Abstract
Background:
Microglia play a central role in neuroinflammation in various CNS diseases.Neonatal microglial culture has been extensively used to in vitro study microglial activation; however, as many neuroinflammatory diseases occur in the elderly, the neonatal microglial culture may not fully replicate the aged microglial activity seen in these diseases.
New method:
Primary microglia from both 18–24-month-old and P0-P4 C57BL/6 mice were cultured simultaneously. Morphology and activation profiles of the two age groups of microglia were examined following ischemic stimulation, by ELISA, RT-PCR, live microscopy, immunocytochemistry, and Western blotting.
Results:
We showed that aged microglia had larger cell bodies, more cytoplasmic inclusions, and enhanced phagocytosis than neonatal microglia. Cytokine production in these cells exhibited heterogeneity either after or before ischemic stimulation. The baseline expression of microglial marker CD11b was significantly higher in aged vs. neonatal cells; ischemic stimulation increased the expression in neonatal vs. aged microglia only in males but not in females.
Comparison with existing methods:
Previous primary microglia cultures have been limited to using neonatal/adult cells. This method is complementary to exiting methods and works for aged microglia, and does not suffer from potential limitations due to filtering artifacts. The protocol renders microglial culture no need for meningeal/hippocampal removal prior to brain tissue dissociation, and compares microglia between males vs. females, and between the aged vs. neonates.
Conclusions:
We concluded that neonatal microglial culture is not appropriate for those in vitro studies that mimic the neuroinflammatory central nervous system disorders occurring in the elderly, in which case the aged microglial culture should be applied, and sex differences should be considered.
Keywords: Age, Cytokines, inflammation, Ischemia, Phagocytosis
1. INTRODUCTION
There are increasing research interests in how age influences microglia due to the importance of the cell in development, phagocytosis, plasticity, homeostasis, sex differentiation, and immune surveillance (Fenn, Henry, Huang, Dugan, & Godbout, 2012; Giulian, 1999; Gordon et al., 2011; Lenz & McCarthy, 2015; Nguyen, Julien, & Rivest, 2002; Stansley, Post, & Hensley, 2012). In vitro models using embryonic (Gingras, Gagnon, Minotti, Durham, & Berthod, 2007) and neonatal (neo) (A. Al Mamun et al., 2020; Chen, Oyarzabal, & Hong, 2013; Lai, Dibal, Armitage, Winship, & Todd, 2013; Moussaud & Draheim, 2010; Njie et al., 2012; Rustenhoven et al., 2016) primary microglial cultures have been employed to study microglial pathology in age-related neurodegenerative or neuroinflammatory central nervous system (CNS) diseases. However, embryonic or neo primary microglia behave differently than the aged microglia (Njie et al., 2012; Sierra, Gottfried-Blackmore, McEwen, & Bulloch, 2007; Wai et al., 2002; Z. Wu, Tokuda, Zhang, & Nakanishi, 2008) especially when modeled in vitro to mimic age related CNS disorders, resulting in incomplete and confusing data (Helena W. Morrison & Filosa, 2016). Compared to neo cells, microglia from aged mice present increased neuroinflammatory profiles, neurodegenerative dysfunction (Gao & Hong, 2008; D. M. Norden & J. P. Godbout, 2013; Tansey et al., 2008), and loss of beneficial microglial effects from anti-inflammatory cytokines on neurons (Nolan et al., 2005; Streit & Xue, 2009; Wynne, Henry, Huang, Cleland, & Godbout, 2010). Therefore, there is a need for age-matched primary microglia cultures for translational research. Nevertheless, it has been a challenge to use adult or aged primary microglia for culture due to some technical difficulties to keep cells alive and sufficient. Microglia prepared from adult animals have been reported (Cardona, Huang, Sasse, & Ransohoff, 2006; De Groot et al., 2000; De Haas, Boddeke, Brouwer, & Biber, 2007; Ford, Goodsall, Hickey, & Sedgwick, 1995; Njie et al., 2012; Sedgwick et al., 1991; Yip, Kaan, Fenesan, & Malcangio, 2009), but these methods are limited to the direct use of the collected cells for RNA or protein extraction (Cardona et al., 2006) or for biological marker examination by flow cytometry (Ford et al., 1995). Up to date, the characteristics of aged microglia culture have not been well studied.
A better understanding of microglial function in aged animals in vitro could identify previously unknown molecular mechanisms that underlie age-related neurological disorders. With this goal, we here introduce a method for isolation, culture, and characterization of microglia from 18–24-month-old mice. By comparison with neo microglia from P0-P4 mice, we explored age and sex differences in cell physiology/morphology, microglial phenotype variations, and inflammatory characteristics.
2. MATERIALS AND METHODS
2.1. Microglia dissociation, purification, and culture
Primary microglia were obtained from aged (18–24-month-old) and neonatal (neo, postnatal 0–4 days) C57BL/6 mice that were kept in barrier-reared conditions in a pathogen-free facility. All mice used in the study were in accordance with the ethical standards of the University of Texas Health (UTHealth) Science Center at Houston. All work was performed under aseptic conditions.
Figure 1 shows workflow for the culture of microglia. First, brains were excised from skulls, washed, minced, and then incubated in C-tubes containing cell dissociation buffer, and further loaded on a gentleMACS Octo Dissociator with Heaters (cat # 130092628; Miltenyi Biotech, Auburn, CA, USA) to run for 30 min at 37°C (Fig. 1.1, steps a-d). These steps help convert a brain to a cell-and-debris suspension. Next, myelin, debris and dead cells were removed from single live cells by discontinuous gradient density centrifugation and suction filtration (Fig. 1.2, steps e-g). Live cells were in the bottom part of the tube (L in Fig.1.2-g); meanwhile the middle (M) and upper (U) parts (Fig.1.2-g) contained dead cells, myelin and debris. Cells from aged brain tissues formed a pellet, but cells from neo brains formed a clog, due to the age difference in the brain tissue density. Both aged and neo brain cells were re-suspended in DPBS buffer by gentle trituration (10–15x), after removal of the debris and dead cells. Red blood cells (RBCs) were lysed after the debris removal step and cells collected by centrifugation and suction filtration. Live brain cells were then plated on poly-D-lysine (PDL) coated cell culture flasks (Fig. 1.2-h). Mature microglia cells were harvested as of division day 12 (DIV 12) by gently shaking of culture flask, then plated/seeded on suitable vessels for downstream experiments. On a microglia harvest day (every 7 days), flasks were shaken gently at 250 RPM for 3 h, and at 37°C. Floating cells from flasks were pooled together, washed with basic culture media, counted with a hemocytometer, and plated in culture vessels containing DMEM_cat # 11965118 (for regular assays) or cat # 31053036 (for sex differences). These media were supplemented with 10% FBS_cat # FBL02–500ML (for regular assays) or cat # A3382101 (for sex difference), 1% penicillin/streptomycin (P/S), and 30% L929 conditioned medium. As phenol red is an estrogen receptor agonist (Berthois, Katzenellenbogen, & Katzenellenbogen, 1986) and FBS contains sex steroid hormones (Milo, Malarkey, Powell, Blakeslee, & Yohn, 1976), we used phenol red-containing medium and FBS for regular assays (no sex difference studies). For sex difference assays, phenol red-free media and charcoal-stripped FBS was used to minimize the effects of these factors on the sex specific characteristics of primary microglia. For downstream experiments, we plated 50,000 cells per well of 96-well plate for microplate reader experiments, 1 × 105 cells per well of 24-well plate or 8-chambered cell culture slide for ICC or morphology examination, 2–4 × 105 cells per well of 24-well plate for ELISA or PCR experiments, and 1.5 × 106 cells per well of 6-well plate for Western blotting. Of note, we isolated astrocytes/microglia from whole brain homogenate, and did not free meninges from hemispheres prior to astrocytes/microglia isolation. The meninges in aged mice are meshed all over the brain parenchyma and not easily removed due to the aging when compared to the neonates. Our method also incorporates steps that can get rid of red blood cells (using red blood cell lysis buffer) and debris removal (using cell debris removal buffer), thereby increasing the purity and yield of microglial culture. In addition, at the harvesting step, we shook off microglia at 250 RPM instead of traditional 150 RPM due to the strong binding of aged microglia with the astrocytic feeder layer. As a result, we harvested microglia once a week instead of twice a week, as the 250 RPM disturbed the astrocytic feeder layer which needs time to be restored to maintain a high cell yield.
