Abstract
Studies of Staphylococcus aureus nasal carriage have distinguished three carriage patterns: persistent, intermittent, and noncarriage. The criteria used to identify these carriage patterns have been inconsistent. In 1988 the S. aureus nasal carrier index, i.e., the proportion of nasal swab specimen cultures yielding S. aureus, was determined for 91 staff members of various departments of a large university hospital by obtaining weekly nasal swab specimens for culture over a 12-week period. Thirty-three (36%) persons had carrier indices of 0.80 or higher, 15 (17%) had indices between 0.1 and 0.7, and 43 (47%) had indices of zero. In 1995, 17 individuals with carrier indices of 0.80 or higher in 1988 were available for reexamination. For 12 (71%) of these individuals, S. aureus was again isolated from a single nasal swab, i.e., from each individual with a 1988 carrier index of 1.0 but from only half of those with indices below 1.0. Genotyping (by randomly amplified polymorphic DNA analysis and pulsed-field gel electrophoresis) of all S. aureus strains showed that strains isolated from only three individuals, all with 1988 carrier indices of 1.0, in 1988 and 1995 showed genetic similarity. In conclusion, persistent S. aureus nasal carriage is a unique characteristic of a fraction of the population, and the attribute “persistent” should be confined to those individuals for whom serial nasal swab specimen cultures consistently yield S. aureus.
Staphylococcus aureus nasal carriage has been extensively studied in patients and healthy individuals (22, 54). Cross-sectional surveys of S. aureus nasal carriage have designated individuals as either carriers or noncarriers (10, 15, 17, 20, 21, 25, 29–32, 34, 35, 40, 55) (Table 1). In longitudinal studies, however, the carrier state has been shown to change over time in some individuals. Three carriage patterns can be distinguished: persistent carriage, intermittent carriage, and noncarriage (1, 14, 15, 19, 20, 24, 28, 30, 31, 38). However, the criteria used to identify these carriage patterns have varied from study to study with respect to the number of nasal specimen cultures that are performed, the follow-up period, and the interpretation of the culture data that are obtained (Table 2). Despite this lack of consistency, several studies have shown the importance of distinguishing persistent from intermittent nasal carriage. The mean number of CFU of S. aureus that can be isolated from the anterior nares is higher in persistent carriers than in intermittent carriers (49, 52), resulting in more extensive dispersal of the staphylococci in the environment (50) and in an increased risk of S. aureus infections (4, 5, 51). Moreover, the number of S. aureus phage types or genotypes that are isolated in repeated cultures is significantly lower for persistent carriers than for intermittent carriers (15, 38, 46), indicating that the basic determinants of persistent and intermittent carriage may be different.
TABLE 1.
S. aureus nasal carriage rates in healthy adults reported in cross-sectional surveys
| Referencea | Study population | No. of subjects | Reported carriage rate (%) |
|---|---|---|---|
| Hallman, 1937 (17) | College students (United States) | 109 | 37 |
| Miles et al., 1944 (30) | Hospital personnel (United Kingdom) | 74 | 64 |
| Findlay and Abrahams, 1946 (10) | European medical personnel (West Africa) | 100 | 38 |
| African soldiers and villagers (West Africa) | 300 | 23 | |
| Gould and McKillop, 1954 (15) | Blood donors (Scotland) | 86 | 52 |
| Medical students (Scotland) | 520 | 19 | |
| Rountree, 1956 (40) | Unselected population (New Guinea) | 120 | 19 |
| Millian et al., 1960 (32) | Caucasian job applicants (United States) | 418 | 37 |
| Negroid job applicants (United States) | 139 | 14 | |
| Jacobs et al., 1961 (21) | Nurses (United Kingdom) | 178 | 38 |
| Miller et al., 1962 (31) | Army recruits (United Kingdom) | 2,376 | 45 |
| Leedom et al. 1965 (25) | Hospital personnel (United States) | 951 | 23 |
| Noble et al., 1967 (34) | Unselected population (The Netherlands) | 839 | 30 |
| Wilson et al., 1977 (55) | Medical students (United States) | 414 | 25 |
| Paul et al., 1982 (35) | Hospital personnel (Nigeria) | 66 | 56 |
| McAnally et al., 1984 (29) | Hospital personnel (United States) | 664 | 25 |
| Hu et al., 1995 (20) | Medical students (Japan) | 51 | 22 |
Author, year of publication (reference number).
TABLE 2.
S. aureus nasal carriage patterns in healthy adults reported in longitudinal surveys
| Referencea | Study population | No. of subjects | Study design
|
% of cultures that grew S. aureus for the following stateb:
|
Reported carriage rates (%)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up period | Culture interval | Noncarriage | Intermittent carriage | Persistent carriage | Noncarriage | Intermittent carriage | Persistent carriage | |||
| Gould and McKillop, 1954 (15) | Medical students (Scotland) | 520 | 1 yr | 1 wk | 0 | 1–9 (O) | ≥91 | 19 | 57 | 24 |
| 10–90 (I) | ||||||||||
| Goslings and Büchli, 1958 (14) | Family members of discharged patients (The Netherlands) | 243 | 8 wk | 2 wk | 0 | 1–99 | 100 | 51 | 25 | 24 |
| Miller et al., 1962 (31) | Army recruits (United Kingdom) | 515 | 7–8 wk | 7–8 wk | 0 | 1–99 | 100 | 39 | 24 | 37 |
| Maxwell et al., 1969 (28) | Hospital personnel (United States) | 127 | 10 wk–5 yr | ≤2 wk | ≤10 | 11–44 (O) | ≥80 | 44 | 38 | 18 |
| 45–79 (I) | ||||||||||
| Armstrong-Esther and Smith, 1976 (1) | University personnel (United Kingdom) | 50 | 30 wk | 1 wk | ≤9 | 10–69 | ≥70 | 2 | 64 | 34 |
| Höffler et al., 1978 (19) | Nurses and laboratory personnel (Germany and Poland) | 261 | 6 wk | 1 wk | ≤17 | 18–82 | ≥83 | 71 | 20 | 9 |
| Lamikanra and Olusanya, 1988 (24) | Pharmacy students (Nigeria) | 50 | 15 mo | ≤2 wk | ≤10 | 11–44 (O) | ≥80 | 46 | 28 | 26 |
| Riewerts Eriksen et al., 1995 (38) | Laboratory personnel (Denmark) | 104 | 19 mo | 1–2 mo | 45–79 (I) | |||||
| 0 | 1–44 (O) | ≥85 | 16 | 70 | 14 | |||||
| 45–84 (I) | ||||||||||
| Hu et al., 1995 (20) | Medical students (Japan) | 51 | 5 mo | 1–3 mo | 0 | 1–99 | 100 | 69 | 19 | 12 |
Author, year of publication (reference number).
