Abstract
Background:
Depression (DEP) and cognitive impairment (CI) share etiological risk factors, anatomical underpinnings, and interact to produce deleterious treatment outcomes. Both DEP and CI exhibit altered patterns of cortical thickness which may impact the course of antidepressant treatment, though inconsistencies in directionality and affected brain regions have been reported. In this study, we examined the relationship between cortical thickness and treatment outcome in older adults with comorbid DEP-CI.
Methods:
55 patients with DEP-CI received baseline MRI scans as part of a larger clinical trial at NYSPI/Columbia University Medical Center and Duke University Medical Center. Mood was assessed using the Hamilton Depression Rating Scale. Patients received open antidepressant treatment for 8 weeks followed by another 8 weeks of the same medication or switch to another antidepressant for a total of 16 weeks. Cortical thickness was extracted using an automated brain segmentation program (FreeSurfer). Vertex-wise analyses evaluated the relationship between cortical thickness and treatment outcome.
Results:
Remitters exhibited diffuse clusters of greater cortical thickness and reduced cortical thickness compared to non-remitters. Thicker baseline middle frontal gyrus most consistently predicted greater likelihood and faster rate of remission. White matter hyperintensities and hippocampal volume were not associated with antidepressant treatment outcome.
Limitations:
MRI was conducted at baseline only and sample size was small.
Discussion:
Cortical thickness predicts treatment remission and magnitude of early improvement. Results indicate that individuals with DEP-CI exhibit unique patterns of structural abnormalities compared to their depressed peers without CI that have consequences for their recovery with antidepressant treatment.
Keywords: Mild cognitive impairment, cognitive impairment, depression, cortical thickness, antidepressant trial
1. Introduction
Depression (DEP) and cognitive impairment (CI) frequently co-occur, yet their comorbid presentation is understudied, as trials of late-life depression typically exclude patients with amnestic deficits(Alexopoulos, 2005), and trials of mild cognitive impairment (MCI) generally exclude patients with major depressive disorder(Petersen, 2004). DEP-CI is associated with adverse outcomes to a greater degree than either disorder in isolation. Most studies show that patients with DEP-CI are more likely to convert to dementia(Mourao et al., 2016) and at a faster rate than patients with CI alone(Chung et al., 2016). This comorbid presentation is also associated with poorer likelihood of recovery following antidepressant treatment(Sneed et al., 2010).
Identifying factors involved in antidepressant outcome allows for better understanding of the antidepressant mechanism of action and the underlying pathology driving the disorder itself. Multiple anatomical predictors of reduced remission have been identified, such as white matter hyperintensities (WMH)(Gunning-Dixon et al., 2010), reduced hippocampal volume(Sheline et al., 2012), and reduced frontal lobe volume(Ribeiz et al., 2013). Cortical thickness has also been examined as a predictor of treatment outcome. Cortical thickness is a measure of grey matter morphometry found to be reduced in patients with MCI and in older adults with depression. In a 12-week treatment trial using the SSRI sertraline for patients with late-life depression, nonremitters had thinner frontal poles at baseline than remitters(Sheline et al., 2012). Another longitudinal trial found patients with depression had thinner orbitofrontal cortex, superior temporal lobe and insular cortex compared to healthy controls(Jarnum et al., 2011). Additionally, nonremitters at 6 months had thinner posterior cingulate cortices than remitters. In a more recent study of patients with treatment-resistant depression, thicker baseline anterior cingulate cortex was associated with greater symptom improvement. Patients who achieved sustained 6-month remission had increased hippocampal volume and increased cortical thickness in the rostral middle frontal gyrus, orbitofrontal cortex, and inferior temporal gyrus compared to baseline. Nonremitters had decreased volume and thickness in the same areas at follow-up(Phillips et al., 2015). Cortical thickness predicts the effectiveness of psychotherapy for late-life depression as well. Non-responders to problem-solving therapy demonstrated thinner bilateral posterior cingulate cortices and parahippocampal cortices, left precuneus, cuneus, and insular cortices, and the right medial orbitofrontal and lateral occipital cortices relative to responders(Mackin et al., 2013). Taken together, reduced cortical thickness is related to reduced efficacy of interventions for depression. Critically, successful treatment appears to reverse atrophy in fronto-limbic areas. This holds particular relevance for DEP-CI, where cortical thinning in these areas is predictive of more rapid dementia onset(Sacuiu et al., 2016).
Cortical thickness is reduced in patients with MCI and to an even greater extent in patients with Alzheimer’s disease (AD) (Julkunen et al., 2010) when compared to healthy controls. For patients with MCI, several cortical regions have been reported as reduced in thickness compared to healthy controls, possibly owing to the diverse etiologies that may contribute to the development of MCI. The regions with the most consistently reduced thickness are the frontal lobe, temporal lobe, and limbic regions(Li et al., 2011). Such thinning is linked with reduced cognitive functioning(Eliassen et al., 2017) and confers increased risk of conversion to dementia(Julkunen et al., 2010; Peters et al., 2014). The presence of depressive symptoms in patients with MCI is also associated with reduced thickness, and additionally may serve as markers of longitudinal atrophy, with greater depressive symptoms being associated with accelerated atrophy over time(Zahodne et al., 2013).
Given the spatial overlap of regions previously implicated in antidepressant treatment outcome with regions affected by MCI and AD pathology, we may expect DEP-CI patients with reduced fronto-temporal-limbic cortical thickness to exhibit worse treatment outcome. To date, no study has examined cortical thickness patterns of patients with DEP-CI, nor their impact on treatment outcome. The purpose of this study in the sample of patients with DEP-CI is: 1) to examine previously identified MRI predictors of antidepressant outcome (WMH and hippocampal volume); and 2) to evaluate the relationship between remission on antidepressant treatment and cortical thickness.
2. Methods
Participants
The present study is concentrated on the acute antidepressant phase of the ‘Donepezil Treatment of Cognitive Impairment and Depression (DOTCODE) trial. The full DOTCODE protocol has been described elsewhere(Devanand et al., 2018; Pelton et al., 2014). The study was approved by the New York State Psychiatric Institute/Columbia University Institutional Review Board (IRB #6459) and the Duke University Medical Center Institutional Review Board. The study is registered at ClinicalTrials.gov (NCT01658228). Patients were eligible if they were age 55 to 95, had 8 years minimum of education, met diagnosis for major depression or dysthymic disorder (based on SCID‐P evaluation), had a minimum score of 14 on the 24‐item Hamilton Depression Rating Scale (HDRS), presented with subjective memory or other cognitive complaints, met criteria for CI (defined as a score ≤11 for delayed recall on the Wechsler Memory Scale‐Revised Logical Memory II test), had a Folstein Mini Mental State Exam (MMSE) score ≥21, received a Clinical Dementia Rating of 0.5, and were willing and capable of giving informed consent. Exclusion criteria were other major pre-existing psychiatric and neurological disorders including dementia, acute medical illness, active suicidal ideation or suicide attempt, alcohol or substance abuse or dependence in the past 6 months, use of medication rated as the likely cause of cognitive impairment, uncontrolled hypertension, current use of effective antidepressants, current use of cholinesterase inhibitors (CheI) or memantine, and ECG QTc interval > 460 msec.
