Highlights
-
•
Patients who develop RCC bone metastases continue to have a poor prognosis.
-
•
RCC bone metastasis treatment failure is more prevalent, likely in part because of longer patient survival.
-
•
RCC bone metastases frequently cause bone destruction that requires surgical intervention.
-
•
RCC bone metastases develop by a vicious cycle in bone that differs from breast cancer and prostate cancer, and more closely resembles multiple myeloma.
Keywords: Renal cell carcinoma, Bone, Metastasis, Bisphosphonate, Denosumab, Anabolic failure
Abstract
Renal cell carcinoma (RCC) is the most common malignancy of the kidney, representing 80–90% of renal neoplasms, and is associated with a five-year overall survival rate of approximately 74%. The second most common site of metastasis is bone. As patients are living longer due to new RCC targeting agents and immunotherapy, RCC bone metastases (RCCBM) treatment failure is more prevalent. Bone metastasis formation in RCC is indicative of a more aggressive disease and worse prognosis. Osteolysis is a prominent feature and causes SRE, including pathologic fractures. Bone metastasis from other tumors such as lung, breast, and prostate cancer, are more effectively treated with bisphosphonates and denosumab, thereby decreasing the need for palliative surgical intervention. Resistance to these antiresportives in RCCBM reflects unique cellular and molecular mechanisms in the bone microenvironment that promote progression via inhibition of the anabolic reparative response. Identification of critical mechanisms underlying RCCBM induced anabolic impairment could provide needed insight into how to improve treatment outcomes for patients with RCCBM, with the goals of minimizing progression that necessitates palliative surgery and improving survival.
1. Introduction
In 2021, The American Cancer Society estimates that 73,700 patients will be newly diagnosed with a renal cortex or renal pelvis cancer and that 14,830 will succumb to their disease [17]. Renal cell carcinoma (RCC) is the most common malignancy of the kidney, representing 80–90% of renal neoplasms, and is associated with a five-year overall survival (OS) rate of approximately 74% [18], [19]. One-third of patients will present with locally advanced or metastatic disease and an additional one-third will develop metastatic disease following nephrectomy [20]. The second most common site of metastasis is bone; lung is the most common. Skeletal involvement is found in 20–39% of patients [20], [21], [22], [23], [24], [25], [26]. As patients are living longer because of targeted treatments and immunotherapy, RCC bone metastases (RCCBM) are becoming more prevalent [27]. Bone involvement results in skeletal related events (SRE) such as increased pain, hypercalcemia, nerve compression, and pathologic fractures. Surgical intervention is undertaken for impending or completed pathologic fracture, and has become more prevalent in recent years.
Bone metastasis formation is an independent risk factor for decreased survival [28] and is a major contributor to morbidity and mortality. In this regard, the International Kidney Cancer Working Group (IKCWG) has identified the presence of either bone or liver metastases as conferring significantly worse overall survival (OS) compared to other metastatic sites [29]. While there have been advances in medical and surgical therapy for primary RCC, the overall survival among patients after developing RCCBM is only 19.7 months [30], [31]. RCCBM are most commonly found in the pelvis, sacrum, spine, and proximal extremities [32]. Lesions are predominantly osteolytic (79% osteolytic, 7% osteoblastic, 13% mixed) and are driven by the predominance of bone resorption over anabolic activity [23]. Over 70% of patients with bone metastasis present with multiple site involvement [27], exposing the majority of patients to the risk of SREs and associated morbidities. The number of bone metastases correlates with the OS: patients with a solitary bone metastasis have a median survival of 28 months; OS is 18 months with 2–5 bone metastases; and OS is reduced to 9 months with > 5 bone metastases [33], [34]. Surgical intervention (metastasectomy) is the only known treatment for improving survival in patients with solitary (but not multiple) bone metastasis; suggesting that the osteogenic niche promotes secondary metastatic dissemination to other organs.
Treatment resistance with RCCBM presents a vexing dilemma for changing the prognosis of RCC. Because of the comparatively low response rates to bone targeted therapy such as bisphosphonates and denosumab [19], [20], additional interventions for the purposes of palliation (rather than cure) are often the only remaining option. These nonspecific treatments do not effectively target critical steps in RCCBM progression in bone. Most often, they include radiation for bone pain (in nearly 80%), and surgical intervention to treat or prevent an impending fracture (28%) [20], [35], both of which are temporally late events in a process that culminates in progressive osteolysis. Antiresorptive agents such as bisphosphonate or denosumab treatment have not changed overall survival for RCCBM patients [36]. The reasons for this remain incompletely understood, however, accumulating evidence indicates unique aspects of RCC growth and progression in bone make it less dependent on osteoclast activity in early stages. Indeed, recent evidence by our group indicates that in earlier steps, RCC inhibits the bone anabolic reparative response via a paracrine mechanism mediated by BIGH3/TGFBI [37] that targets osteoblasts and osteocytes. Invading RCC tumor cells dysregulate bone by inhibiting osteoblast differentiation and inducing osteocyte apoptosis. This creates a pro-osteolytic environment unique to RCCBM that does not initially depend on osteoclast amplification, and consequently is resistant to antiresorptive agents. As shown in Fig. 1, the RCCBM impairment of bone anabolic response is a distinct mechanism for progression in the osteogenic niche, that differs from the ‘vicious cycle’ induced by breast cancer bone metastasis (and others, see below). In comparison to the other solid tumors, patients with RCC (see Table 1):
-
1.
Are more than twice as likely to have distant disease at diagnosis compared with prostate, breast and thyroid cancer.
-
2.
Have a 5-year OS that is worse in comparison to other solid tumors (breast, prostate, thyroid), with the exception of lung cancer.
-
3.
Have a higher incidence of surgery for bone metastasis (only thyroid cancer has a higher rate), despite having a lower incidence of bone metastasis compared with breast, prostate, and lung cancer patients.
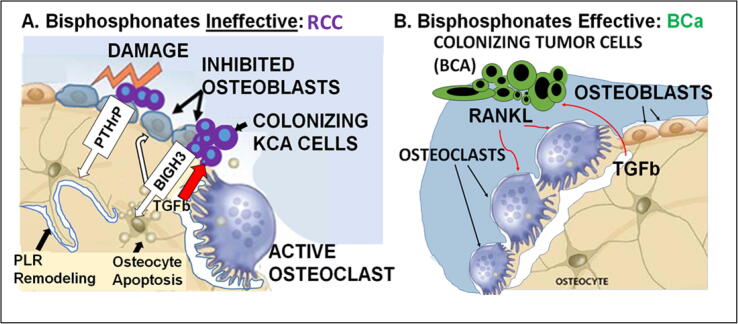
Fig. 1.
A. Colonization of bone by metastatic RCC cells triggers osteocyte amplified osteolysis via osteocyte dysregulation/apoptosis and osteoblast inhibition rather than direct osteoclast activation. As a result, osteolysis is resistant to bisphosphonate treatment. B. In contrast, breast cancer cells in bone directly stimulate osteoclast activity, establishing a ‘vicious cycle’ that is inhibited by bisphosphonates.
Table 1.
