Summary
Immunocompetent pet dogs develop spontaneous, human-like cancers, representing a parallel patient population for the investigation of chimeric antigen receptor (CAR) therapies. We have optimized a retrovirus-based protocol to efficiently CAR transduce primary T cells from healthy and tumor-bearing dogs. While transduction efficiencies and CAR-T expansion vary among dogs, CAR expression is typically higher and more stable compared with previous protocols, thus enabling human and comparative oncology researchers to use the dog as a pre-clinical model for human CAR-T cell research.
For complete details on the use and execution of this protocol, please refer to Panjwani et al. (2020).
Subject areas: Cancer, Immunology, Model Organisms, Biotechnology and bioengineering
Graphical abstract
Highlights
-
•
Canine cancer patients are a valuable immunocompetent resource for CART evaluation
-
•
RD114 retrovirus and dog-specific beads enable high and stable CAR expression
-
•
Canine CART cells can be effectively expanded for clinical use in canine trials
-
•
Canine CART clinical trials are feasible and can inform human CART protocol design
Immunocompetent pet dogs develop spontaneous, human-like cancers, representing a parallel patient population for the investigation of chimeric antigen receptor (CAR) therapies. We have optimized a retrovirus-based protocol to efficiently CAR transduce primary T cells from healthy and tumor-bearing dogs. While transduction efficiencies and CAR-T expansion vary among dogs, CAR expression is typically higher and more stable compared with previous protocols, thus enabling human and comparative oncology researchers to utilize the dog as a pre-clinical model for human CAR-T cell research.
Before you begin
The steps below describe how to generate a clinical scale canine CAR T product from canine peripheral mononuclear cells. Stable and efficient CAR transduction and expression is achieved by means of retroviral vectors. Before starting, dog-specific activating beads should be prepared. The dog-specific activating beads recapitulate the functional features of the equivalent human reagent used in human CAR T trials and enable easy and effective activation of canine lymphocytes required for retroviral transduction.
Manufacturing of canine T cell activating beads
Timing: 2 days
-
1.Prepare Buffer 1 and 2.
-
a.Buffer 1: Dilute 1 mL Sodium Borate Buffer (0.5 M, pH 8.5) with 4 mL H2O (final concentration 0.1 M). Filter buffer 1 through sterile 0.22 μm unit and use immediately.
-
b.Buffer 2: Dissolve 0.1 g bovine serum albumin (BSA) in 10 mL PBS without Ca2+ and Mg2+ to obtain a 1% w/v BSA stock solution. Dilute 1 mL BSA stock solution with 9 mL PBS without Ca2+ and Mg2+ to make a 0.1% w/v BSA blocking solution. Add 40 μL of 0.5 M EDTA (pH 8.0) to a final concentration 2 mM EDTA, pH 7.4. Filter buffer 2 through sterile 0.22 μm unit and use within 24 h. Store at 4°C until use.
-
a.
-
2.
Vortex DynabeadsTM M-450 Tosylactivated vial for at least 60 s to completely resuspend the beads.
-
3.
Transfer 1 mL beads, containing 4 × 108 beads, to a 2 mL sterile microcentrifuge tube.
-
4.
Wash the beads by adding 1 mL of Buffer 1 and carefully pipette them up and down 15 times.
-
5.
Place the washed beads on the DynaMag magnet for at least 1 min. Remove and discard supernatant when it appears clear. Aspiration of the supernatant must happen without moving the tube or disturbing the beads. Remove the tube from the magnet.
-
6.
Repeat steps 4 and 5 for a total of two washes.
-
7.
Resuspend the beads in 600 μL Buffer 1 and while mixing add 100 μL anti-CD28 (clone 1C6) and 100 μL anti-CD3 (clone CA17.2A12) antibodies, i.e., 100 μg of each antibody, up to 800 μL total volume.
Note: Anti-CD28 clone 5B8 can be used in place of anti-CD28 clone 1C6. The volume of antibodies and Buffer 1 should be adjusted to have 100 μg of each antibody in Buffer 1 up to 800 μL final volume.
-
8.
Incubate for 15 min at 20°C–25°C. During the incubation, prepare 10 mL Blocking Buffer by dissolving BSA in Buffer 1 to a final concentration of 0.1% w/v. Filter Blocking Buffer through sterile 0.22 μm unit. At the end of the incubation, add 200 μL of blocking buffer to the bead-antibody mixture and mix by gently pipetting up and down 15 times.
Note: Blocking buffer can be stored at 4°C for 2–7 days.
-
9.
Incubate for 24 h at 37°C on a rotator with gentle tilting and rotation.
Note: This can be incubated at 20°C–25°C, but lower temperatures may require longer incubations.
-
10.
At the end of incubation, remove unbound antibodies by placing the tube on DynaMag magnet for at least 1 min. When the supernatant is clear and without moving the tube or disturbing the beads, aspirate and discard the supernatant. Then, remove the tube from the magnet.
-
11.Wash by adding in 1 mL Buffer 2 and pipetting up and down 15 times to mix. Incubate for 5 min at 2°C–8°C with gentle tilting and rotation. Remove washing buffer as in step 11. Repeat for a total of two washes.
-
a.During the last incubation, prepare 10 mL Buffer 3 by dissolving BSA in 0.2 M Tris (pH 8.5) to a final concentration of 0.1% w/v. Filter buffer 3 through sterile 0.22 μm unit and use immediately.
-
a.
Note: Unused buffer can be stored at 4°C for 2–7 days.
-
12.
After removal of the last wash buffer, resuspend beads in 1 mL Buffer 3 and incubate for 4 h at 37°C to deactivate remaining free tosyl groups.
-
13.
Remove Buffer 3 by placing the tube on DynaMag magnet as described in step 11.
-
14.
Wash once with 1 mL Buffer 2 as described in step 12.
-
15.
Resuspend the Dynabeads in 1 mL Buffer 2 to obtain 4 × 108 beads/mL.
-
16.
Transfer to 50 mL conical tube and dilute 1-in-10 adding 9 mL of Buffer 2. Make sure you rinse the previous microcentrifuge tube to avoid loss of beads.
-
17.
Mix diluted beads by vortexing for at least 60 s.
Optional: Immediately divide into 1–2 mL aliquots and remove 20 μL for counting on hemocytometer to confirm concentration of 4 × 107 beads/mL.
-
18.
The beads are coated and ready for use.
Optional: As preservative, Sodium Azide (NaN3) can be added to the ready-to-use beads to a final concentration of 0.02%, e.g., 200 μL of 1% NaN3 in 10 mL beads.