Figure 1:
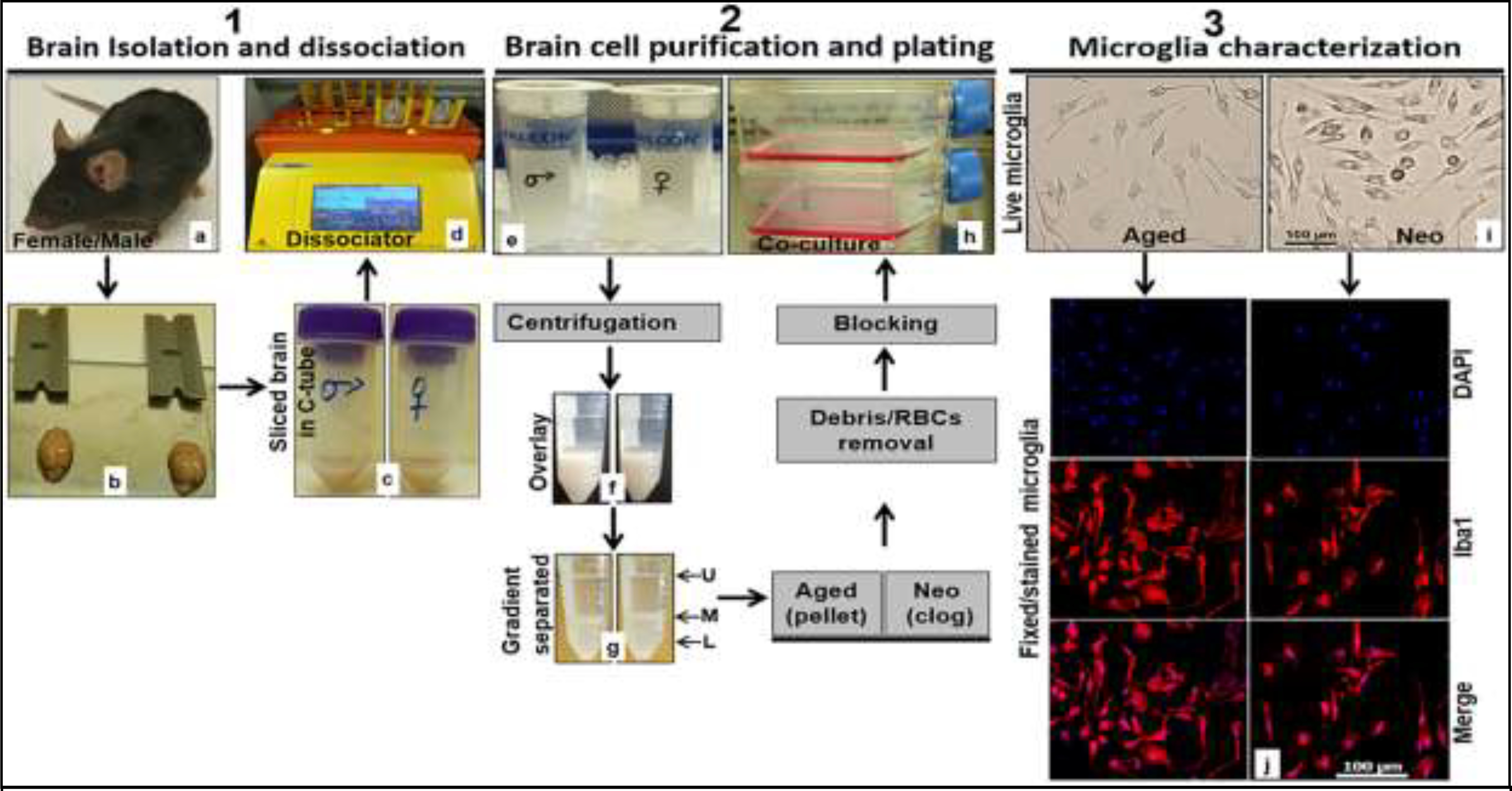
Workflow chart to isolate, culture and characterize microglia from aged and neonatal C57BL/6 mice. 1.1. (a) C57BL/6 mouse, (b) brain isolated from mouse skull ready for physical slicing, (c) sliced brain in C-tube containing neural tissue dissociation buffer, (d) gentleMACS Octo Dissociator with Heaters loaded with 2 C-tubes containing sliced brains and in neural tissue dissociation buffer. 1.2. (e) Straining of dissociated cells into single cells using a cell strainer, (f) gradient density overlaying of dissociated brain tissue, (g) phase separated cell suspension (L lower phase, M middle phase, and U upper phase) after centrifugation, (h) seeding of brain cells in growth medium after debris and red blood cell removal steps. 1.3. (i) Representative live microglial cells taken in EVOS FL Auto 2 light microscope at 20x, (j) representative formaldehyde fixed and dye-stained microglia taken in Leica DMi8 Confocal Microscope at 20x (DAPI blue, Iba1 Red). RBCs, red blood cells.
2.2. Oxygen-glucose deprivation and LPS stimulation
Microglia were seeded onto 6-well culture plates at a density of 0.6 ×106/well and rested until cells become approximately 80–90% confluence. Oxygen-glucose-deprivation (OGD) was performed as previously reported (Ngwa, Mamun, Xu, Sharmeen, & Liu, 2021; Tasca, Dal-Cim, & Cimarosti, 2015). Briefly the culture medium was replaced with glucose-free DMEM_A1443001 (Thermo Fisher Scientific), and then the plates were put in a sealed chamber, followed by expiring oxygen for 10 min via flowing in 95% N2 and 5% CO2 mixture (Airgas-Southwest Inc.) persistently at a low flow. The chamber was transferred into a 37°C incubator after clamping the inlet and outlet, for 4 h to mimic OGD. In the course of OGD, O2 levels dropped to <2% after 2 h, and <1% at 4 h, as shown by a change in color of BD_271051 anaerobic indicator strips from blue (aerobic) to white (anaerobic) coloration. The medium was changed to normal feeding DMEM_31053036 (ThermoFisher Scientific) and cells restored to a normoxic atmosphere by incubation at 95% oxygen, 5% CO2 and at 37°C as reperfusion for 24 h. Microglia were further stimulated with lipopolysaccharide (LPS)_L2630 (Sigma-Aldrich) at 100 ng/mL during the re-perfusion step if applicable. All experiments were performed paralleled with normoxic controls and in replicates.
2.3. Inflammatory mediator measurement by ELISA or qRT-PCR
Levels of TNF-α, IL-6, IL-1β, and IL-10 in the culture medium were measured by Enzyme-linked immunosorbent (ELISA) using mouse ELISA MAX Deluxe or LEGEND MAX (TNF-α_430904, IL-6_431304, IL-1β_432604, IL-4_ 431104, IL-10_431414 (BioLegend), according to the manufacturer’s instructions. Signals were read at 450 nm in EnSpire™ Multimode Plate Reader (PerkinElmer, Inc.) equipped with version 4.13.3005.1482 software. iNOS, CD68, Ym1/2, and CD206 were examined in cell lysates for mRNA levels as they were not detectable in the medium by ELISA. First, total RNA was extracted from OGD+LPS treated cells using RNeasy Mini Kit_74104 (QIAGEN) according to the manufacturer’s protocol. The total RNA eluted from columns was quantified using NanoDrop (ThermoFisher Scientific) and 0.5 −1 μg used for RT-PCR on a C1000 Touch Thermal Cycler. To convert RNA to cDNA we used iScript™ Reverse Transcription Supermix_1708841. C1000 Touch (Thermal Cycler) CFX96 Real-Time System (Bio-Rad) was used and real-time (q) PCR performed with the SsoAdvanced Universal SYBR Green Supermix_1725274 (Bio-Rad) to determine the mRNA levels. The following gene primers from Integrated DNA Technologies (IDT) were used for q-PCR: iNOS F_CAAGCACCTTGGAAGAGGAG, iNOS R_AAGGCCAAACACAGCATACC; CD68 F_ACTTCGGGCCATGTTTCTCT, CD68 R_GCTGGTAGGTTGATTGTCGT; Ym-1/2 F_CAGGGTAATGAGTGGGTTGG, Ym1/2 R_CACGGCACCTCCTAAATTGT; CD206 F_CAA GGA AGG TTG GCA TTT GT, CD206 R_CCT TTC AGT CCT TTG CAA GC; and the housekeeping gene GAPDH F_GTGTTCCTACCCCCAATGTGT, GAPDH R_ ATTGTCATACCAGGAAATGAGCTT. The results were reported as normalized fold changes in mRNA, which were calculated via the ΔΔCt method using the threshold cycle (Ct) value for respective gene of interest and for the housekeeping gene (GAPDH).
2.4. Immunocytochemistry and BrdU assay for microglia proliferation
Immunocytochemistry (ICC) was performed as previously described (Abdullah Al Mamun et al., 2020; Ngwa et al., 2021). Microglia proliferation was assayed by bromodeoxyuridine (BrdU) staining of treated microglia with BrdU (1 mM) vs. controls (vehicle) for 24 h. We used FITC BrdU Flow Kit_cat # 559619 (BD Pharmingen) according to Mayle et al.,(Mayle, Luo, Jeong, & Goodell, 2013) with modification. Briefly, 100,000 primary microglial cells were rested in 8-well chamber slides for 48 h, in a Steri-Cycle CO2 incubator at 95% humidity, 5% CO2 and at 37°C. The complete cell culture medium was DMEM_cat # 11965118 supplemented with 10% FBS, 1% P/S, and 30% L929 conditioned medium. To incorporate BrdU into proliferating cells’ DNA, cells were treated with 1 mM BrdU prepared in DMEM_cat # 11965118 without supplements for 24 h, and then washed with 1xPBS. Cells were fixed with 4% paraformaldehyde (PFA) for 15 min and then washed with PBS. After permeabilizing cells with 0.3% triton X-100 in TBST (0.05%) for 10 mins, blocking buffer (3% BSA in TBST (0.05%)) was applied for 1 h and then cells incubated with anti Iba1 primary antibody for 1 h at room temperature (RT) and overnight at 4°C, followed by a wash with 1xDPBS and incubation with BD Cytoperm permeabilization buffer plus for 10 min at RT, and then another wash with 1x BD Perm/wash buffer. DNase (300 μg/mL) was added to cells and incubated at 37°C for 1 h, to expose binding sites of incorporated BrdU. After a wash with 1x BD Perm/wash buffer, cells were stained with anti Iba-1, anti BrdU antibodies prepared in 1x BD Perm/Wash buffer, for 1 h and at RT. After another wash, cells were counterstained with DAPI for 10 min. The following primary antibodies were used at 1:200: anti Iba1 rabbit_01919741 (FUJIFILM Wako Chemical Corporation), anti CD11b rabbit_NB110–89474 (Novus Biologicals), anti TMEM119 mouse_66948–1, 1:200 (Proteintech). The dye-conjugated secondary antibodies were used at 1:400 including Donkey anti-rabbit Alexa Fluor 594_A-21207, and Donkey anti-mouse Alexa Fluor 488_A-32766 (Thermo Fisher scientific); with the exception of anti BrdU that was used in the ratio 1:100 according to vendor’s instruction.