Measures
At week 0, participants completed an MRI scan, clinical evaluation, and a neuropsychological test battery. At weeks 8 and 16, the clinical evaluation was repeated. The HDRS was the primary mood outcome measure. Blood samples were processed by the Columbia University Human Genetics Resource Core and apolipoprotein E (ApoE) genotype was determined by Prevention Genetics.
Antidepressant Treatment
Patients already receiving antidepressant treatment underwent a medication washout lasting 0–14 days, determined by clinician’s judgement and known half-life of the antidepressant. Patients initially received open antidepressant treatment with either citalopram or venlafaxine. Patients with prolonged QTc received venlafaxine as the initial treatment. Other antidepressants were prescribed for patients with non-response or intolerability to citalopram and venlafaxine. Both at baseline and during the trial, prior non-response or intolerability to a specific antidepressant led to that antidepressant not being prescribed. During the first week, all patients on citalopram began with 10mg/day. This increased to 20mg/day during the second week, and then to a maximum of 40mg/day at week 4 as tolerated and based on clinical response; this study was conducted before the FDA warning about the risk of prolonged QTc interval on the ECG in older patients receiving citalopram doses above 20 mg daily. All patients on venlafaxine began with 37.5mg/day. This increased weekly, as tolerated and based on clinical response, to a maximum of 225mg/day by 3 weeks. At weeks 8 and 16, medication outcome was evaluated. Patients were classified as remitters if they obtained a score of 0–7 on the HDRS, or as non-remitters if they obtained a score on the HDRS of 8 or higher, which is based on a similar approach used in published trials in major depression(Mackin et al., 2013; Sheline et al., 2012). Patients were classified as responders if they achieved a 50% or greater reduction from baseline HDRS scores.
MRI
Images were acquired on a GE Signa 3 Tesla whole body MRI scanner using an identical model at the two sites. As described elsewhere in detail(Pelton et al., 2014), sequences included a 3-Plane localizer and 3D SPGR anatomical acquisition. Cortical thickness was extracted from the 3D SPGR acquisition using FreeSurfer version 6.0, an automated brain segmentation free software program which constructs boundaries between white matter, cortical grey matter, and pial surface(Dale et al., 1999; Fischl et al., 1999). T2 FLAIR deep white matter hyperintensity (DWMH) and periventricular hyperintensity (PVH) volume were measured on a semiautomated version of the Fazekas-modified Coffey Rating Scale(Rorden and Brett, 2000) using MRIcro. Our group has published using these methods and raters using this semiautomated approach have achieved excellent interrater reliability (ICC = .98)(Pimontel et al., 2013). All WMH volume measures were converted to dichotomous variables based on the median value for each measure. A single trained, experienced technician, who established high interrater reliability with expert raters (ICC=0.90–0.96) and showed high intrarater reliability (ICC=0.97–0.99)(Devanand et al., 2007), drew the hippocampus and entorhinal cortex ROIs using atlas-based approaches.
Statistical Analyses
Vertex-wise analysis has high sensitivity, allows for whole-brain exploratory data analysis, and enables detection of clusters of cortical thickness differences that do not conform to the boundaries of predefined cortical parcellations(Woo et al., 2014). Given these advantages, the inconsistency of ROI selection and findings in the depression and CI literature, and the absence of any previous studies on cortical thickness for comorbid DEP-CI, the vertex-wise analysis was chosen over ROI-based analysis to evaluate the cortical thickness hypothesis. Principal component analysis of the vertices yielded 33 principal components (PCs) that explain 80% of the total variance. Penalized generalized mixed effect models were constructed to predict response and remission at 8 and 16 weeks with 33 PCs, time (two levels: 8 and 16 weeks) and the time-by-PC interactions as the fixed effects and random intercept to account for within-subject correlation due to the repeated measure. Generalized mixed effect models can simultaneously model the binary outcomes while accounting for within-subject correlations introduced by repeated measures. Under the missing at random assumption, this approach can infer the overall effect of the independent variables on the outcome. The time interaction with PCs was selected via regularization to ensure marginality condition(Hao et al., 2018). Following the regularization path, we used forward selection for main effects and their interactions, and if an interaction term was included, its parent main effects were included. The model with minimum Bayesian Information Criteria was considered as the best model. For the primary analysis, we used principal components with 80% threshold and sensitivity analysis for different cuts (e.g. 70% and 90%). Principal component-derived clusters larger than 1000 mm2 were retained. For visualization, bootstrapped vertex-wise z-score maps were created to estimate the effect of cortical thickness on the outcomes at 8 and 16 weeks, and the interaction terms from 5000 resampling. For each bootstrapped sample, we fit the selected model and multiply the estimated fixed effects with the PC loading matrices. Separate response/remission models were evaluated with cortical thickness, DWMH, PVH, and hippocampal volumes. For covariate selection, remission/responses models were run without anatomical variables as predictors. Site, sex, age, education, and baseline HDRS were evaluated as covariates.
3. Results
Descriptive statistics
81 patients were recruited, and two patients did not complete all baseline procedures. Of the 79 patients enrolled, 55 completed baseline MRI scans and were included in the current analyses. Patients had a mean HDRS of 23.2 (SD = 5.4), consistent with moderate depression severity. The mean age was 68.4 (SD = 8.5) years, 58.2% were male and 41.8% were female. There was no difference between the two sites in age, sex, or education. The sample’s mean HDRS was 23.2 (SD = 8.5) at baseline and 11.1 (SD =5.5) at week 8. Fifteen patients were classified as remitters and 40 as non-remitters; 35 patients were classified as responders and 20 as non-responders. Non-responders were significantly older (t(54)=2.1, p=.037) and had significantly lower baseline HDRS (t(54)=−3.2, p=.003) than responders (Table 1; Supplementary Table 1). At week 16, the sample’s mean HDRS was 9.6 (SD = 5.2). 20 patients were classified as remitters and 29 as non-remitters; 34 patients were classified as responders and 15 as non-responders (Supplementary Tables 2–3). There were no significant differences between remitters and non-remitters on baseline demographic or clinical characteristics. There were no significant differences between antidepressant treatment groups on demographic or clinical characteristics (Supplementary Table 4).
Table 1.
