Abstract
SARS-CoV-2 variants of concern, demonstrating higher infection rate and lower vaccine effectiveness as compared with the original virus, are important factors propelling the ongoing COVID-19 global outbreak. Therefore, prompt identification of these variants in the environment is essential for pandemic assessment and containment efforts. One well established tool for such viral monitoring is the use of wastewater systems. Here, we describe continuous monitoring of traces of SARS-CoV-2 viruses in the municipal wastewater of a large city in Israel. By observing morbidity fluctuations (during three main COVID-19 surges) occurring in parallel with Pfizer-BioNTech COVID-19 vaccine vaccination rate, compromised immunity was revealed in the current morbidity peak. RT-qPCR assays for the Original (D614G), Alpha and Beta variants had been previously developed and are being employed for wastewater surveillance. In the present study we developed a sensitive RT-qPCR assay designed for the rapid, direct detection of Gamma and Delta variants of concern. Sensitive quantification and detection of the various variants showed the prevalence of the original variant during the first morbidity peak. The dominance of the Alpha variant over the original variant correlated with the second morbidity peak. These variants decreased concurrently with an increase in vaccinations (Feb-March 2021) and the observed decrease in morbidity. The appearance and subsequent rise of the Delta variant became evident and corresponded to the third morbidity peak (June-August 2021). These results suggest a high vaccine neutralization efficiency towards the Alpha variant compared to its neutralization efficiency towards the Delta variant. Moreover, the third vaccination dose (booster) seems to regain neutralization efficiency towards the Delta variant. The developed assays and wastewater-based epidemiology are important tools aiding in morbidity surveillance and disclosing vaccination efforts and immunity dynamics in the community.
Keywords: SARS-CoV-2, Variants of concern, Reverse transcriptase quantitative polymerase chain reaction, Vaccination, Wastewater-based epidemiological, COVID-19
Graphical abstract
1. Introduction
Since declaring COVID-19 a world pandemic in March 2020, the disease continues to evolve and affect significant portions of the world population. Increasing circulation of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus within the world population, results in a continual natural processes of random mutations and evolution (Harvey et al., 2021). In some cases, a mutation will provide the virus an evolutionary advantage and create a new viral lineage that will overpower previous forms. First evidence for such an evolutionary event for the SARS-CoV-2 virus occurred in April 2020, when the D614G substitution enhanced infectivity and transmission. The D614G mutation lineage became the dominant SARS-CoV-2 variant form (Hou et al., 2021; Korber et al., 2020). By late 2020, the term 'variants of concern’ emerged with regards to the high-numbered mutations in a single variant and its increased pathogenicity (Davies et al., 2021).
Variants of concern are mainly characterized through mutations in their spike protein gene (S gene). Such mutations affect the receptor biding domain affinity (Thomson et al., 2021) and antibody neutralization efficiency (Weisblum et al., 2020). Antibody neutralization efficiency is lower when antibodies generated by vaccination or by prior infection are less compatible to the new variants. Previous studies found that the Alpha variant (B.1.1.7), associated with receptor binding motif replacements, does not significantly reduce the neutralizing antibodies capability, despite its increased infectivity compared to the original strain (Meng et al., 2021). Mutations in the Beta (B.1.351), Gamma (P.1) and Delta (B.1.617) variants proved to be less susceptible to antibody neutralization (Mlcochova et al., 2021; Planas et al., 2021; Wibmer et al., 2021). High transmission rates and lower vaccine efficacy towards these variants (Bernal et al., 2021; Hoffmann et al., 2021; Z. Wang et al., 2021) resulted in a greater threat of infectivity and morbidity compared to the original SARS-CoV-2 virus. Therefore, quick detection and quantification of variants of concern, are vital for initiating response and for proper policy-making for pandemic containment.
Currently, SARS-CoV-2 variant detection mainly relies on costly next generation sequencing, where results are obtained within 3–5 days at best (Andrés et al., 2020; Khan et al., 2020). With new variants of interest constantly emerging, sequencing-based approaches are important for their initial identification, however they present difficulties considering the need for special instruments and resources, including data analysis skills. Thus, to ease detection, a reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)-based assay can provide rapid results for detection of known variants of concern. Such an assay can help assess a variant's frequency within a population and contribute to policy guidelines. Indeed a number of publications targeting variants of concern detection using RT-qPCR (Barreto et al., 2020; Bivins et al., 2021; Gand et al., 2021; Vega-Magaña et al., 2021; Vogels et al., 2021; Wang et al., 2021b; Yaniv et al., 2021) as well as available commercial kits (e.g., TaqPath ThermoScientific, GT molecular, PerkinElmer) have emerged for individual assessments.
Population morbidity monitoring through wastewater or wastewater-based epidemiology is well described and is considered an important method (Martin et al., 2020). Its advantage is that it can provide critical information regarding the spread of pathogens in the population in a given area, without relying on individual testing tendencies or testing capacity. Since the beginning of the pandemic, the SARS-CoV-2 virus has been monitored in wastewater around the world (Ahmed et al., 2020; Bar-Or et al., 2020; Claro et al., 2021; La Rosa et al., 2020; Sherchan et al., 2020; Trottier et al., 2020; Westhaus et al., 2021; Wu et al., 2021, 2020). Despite this, in order to detect specific SARS-CoV-2 variants such as the Gamma and Delta variants of concern, there is a need for a direct and sensitive probe-based RT-qPCR assay with detection ability in wastewater samples. Such a quick and accurate method could thus help generate better health policies in a given region.
In this study, we monitored SARS-CoV-2 traces in the wastewater of Beer-Sheva, Israel's 4th largest city (with population of approximately 220,000). Beer-Sheva city is considered a high vaccination area with fluctuating morbidity and was therefore chosen as a model. We developed a novel sensitive RT-qPCR assay design for the direct detection of Gamma and Delta variants of concern. The new assay was later employed for wastewater-based epidemiology, together with the RT-qPCR assays developed previously by our group for quantification of the various variants of SARS-CoV-2. These results were then compared to morbidity levels in the population, allowing us to speculate the immunity level in the city population.
