Graphic abstract
Keywords: Ultrasound-assisted enzymatic method, Pecan protein, Secondary and tertiary structures of protein, Emulsifying activity, Dispersion
Highlights
-
•
Combination of ultrasound and enzymatic method improved extraction yield of pecans protein.
-
•
Ultrasound-assisted enzymatic method promote the tryptophan residues in protein to be more easily exposed to the protein surface.
-
•
Ultrasound reduce the diameter of protein particles to improve the dispersion of pecans protein.
-
•
Ultrasound-assisted enzymatic method changed the unfolding of protein and exposure of hydrophobic groups and sulfhydryl groups.
Abstract
To enhance the extraction yield of pecan protein and modify its functional properties, this study investigated whether both ultrasound and enzyme have a synergistic impact on the extraction of pecan (Carya illinoinensis (Wangenh.) K. Koch) protein. The highest protein extraction rate (25.51%) was obtained under the conditions of 1415.43 W.cm−2, 15 min, pH 10.0, 50 °C, and 1% (w/w) alkaline proteinase. Owing to its high shear, mechanical energy and cavitation, the ultrasound process increased the solubility of the substrate making it readily accessible to the enzyme, thereby accelerating the chemical reaction and improving the yield of the protein. The optimized ultrasound-assisted enzymatic method (400 W, 20 kHz, 5 s/3s) effectively changed the secondary and tertiary structure of the pecan protein. The results of surface hydrophobicity, intrinsic fluorescence spectra, sulfhydryl content and scanning electron microscopy all indicated the unfolding of protein and exposure of hydrophobic groups and sulfhydryl groups. Moreover, the protein obtained by this method showed higher solubility (70.77%), higher emulsifying activity (120.56 m2/g), smaller particle size (326.7 nm), and better dispersion (0.305) than single ultrasound and non-ultrasound methods (p < 0.05). To conclude, ultrasound-assisted enzymatic method could be an appropriate technique to improve the yield and quality of the pecan protein. The study also provides a theoretical basis for the application of pecan protein in food processing.
1. Introduction
Pecans [Carya illinoinensis (Wangenh.) K. Koch], belonging to the Juglandaceae family are native to the northern Mexico and United States of North America. They are also grown for their seeds in Zhejiang, Jiangsu, Hunan, and Anhui province of China [1], [2]. Pecan seeds (nuts) are rich in proteins, various amino acids, mono and polyunsaturated fatty acids, phenolic compounds and vitamin E, which exhibit prominent antioxidant activity [2], [3]. Therefore, pecan nuts have excellent dietary and healthcare value, especially associated with their beneficial effects against chronic non-communicable diseases [3], [4]. Owing to its nutritional value and excellent sensory properties, pecan protein could have a high potential application in the food, cosmetic and pharmaceutical industries. Thus, a deeper knowledge on the extraction, and structure and functional characteristics of protein from pecans is required for its possible applications.
Numerous methods have been investigated to extract the plant proteins, including organic solvents extraction, alkaline extraction, ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzymatic extraction. Owing to their simplicity, organic solvents and alkaline extraction are commonly used methods for protein extraction and are also suitable for mass production [5]. However, the extraction of proteins with solvents have the disadvantages of a low protein recovery [6], large processing times and contamination by solvents. Although alkaline extraction is a more environment friendly than organic solvent extraction, it may affect the quality of the protein and the extraction rate is also low. Besides, extremely alkaline solution may still cause some possible safety or nutritional problems of protein, which preferably has to be avoided [7]. Recently, more and more new methods have been developed for protein extraction, such as ultrasound-assisted enzymatic extraction. Enzyme-assisted extraction is considered an environmental friendly method, it can improve the release of proteins from the plant matrix as well as their purity and yield [8]. Ultrasound-assisted extraction is a new non-thermal physical processing technology [9], which helps to reduce the extraction time and the number of protein aggregates. High shear force and mechanical energy generated by ultrasound can cause cavitation, the violent implosion of cavitational bubbles on the surface of the material may cause micro-jets, resulting in surface peeling, erosion and particle breakage [10]. These effects may contribute to an increase in the solubility of the substrate, making it more accessible to the enzyme, to accelerate chemical reaction and increase yield. Thus, ultrasound and enzyme could form a synergistic potentiation. Yang et al. [11] reported that ultrasound-assisted α-amylase method could be utilized to increase the extraction, solubility, and emulsifying activity of rice protein. However, there is relatively little research on the combination of enzyme and ultrasound for the extraction of pecan protein.
The aim of this study was to investigate the synergistic effect between ultrasound and enzyme on the extraction of pecan protein, and to evaluate structural and functional properties of pecan protein extracted by three methods: ultrasound-assisted enzymatic, ultrasound-assisted, and enzyme-assisted method. With these investigations, current study aimed to provide a reference for the potential utilization of pecan protein.
2. Materials and methods
2.1. Material
The pecan nuts (Carya illinoinensis (Wangenh.) K. Koch, Pawnee variety) were purchased from Anhui Jiayu Agricultural Co., Ltd., Anhui, China. Alkaline proteinase (powder, 200 U/mg, CAS number 9014-01-1) from Bacillus Subtilis, bovine serum albumin (BSA), 1-Anilino-8-naphthalene-sulfonate (ANS) and 5, 5′-dithio-bis-2-nitrobenzoic acid (DTNB) were obtained from Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China. Pecan nuts and chemicals were stored at 4 °C.
2.2. Methods
2.2.1. Ultrasound-assisted enzymatic extraction
Pecans were treated for their protein extraction using the method of Dong et al. [12] with some modifications. The hulled pecans were crushed into pulp, then suspended in distilled water in a beaker, and immersed in the ultrasonic bath (Ultrasonic cell grinder, SCIENTZ -IID, 20 kHz, Ningbo Xinzhi Biotechnology Co., Ltd., Ningbo, China) for ultrasonic treatment. The probe was located at 1/3 of the liquid volume. The parameters were set as follows: the raw material of 50 g, the ultrasonic temperature of 40, 45, 50, 55 and 60 °C; the ultrasonic power of 100 W, 200 W, 300 W, 400 W, 500 W with calorimetric power density of 353.85 W.cm−2, 707.14 W.cm−2, 1061.57 W.cm−2, 1415.43 W.cm−2 and 1769.29 W.cm−2, respectively; the ultrasonic time of 5, 10, 15, 20 and 25 min with pulse duration of on-time 5 s and off-time 3 s.
Alkaline proteinase was used for enzymatic hydrolysis. Certain ratios of proteinase were added to the mixtures obtained from ultrasonic treatment, and the pH and temperature were adjusted to the conditions required for enzymatic hydrolysis. The following parameters were used: the enzyme addition of 50000U, 100000U, 150000U, 200000U and 250000U; the pH of 8.5, 9.0, 9.5, 10.0 and 10.5; the enzymolysis temperature of 35, 40, 45, 50 and 55 °C; the enzymolysis time of 2.5, 3.0, 3.5, 4.0 and 4.5 h. After the incubation, the solution mixtures were kept for 15 min at 80 °C in order to stop the enzyme activity. The mixtures were rapidly cooled to room temperature, then centrifuged for 30 min at 8000 r/min (Sigma 2–16 k, Boli Instruments Co., Ltd., Beijing, China). The residue and upper oil layer were discarded. The supernatants were freeze-dried to get the protein (marked as UE).