Note: Coated dynabeads should be stored at 2°C–8°C and can be used for up to 2 years. While the use of BSA and/or EDTA and/or NaN3 may be omitted, their omission could reduce the beads’ shelf life, in which case we recommend using them within 6 months of preparation.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Dog CD28, clone 1C6, Functional Grade (100 μg /1 mL (4 × 108) beads) | eBioscience | Cat# 16-0283-82 |
| Mouse anti-Dog CD3, Clone CA17.2A12, Purified (100 μg /1 mL (4 × 108) beads) | Bio-Rad | Cat# MCA1774GA |
| Mouse anti-Dog CD11b, Clone CA16.3E10, Purified (1 μg / 1 × 107 cells) | Bio-Rad | Cat# MCA1777S |
| Mouse anti-Dog CD11c, Clone CA11.6A1, Purified (1 μg / 1 × 107 cells) | Bio-Rad | Cat# MCA1778S |
| Mouse anti-Human CD14, Clone TuK4, Purified (1 μg / 1 × 107 cells) | Bio-Rad | Cat# MCA1568GA |
| Mouse anti-Dog CD21, Clone CA2.1D6, Purified (1 μg / 1 × 107 cells) | Bio-Rad | Cat# MCA1781R |
| Goat anti-Mouse IgG Microbeads (20 μL / 1 × 107 cells) | Miltenyi Biotech | Cat# 130-048-401 |
| Dog Gamma Globulin (100 μg /100 μL) | Jackson ImmunoResearch | Cat# 004-000-002 |
| Biotin-SP (long spacer) AffiniPure Rabbit Anti-Mouse IgG (H+L) (0.5–0.75μg/100μL) | Jackson ImmunoResearch | Cat# 315-065-003 |
| Streptavidin, APC (0.2μg/100μL) | BD Biosciences | Cat# 554067 |
| Rat Anti-Dog CD5, Clone YKIX322.3, APC-eFluor780 (0.025μg/100μL) | eBioscience | Cat# 47-5050-42 |
| Rat Anti-Dog CD4, Clone YKIX302.9, Pe-Cy7 (0.006μg/100μL) | eBioscience | Cat# 25-5040-42 |
| Rat Anti-Dog CD8, Clone YCATE55.9, eFluor450 (0.2μg/100μL) | eBioscience | Cat# 50-112-9324 |
| Mouse Anti-Dog CD3, Clone CA17.2A12, FITC (0.4μg/100μL) | Bio-Rad | Cat# MCA1774F |
| Biological samples | ||
| Canine Peripheral Blood Mononuclear Cells | PennVet Cancer Center Ryan Hospital | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Sodium Borate Buffer (0.5 M, pH 8.5) | Alfa Aesar | Cat# AAJ62902AK |
| Bovine serum albumin Stock Solution | Miltenyi | Cat# 130-091-376 |
| 1 M Tris-HCl (pH 8.5) | Jena Bioscience | Cat# BU-124S-85 CAS#: 1185-53-1 |
| 1% Sodium Azide (NaN3) | G-Biosciences | Cat# 786-299 CAS#: 26628-22-8 |
| Lipofectamine 2000 | Invitrogen | Cat# 11668019 |
| ACK lysing buffer | Gibco | Cat# A1049201 |
| Sterile Filtered DMSO | GoldBio | Cat# D-361-10 CAS# 67-68-5 |
| Trypan Blue Solution, 0.4% | Gibco | Cat# 5250061 |
| Recombinant human IL-2 | PeproTech Inc. | Cat# 200-02 |
| Recombinant human IL-15 | PeproTech Inc. | Cat# 200-15 |
| Recombinant human IL-7 | PeproTech Inc. | Cat# 200-07 |
| Recombinant human IL-21 | PeproTech Inc. | Cat# 200-21 |
| RetroNectin Recombinant Human Fibronectin Fragment | Takara | Cat# T100A |
| 7-AAD Viability Staining Solution | BioLegend | Cat# 420404 |
| Critical commercial assays | ||
| MycoAlert Mycoplasma Detection Kit | Lonza | Cat# LT07-118 |
| Experimental models: Cell lines | ||
| HEK 293T | ATCC | Cat# CRL-3216 |
| K-562 | ATCC | Cat# CCL-243 |
| Recombinant DNA | ||
| Gag-Pol plasmid | Dr. Andrei Thomas-Tikhonenko | N/A |
| RD114 plasmid | Dr. Daniel Powell | N/A |
| MSGV1-cCAR20.28Zplasmid | This paper | N/A |
| Software and algorithms | ||
| FlowJo v10.7 | BD | https://www.flowjo.com/solutions/flowjo/downloads |
| Prism 9.1.0 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Dynabeads® M-450 Tosylactivated | Invitrogen | Cat# 14013 |
| LS columns | Miltenyi | Cat# 130-042-401 |
| Ficoll-Paque Plus | Cytiva | Cat# 17144002 |
| Dulbecco's Modified Eagle Medium (DMEM), high Glucose (4500 mg/mL) | Gibco | Cat# 11965092 |
| Iscove's Modified Dulbecco's Medium (IMDM), 25 mM Hepes | Gibco | Cat# 12440053 |
| Roswell Park Memorial Institute Medium (RPMI) 1640 | Corning | Cat# 10-040-CV |
| Heat Inactivated Fetal Bovine Serum (FBS) | Biotechne | Cat# S11150 |
| Penicillin/Streptomycin solution | Invitrogen | Cat# 15140122 |
| Sodium Pyruvate 100 mM | Invitrogen | Cat# 11360070 |
| Glutamax 100× | Invitrogen | Cat# 35050061 |
| Non-essential Amino Acids (MEM NEAA), 100× | Invitrogen | Cat# 11140050 |
| 1 M Hepes | Gibco | Cat#15630130 |
| 2-Mercaptoethanol (50 mM) | Gibco | Cat# 31350010 |
| Dulbecco's phosphate-buffered saline (DPBS), 1×, no calcium, no magnesium | Gibco | Cat# 14190250 |
| Dulbecco's phosphate-buffered saline (DPBS), 1× with Ca++ & Mg++ | Mediatech | Cat# MT21-030-CV |
| Midi MACS separator | Miltenyi | Cat# 130-042-302 |
| Multistand | Miltenyi | Cat# 130-042-303 |
| LS columnsa | Miltenyi | Cat# 130-042-401 |
| 100 mm TC-treated Cell Culture Dishb | Falcon | Cat# 353003 |
| Non-Treated Plate, 96 well, Flat Bottomb | Falcon | Cat# 07-000-108 |
| 1.5mL tubes | Eppendorf | Cat# 022431021 |
| 5 mL conical tubes | MTC Bio | Cat# C2540 |
| 15 mL conical tubes | Nunc | Cat# 339651 |
| 50 mL conical tubes | Nunc | Cat# 339653 |
| 0.45 μm filter units | Fisherbrand | Cat# 09-720-005 |
| 10 mL sterile syringes | Becton Dickinson | Cat# 303134 |
| 30 mL sterile syringes | Becton Dickinson | Cat# 302833 |
| DynaMag-15 Magnet | ThermoFisher | Cat# 12301D |
| DynaMag-50 Magnet | ThermoFisher | Cat# 12302D |
| Non-Treated Plate, 48 well, Flat Bottomb | Falcon | Cat# 351178 |
| Non-Treated Plate, 24 well, Flat Bottomb | Falcon | Cat# 351147 |
| Non-Treated Plate, 6 well, Flat Bottomb | Falcon | Cat# 351146 |
| Non-treated T25 flasks, vent capb | Greiner Bio-one | Cat# 690195 |
| Non-treated T75 flasks, vent capb | Greiner Bio-one | Cat# 658195 |
Alternatives: for MACS negative selection, LD columns can also be used (Cat# # 130-042-901). Sample, hydration, rinse and elution volumes should be adjusted accordingly.