O, occasional carriage; I, intermittent carriage.
In the present study the S. aureus nasal carrier state was determined for 91 healthy adults during a 12-week follow-up period. Eight years later, persistent carriers were reexamined to investigate their current S. aureus nasal carrier state. Different genotyping techniques were applied to identify any genetic similarity of the S. aureus strains isolated over this 8-year period. The results provide unique evidence for the redefinition of the persistent nasal carrier state.
MATERIALS AND METHODS
Study population.
In 1988 a screening for S. aureus nasal carriage was performed among 91 staff members of the Departments of Bacteriology, Virology, Dermatology, Immunology, and Epidemiology of the Erasmus University Medical Center, Rotterdam, The Netherlands. All 51 male and 40 female participants were healthy adults. Nasal swab specimen cultures were performed weekly for 10 to 12 weeks. For each person the S. aureus carrier index was calculated. The carrier index was defined as the number of nasal swab specimen cultures that grew S. aureus divided by the total number of nasal swab specimen cultures performed for that person. Persistent nasal carriers comprised those persons with carrier indices of 0.80 or higher, intermittent carriers were those with carrier indices between 0.1 and 0.70, and noncarriers were those with indices of zero. In 1995 single nasal swab specimens were obtained from 17 (52%) of the 33 persistent carriers that had been identified in 1988 and were available for renewed determination of their S. aureus nasal carrier state.
Nasal swabbing and isolation of S. aureus.
Nasal swab specimens were obtained by using sterile dry cotton-wool swabs (Transwab; Medical Wire & Equipment Co. Ltd., Corsham, United Kingdom). Both the left and right anterior nares were swabbed by rubbing the swab four times around the inside of each nostril while applying an even pressure and rotating the swab without interruption. The swabs were immediately placed in Stuart’s transport medium (Transwab; Medical Wire & Equipment Co. Ltd.) and kept at 4°C until inoculation.
In 1988 nasal swabs were inoculated within 24 h onto Columbia blood agar plates (Becton-Dickinson B.V., Etten-Leur, The Netherlands) and phenol-red mannitol salt agar plates (Difco-Brunschwig B.V., Amsterdam, The Netherlands). The plates were incubated at 37°C for 48 h. Identification of S. aureus was based upon colony morphology, a free coagulase (tube) test (Difco-Brunschwig B.V.), and a bound coagulase (agar) test (47) applied to suspected colonies after demonstration of catalase positivity. For persistent carriers S. aureus isolates of the first, sixth, and last nasal swab cultures were phage typed (National Institute for Public Health and Environmental Hygiene, Bilthoven, The Netherlands). One isolate per phage type was stored as a freeze-dried sample. In 1995, the identification of all isolates obtained in 1988 was confirmed by a rapid S. aureus-specific latex agglutination test (Staphaurex Plus; Murex Diagnostics Benelux B.V., Utrecht, The Netherlands) (26).
In 1995 nasal swabs were inoculated within 24 h onto Columbia blood agar plates (Becton-Dickinson B.V.) and phenol-red mannitol salt agar plates (Difco-Brunschwig B.V.). After inoculation the swabs were placed in brain heart infusion medium, incubated at 37°C for 24 h, and subsequently inoculated onto solid media. All solid media were incubated at 37°C for 48 h. Identification of S. aureus was based upon colony morphology and a rapid S. aureus-specific latex agglutination test (Staphaurex Plus; Murex Diagnostics Benelux B.V.) (26), which was applied to suspected colonies after demonstration of catalase positivity. For each carrier up to three colonies per colony morphotype per inoculated plate were stored in glycerol stocks at −80°C.
Genotyping. (i) RAPD.
Bacteria were grown overnight on Columbia blood agar plates (Becton-Dickinson B.V.). Two to three discrete colonies were resuspended in 150 μl of 25 mM Tris · HCl (pH 8.0)–10 mM EDTA–50 mM glucose. Lysostaphin (75 μl of a 100-μg/ml solution; Sigma Chemical Co., St. Louis, Mo.) was added, and the mixture was incubated at 37°C for 1 h. DNA isolation was performed by the method of Boom et al. (3). Stock solutions of DNA were adjusted to a concentration of 0.5 ng/μl and were stored at −20°C until use. PCR and subsequent electrophoresis of the amplification products was performed essentially as described previously (45). The primers used to discriminate S. aureus strains were RAPD1 (5′-GGTTGGGTGAGAATTGCACG-3′), RAPD7(5′-GTGGATGCGA-3′), and ERIC2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) (45, 48). Randomly amplified polymorphic DNA (RAPD) banding patterns were interpreted visually by two independent observers and were indexed with Roman numerals. Differences in band staining intensities and single band differences were neglected. In this way all S. aureus isolates were identified by a three-letter code.
(ii) PFGE.
