Abstract
Background:
Individuals with depression often demonstrate an altered peripheral inflammatory profile, as well as emotion perception difficulties. However, correlations of inflammation with overall depression severity are inconsistent and inflammation may only contribute to specific symptoms. Moreover, measurement of the association between inflammation and emotion perception is sparse in adolescence, despite representing a formative window of emotional development and high-risk period for depression onset.
Method:
Serum interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β were measured in 34 adolescents aged 12–17 with DSM-IV depressive disorders (DEP) and 29 healthy controls (HC). Participants were evaluated using the Children’s Depression Rating Scale-Revised (CDRS-R) and symptom subscales were extracted based on factor analysis. Participants also completed a performance-based measure of emotion perception, the Facial Emotion Perception Test (FEPT), which assesses the accuracy of categorizing angry, fearful, sad, happy, and neutral facial emotions.
Results:
IL-6 and TNF-α correlated with reported depressed mood and somatic symptoms, respectively, but not total CDRS-R score, anhedonia or observed mood, across both DEP and HC. DEP demonstrated lower accuracy for identifying angry facial expressions. Higher IL-6 was inversely related to accuracy and discrimination of angry and neutral faces across all participants. IL-1β was associated with reduced discrimination of fearful faces.
Conclusion:
Inflammatory markers were sensitive to affective and somatic symptoms of depression and processing of emotional threat in adolescents. In particular, IL-6 was elevated in depressed adolescents and therefore may represent a specific target for modulating depressive symptoms and emotion processing
Keywords: Depression, Adolescents, Inflammation, Emotion Perception
Introduction
Clinical and animal studies implicate inflammation in the pathophysiology of depression. In humans, experimental manipulation of systemic infection triggers sickness behaviors (Konsman et al., 2002), which bear striking resemblance to many symptoms of depression (Dantzer et al., 2008). Inflammatory cytokines play a central role in mediating sickness behaviors by communicating peripheral inflammation to the brain (D’Mello and Swain, 2017). Accordingly, serum cytokines are extensively studied in depression (Dowlati et al., 2010; Haapakoski et al., 2015; Kohler et al., 2017a; Kohler et al., 2017b; Wiedlocha et al., 2018). The most consistent findings are elevated circulating interleukin 6 (IL-6) levels and its membrane-bound receptors in depressed individuals (Dowlati et al., 2010; Kohler et al., 2017a; Pandey et al., 2015; Rizavi et al., 2016; Wang and Miller, 2018) and IL-6 reductions with successful antidepressant treatment (Haapakoski et al., 2015; Kohler et al., 2017b; Strawbridge et al., 2015). Basal IL-6 is also predictive of subsequent symptoms (Moriarity et al., 2019). Elevations in tumor necrosis factor (TNF)-α and IL-1β also occur in depression, but more variably (Dowlati et al., 2010; Haapakoski et al., 2015).
Nevertheless, measured effect sizes of the association between various inflammatory markers and depressive symptoms are often quite small (Valkanova et al., 2013). There are several possible sources of variance. For one, depression is heterogeneous and inflammation might only relate to certain symptom clusters or dimensions (Haroon et al., 2012; Raison and Miller, 2011). For example, primarily subjective reports of depressed mood and somatic symptoms develop from cytokine-induced depression with interferon-α administrations (Capuron et al., 2002). Second, allostatic load, chronic medical co-morbidities, and obesity can distort inflammation measurement over prolonged illness duration (Berk et al., 2014; Lopresti and Drummond, 2013). Third and related, there are developmental differences in cytokine production and the inflammatory profile of adult depression may differ from that during sensitive developmental risk periods, like adolescence. Fourth, because antidepressant medications can inhibit inflammation (Haapakoski et al., 2015; Kohler et al., 2017b; Strawbridge et al., 2015), ongoing treatment may suppress correlations. Moreover, symptoms and inflammation measures may fluctuate in different timescales, including time-lagged associations. To reduce many of these sources of extraneous noise and variance, it is important to measure inflammatory markers and symptom dimensions in adolescence, prior to the onset of chronic co-morbidities, recurrent depressive episodes, chronic morbidity, disruption to occupational, educational, and social milestones, and initiation of pharmaco-treatment.
There is also increasing appreciation regarding a role of chronic inflammation in altered emotional processing (Bollen et al., 2017; Muscatell and Eisenberger, 2012), which is both a risk factor for, and often biased during, depression (Bourke et al., 2010; Watters and Williams, 2011). Evolutionarily (Eisenberger et al., 2017), cytokines prepare the body for defense and repair when confronted with social-emotional signals of threat or aggression (e.g., anger or fear), but this adaptative inflammatory response is temporary and down regulated after threat resolution (Eisenberger and Cole, 2012; Irwin and Cole, 2011). However, if inflammation does not adequately resolve and becomes chronic or recurrent, it may relate to diminished efficiency and accuracy in identification and discrimination of both actual and perceived threats (Eisenberger and Cole, 2012; Irwin and Cole, 2011). Depressed individuals may be particularly vulnerable to the effects of this hypothesized cycle, as depressive symptoms may be coded as a threat in the body and perpetuate low-level inflammation (Slavich and Irwin, 2014). However, few studies have directly interrogated the association between emotional threat perception and inflammation in depressed individuals (Kohler et al., 2011). Adolescence is a unique and important window to do so because emotional processing skills are still undergoing development through salient social interactions (Silk et al., 2012) and inflammation could interfere with consolidation of these social-emotional skills.