Baseline demographic and clinical characteristics for week 8 outcomes
| Demographic Variable | All Patients(n=55) | Non-remitters (n=40) vs Remitters (n=15) | Non-responders (n=20) vs Responders (n=35) |
|---|---|---|---|
| Site NYSPI DU | 28 (50.9%) 27 (49.1%) | χ2=.5, p=.826 | χ2=.2, p=.646 |
| Age | 68.4 (8.5) | t=1.4, p=.179 | t=2.1, p=.037 |
| Sex Male Female | 32 (58.2%) 23(41.8%) | χ2=1.1, p=.289 | χ2=.6, p=.438 |
| Education | 15.4 (3.0) | t=−.9, p=.392 | t=−.3, p=.799 |
| Race Caucasian Black/African American Hispanic | 40 (72.7%) 8 (14.5%) 7 (12.7%) | χ2=1.7, p=.419 | χ2=4.7, p=.094 |
| Marital Status Single/never married Divorced/separated Married/living with partner Widowed | 23 (41.8%) 11 (20.0%) 18 (32.7%) 3 (5.5%) | χ2=1.7, p=.648 | χ2=1.7, p=.646 |
| Family History of Depression No Yes | 26 (47.3%) 29 (52.7%) | χ2=1.3, p=.247 | χ2=.7, p=.799 |
| Family History of Dementia No Yes | 29 (52.7%) 26 (47.3%) | χ2=.0, p=.956 | χ2=.1, p=.759 |
| Family History of Alzheimer’s No Yes | 47 (85.5%) 8 (14.5%) | χ2=.5, p=.482 | χ2=.5, p=.470 |
| HDRS | 23.2 (5.4) | t=.5, p=.599 | t=−3.2., p=.003 |
| ApoE genotype ApoE ε4 allele not present ApoE ε4 allele present | 39 (72.2%) 15 (27.8%) | χ2=3.7, p=.055 | χ2=.7, p=.416 |
All values expressed as mean (SD) or n (%). Abbreviations: NYSPI=New York State Psychiatric Institute; DU=Duke University; HDRS=Hamilton Depression Rating Scale
WMH, hippocampus, and Treatment Outcome
In the initial covariate-only model, older age and male sex were associated with significantly increased remission at week 16 (Table 2). Higher baseline HDRS was associated with increased response at week 8. Site and education were not significantly associated with treatment outcome at any time point and were not included in later models. DWMH, PVH, and ApoE e4 genotype did not significantly predict remission or response at either timepoint when entered alone alongside covariates. There was a nonsignificant trend towards greater total hippocampal volume being associated with increased chance of week 16 remission (p=0.069). When considered separately, there was no association between right or left hemisphere hippocampal volume and week 16 remission. Hippocampus volume (right, left, and total) was not significantly associated with week 8 remission or response at either timepoint.
Table 2.
Treatment Outcome Models Using Demographic and Clinical Covariates
| Predictors | Odds Ratio | 95% CI | p | Odds Ratio | 95 % CI | p |
|---|---|---|---|---|---|---|
| HDRS Remission Week 8 | HDRS Remission Week 16 | |||||
| (Intercept) | 12.34 | 0.01 – 40326.06 | 0.526 | 270.54 | 0.06 – 3190983 | 0.209 |
| Site (NYSPI) | 1.06 | 0.29 – 3.79 | 0.933 | 2.54 | 0.72 – 9.81 | 0.158 |
| Age | 0.95 | 0.87 –1.03 | 0.238 | 0.9 | 0.79 – 0.99 | 0.044 |
| Education | 1.08 | 0.87 – 1.36 | 0.501 | 1 | 0.80 –1.23 | 0.996 |
| Sex (Male) | 0.66 | 0.18 – 2.47 | 0.536 | 6.82 | 1.51 – 41.81 | 0.021 |
| Baseline HDRS | 0.95 | 0.84 –1.07 | 0.447 | 1 | 0.88 –1.13 | 0.949 |
| Observations | 55 | 49 | ||||
| R2 (Tjur) | 0.058 | 0.165 | ||||
| HDRS Response Week 8 | HDRS Response Week 16 | |||||
| (Intercept) | 0.28 | 0.00 – 549.14 | 0.739 | 37.24 | 0.01 – 490266.55 | 0.419 |
| Site (NYSPI) | 1.3 | 0.36 – 5.05 | 0.692 | 1.36 | 0.34 – 5.86 | 0.668 |
| Age | 0.95 | 0.87 – 1.02 | 0.168 | 0.91 | 0.81 – 1.00 | 0.071 |
| Education | 1.06 | 0.86 – 1.3 | 0.587 | 0.99 | 0.78 – 1.25 | 0.946 |
| Sex (Male) | 0.89 | 0.22 – 3.59 | 0.864 | 7.19 | 1.45 – 53.15 | 0.027 |
| Baseline HDRS | 1.23 | 1.06 – 1.48 | 0.012 | 1.12 | 0.98 –1.32 | 0.118 |
| Observations | 55 | 49 | ||||
| R2 (Tjur) | 0.225 | 0.224 | ||||
For categorical variables of site and sex, parentheses denote reference group. Abbreviations: CI=confidence interval; NYSPI=New York State Psychiatric Institute; Hamilton Depression Rating Scale
Cortical Thickness and Treatment Outcome
Principal components analyses of cortical thickness yielded 33 principal components. Of these, two components produced a significant main effect on treatment remission, such that they predicted remission at both week 8 and week 16 (Table 3). Clusters within these principal components are listed in Table 4. Treatment remission was associated with greater cortical thickness in the right hemisphere caudal middle frontal gyrus and isthmus of the cingulate gyrus, and left hemisphere rostral middle frontal gyrus, lateral occipital cortex, and pars triangularis of the inferior frontal gyrus (Figure 1). Treatment remission was also associated with reduced cortical thickness in the right hemisphere inferior temporal gyrus, superior parietal cortex, and precentral gyrus, and left hemisphere lateral occipital cortex, transverse temporal cortex, postcentral gyrus, pericalcarine cortex, superior parietal cortex, lateral orbitofrontal gyrus, and paracentral lobule (Table 4). An additional principal component significantly interacted with time, such that clusters within this component were associated with greater speed of remission from week 0–8 than 8–16 (Table 5). Speed of remission was associated with greater cortical thickness in the right hemisphere caudal middle frontal gyrus, insular cortex, and lingual gyrus, and left hemisphere precuneus cortex, fusiform gyrus, and rostral middle frontal gyrus. Speed of remission was additionally associated with reduced cortical thickness in the right hemisphere middle temporal gyrus, precentral gyrus, superior parietal cortex, inferior temporal gyrus, and lateral occipital cortex, and left hemisphere transverse temporal cortex, lateral orbitofrontal gyrus, postcentral gyrus, and precentral gyrus. No principal components were significantly associated with response (Table 6).