Protein was extracted by classic alkaline dissolving and acid precipitating method as control. In briefly, pecan pulp (50 g) were firstly defatted with petroleum (1:5, w/v) for 6 h using a Soxhlet apparatus. The defatted pecan were then dispersed in distilled water (1:10, w/v) and adjusted pH 10.0 by 1 mol/L NaOH. After that, the solution was continuously stirred well in a water bath (HH-6B, Guohua Electrical Co., Ltd, Shanghai, China) at 30 °C for 3 h and centrifuged for 30 min at 8000 r/min (Sigma 2–16 k, Boli Instruments Co., Ltd., Beijing, China). The extraction procedure was repeated three times. The collected supernatants were subjected to precipitate at pH 4.5 using 1 mol/L HCl adjusted and kept at 4 °C for 24 h. The precipitates were collected by centrifugation for 30 min at 8000 r/min and freeze-dried to get the protein.
2.2.2. Ultrasound-assisted extraction
The extraction of pecan protein was performed under the optimal conditions obtained in the above method of ultrasonic treatment. Pecans powder and distilled water were first mixed and extracted in the ultrasonic bath for 15 min at 400 W and 55 °C. Then, the mixture was adjusted to pH 10 and kept at 50 °C for 3 h. Finally, it was centrifuged 30 min at 8000 r/min, the supernatants were collected and freeze-dried to obtain the protein sample (marked as U) for further analysis.
2.2.3. Enzyme-assisted extraction
The optimum enzyme treatment conditions obtained in section 2.2.1 were applied for the enzyme-assisted extraction process. The pecans powder was first mixed with distilled water and the pH value of the mixture was adjusted to 10.0. Just after addition of 1.0% (w/w) enzyme it was kept at 50 °C for 3 h. After that, the mixture was kept at 80 °C for 15 min, then rapidly cooled to room temperature, and centrifuged for 30 min at 8000 r/min. Finally, the supernatant fraction was freeze-dried to acquire the protein (marked as E).
2.2.4. Protein content
The protein content in the supernatant was measured as described by Dong et al. [12] with some modifications. BSA was used as the standard. Extraction yield was calculated according to following Eq. (1):
| (1) |
Where, c is the protein concentration in supernatant fraction (g/mL); m is the quantity of raw material (g); V is the volume of supernatant fraction (mL).
2.2.5. Surface hydrophobicity (H0)
The H0 of protein was measured as described by Yang [13]. ANS was used as a fluorescent probe. Protein powder was mixed with phosphate buffer (pH 7.0, 0.01 mol/L), then centrifuged for 10 min at 5000 g. After determining the protein concentration in the supernatants by the biuret method, each supernatant was diluted to 0.5 mg/mL. Afterwards, 20 μL of 8.0 mmol/L of ANS (in phosphate buffer) were added to protein solution (4 mL), shaked and allowed to stand for 15 min in the dark. The intensity was then determined by multifunctional microplate reader (I3X., Miguchi Molecular Instruments Co., Ltd, Shanghai, China) at an excitation wavelength of 365 nm with a slit of 5.0 nm, and emission wavelength of 450–600 nm. The scanning speed was 60 nm/min.
2.2.6. Intrinsic fluorescence spectra
The intrinsic fluorescence spectra were studied as described by the modified protocol of Wen, et al. [14]. The protein solution was standardized with phosphate buffer saline to reach a concentration of 1.0 mg/mL. The intrinsic fluorescence spectra were measured using a multifunctional microplate reader. The excitation wavelength was set at 295 nm with a slit of 1.0 nm, and the emission wavelength was set between 320 and 500 nm.
2.2.7. Free sulfhydryl (SH) groups
A modified version of Liu, et al. [15] was employed to determine the concentration of free SH groups of pecan protein. Each protein solution was standardized to the same concentration. Two milliliters of protein solution were mixed with 50 μL of DTNB (10 mmol/L) rapidly, then the absorbance was recorded at 412 nm after 15 min. The concentration of free SH groups of pecan protein was calculated using the following Eq. (2):
| (2) |
A = Absorbance measured at 412 nm, D = Dilution factor, ε = 13600 M−1 cm−1, C = Protein concentration (mg/mL).
2.2.8. Mean diameter and particle size distribution (PSD)
The mean diameter of pecan protein and PSD were determined with a particle size analyzer (Zetasizer Nano-ZS, Malvern, MA) based on the dynamic light scattering (DLS). Each protein solution was diluted to 0.5 wt% for measurement. The whole measurement was performed at 25 °C.
2.2.9. Scanning electron microscopy (SEM)
The surface microstructure of the protein was observed by the SEM (Quanta 200, FEI, USA) as described by Zhang et al. [16]. Using double-sided adhesive tapes, the freeze-dried protein powder was sticked onto the sample stub, then coated with gold to achieve the surface reflectance.
2.2.10. Fourier transform infrared (FTIR) spectra
The FTIR of protein were recorded by spectrum instrument (VERTEX 80 V, Bruker, German). Samples were freeze-dried and pressed into 1–2 mm slices and analyzed. The FTIR spectra were recorded between 400 and 4000 cm−1 with a resolution of 4 cm−1.
2.2.11. Differential scanning calorimetry (DSC)
The peak denaturation temperature of protein samples was determined by thermograms obtained by DSC. Samples were freeze-dried and then heated from 25 °C to 180 °C respectively at a rate of 10 °C/min during analysis. The temperature of the peak apex in the DSC curve was the denaturation temperature. The area covered by the peaks formed by the curve was theoretically the calories absorbed for the denaturation of the protein.
2.2.12. Solubility
Protein solubility was determined as described by Jain, et al. [17] with some modifications. A mixture of protein and phosphate buffer was shaked for 2 h, then centrifuged for 10 min at 5000 g. The supernatant was collected to determine the soluble protein concentration. Protein solubility was calculated using the following Eq. (3):
| (3) |
2.2.13. Emulsifying properties
The emulsifying activity index (EAI) and the emulsion stability index (ESI) were evaluated as described by Pearce, et al. [18] with little modification. A mixture of soybean oil (5 mL) and protein solution (15 mL) was homogenized for 2 min at 12,000 r/min by a homogenizer (AH-100B, ATS Industrial Systems Ltd, Shanghai, China). An aliquote (25 μL) was then pipetted from the bottom solution of the tubes and dispersed in sodium dodecyl sulfate (5 mL, 0.1% SDS (w/v)) at 0 and 30 min. The absorbances read at 500 nm for 0 min (A0) and 30 min (A30) samples were used to calculate the EAI and ESI by Eqs. (4), (5):
| (4) |
| (5) |
T = 2.303, N = Dilution multiple, (250), A0 = Absorbance at 0 min, A30 = Absorbance at 30 min, C = Protein concentration (g/mL), φ = Volume fraction of oil in emulsion (25%).
2.2.14. Statistical analysis
All experiments were performed in triplicate to reduce the error of the study and maintain accuracy and values were reported as means with standard deviations. Statistical analysis (ANOVA) was conducted based on experimental data. The comparison of significant differences between the mean values was established at p < 0.05.