Comparative survival and surgery statistics for various cancers. Sources: SEER (https://seer.cancer.gov/), [4], [5], [6], [7], [12].
| Primary Tumor | Overall 5 yr survival (%) | Overall 5 yr survival with distant disease at diagnosis (%) | OS Decrease with distant disease (% of 5y OS) | Distant disease at diagnosis (%) | Incidence of Bone Metastases (%) | Frequency of SRE (%) | Incidence of Surgery for Bone Metastases (%) |
|---|---|---|---|---|---|---|---|
| Renal | 73.9 | 12.3 | 61.6 (83%) | 13.5 | 20–25 | 34 | 29 |
| Prostate | 98.4 | 30.1 | 68.3 (69%) | 5.8 | 65–75 | 49 | 4 |
| Breast | 87.5 | 31.5 | 56 (64%) | 5.7 | 65–75 | 68 | 11 |
| Lung | 17.8 | 4.5 | 13.3 (74%) | 50.8 | 30–40 | 53 | 9 |
| Thyroid | 97.8 | 54.8 | 43 (44%) | 3.6 | 60 | 5 | 40 |
| Multiple Myeloma | 47.7 | 46.5 | 1.2 (2.5%) | 95.2 | N/A | 51 | 4 |
Evidence determining if metastasectomy is effective for managing oligometastatic disease remains inconclusive. Consistent with SEER data (Table 1), in recent years the frequency of surgical intervention for RCCBM at our institution has increased, such that surgery for RCCBM has become more common than surgery for bone metastases of cancers of the breast, prostate, or lung (submitted, under review). Consequently, we hypothesized that resistance to bisphosphonate therapy contributes to the increased need for surgical intervention in patients with RCCBM [37]. This idea was motivated in part by a need to further study the role of earlier heterotypic interactions between RCC cells and the bone microenvironment. If correct, there is promise for identifying novel therapeutic targets to reduce RCCBM progression and the consequent need for palliative surgery.
In this review, we further examine the possible roots for treatment resistance in RCCBM by comparing pathophysiologic mechanisms (Table 2, Table 3) and the data for bisphosphonate efficacy to BM from breast, prostate, and lung cancers, which are the most common solid tumors causing bone metastases. In addition, contributing factors from new generation primary tumor targeted therapies are reviewed [4], [5], [6], [12]. Preclinical evidence supports treating patients with RCCBM using bone anabolic agents, such as cabozantinib, in addition to anti-resorptive treatments [38], [39]. We also summarize current treatment strategies that target RCCBM and compare them to treatments for bone metastasis from prostate, breast, and lung cancers. We contend that although all of these tumor types respond to the bone microenvironment, innate differences in the cancer cells themselves make each a unique metastatic disease that needs a specifically tailored treatment strategy. This idea suggests the title of this article.
Table 2.
Cytokines in bone microenvironment and bone targeted treatment in different primary tumors.
| Cytokines Involved | References | Osteoblastic/Osteolytic | Bisphosphonates/Denosumab Recommended | |
|---|---|---|---|---|
| Renal Cancer | TGF-β, PDGF, FGF, ILGF, BMP1-3 BIGH3 (Pan et. al., 2018) | [1], [2], [3] | Osteolytic | Bis: Zolendronate (Zol) Denosumab |
| Prostate Cancer | GDF15, FGF, endothelin 1 | [8] | Osteoblastic | Bis: Zol Denosumab |
| Breast Cancer | IL-11, CTGF, CXCR4, MMP1 | [9], [10], [11], [12] | Mixed | Bis: Zol, Pamidronate, Clodronate, Ibandronate Denosumab |
| Lung Cancer | DKK1, IGFBP3, PTHrP, IL-11, TGF-β | [6], [13], [14] | Osteolytic (non-small cell), osteoblastic (small cell) | Zol Denosumab |
| Multiple Myeloma | RANKL, MIP1alpha, IL-3, IL-11, TNF, HGF | [12], [15], [16] | Osteolytic | Bis: Zol, Pamidronate Denosumab |
Table 3.
Bone metastasis impact on the osteogenic niche in different primary tumors.
| Bone metastasis phenotype | Osteogenic niche interaction |
|||
|---|---|---|---|---|
| Early | Late | DTC survival | ||
| Renal Cancer | Osteolytic | Osteoblast inhibition + Osteocyte apoptosis | Osteoclast activation | Short term Unknown Long term |
| Prostate Cancer | Osteoblastic | Osteoblast activation + tumor cell osteomimicry + Osteoclast activation | Osteoclast activation | Short term and long term DTC survival |
| Breast Cancer | Osteolytic/Mixed | Osteoclast activation + osteoblast inhibition | Osteoclast activation | Short term and long term DTC survival |
| Lung Cancer | Osteolytic (non small-cell) Osteoblastic (small cell) |
Osteoclast activation | Osteoclast activation | Short term Unknown Long term |
2. Renal cell carcinoma bone metastasis pathogenesis
Important aspects of the pathogenic mechanism for RCCBM progression in bone remain unexplored and are the focus of current research by our group and others. The relative roles of dormancy, migration, and proliferation in the clinical manifestations of RCCBM, including SRE, treatment response/resistance, and survival are important questions that need further study. In RCC, the colonizing cancer cells simultaneously inhibit the reparative response of osteoblasts, induce osteocyte apoptosis, and in the terminal phase of the process, enter a vicious cycle that activates osteoclasts, creating an uncoupling of homeostatic processes that tips the balance towards resorption and induces progressive bone destruction (Fig. 1) [37], [40]. Osteolysis releases bone-derived growth factors and cytokines that further potentiate cancer cell proliferation and tumor growth, creating a vicious cycle that clinically is manifested as SRE. Factors that are released (Table 2) include transforming growth factor–β (TGF-β), platelet derived growth factor (PDGF), fibroblast growth factor (FGF), insulin like growth factors, and bone morphogenic protein (BMP) [1], [2], [3]. These factors stimulate growth of RCC cells within the bone as well as promoting cell migration, and changes in phenotype (possibly by epigenetic reprogramming and adaptation) that lead to secondary dissemination to other organs. Invading RCC tumor cells interact in the osteogenic niche both via paracrine factors and direct heterotypic contact with bone resident cells. Tumor cells secrete factors such as parathyroid hormone-related peptide (PTHrP), TGF-β and vascular endothelial growth factor (VEGF) [41], [42]. These factors stimulate osteoblasts, increasing production of receptor activator of nuclear factor κB ligand (RANKL). The increased RANKL promotes osteoclast recruitment and activation. So why don’t antiresorptives work more effectively? Recent evidence suggests that RCC cells in bone accelerate osteolysis by simultaneously inhibiting the osteoblast reparative response, inducing osteocyte apoptosis, and tipping osteocyte regulation towards osteolysis via the tumor derived paracrine factor BIGH3 (Fig. 1A) [37], [40]. In recent work, our group explored the role of BIGH3, a paracrine factor secreted by RCC cells invading bone that inhibits osteoblast differentiation, in promoting RCCBM progression. Blocking BIGH3 reduces osteolysis from RCCBM in vivo[37]. Moreover, bone anabolic agents, such as BMPs, and the small molecule TKI cabozantinib (which is active in promoting osteoblastic differentiation), similarly reduce RCCBM progression in preclinical models [37]. In contrast to other solid tumors, osteolytic RCCBM do not seem to amplify osteoclast activity locally [37], [40]. This evidence points to a mechanism that is distinct from the osteoclast centered vicious cycle caused by breast cancer (BCa) (Fig. 1B). As such, it is an alternative pathway that might create resistance to treatment with antiresorptives that target osteoclasts, such as bisphosphonates and denosumab.