2.5. Phagocytosis
Phagocytic activity of aged and neo microglial cells was determined by quantifying the uptake of FITC conjugated E. coli-derived (K-12 strain) bioparticles (A. M. Floden & Combs, 2006), with modifications. Briefly, microglia were stimulated with OGD plus E. coli (K-12 strain) BioParticles (BPs)_E2861 (Thermo Fisher scientific) for 4 h, in a concentration dependent manner, paralleling with normoxic controls. After OGD, cells were re-perfused for 24h. Next, cells were rinsed with PBS (x3) containing 0.001 mg/mL trypan blue to quench extracellular fluorescent BPs. Internalized FITC signals in live cells were quantified at 480 nm (excitation) and 520 nm (emission) wavelengths with EnSpireTM Multimode fluorescence Plate Reader (PerkinElmer, Inc.). For ICC, OGD treated cells were re-perfused and then washed with PBS (x3) and then fixed with PFA (4%) for 15 minutes. Anti Iba-1_01919741 at 1:200 (FUJIFILM Wako Chemical Corporation, Richmond, VA, USA) primary and Donkey anti-rabbit Alexa Fluor 594_A-21207 at 1:400 (Thermo Fisher Scientific) secondary antibodies, were used for co-localization of Iba-1 and FITC BPs. Images were captured in Leica DMi8 Confocal Microscope and BPs fluorescence intensities quantified by ImageJ.
2.6. Western blotting
Western blotting was performed on 4–15% Mini-PROTEAN™ TGX Gels_5671085, 4561086, 5671084, or 4561084 (BioRad, Hercules, CA) depending on the protein quantity (volume) and the number of assays. Briefly, 15 μg protein was loaded in each well of gel and ran for 1.5 h at 75–120 V. Proteins were transferred onto a GE Healthcare nitrocellulose membrane_45004003 at 150 mA, for 2 h and at 4°C. The nitrocellulose membrane was blocked with 5% blotting grade blocker (BGB)_ 1706404 (Bio-Rad) for 1 h and then incubated with the primary antibody for 1 h at RT, and overnight at 4°C. After the membrane was washed with 1xTBST (0.1%), the secondary antibody was added and incubated for 1 h at RT; after another wash the membrane was exposed to Pierce™ ECL western blotting substrate_32209 following the vendor’s procedure (ThermoFisher Scientific). The membrane was then imaged by autoradiography (Bio-Rad) and optical density signals quantified by ImageJ (NIH, Bethesda, MD, USA). Primary antibodies: Rabbit anti-CD11b_NB110–89474 1:500 (Novus Biologicals), Rabbit anti-β-actin_4970 1:1000 (Cell Signaling Technology); secondary antibody: peroxidase anti-Rabbit_PI-1000 1:4000 (VECTOR Laboratories).
2.7. Statistical analysis
Statistical data analysis was performed using Prism 8.0.2 (GraphPad) with p<0.05 considered statistically significant. Unless otherwise indicated, all data are presented as the mean ± standard error of the mean (SEM), and analyzed with Two-way ANOVA plus Sidak’s test with multiple comparisons. Two-tailed Student’s t-tests (unpaired) was also used for two-group data. All n and p values and statistical tests were indicated in figure legends.
3. RESULTS
3.1. Characterization of aged/neo microglia.
Fig. 1.1&.2 show the workflow of microglial isolation and culture (see Method 2.1). The cultured microglia were confirmed with EVOS FL Auto 2 light microscope_AMAFD2000 (Thermo Fisher Scientific) for live cells and with Leica DMi8 Confocal Microscope_S/N 409976 (Leica Microsystems CMS GmbH) for immunofluorescence staining on paraformaldehyde (PFA) fixed cells. Live aged and neo microglial exhibited typical spindled or ramified shapes in resting state (Patel, Ritzel, McCullough, & Liu, 2013) (Fig. 1.3-i). By Iba1 staining (Fig1.3-j), we confirmed that the purity of microglia was 99% for either aged or neo microglial culture.
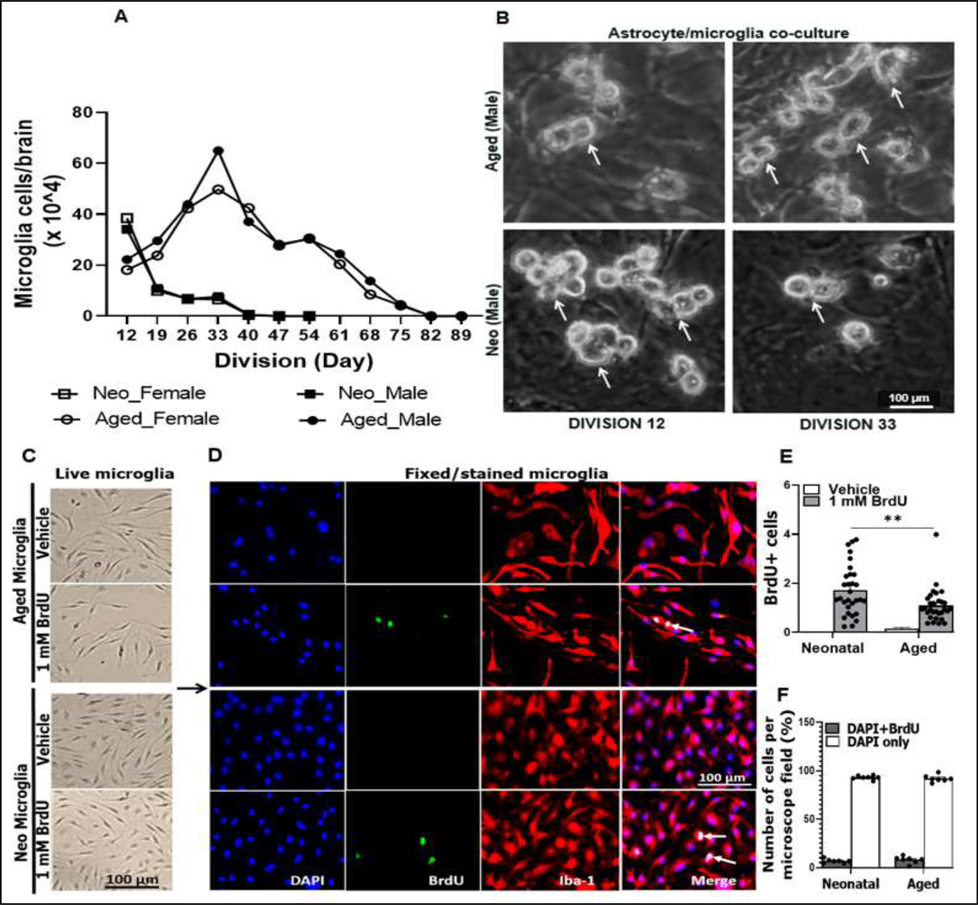
Figure 2.
Division, yield, and proliferation of microglia. (A) Division curve showing average microglial cells per brain/sex, counted by trypan blue exclusion. (B) Representative images of live aged and neonatal (neo) microglia grown on the astrocyte feeder layer. (C) Representative live microglia rested for 48 h following dissociation from astrocyte/microglia co-culture, and then were treated with 1 mM BrdU. (D) Representative fixed/stained microglia for BrdU and Iba1. (E) Quantified BrdU+ cells. (F) Percentage DAPI+BrdU+ (proliferating) vs. DAPI+ only (non-proliferating) cells. White arrows (Fig. 2B) point to mature microglia (ready to dissociate), and white arrows in Fig. 2D point to cells with nuclei incorporating BrdU (proliferating cells). N = 12 flasks each containing cells from 3 neo-brains, and n = 18 flasks each containing cells from 2 aged-brains (Fig. 2A&B). Quantified data for BrdU (Fig. 2E) are from 10 microscope fields and 3 independent experiments. Data of Fig. 2F are from 2–3 microscope fields and 3 independent experiments. **P = 0.0012, (2way ANOVA with Sidak’s multiple comparison’s test).