Table 3.
Principal Components of Cortical Thickness Clusters Predicting Treatment Remission
| Parameter | Mean | SE | 95% CI | z | p |
|---|---|---|---|---|---|
| (Intercept) | 3.4 | 3.3 | −2.7 – 10.3 | 1.0 | 0.311 |
| Age | −0.1 | 0.0 | −0.2 – 0.0 | −1.8 | 0.066 |
| Sex (Male) | 0.2 | 0.8 | −1.2 – 1.7 | 0.3 | 0.744 |
| Baseline HDRS | 0.0 | 0.1 | −0.1 – 0.1 | −0.1 | 0.886 |
| PC 4 | 1.1 | 4.0 | −5.1 – 11.5 | 0.3 | 0.785 |
| PC 10 | 4.9 | 2.9 | 0.0 – 11.5 | 1.7 | 0.093 |
| PC 12 | 7.3 | 2.6 | 2.6 – 13.1 | 2.8 | 0.005 |
| PC 19 | 6.1 | 3.0 | 1.1 – 12.8 | 2.0 | 0.042 |
| Time × PC 4 | 16.6 | 7.7 | 5.5 – 36.4 | 2.2 | 0.031 |
| Time | 1.5 | 0.8 | 0.0 – 3.1 | 2.0 | 0.045 |
Abbreviations: PC=principal component, SE=standard error, CI=confidence interval; HDRS= Hamilton Depression Rating Scale
Table 4.
Cortical Thickness Clusters Predicting Treatment Remission at Week 16
| Cluster # | Max | VtxMax | Size(mm^2) | TalX | TalY | TalZ | NVtxs | Region |
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | ||||||||
| 1 | 3.5 | 8156 | 11258.3 | 33.3 | 16.3 | 45.8 | 19854 | Caudal middle frontal |
| 2 | −3.5 | 162940 | 10322.0 | 52.2 | −35 | −14.7 | 22847 | Inferior temporal |
| 3 | −3.5 | 3766 | 4264.1 | 17.9 | −68.8 | 44.5 | 5898 | Superior parietal |
| 4 | −3.5 | 150865 | 2256.0 | 34.5 | −44.5 | 58.8 | 5621 | Superior parietal |
| 5 | 3.5 | 51666 | 1736.1 | 4.8 | −46.8 | 22.2 | 2929 | Isthmus cingulate |
| 6 | −3.5 | 56262 | 1151.1 | 31 | −10.4 | 52 | 2366 | Precentral |
| Left Hemisphere | ||||||||
| 1 | 3.5 | 145856 | 7133.2 | −32.4 | 49.4 | 1.1 | 11539 | Rostral middle frontal |
| 2 | −3.4 | 41902 | 3660.1 | −38.4 | −84.9 | −3 | 5717 | Lateral occipital |
| 3 | −3.5 | 73556 | 3342.3 | −42.5 | −27.5 | 8.8 | 7513 | Transverse temporal |
| 4 | −3.5 | 4531 | 2356.7 | −61.9 | −11.2 | 23.5 | 5705 | Postcentral |
| 5 | 3.5 | 49629 | 2104.5 | −34.2 | −79.2 | −7.9 | 3229 | Lateral occipital |
| 6 | −3.4 | 137272 | 2097.1 | −19.1 | −73 | 6.6 | 4252 | Pericalcarine |
| 7 | 3.5 | 84798 | 1744.4 | −29.1 | 29 | 3.7 | 4304 | Pars triangularis |
| 8 | −3.5 | 81263 | 1611.9 | −22.5 | −56.4 | 59.1 | 3794 | Superior parietal |
| 9 | −3.5 | 74949 | 1549.0 | −27.4 | 22.2 | −17.5 | 2994 | Lateral orbitofrontal |
| 10 | −3.5 | 140201 | 1065.0 | −6 | −12.6 | 59.2 | 2431 | Paracentral |
All analyses were additionally covaried for sex, age, and baseline HDRS. X, Y, Z coordinates represent peak Talairach coordinates. Abbreviations: VtxMax=vertex maximum; NVtxs=number of vertices.
Figure 1. Cortical Thickness Clusters in Week 16 Remitters vs. Nonremitters.
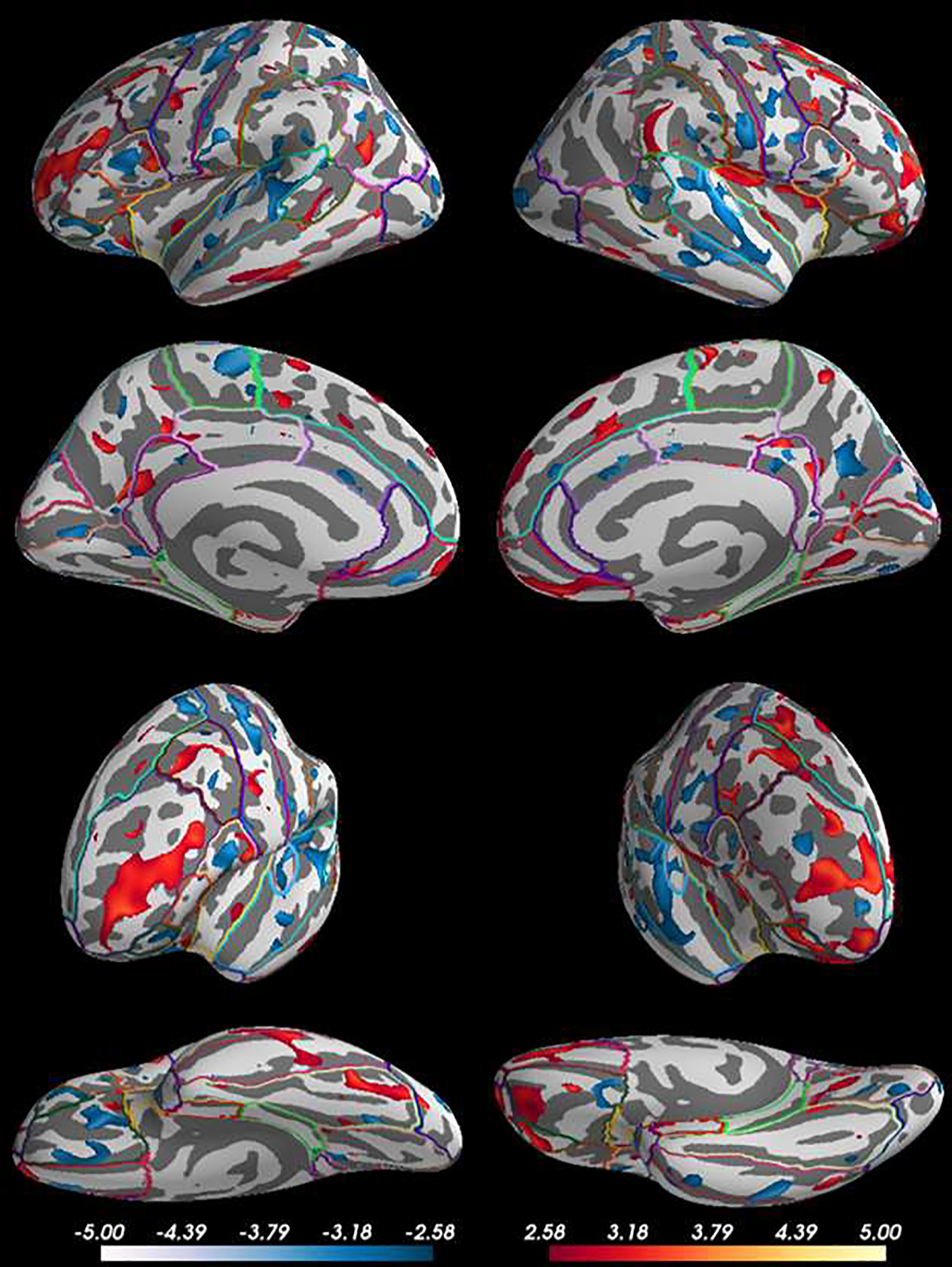
Lateral, medial, anterior, and inferior view of clusters containing greater (red) and reduced (blue) cortical thickness for remitters compared to nonremitters after adjusting for age, sex, and baseline depressive symptoms. Vertex-wise analyses revealed remitters had clusters of greater thickness in predominantly frontal (middle frontal & inferior frontal gyri) and limbic areas (isthmus of the cingulate) and reduced thickness in primarily temporal (inferior temporal, transverse temporal) and parietal lobes (superior parietal and post central gyrus) compared to non-remitters.