Other examples of osteoblast inhibition by tumor cells invading the osteogenic niche have been observed, although the temporal relationship of osteoblast inhibition to osteoclast activation may differ from RCCBM pathophysiology in ways that are incompletely defined (Table 3). In preclinical models, BCa cells were shown to inhibit osteoblast differentiation via the Runx2 and CBFβ (core binding factor beta) pathways [43]. Runx2 was also shown to simultaneously induce overexpression of GM-CSF, an osteoclast activator, by metastatic BCa cells [43]. Similarly, the interactions between myeloma cells and bone-residing cells lead to both increased bone resorption and suppressed osteogenesis. Metastatic multiple myeloma cells are able to suppress the differentiation of osteoblasts, causing to decreased bone deposition. This occurs via IL-3, sclerostin, TGFB, IL-7, TNF-alpha, DKK1, and zinc finger protein GFI1 [7], [15], [16]. Moreover, metastatic myeloma cells also induce epigenetic changes at Runx2 (the same pathway as metastatic BCa), preventing osteoblasts from terminally differentiating [44]. Myeloma cells also promote apoptosis of osteocytes (a mechanism proposed to be active with RCCBM –see Fig. 1A); and simultaneously promote osteolysis via cross-talk in the bone microenvironment that induces release of pro-osteoclastogenic factors, including RANKL, IL6, Activin A, MCSF, and MIP-1α. Because MM is not a solid tumor and arises in the bone marrow, it has unique immunologic aspects that distinguish it from BCa and RCC bone metastasis. However, because some aspects of the pathologic process of osteolysis appear to overlap with mechanisms for RCCBM, they deserve consideration for developing treatment strategies for RCCBM. In particular, the temporal relationship between inhibition of the anabolic response (via osteoblast suppression and osteocyte apoptosis), to the promotion of osteoclast activity, may be an important determinant of observed differences between RCCBM and BCa or MM in response to antiresorptive treatments.
Another important pathway for RCCBM involves cadherin-11 [40]. In order for tumor cells to adhere to bone, adhesion factors, including cadherin-11, are expressed on the surface of bone metastatic cells. Cadherin-11 is a calcium-dependent cell–cell adhesion molecule. Studies have shown that cadherin-11 upregulation is required during RCC bone colonization steps[45], [46], [47], [48]. Cadherin-11 knockout also inhibits RCC migration, reducing metastatic potential [49]. It is not known if targeting these early heterotypic cell adhesions in RCCBM could be therapeutically advantageous for OS or PFS. More work is needed to identify critical aspects of colonization and progression that induce treatment resistant osteolysis.
3. Current treatment strategies for RCCBM
Current treatments for RCCBM include the antiresorptive bone targeting agents including the bisphosphonates and denosumab [50], [51]. Bisphosphonates inhibit resorption by interfering with osteoclast attachment to the bone mineral surface [52]. Bipshosphonates have been shown to prevent both the onset of SRE, and SRE progression in patients with RCCBM [53]. There have also been subsequent reports of seemingly contradictory results regarding SRE in RCC patients [36]. However these retrospective studies were neither controlled nor designed to examine SREs or specific bisphosphonate treatments, and as such are not comparable because of selection bias, etc. Although bisphosphonates have also reduced skeletal complications in many solid tumors [54], pooled analysis in RCC patients found that they did not improve PFS or OS [36]. Denosumab has similarly been shown to reduce SRE, without impacting PFS or OS [55]. Denosumab is a humanized monoclonal antibody that inhibits bone resorption by binding and inhibiting soluble RANKL, which is essential for osteoclast formation and function [56]. Denosumab is FDA-approved for the prevention of SREs in patients with bone metastases from solid tumors [57], [58]. In a trial of over 1700 bone metastatic patients (including > 100 RCC patients) denosumab was found to be nonequivalent (trending towards superiority) to the zoledronic acid in preventing or delaying SRE [39]. More recent analysis of pooled phase III trials confirmed the superiority of denosumab to zolendronic acid in preventing SREs from solid cancer bone metastases, regardless of bone metastasis number, visceral metastasis, or ECOG patient status [55]. These therapies have not been compared specifically in RCC patient cohorts. However, the efficacy of these bone targeting treatments in reducing SRE in patients with RCCBM highlights the importance of the RANK/RANKL pathway for progression to bone destruction that is clinically significant [53], [55].
The lack of impact on survival (OS and PFS) in RCC patients receiving antiresorptives suggests that overall disease progression is not improved by preventing SRE. When coupled with the observation that surgical excision of solitary RCCBM not only prevents local recurrence, but also is favorable for OS and PFS [33], a hypothesis emerges that the process of RCCBM progression in the osteogenic niche may have temporally targetable stages. In this scenario, a SRE represents the end stage of bone destruction, which is preceeded by critical tumor growth and evolution that causes clinical progression via secondary dissementation to other organs. In addition, uptake of bisphosphonates and denosumab in the advanced, highly osteolytic, end-stage RCCBM may be suboptimal, due to both large scale and microscopic destructive changes to the osteogenic niche. More work is needed to explore this possibility. If correct, earlier interventions with agents that target critical steps in the pathophysiology of RCC progression in bone (prior to SRE that are clinically apparent) may be necessary to improve survival outcomes.
Given that RCC primary tumors are hyper-vascular and overexpress VEGF encoding transcripts compared to normal adjacent tissues [59] use of FDA-approved tyrosine kinase inhibitors (TKIs) that target VEGF receptors (VEGFRs) offer an intriguing treatment study focus. Unfortunately, many of these targeted therapy trials in RCC have excluded patients with bone-only metastatic disease, ostensibly because lytic lesions require a measurable soft tissue component in order to use the Response Evaluation Criteria in Solid Tumors (RECISTv1.1) criteria [60]. As a result, the majority of clinical data for patients with RCCBM is retrospective. Sunitinib is a multi-tyrosine kinase inhibitor that broadly targetsVEGF1/2/3, PDGFα/β, KIT, FLT3, RET, and CSF1R receptors. It is one of the first line TKIs with efficacy in preventing bone metastasis progression. Although this drug has shown limited success in treating RCCBM patients, it is more effective than sorafenib [61] another first line, small molecule TKI for RCCBM), or interferon alpha in preventing formation and prolonging the time to occurrence of new bone lesions [62].
A new generation of targeted therapies have supplanted the use of earlier agents. Everolimus is FDA-approved treatment for patients with RCCBM. Pre-clinical data has indicated mTOR signaling is important to bone homeostasis and dysregulation of mTORC1 could contribute to various skeletal diseases including osteoarthritis and osteoporosis [63]. Everolimus inhibits differentiation of osteoclasts in vitro [64], [65]. And interestingly, combining everolimus with antiresorptives enhances the efficacy in bone. Phase II clinical trial data in patients with RCCBM found the addition of zoledronic acid to everolimus reduced bone resorption markers, increased PFS, and increased time to first SRE [50]. This suggests that combining antiresportives with anti-tumor agents (like Everolimus) that also target the osteogenic niche should be further explored. More evidence in support of this strategy has recently emerged with the discovery and approval of RCC treatments that simultaneously enhance the bone anabolic response.