3. Results and discussion
3.1. Ultrasound-assisted enzymatic extraction of pecan protein
In plant cells, the accessibility of protein molecules is one of the most effective factors for the extraction of protein. The ionic interaction between the cells, the protein molecules, intracellular oil, and polysaccharides all affect protein extraction [19]. Therefore, plant cell wall destruction and inclusion of specific reagents during the extraction process have become the key steps to improve the protein extraction efficiency [11], [20], [21], [22]. In order to obtain the protein in the cells, ultrasound-assisted enzymatic extraction method was used in this study. Plant cell walls would be disrupted by ultrasound [23], then, the alkaline proteases which have a degrading effect on lipoprotein complexes would act on plant cells to release the protein better.
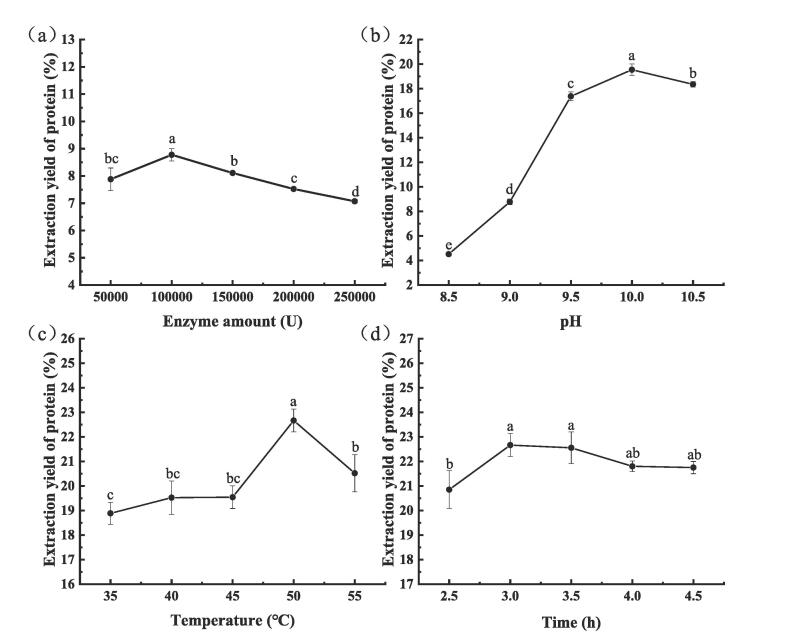
The pecan protein was extracted by ultrasound-assisted enzymatic method (UE). The protein extraction efficiency was investigated by enzyme amount, pH, enzymatic temperature, enzymatic time, ultrasonic temperature, power and time as variables. Fig. 1 showed the effects of enzymatic treatment on the extraction yield of pecan protein. Fig. 1a showed that an increase in the amount of enzyme from 50,000 U to 100,000 U improved the extraction yield. This might be caused by the greater interaction between the substrate and the enzyme due to the increase in the enzyme content, which could promote cell wall degradation [24]. However, the protein extraction yield decreased with the enzyme amount from 100,000 U to 250,000 U, this could be associated with the appearance of turbidity during the extraction process and the formation of complexes between proteins as inhibitors and enzymes, which could prevent its activity (competitive inhibition phenomenon) [25]. In addition, the presence of free enzymes in solvents may result in the degradation of protein molecules because free enzymes could interact with the protein [26]. The appropriate pH and temperature for the extraction of pecan protein were 10.0 and 50 °C, respectively (Fig. 1b and c). Other pH and temperature values led to lower extraction yield of pecan protein. This behavior indicated that a high extraction temperature may lead to irreversible denaturation of the enzyme, thereby reducing the enzyme activity. Fig. 1d shows that the protein extraction yield gradually increased and reached the maximum at 3 h and then slightly decreased. As the reaction progressed, the substrate decreased and the reaction speed gradually slowed down. At the same time the long time heating may lead to several adverse effects such as the damage to some nutritional ingredients and the denaturation of protein [27]. Therefore, the optimal protein extraction conditions for enzyme treatment were enzyme amount, pH, enzymatic temperature, and enzymatic time of 1.0%, 10.0, 50 °C and 3 h, respectively.
Fig. 1.
Effects of enzyme amount (a), pH (b), enzymatic temperature (c) and enzymatic time (d) on the extraction yield of pecan protein. Values are means ± standard deviations (n = 3). Different letters above each of the data points indicate statistical significantly different values (p < 0.05).
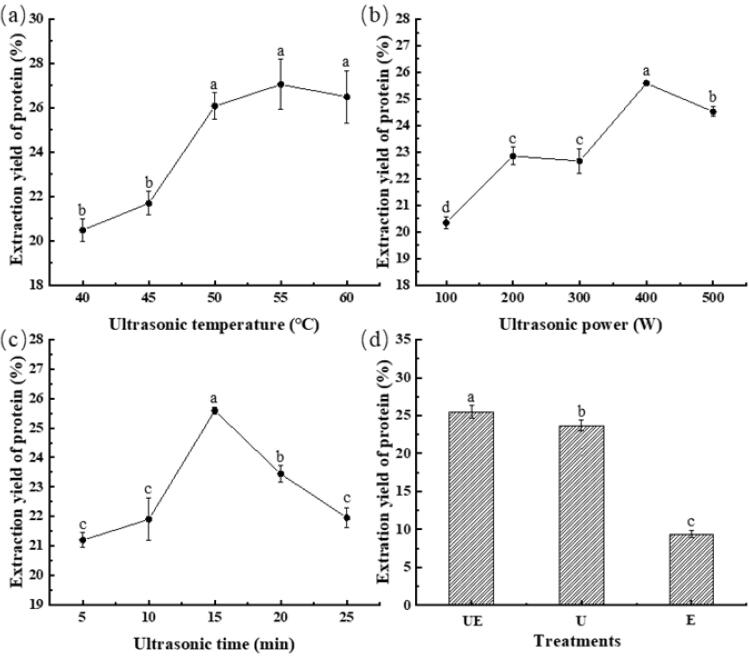
Under the optimal conditions of protein extraction by enzyme treatment, the effects of ultrasonic temperature, power and time on protein extraction rate was shown in Fig. 2a–c. The protein extraction yield gradually increased with the increase of ultrasonic temperature (Fig. 2a), and the highest yield (27.05 ± 1.14 %) was obtained at 55 °C. At an elevated temperature, the protein molecular structure could be more stretchable, thereby promoting the interaction between water and protein molecules [12]. The protein obtained by this method would have higher solubility. When the extraction temperature continues to raise until a point (60 °C) where the chemical bonds begin to break, causing the molecular structure to collapse and then precipitate, leading to a decrease in the extraction yield [12]. Therefore, the optimal ultrasonic temperature for pecan protein extraction was chosen as 55 °C.
Fig. 2.
Effects of ultrasonic temperature (a), power (b) and time (c) on the extraction yield of pecan protein. Fig. 2d shows the difference of extraction yield of protein prepared by different extraction methods. Values are means ± standard deviations (n = 3). Different letters above each of the data points indicate statistical significantly different values (p < 0.05).