Note: For HEK 293T cells, TC-treated vessels are required. For suspension cells, we use non-treated plates. Retronectin requires non-treated plates. Manufacturers and catalog numbers shown are indicative. Any suitable alternative can be used at discretion of protocol users.
Materials and equipment
HEK 293T medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM high glucose (4500 mg/mL) | 86% | 430 mL |
| Heat Inactivated FBS | 10% | 50 mL |
| Penicillin/Streptomycin | 1% | 5 mL |
| 100 mM Sodium Pyruvate | 1% | 5 mL |
| Glutamax | 1% | 5 mL |
| Non-essential Amino Acids, 100× | 1% | 5 mL |
| Total | n/a | 500 mL |
Store at 2°C–4°C for up to 1 month
Transfection medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM high glucose (4500 mg/mL) | 97% | 1,358 μL / dish |
| 100 mM Sodium Pyruvate | 1% | 14 μL / dish |
| Glutamax | 1% | 14 μL / dish |
| 1 M Hepes | 1% | 14 μL / dish |
| Total | n/a | 1,400 μL / dish |
Prepare when required and use immediately
K562 medium
| Reagent | Final concentration | Amount |
|---|---|---|
| RPMI-1640 | 87% | 435 mL |
| Heat Inactivated FBS | 10% | 50 mL |
| Penicillin/Streptomycin | 1% | 5 mL |
| Glutamax | 1% | 5 mL |
| Sodium Pyruvate 100 mM | 1% | 5 mL |
| Total | n/a | 500 mL |
Store at 2°C–4°C for up to 1 month
T cell medium (TCM)
| Reagent | Final concentration | Amount |
|---|---|---|
| IMDM, 25 mM Hepes | 86% | 430 mL |
| Heat Inactivated FBS | 10% | 50 mL |
| Penicillin/Streptomycin | 1% | 5 mL |
| Glutamax | 1% | 5 mL |
| Non-essential Amino Acids | 1% | 5 mL |
| Sodium Pyruvate 100 mM | 1% | 5 mL |
| Total | n/a | 500 mL |
Store at 2°C–4°C for up to 1 month. Primary canine T cells benefit from the supplementation of 2-Mercaptoethanol (BME). We use BME at 50 μM for canine T cells. As 2-Mercaptoethanol is not stable in solution, we supplement it fresh by adding 1μL/mL media of BME 50 mM (1000×) directly to the cells whenever we handle the canine T cell cultures.
FACS buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 × PBS without Ca2+ and Mg2+ | 90–98% | 450–490 mL |
| Heat Inactivated FBS | 2–10% | 10–50 mL |
| Total | n/a | 500 mL |
Store at 2°C–4°C, use within 6 months
Step-by-step method details
The method below provides step-by-step details to effectively engineer primary canine T cells. Compared to previously published methods (Panjwani et al., 2020; Sakai et al., 2020), our implemented protocol based on use of RD114 pseudotyped viral vectors and activation of canine T lymphocytes via anti-canine CD3/CD28-coated beads enables high transduction efficiencies using a single infection. Of note, we have also successfully employed chimeric RD114 and VSVG pseudotyped retrovirus for canine T cell transduction. While chimeric pseudotyped retrovirus is not necessary to efficiently engineer canine primary T cells, this strategy allows more efficient transduction of human cells compared with RD114-only pseudotyped virus, thus enabling side-by-side comparative, mechanistic studies in dog and human cells. For canine clinical trials, our retroviral vector of choice is MSGV1, which has been safely employed to generate CAR T cell products used in the treatment of human cancer patients without evidence of replication-competent retrovirus (RCR) production.
Production of RD114 pseudotyped retrovirus
Timing: 4 days
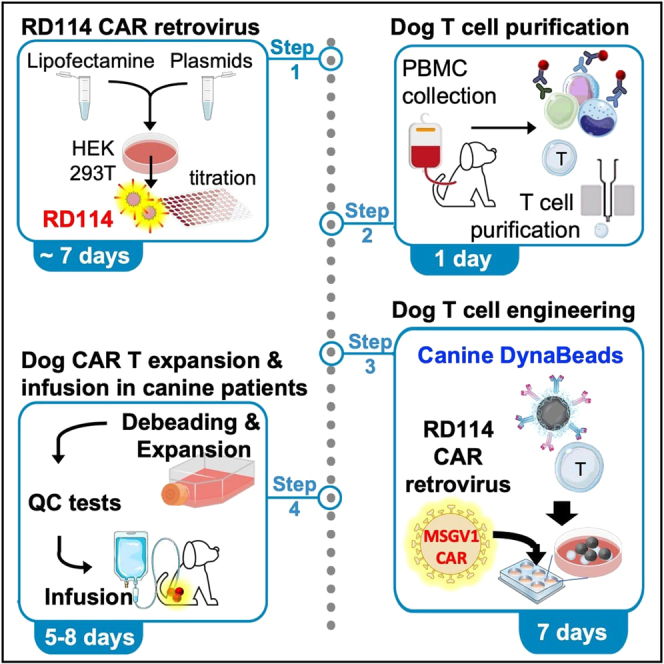
High titers of RD114 retrovirus can be produced by transfecting HEK 293T cells in 10 cm dishes. The final yield is usually 10–12 mL per dish. Several dishes can be transfected to produce clinical scale virus stocks, which can be stored as supernatants at -80°C in the long term and for at least 2 years without loss of their transduction potential. No special producing cell lines, ultracentrifuges or concentrating agents are required, making this method versatile and easy to perform in most laboratories. Figure 1 outlines the main steps of our procedure to produce RD114 pseudotyped virus supernatants.
-
1.
12–18 h prior to transfection (early afternoon on the day before), seed HEK 293T cells in 10 cm dishes at 4 × 106 cells per dish in 10 mL HEK 293T media. Gently rock the dish to ensure even cell distribution and return to 37°C incubator.