Cabozantinib, a small molecule MET/HGF inhibitor, is hypothesized to have greater effect on bone metastases as it targets receptors for VEGF, upregulated in RCC, and MET, an important signaling pathway in both osteoblasts and osteoclasts [66]. In preclinical studies, cabozantinib reduced osteolysis from RCCBM [40]. Retrospective subgroup analyses of patients with bone metastases in the phase II front-line study comparing cabozantinib and sunitinib and the second-line phase III study comparing cabozantinib and everolimus (an mTOR inhibitor) both found improved PFS with cabozantinib (respectively: HR 0.54, 95% CI 0.31–0.95 [67]; HR 0.33, 95% CI 0.21–0.51 [68]). Cabozantinib also had better OS (HR 0.54, 95% CI 0.34–0.84) and a 6% lower SRE rate than everolimus [68]. Cabozantinib targets VEGF1/2/3, c-MET, AXL, FLT3, RET, TRKB, and TIE2. In both three-dimensional (3D) bone metastasis co-culture models and preclinical mouse models, cabozantinib is anabolic for osteoblasts, and inhibits osteoclast differentiation [37], [40]. Additionally, of note, in patients with metastatic papillary RCC, a subtype accounting for 10–15% (making it the second most common subtype of RCC), the presence of bone metastases is an unfavorable prognostic factor associated with decreased median PFS (4 vs 7 months) and median OS (7.5 vs 19 months) even when receiving VEGF-TKI therapy [69]. These results are supportive of pursuing a strategy that includes enhancing the anabolic response at earlier time points in RCC bone metastasis progression, in addition to treating the tumor and inhibiting osteoclasts with antiresorptives.
Data is sparse for immunotherapy. Subgroup analysis of patients with bone metastases treated with nivolumab on CheckMate-025 in the non-front-line setting showed increased overall response rates compared to patients treated with everolimus (26 vs 6%) [70], [71]. However, in a large tumor agnostic study, bone metastases were associated with decreased response to immunotherapy [72]. This is an urgent area for future study.
4. Surgical management of RCCBM
Osteolytic RCCBM destroy cortical bone without periosteal reaction, indicative of suppression of the repair response. Bone erosion often results in pathologic fractures with little potential for spontaneous union without surgical intervention. Surgery is necessary in a large proportion of RCCBM patients, including those who present with: 1) intractable pain; 2) pathological fracture or impending fracture, 3) spinal instability, or 4) spinal cord compression. In the case of solitary bone metastasis, there is a possibility of a curative surgery [19], [73]. Complete resection of solitary RCCBM is prognostically favorable and potentially curative, indicating that the biology for RCCBM progression in bone has unique features, distinct from cancers in the prostate and breast [33], [34]. Secondary dissimenation from bone to other organs may be important in this regard, and deserves further investigation. Surgical procedures commonly used include excision, reconstruction, internal fixation, and/or neural decompression [19], [74]. Surgical intervention that introduces mechanical stabilization allowing immediate weightbearing produces the best outcomes. Fixation can be achieved with internal fixation or with a prosthesis combined with polymethyl methacrylate (PMMA) bone cement [18]. During surgery, local cryotherapy and or adjuvants also can be considered [75]. Prosthetic replacement of affected bone segments is used for large RCCBM osteolytic defects [76]. Operations for additional synchronous and metachronous bone metastases have become more common as patient survival improves [73].
Dormancy is poorly understood in RCCBM; however, the overall favorable effect on survival after surgical resection implies that it is less of a factor in RCC than in breast or prostate cancer (PCa), where bone metastasis can form after many years of latency. Following surgery, good overall prognoses are associated with tumor-free surgical margins. A negative wide surgical margin improves the 5-year recurrence free survival from 11% to 31%, compared to intralesional margins [34]. A recent study evaluated surgical outcomes in 45 patients, finding that pain relief was achieved in 91% and good-to-excellent functional outcome was achieved in 89%. However, overall survival following surgery remains low, at 47% and 11% at one a five years respectively [33], highlighting the palliative nature of these surgical interventions, and the need for novel, efficacious bone targeting treatments.
As the mechanistic pathways for pathologic progression for bone metastasis have been determined for a variety of tumors, it has become increasingly apparent that there are unique and critical mechanisms for each that account for the differential responses to bone targeting therapies. In what follows, an examination of these mechanisms is covered for the most frequently treated bone metastasis (prostate, breast, and lung cancers) in order to highlight critical differences between solid tumors, and to identify potential treatment strategies that are more specific for RCCBM.
5. Prostate cancer bone metastasis
Prostate cancer bone metastases (PCaBM) have a predominantly blastic phenotype, due to osteomimicry. Invading PCa cells secrete growth factors such as TGFβ, BMPs, FGF, and Wnt, which promote osteoblastic differentiation. Moreover, PCa cells also secrete ET-1 and PSA, which can inhibit bone resorption, thereby shifting the balance of bone homeostasis towards osteogenesis [8]. In addition to these distinguishing characteristics, additional processes have been identified that likely uniquely contribute to osteogenesis from PCaBM (in contrast to RCCBM), including: tumor cell reactivation from dormancy, and induction of endothelial cell conversion to osteoblasts via BMP4 [77]. The dual-directional feedback also can produce a mixed sclerotic/lytic picture within the bone. Mechanistically, tumor cells secrete parathyroid hormone-related protein (PTHrP), which induces the activation of NF-κB secretion by osteoblasts. This stimulates the maturation of monocytes into osteoclasts. Osteolysis releases calcium along with many growth factors such as TGFβ that bind to tumor cells, inducing additional production of metastases’ promoting factors such as PTHrP and Jagged1 [31].
For patients with PCaBM, bisphosphonate and denosumab treatments have reduced the risk of SRE, and hence the need for surgical intervention. Bisphosphonates have dual bone anabolic effects in PCa patients: reducing osteoporosis from ADT, and reducing the risk of SREs in patients with PCaBM. In one study, zoledronic acid (4 mg intravenous every three months) in combination with ADT increased BMD at all skeletal sites after one year of treatment [78]. In fact, one dose at the start of ADT improved BMD [79]. Zoledronic acid is FDA-approved for the treatment of patients with PCa that have progressed after treatment with one hormone therapy [80]. In a large phase III trial, patients with metastatic castration-resistant PCa (mCRPC) to bone were randomized to receive either zoledronic acid 8 mg, 4 mg, or placebo every three weeks for 15 months [81]. The 4 mg dose was the most efficacious with median time to SRE of 488 days compared to 363 days for patients receiving 8 mg, and 231 days for patients in the placebo group [81], [82]. Long-term follow up in patients who completed 24 months on study found that 40% patients in the 4 mg group had SREs compared to 49% in the placebo group [82]. In the TRAPEZE trial, when combined with docetaxel, zoledronic acid increased median SRE-free interval, but did not increase overall survival or clinical PFS (defined as pain progression, SRE, or death) in patients with mCRPC when compared to docetaxel (standard of care), strontium 89, or both [83]. In addition, the oral bisphosphonate clodronate increased OS in metastatic patients on ADT (HR 0.77, p = 0.032), though there was no OS benefit in patients with non-metastatic disease [84].