We measured pro-inflammatory cytokines, IL-6, (TNF)-α, and IL-1β, depressive symptoms, and emotion perception, amongst adolescents with depressive mood disorders (DEP), naïve to psychiatric treatment for their mood disorder, and healthy controls (HC), defined as having no history of any psychiatric disorder. The first aim was to assess correlations between inflammatory markers and depressive symptoms, including depression subscales. We expected the cytokines to positively correlate with reported affective and somatic, but not other symptom dimensions in DEP. The second aim was to compare groups in facial emotion perception. We predicted deficits in accuracy and discrimination of emotions conveying threat (anger and fear) among DEP relative to HC. The third aim was to evaluate associations between cytokines and emotion perception in DEP and HC. We anticipated that IL-6 would inversely relate to anger and fear accuracy and discrimination in DEP. We did not expect cytokines to correlate with depressive symptoms, depressive symptom subscales, or accuracy and discrimination of emotions conveying threat in HC.
Method
Participants
The Institutional Review Board where the research was conducted approved all study procedures. Participants were assenting adolescents (with consenting parent), ages 12–17 with DEP (depression, dysthymia, adjustment disorder with depressed mood, sub-threshold and unspecified depressive symptoms [n = 34]) and HC (n = 29) adolescents with no psychiatric history, comparable in age and sex with complete data on all study variables. The sampling strategy for the DEP participants was to capture the full range of negative mood disturbance, consistent with the Research Domain Criteria Framework (RDoC). DEP adolescents were recruited based on self- or clinician-report of depressive symptoms lasting at least one week, which were confirmed in a structured clinical interview. DEP participants were recruited from outpatient psychiatry clinics in a large, urban, academic medical center and the surrounding community. HC participants were recruited both from community populations through train advertisements and from involvement in previous studies. HC status, defined as having no history of psychiatric disorder, was confirmed in a structured clinical interview.
Initial eligibility was determined via a semi-structured telephone screening. At in-person screening, all participants demonstrated an estimated Verbal IQ in ‘borderline’ or higher range [T-score >30, Wechsler Abbreviated Scale of Intelligence - 2nd Edition (WASI-II) on the Vocabulary Test (Wechsler, 1999)], and English fluency. Participants were excluded for: 1) psychiatric medication (except for stimulant use, which was permitted among DEP with co-morbid ADHD in order to provide an ideal testing environment for neurocognitive tasks, as stimulants are not known to interfere with measurement of inflammation (Baumeister et al., 2016; Kittel-Schneider et al., 2016); 2) chronic neurological or medical illness affecting cognition, 3) active suicidality with plan or intent requiring acute intervention, 4) history of head injury with loss of consciousness >10 minutes, 5) current substance or alcohol use disorder 6) nicotine use, 7) participation in an investigational medication research protocol, or 8) active virus or infection within two weeks prior to enrollment. Additionally, participants screened as HC were deemed ineligible if they met current or past criteria for any DSM-IV psychiatric disorder on structured interview.
Procedures
Eligible participants completed a semi-structured clinical assessment, including diagnostic interview and clinician-rated symptom scales; a facial emotion perception task, self-reports of demographics and medical history, including height and weight for calculation of body mass index (BMI); and a non-fasting blood sample obtained by venipuncture (see Cytokine Assay for detail). Consistent with university standards, participants were compensated $10 per hour.
Measures
Clinical Assessment
Clinical interviews were conducted by masters-level mental health professionals using the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (KSADS) (Kaufman et al., 1997) to yield current and lifetime DSM-IV psychiatric diagnoses. Enrollment into the present study required meeting any diagnosis of a depressive disorder involving symptoms lasting at least one week and significant functional impairment, rather than a pre-specified symptom severity cut-off, consistent with an agnostic RDoC approach. The KSADS was also used to diagnose psychiatric co-morbidities, screen for substance use, and evaluate for suicidality. Eligible HCs did not meet criteria for any psychiatric disorder as assessed on the KSADS. Functional impairment was rated using the Children’s Global Assessment Scale (CGAS) (Shaffer et al., 1983).
Clinicians also rated depression symptom severity on the Children’s Depression Rating Scale – Revised (CDRS-R) (Poznanski et al., 1984). In order to test the hypothesis that inflammatory markers would correlate with specific clusters of heterogeneous depression symptoms (i.e., proclivity for association with depression/withdrawal and somatic symptoms), depression subscales which optimally represent these dimensions were derived based on the CDRS-R factor structure identified in the largest published (n = 314) sample of English-speaking children and adolescents (Guo et al., 2006). Specifically, ‘reported mood’ and ‘somatic symptoms’ subscales were computed by summing CDRS-R individual item ratings according to the reported factor loading for each factor. Factor loadings for ‘reported mood’ included: self-esteem (.38), depressed feelings (.45), and excessive weeping (.63), and achieved α = .80 in this sample. Factor loadings for somatic symptoms included: sleep disturbance (.43), excessive fatigue (.58), physical complaints (.42), and impaired schoolwork (.40), and achieved α = .83 in this sample.