Pulsed-field gel electrophoresis (PFGE) was carried out on the basis of protocols previously described for S. aureus (6, 36). The bacteria were grown overnight on Columbia blood agar plates (Becton-Dickinson B.V.). Two to three discrete colonies were suspended in a 1:1 ratio in 1% InCert agarose (FMC Bioproducts, Rockland, Maine). Agarose plugs were prepared with Bio-Rad casting forms (Bio-Rad Inc., Veenendaal, The Netherlands) and were incubated with lysostaphin (Sigma Chemical Co.). Spheroplasts were lysed by incubating the plugs in buffer containing 1% sodium dodecyl sulfate and 1 mg of proteinase K (Boehringer Mannheim, Mannheim, Germany) per ml. The plugs were washed six times for 30 min each time in 10 mM Tris · HCl (pH 8.0)–1 mM EDTA and were stored at 4°C. DNA was digested with the restriction enzyme SmaI (Boehringer Mannheim), and PFGE was carried out in 1% SeaKem GTG agarose gels (FMC Bioproducts) in a 0.5× TBE (Tris-borate-EDTA) buffer at a temperature of 14°C. Electrophoresis was performed in a Bio-Rad CHEF Mapper. The running time was 22 h, with linear ramping from 2.16 to 44.69 s at an angle of 120°C (60°C and −60°C), at a voltage of 6 V/cm. Banding patterns were interpreted by two independent observers according to the guidelines provided by Tenover et al. (44). Types were defined on the basis of the identity or nonidentity of the banding patterns and were indexed with Roman numerals.
(iii) Protein A gene PCR.
Protein A gene polymorphisms were determined by PCR as described previously (12). The repetitive X region within the spa gene was amplified with oligonucleotide primers with the following DNA sequences: 5′-TGTAAAACGACGGCCAGTGCTAAAAAGCTAAACGATGC-3′ and 5′-CAGGAAACAGCTATGACCCCACCAAATACAGTTGTACC-3′. The PCR product was digested with the restriction endonuclease RsaI (Boehringer Mannheim), resulting in two fragments composed of 214 and 35 bases, respectively, and a third fragment containing the variable-length repetitive DNA. The restriction fragment length polymorphisms (RFLPs) of this third fragment were determined by electrophoresis. RFLP patterns were visually interpreted by two independent observers. The number of 24-bp repeats present was determined in comparison with a 100-bp molecular length marker (Pharmacia, Gouda, The Netherlands).
(iv) Coagulase gene PCR.
Coagulase gene polymorphism was determined by PCR as described previously (42). The primers used for amplification of the coagulase gene were COAG2 (5′-CGAGACCAAGATTCAACAAG-3′) and COAG3 (5′-AAAGAAAACCACTCACATCA-3′) (13). The PCR product (10 μl) was digested with the restriction endonuclease AluI (Boehringer Mannheim). RFLP patterns were visually interpreted by two independent observers and were indexed with Roman numerals.
Statistical analysis.
Differences in the distributions of continuous and categorical variables between groups were tested by one-way analysis of variance and the chi-square test, respectively.
RESULTS
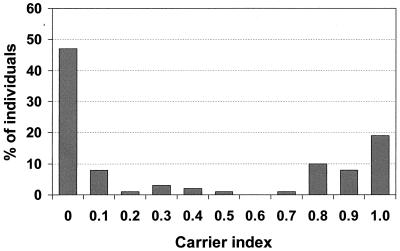
The carrier indices for the 91 persons screened in 1988 are presented in Fig. 1. According to our 1988 definitions for the nasal carrier state (see Materials and Methods), 43 (47%) noncarriers, 15 (17%) intermittent carriers, and 33 (36%) persistent carriers were identified. Population characteristics are shown in Table 3. Sex, age, and department of employment were not statistically significantly different for the three carrier states.
FIG. 1.
Distribution of S. aureus nasal carrier indices among 91 healthy adults repeatedly cultured over a 10- to 12-week period in 1988. The carrier index is defined as the proportion of cultures yielding S. aureus for a given individual.
TABLE 3.
Distribution of sex, age, and department of employment by S. aureus nasal carrier state among 91 healthy adults repeatedly sampled for culture over a 10- to 12-week period in 1988
| Characteristic |
S. aureus nasal carrier statea
|
P | |||
|---|---|---|---|---|---|
| Noncarriage (n = 43) | Intermittent carriage (n = 15) | Persistent carriage (n = 33) | Overall (n = 91) | ||
| Male/female ratio | 0.6 | 0.7 | 1.2 | 0.8 | 0.30b |
| Age (yr) (mean [SD]) | 30.3 (9.5) | 28.2 (7.2) | 33.4 (8.2) | 31.0 (8.8) | 0.13c |
| Department (no. [%] of subjects) | |||||
| Bacteriology | 7 (16) | 5 (33) | 13 (40) | 25 (27) | 0.60d |
| Virology | 4 (9) | 1 (7) | 2 (6) | 7 (8) | |
| Dermatology | 6 (14) | 1 (7) | 2 (6) | 9 (10) | |
| Immunology | 18 (42) | 6 (40) | 10 (30) | 34 (37) | |
| Epidemiology | 8 (19) | 2 (13) | 6 (18) | 16 (18) | |
The nasal carrier state was defined according to the carrier index (i.e., the proportion of cultures yielding S. aureus for a given individual). Noncarriage, carrier index of 0; intermittent carriage, carrier indices of 0.1 to 0.7; persistent carriage, carrier indices of 0.8 to 1.0.
χ2 = 2.392 (2 degrees of freedom).
F = 2.096 (degrees of freedom, between and within, 2 and 88, respectively).
χ2 = 6.387 (8 degrees of freedom).
Seventeen (52%) of the 33 persistent carriers in the 1988 screening were available for nasal swab specimen culture in 1995. The distributions of sex, age, and department of employment in 1988 for individuals available for reexamination were comparable to those for individuals not available for reexamination (data not shown). In 12 (71%) of these 1988 carriers, S. aureus was again isolated from a single nasal swab specimen. S. aureus isolation in 1995 was significantly more frequent in carriers with 1988 carrier indices of 1.0 (100%) than in carriers with 1988 indices below 1.0 (50%) (P = 0.04). The isolation rate for the latter group was significantly lower than the expected isolation rate of 85% (95% confidence interval, 78 to 92%) on the basis of the previous carrier indices.