3.2. Division and proliferation of microglia
A challenging aspect in microglia culture has been the low yield, and it is not easy to maintain cultures for weeks (A. M. Floden & Combs, 2007). This challenge has made in vitro experiments that require high number of cells difficult, especially for aged microglia culture due to the cell senescence (Angelova & Brown, 2019; Diana M Norden & Jonathan P Godbout, 2013) and the decline in homeostatic functions that make cells susceptible to deterioration (Lourbopoulos, Ertürk, & Hellal, 2015). The protocol we developed for aged microglial culture can keep cells alive and productive for up to 89 days (Fig. 2A). The number of harvested aged microglia increased with days for cells to divide, and peaked at division day 33 (DIV 33) in both sexes (Fig. 2A, ascending curves). After DIV 33, the cell number started to decrease and became zero at DIV 82 to 89. We were able to obtain averagely 625,000 (male) and 500,000 (female) aged microglial cells per brain at the peak point (DIV 33). However, the number of cultured neo microglia peaked at an early time point (DIV 12) with averagely 380,000 (male) and 390,000 (female) cells per brain, and then decreased and became zero at DIV 40 (Fig. 2A, descending curves) in both sexes. Figure 2B shows representative bright fields of live male microglia taken with EVOS FL Auto 2 light microscope on DIVs 12 and 33, the peak time for neo and aged microglia numbers respectively. We also compared neonatal vs. aged microglia proliferation by treating the harvested cells with bromodeoxyuridine (BrdU). Figure 2C shows representative bright field images of treated live cells vs. controls. Immunofluorescence staining showed both neonatal and aged microglia had low proliferation potential (<10%) (Fig. 2D&F), although the neonatal microglia were more proliferative (Fig. 2E).
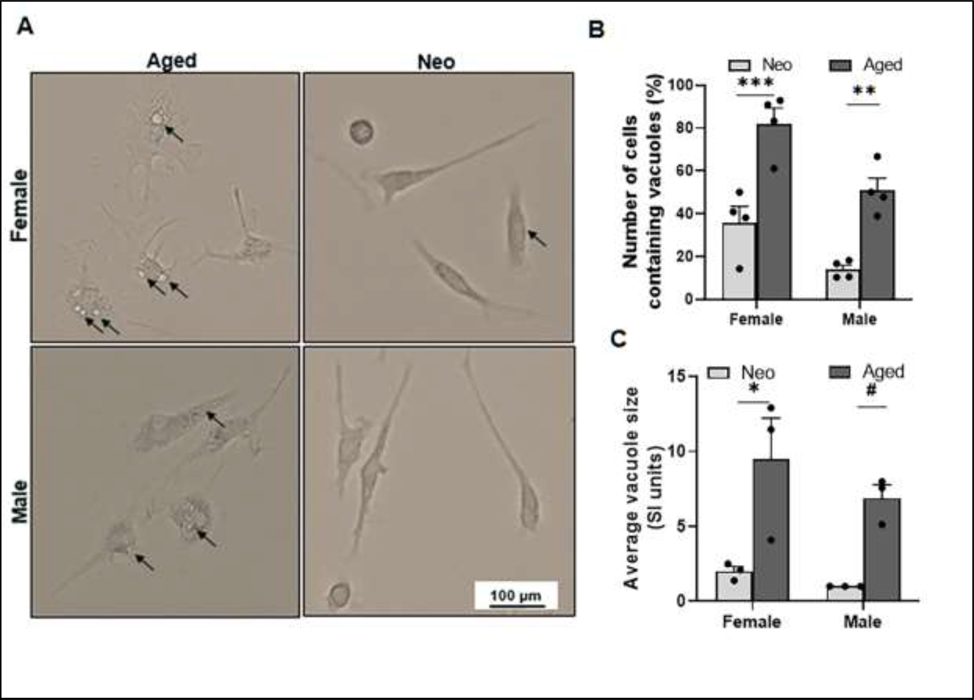
3.3. Aged microglia have more cytoplasmic inclusions than neo microglia.
Microglial morphology changes with age (Angela M Floden, Li, & Combs, 2005; Perry, Matyszak, & Fearn, 1993; Sierra et al., 2007); next we examined the morphology of cultured microglia. By the bright fields of live cells under the light microscope, we found aged microglia not only exhibited bigger cell bodies, but also had significantly more cells containing cytoplasmic inclusions (vacuoles) compared to neo microglia (Fig. 3A&B). The average vacuole size was also significantly larger in aged vs. neo cells of either sex (Fig. 3A&C). The vacuole formation usually indicates the activation of phagocytes (Petricevich, Reynaud, Cruz, & Possani, 2008; Shubin, Demidyuk, Komissarov, Rafieva, & Kostrov, 2016). Our data suggest that the aged microglia are primed to activation; whereas most neo microglia are in the resting state.
Figure 3.
Aged microglia exhibit bigger cell bodies and more/larger cytoplasmic inclusions than neo microglia. (A) Representative EVOS FL Auto 2 light microscope images at 20x showing vacuoles (inclusions) in the cell cytoplasm (dark arrows). (B) Percentage of vacuolated cell numbers in total neo or aged microglia. Cells per microscopic field were counted using ImageJ software. (C) Average vacuole size in microglia. N = 15–20 cells per microscopy field of independent experiments; *P = 0.0127, **P = 0.0021, ***P = 0.0003, #P = 0.0403 (2way ANOVA with Sidak’s multiple comparison’s test).
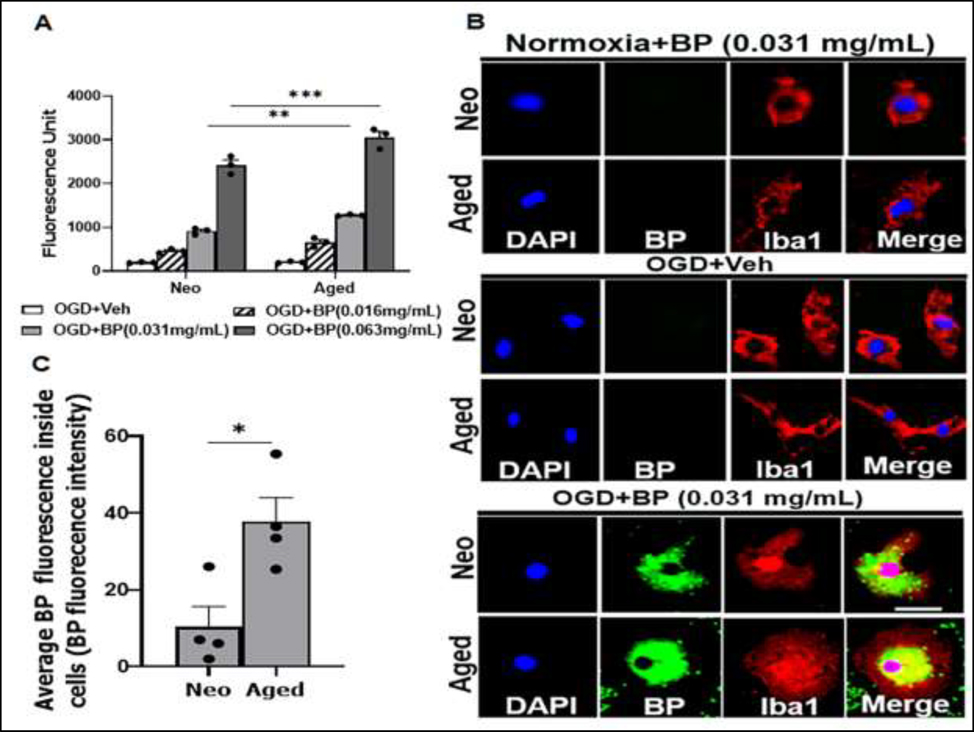
3.4. Microglia phagocytosis
Microglia are brain resident macrophages, and have the ability to phagocytose pathological debris after brain injury or infection including damaged cells or pathogens (Bolós et al., 2017; H. W. Morrison & Filosa, 2013; Mosley & Cuzner, 1996; Peters & Sethares, 2002). We examined this important homeostatic function in neo vs. aged microglia exposed to OGD and FITC labeled E-coli bioparticles (BPs). First, we performed dose gradient test by incubating cells with different concentrations of BPs (0.016, 0.031, and 0.063 mg/mL). With the phagocytosis assay, we found neo and aged microglia both phagocytosed BPs in a dosage-dependent manner by measuring the fluorescence of internalized BPs in these cells (Fig. 4A). Interestingly, at both doses 0.031 mg/ml and 0.063 mg/mL, aged microglia phagocytosed significantly more BPs than neo microglia. ICC was performed to confirm the age difference in phagocytosis using 0.031 mg/mL BPs. In normoxia+BP and OGD+BP groups, no BP signal was seen in Iba-1+ cells; however, co-localized Iba-1 and BP were very evident in OGD+BP group (Fig. 4B), and the quantification data showed that the uptake of BPs was significantly increased in aged vs. neo microglia (Fig. 4C).
Figure 4.
