ||
| 7. Drug | 6 (13%) | 5 (20%) | 0.50 | ||
| 8. Post ERCP | 1 (2%) | 0 (0%) | 1.00 | ||
| Index case | 37 (80%) | 21 (84%) | 1.00 | ||
| SAP * | 10 (22%) | 6 (24%) | 0.83 |
Median (25th-75th percentile) or n (%). NA = Not applicable
SAP = Combined moderately severe and severe AP;
Not all patients received genetic testing
Table 3:
Clinically Obtained Biochemical Characteristics
| Derivation (n=46) | Validation (n=25) | P-value | |
|---|---|---|---|
| Lipase x ULN | 7.4 (4.4-21.8) | 8.7 (5.8-32.5) | 0.18 |
| Amylase x ULN | 1.7 (0.9-6.5) n=32 | 2.2 (1.5-5.5) n=16 | 0.37 |
| Albumin, g/dL | 3.6 (3.2-4.1) n=45 | 3.9 (3.4-4.2) | 0.20 |
| WBC, 103/microL | 9.3 (5.9-13.5) n=41 | 8.1 (6.7-11.4) | 0.93 |
| Creatinine, mg/dL | 0.5 (0.4-0.7) n=42 | 0.6 (0.4-0.6) | 0.81 |
| Hematocrit, % | 38.7 (31.9-42.9) n=40 | 38.9 (37.1-41.6) | 0.63 |
| Hemoglobin, g/dL | 13.2 (11.1-15.0) n=41 | 13.0 (12.4-13.7) | 0.69 |
| BUN, mg/dL | 10.0 (7.0-15.0) n=42 | 12.0 (8.0-17.0) | 0.25 |
Data presented as median (25th-75th percentile).
Investigating roles of novel biomarkers:
The first step of analysis was to compare all patients with AP to control subjects, and to look for any significant differences between the two groups based on the heat map as determined by t-tests. Subsequently the difference between mild disease and non-mild (combined moderately severe and severe, labeled collectively as SAP) disease was examined (Figure 1, A and B). The initial heatmap analysis identified 48 statistically significant markers between control and AP (Figure 1, A). Levels of Interleukin-6 (IL-6) and Monocyte chemoattractant protein-1 (MCP-1/CCL2) were statistically different between the mild AP and SAP groups in the derivation cohort based on t-tests (P = 0.02 for both) (Figure 1, B). This comparison of mild and SAP groups was then repeated in the validation cohort, which again showed significantly different values for each marker (IL-6; P = 0.02) utilizing the non-parametric Wilcoxon analysis and (MCP-1; P = 0.007) (Figure 4; available at www.jpeds.com). With the targets identified, the SAP group was separated into its components (moderately severe and severe groups), and there remained a significant, progressive difference amongst the groups (P≤0.01 across all groups) (Figure 1, C–F).
Figure 1.
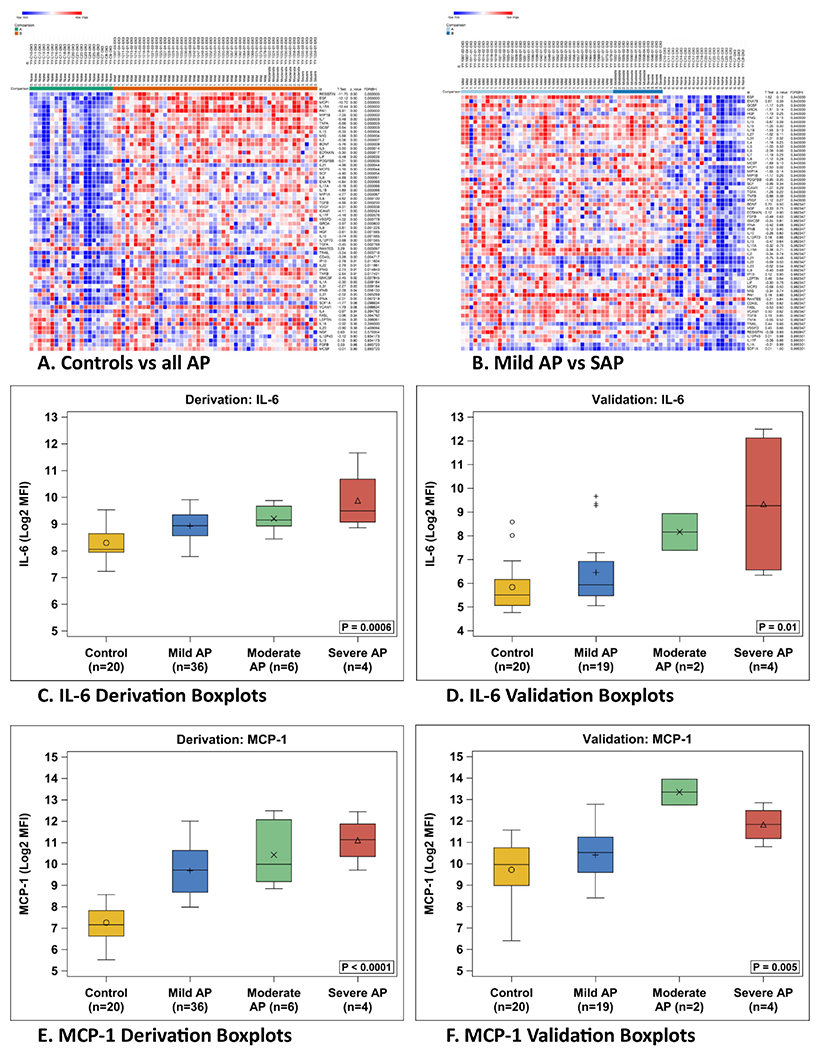
Heat map of original derivation sample, comparisons calculated via t-test. Boxplots of derivation and validation cohorts of identified target cytokines.