Other treatments that inhibit osteoclast resorption are efficacious in treating PCaBM, indicative of osteoclast dependent mechanisms that are targetable when bone metastasis are clinically apparent (in contrast to RCCBM). In a randomized phase III trial of 1904 patients, denosumab outperformed zoledronic acid in patients with castration-resistant metastatic PCa [85]. Median time to first skeletal-related event (SRE) was 20.7 months with denosumab compared to 17.1 months with zoledronic acid (p = 0.0002). Additional studies validated denosumab’s superiority at preventing SREs or symptomatic skeletal events (SSEs) from PCaBM, similar to other solid tumors [86].
For PCaBM, targeting pathologic sclerosis also is effective. Radium-223 dichloride is a targeted alpha radiation emitter that acts as a calcium mimetic and accumulates in the hydroxyapatite bone matrix at sites of bone remodeling, such as occur in osteoblastic and sclerotic metastases [87]. The radiation induces double-stranded DNA breaks and has a local cytotoxic effect [88]. A phase III trial of 921 patients with PCaBM found that radium-223, as compared to placebo, improved OS (median 14.9 months vs 11.3 months, p < 0.001) [89]. Treatment was well tolerated with low rates of myelosuppression. Given the survival benefit, radium-223 is FDA-approved for patients with mCRPCa with symptomatic bone metastases and no known visceral disease [90].
The efficacy of these additional, PCa specific interventions for treating PCaBM also is demonstrated by a reduction in surgical intervention rates compared to BCa and RCC patients with bone metastases (Table 1). A similar, mechanism based strategy for treating RCCBM has not been identified nor implemented, but potentially can improve outcomes.
6. Breast cancer bone metastasis pathogenesis
BCa bone metastasis (BCaBM) are predominantly osteolytic. The pathologic processes induced by bone invasion, colonization, progression, and further dissemination, are well characterized, and serve as an accepted basis for comparison with other tumors. The ‘vicious cycle’ feedback-loop that produces BCaBM is established when tumor cells either arrive in bone or are reactivated from dormancy. BCa cells proliferate in the osteogenic niche, undergoing local expansion and activating reciprocal stimulations with osteoblasts and osteoclasts. This cell–cell crosstalk is amplified by tumor cells that secrete pro-osteoclastogenic cytokines such as RANKL to stimulate bone resorption (Fig. 1B). In contrast to RCC, BCa cells directly activate osteoclasts by paracrine secretion of RANKL, thereby accelerating osteolysis (Fig. 1B). TGFβ released from the bone matrix induces the expression of osteolytic factors such as PTHrP and Jagged1 from tumor cells [9], [10], [11]. Similarly, pro-osteoblastogenic factors can be released by tumor cells, resulting in the development of sclerotic lesions, and a mixed pattern. BCa cells also inhibit osteoblast differentiation, which impairs the compensatory repair response [43]. Hence, in a majority of cases, osteolysis predominates as a tipping point that when reached, pathologically harnesses bone homeostasis to amplify the resorptive phenotype. Consequently, antiresorptive therapies are effective for treating BCaBM.
Some of the first studies that found significant clinical benefit from bisphosphonates for the treatment of bone metastases were in BCa. A large proportion (65–75%) of BCa patients develop bone metastases, providing a large population to determine treatment efficacy of bone targeting agents. In contrast to RCC, bisphosphonate treatment is associated with a survival benefit in BCa patients. In clinical trials, bisphosphonates also reduce the rates of SRE for up to two years following initiation of treatment [91], [92] and decrease bone pain [93] when compared to placebo. A pooled analysis of two randomized studies in BCa patients found that those receiving pamidronate had significantly lower rates of SRE compared to the placebo group (2.4 vs. 3.7, p-value < 0.001) [93]. Moreover, patients in the treatment group had significantly less pain compared to the placebo group after 24 months of follow-up (p-value < 0.015) [93]. The dosing interval of zoledronic acid was investigated, demonstrating no difference between a 4- or 12-week dosing schedule for reducing SRE [94]. Finally, bisphosphonates were found to increase the length of time before BCa metastasizes to the bone [91], [95], [96], [97], [98].
Clinical trials with antiresorptives also have examined the best strategies for preventing BCaBM formation. In the D-CARE trial, among patients with stage II/III BCa, treatment with denosumab did not improve bone metastases free survival [99]. The AZURE trial studied post-menopausal women and examined the effects of zoledronic acid in early high-risk BCa treatment. The trial concluded that zoledronic acid reduced the incidence of bone metastases, even though the findings were limited to hormone-receptor positive BCa [100], [101].
Current recommendations and guidelines from Europe and Canada recommend using a bone modifying agent in patients with bone metastatic disease, and specifically state that either zoledronic acid or denosumab are appropriate choices [102]. Consequently, the use of these bone modifying and targeting agents to treat bone metastases is widespread among BCa patients. A retrospective review found that 56% of BCa patients received a bisphosphonate during treatment [39]. Despite these recommendations, approximately 30% of BCa patients have an SRE prior to starting bisphosphonate therapy, indicating that more aggressive and earlier management would be beneficial [38].
In a recent review of the surgical experience at a large cancer center, it was noted that the frequency of surgical intervention for impending or presenting pathologic fractures has decreased for BCa patients since the introduction of bisphosphonates (Fosomax), following FDA approval in 1996 (submitted, under review). Moreover as found in this study, 2018 was the first year that more surgeries were performed for bone metastasis in RCC patients than in BCa patients—a trend that has subsequently continued in agreement with nationwide cancer data (Table 1) (SEER (https://seer.cancer.gov/)).
7. Lung cancer bone metastasis pathogenesis
Lung cancer patients with bone metastasis continue to have the poorest prognosis, with an even worse OS than RCC patients (Table 1). The OS decrease (as a % of 5 y OS) with the development of distant disease is similar to RCC patients (Table 1). Tumor dissemination occurs early in lung cancer (LCa) and is unrelated to the size of the primary tumor [13], [14]. Osteoblasts attract cancer cells via expression of stromal derived factor-1 (SDF-1) and annexin II (Anxa2) receptors [13]. In addition, physical factors within the bone such as hypoxia, acidic pH, extracellular calcium activate tumor expression of osteoblast stimulatory factors including BMPs, VEGF, and ET-1. Osteoblasts are induced to release CCL2 and CXCL8 that stimulate osteoclasts to cause osteolysis in the bone [14].
Studies examining the efficacy of bisphosphonates in LCaBM showed promising results for preventing metastasis formation, associated pain, and OS [38], [103]. A meta-analysis of 7 studies showed a 19% risk reduction for developing new skeletal related events (SRE) within the first two years of treatment with zoledronic acid (RR = 0.81, 95% CI: 0.67–0.97) [54], [104], [105], [106], [107]. In addition, pain control was superior when bisphosphonates were given concurrently with chemotherapy or radiation therapy [107], [108], [109], [110]. Moreover, OS was increased by a median of 72 days among patients that received zoledronic acid [54], [104], [105], [106], [107], [111]. Additional studies are needed to determine if the benefits of bisphosphonates in LCaBM are limited to reducing SRE, and if this treatment reduces the need for surgical intervention.