CRITICAL: Health of HEK 293T is a key determinant for high retroviral titers. When seeding them for transfection, HEK 293T cells should be a single cell suspension grown to 70%–80% confluency, >90% viable and not passaged more than 25 times (see troubleshooting, problem 1 for further details). The number of HEK 293T can be modified based on the time of transfection on the following day. If transfection is performed after >18h, <4 × 106 cells per dish should be plated to avoid overgrowth.
-
2.On the day of transfection, check HEK 293T under an inverted microscope to confirm that they are evenly distributed and 70%–90% confluent. If so, proceed to transfection as follow:
-
a.transfer 4 mL HEK 293T media per dish into a sterile 50 mL conical tube and let warm to 37°C in a water bath.
-
b.For each virus to be produced, transfer 15 μL Lipofectamine 2000/dish into a sterile conical tube containing 1.4 mL transfection media/dish. Mix by gently flicking the tube, without pipetting or vortexing, and avoiding bubbles, as this may cause oxidation of lipofectamine cationic lipids and reduced transfection efficiency. Incubate the lipofectamine dilution at 20°C–25°C for ≤14 min.Note: If transfecting >3 dishes/virus, consider preparing 1 tube for up to 3 dishes to avoid prolonged incubations between the mix preparation and the last transfections.
-
c.During the lipofectamine/media incubation, prepare the plasmid master mix by combining the Gag/Pol (GP) packaging plasmid, the RD114 (R) envelope plasmid and the MSGV1.CAR (M) plasmid in a sterile microcentrifuge tube. We use a molar ratio of GP:R:M = 1:1.5:3–5 for a total of 21–23 μg plasmid DNA/dish in up to 100 μL transfection media/dish.
 CRITICAL: Use endotoxin-free maxiprep kits to produce plasmid DNA. Verify that DNA is predominantly supercoiled after maxiprep on an agarose gel, as relative increases in the proportion of nicked/linear DNA significantly impair viral titers.
CRITICAL: Use endotoxin-free maxiprep kits to produce plasmid DNA. Verify that DNA is predominantly supercoiled after maxiprep on an agarose gel, as relative increases in the proportion of nicked/linear DNA significantly impair viral titers. -
d.Once Lipofectamine 2000 incubation is complete, carefully add plasmid mixtures to the center of the tube(s), gently flick to mix and incubate for ≤25 min at 20°C–25°C.
-
e.At the end of the incubation, take HEK 293T dish(es) from the incubator and completely remove media. Using a 2 mL pipette, immediately and carefully add the lipofectamine-plasmid mix dropwise (∼ 1.5 mL). Cover the entire layer of cells, then gently rock the dish back and forth for 30–60 s to evenly distribute the transfection complexes.Note: Handle 293T gently but quickly. To avoid detaching, HEK 293T should not be kept out of the incubator and/or without media and/or in direct contact with the lipofectamine-plasmid mix for too long. If transfecting several dishes, proceeding with set of 3 transfections at a time may help prevent unwanted delays.
-
f.Gently tilt the dish and slowly add ∼3.5 mL warm HEK 293T media without dislodging the cells. Tilt the dish back, then gently rock back and forth to ensure even distribution of the media/transfection complexes and return to the incubator for 6 h.Note: After addition of the warm media, the total volume should not exceed 6 ml per dish.
-
a.
-
3.
6 h after transfection, completely replace the transfection media with 6 mL pre-warmed HEK 293T media and return the plate to the 37°C incubator.
-
4.48 h after transfection, harvest supernatant containing retroviral vectors
-
a.30 min before harvest, warm 6 mL of HEK 293T media per plate
-
b.Carefully tilt the dish and collect the media into a sterile 50 mL conical tube. Pool supernatants if multiple dishes have been transfected with the same plasmid mix.
-
c.Carefully replace supernatant with 5.5 mL of warmed HEK 293T media without dislodging the cells and return cells to the incubator.
-
d.Centrifuge tubes at 500 × g for 5 min at 20°C–25°C to remove cellular debris. Harvest and filter supernatants with 0.45 μM PES filters to remove contaminating cells and large cell debris.
-
e.Aliquot supernatants into 1.5 mL screw-cap tubes and snap-freeze on dry ice for 30 min before storing at −80°C.
-
a.
-
5.
Repeat harvest of vector supernatant 24 h later (72-h harvest) as described in step 4b-e.
Figure 1.
Workflow to produce RD114 pseudotyped retrovirus
Titration of RD114 retrovirus batches on K562 cells
Timing: 3 days
-
6.Titrate supernatants on K562 cells.
-
a.Thaw virus on ice for 20 min, then complete thawing at 20°C–25°C.
-
b.In a 96-well flat-bottom plate (K562-titration plate) seed K562 cells in two or three 8-well columns for technical duplicates or triplicates respectively, at 4 × 104 K562 cells in 100 μL of K562 media per well. Repeat this for each virus batch to be titrated.
-
c.On a parallel 96-well round-bottom plate (virus-dilution plate), pipette 125 μL K562 media per well following the same pattern. Leave the first row empty. Then, add 250 μL of the virus to the top well of each column.
-
d.With a multichannel pipette, prepare a 2-fold dilution series by transferring 125 μL of viral supernatant from row-1 wells to row-2 wells of the virus-dilution plate. Gently pipette up and down ∼6 times to mix the virus supernatant and K562 media. After the last mix, without discarding the tips, carry 125 μL down from row-2 to row-3 wells. Follow down to row-7 wells. Do not add any virus into row-8 wells.
-
e.Transfer 100 μL of virus and dilution series from each well of column A of the virus-dilution plate to the corresponding K562 cell column of the K562-titration plate. Repeat for each column and, when done, incubate at 37°C for 72 h.
-
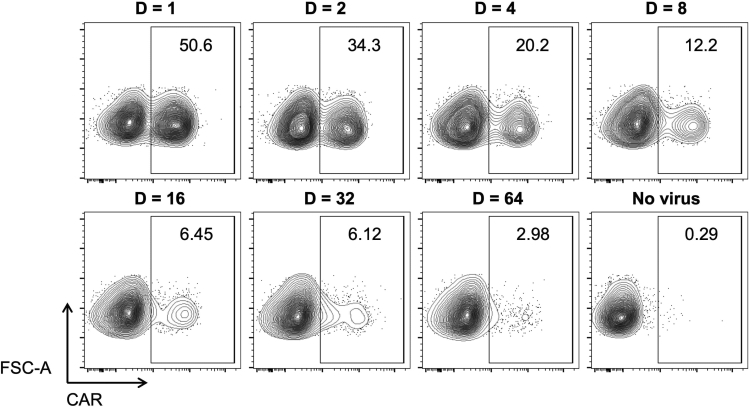
f.After 72 h, transfer cells to FACS tube, proceed to CAR labeling as required (see below step 36 for details on our CAR labeling reagents and protocol) and determine transduction efficiency (TE) by flow cytometry. A representative K562 TE profile is shown in Figure 2.