Figure 4.
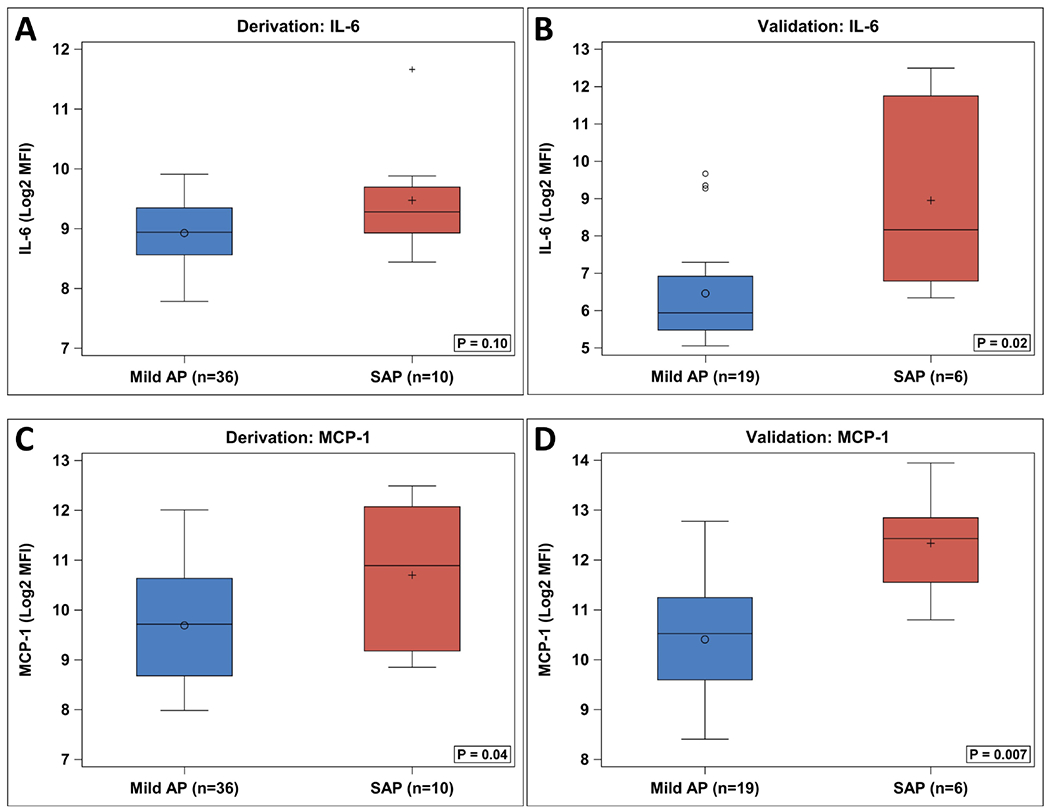
Boxplot comparison of target cytokines from derivation and validation cohorts. Due to small sample size, Wilcoxon analysis was used for comparison.
Additional 53 remaining aliquots from the same patients were run in our clinical laboratory to detect CRP levels separately as it was not included in the cytoplex assay. Table 4 (available at www.jpeds.com) demonstrates the results of the CRP study, as well as the results of the IL-6 and MCP-1 assays; increased IL-6 and MCP-1 has been associated with increased neutrophils and monocytes and decreased lymphocyte count. CRP, IL-6 and MCP-1 were significantly different between the three groups (P≤0.01 for all comparisons) (Table 4). Within the validation cohort, the absolute neutrophil count (P=0.03), the absolute lymphocyte count (P=0.03), and the absolute monocyte count (P=0.04) were also significantly different.