2. Methodology
2.1. Primers and probes
The original sequence of SARS-CoV-2 (NC_045512.2) was taken from NCBI database. Gamma variant (P.1, Brazilian, EPI_ISL_981,709) and Delta variant (B.1.617, Indian, EPI_ISL_1,704,637) sequences were taken from GISAID database (Shu and McCauley, 2017). All primers and probe used in this study are listed in Table S1. For the Gamma variant, the newly probe design focused on the end of ORF8 and the beginning of N gene 28,192–28,244 bp location that includes it's specific insertion, or S gene 21,964–22,017 bp location that includes the Delta variant deletion 157–158. All primers and probes were purchased through Integrated DNA Technologies (IDT). ZEN Quencher was added to the probes as a second, internal quencher in qPCR 5′-nuclease assay. Probes were assigned a 6-carboxy-fluorescein (FAM) fluorophore.
2.2. Wastewater collection
Wastewater samples were collected in the cities of Modi'in and Beer Sheva, Israel for 24 h composite sewage samples. In Modi'in, samples were taken from different manholes (located at the different neighborhoods) and immediately transferred to the lab under chilled conditions. The automated composite samplers (KANDO, Israel) were activated during June 23, 2021. Sampling was carried out for 18 h from 5 AM to 11 PM at each point and collected the next morning. The automated sampler pumped 0.3 L every half an hour (total of 10 L approximately). In Beer- Sheva composite samples were collected by the WWTP facility twice (or three times) a week and transferred under chilled conditions as well. The WWSP'S automated composite sampler was activated for 24 h and pumped 0.25 L of untreated wastewater every half an hour. A temperature of 4 °C maintained during sample transport to the laboratory.
2.3. RNA extraction
Direct RNA was extracted twice for each sample for duplicates. The RNA extraction procedure was executed according to Zymo Environmental Water RNA (Zymo Research R2042) manufacture protocol including their Viral Enrichment protocol for 5 mL sample volume. For internal control, we added 104 or 105 copies MS2 phage to the lysis buffer in each RNA extraction. RNA was eluted with 50 μL of RNase free water. The fresh wastewater samples were kept at 4 °C until processed. RNA samples were kept at –80 °C. In the case of Not Detected result after RT-qPCR, the wastewater sample was concentrated using MCE electronegative membrane as described before (Bar-Or et al., 2021) with minor change. 45 ml of wastewater was centrifuged at 3000 g for 5 min and the supernatant was collected into separate 50 ml tube containing 1 g of MgCl2. The mixture was gently shaken for 5 min and was then split into a duplicate, 20 mL each. Every 20 mL were vacuum pumped through 0.45 μm 47 mm diameter electronegative MCE membrane (Merck Millipore Ltd). The membrane was immediately transferred into a petri dish containing 2 ml lysis buffer of the NucleoSpin RNA extraction kit (Macherey Nagel, Germany) and was gently shaken for 45 min. Following this step, RNA was extracted using the manufacture protocol. Sample processing (raw or concentrated) are color coded in Table S4. Calculation for RNA copy number per Liter of wastewater was performed Eq. (1) for raw sample or Eq. (2) for concentrated samples.
| (1) |
| (2) |
2.4. RT-qPCR
RT-qPCR was executed as previously described (Yaniv et al., 2021). Reaction final volume is 20 µL with primers concentration of 0.5 µM and probe concentration of 0.2 µM. The reaction contained 5 µL of RNA sample and ROX as a reference dye. Reaction steps were executed according to manufacture recommended protocol (One Step PrimeScript III RT-qPCR mix RR600 TAKARA, Japan) using Applied Biosystems Thermocycler (Thermo Scientific). For reverse transcription reaction, the reaction holding stage carried out for 7 min at 52 ℃ followed by 10 s at 95 ℃. For PCR reaction, 45 cycles were performed at 95 ℃ for 5 s followed by 30 s at 60 ℃. Each RT-qPCR run included relevant quality controls, Non template control (NTC) and MS2 phage detection for wastewater RNA sample (Dreier et al., 2005). MS2 addition was meant for inhibitors assessment through calculated recovery percentage (appear in Table S4, values were calculated using Fig. S1). Ct value initially converted to RNA copies per μL (in the RNA sample) based on the standard curves (as described in the next section). Duplicate were averaged for each target gene separately. The gene copies per L of wastewater sample were calculated according to Eqs. (1) and 2 for Raw or Concentrated sample respectively.
2.5. Calibration curves and limit of detection determination
Calibration curves were performed on a known-positive double-strands DNA template. Three different templates were used and full sequences are reported in Table S3. (1) gBlocks™ Gene Fragments (double-stranded DNA fragments) from IDT Technologies, containing SARS-CoV-2 S gene sequence as reported for Wuhan-Hu-1 (NC_045512.2). (2) pUC-GW-kan plasmid from GENEWIZ, cloned with the end of ORF8 until the beginning of N gene matching the reported 4 nucleotides insertion of the Gamma variant. (3) gBlocks™ Gene Fragments (double-stranded DNA fragments) from IDT Technologies, containing S gene sequence matching the reported 157–158 deletion of the Delta variant. Calibration of P.1 probe was performed using the second gene block, while calibration of S∆157 probe was performed with the third gene block. Negative control for the P.1 probe was performed using Wastewater samples confirmed as positive for the original variant or Alpha variant of concern, and negative control for the S∆157 probe was performed using the first gene block. Serial dilutions for the relevant gene block were prepared based on copy number calculations at an initial concentration of 106 copies per µL down to 10° copies per µL. The resulting Ct values were plotted against the log copy number of the gene block template. Each concentration was examined by six repetitions and a standard deviation was calculated. The lowest concentration tested (i.e., 10° copies per µL) was verified for at least 10 times to ensure more than 95% success in positive detection. Linear regression was performed between the log copy number and the Ct values from the RT-qPCR results. In addition, the coefficient of variants between replicates was < 3.35% for Gamma assay and < 2.94% for the Delta assay.
2.6. Complex matrix detection
Wastewater matrix consist of various PCR inhibitors that are likely to affect the assay performance, therefore there was a need to assess the primers-probe detection abilities in an environment as complex as that. In order to validate the RT-qPCR designed assay's sensitivity in a wastewater matrix, RNA extracted from pre-determined negative wastewater sample for SARS-CoV-2 was examined using standard CDC's detection sets (Centers for Disease Control and Prevention, 2019). The negative RNA sample was supplemented with known concentrations of a desired gene block to reach 10°,101 and 102 copies per µL. All experiments were executed with a pre-determined negative matrix for additional verification of the sample as negative. Eight repetitions were performed for each viral concentration or control. Ct results were plotted to represent the new probes limit of detection in a complex environment.