-
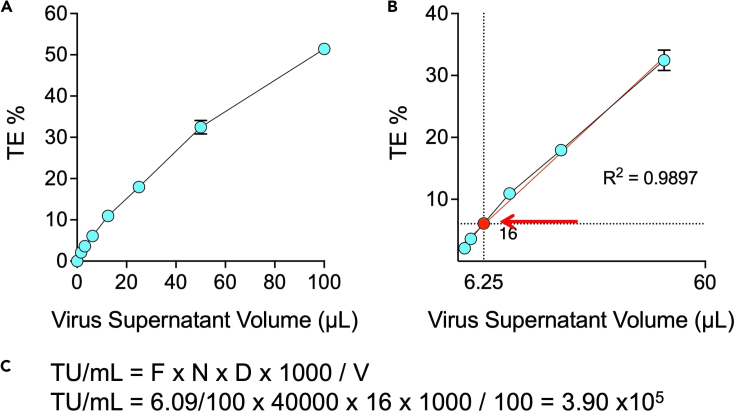
g.Graph titration results on a XY scatter chart by plotting the μL of virus supernatant on the x axis (e.g., 0, 1.5625, 3.125, 6.250, etc) and the TE percentages on the y axis (Figure 3A). The virus supernatant volumes should approximate a linear correlation particularly with intermediate TE values (Figure 3B, red line).
-
h.To determine the functional titer of the virus batch, select the point that most accurately represents the number of transduced cells. For TE<1%, flow cytometry may not be accurate enough. For TE>20%, multiple vectors are more likely to infect each positive cells, leading to underestimation of the number of transducing particles. TE values within the range of 1%–20% are usually associated with the most accurate estimate (Giry-Laterrière et al., 2011). Once chosen the appropriate point, use the corresponding percentage of positive cells (F) and virus dilution (D) to determine the number of viral vectors per mL (TU/mL) as detailed in Figure 3C.
-
a.
Note: Titration could be performed using different cells lines (e.g., 293T, Jurkat cells), vessels (U bottom 96-well plates), cell numbers (e.g., 100,000 cells), fold-dilutions (e.g., 3-fold), and strategies (e.g., additional polybrene or spinoculation). Such variations will result in different estimated TU/mL values and may require further trial transductions of primary canine T cells to identify and validate the most appropriate multiplicity of infection (MOI).
Figure 2.
Titration of RD114 pseudotyped virus supernatants on K562 cells
Representative TE profile of K562 cells infected with the indicated virus dilutions (D). No polybrene or other strategies to enhance transduction were employed.
Figure 3.
Determination of functional titer
(A) Representative XY scatter chart of virus supernatant volumes and TE percentages (average TE of duplicates) from the same experiment shown in Figure 2. Data are represented as mean with range.
(B) Selection of the best point for determination of functional titer. The same series as in A is shown, without extreme points, i.e., x=100 (no dilution) and x=0 (no virus). Linear regression modeling (superimposed red line) confirmed a good correlation between the virus volumes used and the resulting TE percentages (R2 >0.95). The point obtained using a dilution (D) 16 was chosen as the most appropriate for determination of the functional titer (average TE=6.09%). Data in Figures 3A and 3B are represented as mean with range.
(C) Equation to calculate the number of virions per mL. TU/mL: number of transducing particles (viral vectors) per mL. F: fraction of CAR positive K562 cells (% of positive cells/100). N: number of K562 at the time of infection (4 × 104). D: dilution factor. 1000: correction factor to provide the number of TU per mL. V: volume of diluted virus added to each well (100 μL).
Isolation of primary canine T cells
Timing: 1 day
The goal of the following steps is to purify canine T lymphocytes from peripheral blood mononuclear cells (PBMCs), i.e., monocytes, granulocytes, B lymphocytes and any potential circulating myeloid derived suppressor cell (MDSC), that can reduce efficiency of CAR engineering particularly in samples obtained from canine cancer patients. We routinely perform a negative selection to enrich for T cells. However, we have also successfully purified T cells by positive selection using anti-CD5 primary antibody, followed by labeling with appropriate MicroBead-conjugated secondary antibody.
-
7.
Obtain peripheral blood sample from healthy dog or canine cancer patient and isolate PBMCs by gradient centrifugation using standard, sterile media and protocols optimized for isolating human lymphocytes, such as Ficoll-Paque Plus or Histopaque®-1077 (see manufacturer’s recommendations for Ficoll-Paque and Hstopaque-1077). If available, leukapheresis products may provide higher initial numbers of T cells, which may be particularly useful for clinical scale manufacturing of canine patient derived, autologous CAR T cells.
-
8.
To remove any residual isolation media, resuspend isolated mononuclear cells in at least an equal volume of 1× PBS without Ca++ and Mg++, e.g., 25 mL cell suspension and 25 mL PBS. Centrifuge at 500 × g for 10 min, and carefully aspirate supernatant without disturbing the pellet. Repeat twice.
-
9.
After the second wash resuspend in up to 50 mL T cell medium (TCM) and obtain cell count and viability using Trypan Blue.
-
10.
Calculate the amount of required antibodies (mAb), FcR blocking reagent and TCM in which to label the cells to perform T cell enrichment, as shown in Table 1. Put TCM on ice to keep it cold throughout the selection.
-
11.
Centrifuge cell suspension at 500 × g for 10 min, aspirate supernatant and resuspend cell pellet in the required volume of TCM as determined above.
-
12.
Add the required volume of dog gamma-globulins, gently mix and incubate for 10–15 min at 20°C–25°C.
-
13.
Add the primary antibodies, gently mix and incubate for 30 min at 2°C–8°C.
-
14.
At the end of the incubation, wash out the excess of unbound mAb by adding at least 2 mL TCM / 10⁷ cells and up to 50 mL total TCM. Centrifuge at 500 × g for 10 min and completely remove the supernatant by aspiration.
Optional: Repeat this step once. While centrifuging the cells, calculate the volumes of TCM and anti-Mouse IgG MicroBeads required to resuspend the cell suspension in 80 μL TCM / 10⁷ cells and provide 20 μL microbeads / 10⁷ cells.
-
15.
Resuspend cell pellet in 80 μL TCM / 10⁷ total cells.
-
16.
Resuspend anti-Mouse IgG MicroBeads by vortexing for 30 s. Immediately, add 20 μL of Anti-Mouse IgG MicroBeads per 10⁷ cells.
-
17.
Mix well and incubate for 15 min at 2°C–8°C.
-
18.Wash twice with TCM up to 50 mL, remove 200 μL for further flow cytometry analysis at the end of the selection. This will serve as the pre-selection control sample. Then, centrifuge at 500 × g for 10 min. During this time,
-
a.Sterilize MACS multistand and MidiMACS separator with ethanol and set them in the hood with a rack for collection tubes. Let dry.
-
b.Label 2 FACS tubes as “flow-through”, “antibody-bound”.