Increase in ultrasonic power from 100 to 400 W significantly enhanced the extraction yield (Fig. 2b). This could be caused by the enhancement of ultrasonic cavitation in the extraction system due to the strengthening of ultrasonic power [28], causing the disruption of cell wall, resulting in a large amount of solvent to penetrate into the cellular material, improve mass transfer, and release the cell content [29], thereby promoting the binding of the enzyme to the substrate. As a consequence, the protein extraction yield was improved. These results indicated that the ultrasonic power was one of the crucial factors affecting protein extraction yield, which were supported by the previous studies [23], [30]. Chittapalo et al. [30] reported that the yield of the rice bran protein increased with the increase of ultrasonic power. Görgüç et al. [23] reported that the results revealed that the ultrasound-assisted extraction at the elevated ultrasound power facilitated the recovery of protein from sesame bran. This could be explained by the mechanical vibration effect of the ultrasound waves which provide wider surface area between solid matrix and liquid solvent to contact. While Fig. 2b showed that the protein yield began to decline with the ultrasonic power from 400 to 500 W, that might be on account of the protein hydrolyzation, denaturation and aggregation under high ultrasonic power [31]. Ultrasound treatment also can significantly reduce the size of protein aggregates and significantly reduced the aggregate size of leguminous plant-derived proteins, including pea protein, soy protein, black bean protein and mung bean protein, among others [14]. Barteri et al. [32] reported that hydroxyl-free radicals generated by ultrasound promoted the oxidation of cysteine residues; therefore, it could be found that the aggregation of protein molecules was caused by the formation of intermolecular disufide bridges. These phenomena could reduce the soluble protein content in the extraction. Upon comprehensive consideration, an ultrasonic power of 400 W was selected for further experiments.
The protein extraction yield first increased significantly and then decreased steeply with the ultrasonic time from 5 to 25 min, the maximum yield (25.59 ± 1.14%) was obtained at 15 min (Fig. 2c). Sonication treatment resulted in protein release though physical, mechanical and chemical effects induced by acoustic cavitation [14]. This indicated that the protein extraction rate could be improved with an appropriate length of ultrasonic treatment time. However, the too long ultrasonic time would hinder the mass transfer effect owing to the enhancement of ultrasonic cavitation effect. The protein active sites may be exposed and destroyed, leading the extraction yield to be lower [12]. The optimal conditions for protein extraction by ultrasonic treatment were set as ultrasonic temperature, power, and time of 55 °C, 400 W, and 15 min, respectively.
The pecan protein was extracted by UE under the mentioned optimal conditions. E and U were employed separately under their optimal conditions and the results of the three methods were compared. From Fig. 2d, it is obvious that combination of ultrasound and enzyme significantly improved the extraction yield of pecan protein (p < 0.05) in comparison with E and U. Ahmet et al. [23] tested four different protein extraction methods and the combined ultrasound-assisted enzymatic extraction led to the highest protein yield of sesame bran. The violent implosion of cavitational bubbles provided by ultrasound on materials’ surface may result in micro-jets, shear forces and turbulences, generating surface peeling, erosion as well as a fragmentation of material [10]. The increase of material surface area led to higher mass transfer and improved release of cell content. These effects may contribute to make the substrate more accessible to the enzyme and accelerate biochemical reaction leading to the increased extraction rate and yield [23]. Thus, ultrasound and enzyme could form a synergistic potentiation.
Based on the above discussion, schematic structural changes of different methods on the extraction of pecan protein were proposed as shown in Fig. 3.
Fig. 3.
Schematic structural changes of different methods on the extraction of protein.
3.2. Physicochemical characteristics of pecan protein
A large difference in the extraction rate of pecan protein was observed as extracted by different methods (UE, U, E, and Control). The morphological and structural analyses were performed on the extracted protein to better understand its functionalities and reliability of the schematic structural changes by different methods on the extraction of pecan protein.
3.2.1. Morphology
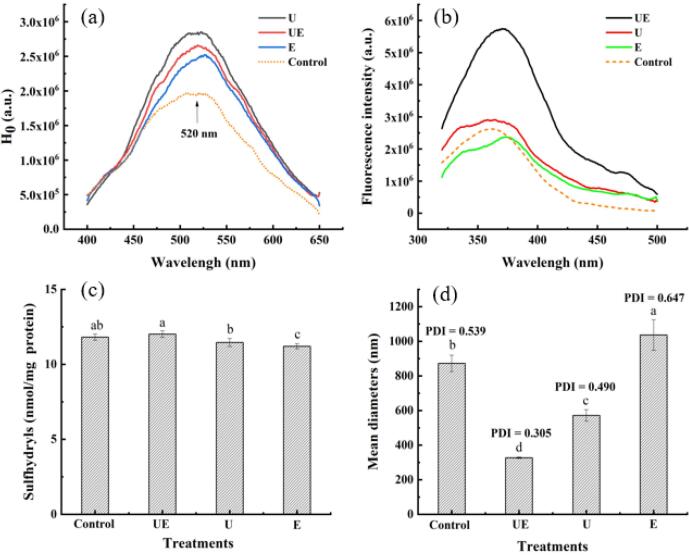
Protein surface hydrophobicity, is a property of a protein showing the number of hydrophobic groups exposed on the surface of a protein molecule. It is often used to evaluate the protein conformational changes and has a great correlation with its functional properties [33]. The H0 expressed by the relative fluorescence intensity of pecan protein is shown in Fig. 4a. The proteins obtained by the four methods all had the maximum fluorescence intensity absorption peak at 520 nm. The H0 of control group was significantly lower (p < 0.05) than the U, E and UE groups, indicating that ultrasonic and enzyme treatment can significantly improve the surface hydrophobicity of proteins. The intensity of the protein peaked at 520 nm, and the protein was significantly increased by ultrasound treatment. This increase in H0 indicates the unfolding of protein molecule and exposure of the hydrophobic groups due to the action of ultrasound [34]. Under the ultrasonic cavitation, the complex structure of protein got destroyed such as external physical field loosened the protein structure. This may lead the hydrophobic amino acids to release, that were originally buried inside the molecules. Their exposure could be the best reason for the increased surface hydrophobicity in the protein extracted by UE compared to that extracted by control method. And the H0 of the protein increased with the stretching of the protein molecules [35]. These results also supported the microstructure observed in the Fig. 5a.
Fig. 4.
Difference of the surface hydrophobicity (a), fluorescence emission spectra (b), SH groups (c), mean diameters of particles and polydispersity index (PDI) of particle size (d) of pecan protein prepared by different extraction methods. Different letters above each of the data points indicate statistical significantly different values (p < 0.05).