Phagocytic activity of neo and aged microglia. (A) Fluorescence of internalized BPs measured in EnSpire™ Multimode Plate Reader at 480 nm (excitation) and 520 nm (emission) wavelengths; n = 3 independent experiments assayed in duplicates. (B) Representative ICC images showing uptake of BPs in OGD+BP (0.031 mg/mL) treated microglia relative to untreated (OGD+Veh) and normoxia (normoxia+BP) cells. (C) Quantification data for confocal images in B (at 0.031 mg/mL treatment concentration); data were obtained from 4 cells per condition. **P = 0.0075, ***P < 0.0001 (2way ANOVA with Sidak’s multiple comparison’s test); *P = 0.0166 (unpaired two-tailed t test). Veh, vehicle (phosphate buffer saline); BP, FITC conjugated E. coli-derived (K-12 strain) bioparticles; DAPI (blue), Iba1 (red). Scale bar = 100 μm.
3.5. Release of inflammatory mediators after pathogenic stimulation.
Next, we explored whether age differences also exist in the production of inflammatory mediators after the cultured microglia were exposed to pathogenic stimulations. OGD is an in vitro ischemia model, and lipopolysaccharide (LPS) has been widely used to activate microglia in vitro (Abdullah Al Mamun et al., 2020; Lively & Schlichter, 2018; Ngwa et al., 2021; Pannell, Szulzewsky, Matyash, Wolf, & Kettenmann, 2014). We subjected microglia to OGD for 4 h, followed by LPS stimulation for 24 h, and then inflammatory mediator levels were detected in the culture media by ELISA or in cell homogenates by RT-PCR for mRNA levels if the ELISA could not detect an inflammatory mediator. We used OGD alone, and OGD+LPS combined stimulation because OGD alone cannot induce detectable inflammatory mediator levels in our previous study (Ngwa et al., 2021). Pro-inflammatory (TNF-α, IL-6, IL-1β, iNOS, CD68) and anti-inflammatory (IL-10, Ym1/2, CD206) mediators were examined. As shown in Figure 5, the protein levels of TNF-α/IL-10, and mRNA levels of iNOS/CD68/Ym1/2 were significantly different in aged vs. neo microglia after OGD+LPS treatment. For IL-6, aged microglia had a significant higher baseline level than the neonates, although OGD+LPS attenuated the difference (Fig. 5B). OGD alone stimulation did not induce upregulation of these inflammatory mediators compared to normoxia controls, consistent with our previous studies (Ngwa et al., 2021).
Figure 5.
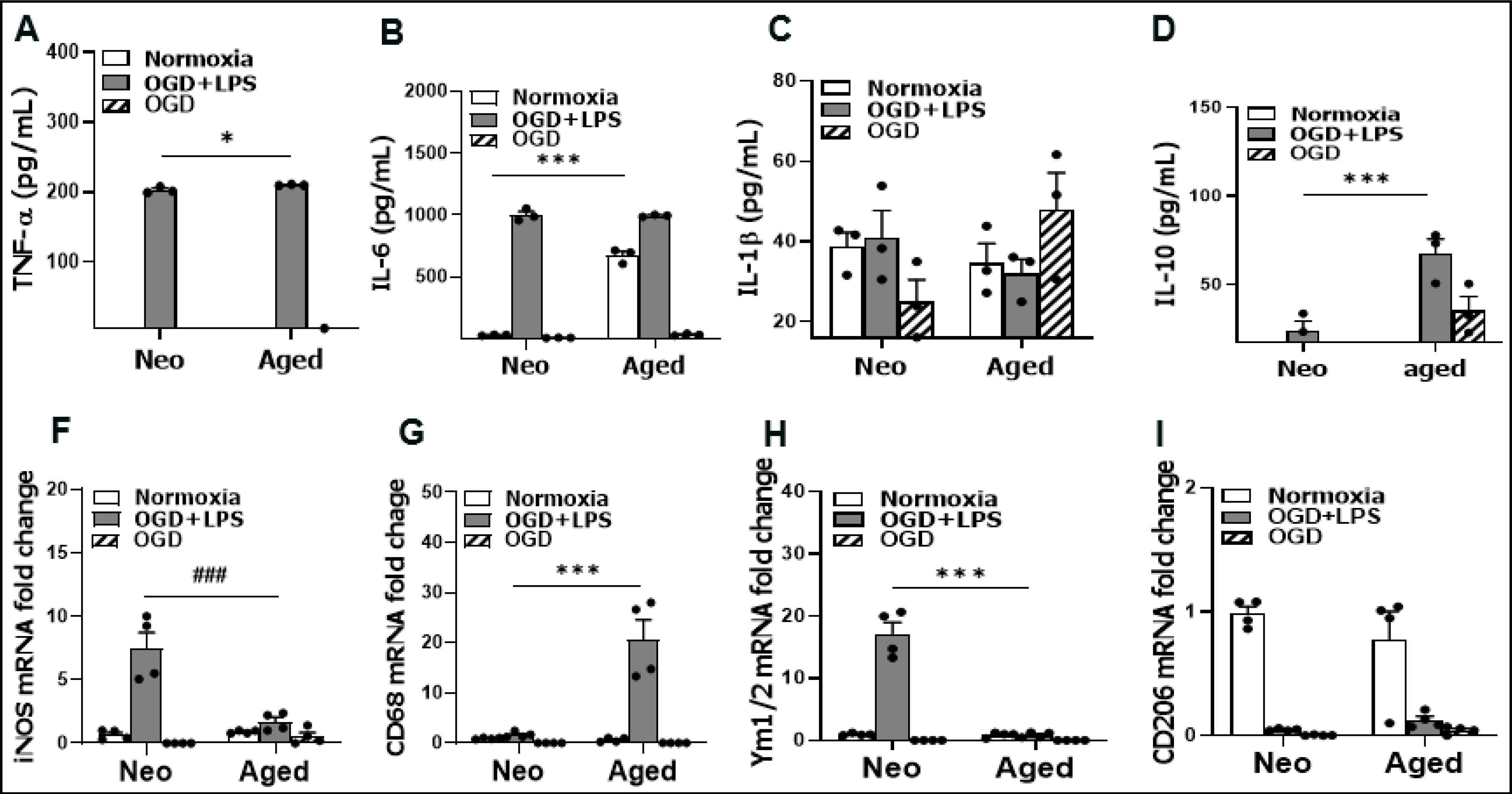
Levels of inflammatory mediators. (A-D) Protein levels of pro-inflammatory (TNF-α, IL-6, IL-1β) and anti-inflammatory (IL-10) cytokines in cell culture media by ELISA. (F-I) mRNA fold changes for pro-inflammatory (iNOS and CD68) and anti- inflammatory (Ym1/2 and CD206) mediators by RT-PCR. N = 3–4 independent experiments assayed in duplicates. *P = 0.0326, ***P < 0.0001, ###P = 0.0001 (2 way ANOVA with Sidak’s multiple comparison’s test).
3.6. Microglial cell surface marker expression
Microglia express CD11b and TMEM119 on cell membrane (Lee et al., 2014; Wake, Moorhouse, Jinno, Kohsaka, & Nabekura, 2009). We examined the expression of the two microglial markers with ICC and western blots. The immunofluorescence data showed aged male and female microglia express significantly higher levels of CD11b than their neo counterparts in normoxic condition (Fig. 6A&E; and 6B&F), an age-related difference that was not seen in TMEM119 expression. We further confirmed the age dependent increase in CD11b expression with Western blots in cell homogenates (Fig. 6G&H), and data showed the same pattern as in ICC. After OGD exposure, CD11b levels increased in neo microglia of both sexes but not in aged microglia (Fig. 6C&I; and 6D&J). In male neo microglia, the CD11b expression increased so much so that the level was significantly higher than male aged microglia, which was not seen in female microglia.
Figure 6.