Table 4:
Biomarkers Comparison
| Derivation Cohort | Validation Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Severe AP (n=4) | Moderately Severe (n=6) | Mild AP (n=36) | Controls (n=20) | P-value* | Severe AP (n=4) | Moderately Severe (n=2) | Mild AP (n=19) | Controls (n=20) | P-value* | |
|
CRP <0.4 0.4-2.5 >2.5 |
1/2 (50%) 1/2 (50%) 0/2 (0%) |
1/4 (25%) 2/4 (50%) 1/4 (25%) |
12/22 (55%) 6/22 (27%) 4/22 (18%) |
18/19 (95%) 1/19 (5%) 0/19 (0%) |
0.01 |
0 (0%) 1 (25%) 3 (75%) |
0 (0%) 0 (0%) 2 (100%) |
9 (47%) 7 (37%) 3 (16%) |
20 (100%) 0 (0%) 0 (0%) |
<0.0001 |
| IL6 (Log2) | 9.5 (9.1-10.7) | 9.1 (8.9-9.7) | 8.9 (8.6-9.3) | 8.1 (7.9-8.6) | 0.0006 | 9.3 (6.6-12.1) | 8.2 (7.4-8.9) | 5.9 (5.5-6.9) | 5.5 (5.1-6.2) | 0.01 |
| MCP-1 (Log2) | 11.1 (10.3-11.9) | 10.0 (9.2-12.1) | 9.7 (8.7-10.6) | 7.2 (6.6-7.8) | <0.0001 | 11.8 (11.2-12.5) | 13.3 (12.7-13.9) | 10.5 (9.6-11.2) | 10.0 (9.0-10.8) | 0.005 |
| NLR | 1.5 (1.1-16.1) | 4.3 (2.4-6.8) | 3.2 (2.0-5.6) n=29 | NA | 0.46 | 6.2 (3.0-16.0) | 6.4 (5.3-7.6) | 2.5 (1.9-4.7) | NA | 0.16 |
| MLR | 0.2 (0.1-0.6) | 0.3 (0.1-1.1) | 0.4 (0.2-0.8) n=29 | NA | 0.52 | 0.8 (0.5-2.3) | 0.2 (0.0-0.4) | 0.4 (0.2-0.6) | NA | 0.13 |
| Absolute neutrophil | 5.7 (2.6-11.9) | 5.8 (3.4-8.8) | 5.6 (3.7-9.5) n=29 | NA | 1.00 | 10.5 (5.0-16.9) | 0.5 (0.2-0.8) | 5.6 (4.1-7.4) | NA | 0.03 |
| Absolute lymphocyte | 2.0 (1.1-3.9) | 1.2 (0.9-2.3) | 1.8 (1.2-2.6) n=29 | NA | 0.56 | 1.6 (1.1-1.8) | 0.1 (0.0-0.1) | 2.1 (1.2-2.7) | NA | 0.03 |
| Absolute monocyte | 0.5 (0.4-0.6) | 0.4 (0.1-1.4) | 0.7 (0.5-1.1) n=29 | NA | 0.31 | 1.0 (0.6-3.5) | 0.0 (0.0-0.0) | 0.7 (0.6-0.9) | NA | 0.04 |
| RDW | 14.4 (12.0-17.3) | 12.9 (12.3-13.7) | 12.2 (11.7-13.0) n=31 | NA | 0.52 | 13.8 (13.4-14.0) | 14.4 (14.2-14.6) | 13.0 (11.9-13.9) | NA | 0.17 |
Data presented as n (%) or median (25th-75th percentile)
P-values for all 4 groups if available or the AP groups if NA for controls
Figure 2, A shows the boxplot distribution of the CRP values, and shows a clear trend toward increased CRP levels in severe disease. We stratified CRP into 3 distinct groups, <0.4 mg/dL, 0.4 −2.5 mg/dL, and >2.5 mg/dL and used this to generate a ROC curve which nearly reached statistical significance (AUROC 0.72 [95%CI, 0.57-0.88], P=0.057) (Table 4 and Figure 2, B).
Figure 2.