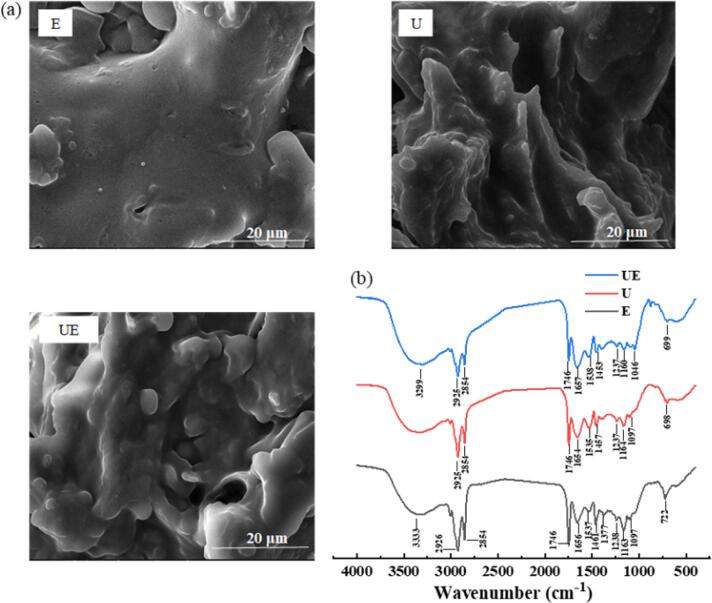
Fig. 5.
The results of SEM (a) and FTIR spectroscopy (b) of pecan protein prepared by different extraction methods.
Intrinsic fluorescence spectra can sensitively characterize protein and its conformations. Tyrosine (Tyr), phenylalanine (Phe) and tryptophan (Trp) residues in a protein, especially Trp residue [36] can critically fluoresce according to the protein folding, hence acting as a sensitive monitor for conformational changes of tertiary structure. It could be seen in Fig. 4b that the emission fluorescence intensities (320–500 nm) of sonicated protein (excited at 295 nm) were peaked at 370 nm. The characteristic fluorescence intensity of proteins extracted from UE was significantly higher (p < 0.05) than that of U, E and control group, indicating that the tertiary structure or aggregation state of proteins was changed by ultrasound-assisted enzymatic method. A reasonable explanation might be that ultrasound treatment destroyed hydrophobic interactions, leading to the unfolding of protein molecular structure, subsequently triggered hydrophobic regions and groups to be more exposed on the surface of protein molecule [37], [38]. In conclusion, compared with the control method (alkali extraction), UE can promote the tryptophan residues in protein to be more easily exposed to the protein surface, thus improving the hydrophobic ability of protein.
The changes in SH groups of protein due to sonication were presented in Fig. 4c. It can be seen the SH groups of protein extracted by UE increased significantly (p < 0.05). The increase might be due to the destruction of disulfide bonds (SS) by ultrasound, leading the SS to form SH groups and the SH groups were exposed towards outside [38]. Zhang et al. [39] reported that ultrasound significantly decreased mean particle size of peanut protein isolate. Thus it could be that during the reduction process of protein size, the buried SH groups were exposed to the surface of protein molecules under the action of turbulence, shear forces, high pressure, and shock waves by the cavitation. The gas nucleus present in the fluid can form cavitation bubbles. These cavitation bubbles continuously reduce until they reach a critical size, and then collapse violently. Moreover, the collapse of cavitation bubbles produces strong hydrodynamic turbulence, shear forces, high pressure and shock waves [38]. The combination of turbulence, shear forces, high pressure and shock waves effects further increases the mass transfer of ultrasound, which accelerates the effects of ultrasound on the chemical structure of proteins [14], which in turn induce strong changes in protein function.
The sizes of soluble pecan protein aggregates were determined by DLS and expressed as hydrodynamic diameter. The diameter of protein particles obtained by the four methods was significantly different (Fig. 4d). The diameter of protein particles extracted by enzyme and alkali solution was 1036.3 nm and 874.2 nm, respectively. After ultrasonic treatment, the mean diameters of pecan protein were obviously decreased (p < 0.05), and the mean hydrodynamic diameters were recorded as 572.1 and 326.7 nm as extracted by U and UE, respectively. A wide PSD (PDI = 0.539–0.673) was detected for the protein extracted by enzymic method and control method, which confirmed the poor dissociation of protein, along with the presence of large aggregates in the solution [40]. By contrast, the PSD of protein extracted by U and UE was inclined to be uniform and narrow. The narrowest PSD turned up in the protein extracted by UE (PDI = 0.305) showed a good dispersion. The reduction in PSD and improvement in PDI could be on account of the ultrasonic cavitation and mechanical vibration that leaded to the dissociation of protein aggregates [41]. Therefore, ultrasound reduced the diameter of protein particles to improve the dispersion of protein, which is better than alkali extraction method.
3.2.2. Amino acid composition of proteins
Table 1 showed the amino acid composition of pecan protein extracted by different methods. The ratio of acidic amino acid and hydrophobic amino acid was the highest among the 17 amino acid compositions, including aspartic acid, arginine and glutamic acid. Compared with enzymic method (E) and control groups, UE method could significantly increase (p < 0.05) the contents of total amino acids (TAA) and hydrophobic amino acids in fructus pecoris protein. We speculated the possible reason was that UE method could dissociate protein aggregates, thus improving the protein extraction rate. Combined with the results of surface hydrophobicity, we further confirmed that UE can better modify the protein structure and expose the hydrophobic groups originally buried inside the protein molecule, which increasing the surface hydrophobicity of the protein.
Table 1.
Effect of different extraction methods on the amino acid content of pecan protein (g/100 g protein).
| Amino acid | U | E | UE | Control |
|---|---|---|---|---|
| Asp | 43.25 ± 2.57a | 15.84 ± 1.05b | 15.84 ± 0.88b | 6.34 ± 0.48c |
| Thr | 13.4 ± 1.11a | 5.132 ± 0.35b | 5.40 ± 0.32b | 4.27 ± 0.23c |
| Ser | 21.53 ± 1.71a | 7.30 ± 0.49b | 8.38 ± 0.46b | 7.02 ± 0.59b |
| Glu | 103.60 ± 8.32a | 27.65 ± 1.85c | 48.96 ± 2.84b | 30.07 ± 1.85c |
| Gly | 23.6 2 ± 1.49a | 8.81 ± 0.45c | 11.76 ± 0.76b | 13.52 ± 0.94b |
| Ala | 21.39 ± 1.76a | 7.46 ± 0.42b | 8.28 ± 0.53b | 8.03 ± 0.56b |
| Cys | 4.78 ± 0.35a | 0.51 ± 0.02d | 1.70 ± 0.07c | 2.14 ± 0.11b |
| Val | 20.85 ± 1.24a | 7.47 ± 0.31c | 8.02 ± 0.31bc | 9.55 ± 0.54b |
| Met | 4.36 ± 0.84a | 1.50 ± 0.1b | 1.29 ± 0.06b | 1.61 ± 0.08b |
| Ile | 19.10 ± 0.16a | 6.56 ± 0.25b | 7.23 ± 0.34b | 5.92 ± 0.27b |
| Leu | 32.51 ± 1.85a | 11.71 ± 0.07b | 12.46 ± 0.75b | 12.37 ± 0.96b |
| Tyr | 12.52 ± 0.62a | 3.06 ± 0.15d | 4.46 ± 0.19c | 6.23 ± 0.25b |
| Phe | 24.05 ± 1.77a | 8.50 ± 0.34bc | 9.39 ± 0.27b | 7.42 ± 0.43c |
| His | 16.59 ± 0.81a | 8.14 ± 0.38c | 9.86 ± 0.33c | 13.21 ± 0.98b |
| Lys | 14.52 ± 0.98a | 4.90 ± 0.24d | 7.95 ± 0.18c | 9.85 ± 0.36b |
| Arg | 71.49 ± 5.39a | 22.98 ± 1.52d | 31.42 ± 0.23c | 37.26 ± 1.53b |
| Pro | 9.34 ± 0.52a | 4.40 ± 0.16b | 4.44 ± 0.13b | 4.02 ± 0.21b |
| Total amino acids | 456.96 ± 24.17a | 151.98 ± 7.74d | 196.90 ± 11.24b | 178.83 ± 10.54c |
| Hydrophobic amino acids | 155.25 ± 8.93a | 56.43 ± 3.26c | 62.90 ± 4.36b | 62.44 ± 4.32b |
| Hydrophilic amino acids | 52.24 ± 2.55a | 16.02 ± 0.25c | 19.95 ± 1.26b | 19.66 ± 1.39b |
| Acidic amino acid | 146.86 ± 10.63a | 43.49 ± 1.48c | 64.80 ± 3.72b | 36.41 ± 2.18d |
| Basic amino acid | 102.61 ± 7.46a | 36.04 ± 1.95d | 49.24 ± 2.93c | 60.32 ± 4.21b |
Hydrophobic amino acids: Ala, Ile, Leu, Met, Phe, Val, Gly, Pro; Hydrophilic amino acids: Ser, Thr, Cys, Tyr; Acidic amino acid: Asp, Glu; Basic amino acid: Lys, Arg, His.