Though the benefit of bisphosphonates in patients with LCaBM is established, the benefit is partly dependent on the type of bisphosphonate given, and if there are additional treatments [107], [109], [111], [112], [113]. As a consequence, bisphosphonates are used less commonly in patients with lung cancer bone metastases (LCaBM) compared with BCa and PCa patients, despite recent recommendations [114], [115]. Radiotherapy can be used synergistically. The bisphosphate clodronate combined with radiotherapy delays BM progression three months compared to treatment with radiotherapy alone [107], [109]. However, after two completed treatment cycles, it was determined that there was no difference in overall disease progression between the two groups. Moreover, most studies reported no difference in survival when comparing bisphosphonates plus chemotherapy to chemotherapy alone [39], [58], [104], [107], [111], [116], [117].
As patient survival has improved, the incidence of patients with BM and those requiring surgical intervention has increased. In comparison to RCC the incidence of surgery is currently lower, although this may change as patient survival continues to improve. Surgically, the strategy for stabilizing actual or impending pathologic fractures in LCaBM patients is similar to the techniques used in patients with PCa and BCa. The bone metastasis from LCa are almost exclusively osteolytic. However, in contrast to RCC, the response to radiation is more consistent. Thus, an intralesional procedure (e.g. intramedullary nailing), rather than metastectomy, is usually preferred.
Overall, and similar to RCCBM, the pathophysiology of LCaBM deserves further study. More insight on the specific changes in the oteogenic niche that create the vicious cycle of bone destruction will help in identifying treatments that are more effective.
8. Summary and discussion
This study provides an overview of the treatment landscape for RCCBM in comparison with bone metastasis from other solid tumors that frequently metastasize to bone. RCCBM resistance to current treatment modalities results in more frequent palliative surgery. The differences in the pathogenesis of bone metastasis formation are summarized in Table 2. Amongst solid tumors, RCC consistently causes bone metastases that are highly destructive, frequently cause SRE, and portend a poor prognosis which has not significantly improved with antiresorptive therapy use . In recent years, surgery for patients with RCCBM has increased relative to other solid tumors (PCa, BCa, and LCa). Translational research to understand the pathophysiology of RCCBM is underway, and remains highly worthy of future work as we move toward bone targeting medicine for metastatic disease.
For treatment strategy, surgery and radiation for RCCBM are traditionally the last and only options after medical and non-invasive modalities have failed. They are rarely curative. The example of RCCBM highlights an important limitation for antiresorptive therapy. Bone targeting treatments have been in widespread use for the last decade. Overall, this has contributed to the improved survival in patients with bone metastases from BCa and PCa, with slower progression to SRE. RCCBM impact the osteogenic niche in unique ways, including inhibition of the osteoanabolic response, as well has dysregulation of bone homeostasis via osteocyte apoptosis, rather than direct osteoclast stimulation (as occurs with BCa), as shown in Fig. 1. The critical, early, RCC-bone cell interactions underlying these processes (i.e. tumor-osteoblast and tumor-osteocyte) are not directly impacted by antiresorptive treatments such as bisphosphonates, and may be the reason for treatment resistance as evidenced by the lack of impact on survival. However, combined therapy approaches which include bone anabolic agents have shown early promise. In pre-clinical models, Pan et al. [40] demonstrated that agents that promote an osteoanabolic response are efficacious in limiting osteolysis from RCC that is otherwise unresponsive to antiresorptives.
Future translational studies for RCCBM are needed to further explore the cross-talk dynamics between metastasizing cells and cells in the bone microenvironment, in order to identify novel targets and to create efficacious treatments that overcome resistance to bisphosphonates and denosumab.
9. Conclusions
Bone metastases in patients with RCC is indicative of more aggressive disease and worse prognosis than many other solid cancers that metastasize to bone. Not only do RCCBM patients have poorer OS and increased surgical intervention rates, but they also have significant morbidity in the form of pathologic fractures and SRE. Data show a trend toward increasing incidence of clinically apparent bone metastasis in RCC, as well as higher rates of SRE, compared to other solid cancers. The increasing prevalence of treatment resistance in the late stages of RCCBM reflects unique cellular and molecular interactions in the bone microenvironment that promote progression. Additional studies are urgently needed to better understand the unique RCC-bone interactions that lead to pathologic osteolysis and SRE. Discovery of these mechanisms could provide much needed insight into how to better treat patients with RCCBM, with the goal to minimize progression that necessitates surgical intervention, and improve survival.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported research grants from: DOD CDMRP Kidney Cancer Research Program (KCRP)- Award W81XWH-20-1-0895; Knowledge Gap Award (MD Anderson Cancer Center); and Institutional Research Award (MD Anderson Cancer Center).
References
- 1.Kominsky S.L., Doucet M., Brady K., Weber K.L. TGF-beta promotes the establishment of renal cell carcinoma bone metastasis. J. Bone Min. Res. 2007;22:37–44. doi: 10.1359/jbmr.061005. [DOI] [PubMed] [Google Scholar]
- 2.Kakonen S.M. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 2002;277:24571–24578. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 3.Dallas S.L., Rosser J.L., Mundy G.R., Bonewald L.F. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J. Biol. Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 4.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 6.Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich L.A. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 8.Cook L.M., Shay G., Araujo A., Lynch C.C. Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev. 2014;33:511–525. doi: 10.1007/s10555-014-9494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 10.Mundy G.R. Bisphosphonates and tumor burden. J. Clin. Oncol. 2002;20:3191–3192. doi: 10.1200/JCO.2002.20.15.3191. [DOI] [PubMed] [Google Scholar]
- 11.Roodman G.D. Mechanisms of bone metastasis. New Engl. J. Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 12.Zekri J., Ahmed N., Coleman R.E., Hancock B.W. The skeletal metastatic complications of renal cell carcinoma. Int. J. Oncol. 2001;19:379–382. doi: 10.3892/ijo.19.2.379. [DOI] [PubMed] [Google Scholar]
- 13.Shiozawa Y. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J. Cell. Biochem. 2008;105:370–380. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.S.J. Coniglio. Role of tumor-derived chemokines in osteolytic bone metastasis. Front. Endocrinol. (Lausanne)9, 313, doi:10.3389/fendo.2018.00313 (2018). [DOI] [PMC free article] [PubMed]
- 15.Bataille R. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J. Clin. Oncol. 1989;7:1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- 16.R. Silbermann. et al. Bone marrow monocyte-/macrophage-derived activin A mediates the osteoclastogenic effect of IL-3 in multiple myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K28, 951-954, doi:10.1038/leu.2013.385 (2014). [DOI] [PMC free article] [PubMed]
- 17.R.L. Siegel, K.D. Miller, A. Jemal. Cancer statistics, 2020. CA: a cancer journal for clinicians70 2020 7-30 doi:10.3322/caac.21590. [DOI] [PubMed]
- 18.Umer M., Mohib Y., Atif M., Nazim M. Skeletal metastasis in renal cell carcinoma: a review. Ann. Med. Surg. (Lond) 2018;27:9–16. doi: 10.1016/j.amsu.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood S.L., Brown J.E. Skeletal metastasis in renal cell carcinoma: current and future management options. Cancer Treat. Rev. 2012;38:284–291. doi: 10.1016/j.ctrv.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Woodward E. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48:160–166. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Ruatta F. Prognosis of renal cell carcinoma with bone metastases: experience from a large cancer centre. Eur. J. Cancer. 2019;107:79–85. doi: 10.1016/j.ejca.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Weber K., Doucet M., Kominsky S. Renal cell carcinoma bone metastasis–elucidating the molecular targets. Cancer Metastasis Rev. 2007;26:691–704. doi: 10.1007/s10555-007-9090-y. [DOI] [PubMed] [Google Scholar]