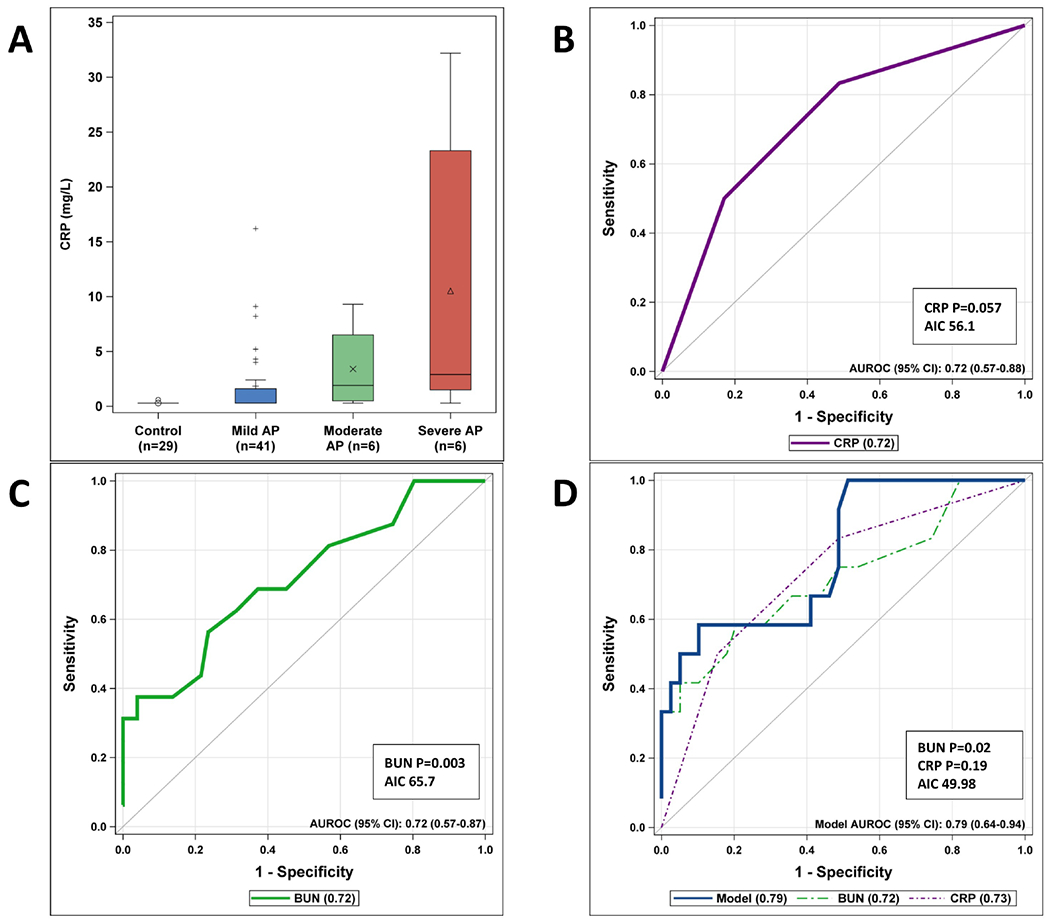
CRP and BUN as a predictor of severity.
Combining and comparing markers in models:
We examined BUN levels on admission for the combined cohort (derivation and validation) and found they were significantly lower in patients who developed mild disease compared with those who progressed to severe disease, and performed well on the ROC curve (AUROC 0.72 [95%CI, 0.57-0.87], P=0.003) (Figure 2, C). When BUN and CRP were combined into a ROC curve for the 51 patients who had both BUN and CRP values, the model improved (AUROC 0.79 [95%CI, 0.64-0.94]), and had the lowest AIC (49.98), but although BUN remained significant in the combined model (P=0.02), CRP was not (P=0.19) (Figure 2, D).
We completed ROC curves for the derivation and validation cohorts based on IL-6 and MCP-1, and also combined them with BUN to see how they performed (Figure 3 and Figure 5 [available at www.jpeds.com]). Due to the nature of the cytoplex assay results, we did not combine the derivation and validation cohorts for the final analysis. In the validation cohort, IL-6 by itself resulted in a significant ROC curve (AUROC 0.83 [95%CI, 0.67-1.00], P=0.03), and when combined with BUN, and the AIC improved (22.1), the model was very similar (AUROC 0.82 [95%CI, 0.56-1.00]) but neither IL-6 (P=0.18) nor BUN remained significant (P=0.09) (Figure 3, A and B). MCP-1 by itself was also a significant predictor of progression to severe disease (AUROC 0.88 [95%CI, 0.73-1.00], P=0.02) (Figure 3, C). However, when combined with BUN, even though the AIC improved (20.3), the AUC results were similar (AUROC 0.88 [95%CI, 0.71-1.00]) but neither MCP-1 (P=0.10) nor BUN remained significant (P=0.18) (Figure 3, D).