Table 5.
Cortical Thickness Clusters Predicting Faster Rate of Remission From Week 0–8 than 8–16
| Cluster # | Max | VtxMax | Size(mm^2) | TalX | TalY | TalZ | NVtxs | Region |
|---|---|---|---|---|---|---|---|---|
| Right Hemisphere | ||||||||
| 1 | 3.4 | 145351 | 8782.3 | 33.2 | 15.7 | 46.2 | 16030 | Caudal middle frontal |
| 2 | −3.4 | 128496 | 2332.1 | 51.2 | −15.1 | −15.7 | 4768 | Middle temporal |
| 3 | −3.4 | 55113 | 1647.9 | 47.8 | 0.9 | 35.7 | 3900 | Precentral |
| 4 | −3.4 | 3766 | 1499.1 | 17.9 | −68.8 | 44.5 | 2089 | Superior parietal |
| 5 | 3.2 | 109813 | 1122.5 | 34.3 | −8.3 | 15.7 | 3395 | Insula |
| 6 | 3.4 | 41290 | 1099.7 | 24.3 | −59.8 | 3.5 | 2000 | lingual |
| 7 | −3.4 | 114748 | 1099.1 | 53.3 | −38.1 | −16 | 1726 | Inferior temporal |
| 8 | −3.3 | 16247 | 1013.3 | 31.8 | −82.8 | 4.1 | 1282 | Lateral occipital |
| Left Hemisphere | ||||||||
| 1 | −3.4 | 73556 | 3853.0 | −42.5 | −27.5 | 8.8 | 7985 | Transverse temporal |
| 2 | 3.4 | 159436 | 2277.2 | −9 | −56.1 | 15.3 | 5107 | Precuneus |
| 3 | −3.4 | 34014 | 1953.3 | −27.8 | 21.4 | −18.1 | 3898 | Lateral orbitofrontal |
| 4 | 3.4 | 127984 | 1761.7 | −41.1 | −51.5 | −12.2 | 2746 | Fusiform |
| 5 | 3.4 | 34946 | 1697.8 | −31.6 | 48.9 | 0.3 | 2644 | Rostral middle frontal |
| 6 | −3.4 | 123471 | 1139.6 | −62.2 | −9.7 | 24.4 | 2839 | Postcentral |
| 7 | −3.4 | 28806 | 1113.8 | −40.4 | −8.2 | 56.1 | 2587 | Precentral |
All analyses were additionally covaried for sex, age, and baseline HDRS. X, Y, Z coordinates represent peak Talairach coordinates. Abbreviations: VtxMax=vertex maximum; NVtxs=number of vertices.
Table 6.
Principal Components of Cortical Thickness Clusters Predicting Treatment Response
| Parameter | Mean | SE | 95% CI | z | p |
|---|---|---|---|---|---|
| (Intercept) | 6.8 | 9.6 | −9.7 – 30.6 | 0.7 | 0.477 |
| PC 4 | 6.6 | 9.1 | −6.4 – 29.3 | 0.7 | 0.47 |
| PC 5 | 6.7 | 7.5 | −3.9 – 23.8 | 0.9 | 0.37 |
| PC 7 | 6.1 | 9.4 | −7.5 – 31.1 | 0.6 | 0.517 |
| PC 10 | 15.1 | 8.7 | 2.1 – 34.5 | 1.7 | 0.084 |
| PC 12 | 17.2 | 9.8 | 3.8 – 41.0 | 1.7 | 0.081 |
| PC 14 | 16.8 | 10.0 | 1.1 – 41.1 | 1.7 | 0.094 |
| PC 15 | 1.5 | 8.1 | −14.5 – 20.6 | 0.2 | 0.852 |
| PC 20 | 12.0 | 7.1 | 1.2 – 28.9 | 1.7 | 0.091 |
| PC 25 | 16.5 | 8.7 | 2.4 – 33.8 | 1.9 | 0.059 |
| PC 27 | −10.9 | 8.3 | −29.3 – 3.3 | −1.3 | 0.191 |
| PC 28 | 1.5 | 6.3 | −10.4 – 14.4 | 0.2 | 0.809 |
| PC 29 | 11.6 | 11.6 | −4.7 – 37.8 | 1.0 | 0.314 |
| PC 30 | 17.8 | 9.2 | 4.7 – 38.1 | 1.9 | 0.052 |
| Time × PC 4 | 21.6 | 12.9 | 0.0 – 53.6 | 1.7 | 0.095 |
| Time × PC 7 | −14.2 | 11.7 | −38.6 – 6.8 | −1.2 | 0.225 |
| Time × PC 10 | −15.2 | 11.9 | −44.7 – 2.2 | −1.3 | 0.204 |
| Time × PC 14 | −21.8 | 12.2 | −50.4 – −0.1 | −1.8 | 0.073 |
| Time × PC 15 | −26.0 | 13.7 | −56.7 – −5.8 | −1.9 | 0.057 |
| Time × PC 25 | −12.1 | 10.3 | −37.8 – 4.3 | −1.2 | 0.24 |
| Time × PC 28 | 21.3 | 11.2 | 2.4 – 43.0 | 1.9 | 0.057 |
| Time × PC 29 | −14.5 | 12.0 | −41.9 – 4.2 | −1.2 | 0.224 |
| Time | 1.3 | 1.5 | −1.1 – 4.7 | 0.8 | 0.396 |
| Age | −0.2 | 0.1 | −0.5 – 0.0 | −1.6 | 0.119 |
| Sex (Male) | 3.0 | 2.2 | 0.0 – 7.7 | 1.3 | 0.181 |
| Baseline HDRS | 0.3 | 0.2 | −0.1 – 0.8 | 1.6 | 0.112 |
Abbreviations: PC=principal component, SE=standard error, CI=confidence interval; HDRS= Hamilton Depression Rating Scale
DWMH and hippocampal volume were added to the remission model to determine if cortical thickness remained a significant predictor of remission after adjusting for these anatomical variables. The main effect of cortical thickness on remission remained significant (Mean=5.7, SE=2.4, 95% CI=1.2–11.3, z=2.4, p=0.018), but the principal component x time interaction was no longer significant (Mean=5.8, SE=5.6 95% CI=−1.9–17.8, z=1.0, p=0.306). DWMH and hippocampal volume remained not significantly associated with remission.