- 23.Santini D. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis survey. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchi M. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann. Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 25.Huang J.F. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann. Transl. Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehne J., Tsagozis P. Current concepts in the surgical treatment of skeletal metastases. World J. Orthop. 2020;11:319–327. doi: 10.5312/wjo.v11.i7.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunwald V. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat. Rev. Urol. 2018;15:511–521. doi: 10.1038/s41585-018-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kankuri M. Feasibility of prolonged use of interferon-alpha in metastatic kidney carcinoma: a phase II study. Cancer. 2001;92:761–767. doi: 10.1002/1097-0142(20010815)92:4<761::aid-cncr1380>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.McKay R.R. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur. Urol. 2014;65:577–584. doi: 10.1016/j.eururo.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heng D.Y. Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer. 2011;117:2637–2642. doi: 10.1002/cncr.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito M., Guise T., Kang Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forbes G.S., McLeod R.A., Hattery R.R. Radiographic manifestations of bone metastases from renal carcinoma. AJR Am. J. Roentgenol. 1977;129:61–66. doi: 10.2214/ajr.129.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Lin P.P. Patient survival after surgery for osseous metastases from renal cell carcinoma. J. Bone Joint Surg. 2007;89:1794–1801. doi: 10.2106/JBJS.F.00603. [DOI] [PubMed] [Google Scholar]
- 34.Fottner A. Bone metastases from renal cell carcinoma: patient survival after surgical treatment. BMC Musculoskelet Disord. 2010;11:145. doi: 10.1186/1471-2474-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinnane N. Burden of bone disease. Eur. J. Oncol. Nurs. 2007;11(Suppl 2):S28–S31. doi: 10.1016/j.ejon.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.McKay R.R. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur. Urol. 2014;66:502–509. doi: 10.1016/j.eururo.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan T. Cabozantinib reverses renal cell carcinoma-mediated osteoblast inhibition in three-dimensional coculture in vitro and reduces bone osteolysis in vivo. Mol. Cancer Ther. 2020;19:1266–1278. doi: 10.1158/1535-7163.MCT-19-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oster G. Use of intravenous bisphosphonates in patients with breast, lung, or prostate cancer and metastases to bone: a 15-year study in two large US health systems. Supportive Care Cancer. 2014;22:1363–1373. doi: 10.1007/s00520-013-2094-y. [DOI] [PubMed] [Google Scholar]
- 39.Henry D.H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 40.Pan T. BIGH3 promotes osteolytic lesions in renal cell carcinoma bone metastasis by inhibiting osteoblast differentiation. Neoplasia. 2018;20:32–43. doi: 10.1016/j.neo.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dougall W.C. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 2012;18:326–335. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 42.Mikami S. Invasion and metastasis of renal cell carcinoma. Med. Mol. Morphol. 2014;47:63–67. doi: 10.1007/s00795-013-0064-6. [DOI] [PubMed] [Google Scholar]
- 43.Mendoza-Villanueva D., Zeef L., Shore P. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFbeta-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13:R106. doi: 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marino S., Roodman G.D. Multiple myeloma and bone: the fatal interaction. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng S.L. Human osteoblasts express a repertoire of cadherins, which are critical for BMP-2-induced osteogenic differentiation. J. Bone Miner. Res. 1998;13:633–644. doi: 10.1359/jbmr.1998.13.4.633. [DOI] [PubMed] [Google Scholar]
- 46.Okazaki M. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 1994;269:12092–12098. [PubMed] [Google Scholar]
- 47.Huang C.F. Cadherin-11 increases migration and invasion of prostate cancer cells and enhances their interaction with osteoblasts. Cancer Res. 2010;70:4580–4589. doi: 10.1158/0008-5472.CAN-09-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura D., Hiraga T., Myoui A., Yoshikawa H., Yoneda T. Cadherin-11-mediated interactions with bone marrow stromal/osteoblastic cells support selective colonization of breast cancer cells in bone. Int. J. Oncol. 2008;33:17–24. [PubMed] [Google Scholar]
- 49.Satcher R.L. Cadherin-11 in renal cell carcinoma bone metastasis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broom R.J. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin. Genitourinary Cancer. 2015;13:50–58. doi: 10.1016/j.clgc.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Alcaraz A. Biochemical markers of bone turnover and clinical outcome in patients with renal cell and bladder carcinoma with bone metastases following treatment with zoledronic acid: The TUGAMO study. Br. J. Cancer. 2013;109:121–130. doi: 10.1038/bjc.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodan G.A., Fleisch H.A. Bisphosphonates: mechanisms of action. J. Clin. Investig. 1996;97:2692–2696. doi: 10.1172/jci118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipton A., Zheng M., Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98:962–969. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 54.Rosen L.S. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 55.Lipton A. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer. 2016;53:75–83. doi: 10.1016/j.ejca.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 56.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 57.von Moos R. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Supportive Care Cancer. 2013;21:3497–3507. doi: 10.1007/s00520-013-1932-2. [DOI] [PubMed] [Google Scholar]
- 58.Henry D. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Supportive Care Cancer. 2014;22:679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi K. Challenges for 1994 by hospital nursing departments described by directors of nursing. Programs for reaching out to community. Kango. 1994;46:79–83. [PubMed] [Google Scholar]
- 60.E.A. Eisenhauer. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer (Oxford, England: 1990)45, 228-247, doi:10.1016/j.ejca.2008.10.026 (2009). [DOI] [PubMed]
- 61.H. Deng et al. Comparative Efficacy, Safety, and costs of sorafenib vs. sunitinib as first-line therapy for metastatic renal cell carcinoma: a systematic review and meta-analysis. Front. Oncol.9 479, doi:10.3389/fonc.2019.00479 (2019). [DOI] [PMC free article] [PubMed]
- 62.Zolnierek J. Efficacy of targeted therapy in patients with renal cell carcinoma with pre-existing or new bone metastases. J. Cancer Res. Clin. Oncol. 2010;136:371–378. doi: 10.1007/s00432-009-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J., Long F. mTOR signaling in skeletal development and disease. Bone Res. 2018;6:1. doi: 10.1038/s41413-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simone V. Everolimus restrains the paracrine pro-osteoclast activity of breast cancer cells. BMC Cancer. 2015;15:692. doi: 10.1186/s12885-015-1717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercatali L. The effect of everolimus in an in vitro model of triple negative breast cancer and osteoclasts. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17111827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grano M. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. PNAS. 1996;93:7644–7648. doi: 10.1073/pnas.93.15.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyseng-Williamson K.A. Cabozantinib as first-line treatment in advanced renal cell carcinoma: a profile of its use. Drugs Ther. Perspect. 2018;34:457–465. doi: 10.1007/s40267-018-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choueiri T.K. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 69.Haaker L. Bone metastasis is associated with poor prognosis in metastatic papillary renal cell carcinoma patients treated with first agent angiogenesis inhibitors. Urol. Oncol. 2020 doi: 10.1016/j.urolonc.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 70.Motzer R.J. Nivolumab versus everolimus in advanced renal-cell carcinoma. New Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Escudier B. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for Nivolumab versus Everolimus in advanced renal cell carcinoma. Eur. Urol. 2017;72:962–971. doi: 10.1016/j.eururo.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Botticelli A. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines. 2020;8 doi: 10.3390/vaccines8020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Motzer R.J. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2017;15:804–834. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 74.Kollender Y. Metastatic renal cell carcinoma of bone: indications and technique of surgical intervention. J. Urol. 2000;164:1505–1508. [PubMed] [Google Scholar]