3.2.3. Microstructures
To better illustrate the physicochemical characteristics of pecan protein and confirm the changes of proposed protein structural, SEM and FTIR were used to characterize the structures of pecan protein, respectively.
The microstructure of pecan protein was visualized by SEM (Fig. 5a). Noticeably, the microstructure of pecan protein extracted by UE, U, and E showed obvious differences. The pecan protein extracted by E exhibited a tight microstructure, presenting relatively flat surfaces, while the microstructures of pecan protein extracted by UE and U were looser like a spongy and porous material; the texture became dispersive, and exhibited more irregular fragments with disordered arrangement. Therefore, the acceleration of protein extraction was connected with the changes of protein microstructure induced by ultrasound. And this effect was also observed in ultrasound-assisted treatment systems involving walnut protein [42]. Microstructure influenced the chemical and physical properties, and functionality of the protein. The bulk density of the loose structure of the protein caused by the synergistic effect between ultrasound and enzyme was smaller than that of the dense structure of the sample extracted by E, which instead helped to improve the solubility of the protein [43]. These findings agreed with the protein solubility in Table 2.
Table 2.
Functional properties of pecan protein.
| Functional property | Extraction method |
|||
|---|---|---|---|---|
| E | U | UE | Control | |
| Solubility (%) | 47.98 ± 1.36b | 40.43 ± 0.68c | 70.77 ± 1.01a | 33.64 ± 1.22d |
| EAI (m2/g) | 82.87 ± 1.37c | 97.62 ± 1.59b | 120.56 ± 2.27a | 76.27 ± 1.65d |
| ESI (min) | 120 ± 0.003c | 424 ± 0.002a | 202 ± 0.001b | 103 ± 0.010d |
Values are means ± standard deviations (n = 3).
Different letters within the same row indicate statistical significantly different values (p < 0.05).
The structures of pecan protein extracted by different extraction treatments at optimum time of 3 h were observed by FTIR as shown in Fig. 5b. The overall FTIR chromatogram of the pecan protein for all the extraction methods was similar. The bands at wavenumbers of 3299 and 3333 cm−1 for the proteins extracted by UE and E showed peaks corresponding to the stretching vibration of amino and hydroxyl groups, respectively. It was found that the peak strength of the ultrasonic sample shifted to the lower wavenumber. When the intramolecular or intermolecular hydrogen bonds are broken, the infrared spectrum will move to a higher wavenumber. This indicated that the hydrogen bonding force within the protein was increased by UE compared to E. This difference was caused by the shear forces under the action of ultrasonic effect, which disrupted the protein molecules interactions and affected their internal structure. Qu et al. [44] found the same phenomenon in their study of rapeseed protein isolate-dextran conjugates that the ultrasonic treatment could increase hydrogen bonding force. It is well known that the peak at 1700–1600 cm−1 corresponds to amide I vibration, the peak at 1600–1500 cm−1 corresponds to amide II vibration, and the peak at 1330–1200 cm−1 corresponds to amide III vibration [45]. The characteristic peak of amide I band can reflect changes of protein secondary structure [46]. The bands in the pecan protein at 1656, 1537 and 1238 cm−1 (by E) shifted to 1657, 1538 and 1237 cm−1 (by UE). These results indicated that ultrasound changed the secondary structure of pecan protein.
3.2.4. Thermal stability
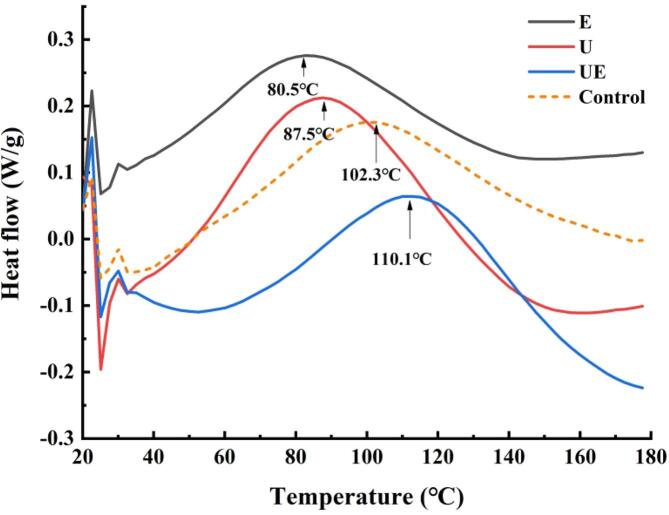
DSC is widely used in the study of thermal denaturation of proteins [47]. In the DSC curves, the temperature corresponding to the maximum peak represents the transformation temperature during protein denaturation, and the denaturation temperature can reflect the thermal stability and the aggregation degree of protein molecules. As shown in Fig. 6, the denaturation temperature of the protein extracted by UE was changed compared with that by E (from 80.5 °C to 110.1 °C), indicating a stable structure of protein extracted by the synergistic effect between ultrasound and enzyme and that the molecular structure tended to be looser. More hydrophobic groups are exposed the surface of the protein molecule by using ultrasound treatment, which could reduce the viscosity, the surface tension of the solution and rigidity of the protein. These reasons cause the structure of polymer more stable. Therefore, the protein extracted by the UE method showed higher denaturation temperature and more thermally stable.
Fig. 6.
Effect of four extraction methods on thermal transition curve of pecan protein.
The results of the SEM and FTIR analysis combined with those of the surface hydrophobicity, fluorescence emission spectra, SH groups, and particle size distribution determination indicated that ultrasound could open the compact structure of protein and lead to conformational changes. Consequently, the protein structure became more flexible, exposing many groups that were buried inside the molecules. These changes in protein structure may greatly influence the functional properties.