Figure 3.
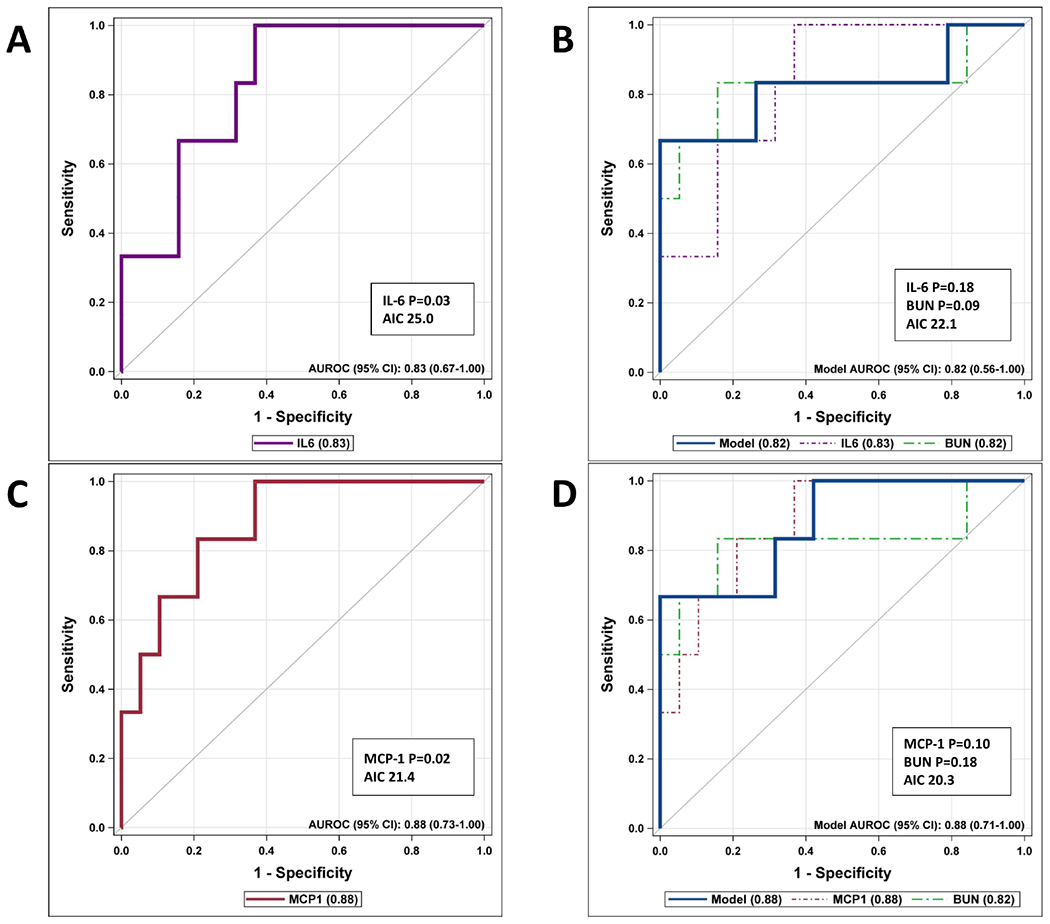
ROC curves for Validation cohort.