-
c.Place LS column(s) in the separator and 50 mL tube(s) in a rack under the column(s) without touching the tip(s). Note: LS columns bind up to 1 × 108 cells. One column every 108 cells will be required.
-
d.Rinse with 3 mL cold TCM and discard the flow-through.
-
e.Replace the collection tube(s) with new 50mL tube(s), that will receive the flow-through enriched for the T cells.
-
a.
-
19.
Aspirate the supernatant from centrifuged cells and resuspend the cell pellet in TCM at 1.0 × 108 cells / 500μL.
-
20.
Apply the cell suspension onto the column and collect flow-through containing unlabeled cells.
-
21.(Optional) Close the “flow-through” tube with the unlabeled cells. If the antibody-bound fraction is needed for further studies, keep the “flow-through” tube in ice or at 4°C and retrieve the antibody-bound fraction as follows:
-
a.Once the upper chamber is empty and after the column stops dripping, add 3 mL of TCM. Repeat twice for a total of 3 washes.
-
b.Remove LS column from the separator, place it on a new 15 mL conical tube, immediately pipette 5 mL TCM in the column chamber and firmly push the plunger provided with the column to flush out the antibody-bound cells.
-
a.
-
22.
Combine the unlabeled cells from all “flow-through” tubes in a 50 mL conical tube. Add 10 mL TCM. Gently mix by pipetting up and down and remove 20 μL for counting and viability check by Trypan Blue.
-
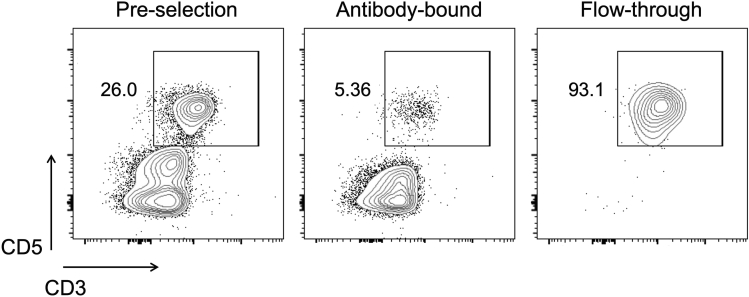
23.
Remove 100,000 cells and check purity by flow cytometry. More than 90% of mononuclear cells in the flow-through should be T lymphocytes (Figure 4).
-
24.Centrifuge cells in the collection tubes at 500 × g for 5 min, resuspend at 2–4 × 106/mL TCM for immediate use (if virus has been already produced and titrated). Alternatively, resuspend at 20–50 × 106/mL and cryopreserve as follow:
-
a.Pre-cool a cell freezing container and cryovials at 2°C–8°C or on ice for a minimum of 30 min.
-
b.Resuspend cells in cold TCM at 20 × 106/mL and leave in ice while labeling the cryovials.
-
c.Add an equivalent volume of chilled 2× freezing medium (20% DMSO/FBS) to achieve a concentration of 10 × 106 cells/mL. Mix gently.
-
d.Dispense cell suspension aliquots into the pre-cooled cryovials, immediately transfer to the cell freezing container and store at −80°C.
-
e.After ≥4 h, transfer the cryovials into liquid nitrogen storage. We usually transfer them on the following day.
-
a.
Table 1.
Reagent volumes for canine T cell isolation by MACS negative selection
| Primary antibodies (mAb) | |
|---|---|
| purified mouse anti-dog CD11b (clone CA16.3E10) | 1 μg / 1 × 107 cells |
| purified mouse anti-dog CD11c (clone CA11.6A1) | 1 μg / 1 × 107 cells |
| purified mouse anti-dog CD21 (clone CA2.1D6) | 1 μg / 1 × 107 cells |
| purified mouse anti-human CD14 (clone TuK4) | 1 μg / 1 × 107 cells |
| Dog gamma-globulins (blocking reagent) | 10 μL / 1 × 107 cells |
| TCM | (90 μL / 1 × 107 cells) – mAb Volume |
| Total Volume | 100 μL / 1 × 107cells |
mAb: monoclonal antibody; TCM: T cell medium
Figure 4.
Purity check after canine T cell selection
Representative plots showing T cell enrichment after MACS negative selection. Cells were labeled with anti-canine CD3, CD5, CD4 and CD8a mAb for 30 min at 2°C–8°C. At the end of incubation, 1mL ACK lysing buffer was added for 1–2 min to lyse any contaminating red blood cells, followed by addition of 3 mL TCM and 5-min centrifugation at 500 ×g. Supernatants were eliminated by decanting and cells resuspended in 300 μL FACS buffer with 2 μL 7AAD prior to acquisition by flow cytometer. All cells were alive and >90% were CD5+CD3+ cells. Gated on singlets.
Activation and transduction of canine T cells
Timing: 7 days
Retroviral transduction requires rapidly dividing T cells. The steps below describe effective activation of T cells for ∼44–48 h with anti-canine CD3 and anti-canine CD28 mAb-coated beads in the presence of IL-2. We use cross-reactive recombinant human IL-2. Other cytokines such as IL-15, IL-7 and IL-21 are not necessary to successfully transduce canine T cells, but they may be included from the first day of activation to promote better recovery after thawing and preserve a more favorable T cell memory profile in the long term. Activation of 1.5 ×107 cells is usually sufficient to achieve clinically relevant products. 1–2 ×106 cells are suitable for trial transductions and/or small-scale studies aiming to test the ability of a donor/patient specific cell preparation to be gene engineered and to perform pilot experiments, such as screening of new CAR constructs and immunotherapy combinations.
Activation: day −2 and −1
Transduction: day 0
Recovery post infection: day +1 and +2
Inspection, media supplementation or subculturing: day +3 and +4
-
25.If using fresh cells, skip this step and proceed to step 26. If transducing cryopreserved cells, thaw T cells as follow:
-
a.Remove cryovials from storage and immediately thaw in the water bath at 37°C for 2–5 min.
-
b.In a biosafety hood, gently transfer thawed cells to a 50-mL conical tube, rinse the cryovial with 1 mL of warm TCM and add the rinse dropwise (1 drop per 5 s) to the 50-mL conical tube while gently swirling the tube.
-
c.Sequentially dilute cells in the 50-mL conical tube by slowly adding 2 mL, 4 mL, 8 mL media, approximately 1 mL/3–5 s with ∼1 min wait between additions. The final volume will be 16 mL. Slow, sequential dilutions, while swirling the tube, help minimize osmotic stress and preserve viability.
-
d.Centrifuge at 300 × g for 5 min at 20°C–25°C, completely remove the supernatant and resuspend cell pellet in 1 mL of TCM. Add an additional 9 mL TCM at a speed of 1 mL/3–5 s.
-
e.Repeat centrifugation at 300 × g for 5 min at 20°C–25°C, completely remove the supernatant and resuspend cell pellet in 1 mL of TCM. Determine the cell concentration and viability.