3.3. Functionality properties
3.3.1. Solubility
Solubility is an important index of protein denaturation aggregation, which can reflect its functional characteristics and application potential. The protein solubility measurements by different extraction methods are shown in Table 2. It can be seen that the solubility of protein extracted by UE was obviously higher than that extracted by E, U and control group (p < 0.05), indicating the ultrasonic treatment can transform the originally less soluble proteins into more soluble forms [48]. A great quantity of cavitation bubbles generated by ultrasound may raise the partial pressure and temperature, resulting in the unfolding of the protein. In addition, ultrasonic treatment can reduce the protein particle size [39]. As a result, the conformation of protein may change, resulting in more hydrophilic amino acid residues towards water, and increases protein–water interactions and promotes less-soluble protein to form soluble protein, leading to an increased protein solubility [49], [50].
3.3.2. Emulsifying properties
Emulsifying properties are of great importance for many food applications of proteins, and characterize the ability of a protein to get adsorbed to the water–oil interface. The protein emulsifying property is regularly evaluated by EAI and ESI [12], which is used to measure the ability of proteins to form emulsions and describe the stability, respectively. The effects of different methods on the EAI and ESI of pecan protein are presented in Table 2. The protein from U, E and UE methods significantly increased the EAI and ESI (p < 0.05), compared with the control sample. The protein from UE method exhibited the highest EAI value. The results indicated the synergistic effect between ultrasound and enzyme could significantly increase the emulsifying activity (p < 0.05), which agreed with the previous report that the ultrasound modified the emulsifying properties [11]. This might be attributable to the cavitation by ultrasound, which caused the unfolding of conformational structures of protein and improved the molecular flexibility, thus more internal hydrophobic groups were exposed on the surface of pecan protein, finally promoting the interaction between oils and proteins. As a consequence, protein molecules could be more effectively adsorbed on the water–oil interface on account of structural variations [51], furthermore, the mechanical effects produced by ultrasound could reduce the particle size of the pecan protein, thus improving the molecular fluidity and emulsifying ability.
4. Conclusion
This research demonstrates that ultrasound-assisted enzymatic method to increase the extraction yield of pecan protein and modified its functional properties. Compared with alkali extraction or enzyme-assisted extraction, ultrasound-assisted enzymatic method changed the secondary and tertiary structure of protein, which caused the unfolding of protein and exposure of hydrophobic groups and sulfhydryl groups. The protein obtained by this method had smaller particle size, better dispersion, and better functional properties including higher solubility and emulsifying properties, which are important functions of protein in food applications. Therefore, this study provided an appropriate technique to improve the pecan protein yield and a theoretical basis for the application of pecan protein in food processing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by Beijing Advanced Innovation Center for Food Nutrition and Human Health (20171044), the Natural Science Foundation of China (31871728), Project of Science and Technology Research Program of Chongqing Education Commission of China (KJZD-M202001601), Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJB550008), National Natural Science Foundation of China for Young Scholars (31401679).
Contributor Information
Qiang Wang, Email: gogo1443@sina.com.
Fuping Zheng, Email: zhengfp@btbu.edu.cn.
References
- 1.Wang M., Xi D., Chen Y., Zhu C., Zhao Y., Geng G. Morphological characterization and transcriptome analysis of pistillate flowering in pecan (carya illinoinensis) Sci. Hortic.-Amsterdam. 2019;257:108674. [Google Scholar]
- 2.Hu Y., Yi R., Sun P., Li G., Zhao X., Wang Q. Tartary Buckwheat Flavonoids Ameliorate High Fructose-Induced Insulin Resistance and Oxidative Stress Associated with the Insulin Signaling and Nrf2/HO-1 Pathways in Mice. Food Func. 2017;8:2803–2816. doi: 10.1039/c7fo00359e. [DOI] [PubMed] [Google Scholar]
- 3.Atanasov A.G., Sabharanjak S.M., Zengin G., Mollica A., Szostak A., Simirgiotis M. Pecan nuts: a review of reported bioactivities and health effects. Trends. Food. Sci. Tech. 2018;71:246–257. [Google Scholar]
- 4.Chang S.K., Alasalvar C., Bolling B.W., Shahidi F. Nuts and their co-products: the impact of processing (roasting) on phenolics, bioavailability, and health benefits-a comprehensive review. J. Funct. Foods. 2016;26:88–122. [Google Scholar]
- 5.Capellini M.C., Giacomini V., Cuevas M.S., Rodrigues C.E.C. Rice bran oil extraction using alcoholic solvents: physicochemical characterization of oil and protein fraction functionality. Ind. Crop. Prod. 2017;104:133–143. [Google Scholar]
- 6.Sawada M.M., Venâncio L.L., Toda T.A., Rodrigues C.E.C. Effects of different alcoholic extraction conditions on soybean oil yield, fatty acid composition and protein solubility of defatted meal. Food. Res. Int. 2014;62:662–670. [Google Scholar]
- 7.Hou F., Ding W., Qu W., Oladejo A.O., Xiong F., Zhang W. Alkali solution extraction of rice residue protein isolates: influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food. Chem. 2017;218:207–215. doi: 10.1016/j.foodchem.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 8.Fetzer A., Herfellner T., Stäbler A., Menner M., Eisner P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crop. Prod. 2018;112:236–246. [Google Scholar]
- 9.Chemat F., Zill-e-Huma M.K. Khan, Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem. 2011;18:813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Chemat F., Rombaut N., Sicaire A., Meullemiestre A., Fabiano-Tixier A., Abert-Vian M. Ultrasound assisted extraction of food and natural products, Mechanisms, Techniques, Combinations, Protocols and applications A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Yang X., Li Y., Li S., Oladejo A.O., Wang Y., Huang S. Effects of ultrasound-assisted α-amylase degradation treatment with multiple modes on the extraction of rice protein. Ultrason. Sonochem. 2018;40:890–899. doi: 10.1016/j.ultsonch.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Dong Z.Y., Li M.Y., Tian G., Zhang T.H., Ren H., Quek S.Y. Effects of ultrasonic pretreatment on the structure and functionality of chicken bone protein prepared by enzymatic method. Food. Chem. 2019;299:125103. doi: 10.1016/j.foodchem.2019.125103. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., Li Y., Li S., Oladejo A.O., Ruan S., Wang Y. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason. Sonochem. 2017;38:19–28. doi: 10.1016/j.ultsonch.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Wen C., Zhang J., Yao H., Zhou J., Duan Y., Zhang H. Advances in renewable plant-derived protein source: the structure, physicochemical properties affected by ultrasonication. Ultrason. Sonochem. 2019;53:83–98. doi: 10.1016/j.ultsonch.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Liu G., Xiong Y.L., Butterfield D.A. Chemical, physical, and gel-forming properties of oxidized myofibrils and whey-and soy-protein isolates. J. Food. Sci. 2000;65:811–818. [Google Scholar]