3. Results and discussion
3.1. Wastewater-based epidemiology immunity trends
Throughout the COVID-19 outbreak, we monitored wastewater samples from Beer-Sheva city and several other locations in Israel, for the presence of SARS-CoV-2 virus using RT-qPCR. Considering that the different SARS-CoV-2 variants share very high similarity in their N gene, we employed N gene detection in prospect of general detection of all the SARS-CoV-2 RNA traces in the wastewater. Using a CDC's standard detection set (CDC's N2) and our previously published designed primers-probe set (Yaniv et al., 2021) (Improved N3), we documented the SARS-CoV-2 RNA levels in wastewater and monitored these values together with the corresponding reported active cases and percent vaccination in the city populations (Fig. 1 ). Looking at Fig. 1, it is evident that an increase in detected SARS-CoV-2 RNA levels in the city wastewater appeared just prior to every one of the three peaks in reported active cases. Thus, the appearance in the city's wastewater foresaw the increase in reported clinical cases of COVID-19. This result was unsurprising as it is supported by other studies showing that the use of wastewater-based epidemiology is an effective tool for population morbidity monitoring (Ahmed et al., 2020; Bar-Or et al., 2020; La Rosa et al., 2020; Trottier et al., 2020; Westhaus et al., 2021; Wu et al., 2020). We then wanted to ascertain if the effects of massive vaccinations could also be assessed using wastewater-epidemiology.
Fig. 1.
Beer-Sheva city, Israel SARS-CoV-2 monitoring through wastewater epidemiology, reported active cases per 10 K people in the population and vaccination percentages during mid-September 2020 until October 2021. SARS-CoV-2 RNA levels in wastewater detected through RT-qPCR on the N gene (Blue columns). Detection was achieved using an average value from CDC's N2 and Improved N3 sets. Reported active cases per 10 K people in the population (Orange line), and second dose vaccination percentages (Black dashed line) were extracted from the Ministry of Health database (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
In December 2020, Israel began vaccinating its population with the Pfizer-BioNTech BNT162b2 COVID-19 vaccine, with a two dose, three-weeks interval regimen. Individuals were considered fully vaccinated two weeks after receiving the second dose, in accordance with the clinical trial data (Polack et al., 2020). Concurrently to the beginning of the vaccination efforts, a meaningful morbidity peak was apparent (Fig. 1) with values of 55 to 71 SARS-CoV-2 active cases per 10 K people. Subsequently when over 50% of the population was vaccinated, a steady and consistent decrease appeared in the level of SARS-CoV-2 RNA detected in wastewater and in the concurrent reported active cases per 10 K people in the population. The declines in SARS-CoV-2 RNA levels in wastewater reached the lowest values by the end of May 2021 (Fig. 1) when reported active cases decreased below 10 diagnosed patients per 10 K of the population. At this time, RNA levels in wastewater decreased below detectable concentrations (resulted in Not Detected value).
One month later, by the end of June 2021, SARS-CoV-2 RNA levels in wastewater reappeared. This was followed by an increase in reported active Cases 2–3 weeks later (Fig. 1). Another analysis, zooming on the appearance of the third morbidity peak, was done for observing new SARS-CoV-2 verified cases alongside our wastewater N gene RNA detection (Fig. S2). Wastewater N gene detection was not visible at a rate of lower than two new cases per day in the city and remained undetected in five measurements between June 1–17. On June 22, the first positive wastewater result was obtained, while notedly, a meaningful increase in cases was seen only a week later. This reappearance of increased morbidity continues throughout September 2021, despite vaccination percentages (second dose) of nearly 70% of the city's population. Previously, we reported the emergence of the Alpha variant of concern (B.1.1.7) in Israel by the end of January 2021 using RT-qPCR assay we developed (Yaniv et al., 2021). Soon after appearance, the alpha variant dominated the original variant to such an extent that within one month all SARS-CoV-2 RNA traces in wastewater corresponded solely to the Alpha variant. Moreover, the observed decline in morbidity, suggest the effectiveness of the Pfizer-BioNTech COVID-19 vaccine against the Alpha variant (Meng et al., 2021). The reappearance of COVID-19 morbidity in June 2021 raised questions regarding the efficiency of the Pfizer-BioNTech COVID-19 vaccine. Is the vaccine less efficient over-time or it is compromised due to lack of compatibility to other variants of concerns?
3.2. Developed RT-qPCR assays for Gamma (P.1) and Delta (B.1.617) detection
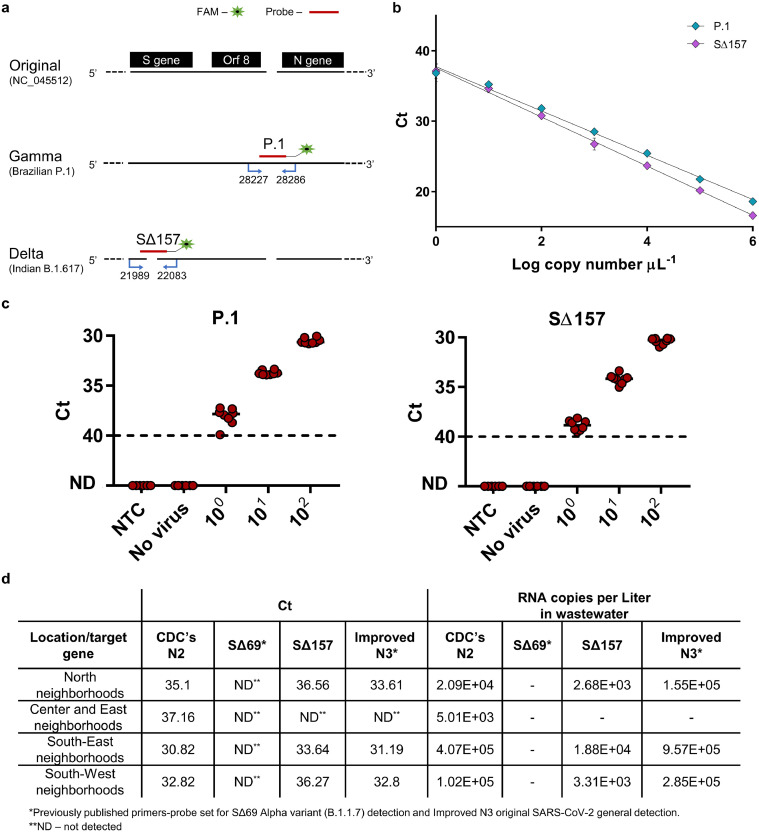
Previously we developed direct RT-qPCR detection assays for the Alpha variant (B.1.17, British) and Beta variant (B.1.315, South Africa) (Yaniv et al., 2021). The possibility that the new outbreak is a result of the invasion of a novel variant of concern that differs from the Alpha or Beta was suggested. The most prominent variants of concern to invade Israel were the Gamma variant (P.1, Brazilian) or the Delta variant (B.1.617, Indian), therefore they were the most urgent variants in need for rapid detection. We therefore developed direct RT-qPCR detection assays for these two variants of concern. Our design for detection of these two variants is based on the differences in gene sequence from the original SARS-CoV-2 sequence (NC_045512.2). The Gamma variant contains an insertion in the ORF8 region, and the Delta variant S gene contains a deletion known as Δ157–158. Accordingly, our primers-probe sets were designed with a focus on these regions (Fig. 2 a -sequences available in Table S1).