All S. aureus strains isolated in 1988 and 1995 were genotyped by RAPD analysis and PFGE and were screened for polymorphisms in the protein A and coagulase genes (Table 4; Fig. 2 to 5). The results of the two whole-genome typing methods, RAPD analysis and PFGE, were quite concordant, with the methods detecting 15 and 16 different genotypes, respectively. RAPD analysis and PFGE revealed the genetic similarity of the 1988 and 1995 S. aureus isolates in four and three individuals, respectively. PFGE types G and K, isolated from carrier 11 in 1995, and types A and N, isolated from carrier 13 in 1995, differed by two and three bands, respectively. According to the guidelines provided by Tenover et al. (44), these isolates should be considered closely related, indicating that the genomes of persistent S. aureus strains may slowly evolve in an individual over time. The numbers of protein A repeat units in the 1988 and 1995 isolates were identical in six carriers, but identical numbers of protein A repeat units were detected in isolates from only two of the three individuals for whom the genetic similarity of the S. aureus isolates was identified by RAPD analysis and PFGE, indicating variability within the protein A gene of S. aureus strains that otherwise showed constant overall genotypic characteristics. The RFLP patterns of the coagulase genes of the 1988 and 1995 isolates were identical in all three individuals in which the genetic similarity of the S. aureus isolates was identified by RAPD analysis and PFGE. In one additional carrier (carrier 15) the coagulase gene typing patterns of the 1988 and 1995 isolates were identical, but this result could not be confirmed by RAPD analysis or PFGE. This observation can be explained by the limited resolution of coagulase gene typing, which is illustrated by the data in Table 4. Only 7 coagulase gene RFLP patterns were observed; this is in contrast to the 16 different genotypes that were detected by PFGE. When RAPD analysis and PFGE, the typing techniques with the highest discriminatory powers, were combined, three carriers of S. aureus isolates with genetic similarity in 1988 and 1995 were identified. Genetic similarity was seen only for isolates from carriers with carrier indices of 1.0 in the original screening in 1988. Renewed isolation of S. aureus strains in 1995 did not seem to be associated with the number of protein A repeats or with the coagulase gene RFLP pattern, as the patterns observed for these strains were also found for strains that were isolated only in 1988 (Table 4).
TABLE 4.
Genotyping of S. aureus strains isolated in nasal carriers in 1988 and 1995
| Carrier no. | Carrier indexa | Yr of culture | Isolation of S. aureus | Genotyping
|
|||
|---|---|---|---|---|---|---|---|
| RAPD | PFGE | No. of protein A repeatsb | Coagulase gene | ||||
| 1 | 0.80 | 1988 | Yes | NDc | ND | ND | ND |
| 1995 | No | ||||||
| 2 | 0.83 | 1988 | Yes | AAA | A | 3 | A |
| 1995 | Yes | BBB | B | 9 | B | ||
| CBB | C | 13 | C | ||||
| 3 | 0.83 | 1988 | Yes | ND | ND | ND | ND |
| 1995 | No | ||||||
| 4 | 0.83 | 1988 | Yes | DCC | D | 10d | D |
| 1995 | Yes | EAA | E | 3 | A | ||
| BBD | B | 10d | B | ||||
| 5 | 0.83 | 1988 | Yes | BBB | B | 10d | B |
| 1995 | Yes | BBD | F | 11 | E | ||
| BBD | G | 11 | E | ||||
| BBD | F | 10d | E | ||||
| 6 | 0.83 | 1988 | Yes | FDEd | H | 12 | F |
| 1995 | Yes | FDEd | I | 7 | B | ||
| 7 | 0.83 | 1988 | Yes | ND | ND | ND | ND |
| 1995 | No | ||||||
| 8 | 0.92 | 1988 | Yes | ND | ND | ND | ND |
| 1995 | No | ||||||
| 9 | 0.92 | 1988 | Yes | AAA | J | 1 | A |
| 1995 | Yes | FDF | G | 11 | E | ||
| 10 | 0.92 | 1988 | Yes | ND | ND | ND | ND |
| 1995 | No | ||||||
| 11 | 1.00 | 1988 | Yes | AAA | A | 9 | A |
| 1995 | Yes | GDE | Ge | 13 | E | ||
| GDE | Ke | 13 | E | ||||
| 12 | 1.00 | 1988 | Yes | HCCd | Ld | 9 | Ed |
| 1995 | Yes | HCCd | Ld | 5 | Ed | ||
| ICC | M | 5 | Ed | ||||
| 13 | 1.00 | 1988 | Yes | AEAd | Ad | 7d | Ad |
| 1995 | Yes | AEAd | Ad,f | 7d | Ad | ||
| AEAd | Nf | 7d | Ad | ||||
| 14 | 1.00 | 1988 | Yes | DCCd | Od | 9d | Gd |
| 1995 | Yes | DCCd | Od | 9d | Gd | ||
| 15 | 1.00 | 1988 | Yes | HCC | D | 10d | Ed |
| 1995 | Yes | JBB | B | 10d | Ed | ||
| 16 | 1.00 | 1988 | Yes | GCG | C | 10 | E |
| 1995 | Yes | FBE | P | 9 | F | ||
| 17 | 1.00 | 1988 | Yes | FDF | G | 8d | H |
| 1995 | Yes | BBB | B | 8d | B | ||
| Total no. of genotypes | 15 | 14 | 10 | 8 | |||
The carrier index is defined as the proportion of nasal cultures yielding S. aureus for a given individual.
The number of protein A repeats as assessed by RFLP analysis.
ND, not done.
Long-term persistence of molecular type.
Closely related genotypes; only a two-band difference.
Closely related genotypes; only a three-band difference.