To test the specificity of our hypotheses, we also conducted exploratory analyses of correlations between the inflammatory markers and the other symptom factors identified by Guo et al. Specifically an ‘anhedonia’ factor included: social withdrawal (.81) and capacity to have fun (.60) and achieved α = .91 in this sample. An ‘observed depressive mood’ factor included: depressed affect (.58), tempo of speech (.86), and hypoactivity (.69) and achieved α = .91 in this sample. A ‘morbid thoughts’ factor included: morbid ideation (.87) and suicidal ideation (.44) and achieved α = −.04 in this sample. This poor reliability was largely a function of infrequent endorsement of morbid ideation in the present sample, but given the poor psychometric properties, we opted not to utilize the morbid thoughts factor in further analyses.
Facial Emotion Perception.
The Facial Emotion Perception Test (FEPT) (Langenecker et al., 2005; Rapport et al., 2002) is a 7-minute task that assesses emotion perception accuracy, an area of impaired functioning in depression (Bourke et al., 2010; Stuhrmann et al., 2011). Participants were presented with and asked to rapidly categorize adult faces (Ekman, 1976) and animals (control condition). For the face trials, participants categorized the facial expression into one of five possibilities, with one response option per finger/button: angry, fearful, happy, sad or neutral. For the animal trials, participants categorized the animal into one of five possibilities: dog, cat, primate, fish, or bird. A stimulus is presented for 300 ms, followed by a mask for 100 ms, and then 2600 ms are provided as a response window. Trials are separated by the presentation of a cross for 500 ms. Percentage of accurately identified facial expressions are calculated for each emotion. We also calculated d’, the discrimination of each emotion stimulus from noise (e.g., angry hit rate – angry false alarm rate).
Cytokine Assay
Serum was separated, centrifuged at 6,000 rpm for 10 min, and stored at −80°C before batch analysis. Laboratory staffs measuring IL-6 and additional cytokines were blinded to participant diagnosis. Cytokine level was determined in plasma/serum aliquots by enzyme-linked immunosorbent assay using Quantakine® kits (R&D Systems, Inc., Minneapolis, MN, USA) for human IL-6, IL-1β, and TNF-α. Briefly, 100 μL of incubation buffer and 100 μL of serum/plasma or standard is added to each well and incubated for 3 h at room temperature (RT) on the orbital shaker. After washing wells six times with Wash Buffer, 200 μL of Conjugate is added to each well, incubated for 2 h at room temperature, washed using Wash Buffer as before, 50 μL of Substrate Solution is added to each well and incubated for 60 min. at room temperature. Following this, 50 μL of Amplifier Solution is added to each well, incubated for 30 min. at room temperature and 50 μL of Stop Solution is added to each well. The optical density of each well is determined within 30 min using a microplate reader set to 490 nm, and wavelength correction is set to 650 nm, and cytokine levels are calculated. Standard curve was generated by plotting the mean absorbance for each standard, and data points were linearized. The cytokine concentration in each sample was determined by reading it against the standard curve.
Statistical Analyses
Differences between HC and DEP participants in demographics and clinical characteristics were compared using one-way analysis of variance and chi-squared tests, as appropriate. Inflammatory markers values were not normally distributed and subjected to log-transformation. Log-transformed cytokine values were used in subsequent analyses. Inflammatory cytokines and emotion perception accuracy and discrimination were compared between DEP and HC using multivariate analysis of variance. Pearson’s correlations were used to examine the association of inflammatory cytokines with measures of depression severity and emotion perception across the full sample, and then within each DEP and HC alone. In sensitivity analyses, significant bivariate associations were subjected to multivariate modeling to adjust for potential confounders to inflammation or emotional processing such as age, sex, race (white vs. all other racial categories combined), body mass index, and verbal IQ estimate.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the sample are shown in Table 1. HCs were comparable to DEP on age, sex, verbal IQ estimate, race/ethnicity, and BMI. Of DEP participants, 41.2% (n = 14) met criteria for major depression, 17.6% (n = 6) for dysthymia, 35.3% (n=12) for depressive disorder not otherwise specified (NOS), and 5.9% (n=2) for adjustment disorder with depressed mood. Of participants with current dysthymia or depression NOS (n = 18), 38.9% (n=7) met criteria for a past major depressive episode. Mean CDRS-R total score in DEP subjects was 43.56 (SD = 11.29), consistent with moderate overall depression severity (Poznanski and Mokros, 1996).
Table 1.