4. Discussion
Diffuse clusters of greater cortical thickness and reduced cortical thickness were predictive of remission of depressive symptoms. Clusters were distributed bilaterally and across the lobes of the cortex. Greater cortical thickness was associated with increased remission in the frontal lobe (middle frontal & inferior frontal gyri), limbic region (isthmus of the cingulate), and occipital lobe (lateral occipital gyrus). Reduced cortical thickness was associated with increased remission in clusters spanning the frontal lobe (precentral and orbitofrontal gyri), temporal lobe (inferior temporal and transverse temporal gyri), parietal lobe (superior parietal and post central gyri), and occipital lobe (pericalcarine cortex and lateral occipital gyrus). The heterogenous distribution of clusters, both by regional allotment and directionality of effect, limits clinical interpretability and makes it doubtful that a single mechanism is involved.
The anatomical region most consistently predictive of treatment outcome across analyses was the middle frontal gyrus, wherein greater thickness in each hemisphere predicted both overall remission (across all 16 weeks) and faster rate of remission (by week 8). Clusters in this region tended to be larger than clusters located in other regions. The relationship between middle frontal gyrus and depression prognosis is in line with previous studies in the literature. Patients with late-life depression have reduced thickness in the middle frontal gyrus compared to healthy controls(Lim et al., 2012; Mackin et al., 2013; Sheline et al., 2012). In one antidepressant trial, rostral middle frontal gyrus thickness increased over time in remitters and decreased in non-remitters(Phillips et al., 2015). The middle frontal gyrus contains the dorsolateral prefrontal cortex (DLPFC), which governs working memory and inhibition(Milham et al., 2003). Together with the anterior cingulate cortex, the DLPFC is a part of the cognitive control network, which performs error detection and resolution(Langenecker et al., 2007), as well as emotional regulation(Fales et al., 2009). Structural damage and reduced functional connectivity in the cognitive control network is related to poor antidepressant outcome(Alexopoulos et al., 2012; Taylor et al., 2013), and successful antidepressant treatment appears to normalize cognitive control network functioning(Aizenstein et al., 2009; Fales et al., 2009).
This finding of a relationship between middle frontal gyrus thickness and remission has not been reported in antidepressant and psychotherapy trials for late-life depression(Mackin et al., 2013; Sheline et al., 2012). There are several possible reasons for the disparate findings. First, the present trial included MCI patients, who have reduced middle frontal gyrus thickness compared to controls(Li et al., 2011), thus providing an additional pathway by which mood regulation may be disrupted. Second, the present analysis is vertex-based instead of ROI based, allowing for detection of intraregional clusters. Finally, factors including sex, age, education, and site differences account for as much as a fifth of the total individual differences in cortical thickness, exceeding the variance accounted for by depression severity and group differences in remission/response status(Perlman et al., 2017). Therefore, the lack of agreement in covariate selection between the studies may have contributed to the discrepant findings. Altered cortical thickness may be conceptualized as one of multiple possible structural routes by which brain networks mediating antidepressant action can be disrupted. Curiously, clusters of cortical thickness were associated with rate of remission. This may also indicate a role of these cortical regions in modulating the speed with which antidepressant mechanisms operate, rather than hindering their effect entirely. Future follow-up studies with larger sample sizes and repeat MRI may clarify these issues.
The process behind cortical thinning in depression remains unclear, as does the mechanism driving the relationship between reduced cortical thickness and poor treatment outcome. One possibility involves glucocorticoid-mediated atrophy, whereby a hyperactive hypothalamic-pituitary-adrenal axis and elevated cortisol levels produce neurotoxic effects. Indeed, cortisol levels are negatively correlated with cortical thickness in the orbitofrontal cortex of depressed patients(Liu et al., 2015). Another potential mechanism is that cortical thickness-mediated antidepressant outcomes may relate to elevated serotonin 1A (5-HT1A) receptor density, which results in deficient synaptic serotonin levels, and in turn lowers the probability of post-synaptic signaling, creating loss of dendrites on the pre and post synaptic neurons(Azmitia, 2001). Lastly, cortical thickness changes may also be partially driven by WMH burden, which disrupts axons corresponding to cortical neurons and leads to cellular disruption by the processes of anterograde and retrograde degeneration(Mortamais et al., 2013). Collectively, these mechanisms produce atrophy in frontal areas, that in turn inhibits mood regulation, thus potentially interfering with the restorative actions of antidepressants. Notably, results of this study also implicate clusters of reduced thickness with increased treatment remission. A mechanism driving this relationship is not understood, though inflammatory responses driven by cytokines, cellular hypertrophy, and astrocyte proliferation has been posited in late-life depression(Dowlati et al., 2010; Szymkowicz et al., 2016). If inflammation is indeed involved in producing greater cortical thickness in some patients, and in turn lower probability of treatment remission, then individuals lacking inflammatory processes in select regions (thereby having reduced cortical thickness by comparison) may have improved prognosis. Inflammation alone is likely not sufficient to fully account for the current findings, as the observed bidirectional cortical thickness/remission clusters would necessitate a high degree of regional specificity in inflammation, or inflammation only affecting a subset of patients with a separate mechanism driving cortical thinning in other patients.