Fig. 2.
RT-q-PCR primers-probe sets designed for the detection of SARS-CoV-2 Gamma and Delta variants. a, Detection primer sets designed for differentiation and identification of SARS-CoV-2 Gamma variant (P.1) and Delta variant (B.1.617). Design was based on sequence changes between the original SARS-CoV-2 virus and variants of concern, with insertion after ORF8 for Gamma variant and deletion in the S gene of the Delta variant b, Calibration curves for primers-probe sets developed for variants of concern detection. Resulted Ct value plotted against the tested Log copy number. Error bars present standard deviation for ten replicates. c, Lower detection limit of P.1 and S∆157 primers–probe sets in wastewater matrix. RNA extracted from negative detection wastewater sample (No virus) spiked with known concentrations of positive template corresponding to each probe accordingly (10°–102 S gene template copies per μL) and Non-Template Control (NTC, water). ND - not detected. Solid lines indicate the median and dashed lines indicate the detection limit as decided by clinical guidelines. d, Modi'in city manhole detection for SARS-CoV-2 N gene (CDC's N2, Improved N3), Alpha variant S gene (SΔ69) and Delta variant S gene (S∆157). These samples were taken on June 23, 2021.
For Gamma variant detection, the designed forward primer is located at the end of ORF8 gene and the reverse primer is located at the beginning of the N gene, meaning that 28,227–28,286 bp of the original sequence are included. Within this range, the original SARS-CoV-2 and Gamma variant sequences are identical, apart from a four-nucleotide insertion in the Gamma lineage (Fig. 2a). Using a detection set comprised of two primers meant to amplify the target region, a single probe (P.1 probe) was designed for the detection of the Gamma variant only (corresponding to the insertion). For the detection of the Delta variant, the designated detection region was chosen from within the S gene. Focusing on S gene's 21,989–22,083 bp of the original sequence, the original SARS-CoV-2 sequence is identical to the Delta variant sequence with the exception of a 6 nucleotides deletion in the Delta lineage (Fig. 2a). Using a detection set comprised of two primers meant to amplify the target region, a single probe (S∆157 probe) was designed for the area corresponding to the deletions, resulting in the detection of the Delta variant only.
To ensure functionality, the described sets of primers and probes underwent characterization. Initially, a calibration curve was generated for the two primers-probe sets, using dsDNA as a template. A detection range of between 106 copies and 10° copies per µL was tested for each set (Fig. 2b). Linear regression performed for the two probes demonstrated strong correlation. A limit of detection (LOD) could be determined for each primers-probe set and was identified as 10° copies per µL for the two sets (Fig. 2b, Table S2). A LOD of 10° copies per µL, is equivalent to a LOD calculated as 2000 copies per L wastewater in wastewater samples that were extracted directly (defined as Raw in equation S1). Additionally in wastewater samples that were concentrated and then extracted for RNA (defined as Concentrated in equation S2), 10° copies per µL LOD is equivalent to a calculated LOD of 500 copies per L wastewater. This indicates highly sensitive detection. Specificity of the described sets was examined with either dsDNA fragments from the original SARS-CoV-2, relevant to the assigned detection regions, or using wastewater found positive for the original SARS-CoV-2 and for the Alpha variant. Both sets did not manifest a signal when tested with such negative controls and were therefore found to be highly specific.
Wastewater matrix consists of various PCR inhibitors that are likely to affect the RT-qPCR performance, therefore there was a need to assess the primers-probe detection abilities in such a complex environment. Hence, primer-probe sets were further characterized within a more intricate matrix. Wastewater from a rural area in Binyamina, Israel served as a more complex environment for the RT-qPCR assay. Wastewater samples were pre-determined as negative for SARS-CoV-2 using N gene detection. After confirming that the wastewater samples were negative, relevant dsDNA template copies were added exogenously at known concentrations (Fig. 2c). These samples were then used for SARS-CoV-2 detection by the examined primers-probe sets. As can be seen in Fig. 2c, despite the wastewater matrix, P.1 and S∆157 probes maintained high functionality with a LOD of 10° copies per µL, proving their sensitivity and stability.
After fully characterizing the developed primers-probe sets, revealing their specificity, sensitivity and durability, we employed them on recently collected wastewater samples. Until recently, the Alpha variant was the most dominant in Israel, however a noticeable decrease in this variant was observed in the past months. In May-June 2021, clinical reports indicated that the Delta variant had reached Israel and was responsible for the majority of new morbidity cases. Taking this into account, samples from different locations in the city of Modi'in were collected on June 23rd, 2021 and tested for general SARS-CoV-2 presence, using N gene detection, as well as for the presence of the Alpha and Delta variants, using relevant S gene detection (Fig. 2d). As can be seen in Fig. 2d, all of the tested samples were positive to SARS-CoV-2 through N gene detection. However, none of the samples resulted in a positive detection for the Alpha variant. This corresponded to the disappearance of this variant in clinical tests as reported by health officials. Furthermore, all samples, except for one, resulted in a positive signal for the Delta variant using the specific designed primers-probe set described above. These field results confirmed the new primers-probe sets’ ability to detect the Delta variant in a quantitative sensitive manner, even within a complex environment. This technology thus, can provide an assessment for variant of concern's geographical spread in the population. A Gamma variant primer-probe set was also employed on numerous wastewater samples; however, none resulted in a positive signal as yet. Considering the importance of wastewater monitoring, as well as the possibility of their use in clinical settings, these new tools may serve for enhancing SARS-CoV-2 variants of concern detection in a population.