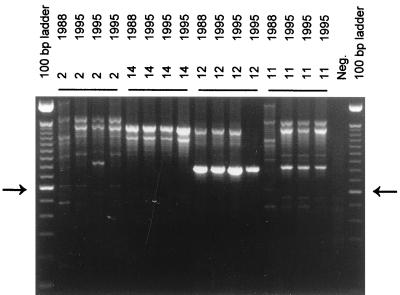
FIG. 2.
RAPD analysis with primer ERIC2 of S. aureus strains isolated in 1988 and 1995 from carriers 2, 11, 12, and 14. The arrows on the left and right identify the molecular length marker that is 600 bp long in the 100-bp ladder. The other fragments differ in size by multiples of 100 bp in length. Neg., negative control.
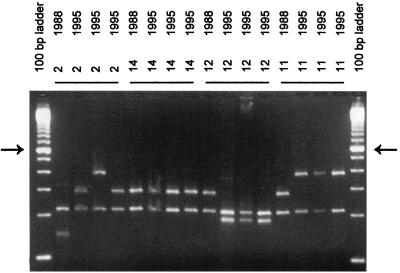
FIG. 5.
Coagulase gene PCR of S. aureus strains isolated in 1988 and 1995 from carriers 2, 11, 12, and 14. The arrows on the left and right identify the molecular length marker that is 600 bp in the 100-bp ladder. The other fragments differ by multiples of 100 bp in length. Neg., negative control.
DISCUSSION
In the initial 12-week screening of 91 healthy adults we identified 33 (36%) persons with S. aureus carrier indices of 0.80 or higher, 15 (17%) with carrier indices between 0.1 and 0.7, and 43 (47%) with carrier indices of zero. Eight years later, reexamination of carriers with indices of 0.8 or higher demonstrated that S. aureus was still present in the nares of each individual with a carrier index of 1.0 during the initial 12-week screening but in only half of those with 1988 indices below 1.0. Moreover, only the 1988 and 1995 S. aureus isolates observed in carriers with indices of 1.0 were found to be genetically similar.
The anterior nares have proven to be the primary reservoir of S. aureus in humans (7, 33, 53), and S. aureus nasal carriage has been established as a major risk factor for the development of both community-acquired and nosocomial infections (8, 9, 23, 27). S. aureus nasal carriage has been extensively studied in patients and healthy individuals (22, 54). Cross-sectional surveys of healthy adult populations have reported S. aureus nasal carriage rates between 20 and 55% (10, 15, 17, 20, 21, 25, 29–32, 34, 35, 40, 55). Longitudinal studies, however, indicated that carriage patterns differ between individuals, and that 10 to 35% of individuals carry S. aureus persistently, 20 to 75% carry S. aureus intermittently, and 5 to 70% are persistently free of S. aureus (noncarriers) (1, 14, 15, 19, 20, 24, 28, 30, 31, 38). The variation in reported rates results, at least partly, from differences in study populations, sampling and culture techniques, and criteria for the definition of persistent or intermittent carriage. First, many studies of S. aureus nasal carriage rates in healthy adults have been performed in selected populations, including medical students, hospital personnel, job applicants, and blood donors (Tables 1 and 2); the reported rates may therefore differ from those for unselected populations (34). Second, differences in the procedures of nasal swabbing and isolation of S. aureus may account for some variation in carriage rates. It has been documented that swabs from the anterior nares (i.e., the vestibulum nasi) yield higher carriage rates than swabs taken from sites beyond this region (21, 33). A recent study has shown that the number of carriers in a given population is also dependent on the swab material, the transport medium, the medium for cultivation, and the incubation period (37). However, this was not true for the identification of persistent carriers, which was confirmed by our finding that broth enrichment of the 1995 nasal swabs did not result in the detection of additional carriers. Thus, noncarriage in some individuals was not due to the presence of only small numbers of bacteria in the anterior nares. Third, and perhaps most important, differences in reported carriage rates are also due to the various definitions used to assign the persistent and intermittent carrier state. The criteria used to assign an individual to either carriage pattern have varied from study to study in terms of both the interval and the number of cultures performed and the required proportion of cultures that grow S. aureus (Table 2). Persistent carriage rates as well as noncarriage rates tend to decrease with increasing follow-up periods and decreasing culture intervals, indicating that intermittent carriers may be misclassified as either persistent carriers or noncarriers if the follow-up period is short or when only a few specimens are cultured. Obviously, persistent carriage rates decrease if the required proportion of cultures that grow S. aureus, i.e., the carrier index, increases. In the present follow-up of S. aureus carriers with 1988 carrier indices of ≥0.8, the observed S. aureus carriage rate in 1995 was significantly lower than that expected for individuals with initial carrier indices of 0.8 or 0.9. In carriers with 1988 indices of 1.0, however, S. aureus was again isolated from all individuals, suggesting that the starting point in the identification of persistent S. aureus nasal carriage during a relatively short follow-up period should be the isolation of S. aureus in 100% of the nasal swab specimen cultures. The correct separation of the population into persons who are true persistent carriers versus those who carry S. aureus only intermittently may be highly relevant, since it allows studies into the molecular and genetic basis of S. aureus nasal carriage to become better focused. Moreover, it may have a direct clinical impact when one is designing intervention strategies because of the risks of infection associated with S. aureus nasal carriage. As the risks of S. aureus nasal carriage differ for intermittent and persistent carriers, the distinction between intermittent and persistent carriage enables the differential application of elimination strategies, which reduces costs and diminishes the risk of the development of antibiotic resistance. To our knowledge no data on the long-term persistence of S. aureus nasal carriage in healthy persons are available. Follow-up periods in longitudinal studies of S. aureus nasal carriage in healthy individuals have varied from 6 weeks to 5 years (1, 14, 15, 19, 20, 24, 28, 31, 38) (Table 2). In the present study, 71% of persistent S. aureus nasal carriers, as defined in the initial 12-week screening in 1988, were again identified as nasal carriers 8 years later. The finding of genetic similarity of strains isolated over such a long time frame in one-third of these carriers suggests that nasal carriage may persist for years, although intermittent colonization with these S. aureus strains over the 8-year period cannot be excluded. The persistence of single S. aureus clones in some of the carriers confirms previous reports on the exchange of S. aureus strains over time in nasal carriers (15, 28, 38, 46). A recent study noted that the S. aureus exchange rate was significantly higher in intermittent carriers than in persistent carriers (46), which would agree with our finding that the persistence of single S. aureus clones occurred only in carriers with carrier indices of 1.0.