WMH burden and hippocampal volume were expected to be linked with worse antidepressant treatment outcome, as these relationships have been reported in previous trials with non-amnestic MCI patients(Gunning-Dixon et al., 2010; Sheline et al., 2012; Sneed et al., 2011). Contrary to expectation, neither of these factors predicted treatment outcome. However, it is premature to conclude that there is no involvement of the hippocampus in governing treatment effectiveness in aMCI samples, given that there was some indication of a trend-level association between hippocampal volume and remission, and that this sample was selected on the basis of a mild amnestic deficit, thereby restricting the range of potential hippocampal volumes to individuals who may have already experienced some atrophy. This, in turn, may have limited its predictive utility. Regardless, the present findings suggest that these anatomical and clinical features may behave differently in aMCI and naMCI samples, and that cortical thickness is a better measure for remission prediction in aMCI samples. Cognitive deficits in other domains, such as executive functioning and processing speed, have been linked with poor antidepressant outcomes(Sheline et al., 2012). ApoE genotype was unrelated to both thickness and treatment outcome, though accelerated thinning has been previously reported in healthy carriers of ApoE ε4(Espeseth et al., 2008).
This study’s limitations are balanced by its unique strengths. MRI was collected at baseline only, preventing any inference on the longitudinal impact of antidepressants on regional thicknesses/volume. The sample size limits the ability to detect small effects. However, the use of broad inclusion/exclusion criteria enhances generalizability to clinical settings. Additionally, it is possible that some participants’ performance on memory tests during enrollment fell 1.0 SD below the mean without this representing a true decline from their premorbid abilities. Several methodological features of this study improve upon previous reports in crucial ways. The present analysis is vertex-based instead of ROI based, allowing for detection of intraregional clusters. We also adjusted for multiple comparisons. Conservative extent-level thresholding also limited the probability of type I errors.
In summary, the current study represents an assessment of predictors of acute antidepressant outcome. Diffuse clusters of cortical thickness predicted treatment outcome, though bidirectionality and limited size of effect limit direct clinical application. DWMH and hippocampal volume were not as useful in predicting treatment outcome as compared to their application in naMCI populations. Taken together, individuals with DEP-CI exhibit unique patterns of structural abnormalities compared to their depressed peers without CI that have consequences for their recovery with antidepressant treatment and may affect their risk of progression when clinical manifestations of neurodegenerative diseases develop.
Supplementary Material
Supplementary Table 1. Week 8 treatment outcome crosstabs
Supplementary Table 2. Baseline demographic and clinical characteristics for week 16 outcomes
Supplementary Table 3. Week 16 treatment outcome crosstabs
Supplementary Table 4. Antidepressant assignment by demographics and treatment outcome
Highlights:
Patients with depression and cognitive impairment received antidepressants for 16 weeks.
Clusters of cortical thickness predicted treatment remission and rate of remission.
Middle frontal gyrus thickness was associated with positive treatment outcome.
Hippocampus and MRI cerebrovascular disease were unrelated to treatment outcome.
Depression with cognitive impairment manifests with unique prognostic indicators.
Role of Funding Source
This work was supported by the National Institute of Aging [grant number R01AG040093-01] and the National Institute of Mental Health [grant number 2T32MH020004-21]. Funding sources had no role in the writing of this report or decision to submit the article for publication.
Conflicts of Interest Statement
JNM: Research support from NIMH. SL: Research support from NIMH, NIA, NICHD, and DOD. PMD: PMD has received grants from and/or served as an advisor to several health and technology companies for other projects. PMD is a shareholder and/or board member in health and technology companies whose products are not discussed here. PMD is a co-inventor on unrelated patents through Duke University. GHP: Research and travel support to meetings from NIMH, NIA, DOD, and Avanir Pharmaceuticals. JRP: Research support from NIA. DPD: Consultant to Eisai, Genentech, Axovant, Astellas, Acadia. Research support: NIA, DOD, Avanir.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, Becker JT, Reynolds CF 3rd, Carter CS, 2009. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 17, 30–42. 10.1097/JGP.0b013e31817b60af [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, 2005. Depression in the elderly. Lancet 365, 1961–70. 10.1016/S0140-6736(05)66665-2 [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM, 2012. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders 139, 56–65. 10.1016/j.jad.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, 2001. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Research Bulletin 56, 413–424. 10.1016/S0361-9230(01)00614-1 [DOI] [PubMed] [Google Scholar]
- Chung JK, Plitman E, Nakajima S, Chakravarty MM, Caravaggio F, Takeuchi H, Gerretsen P, Iwata Y, Patel R, Mulsant BH, Graff-Guerrero A, 2016. Depressive Symptoms and Small Hippocampal Volume Accelerate the Progression to Dementia from Mild Cognitive Impairment. Journal of Alzheimer’s disease : JAD 49, 743–54. 10.3233/jad-150679 [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9, 179–94. 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pelton GH, D’Antonio K, Ciarleglio A, Scodes J, Andrews H, Lunsford J, Beyer JL, Petrella JR, Sneed J, Ciovacco M, Doraiswamy PM, 2018. Donepezil Treatment in Patients With Depression and Cognitive Impairment on Stable Antidepressant Treatment: A Randomized Controlled Trial. Am J Geriatr Psychiatry 26, 1050–1060. 10.1016/j.jagp.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ, 2007. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology 68, 828–36. 10.1212/01.wnl.0000256697.20968.d7 [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biological psychiatry 67, 446–57. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Eliassen CF, Reinvang I, Selnes P, Fladby T, Hessen E, 2017. Convergent Results from Neuropsychology and from Neuroimaging in Patients with Mild Cognitive Impairment. Dementia and geriatric cognitive disorders 43, 144–154. 10.1159/000455832 [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I, 2008. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiology of aging 29, 329–40. 10.1016/j.neurobiolaging.2006.10.030 [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI, 2009. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. Journal of affective disorders 112, 206–11. 10.1016/j.jad.2008.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM, 1999. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage 9, 195–207. 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, Brickman AM, Hoptman MJ, Young RC, Alexopoulos GS, 2010. MRI signal hyperintensities and treatment remission of geriatric depression. Journal of affective disorders 126, 395–401. 10.1016/j.jad.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao N, Feng Y, Zhang HH, 2018. Model Selection for High-Dimensional Quadratic Regression via Regularization. Journal of the American Statistical Association 113, 615–625. 10.1080/01621459.2016.1264956 [DOI] [Google Scholar]