3.3. Variants of concern detection discloses compromised immunity
With the new RT-qPCR tool for detection of variants of concern in hand, we set out to identify and quantify the various variants of concern in Beer-Sheva's wastewater during the past year. Fig. 3 illustrates the dynamics of the different SARS-CoV-2 variants in Beer-Sheva's wastewater. Prior work by our group (Yaniv et al., 2021) revealed that until January 2021, the original SARS-CoV-2 was apparently the only version of SARS-CoV-2 in Israel and was detected by the S1 primers-probe set (primers-probe set details in the SI). During January 2021, a shift occurred, and the Alpha variant, detected by SΔ69 primers-probe set (primers-probe set details in the SI) manifested in the wastewater samples. Within a month, quickly taking over the original variant, the Alpha variant was the only detectable variant in the city's wastewater. It is important to note, that the S1 detection set was relevant for original SARS-CoV-2 detection until around March 2021. However, with the emergence of new variants of concern it could no longer serve as an indication for the original variant as it relied strictly on differences between the original and the Alpha variant. The high vaccination values allowed the containment of this variant by the end of May, and resulted in “Not Detected” values until June 24th. Concurrent to the latest emergence of SARS-CoV-2 at end of June 2021 (Fig. 1), we employed our previously developed detection primer-probe sets for the Alpha and Beta variants of concern (Yaniv et al., 2021), together with the newly developed detection sets for the Gamma and Delta variants of concern.
Fig. 3.
RT-qPCR SARS-CoV-2 variants of concern analysis in the city of Beer-Sheva's wastewater and vaccination percentages during mid-November 2020 until October 2021. Original variant detected using S1 set (Blue columns), Alpha variant detected using SΔ69 set (Orange columns) and Delta variant detected using SΔ157 set (Green columns). Second dose vaccination (Black dashed line) and third dose vaccination (Magenta dashed line) percentages were extracted from the Ministry of Health database.
The results from the RT-qPCR assays on wastewater samples indicated that the Delta variant had arrived in Beer-Sheva city (Fig. 3) by the end of June 2021. None of the other variants of concern manifested a signal during late-June, July, August, September and October 2021, apart from the Delta variant. It is important to note, that as in our previous study (Yaniv et al., 2021), the RNA detection levels of the S gene in wastewater samples are generally lower than the N gene. Here as well, positive samples for SARS-CoV-2 RNA demonstrated detection levels of the S gene lower than the detection levels of the N gene, despite the demonstrated sensitivity of used designed sets. This was suggested to be related to different gene transcription levels between the N gene and S gene in the SARS-CoV-2 (Emanuel et al., 2021; Kim et al., 2020), however such a subject requires further research.
Since first being documented in India, the Delta variant is known to be less responsive to the Pfizer-BioNTech COVID-19 vaccine due to S gene mutations (Mlcochova et al., 2021). Antibodies generated by the vaccine are apparently less compatible for neutralization of the Delta variant. In fact, the wastewater epidemiology results presented in this study clearly reflects this lower immunity despite high vaccination percentages. Additionally, finding the Delta virus strain in wastewater may also reflect that despite vaccination, vaccinated people may still be infected and carry the virus while being asymptomatic. Supporting this notion is the Health ministry report in which ∼50% of the clinically infected people in the current morbidity peak are vaccinated. Furthermore, a decrease in immunity as reflected by an increase in morbidity observed until the end of September could also be a result of decrease in antibodies in vaccinated and recovering people over time. Despite this, our results provide strong evidence for the connection of the presence of the Delta variant in wastewater to the observed morbidity, and this can serve as a partial explanation regarding the latest morbidity trend in Beer-Sheva with relation to the level of vaccination and immunity in its population.
Nevertheless, in an effort to regain population-immunity, the booster vaccination efforts (third dose) began in the first week of August 2021. Only two months later, when over 40% of the population was vaccinated with the third dose, a moderated decrease emerged in SARS-CoV-2 RNA detection levels in the city's wastewater and a sharp decrease in reported morbidity cases occurred (Figs. 1 and 3). More data is needed to determine the possible effects of the third vaccination dose on the current morbidity peak. Yet, these results emphasize the importance and reliability of this study to use wastewater as an epidemiology tool for early warning and policy decision-making in regards to pandemic containment.
4. Conclusions
The study presented here, consolidate data gathered on a single model city throughout the past year. The targeted research, demonstrated how wastewater-based epidemiology can serve as a tool for morbidity surveillance within the population, given a specific geographical area. Wastewater monitoring was able to provide early alerts of 2–3 weeks before three observed morbidity peaks during this study's time period. Combining our SARS-CoV-2 wastewater results with vaccination data, raised questions regarding vaccine induced immunity. Out of three observed morbidity peaks, as vaccine percentages increased to above 50%, the second peak's infection was lowered. Despite this, the infection reemerged into the third peak a month later, regardless of almost 70% vaccine coverage. An explanation to these observed results could be found in the exposure of the population to a new variant. We aimed to prove that our RT-qPCR assessment of SARS-CoV-2 variants for the Gamma (P.1) and Delta (B.1.617) can be used as an expansion to the previously published Alpha (B.1.1.7) and Beta (B.1.351) variants (Yaniv et al., 2021) probe sets and novel sets may be needed as more variants appear.
RT-qPCR primers-probe sets were designed based on sequence differences from the original SARS-CoV-2 and other variants. The new sets for Gamma and Delta variants detection, were found specific as well as sensitive, with a LOD of 10° both in sterile conditions and in sewage matrix. Employment of the sets on Israel's wastewater was positive for the Delta variant and negative for the Gamma variant, corresponding to health officials reports for infections during the third peak. Having 4 different primers-probe sets for detection of the variants of concern (2 of which are presented for the first time here), enabled the identification and quantification of the SARS-CoV-2 variants presence the city's wastewater.