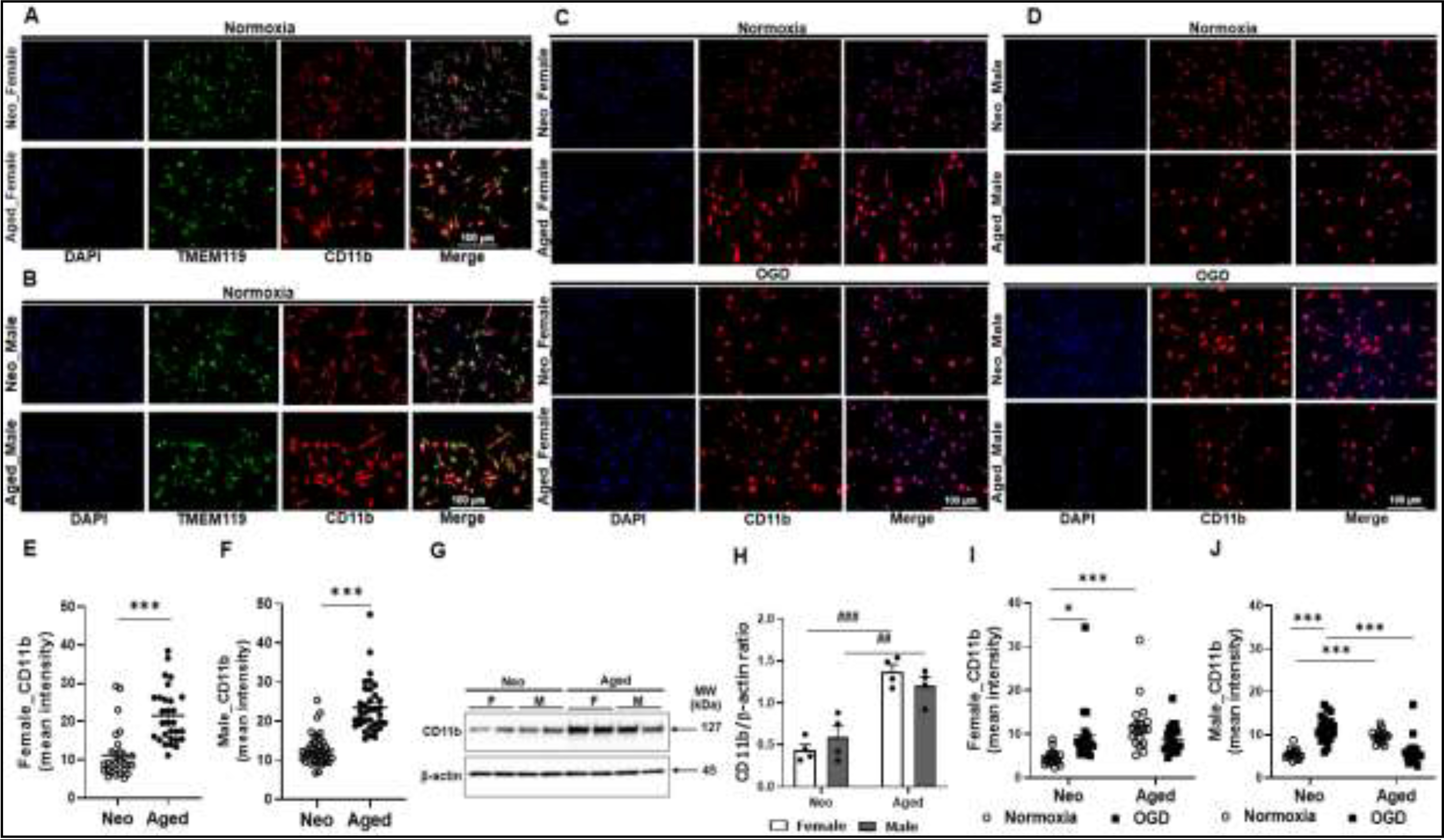
CD11b and TMEM119 expression in neo and aged microglia. (A, B) Normoxic female (A) and male (B) neo/aged microglia were stained for CD11b/TMEM119 expression. (C) Female neo/aged microglia exposed to OGD vs. normoxia were stained for CD11b. (D) Male neo/aged microglia exposed to OGD vs. normoxia were stained for CD11b. (E, F) ImageJ quatification of CD11b intensity in (A) and (B) respectively. (G) Representative Western blot for CD11b expression in normoxic neo/aged microglia. (H) Optical density ratio of CD11b over β-actin from Western blots in (G). (I, J) ImageJ quantification of CD11b intensity in (C) and (D) respectively. Western blot data were from four independent experiments conducted per sex/age. For ICC, 25–50 cells in a microscopy field were quantified using ImageJ for CD11b expression. ***P < 0.0001 (unpaired two-tailed t test); *P = 0.0101, ***P < 0.0001, ##P = 0.0058, ###P = 0.0001 (two-way ANOVA with Sidak’s multiple comparison’s test).
4. DISCUSSION
Microglia are responsible for the induction of innate immune responses by receiving and propagating inflammatory signals (Nguyen et al., 2002) and perform macrophage-like functions including phagocytosis, inflammatory damage and antigen presentation after activation (Garden & Möller, 2006). In this study we isolated and cultured neo and aged microglia from C57BL/6 mice, and explored inflammatory and homeostatic functions in these cells. We found aged microglia had distinct inflammatory characteristics compared to neo microglia, which underlines the importance of the age selection of microglia when neuroscientists conduct cell cultures. Aged microglia culture not only show a delayed cell number peak time, but also a longer lifetime compared to neo microglia culture (Fig. 2A). Aged microglia are primed to activation and once activated, have a more robust phagocytosis function than their neo counterparts (Fig. 3&4). Inflammatory profiles defined by inflammatory mediators or cell membrane markers are very different between aged and neo microglia, exhibiting age-related heterogeneity either before or after cell activation (Fig. 5&6).
Microglia are usually co-cultured with astrocytes before they are separated from the astrocytic feeder layer (Ngwa et al., 2021; Santambrogio et al., 2001; Zhang et al., 2020). In our study we found the astrocytic feeder layer for aged glia co-culture develop and mature later and last longer than the neo glia culture, which has not been reported before in literature. Microscopic observation revealed that the neo astrocyte feeder layer developed as early as DIV 5 and matured at DIV 12 (~80% confluence), which is different from the aged glia co-culture showing ~85% confluence of the astrocyte layer at DIV 33 (Fig. 2A, B). Astrocytes promote microglia developmental processes (Erblich, Zhu, Etgen, Dobrenis, & Pollard, 2011; Santambrogio et al., 2001; Zhang et al., 2020) and were reported to enhance purinergic signaling in microglia by enhancing activation of P2Y6 and P2Y12 receptors to promote phagocytosis and processes extension in rats (Koizumi et al., 2007). Bohlen et al.(Bohlen et al., 2017) reported that astrocyte-derived factors such as CSF-1/IL-34 (Easley-Neal, Foreman, Sharma, Zarrin, & Weimer, 2019; Węgiel et al., 1998), TGF-β2 (Bureta et al., 2019; Pratt & McPherson, 1997), and cholesterol (Goshi, Morgan, Lein, & Seker, 2020), prevent microglial death ex vivo. These previous and our present data suggest that astrocyte feeder layer is responsible for the delayed cell number peak time and the longer lifetime in aged microglia culture. The reason for the difference in astrocyte feeder layer between aged vs. neo glia culture is not clear, and may be due to the intrinsic, age related tissue developmental difference. We observed a low proliferative potential for both neo and aged microglia after the cells were harvested and cultured alone without astrocytic feeder layer, which is consistent with previous studies (Woolf et al., 2021). This again highlighted the importance of the astrocytic feeder layer in microglial culture.
Cytoplasmic vacuoles have been frequently found in microglia that are phagocytic and activated (Ohmi et al., 2003). Our data showed aged microglia had more and larger vacuoles than their neo counterparts, suggesting aged microglia are primed to be activated, which is consistent with previous reports (Diana M Norden & Jonathan P Godbout, 2013; Norden, Muccigrosso, & Godbout, 2015). The presence of cytoplasmic inclusions (vacuoles) in microglia alter their morphology (Santambrogio et al., 2001; Wake et al., 2009; Zhang et al., 2020) and were predominant in major histocompatibility complex (MHC) II-positive microglia in aging rats (Erblich et al., 2011; Koizumi et al., 2007; Pannell et al., 2014). It has been proposed that the amplified microglial activation with age may be related to the decreased activities of several key regulatory signaling pathways, such as CD200-CD200R signaling (Frank et al., 2006; Lyons et al., 2007), fractalkine signaling (Bachstetter et al., 2011; Vukovic, Colditz, Blackmore, Ruitenberg, & Bartlett, 2012), and some anti-inflammatory cytokine activities (Nolan et al., 2005; Wynne et al., 2010), resulting in weakened microglial capability to resolve the inflammatory activation. A recent study has found aged mice brains develop cerebral microbleeds starting at 18 months old (Taylor et al., 2020), which may also stimulate aged microglia to activation. Consistent with the data of more and larger cytoplasmic vacuoles in aged microglia, our phagocytosis assay did show aged microglia had more robust phagocytic function than their neo counterparts after OGD stimulation (Fig. 4). However, the increased phagocytic capability with aging does not necessarily mean aged microglia can better digest pathogens or debris after tissue injury than younger microglia. Peters et al. (Peters, Josephson, & Vincent, 1991) found microglia of aged rhesus monkeys displayed heterogeneous intracellular inclusions indicative of increased phagocytosis but with reduced capacity to digest engulfed particles (Choudhury, Kigami, & Tanaka, 2021; Paresce, Chung, & Maxfield, 1997), due to a decline in Triggering Receptor Expressed on Myeloid cells-2 (TREM-2) with age in the cell (Hickman et al., 2013; Mecca, Giambanco, Donato, & Arcuri, 2018). Therefore, modulating the digestive function in aged microglia might be a potential cure for aging diseases including ischemia. Of note, our data showed that aged microglia had larger cell bodies than neo microglia (Fig. 3), and the dystrophic morphology may reflect the cell senescence of aged microglia. Dystrophy is a common phenomenon in aging microglia that undergo senescence (Bohlen et al., 2017). It is characterized by cell processes that appear stripped of fine ramifications, and show excessive beading and formation of spheroidal swellings, as well as fragmentation or tortuosity, which could be different from the morphological changes that occur during microglial activation as observed in rodents after acute CNS injury (Streit, Sammons, Kuhns, & Sparks, 2004). In human beings dystrophic morphology is much more prevalent in aged vs. younger microglia (Streit et al., 2004).