Figure 5:
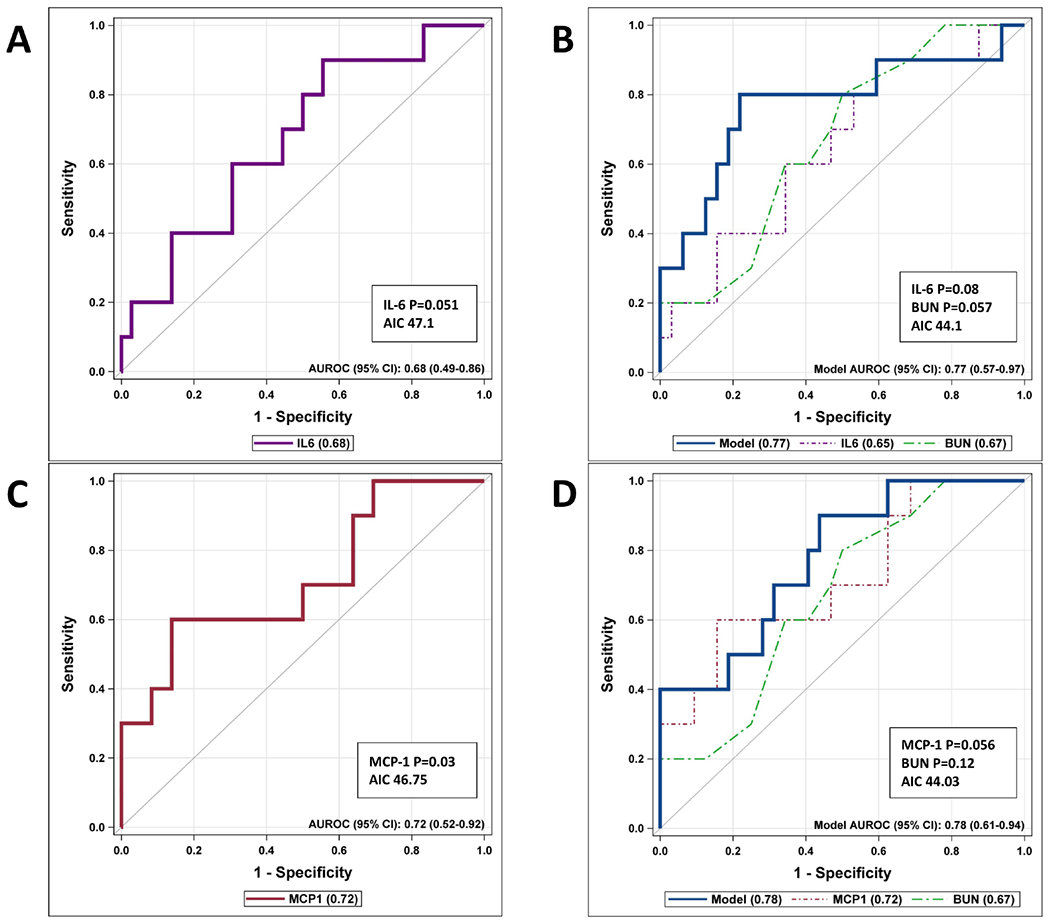
ROC Curves for Derivation Cohort
Discussion
Our study showed that elevations in IL-6, MCP-1 and CRP on admission are independently associated with progression to severe disease course. IL-6 and MCP-1 have been studied using two different multiplex different assays, as well as unique derivation and validation cohorts of patients and controls. Our study investigated pediatric AP utilizing the standard definitions of severity as defined by NASPGHAN.
Our previous work identified that the commonly obtained BUN as part of a standard initial clinical biochemical evaluation on admission, as well as the response of the patient’s BUN to fluid resuscitation can help predict disease severity with high specificity and a high Negative Predictive Value which can help to determine those patients least likely to progress to severe disease.(6, 7) We demonstrated that elevated BUN increased in patients who progressed to developing SAP, and we attempted to determine the utility of CRP, IL-6, and MCP-1 as useful predictive markers alone and in addition to this predictive model, and compared the performances within those models.
The field of pediatric pancreatology is in need of a tool for identifying patients at highest risk of progressing to severe disease at time of presentation to aid in delineating a treatment strategy, offer risk stratified interventions for different populations, or even targeted pathways to prevent severe disease. Facilitating transfer of appropriate high risk patients from community sites to tertiary centers (and avoiding unnecessary transfers) is a first step, but we must develop targets for meaningful therapy. Our study identified IL-6 and MCP-1 as potential targets for such therapy.
This is a North American study in pediatric AP to identify IL-6 elevation in AP; it has been studied in Asia in a study of SIRS in AP, where both CRP and IL-6 were found to be elevated in the children who developed SIRS and progressed to severe disease.(10) There are numerous adult studies that have reported the power of IL-6 in predicting the development of severe disease, including a meta-analysis that suggested IL-6 may be the most important early marker to follow.(11) In addition, there is adult literature showing the benefit of placing patients at highest risk of progression to severe disease on Cox-2 inhibitors and monitoring, among other measures, the response of IL-6. Patients who received the investigational drug had significantly lower levels of IL-6, and the therapy was associated with an almost 50% reduction in the progression of patients to severe disease.(12) This suggests that although IL-6 is a general marker of inflammation in many different disease states, and while not pancreas-specific, suppressing IL-6 levels by targeting either IL-6 itself or the upstream inflammasome in AP may lead to improved outcomes.(13) The mechanism of IL-6 activation is multifold and leads to downstream upregulation of the neutrophil response. These results could explain why different authors investigating severe AP have found that an elevated WBC is useful and others have not. In patients with an elevated WBC, if the response was driven primarily by an increase in neutrophils, this could explain the rise and may be due to the IL-6 response. Alternatively, if the more pronounced feature was lymphopenia, the overall WBC may not have been elevated in severe cases, but there still may have been an exaggerated neutrophil response.