Interestingly, each observed morbidity peak in this study, corresponded to a different SARS-CoV-2 variant indicating the usefulness of this tool. The original variant responsible for the first peak, the Alpha variant responsible for the second peak and the Delta variant responsible for the third peak. This study also demonstrated that a second-dose vaccine percentage of over 50% successfully contained the Alpha variant, as expected based on published in vitro studies. Nevertheless, a second-dose vaccination percentage of almost 70% was not enough to prevent the outbreak and continuous spread of the Delta variant in the population. In order to regain and maintain vaccine-induced immunity for longer period in the population, an additional vaccine dose was given. This study validates that the booster (third dose) vaccine rate of over 40% successfully started to reduce the Delta variant detection. However, further monitoring is required to fully elucidate the booster effect within the population. Overall, the tools used in this study can provide valuable information not only regarding morbidity, but also regarding vaccine induced immunity in the population.
CRediT authorship contribution statement
Karin Yaniv: Conceptualization, Writing – original draft, Visualization, Formal analysis. Eden Ozer: Conceptualization, Writing – original draft, Formal analysis. Yair Lewis: Formal analysis, Writing – original draft. Ariel Kushmaro: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
We thank Electra Company, which holds WWTP of Beer- Sheva management, especially Nikolay Schipunov. We thank KANDO and the Israeli Ministry of Health for providing us with the sewage samples from Modi'in city. We gratefully acknowledge GISAID database for access to SARS-CoV-2 variants sequences. We gratefully acknowledge Esti Kramarsky-Winter assistance for comments and scientific editing of the manuscript.
Funding Sources
We would like to acknowledge funding from Ben Gurion University, The Corona Challenge Covid-19 (https://in.bgu.ac.il/en/corona-challenge/Pages/default.aspx) and funding from the Israeli ministry of Health.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.watres.2021.117808.
Appendix. Supplementary materials
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés C., Garcia-Cehic D., Gregori J., Piñana M., Rodriguez-Frias F., Guerrero-Murillo M., Esperalba J., Rando A., Goterris L., Codina M.G., Quer S., Martín M.C., Campins M., Ferrer R., Almirante B., Esteban J.I., Pumarola T., Antón A., Quer J. Naturally occurring SARS-CoV-2 gene deletions close to the spike S1/S2 cleavage site in the viral quasispecies of COVID-19 patients. Emerg. Microbes Infect. 2020;9:1900–1911. doi: 10.1080/22221751.2020.1806735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Weil M., Indenbaum V., Bucris E., Bar-Ilan D., Elul M., Levi N., Aguvaev I., Cohen Z., Shirazi R., Erster O., Sela-Brown A., Sofer D., Mor O., Mendelson E., Zuckerman N.S. Detection of SARS-CoV-2 variants by genomic analysis of wastewater samples in Israel. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.148002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or I., Yaniv K., Shagan M., Ozer E., Erster O., Mendelson E., Mannasse B., Shirazi R., Kramarsky-Winter E., Nir O., Abu-Ali H., Ronen Z., Rinott E., Lewis Y.E., Friedler E., Bitkover E., Paitan Y., Berchenko Y., Kushmaro A. medRxiv; 2020. Regressing SARS-CoV-2 Sewage Measurements Onto COVID-19 Burden in the population: A proof-Of-Concept For Quantitative Environmental Surveillance; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto H.G., de Pádua Milagres F.A., de Araújo G.C., Daúde M.M., Benedito V.A. Diagnosing the novel SARS-CoV-2 by quantitative RT-PCR: variations and opportunities. J. Mol. Med. 2020;98:1727–1736. doi: 10.1007/s00109-020-01992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C., Amirthalingam G., Edmunds M., Zambon M., Brown K., Hopkins S., Chand M., Ramsay M. medRxiv; 2021. Effectiveness of COVID-19 Vaccines Against the B.1.617.2 Variant; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A., Kaya D., Bibby K., Simpson S.L., Bustin S.A., Shanks O.C., Ahmed W. Variability in RT-qPCR assay parameters indicates unreliable SARS-CoV-2 RNA quantification for wastewater surveillance. Water Res. 2021;203 doi: 10.1016/j.watres.2021.117516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, n.d. CDC 2019 - Novel Coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel [WWW document]. URL https://www.fda.gov/media/134922/download (accessed 8.10.20).
- Claro I.C.M., Cabral A.D., Augusto M.R., Duran A.F.A., Graciosa M.C.P., Fonseca F.L.A., Speranca M.A., Bueno R.de F. Long-term monitoring of SARS-COV-2 RNA in wastewater in Brazil: a more responsive and economical approach. Water Res. 2021;203 doi: 10.1016/j.watres.2021.117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372(80):eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier J., Störmer M., Kleesiek K. Use of bacteriophage MS2 as an internal control in viral reverse transcription-PCR assays. J. Clin. Microbiol. 2005;43:4551–4557. doi: 10.1128/JCM.43.9.4551-4557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel W., Kirstin M., Vedran F., Asija D., Theresa G.L., Roberto A., Filippos K., David K., Katja H., Salah A., Christopher B., Karen H., Anja R., Ivano L., Andranik I., Tommaso M., Simone D.G., Jan P., Samantha P., Meyer Thomas F., Alexander M.M., Daniela N., Andreas H., Matthias S., Altuna A., Nikolaus R., Christian# D., Markus L. Transcriptomic profiling of SARS-CoV-2 infected human cell lines identifies HSP90 as target for COVID-19 therapy. iScience. 