Although aged microglia are primed to activation compared to neo microglia, production of inflammatory mediators including cytokines, enzymes, cell surface or transmembrane, and intracellular markers exhibited heterogeneity in cells of both ages after OGD+LPS stimulation (Fig. 5). The protein levels of inflammatory mediators including TNF-α and IL-10, and the mRNA level of CD68) were higher in aged microglia; whereas the neonates had higher iNOS and Ym1/2 at the transcription level. These data again provide the evidence that the factor age is critical for inflammatory mediator production and microglial inflammatory profiles. Our finding is consistent with a previous study (Njie et al., 2012) that reported microglial activation and purinergic receptor profiles vary non-linearly with developmental age. The heterogeneity in inflammatory mediator production suggests that microglial functions are not exclusively driven by their milieu, but also by the unique properties the cells of different ages possess (Stratoulias, Venero, Tremblay, & Joseph, 2019). We also found some pro- and anti-inflammatory markers (e.g. iNOS and Ym1/2) were both upregulated after OGD+LPS stimulation (Fig. 5), suggesting the two activation states of microglia may coexist and overlap with each other (Orecchioni, Ghosheh, Pramod, & Ley, 2019; H. Wu et al., 2021).
CD11b is an important cell membrane marker that controls myeloid cell polarization and drives the innate immune response (Jurga, Paleczna, & Kuter, 2020; Panni et al., 2019). We not only found an age-related difference in CD11b baseline expression (Fig. 6E–G), but also a sex difference after OGD stimulation as OGD reverses the expression of neo vs. aged microglia only in males but not in females (Fig. 6I&J). The higher level of CD11b in neo vs. aged male (but not female) microglia in OGD condition indicated a more enhanced polarization in neo male microglia after ischemia, and also suggested that female aged microglia may be more activated than the aged males, consistent with our previous in vivo studies (McCullough et al., 2016). All these data point to the importance of consideration of sex and age as biological variables for microglia study in CNS diseases (Shansky & Murphy, 2021).
5. CONCLUSIONS
We cultured microglia from 18–24-month-old and 0–4-day-old C57BL/6 mice, and examined cell yields, morphology, phagocytosis, inflammatory mediator expression in the cultures of the two-age cells. Compared to the neonates, aged microglial culture not only exhibited higher cell yields, but also more robust phagocytosis after ischemia, an age-related difference revealed by our experimental protocol. Heterogeneity exists in the increase of inflammatory mediators’ expression when aged vs. neo microglia were compared, and sex differences were seen in microglial CD11b expression. We conclude that the age and sex are critical factors for microglial culture to study CNS disorders. It is inappropriate to use neo microglial culture to study neurodegenerative and neuroinflammatory diseases that mostly occur in the elderly.
HIGHLIGHTS.
Aged microglial culture has higher yield and longer life time than neonatal microglial culture.
More and bigger cytoplasmic inclusions are seen in aged vs. neonatal microglia.
Aged microglia are more phagocytic than neonatal microglia.
Inflammatory phenotypes of microglia vary with age and sex.
ACKNOLEDGEMENTS:
This work was supported by funding from NIH Grants R01 NS093042/NS108779 to Fudong Liu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, . . . Liu F(2020). Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proc Natl Acad Sci U S A, 117(3), 1742–1752. doi: 10.1073/pnas.1914742117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Mamun A, Chauhan A, Qi S, Ngwa C, Xu Y, Sharmeen R, . . . Liu F (2020). Microglial IRF5-IRF4 regulatory axis regulates neuroinflammation after cerebral ischemia and impacts stroke outcomes. Proceedings of the National Academy of Sciences, 117(3), 1742–1752. doi: 10.1073/pnas.1914742117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova DM, & Brown DR (2019). Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J Neurochem, 151(6), 676–688. doi: 10.1111/jnc.14860 [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, . . . Gemma C (2011). Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging, 32(11), 2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, & Katzenellenbogen BS (1986). Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A, 83(8), 2496–2500. doi: 10.1073/pnas.83.8.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, & Barres BA (2017). Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron, 94(4), 759–773. e758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolós M, Llorens-Martín M, Perea JR, Jurado-Arjona J, Rábano A, Hernández F, & Avila J (2017). Absence of CX3CR1 impairs the internalization of Tau by microglia. Molecular neurodegeneration, 12(1), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureta C, Setoguchi T, Saitoh Y, Tominaga H, Maeda S, Nagano S, . . . Taniguchi N (2019). TGF-β Promotes the Proliferation of Microglia In Vitro. Brain Sci, 10(1). doi: 10.3390/brainsci10010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Huang D, Sasse ME, & Ransohoff RM (2006). Isolation of murine microglial cells for RNA analysis or flow cytometry. Nature protocols, 1(4), 1947–1951. [DOI] [PubMed] [Google Scholar]
- Chen SH, Oyarzabal EA, & Hong JS (2013). Preparation of rodent primary cultures for neuron-glia, mixed glia, enriched microglia, and reconstituted cultures with microglia. Methods Mol Biol, 1041, 231–240. doi: 10.1007/978-1-62703-520-0_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury ME, Kigami Y, & Tanaka J (2021). Dual Roles of Microglia in the Basal Ganglia in Parkinson’s Disease. International journal of molecular sciences, 22(8), 3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot C, Montagne L, Janssen I, Ravid R, Van Der Valk P, & Veerhuis R (2000). Isolation and characterization of adult microglial cells and oligodendrocytes derived from postmortem human brain tissue. Brain Research Protocols, 5(1), 85–94. [DOI] [PubMed] [Google Scholar]
- De Haas AH, Boddeke HW, Brouwer N, & Biber K (2007). Optimized isolation enables ex vivo analysis of microglia from various central nervous system regions. Glia, 55(13), 1374–1384. [DOI] [PubMed] [Google Scholar]
- Easley-Neal C, Foreman O, Sharma N, Zarrin AA, & Weimer RM (2019). CSF1R Ligands IL-34 and CSF1 Are Differentially Required for Microglia Development and Maintenance in White and Gray Matter Brain Regions. Front Immunol, 10, 2199. doi: 10.3389/fimmu.2019.02199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, & Pollard JW (2011). Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PloS one, 6(10), e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, & Godbout JP (2012). Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity, 26(5), 766–777. doi: 10.1016/j.bbi.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, & Combs CK (2006). Beta-amyloid stimulates murine postnatal and adult microglia cultures in a unique manner. J Neurosci, 26(17), 4644–4648. doi: 10.1523/jneurosci.4822-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, & Combs CK (2007). Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. Journal of neuroscience methods, 164(2), 218–224. doi: 10.1016/j.jneumeth.2007.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Li S, & Combs CK (2005). β-Amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor α and NMDA receptors. Journal of Neuroscience, 25(10), 2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, & Sedgwick JD (1995). Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. The Journal of Immunology, 154(9), 4309–4321. [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, & Maier SF (2006). mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging, 27(5), 717–722. doi: 10.1016/j.neurobiolaging.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Gao H-M, & Hong J-S (2008). Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends in immunology, 29(8), 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, & Möller T (2006). Microglia biology in health and disease. J Neuroimmune Pharmacol, 1(2), 127–137. doi: 10.1007/s11481-006-9015-5 [DOI] [PubMed] [Google Scholar]
- Gingras M, Gagnon V, Minotti S, Durham HD, & Berthod F (2007). Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. Journal of Neuroscience Methods, 163(1), 111–118. [DOI] [PubMed] [Google Scholar]
- Giulian D (1999). Microglia and the Immune Pathology of Alzheimer Disease. The American Journal of Human Genetics, 65(1), 13–18. doi: 10.1086/302477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Hogan CE, Neal ML, Anantharam V, Kanthasamy AG, & Kanthasamy A (2011). A simple magnetic separation method for high-yield isolation of pure primary microglia. J Neurosci Methods, 194(2), 287–296. doi: 10.1016/j.jneumeth.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshi N, Morgan RK, Lein PJ, & Seker E (2020). A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. Journal of Neuroinflammation, 17(1), 155. doi: 10.1186/s12974-020-01819-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, & El Khoury J (2013). The microglial sensome revealed by direct RNA sequencing. Nat Neurosci, 16(12), 1896–1905. doi: 10.1038/nn.3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurga AM, Paleczna M, & Kuter KZ (2020). Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Frontiers in cellular neuroscience, 14, 198–198. doi: 10.3389/fncel.2020.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, . . . Inoue K (2007). UDP acting at P2Y 6 receptors is a mediator of microglial phagocytosis. Nature, 446(7139), 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Dibal CD, Armitage GA, Winship IR, & Todd KG (2013). Distinct activation profiles in microglia of different ages: a systematic study in isolated embryonic to aged microglial cultures. Neuroscience, 254, 185–195. doi: 10.1016/j.neuroscience.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT, & Lee HJ (2014). Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int, 2014, 297241. doi: 10.1155/2014/297241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, & McCarthy MM (2015). A starring role for microglia in brain sex differences. The Neuroscientist, 21(3), 306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively S, & Schlichter LC (2018). Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+ TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Frontiers in cellular neuroscience, 12, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourbopoulos A, Ertürk A, & Hellal F (2015). Microglia in action: how aging and injury can change the brain’s guardians. Front Cell Neurosci, 9, 54. doi: 10.3389/fncel.2015.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, & Lynch MA (2007). CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci, 27(31), 8309–8313. doi: 10.1523/jneurosci.1781-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayle A, Luo M, Jeong M, & Goodell MA (2013). Flow cytometry analysis of murine hematopoietic stem cells. Cytometry A, 83(1), 27–37. doi: 10.1002/cyto.a.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, & Liu F (2016). Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY), 8(7), 1432–1441. doi: 10.18632/aging.100997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca C, Giambanco I, Donato R, & Arcuri C (2018). Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. International journal of molecular sciences, 19(1), 318. doi: 10.3390/ijms19010318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo GE, Malarkey WB, Powell JE, Blakeslee JR, & Yohn DS (1976). Effects of steroid hormones in fetal bovine serum on plating ang cloning of human cells in vitro. In Vitro, 12(1), 23–30. doi: 10.1007/bf02832789 [DOI] [PubMed] [Google Scholar]