- 16.Zhang Y., Ma H., Wang B., Qu W., Li Y., He R. Effects of ultrasound pretreatment on the enzymolysis and structural characterization of wheat gluten. Food. Biophys. 2015;10:385–395. [Google Scholar]
- 17.Jain S., Anal A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (esm) by ultrasonic assisted extraction (uae) and enzymatic hydrolysis. LWT – Food Sci. Technol. 2016;69:295–302. [Google Scholar]
- 18.Pearce K.N., Kinsella J.E. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agr. Food. Chem. 1978;26:716–723. [Google Scholar]
- 19.Naseri A., Marinho G.S., Holdt S.L., Bartela J.M., Jacobsen C. Enzyme-assisted extraction and characterization of protein from red seaweed palmaria palmata. Algal. Res. 2020;47:101849. [Google Scholar]
- 20.Vernès L., Abert-Vian M., El Maâtaoui M., Tao Y., Bornard I., Chemat F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019;54:48–60. doi: 10.1016/j.ultsonch.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Roselló-Soto E., Barba F.J., Parniakov O., Galanakis C.M., Lebovka N., Grimi N. High voltage electrical discharges, pulsed electric field, and ultrasound assisted extraction of protein and phenolic compounds from olive kernel. Food. Bioprocess. Tech. 2015;8:885–894. [Google Scholar]
- 22.Stefanovic A.B., Jovanovic J.R., Grbavcic S.Z., Sekuljica N.Z., Manojlovic V.B., Bugarski B.M. Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. Eur. Food. Res. Technol. 2014;239:979–993. [Google Scholar]
- 23.Görgüç A., Bircan C., Yılmaz F.M. Sesame bran as an unexploited by-product: effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food. Chem. 2019;283:637–645. doi: 10.1016/j.foodchem.2019.01.077. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L., Hua D., Wang Z., Xu S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food. Bioprod. Process. 2010;88:233–238. [Google Scholar]
- 25.Vergara-Barberan M., Lerma-Garcia M.J., Herrero-Martinez J.M., Simo-Alfonso E.F. Efficient extraction of olive pulp and stone proteins by using an enzyme-assisted method. J. Food. Sci. 2014;79:C1298–C1304. doi: 10.1111/1750-3841.12499. [DOI] [PubMed] [Google Scholar]
- 26.Shen L., Wang X., Wang Z., Wu Y., Chen J. Studies on tea protein extraction using alkaline and enzyme methods. Food. Chem. 2008;107:929–938. [Google Scholar]
- 27.Li X., Chen L., Hua Y., Chen Y., Kong X., Zhang C. Effect of preheating-induced denaturation during protein production on the structure and gelling properties of soybean proteins. Food. Hydrocolloid. 2020;105:105846. [Google Scholar]
- 28.H. Feng, G.V. Barbosa-Cánovas, J. Weiss, Ultrasound technologies for food and bioprocessing, New York, 2011.
- 29.Karki B., Lamsal B.P., Jung S., van Leeuwen J.H., Pometto A.L., Grewell D. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J. Food. Eng. 2010;96:270–278. [Google Scholar]
- 30.Chittapalo T., Noomhorm A. Ultrasonic assisted alkali extraction of protein from defatted rice bran and properties of the protein concentrates. Int. J. Food. Sci. Tech. 2009;44:1843–1849. [Google Scholar]
- 31.Xu Y., Li Y., Bao T., Zheng X., Chen W., Wang J.A. Recyclable protein resource derived from cauliflower by-products: potential biological activities of protein hydrolysates. Food. Chem. 2017;221:114–122. doi: 10.1016/j.foodchem.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 32.Barteri M., Diociaiuti M., Pala A., Rotella S. Low frequency ultrasound induces aggregation of porcine fumarase by free radicals production. Biophys. Chem. 2004;111:35–42. doi: 10.1016/j.bpc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Chandrapala J., Zisu B., Palmer M., Kentish S., Ashokkumar M. Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011;18:951–957. doi: 10.1016/j.ultsonch.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Regenstein J.M., Zhou P., Yang Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Wali H., Ma M., Shahnawaz K., Hayat J., Xiaong L. Jing, Impact of power ultrasound on antihypertensive activity, functional properties, and thermal stability of rapeseed protein hydrolysates. J. Chem-Ny. 2017;2017:1–11. [Google Scholar]
- 36.Jia J., Ma H., Zhao W., Wang Z., Tian W., Luo L. The use of ultrasound for enzymatic preparation of ace-inhibitory peptides from wheat germ protein. Food. Chem. 2010;119:336–342. [Google Scholar]
- 37.Zhang X., Wang L., Chen Z., Li Y., Luo X., Li Y. Effect of electron beam irradiation on the structural characteristics and functional properties of rice proteins. RSC Adv. 2019;9:13550–13560. doi: 10.1039/c8ra10559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin J., Ma H., Wang K., Yagoub A.E.A., Owusu J., Qu W. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015;24:55–64. doi: 10.1016/j.ultsonch.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q., Tu Z., Xiao H., Wang H., Huang X., Liu G. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food. Bioprod. Process. 2014;92:30–37. [Google Scholar]
- 40.Wang F., Zhang Y., Xu L., Ma H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT. 2020;127:109348. [Google Scholar]
- 41.Zou J., Nguyen N., Biers M., Sun G. Conformational changes of soy proteins under high-intensity ultrasound and high-speed shearing treatments. Acs. Sustain. Chem. Eng. 2019;7:8117–8125. [Google Scholar]
- 42.Golly M.K., Ma H., Duan Y., Wu P., Dabbour M., Sarpong F. Enzymolysis of walnut (juglans regia l.) Meal protein: ultrasonication-assisted alkaline pretreatment impact on kinetics and thermodynamics. J. Food Biochem. 2019;43:e12948. doi: 10.1111/jfbc.12948. [DOI] [PubMed] [Google Scholar]
- 43.Hu H., Wu J., Li-Chan E.C.Y., Zhu L., Zhang F., Xu X. Effects of ultrasound on structural and physical properties of soy protein isolate (spi) dispersions. Food. Hydrocolloid. 2013;30:647–655. [Google Scholar]
- 44.Qu W., Zhang X., Chen W., Wang Z., He R., Ma H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochem. 2018;42:250–259. doi: 10.1016/j.ultsonch.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Zandomeneghi G., Krebs M.R.H., McCammon M.G., FändrichFtir M. Reveals structural differences between native β-sheet proteins and amyloid fibrils. Protein. Sci. 2004;13:3314–3321. doi: 10.1110/ps.041024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta. Bioch. Bioph. Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 47.Taghian Dinani S., Hamdami N., Shahedi M., Havet M., Queveau D. Influence of the electrohydrodynamic process on the properties of dried button mushroom slices: a differential scanning calorimetry (dsc) study. Food. Bioprod. Process. 2015;95:83–95. [Google Scholar]
- 48.Tang X., Wang X., Yang L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. J. Food. Eng. 2009;92:432–437. [Google Scholar]
- 49.Jiang J., Xiong Y.L., Chen J. Ph shifting alters solubility characteristics and thermal stability of soy protein isolate and its globulin fractions in different ph, salt concentration, and temperature conditions. J. Agr. Food. Chem. 2010;58:8035–8042. doi: 10.1021/jf101045b. [DOI] [PubMed] [Google Scholar]
- 50.Arzeni K., Martínez P., Zema A., Arias O.E., Pérez A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food. Eng. 2012;108:463–472. [Google Scholar]
- 51.Yao Y., Liu C., Xiong W., Liang Q., Xuan P., Zeng X.S. Silicon dioxide as an efficient adsorbent in the degumming of rapeseed oil. J. Clean. Prod. 2020;268:122344. [Google Scholar]