Demographic and Clinical Characteristics of HC and DEP Adolescents
|
HC
(n = 29) |
DEP
(n = 34) |
Omnibus Test | ||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | F | p-value | |
|
| ||||||
| Age | 14.72 | 1.36 | 14.47 | 1.67 | .43 | .517 |
| Verbal IQ Estimate a | 56.07 | 8.30 | 51.53 | 9.56 | 3.96 | .051 |
| Body Mass Index | 22.98 | 3.21 | 22.48 | 4.79 | .27 | .636 |
| Global Functioning b | 91.58 | 5.75 | 65.08 | 7.46 | 242.72 | <.001 |
| CDRS Total | 19.45 | 1.74 | 43.56 | 11.29 | 129.20 | <.001 |
|
| ||||||
| N | % | N | % | χ2 | p-value | |
|
| ||||||
| Sex (% Female) | 18 | 62.1 | 19 | 55.9 | .25 | .619 |
| Ethnicity (% Hispanic/Latino) | 5 | 17.2 | 11 | 32.4 | 1.89 | .170 |
| Race | ||||||
| Caucasian | 16 | 55.2 | 19 | 55.9 | 5.46 | .243 |
| Black | 8 | 27.6 | 7 | 20.6 | -- | -- |
| Asian | 5 | 17.2 | 3 | 8.8 | -- | -- |
| American Indian/Alaskan Native | 0 | 0 | 1 | 2.9 | -- | -- |
| Other/Unknown | 0 | 0 | 4 | 11.8 | -- | -- |
| Current DSM-IV Diagnosis | ||||||
| Major Depression | -- | -- | 14 | 41.2 | -- | -- |
| Dysthymia | -- | -- | 6 | 17.6 | -- | -- |
| Depressive d/o NOS | -- | -- | 12 | 35.3 | -- | -- |
| Adjustment d/o Depressed Mood | -- | -- | 2 | 5.9 | -- | -- |
| Comorbid DSM-IV Diagnoses | ||||||
| Panic Disorder | -- | -- | 2 | 5.9 | -- | -- |
| Social Phobia | -- | -- | 7 | 20.6 | -- | -- |
| Agoraphobia | -- | -- | 1 | 2.9 | -- | -- |
| Generalized Anxiety Disorder | -- | -- | 16 | 47.1 | -- | -- |
| Post-Traumatic Stress Disorder | -- | -- | 4 | 11.8 | -- | -- |
| Attention Deficit/Hyperactivity | -- | -- | 5 | 14.7 | -- | -- |
| Oppositional Defiant Disorder | -- | -- | 2 | 5.9 | -- | -- |
Vocabulary subtest T-Score from the WASI-II
Children’s Global Assessment Scale
Inflammatory Cytokines and Depression
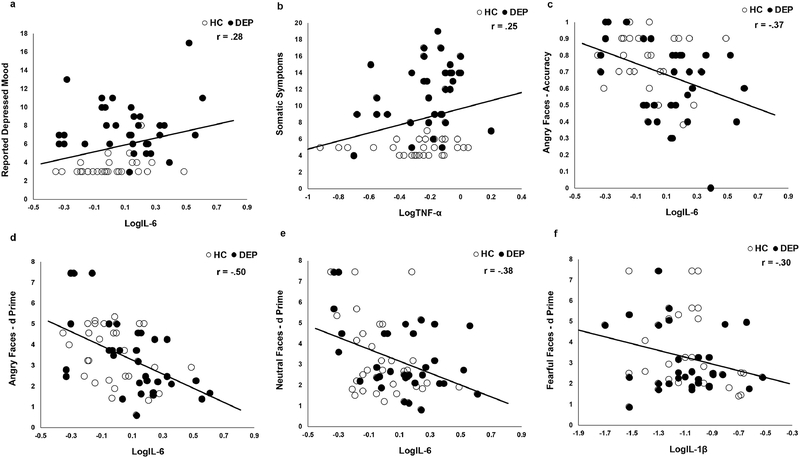
IL-6 was significantly elevated in DEP relative to HC (Table 2). TNF-α and IL-1β did not significantly differ between DEP and HC. IL-6 was positively associated with the reported depressed mood factor, but not the somatic symptoms factor or CDRS total score, across all participants (Figure 1a, Table 3). However, this association was not significant in DEP (r = .08, p = .654) or HC (r = .26, p = .161) alone. TNF-α was positively associated with the somatic symptom factor across all participants, but not the reported depressed mood factor or CDRS total score (Figure 1b, Table 2). This association was not significant in DEP (r = .26, p = .134) or HC (r = .10 p = .609) alone. IL-1β was unrelated to any symptom scales and neither IL-6 nor TNF-α were significantly associated with the anhedonia or observed depressed mood factors (Table 3).
Table 2.
Inflammatory cytokines and facial emotion accuracy and discrimination in DEP vs. HC adolescents
| HC (n = 29) |
DEP (n = 34) |
Analysis of Variance | Effect Size | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| M | SD | M | SD | F | p-value | Cohen’s d | |
| Cytokines (Log Transformed) | |||||||
| IL-6 | −.02 | .18 | .12 | .25 | 6.04 | .017 | .64 |
| TNF-α | −.29 | .23 | −.22 | .21 | 1.69 | .199 | .32 |
| IL-1β | −1.12 | .36 | −1.09 | .27 | .15 | .704 | .09 |
|
FEPT Accuracy (% Correct) | |||||||
| Fear | .84 | .15 | .80 | .20 | .86 | .357 | .23 |
| Anger | .78 | .15 | .64 | .24 | 7.48 | .008 | .70 |
| Sad | .63 | .21 | .54 | .24 | 2.47 | .121 | .40 |
| Happy | .93 | .11 | .91 | .13 | .34 | .565 | .17 |
| Neutral | .76 | .20 | .78 | .19 | .14 | .707 | .10 |
|
FEPT Discrimination (d’) | |||||||
| Feara | 3.62 | 1.87 | 2.98 | 1.50 | 2.22 | .142 | .38 |
| Angera | 3.63 | 1.23 | 3.29 | 1.81 | .73 | .398 | .22 |
| Sada | 2.90 | 1.39 | 2.46 | 1.57 | 1.36 | .248 | .30 |
| Happy | 5.73 | 1.92 | 5.29 | 1.72 | .92 | .341 | .24 |
| Neutral | 3.37 | 1.87 | 3.23 | 1.66 | .09 | .760 | .08 |
n = 62; d-prime not calculated where accuracy for given trial type was 0%
Figure 1.