-
f.Plate thawed T cells at 4 × 106 cells/mL (or 2.1 x 106 cells/cm2) in a suitable non-treated plate (Table 2).
-
a.
Optional: Let T cells recover for 4–12 h at 37°C in TCM supplemented with IL-2 100 IU/mL.
Note: A lower T cell density, e.g., 1–2 × 106 cell/mL, can be used when working with fresh T cells.
-
26.On day -2, activate purified canine T cells with canine activating beads. We generally use a 3:1 ratio to T cells, i.e., 3 beads to 1 T cell, to activate canine T cells prior to CAR-retrovirus infection. However, lower ratios, such as 1:1 and 2:1, have also been successfully employed.
-
a.Vortex tube to resuspend beads, which are at a concentration of 4 × 107 / mL.
-
b.Transfer 75 μL beads / 106 cells to a sterile tube. Scale up or down accordingly.
-
c.Wash with at least 1 mL of TCM or the same volume of beads. Place on DynaMag magnet for 1 min or until supernatant is clear. Aspirate the supernatant without disturbing the beads. Remove tube from magnet.
-
d.Repeat step c for a total of 3 washes
-
e.After the last wash, add the cells to the tube with the bead pellet. Gently resuspend the beads in the cell/TCM suspension by pipetting the mixture up and down until the beads are completely resuspended.
-
i.If working with thawed cells that were plated at a density of 4 ×106 cells/mL in TCM supplemented with cytokines, simply harvest them and use this suspension to resuspend the beads. When done, replate at the same density.
-
ii.If working with fresh T cells that have just been purified (see step 24) use 1 mL TCM to resuspend the bead pellet, then add the required number of cells in TCM, aiming for a final 2–4 × 106 cell/mL density. Add IL-2 100 U/mL (omit this if already added in step 26).
-
i.
-
f.Place in incubator at 38.8°C for 12–16 h.
-
a.
-
27.
On day -1, check T cell health and activation under an inverted microscope. Activated T cells form clumps and blast, acquiring spindle-like morphology (Figure 5). A decrease in the number of T cells might be noticed, likely due to activation-induced cell death (AICD).
Optional: Consider an extra supplementation of IL-2 100 IU/mL if cells do not show morphological changes consistent with activation (see troubleshooting, problem 2 and problem 3 for additional details).
-
28.On the day of transduction (day 0),
-
a.Check cells under the microscope and ∼4 h prior to transduction supplement with extra IL-2 200 IU/mL to ensure that they are dividing as rapidly as possible when exposed to the viral supernatant.
-
b.Prepare RetroNectin coated plate(s) by diluting 10 μL RetroNectin stock in 990 μL PBS and dispensing it into appropriate non-treated, cell culture-grade plate, i.e., 2 mL/well in a 6 well plate for each 1.5 ×106 cells to transduce, or 1 mL/well in a 24 well plate for each 3 ×105 cells to transduce. Allow the plate to stand for 2 h at 20°C–25°C.
-
c.Remove the RetroNectin solution and replace with the same volume of sterile 2% BSA/PBS solution. Allow the plate to stand for 30 min at 20°C–25°C.
 CRITICAL: Use freshly made, sterile BSA. Poor blocking may lead to non-specific binding of RetroNectin to proteins in the media rather than virus and results in lower transduction efficiency.
CRITICAL: Use freshly made, sterile BSA. Poor blocking may lead to non-specific binding of RetroNectin to proteins in the media rather than virus and results in lower transduction efficiency. -
d.During BSA blocking, remove virus aliquot from −80°C. Thaw on ice for ∼20 min, then keep at 20°C–25°C for ∼10 min and determine the volume of virus that will be used to transduce the canine T cells (VT)Note: We have observed that compared to unconcentrated supernatants, RetroNectin concentrated RD114-pseudotyped virus is associated with higher transduction efficiencies of K562 cells, which result in greater functional titers when calculating the TU/mL. On this basis, we empirically correct the TU/mL values, as determined in step 6, by a factor 10, and load RetroNectin-coated wells with the required volume to achieve MOIs of 2–10 following the equation VT (mL) = N × MOI / (10 × TU/mL). N: number of cells per well (1.5 ×106/well in a 6-well plate; 3 ×105/well in a 24-well plate). MOI: number of times that a cell could be theoretically infected (2–10).
-
e.Completely remove the BSA solution and wash the plate once with at least the same volume of sterile 25mM Hepes/PBS solution (2.5% v/v).
 CRITICAL: Residual BSA may result in lower cell viability.
CRITICAL: Residual BSA may result in lower cell viability. -
f.Remove wash solution. Immediately, add the required volume of virus as determined in step 28d plus serum-free IMDM to achieve 3 mL/well if using a 6-well plate, or 1 mL if using a 24-well plate. As control, add only serum-free IMDM to 1 well for side-by-side expansion of untransduced (UTD) T cells.
 CRITICAL: Do not let the wells dry. Remove wash solution immediately before adding virus. If virus is not completely thawed, place the vial in 37°C waterbath for a few seconds. Avoid repeated freeze-thaw cycles as this reduces virus titers.
CRITICAL: Do not let the wells dry. Remove wash solution immediately before adding virus. If virus is not completely thawed, place the vial in 37°C waterbath for a few seconds. Avoid repeated freeze-thaw cycles as this reduces virus titers. -
g.Centrifuge at 2,000 × g for 2 h at 32°C to facilitate binding of virus particles with RetroNectin reagent.
-
h.During the last ∼15 min of centrifugation, harvest the T cells. Gently pipette up and down to disperse clusters and generate a single cell suspension, as this maximizes the number of cells transduced. Dilute to 6 × 105cells/mL with TCM supplemented with IL-2 at 100 IU/mL.
-
i.Discard the supernatant from the RetroNectin plate, but do not allow the plate to dry. Gently and without touching the well bottom with the tip, wash the plate with at least the same volume of 1× PBS.
-
j.Add 2.5 mL cells/well for a total of 1.5 × 106 cells/well in a 6-well plate or 500 μL/well for a total of 3 × 105 cells/well in a 24-well plate.
-
k.Incubate at 38.8°C for 36–48h.
-
a.
-
29.
On day +2, add an equal volume of fresh media supplemented with IL-2 at 100–200 IU/mL (final concentration 50–100 IU/mL).
Note: While cytokines may have a positive impact on cell viability and expandability, extra cytokine supplementation may promote competitive proliferation of residual non-transduced cells.
Table 2.
Seeding Volumes and Numbers for thawed T cells
| Culture plate | Surface area | Volume | Cell number / well |
|---|---|---|---|
| 48-well | 1.1 cm2 | 0.55–0.6 mL | 2.4 × 106 |
| 24-well | 1.9 cm2 | 1.0–1.2 mL | 4.0 × 106 |
| 12-well | 3.5 cm2 | 2.1–2.2 mL | 8.4 × 106 |
| 6-well | 9.6 cm2 | 5.0–5.1 mL | 20.0 × 106 |
Figure 5.