- Jarnum H, Eskildsen SF, Steffensen EG, Lundbye-Christensen S, Simonsen CW, Thomsen IS, Frund ET, Theberge J, Larsson EM, 2011. Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta psychiatrica Scandinavica 124, 435–46. 10.1111/j.1600-0447.2011.01766.x [DOI] [PubMed] [Google Scholar]
- Julkunen V, Niskanen E, Koikkalainen J, Herukka SK, Pihlajamaki M, Hallikainen M, Kivipelto M, Muehlboeck S, Evans AC, Vanninen R, Hilkka S, 2010. Differences in cortical thickness in healthy controls, subjects with mild cognitive impairment, and Alzheimer’s disease patients: a longitudinal study. Journal of Alzheimer’s disease : JAD 21, 1141–51. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK, 2007. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biological psychiatry 62, 1272–80. 10.1016/j.biopsych.2007.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang J, Gui L, Zheng J, Liu C, Du H, 2011. Alterations of whole-brain cortical area and thickness in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD 27, 281–90. 10.3233/JAD-2011-110497 [DOI] [PubMed] [Google Scholar]
- Lim HK, Jung WS, Ahn KJ, Won WY, Hahn C, Lee SY, Kim I, Lee CU, 2012. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37, 838–49. 10.1038/npp.2011.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kakeda S, Watanabe K, Yoshimura R, Abe O, Ide S, Hayashi K, Katsuki A, Umeno-Nakano W, Watanabe R, Ueda I, Moriya J, Nakamura J, Korogi Y, 2015. Relationship between the cortical thickness and serum cortisol levels in drug-naive, first-episode patients with major depressive disorder: a surface-based morphometric study. Depress Anxiety 32, 702–708. 10.1002/da.22401 [DOI] [PubMed] [Google Scholar]
- Mackin RS, Tosun D, Mueller SG, Lee JY, Insel P, Schuff N, Truran-Sacrey D, Arean P, Nelson JC, Weiner MW, 2013. Patterns of reduced cortical thickness in late-life depression and relationship to psychotherapeutic response. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 21, 794–802. 10.1016/j.jagp.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V, 2003. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the stroop task. Brain research. Cognitive brain research 17, 212–22. [DOI] [PubMed] [Google Scholar]
- Mortamais M, Artero S, Ritchie K, 2013. Cerebral white matter hyperintensities in the prediction of cognitive decline and incident dementia. International Review of Psychiatry (Abingdon, England) 25, 686–698. 10.3109/09540261.2013.838151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourao RJ, Mansur G, Malloy-Diniz LF, Castro Costa E, Diniz BS, 2016. Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. International journal of geriatric psychiatry 31, 905–11. 10.1002/gps.4406 [DOI] [PubMed] [Google Scholar]
- Pelton GH, Andrews H, Roose SP, Marcus SM, D’Antonio K, Husn H, Petrella JR, Zannas AS, Doraiswamy PM, Devanand DP, 2014. Donepezil treatment of older adults with cognitive impairment and depression (DOTCODE study): clinical rationale and design. Contemporary clinical trials 37, 200–8. 10.1016/j.cct.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Bartlett E, DeLorenzo C, Weissman M, McGrath P, Ogden T, Jin T, Adams P, Trivedi M, Kurian B, Oquendo M, McInnis M, Weyandt S, Fava M, Cooper C, Malchow A, Parsey R, 2017. Cortical thickness is not associated with current depression in a clinical treatment study. Human brain mapping 38, 4370–4385. 10.1002/hbm.23664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters F, Villeneuve S, Belleville S, 2014. Predicting progression to dementia in elderly subjects with mild cognitive impairment using both cognitive and neuroimaging predictors. Journal of Alzheimer’s disease : JAD 38, 307–18. 10.3233/JAD-130842 [DOI] [PubMed] [Google Scholar]
- Petersen RC, 2004. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine 256, 183–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P, 2015. A Prospective, Longitudinal Study of the Effect of Remission on Cortical Thickness and Hippocampal Volume in Patients with Treatment-Resistant Depression. The international journal of neuropsychopharmacology 18. 10.1093/ijnp/pyv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimontel MA, Reinlieb ME, Johnert LC, Garcon E, Sneed JR, Roose SP, 2013. The external validity of MRI-defined vascular depression. International journal of geriatric psychiatry 28, 1189–96. 10.1002/gps.3943 [DOI] [PubMed] [Google Scholar]
- Ribeiz SRI, Duran F, Oliveira MC, Bezerra D, Castro CC, Steffens DC, Busatto Filho G, Bottino CMC, 2013. Structural Brain Changes as Biomarkers and Outcome Predictors in Patients with Late-Life Depression: A Cross-Sectional and Prospective Study. PLoS ONE 8, e80049. 10.1371/journal.pone.0080049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M, 2000. Stereotaxic display of brain lesions. Behavioural neurology 12, 191–200. [DOI] [PubMed] [Google Scholar]
- Sacuiu S, Insel PS, Mueller S, Tosun D, Mattsson N, Jack CR Jr., DeCarli C, Petersen R, Aisen PS, Weiner MW, Mackin RS, Alzheimer’s Disease Neuroimaging I, 2016. Chronic Depressive Symptomatology in Mild Cognitive Impairment Is Associated with Frontal Atrophy Rate which Hastens Conversion to Alzheimer Dementia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 24, 126–35. 10.1016/j.jagp.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Disabato BM, Hranilovich J, Morris C, D’Angelo G, Pieper C, Toffanin T, Taylor WD, MacFall JR, Wilkins C, Barch DM, Welsh-Bohmer KA, Steffens DC, Krishnan RR, Doraiswamy PM, 2012. Treatment course with antidepressant therapy in late-life depression. The American journal of psychiatry 169, 1185–93. 10.1176/appi.ajp.2012.12010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP, 2010. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 18, 128–35. 10.1097/JGP.0b013e3181c796d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Culang-Reinlieb ME, Brickman AM, Gunning-Dixon FM, Johnert L, Garcon E, Roose SP, 2011. MRI signal hyperintensities and failure to remit following antidepressant treatment. Journal of affective disorders 135, 315–20. 10.1016/j.jad.2011.06.052 [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, McLaren ME, Kirton JW, O’Shea A, Woods AJ, Manini TM, Anton SD, Dotson VM, 2016. Depressive symptom severity is associated with increased cortical thickness in older adults. International journal of geriatric psychiatry 31, 325–33. 10.1002/gps.4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS, 2013. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Molecular psychiatry 18, 963–74. 10.1038/mp.2013.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD, 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–9. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Gongvatana A, Cohen RA, Ott BR, Tremont G, Alzheimer’s Disease Neuroimaging I, 2013. Are apathy and depression independently associated with longitudinal trajectories of cortical atrophy in mild cognitive impairment? The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 21, 1098–106. 10.1016/j.jagp.2013.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Week 8 treatment outcome crosstabs
Supplementary Table 2. Baseline demographic and clinical characteristics for week 16 outcomes
Supplementary Table 3. Week 16 treatment outcome crosstabs
Supplementary Table 4. Antidepressant assignment by demographics and treatment outcome
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