Many different typing methods have been used to study clonal relatedness between S. aureus strains (43). PFGE and RAPD analysis are considered to be among the most reliable and reproducible whole-genome typing procedures (43, 45), with even increased resolution when combined analyses are performed (45). RFLP analyses of the genes encoding S. aureus proteins, such as protein A and coagulase, have also been applied as genotyping techniques (11–13, 18, 41–43). Although it has been suggested that protein A genotyping may be an important tool in the clonal analysis of methicillin-resistant S. aureus isolates (11), our data confirm the findings of recent studies that reported striking heterogeneity in the number of protein A gene repeat units in otherwise genetically highly related S. aureus isolates (18, 46), indicating that the protein A gene behaves in a hypervariable, unstable manner that is unrelated to the overall evolution of the S. aureus genome. Coagulase gene typing has been successful in the identification of outbreak-related strains (13, 18, 42, 43); however, unrelated strains may share identical AluI RFLP patterns (41, 43), suggesting that it should not be used as the sole method for the typing of S. aureus. In this study only 7 coagulase genotypes were observed, whereas 15 were observed by RAPD analysis and 16 were observed by PFGE, confirming the lower discriminatory power of coagulase gene typing compared to those of RAPD analysis and PFGE.
Protein A and coagulase, S. aureus proteins that protrude from the bacterial surface, have been suggested to play a role in the process of adherence of S. aureus to host cell structures (2, 16) and the epidemic behavior of S. aureus (12, 39). As polymorphisms in the genes that encode these proteins might be related to the efficiency of S. aureus adherence, we studied whether there was a relation between the composition of the coagulase genes and/or protein A genes of S. aureus strains and the likelihood of isolation of these specific strains after 8 years. Confirming recent data (46), the results of this study do not provide evidence of such a relation.
In summary, the lack of consistency in defining the S. aureus nasal carrier states has resulted in striking variations in reported carriage rates. Major factors that should be considered when comparing carriage rates are the population studied, the sampling and culture techniques applied, the follow-up period, the number of cultures available, and the criteria used to distinguish intermittent from persistent carriers. The results of this study indicate that persistent carriage of S. aureus is a unique characteristic of a fraction of the population and that the attribute “persistent” should be confined to those individuals for whom serial nasal swab specimen cultures uniformly and consistently yield S. aureus.
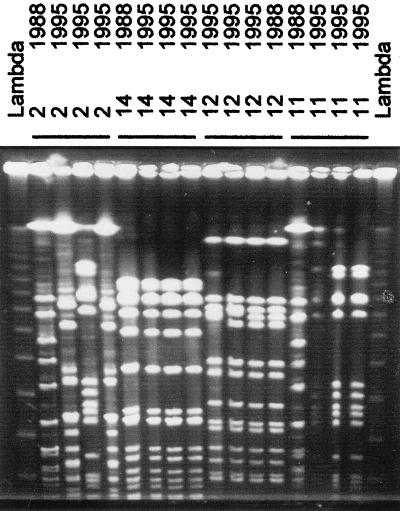
FIG. 3.
PFGE of S. aureus strains isolated in 1988 and 1995 from carriers 2, 11, 12, and 14. The lane marked lambda contains bacteriophage lambda concatemers that differ in size by multiples of 50 kbp.
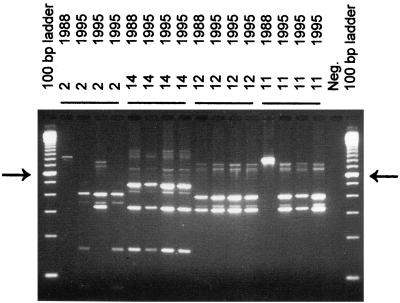
FIG. 4.
Protein A gene PCR of S. aureus strains isolated in 1988 and 1995 from carriers 2, 11, 12, and 14. The arrows on the left and right identify the molecular length marker that is 600 bp long in the 100-bp ladder. The other fragments differ by multiples of 100 bp in length.
ACKNOWLEDGMENTS
We thank Erwin Panken and Miranda Boers for excellent technical assistance with the genotyping.