- 75.Marcove R.C., Sadrieh J., Huvos A.G., Greabstald H. Cryosurgery in the treatment of solitary or multiple bone metastases from renal cell carcinoma. J. Urol. 1972;108:540–547. doi: 10.1016/s0022-5347(17)60797-3. [DOI] [PubMed] [Google Scholar]
- 76.Bickels J., Dadia S., Lidar Z. Surgical management of metastatic bone disease. J. Bone Joint Surg. Am. 2009;91:1503–1516. doi: 10.2106/JBJS.H.00175. [DOI] [PubMed] [Google Scholar]
- 77.S. D'Oronzo, R. Coleman, J. Brown, F. Silvestris. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol.15 2019 004-004, doi:10.1016/j.jbo.2018.10.004. [DOI] [PMC free article] [PubMed]
- 78.Smith M.R. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J. Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 79.Michaelson M.D. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J. Clin. Oncol. 2007;25:1038–1042. doi: 10.1200/jco.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ibrahim A. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin. Cancer Res. 2003;9:2394–2399. [PubMed] [Google Scholar]
- 81.Saad F. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 82.Saad F. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J. Natl. Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 83.James N.D. Clinical outcomes and survival following treatment of metastatic castrate-refractory prostate cancer with docetaxel alone or with strontium-89, zoledronic acid, or both: the TRAPEZE randomized clinical trial. JAMA Oncol. 2016;2:493–499. doi: 10.1001/jamaoncol.2015.5570. [DOI] [PubMed] [Google Scholar]
- 84.Dearnaley D.P., Mason M.D., Parmar M.K., Sanders K., Sydes M.R. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fizazi K. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith M.R. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann. Oncol. 2015;26:368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jadvar H., Quinn D.I. Targeted α-particle therapy of bone metastases in prostate cancer. Clin. Nucl. Med. 2013;38:966–971. doi: 10.1097/rlu.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henriksen G., Breistøl K., Bruland Ø.S., Fodstad Ø., Larsen R.H. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120–3125. [PubMed] [Google Scholar]
- 89.Parker C. Alpha emitter radium-223 and survival in metastatic prostate cancer. New Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 90.P.G. Kluetz et al. Radium Ra 223 dichloride injection: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res.20, 9-14, doi:10.1158/1078-0432.ccr-13-2665 (2014). [DOI] [PubMed]
- 91.Hortobagyi G.N. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J. Clin. Oncol. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 92.Theriault R.L. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. Protocol 18 Aredia Breast Cancer Study Group. J. Clin. Oncol. 1999;17:846–854. doi: 10.1200/JCO.1999.17.3.846. [DOI] [PubMed] [Google Scholar]
- 93.Lipton A. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 94.Himelstein A.L. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2017;317:48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Body J.J. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br. J. Cancer. 2004;90:1133–1137. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Body J.J. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain. 2004;111:306–312. doi: 10.1016/j.pain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 97.Kristensen B. Oral clodronate in breast cancer patients with bone metastases: a randomized study. J. Intern. Med. 1999;246:67–74. doi: 10.1046/j.1365-2796.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 98.Kohno N. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J. Clin. Oncol. 2005;23:3314–3321. doi: 10.1200/JCO.2005.05.116. [DOI] [PubMed] [Google Scholar]
- 99.Coleman R. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 100.Early Breast Cancer Trialists' Collaborative, G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet386 2015 1353-1361, doi:10.1016/S0140-6736(15)60908-4 (2015). [DOI] [PubMed]
- 101.Wang W. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene. 2019;38:4540–4559. doi: 10.1038/s41388-019-0736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coleman, R. et al. Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol.: official journal of the European Society for Medical Oncology/ESMO25 Suppl 3 2014 iii124-137, doi:10.1093/annonc/mdu103. [DOI] [PubMed]
- 103.Winn R.J., Brown N.H., Botnick W.Z. Reproducibility of guidelines. A comparison of the NCCN and ASCO lung cancer guidelines. Oncology (Williston Park) 1999;13:35–39. [PubMed] [Google Scholar]
- 104.Zarogoulidis K. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int. J. Cancer. 2009;125:1705–1709. doi: 10.1002/ijc.24470. [DOI] [PubMed] [Google Scholar]
- 105.Rosen L.S. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 106.Hirsh V. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J. Thorac. Oncol. 2008;3:228–236. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 107.Lopez-Olivo M.A. Bisphosphonates in the treatment of patients with lung cancer and metastatic bone disease: a systematic review and meta-analysis. Support. Care Cancer. 2012;20:2985–2998. doi: 10.1007/s00520-012-1563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li X.X., Zhou T.C., Zhao J. Clinical evaluation of combined-modality therapy for bone metastasis of non-small-cell lung cancer. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:752–753. [PubMed] [Google Scholar]
- 109.Su J., You C., Cai S., Meng Y. (8)(9)SrCl(2) and/or Bonefos in the treatment of bone metastasis from pulmonary carcinoma. Zhongguo Fei Ai Za Zhi. 2002;5:357–359. doi: 10.3779/j.issn.1009-3419.2002.05.11. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L. Predictive significance of bone sialoprotein and osteopontin for bone metastases in resected Chinese non-small-cell lung cancer patients: a large cohort retrospective study. Lung Cancer. 2010;67:114–119. doi: 10.1016/j.lungcan.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Pandya K.J. Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer. 2010;67:330–338. doi: 10.1016/j.lungcan.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 112.Guo Q. Characteristics and treatment of bone metastases in 322 cases non-small cell lung cancer: a retrospective study. Zhongguo Fei Ai Za Zhi. 2014;17:656–662. doi: 10.3779/j.issn.1009-3419.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang C. Nomogram based on homogeneous and heterogeneous associated factors for predicting bone metastases in patients with different histological types of lung cancer. BMC Cancer. 2019;19:238. doi: 10.1186/s12885-019-5445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Marinis F. Bisphosphonate use in patients with lung cancer and bone metastases: recommendations of a European expert panel. J. Thoracic Oncol. 2009;4:1280–1288. doi: 10.1097/JTO.0b013e3181b68e5a. [DOI] [PubMed] [Google Scholar]
- 115.Tolia M. The key role of bisphosphonates in the supportive care of cancer patients. Anticancer Res. 2014;34:23–37. [PubMed] [Google Scholar]
- 116.Scagliotti G.V. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J. Thoracic Oncol. 2012;7:1823–1829. doi: 10.1097/JTO.0b013e31826aec2b. [DOI] [PubMed] [Google Scholar]
- 117.Scagliotti G.V. Zoledronic acid in patients with stage IIIA/B NSCLC: results of a randomized, phase III study. Ann. Oncol. 2012;23:2082–2087. doi: 10.1093/annonc/mds128. [DOI] [PubMed] [Google Scholar]