2021;24(3) doi: 10.1016/j.isci.2021.102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gand M., Vanneste K., Thomas I., Van Gucht S., Capron A., Herman P., Roosens N.H.C., De Keersmaecker S.C.J. Deepening of in silico evaluation of sars-cov-2 detection rt-qpcr assays in the context of new variants. Genes (Basel) 2021;12:1–14. doi: 10.3390/genes12040565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Hofmann-Winkler H., Krüger N., Kempf A., Nehlmeier I., Graichen L., Arora P., Sidarovich A., Moldenhauer A.S., Winkler M.S., Schulz S., Jäck H.M., Stankov M.V., Behrens G.M.N., Pöhlmann S. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021 doi: 10.1016/j.celrep.2021.109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y.J., Chiba S., Halfmann P., Ehre C., Kuroda M., Dinnon K.H., Leist S.R., Schäfer A., Nakajima N., Takahashi K., Lee R.E., Mascenik T.M., Graham R., Edwards C.E., Tse L.V., Okuda K., Markmann A.J., Bartelt L., Silva A.De, Margolis D.M., Boucher R.C., Randell S.H., Suzuki T., Gralinski L.E., Kawaoka Y., Baric R.S. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science. 2021;370(80):1464–1468. doi: 10.1126/science.abe8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.I., Khan Z.A., Baig M.H., Ahmad I., Farouk A.E.A., Song Y.G., Dong J.J. Comparative genome analysis of novel coronavirus (SARS-CoV-2) from different geographical locations and the effect of mutations on major target proteins: an in silico insight. PLoS One. 2020;15:1–18. doi: 10.1371/journal.pone.0238344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. .e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Angyal A., Brown R.L., Carrilero L., Green L.R., Groves D.C., Johnson K.J., Keeley A.J., Lindsey B.B., Parsons P.J., Raza M., Rowland-Jones S., Smith N., Tucker R.M., Wang D., Wyles M.D., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro .G.., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J., Klapsa D., Wilton T., Zambon M., Bentley E., Bujaki E., Fritzsche M., Mate R., Majumdar M. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses. 2020;12:1144. doi: 10.3390/v12101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng B., Kemp S.A., Papa G., Datir R., Ferreira I.A.T.M., Marelli S., Harvey W.T., Lytras S., Mohamed A., Gallo G., Thakur N., Collier D.A., Mlcochova P., et al. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the alpha variant B.1.1.7. Duncan L.M., Carabelli A.M., Kenyon J.C., Lever A.M., De Marco A., Saliba C., et al., editors. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the alpha variant B.1.1.7Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109292. The COVID-19 genomics UK (COG-UK) consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., Pandey R., Brown J., Zhou J., Goonawardane N., Mishra S., Whittaker C., Mellan T., Marwal R., Datta M., Sengupta S., Ponnusamy K., Radhakrishnan V.S., Abdullahi A., Charles O., Chattopadhyay P., Devi P., Caputo D., Peacock T., Wattal C., Goel N., Satwik A., Vaishya R., Agarwal M., Chauhan H., Dikid T., Gogia H., Lall H., Verma K., Dhar M.S., Singh M.K., Soni N., Meena N., Madan P., Singh P., Sharma R., Kabra R., Sharma S., Kumar S., Kumari S., Sharma U., Chaudhary U., Sivasubbu S., Scaria V., Oberoi J.K., Raveendran R., Datta S., Das S., Maitra A., Chinnaswamy S., Biswas N.K., Parida A., Raghav S.K., Prasad P., Sarin A., Mayor S., Ramakrishnan U., Palakodeti D., Seshasayee A.S.N., Thangaraj K., Bashyam M.D., Dalal A., Bhat M., Shouche Y., Pillai A., Abraham P., Potdar V.A., Cherian S.S., Desai A.S., Pattabiraman C., Manjunatha M.V., Mani R.S., Udupi G.A., Nandicoori V., Tallapaka K.B., Sowpati D.T., Kawabata R., Morizako N., Sadamasu K., Asakura H., Nagashima M., Yoshimura K., Ito J., Kimura I., Uriu K., Kosugi Y., Suganami M., Oide A., Yokoyama M., Chiba M., Saito A., Butlertanaka E.P., Tanaka Y.L., Ikeda T., Motozono C., Nasser H., Shimizu R., Yuan Y., Kitazato K., Hasebe H., Nakagawa S., Wu J., Takahashi M., Fukuhara T., Shimizu K., Tsushima K., Kubo H., Shirakawa K., Kazuma Y., Nomura R., Horisawa Y., Takaori-Kondo A., Tokunaga K., Ozono S., Baker S., Dougan G., Hess C., Kingston N., Lehner P.J., Lyons P.A., Matheson N.J., Owehand W.H., Saunders C., Summers C., Thaventhiran J.E.D., Toshner M., Weekes M.P., Maxwell P., Shaw A., Bucke A., Calder J., Canna L., Domingo J., Elmer A., Fuller S., Harris J., Hewitt S., Kennet J., Jose S., Kourampa J., Meadows A., O’Brien C., Price J., Publico C., Rastall R., Ribeiro C., Rowlands J., Ruffolo V., Tordesillas H., Bullman B., Dunmore B.J., Fawke S., Gräf S., Hodgson J., Huang C., Hunter K., Jones E., Legchenko E., Matara C., Martin J., Mescia F., O’Donnell C., Pointon L., Pond N., Shih J., Sutcliffe R., Tilly T., Treacy C., Tong Z., Wood J., Wylot M., Bergamaschi L., Betancourt A., Bower G., Cossetti C., De Sa A., Epping M., Fawke S., Gleadall N., Grenfell R., Hinch A., Huhn O., Jackson S., Jarvis I., Krishna B., Lewis D., Marsden J., Nice F., Okecha G., Omarjee O., Perera M., Potts M., Richoz N., Romashova V., Yarkoni N.S., Sharma R., Stefanucci L., Stephens J., Strezlecki M., Turner L., De Bie E.M.D.D., Bunclark K., Josipovic M., Mackay M., Rossi S., Selvan M., Spencer S., Yong C., Allison J., Butcher H., Caputo D., Clapham-Riley D., Dewhurst E., Furlong A., Graves B., Gray J., Ivers T., Kasanicki M., Le Gresley E., Linger R., Meloy S., Muldoon F., Ovington N., Papadia S., Phelan I., Stark H., Stirrups K.E., Townsend P., Walker N., Webster J., Scholtes I., Hein S., King R., Mavousian A., Lee J.H., Bassi J., Silacci-Fegni C., Saliba C., Pinto D., Irie T., Yoshida I., Hamilton W.L., Sato K., Bhatt S., Flaxman S., James L.C., Corti D., Piccoli L., Barclay W.S., Rakshit P., Agrawal A., Gupta R.K. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021 doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas D., Bruel T., Grzelak L., Guivel-Benhassine F., Staropoli I., Porrot F., Planchais C., Buchrieser J., Rajah M.M., Bishop E., Albert M., Donati F., Prot M., Behillil S., Enouf V., Maquart M., Smati-Lafarge M., Varon E., Schortgen F., Yahyaoui L., Gonzalez M., De Sèze J., Péré H., Veyer D., Sève A., Simon-Lorière E., Fafi-Kremer S., Stefic K., Mouquet H., Hocqueloux L., van der Werf S., Prazuck T., Schwartz O. Sensitivity of infectious SARS-CoV-2 B1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 2021;27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/nejmoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana. U. S. A. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Eurosurveillance. 2017;22:2–4. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E.C., Rosen L.E., Shepherd J.G., Spreafico R., da Silva Filipe A., Wojcechowskyj J.A., Davis C., Piccoli L., Pascall D.J., Dillen J., Lytras S., Czudnochowski N., Shah R., Meury M., Jesudason N., De Marco A., Li K., Bassi J., O'Toole A., Pinto D., Colquhoun R.M., Culap K., Jackson B., Zatta F., Rambaut A., Jaconi S., Sreenu V.B., Nix J., Zhang I., Jarrett R.F., Glass W.G., Beltramello M., Nomikou K., Pizzuto M., Tong L., Cameroni E., Croll T.I., Johnson N., Di Iulio J., Wickenhagen A., Ceschi A., Harbison A.M., Mair D., Ferrari P., Smollett K., Sallusto F., Carmichael S., Garzoni C., Nichols J., Galli M., Hughes J., Riva A., Ho A., Schiuma M., Semple M.G., Openshaw P.J.M., Fadda E., Baillie J.K., Chodera J.D., Rihn S.J., Lycett S.J., Virgin H.W., Telenti A., Corti D., Robertson D.L., Snell G. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell. 2021;184:1171–1187. doi: 10.1016/j.cell.2021.01.037. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Ait Mouheb N., Partiot E., Bakhache W., Deffieu M.S., Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Magaña N., Sánchez-Sánchez R., Hernández-Bello J., Venancio-Landeros A.A., Peña-Rodríguez M., Vega-Zepeda R.A., Galindo-Ornelas B., Díaz-Sánchez M., García-Chagollán M., Macedo-Ojeda G., García-González O.P., Muñoz-Valle J.F. RT-qPCR Assays for rapid detection of the N501Y, 69-70del, K417N, and E484K SARS-CoV-2 mutations: a screening strategy to identify variants with clinical impact. Front. Cell. Infect. Microbiol. 2021;11:1–11. doi: 10.3389/fcimb.2021.672562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels C.B.F., Breban M.I., Ott I.M., Alpert T., Petrone M.E., Watkins A.E., Kalinich C.C., Earnest R., Rothman J.E., de Jesus J.G., Claro I.M., Ferreira G.M., Crispim M.A.E., Singh L., Tegally H., Anyaneji U.J., Hodcroft E.B., Mason C.E., Khullar G., Metti J., Dudley J.T., MacKay M.J., Nash M., Wang J., Liu C., Hui P., Murphy S., Neal C., Laszlo E., Landry M.L., Muyombwe A., Downing R., Razeq J., de Oliveira T., Faria N.R., Sabino E.C., Neher R.A., Fauver J.R., Grubaugh N.D., da Silva Sales F.C., Ramundo M.S., Candido D.S., Silva C.A.M., de Pinho M.C., Coletti T.de M., Andrade P.dos S., de Souza L.M., Rocha E.C., Gomes Jardim A.C., Manuli E., Gaburo N., Granato C., Levi J.E., Costa S., de Souza W.M., Salum M.A., Pereira R., de Souza A., Matkin L.E., Nogueria M.L., Levin A.S., Mayaud P., Alexander N., Souza R., Acosta A.L., Prete C., Quick J., Brady O., Messina J., Kraemer M., Gouveia N., da C., Oliva I., de Souza M., Lazari C., Alencar C.S., Thézé J., Buss L., Araujo L., Cunha M.S., Loman N.J., Pybus O.G., Aguiar R.S., Wilkinson E., Msomi N., Iranzadeh A., Fonseca V., Doolabh D., San E.J., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao M., Korsman S., Davies M.A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Kosakovsky Pond S.L., Weaver S., Giovanetti M., Alcantara L.C.J., Martin D., Bhiman J.N., Williamson C. Multiplex qPCR discriminates variants of concern to enhance global surveillance of SARS-CoV-2. PLoS Biol. 2021;19:1–12. doi: 10.1371/journal.pbio.3001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Miller J.A., Verghese M., Sibai M., Solis D., Mfuh K.O., Jiang B., Iwai N., Mar M., Huang C., Yamamoto F., Sahoo M.K., Zehnder J., Pinsky B.A. Multiplex SARS-CoV-2 genotyping RT-PCR for population-level variant screening and epidemiologic surveillance. J. Clin. Microbiol. 2021 doi: 10.1128/JCM.00859-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., Oliveira T.Y., Yang Z., Abernathy M.E., Huey-Tubman K.E., Hurley A., Turroja M., West K.A., Gordon K., Millard K.G., Ramos V., Da Silva J., Xu J., Colbert R.A., Patel R., Dizon J., Unson-O'Brien C., Shimeliovich I., Gazumyan A., Caskey M., Bjorkman P.J., Casellas R., Hatziioannou T., Bieniasz P.D., Nussenzweig M.C. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C., Muecksch F., Rutkowska M., Hoffmann H.-.H., Michailidis E., Gaebler C., Agudelo M., Cho A., Wang Z., Gazumyan A., Cipolla M., Luchsinger L., Hillyer C.D., Caskey M., Robbiani D.F., Rice C.M., Nussenzweig M.C., Hatziioannou T., Bieniasz P.D. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife. 2020;9:1. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhaus S., Weber F.A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., Rossouw T., Boswell M., Ueckermann V., Meiring S., von Gottberg A., Cohen C., Morris L., Bhiman J.N., Moore P.L. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Bushman M., Chai P.R., Duvallet C., Erickson T.B., Foppe K., Ghaeli N., Gu X., Hanage W.P., Huang K.H., Lee W.L., McElroy K.A., Rhode S.F., Matus M., Wuertz S., Thompson J., Alm E.J. Wastewater surveillance of SARS-CoV-2 across 40 U.S. states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 Titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5:1–9. doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv K., Ozer E., Shagan M., Lakkakula S., Plotkin N., Bhandarkar N.S., Kushmaro A. Direct RT-qPCR assay for SARS-CoV-2 variants of concern (Alpha, B.1.1.7 and Beta, B.1.351) detection and quantification in wastewater. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.