REFERENCES
- 1.Armstrong-Esther C A, Smith J E. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–227. doi: 10.1080/03014467600001381. [DOI] [PubMed] [Google Scholar]
- 2.Bóden M K, Flock J. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989;57:2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruun J N. Post-operative wound infection. Predisposing factors and the effect of a reduction in the dissemination of staphylococci. Acta Med Scand. 1970;514:1–89. [PubMed] [Google Scholar]
- 5.Calia F M, Wolinsky E, Mortimer E A, Jr, Abrams J S, Rammelkamp C H., Jr Importance of the carrier state as a source of Staphylococcus aureus in wound sepsis. J Hyg. 1969;67:49–57. doi: 10.1017/s0022172400041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carles-Nurit M J, Christophle B, Broche S, Gouby A, Bouziges N, Ramuz M. DNA polymorphisms in methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1992;30:2092–2096. doi: 10.1128/jcm.30.8.2092-2096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casewell M W, Hill R L R. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (‘pseudomonic acid’)—a controlled trial. J Antimicrob Chemother. 1986;17:365–372. doi: 10.1093/jac/17.3.365. [DOI] [PubMed] [Google Scholar]
- 8.Chow J W, Yu V L. Staphylococcus aureus nasal carriage in hemodialysis patients. Its role in infection and approaches to prophylaxis. Arch Intern Med. 1989;149:1258–1262. [PubMed] [Google Scholar]
- 9.Corbella X, Domínguez M A, Pujol M, Ayats J, Sendra M, Pallares R, Ariza J, Gudiol F. Staphylococcus aureus nasal carriage as a marker for subsequent staphylococcal infections in intensive care unit patients. Eur J Clin Microbiol Infect Dis. 1997;16:351–357. doi: 10.1007/BF01726362. [DOI] [PubMed] [Google Scholar]
- 10.Findlay G M, Abrahams C. The incidence of staphylococci in the nose and on the skin of Africans and Europeans in West Africa. J Royal Army Med Corps. 1946;87:272–274. [PubMed] [Google Scholar]
- 11.Frénay H M E, Bunschoten A E, Schouls L M, van Leeuwen W J, Vandenbroucke-Grauls C M J E, Verhoef J, Mooi F R. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. Eur J Clin Microbiol Infect Dis. 1996;15:60–64. doi: 10.1007/BF01586186. [DOI] [PubMed] [Google Scholar]
- 12.Frénay H M E, Theelen J P G, Schouls L M, Vandenbroucke-Grauls C M J E, Verhoef J, van Leeuwen W J, Mooi F R. Discrimination of epidemic and nonepidemic methicillin-resistant Staphylococcus aureus strains on the basis of protein A gene polymorphism. J Clin Microbiol. 1994;32:846–847. doi: 10.1128/jcm.32.3.846-847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh S W, Byrne S K, Zhang J L, Chow A W. Molecular typing of Staphylococcus aureus on the basis of coagulase gene polymorphisms. J Clin Microbiol. 1992;30:1642–1645. doi: 10.1128/jcm.30.7.1642-1645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goslings W R O, Büchli K. Nasal carrier rate of antibiotic-resistant staphylococci. Arch Intern Med. 1958;102:691–715. doi: 10.1001/archinte.1958.00260220007002. [DOI] [PubMed] [Google Scholar]
- 15.Gould J C, McKillop E J. The carriage of Staphylococcus pyogenes var. aureus in the human nose. J Hyg. 1954;52:304–310. doi: 10.1017/s0022172400027509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haagen I A, Heezius H C, Verkooyen R P, Verhoef J, Verbrugh H A. Adherence of peritonitis-causing staphylococci to human peritoneal mesothelial cell monolayers. J Infect Dis. 1990;161:266–273. doi: 10.1093/infdis/161.2.266. [DOI] [PubMed] [Google Scholar]
- 17.Hallman F A. Pathogenic staphylococci in the anterior nares: their incidence and differentiation. Proc Soc Exp Biol Med. 1937;36:789–794. [Google Scholar]
- 18.Hoefnagels-Schuermans A, Peetermans W E, Struelens M J, van Lierde S, van Eldere J. Clonal analysis and identification of epidemic strains of methicillin-resistant Staphylococcus aureus by antibiotyping and determination of protein A gene and coagulase gene polymorphisms. J Clin Microbiol. 1997;35:2514–2520. doi: 10.1128/jcm.35.10.2514-2520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Höffler U, Bulanda M, Heczko P B, Pulverer G. A comparison of staphylococcal nasal carrier rates in Germany and Poland. Med Microbiol Immunol. 1978;164:285–290. doi: 10.1007/BF02125497. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, Umeda A, Kondo S, Amako K. Typing of Staphylococcus aureus colonising human nasal carriers by pulsed-field gel electrophoresis. J Med Microbiol. 1995;42:127–132. doi: 10.1099/00222615-42-2-127. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs S I, Williamson G M, Willis A T. Nasal abnormality and the carrier rate of Staphylococcus aureus. J Clin Pathol. 1961;14:519–521. doi: 10.1136/jcp.14.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluytmans J A J W, Mouton J W, Yzerman E P F, Vandenbroucke-Grauls C M J E, Maat A P W M, Wagenvoort J H T, Verbrugh H A. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171:216–219. doi: 10.1093/infdis/171.1.216. [DOI] [PubMed] [Google Scholar]
- 24.Lamikanra A, Olusanya O I. A long-term study of the nasal carriage of Staphylococcus aureus in healthy Nigerian students. Trans R Soc Trop Med Hyg. 1988;82:500–502. doi: 10.1016/0035-9203(88)90177-0. [DOI] [PubMed] [Google Scholar]
- 25.Leedom J M, Kennedy R P, Lepper M H, Jackson G G, Dowling H F. Observations of the staphylococcal nasal carrier state. Ann NY Acad Sci. 1965;128:381–403. doi: 10.1111/j.1749-6632.1965.tb11650.x. [DOI] [PubMed] [Google Scholar]
- 26.Luijendijk A, van Belkum A, Verbrugh H, Kluytmans J. Comparison of five tests for identification of Staphylococcus aureus from clinical samples. J Clin Microbiol. 1996;34:2267–2269. doi: 10.1128/jcm.34.9.2267-2269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luzar M A, Coles G A, Faller B, Slingeneyer A, Dah Dah G, Briat C, Wone C, Knefati Y, Kessler M, Peluso F. Staphylococcus aureus nasal carriage and infection in patients on continuous ambulatory peritoneal dialysis. N Engl J Med. 1990;322:505–509. doi: 10.1056/NEJM199002223220804. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell J G, Ford C R, Peterson D E, Mitchell C R. Long-term study of nasal staphylococci among hospital personnel. Am J Surg. 1969;118:849–854. doi: 10.1016/0002-9610(69)90245-1. [DOI] [PubMed] [Google Scholar]