a) IL-6 is positively correlated with reported depressed mood symptoms; b) TNF-α is positively correlated with somatic symptoms; IL-6 is inversely correlated with c) percent accuracy for angry faces, d) discrimination of angry faces, and e) discrimination of neutral faces; f) IL-1β is inversely correlated with discrimination of fearful faces. `Correlation between IL-6 and accuracy for angry faces (b) remained significant after either exclusion (r = .33, p = .009) of the single subject with 0% accuracy (3 standard deviations below the sample mean) or truncation to 2 standard deviations below the sample mean (r = .36, p = .004).
Table 3.
Bivariate associations between inflammatory cytokines, depressive symptoms, and emotion perception in DEP and HC
| Depressive Symptoms |
FEPT Percent Accuracy |
FEPT Discrimination (d’) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDRS | Reported Mood | Somatic | Fear | Anhedonia | Observed Mood | Anger | Sad | Happy | Neutral | Feara | Angera | Sada | Happy | Neutral | |
| Full Sample | |||||||||||||||
| LogIL-6 | .21 | .28* | .21 | −.15 | .19 | .05 | −.37* | −.20 | −.05 | −.11 | −.17 | −.50* | −.20 | −.01 | −.38* |
| LogTNF-α | .09 | .02 | .25* | .05 | .08 | −.12 | −.15 | −.08 | .14 | .21 | −.12 | −.15 | −.16 | .19 | .05 |
| LogIl-1β | .08 | .10 | .05 | −.11 | .05 | .08 | −.09 | .06 | −.13 | −.17 | −.30* | −.05 | −.02 | −.20 | −.24 |
p < .05
n = 62; d-prime not calculated where accuracy for given trial type was 0%.
Inflammatory Cytokines and Emotion Perception
DEP demonstrated lower accuracy for angry faces compared to HC. DEP and HC did not differ in accuracy for or discrimination (d’) of fearful, sad, happy, or neutral faces (Table 2). IL-6 was inversely associated with accuracy and discrimination of angry faces, as well as discrimination of neutral faces across all subjects (Table 3, Figure 1c–e). Within groups, the correlations in DEP remained significant for accuracy of angry faces (r = −.41, p = .017), as well as discrimination of angry (r = −.59, p < .001) and neutral (r = −.43, p = .012) faces. The correlations in HC were not significant for accuracy of angry faces (r = −.06, p = .741), nor discrimination of angry (r = −.32, p = .079) and neutral (r = −.36, p = .052) faces. Additionally, IL-1β was inversely associated with discrimination of fearful faces (Table 3, Figure 1f). This association was not significant in DEP (r = −.22, p = .220) or HC alone (r = −.34, p = .070). TNF-α was unrelated to emotion perception.
Sensitivity Analyses
Modeling of cytokine associations with symptom factors and emotion processing measures after adjustment for age, sex, race, body mass index, and verbal IQ are reported in Table 4. The associations of IL-6 and TNF-α with the reported mood and somatic symptom factors, respectively, were reduced to a trend level after correction for covariates. The associations of IL-6 with accuracy for angry faces and discrimination of angry and neutral faces, as well as the association of IL-1β with discrimination of fearful faces, all were significant, even with reduced power of adding these covariates.
Table 4.
Multivariate adjustment to cytokine associations with symptom and emotion processing dimensions
| Dependent/Covariate | b | se | t | p | R 2 |
|---|---|---|---|---|---|
| Reported Mood | .12 | ||||
|
| |||||
| LogIL-6 | 332 | 1.75 | 1.83 | .072 | |
| Age | −.20 | .26 | −.77 | .447 | |
| Sex | .69 | .80 | .86 | .393 | |
| Race | −.63 | .79 | −.79 | .431 | |
| BMI | .09 | .10 | .90 | .372 | |
| Verbal IQ Estimate | −.01 | .04 | −.26 | .795 | |
| Somatic Symptoms | .11 | ||||
| TNF-α | 4.70 | 2.60 | 1.81 | .076 | |
| Age | −.29 | .38 | −.75 | .459 | |
| Sex | −.29 | 1.18 | −.24 | .810 | |
| Race | −.35 | 1.14 | −.31 | .760 | |
| BMI | .07 | .15 | .51 | .612 | |
| Verbal IQ Estimate | −.09 | .06 | −1.40 | .168 | |
| Anger Accuracy | .33 | ||||
| LogIL-6 | −.37 | .11 | −3.47 | .001 | |
| Age | .003 | .02 | .19 | .854 | |
| Sex | <.001 | .05 | .01 | .995 | |
| Race | −.09 | .05 | −1.89 | .063 | |
| BMI | .02 | .006 | 3.15 | .003 | |
| Verbal IQ Estimate | .004 | .003 | 1.40 | .168 | |
| Anger Discrimination | .37 | ||||
| LogIL-6 | −3.87 | .79 | −4.90 | <.001 | |
| Age | .06 | .12 | .48 | .633 | |
| Sex | −.06 | .35 | −.18 | .856 | |
| Race | −.27 | .35 | −.78 | .440 | |
| BMI | .13 | .05 | 2.78 | .007 | |
| Verbal IQ Estimate | .01 | .02 | .72 | .475 | |
| Neutral Discrimination | .30 | ||||
| LogIL-6 | −2.66 | .91 | −2.93 | .005 | |
| Age | .18 | .14 | 1.29 | .202 | |
| Sex | −.37 | .41 | .89 | .380 | |
| Race | −.23 | .41 | −.56 | .577 | |
| BMI | .07 | .05 | 1.35 | .182 | |
| Verbal IQ Estimate | .06 | .02 | 2.42 | .019 | |
| Fear Discriminationa | .17 | ||||
| LogIL-1β | −1.47 | .70 | −2.11 | .040 | |
| Age | .20 | .15 | 1.35 | .183 | |
| Sex | .40 | .44 | .90 | .370 | |
| Race | −.31 | .43 | −.72 | .474 | |
| BMI | −.02 | .06 | −.29 | .775 | |
| Verbal IQ Estimate | .03 | .02 | 1.31 | .195 | |
n = 62; d-prime not calculated where accuracy for given trial type was 0%.