- Morrison HW, & Filosa JA (2013). A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation, 10, 4. doi: 10.1186/1742-2094-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HW, & Filosa JA (2016). Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience, 339, 85–99. doi: 10.1016/j.neuroscience.2016.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley K, & Cuzner M (1996). Receptor-mediated phagocytosis of myelin by macrophages and microglia: effect of opsonization and receptor blocking agents. Neurochemical research, 21(4), 481–487. [DOI] [PubMed] [Google Scholar]
- Moussaud S, & Draheim HJ (2010). A new method to isolate microglia from adult mice and culture them for an extended period of time. J Neurosci Methods, 187(2), 243–253. doi: 10.1016/j.jneumeth.2010.01.017 [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, & Rivest S (2002). Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci, 3(3), 216–227. doi: 10.1038/nrn752 [DOI] [PubMed] [Google Scholar]
- Ngwa C, Mamun AA, Xu Y, Sharmeen R, & Liu F (2021). Phosphorylation of Microglial IRF5 and IRF4 by IRAK4 Regulates Inflammatory Responses to Ischemia. Cells, 10(2). doi: 10.3390/cells10020276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, & Streit WJ (2012). Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging, 33(1), 195.e191–112. doi: 10.1016/j.neurobiolaging.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, . . . Lynch MA (2005). Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. J Biol Chem, 280(10), 9354–9362. doi: 10.1074/jbc.M412170200 [DOI] [PubMed] [Google Scholar]
- Norden DM, & Godbout JP (2013). Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol, 39(1), 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, & Godbout JP (2013). Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology, 39(1), 19–34. doi: 10.1111/j.1365-2990.2012.01306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, & Godbout JP (2015). Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology, 96, 29–41. doi: 10.1016/j.neuropharm.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, & Neufeld EF (2003). Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proceedings of the National Academy of Sciences, 100(4), 1902–1907. doi: 10.1073/pnas.252784899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchioni M, Ghosheh Y, Pramod AB, & Ley K (2019). Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol, 10, 1084. doi: 10.3389/fimmu.2019.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell M, Szulzewsky F, Matyash V, Wolf SA, & Kettenmann H (2014). The subpopulation of microglia sensitive to neurotransmitters/neurohormones is modulated by stimulation with LPS, interferon-γ, and IL-4. Glia, 62(5), 667–679. [DOI] [PubMed] [Google Scholar]
- Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, . . . DeNardo DG (2019). Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Science translational medicine, 11(499), eaau9240. doi: 10.1126/scitranslmed.aau9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Chung H, & Maxfield FR (1997). Slow degradation of aggregates of the Alzheimer’s disease amyloid β-protein by microglial cells. Journal of Biological Chemistry, 272(46), 29390–29397. [DOI] [PubMed] [Google Scholar]
- Patel AR, Ritzel R, McCullough LD, & Liu F (2013). Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol, 5(2), 73–90. [PMC free article] [PubMed] [Google Scholar]
- Perry V, Matyszak M, & Fearn S (1993). Altered antigen expression of microglia in the aged rodent CNS. Glia, 7(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Peters A, Josephson K, & Vincent SL (1991). Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. The Anatomical Record, 229(3), 384–398. [DOI] [PubMed] [Google Scholar]
- Peters A, & Sethares C (2002). The effects of age on the cells in layer 1 of primate cerebral cortex. Cerebral Cortex, 12(1), 27–36. [DOI] [PubMed] [Google Scholar]
- Petricevich VL, Reynaud E, Cruz AH, & Possani LD (2008). Macrophage activation, phagocytosis and intracellular calcium oscillations induced by scorpion toxins from Tityus serrulatus. Clinical and experimental immunology, 154(3), 415–423. doi: 10.1111/j.1365-2249.2008.03754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt BM, & McPherson JM (1997). TGF-β in the central nervous system: Potential roles in ischemic injury and neurodegenerative diseases. Cytokine & Growth Factor Reviews, 8(4), 267–292. doi: 10.1016/S1359-6101(97)00018-X [DOI] [PubMed] [Google Scholar]
- Rustenhoven J, Park TI, Schweder P, Scotter J, Correia J, Smith AM, . . . Dragunow M (2016). Isolation of highly enriched primary human microglia for functional studies. Sci Rep, 6, 19371. doi: 10.1038/srep19371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L, Belyanskaya SL, Fischer FR, Cipriani B, Brosnan CF, Ricciardi-Castagnoli P, . . . Riese R (2001). Developmental plasticity of CNS microglia. Proc Natl Acad Sci U S A, 98(11), 6295–6300. doi: 10.1073/pnas.111152498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, & Ter Meulen V (1991). Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proceedings of the National Academy of Sciences, 88(16), 7438–7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, & Murphy AZ (2021). Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci, 24(4), 457–464. doi: 10.1038/s41593-021-00806-8 [DOI] [PubMed] [Google Scholar]
- Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, & Kostrov SV (2016). Cytoplasmic vacuolization in cell death and survival. Oncotarget, 7(34), 55863–55889. doi: 10.18632/oncotarget.10150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, & Bulloch K (2007). Microglia derived from aging mice exhibit an altered inflammatory profile. Glia, 55(4), 412–424. [DOI] [PubMed] [Google Scholar]
- Stansley B, Post J, & Hensley K (2012). A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. Journal of Neuroinflammation, 9(1), 115. doi: 10.1186/1742-2094-9-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratoulias V, Venero JL, Tremblay M, & Joseph B (2019). Microglial subtypes: diversity within the microglial community. Embo j, 38(17), e101997. doi: 10.15252/embj.2019101997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, & Sparks DL (2004). Dystrophic microglia in the aging human brain. Glia, 45(2), 208–212. [DOI] [PubMed] [Google Scholar]
- Streit WJ, & Xue Q-S (2009). Life and death of microglia. Journal of neuroimmune pharmacology, 4(4), 371. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, . . . Tran TA (2008). Neuroinflammation in Parkinson’s disease: is there sufficient evidence for mechanism-based interventional therapy. Front Biosci, 13(5), 709–717. [DOI] [PubMed] [Google Scholar]
- Tasca CI, Dal-Cim T, & Cimarosti H (2015). In vitro oxygen-glucose deprivation to study ischemic cell death. Methods Mol Biol, 1254, 197–210. doi: 10.1007/978-1-4939-2152-2_15 [DOI] [PubMed] [Google Scholar]
- Taylor EN, Huang N, Wisco J, Wang Y, Morgan KG, & Hamilton JA (2020). The brains of aged mice are characterized by altered tissue diffusion properties and cerebral microbleeds. Journal of Translational Medicine, 18(1), 277. doi: 10.1186/s12967-020-02441-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, & Bartlett PF (2012). Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci, 32(19), 6435–6443. doi: 10.1523/jneurosci.5925-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai HY, Go L, Guinn BA, Fraser PE, Westaway D, & McLaurin J (2002). Phenotypic and functional changes in glial cells as a function of age. Neurobiol Aging, 23(1), 105–115. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, & Nabekura J (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci, 29(13), 3974–3980. doi: 10.1523/jneurosci.4363-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Węgiel J, Wiśniewski HM, Dziewiątkowski J, Tarnawski M, Kozielski R, Trenkner E, & Wiktor-Jędrzejczak W (1998). Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain research, 804(1), 135–139. [DOI] [PubMed] [Google Scholar]
- Woolf Z, Stevenson TJ, Lee K, Jung Y, Park TIH, Curtis MA, . . . Dragunow M (2021). Isolation of adult mouse microglia using their in vitro adherent properties. STAR Protocols, 2(2), 100518. doi: 10.1016/j.xpro.2021.100518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zheng J, Xu S, Fang Y, Wu Y, Zeng J, . . . Zhang J(2021). Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation, 18(1), 2. doi: 10.1186/s12974-020-02041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Tokuda Y, Zhang X-W, & Nakanishi H (2008). Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiology of Disease, 32(3), 543–551. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, & Godbout JP (2010). Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain, behavior, and immunity, 24(7), 1190–1201. doi: 10.1016/j.bbi.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PK, Kaan TK, Fenesan D, & Malcangio M (2009). Rapid isolation and culture of primary microglia from adult mouse spinal cord. Journal of Neuroscience Methods, 183(2), 223–237. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Yi S, Jiang X, Qiao Y, Zhang Y, . . . Zhou T (2020). Mouse Astrocytes Promote Microglial Ramification by Releasing TGF-β and Forming Glial Fibers. Frontiers in cellular neuroscience, 14(195). doi: 10.3389/fncel.2020.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]