- 29.McAnally T P, Lewis M R, Brown D R. Effect of rifampin and bacitracin on nasal carriers of Staphylococcus aureus. Antimicrob Agents Chemother. 1984;25:422–426. doi: 10.1128/aac.25.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miles A A, Williams R E O, Clayton-Cooper B. The carriage of Staphylococcus (pyogenes) aureus in man and its relation to wound infection. J Pathol Bacteriol. 1944;56:513–524. [Google Scholar]
- 31.Miller D L, McDonald J C, Jevons M P, Williams R E O. Staphylococcal disease and nasal carriage in the Royal Air Force. J Hyg. 1962;60:451–465. doi: 10.1017/s0022172400020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millian S J, Baldwin J N, Rheins M S, Weiser H H. Studies on the incidence of coagulase-positive staphylococci in a normal unconfined population. Am J Public Health. 1960;50:791–798. doi: 10.2105/ajph.50.6_pt_1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss B, Squire J R, Topley E. Nose and skin carriage of Staphylococcus aureus in patients receiving penicillin. Lancet. 1948;i:320–325. doi: 10.1016/s0140-6736(48)92088-1. [DOI] [PubMed] [Google Scholar]
- 34.Noble W C, Valkenburg H A, Wolters C H L. Carriage of Staphylococcus aureus in random samples of a normal population. J Hyg. 1967;65:567–573. doi: 10.1017/s002217240004609x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul M O, Aderibigbe D A, Sule C Z, Lamikanra A. Antimicrobial sensitivity patterns of hospital and non-hospital strains of Staphylococcus aureus isolated from nasal carriers. J Hyg. 1982;89:253–260. doi: 10.1017/s0022172400070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prevost G, Jaulhac B, Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992;30:967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riewerts Eriksen N H, Espersen F, Rosdahl V T, Jensen K. Evaluation of methods for the detection of nasal carriage of Staphylococcus aureus. APMIS. 1994;102:407–412. doi: 10.1111/j.1699-0463.1994.tb04891.x. [DOI] [PubMed] [Google Scholar]
- 38.Riewerts Eriksen N H, Espersen F, Thamdrup Rosdahl V, Jensen K. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol Infect. 1995;115:51–60. doi: 10.1017/s0950268800058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts J I S, Gaston M A. Protein A and coagulase expression in epidemic and non-epidemic Staphylococcus aureus. J Clin Pathol. 1987;40:837–840. doi: 10.1136/jcp.40.8.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rountree P M. Staphylococci harboured by people in western highlands of New Guinea. Lancet. 1956;i:719–720. doi: 10.1016/s0140-6736(56)90747-4. [DOI] [PubMed] [Google Scholar]
- 41.Schwarzkopf A, Karch H. Genetic variation in Staphylococcus aureus coagulase genes: potential and limits for use as epidemiological marker. J Clin Microbiol. 1994;32:2407–2412. doi: 10.1128/jcm.32.10.2407-2412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarzkopf A, Karch H, Schmidt H, Lenz W, Heesemann J. Phenotypical and genotypical characterization of epidemic clumping factor-negative, oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:2281–2285. doi: 10.1128/jcm.31.9.2281-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenover F, Arbeit R, Archer G, Biddle J, Byrne S, Goering R, Hancock G, Hébert A, Hill B, Hollis R, Jarvis W R, Kreiswirth B, Eisner W, Maslow J, McDougal L K, Miller J M, Mulligan M, Pfaller M A. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol. 1994;32:407–415. doi: 10.1128/jcm.32.2.407-415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tenover F, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Belkum A, Kluytmans J, van Leeuwen W, Bax R, Quint W, Peters E, Fluit A, Vandenbroucke-Grauls C, van den Brule A, Koeleman H, Melchers W, Meis J, Elaichouni A, Vaneechoutte M, Moonens F, Maes N, Struelens M, Tenover F, Verbrugh H. Multicenter evaluation of arbitrarily primed PCR for typing of Staphylococcus aureus strains. J Clin Microbiol. 1995;33:1537–1547. doi: 10.1128/jcm.33.6.1537-1547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Belkum A, Riewerts Eriksen N H, Sijmons M, van Leeuwen W, VandenBergh M, Kluytmans J, Espersen F, Verbrugh H. Coagulase and protein A polymorphisms do not contribute to persistence of nasal colonisation by Staphylococcus aureus. J Med Microbiol. 1997;46:222–232. doi: 10.1099/00222615-46-3-222. [DOI] [PubMed] [Google Scholar]
- 47.van der Vijver J C, Kraayeveld C A, Michel M F. A solid medium for visual demonstration of coagulase production by Staphylococcus aureus. J Clin Pathol. 1972;25:450–452. doi: 10.1136/jcp.25.5.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Versalovic J, Koeuth T, Lupski J R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White A. Quantitative studies of nasal carriers of staphylococci among hospitalized patients. J Clin Invest. 1961;40:23–30. doi: 10.1172/JCI104233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White A. Relation between quantitative nasal cultures and dissemination of staphylococci. J Lab Clin Med. 1961;58:273–277. [PubMed] [Google Scholar]
- 51.White A. Increased infection rates in heavy nasal carriers of coagulase-positive staphylococci. Antimicrob Agents Chemother. 1963;30:667–670. [PubMed] [Google Scholar]
- 52.White A, Hemmerly T, Martin M P, Knight V. Studies on the origin of drug-resistant staphylococci in a mental hospital. Am J Med. 1959;27:26–39. doi: 10.1016/0002-9343(59)90058-0. [DOI] [PubMed] [Google Scholar]
- 53.Williams R E O. Skin and nose carriage of bacteriophage types of Staph. aureus. J Pathol Bacteriol. 1946;58:259–268. doi: 10.1002/path.1700580214. [DOI] [PubMed] [Google Scholar]
- 54.Williams R E O. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;26:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson S Z, Martin R R, Putman M. In vivo effects of josamycin, erythromycin, and placebo therapy on nasal carriage of Staphylococcus aureus. Antimicrob Agents Chemother. 1977;11:407–410. doi: 10.1128/aac.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]