Discussion
In this study, IL-6 was elevated in adolescents with depressive disorders and, across the full sample, associated with increased reported depressed mood symptoms. TNF-α was related to somatic symptoms. Adolescents with depressive disorders also demonstrated lower accuracy for identifying angry faces and increased IL-6 was related to decreased accuracy for and discrimination of angry faces. Higher IL-6 was also related to reduced discrimination of neutral faces and IL-1β was associated with reduced discrimination of fearful faces. Cumulatively, these findings demonstrate a link between cytokines, subjective mood complaints and somatic symptoms, and emotional processing.
Elevated IL-6 in adolescent depression is generally consistent with prior studies (Gabbay et al., 2009a; Henje Blom et al., 2012; Miller and Cole, 2012; Pandey et al., 2012) (but see (Gabbay et al., 2009b)). Here, we also demonstrate that IL-6 positively correlated with reported mood, but not somatic symptoms, anhedonia, observed mood, or the depression composite. Additionally, TNF-a did not differ between groups but was associated with somatic symptoms. These findings offers some specificity to existing variable correlations between cytokines and total depression severity (Howren et al., 2009), implicating a possible function of IL-6 in subjective mood complaints and TNF-α in somatic symptoms. However, these findings were attenuated to the trend level after correction for demographics, BMI, and IQ, which may reflect either shared variance or constrained power for correction of small-to-medium correlations in the current sample. Additionally, it was notable that the correlation between cytokines and symptoms were present across the full spectrum of healthy, sub-threshold, and affected adolescents, which speculatively, implicates cytokines in features of depression risk dimensionally (Giletta et al., 2017). However, it is also noteworthy, that while not significant, the magnitude of the correlations seem to suggest that the association between IL-6 and depressed mood could be driven by a stronger correlation in HC, whereas the association between TNF-α and somatic symptoms could be driven by a stronger correlation in DEP. The reasons for this are unclear and should be explored in future studies. One hypothesis is that IL-6 is implicated in risk for depressive symptoms broadly, but that there may exist a critical threshold where active depressed mood symptoms or chronic stress may actually obscure or suppress certain inflammatory mediators. Somatic symptoms on the other hand, may specifically mount the response of inflammation only once the physical symptoms become clinically significant or chronic, as increases in cytokines generally parallel increases in pain perception (Irwin, 2011).
Increased IL-6 was related to reduced anger perception accuracy, which was impaired in DEP. Importantly, IL-6 was also associated with reduced anger discrimination, which reflects the confluence of both low accuracy in correctly identifying anger and tendency to incorrectly label other emotions as anger. One interpretation is that individuals less accurate at identifying true threat might mount a larger or longer immune response to manage and defend against unclear or unpredictable sources of danger. This hypothesis would also be consistent with finding of IL-6 predicting poorer discrimination of neutral faces, which are often perceived as ambiguous and potentially threatening by individuals with mood disorders (Bourke et al., 2010; Rich et al., 2006). Alternatively, chronic inflammation may bequeath reduced sensitivity to angry faces, consistent with endotoxin manipulations causing impaired theory of mind (Grigoleit et al., 2011; Moieni et al., 2015b), increased subjective feelings of social disconnectedness (Eisenberger et al., 2010b; Moieni et al., 2015a), and hedonic reward responding (Eisenberger et al., 2010a; Harrison et al., 2016). An additional alternative explanation is that those with elevated inflammation might be more frequently subjected to chronic and unpredictable social stressors, with higher stakes emotion perception necessary for safety (Wright et al., 2009). Finally, exposure to inflammation may change developmental pathways in sensitive systems like those important for emotion perception. Longitudinal studies will be paramount for dissociating social-emotional antecedents and sequelae of inflammation.