This study investigated the role of MCP-1 in pediatric AP. There are a few studies that have examined MCP-1 in adult AP and in animal models, and have postulated that this chemokine is associated with the recruitment of leukocytes that can cause the acute local damage in severe AP.(13, 14)
Previous studies in CRP have shown mixed results in the pediatric literature, with some authors suggesting that it may be useful, whereas others have not appreciated the same relationship.(7, 10, 15, 16) It should be noted that most of these efforts did not utilize the most recent AP severity guidelines, so it is difficult to compare those results to our current study. The adult literature on CRP in AP has also shown that there is a role for CRP in predicting progression to severe AP.(17–19) In our study, there was a trend toward increased severity as the CRP increased. The CRP and IL-6 levels appear to be correlated as well (data not shown), which suggests that CRP values may be a valuable clinical marker in current use, although IL-6 may be more relevant for elucidating pathways for drug therapies in the future. Both markers have roles in the inflammatory cascade that help us understand the pathophysiology of AP.
In an investigation into referral trends and etiologies of pediatric AP, previous authors noted an increase in referrals of all cases of pediatric AP to tertiary care centers from community sites within their catchment regions.(20) This can contribute to the volume of patients seen in the ED, and lead to increased direct costs to the family as well as the potential indirect costs of moving the families far away from home (lost wages for parents, missed school for other children, etc), as well as utilization of resources at the tertiary care center. The cost and resources expended in the care of these children is absolutely appropriate if the transfer is needed, but if a system can be created and communicated widely to identify patients who do not require transfer, these real and indirect costs can be spared, and healthcare resources can be applied more appropriately. For example, one study found that the direct cost of just the transfer from a community ED to a pediatric ED was over $4000.(21)
This study is limited by the fact that it occurred within a single academic center, and although the study was relatively large for a pediatric study, it nevertheless had a small number of patients with acute pancreatitis compared with adult studies, with a wide variety of etiologies that may affect the biochemical profiles. For example, there was likely a proportion of these patients who will ultimately carry a diagnosis of a genetic etiology, but this is generally not tested at the time of the first attack of AP. Although etiologies here were diverse, they reflect the reality of clinical pediatric AP, so inference can be developed from this study. The study occurred over a relatively long time-frame, the collection of clinical registry data and biospecimens was over a span of four years without interruption, perhaps reflecting the reality that the conduct of translational studies is slow and challenging in the pediatric AP population. The results, while alluding to possible roles of the cytokines investigated, do not lead to a direct understanding of their cellular effects, as we measure the systemic levels of the cytokines circulating in the plasma but experimental studies suggest important roles for the identified cytokine and chemokine in pancreatitis lethality/severity. (22, 23)
In conclusion, elevation of IL-6, MCP-1, and CRP are associated with progression to severe AP. Adding CRP to commonly obtained laboratory markers such as BUN may allow clinicians to identify patients at highest risk of progression to severe disease. Monitoring IL-6 and MCP-1 levels may offer novel insights into the inflammatory responses and immunological pathways involved in pediatric AP, provide targets to measure for response to therapy, and are potential targets for future medical therapies. Multi-center studies should further investigate the role of these cytokines in the prediction and treatment of pediatric AP in the future.
Acknowledgments
We acknowledge the Human Diabetes Immune Monitoring Core of the Stanford Diabetes Research Center (P30DK116074) for help in running the Luminex assay.
Supported in part by NIH training grant T32 DK007727 awarded to Cincinnati Children’s Hospital Medical Center, from which P.F. received support during fellowship. M.A-E-H. is supported by NIDDK (1K23DK118190). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interest.
Abbreviations:
- IL-6
interleukin 6
- MCP-1
monocyte chemotactic protein-1
- CRP
C-Reactive Protein
- BUN
Blood Urea Nitrogen
- NASPGHAN
The North American Society for Pediatric Gastroenterology, Hepatology and Nutrition
- ROC
Receiver Operating Characteristic
- AIC
Akaike’s Information Criterion
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at Digestive Disease Week, ≪ ≫, 2020 (virtual); and at the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN), ≪ ≫, November, 2020 (virtual).