Aspects of our hypotheses were only partially supported. For instance, depressed adolescents demonstrated reduced anger, but not fear accuracy, and inflammatory markers were more consistently associated with anger than fear processing. Plausibly, anger and fear could evoke different threat attributions in that anger conveys aggression, or the potential for threat, whereas fear may reflect a response to threat, and these gradations may differentially inform immune responding. On the other hand, depressed subjects demonstrated non-significantly lower fear accuracy and discrimination, with small effect sizes (d = .23 and .38, respectively), which we had limited power to detect. It was also somewhat unanticipated that IL-1β was associated with reduced discrimination of neutral faces, though ambiguous facial expressions evoke activation of emotion circuitry in multiple psychiatric disorders, challenging their inherent “neutrality” (Filkowski and Haas, 2017). Finally, it was also somewhat surprising that TNF-α did not relate to emotion processing. IL-6 and IL-1β may specifically relate to emotion processing in adolescence via its effects on serotonin production and integrity (Barkhudaryan and Dunn, 1999), whereas TNF-α is implicated in synaptic pruning and neuroplasticity and maybe more likely to influence cognition (Rosenblat et al., 2014).
This study had several strengths, including a clinically and demographically diverse sample of depressed adolescents, while comparable to HCs in demographics and carefully screened for confounds to inflammation. The primary limitation of this study is that the correlational design precludes causal inference. Related, the cross-sectional nature of the study did not allow for assessment of temporal associations. Second, cytokine physiologies are complex and the effects of anti-inflammatory cells or other mediators (e.g., soluble receptors and their buffers) are unknown. Third, the generalizability of a sample naïve to psychiatric treatment for depression deserves consideration. Some participants initiated pharmacotherapy after the study or were actively engaged in psychotherapy, but neither were systematically recorded, which is noteworthy in light of recent data showing changes in inflammation with psychotherapy (Lopresti, 2017). Fourth, although our findings accounted for demographic factors including sex, we were underpowered to elucidate and interpret three-way interactions between inflammation, sex, and diagnosis (Barrientos et al., 2019) due to the limited sample size. Finally, stage of menstrual cycle was not assessed in females, which could contribute to variance in immune markers (Oertelt-Prigione, 2012), though it is noted that the association of menstrual cycle with immune markers is not necessarily expected to differ in depressed adolescents and demographically matched healthy controls.
In sum, inflammatory markers were associated with increased affective and somatic symptoms of depression and compromised processing of anger, fear, and ambiguous facial emotions across the spectrum of healthy to depressed adolescents. Moreover, IL-6 was elevated in depressed adolescents and therefore may represent a specific target for modulating depressive symptoms and emotion processing dysfunction in adolescents. Future research should evaluate whether IL-6 relates to course of depressive symptoms and affective cognition.
Highlights.
This study assessed pro-inflammatory cytokines, depressive symptoms, and emotion perception, amongst adolescents with and without depressive mood disorders (DEP).
Inflammatory markers were sensitive to affective and somatic symptoms of depression and processing of emotional threat in adolescents.
In particular, IL-6 was elevated in depressed adolescents, related to depressed mood, and perception of angry and neutral faces and may represent a specific target for modulating depressive symptoms and emotion processing dysfunction in adolescents.
Future research should evaluate whether IL-6 relates to course of depressive symptoms and emotion processing.
Acknowledgements
We certify that all authors have seen and approved the final version of the manuscript being submitted. This article is the based on original work and has not received prior publication or is under consideration for publication elsewhere.
Role of the Funding Source
This research was designed under and supported by the National Institute of Mental Health F31 MH108258 (ATP) and the American Psychological Foundation (ATP). Additional laboratory support for the current study was also provided by R01 MH098554 (GNP). ATP is currently supported by K23 MH 122676.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barkhudaryan N, Dunn AJ, 1999. Molecular mechanisms of actions of interleukin-6 on the brain, with special reference to serotonin and the hypothalamo-pituitary-adrenocortical axis. Neurochem Res 24, 1169–1180. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Brunton PJ, Lenz KM, Pyter L, Spencer SJ, 2019. Neuroimmunology of the female brain across the lifespan: Plasticity to psychopathology. Brain Behav Immun 79, 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Ciufolini S, Mondelli V, 2016. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl) 233, 1575–1589. [DOI] [PubMed] [Google Scholar]
- Berk M, Berk L, Dodd S, Cotton S, Macneil C, Daglas R, Conus P, Bechdolf A, Moylan S, Malhi GS, 2014. Stage managing bipolar disorder. Bipolar disorders 16, 471–477. [DOI] [PubMed] [Google Scholar]
- Bollen J, Trick L, Llewellyn D, Dickens C, 2017. The effects of acute inflammation on cognitive functioning and emotional processing in humans: A systematic review of experimental studies. J Psychosom Res 94, 47–55. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R, 2010. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry 44, 681–696. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH, 2002. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26, 643. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Swain MG, 2017. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr Top Behav Neurosci 31, 73–94. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews. Neuroscience 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL, 2010. A meta-analysis of cytokines in major depression. Biol Psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010a. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW, 2012. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature neuroscience 15, 669. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR, 2010b. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, behavior, and immunity 24, 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR, 2017. In Sickness and in Health: The Co-Regulation of Inflammation and Social Behavior. Neuropsychopharmacology 42, 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, 1976. Pictures of facial affect. Consulting Psychologists Press. [Google Scholar]