REFERENCES:
- 1.Sellers ZM, MacIsaac D, Yu H, Dehghan M, Zhang KY, Bensen R, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-Elderly Adults in the United States, 2007-2014. Gastroenterology. 2018;155:469–78.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinath AI, Lowe ME. Pediatric pancreatitis. Pediatrics in review. 2013;34:79–90. [DOI] [PubMed] [Google Scholar]
- 3.Abu-El-Haija M, Lowe ME. Pediatric Pancreatitis-Molecular Mechanisms and Management. Gastroenterol Clin North Am. 2018;47:741–53. [DOI] [PubMed] [Google Scholar]
- 4.Abu-El-Haija M, Kumar S, Quiros JA, Balakrishnan K, Barth B, Bitton S, et al. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. Journal of pediatric gastroenterology and nutrition. 2018;66:159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-El-Haija M, Kumar S, Szabo F, Werlin S, Conwell D, Banks P, et al. Classification of Acute Pancreatitis in the Pediatric Population: Clinical Report From the NASPGHAN Pancreas Committee. Journal of pediatric gastroenterology and nutrition. 2017;64:984–90. [DOI] [PubMed] [Google Scholar]
- 6.Farrell PR, Hornung L, Farmer P, DesPain AW, Kim E, Pearman R, et al. Who’s at Risk? A Prognostic Model for Severity Prediction in Pediatric Acute Pancreatitis. Journal of pediatric gastroenterology and nutrition. 2020;71:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitale DS, Hornung L, Lin TK, Nathan JD, Prasad S, Thompson T, et al. Blood Urea Nitrogen Elevation Is a Marker for Pediatric Severe Acute Pancreatitis. Pancreas. 2019;48:363–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morinville VD, Husain SZ, Bai H, Barth B, Alhosh R, Durie PR, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. Journal of pediatric gastroenterology and nutrition. 2012;55:261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- 10.Zheng W, Zhang L, Long G, Chen B, Shu X, Jiang M. Amalgamation of systemic inflammatory response syndrome score with C-reactive protein level in evaluating acute pancreatitis severity in children. Scand J Gastroenterol. 2018;53:755–9. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; A systematic review and meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al]. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Ma X, Jia X, Wang R, Liu L, Zhang M, et al. Prevention of Severe Acute Pancreatitis With Cyclooxygenase-2 Inhibitors: A Randomized Controlled Clinical Trial. The American journal of gastroenterology. 2020;115:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 Inflammasome Regulates Development of Systemic Inflammatory Response and Compensatory Anti-Inflammatory Response Syndromes in Mice With Acute Pancreatitis. Gastroenterology. 2020;158:253–69.e14. [DOI] [PubMed] [Google Scholar]
- 14.Yang YZ, Xiang Y, Chen M, Xian LN, Deng XY. Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis. Asian Pac J Trop Med. 2016;9:1111–4. [DOI] [PubMed] [Google Scholar]
- 15.Coffey MJ, Nightingale S, Ooi CY. Serum lipase as an early predictor of severity in pediatric acute pancreatitis. Journal of pediatric gastroenterology and nutrition. 2013;56:602–8. [DOI] [PubMed] [Google Scholar]
- 16.Fabre A, Petit P, Gaudart J, Mas E, Vial J, Olives JP, et al. Severity scores in children with acute pancreatitis. Journal of pediatric gastroenterology and nutrition. 2012;55:266–7. [DOI] [PubMed] [Google Scholar]
- 17.Stirling AD, Moran NR, Kelly ME, Ridgway PF, Conlon KC. The predictive value of C-reactive protein (CRP) in acute pancreatitis - is interval change in CRP an additional indicator of severity? HPB : the official journal of the International Hepato Pancreato Biliary Association. 2017;19:874–80. [DOI] [PubMed] [Google Scholar]
- 18.Vasudevan S, Goswami P, Sonika U, Thakur B, Sreenivas V, Saraya A. Comparison of Various Scoring Systems and Biochemical Markers in Predicting the Outcome in Acute Pancreatitis. Pancreas. 2018;47:65–71. [DOI] [PubMed] [Google Scholar]
- 19.Wu BU, Hwang JQ, Gardner TH, Repas K, Delee R, Yu S, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:710–7.e1. [DOI] [PubMed] [Google Scholar]
- 20.Park A, Latif SU, Shah AU, Tian J, Werlin S, Hsiao A, et al. Changing referral trends of acute pancreatitis in children: A 12-year single-center analysis. Journal of pediatric gastroenterology and nutrition. 2009;49:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattu RK, De Fee AS, Lichenstein R, Teshome G. Consideration of Cost of Care in Pediatric Emergency Transfer-An Opportunity for Improvement. Pediatr Emerg Care. 2017;33:334–8. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi T, Zhao H, Kawabe K, Oono T, Egashira K, Suzuki K, et al. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol. 2008;43:79–85. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