- Filkowski MM, Haas BW, 2017. Rethinking the Use of Neutral Faces as a Baseline in fMRI Neuroimaging Studies of Axis-I Psychiatric Disorders. J Neuroimaging 27, 281–291. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, Hirsch GS, Hottinger-Blanc PM, Gonzalez CJ, 2009a. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord 115, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ, 2009b. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol 19, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein MJ, 2017. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J Child Psychol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit J-S, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Engler H, Gizewski E, Oberbeck R, Schedlowski M, 2011. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One 6, e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nilsson ME, Heiligenstein J, Wilson MG, Emslie G, 2006. An exploratory factor analysis of the children’s depression rating scale-revised. J Child Adolesc Psychopharmacol 16, 482–491. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M, 2015. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH, 2012. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37, 137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD, 2016. A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biological psychiatry 80, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henje Blom E, Lekander M, Ingvar M, Asberg M, Mobarrez F, Serlachius E, 2012. Proinflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. J Affect Disord 136, 716–723. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J, 2009. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71, 171–186. [DOI] [PubMed] [Google Scholar]
- Irwin MR, 2011. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr Clin North Am 34, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole SW, 2011. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology 11, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Kittel-Schneider S, Spiegel S, Renner T, Romanos M, Reif A, Reichert S, Heupel J, Schnetzler L, Stopper H, Jacob C, 2016. Cytogenetic Effects of Chronic Methylphenidate Treatment and Chronic Social Stress in Adults with Attention-Deficit/Hyperactivity Disorder. Pharmacopsychiatry 49, 146–154. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, 2017a. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, de Andrade NQ, Morris G, Fernandes BS, Brunoni AR, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, 2017b. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ, 2011. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry research 188, 303–309. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R, 2002. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in neurosciences 25, 154–159. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S, 2005. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol 27, 320–333. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, 2017. Cognitive behaviour therapy and inflammation: A systematic review of its relationship and the potential implications for the treatment of depression. Aust N Z J Psychiatry 51, 565–582. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Drummond PD, 2013. Obesity and psychiatric disorders: commonalities in dysregulated biological pathways and their implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 45, 92–99. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW, 2012. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry 72, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Breen EC, Cho HJ, Arevalo JM, Ma J, Cole SW, Eisenberger NI, 2015a. Trait sensitivity to social disconnection enhances pro-inflammatory responses to a randomized controlled trial of endotoxin. Psychoneuroendocrinology 62, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Breen EC, Eisenberger NI, 2015b. Inflammation impairs social cognitive processing: a randomized controlled trial of endotoxin. Brain, behavior, and immunity 48, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Giollabhui NM, Ellman LM, Klugman J, Coe CL, Abramson LY, Alloy LB, 2019. Inflammatory Proteins Predict Change in Depressive Symptoms in Male and Female Adolescents. Clin Psychol Sci 7, 754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Eisenberger NI, 2012. A Social Neuroscience Perspective on Stress and Health. Soc Personal Psychol Compass 6, 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S, 2012. Immunology and the menstrual cycle. Autoimmun Rev 11, A486–492. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Zhang H, 2015. Abnormal gene expression of proinflammatory cytokines and their receptors in the lymphocytes of patients with bipolar disorder. Bipolar Disord 17, 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y, 2012. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res 46, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R, 1984. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry 23, 191–197. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Mokros HB, 1996. Children’s depression rating scale, revised (CDRS-R). Western Psychological Services; Los Angeles. [Google Scholar]
- Raison CL, Miller AH, 2011. Is depression an inflammatory disorder? Curr Psychiatry Rep 13, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport LJ, Friedman SR, Tzelepis A, Van Voorhis A, 2002. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology 16, 102–110. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E, 2006. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A 103, 8900–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizavi HS, Ren X, Zhang H, Bhaumik R, Pandey GN, 2016. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res 240, 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, Cha DS, Mansur RB, McIntyre RS, 2014. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 53, 23–34. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S, 1983. A children’s global assessment scale (CGAS). Arch Gen Psychiatry 40, 1228–1231. [DOI] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE, 2012. Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychol Med 42, 2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U, 2011. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL, 2013. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 150, 736–744. [DOI] [PubMed] [Google Scholar]
- Wang AK, Miller BJ, 2018. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr Bull 44, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters AJ, Williams LM, 2011. Negative biases and risk for depression; integrating self-report and emotion task markers. Depress Anxiety 28, 703–718. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1999. Wechsler abbreviated scale of intelligence. Psychological Corporation. [Google Scholar]
- Wiedlocha M, Marcinowicz P, Krupa R, Janoska-Jazdzik M, Janus M, Debowska W, Mosiolek A, Waszkiewicz N, Szulc A, 2018. Effect of antidepressant treatment on peripheral inflammation markers - A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 80, 217–226. [DOI] [PubMed] [Google Scholar]
- Wright SL, Langenecker SA, Deldin PJ, Rapport LJ, Nielson KA, Kade AM, Own LS, Akil H, Young EA, Zubieta JK, 2009. Gender-specific disruptions in emotion processing in younger adults with depression. Depress Anxiety 26, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]