Canine T cell activation using canine-specific anti-CD3/CD28 dynabeads
(A) Activation failure: T cells are uniformly rounded and do not clump.
(B) Successful activation: most T cells appear enlarged with spindle-shaped morphology. Cells converge toward each other forming clusters. Scale bars indicate 50 μm.
Day +4: assessment of transduction efficiency
Timing: 4 hours
On day +4, we assess CAR T transduction efficiency by flow cytometry. Prior to flow cytometric analysis, activating beads and bead-bound cells are removed on a magnet separator. Identity and purity of CAR cells are then evaluated by multiparametric flow cytometry using a CAR and T cell specific panel. Our anti-canine CD20 CAR harbors a murine scFv that can be detected via polyclonal anti-mouse immunoglobulin reagents. Different scFv may require different detection reagents. Assessment of co-engineered ‘marker genes’ can also be used as surrogate of CAR expression. The flow cytometry panel and labeling procedure will need to be optimized accordingly.
Bead removal: 1 h
Flow cytometry assessment: 3 h
-
30.
Recover cells from RetroNectin coated wells by gently pipetting up and down several times. Transfer and pool cells into a suitable 15 or 50 mL conical tube. Remove an aliquot for counting and viability check. Ideally, cell density should be >1 ×106/mL prior to debeading.
-
31.
Note down the cell number and label a new flask or plate, that will receive the unbound cell fraction. Place the tube in appropriate DynaMag magnet for 2 min to allow the beads to migrate to the side.
-
32.
With a suitable serological pipette, carefully remove cell suspension from the middle of the tube and transfer to the new, labeled culture vessel.
-
33.
Rinse well(s) with the same volume of TCM to recover any residual cells. Remove tube from magnet and resuspend beads with the media used to wash the well(s). Repeat steps 31–32 and combine the supernatant with the cells already transferred into the new vessel.
-
34.
Gently resuspend cells by pipetting up and down several times and adjust concentration at 1 × 106 cells/mL in warm TCM supplemented with IL-2 to achieve a final concentration of 50–100 IU IL-2/mL.
-
35.
Aliquot 1–2 × 105 cells from both CAR transduced and UTD vessels into 5 mL round-bottom flow cytometry tubes. Collect cells in the tube bottom by spinning down at 500 × g for 5 min. Decant supernatants and treat cells with 10 μL of canine gamma-globulins for 10 min at 20°C–25°C. This will help reduce non-specific binding of mAb.
-
36.
Proceed with CAR labeling as required (see representative CAR labeling procedure for our anti-canine CD20 CAR in Figure 6). Additional mAb can be included, such as anti-canine CD3, CD5, CD4 and CD8a mAb, to characterize canine T cell phenotype, or to detect marker genes co-expressed with the CAR. Viability can be assessed with 7AAD viability dye.
-
37.
Acquire ≥ 50,000 events on a flow cytometer to determine transduction efficiency (see troubleshooting, problem 4 for details on how to improve transduction efficiencies).
Figure 6.
Assessment of anti-canine CD20 CAR expression and transduction efficiency
(A) Reagents used to label UTD and CAR cells. UTD and CAR cells were treated with 10 μL of dog gamma-globulins in ∼ 100 μL for 10 min at 20°C–25°C, followed by incubation with 0.5 μL (0.5μg) of Biotinylated Rabbit anti-mouse (RaM) IgG H+L for 30 min at 20°C–25°C (first step). At the end of incubation, the unbound immunoglobulins were removed by adding 4 mL FACS buffer, followed by 5-min centrifugation at 500 ×g and complete removal of the supernatant by decanting the supernatant and gently touching the rim of the tubes to tissue paper, for a total of two washes. T cells were incubated with anti-dog CD3, CD5, CD4 and CD8 mAb and Streptavidin for 20–30 min at 2°C–8°C (second step). At the end of incubation, cells were washed once as above and resuspended in 300 μL FACS buffer with 2 μL 7AAD prior to data acquisition by flow cytometer.
(B) Representative plots showing CAR transduction efficiency relative to UTD cells.
Canine CAR T cell expansion and final assessment
Timing: 5–8 days
-
38.
Maintain CAR T cells at 38.8°C at ∼ 1 × 106 cells/mL in TCM supplemented with IL-2 to a final concentration of 50–100 IU/mL.
Note: While a greater fold-expansion of total cells may be achieved by using higher doses of cytokines, lower concentrations will prevent the relative expansion of residual non-transduced cells co-existing in the CAR cell vessels.
-
39.
Count and adjust cell concentration every other day. Scale up aiming for 0.53–1.2 × 106 cells / cm2 in T25 flasks (up to 8–10 mL, flat) and T75 flasks (up to 25–30 mL, flat) (see troubleshooting, problem 5 for useful tips for expansion of CAR T cells) .
-
40.
9–12 days after transduction, repeat flow cytometry assessment to confirm CAR expression and CAR T cell purity as on day +4.
-
41.
End-of-expansion CAR T cells can be used for functional in vitro assays, such as flow cytometry-based cytotoxicity assays (see Figure 9 for further details on flow cytometry based cytotoxicity assays). Alternatively, cells can be cryopreserved for later analysis and use as described in step 24 (see troubleshooting, problem 6 for tips on how to preserve functionality of cryopreserved CAR T cell products).
Note: End-of-expansion CAR T cells can be infused in dogs to investigate efficacy and/or safety of canine CAR T cell products in vivo in the clinically relevant setting of immunocompetent pet dogs with spontaneous cancers (see below Ancillary considerations for the use of canine CAR T cells in pet dogs: Dose and QC tests).
Figure 9.
In vitro assessment of canine CAR T cell specific cytotoxic activity
(A) anti-CD20 CAR T cell cytotoxic activity was assessed relative to parent UTD control cells using a flow cytometry-based cytotoxicity assay (Rotolo et al., 2018), according to the following method. In a U bottom 96-well plate, canine CAR T cells or control UTD (effectors, E) were co-cultured for 3.5 h at the indicated E/T ratios with 10,000 cell-trace violet (CTV)-labeled CLBL cells, a CD20+ canine lymphoma cell line (targets [T]). Targets alone were plated as reference control for spontaneous lysis. At the end of co-culture, 7AAD was added to each well and the proportion of dead targets was determined by flow cytometry. Spontaneous lysis of control targets alone was defined as percentage of CTV+, 7AAD+ events. Anti-CD20 CAR T specific cytotoxic activity was determined based on the percentage of dead targets in the co-culture wells relative to wells with targets alone, using the following equation: (% dead targets in co-culture wells - % spontaneous lysis in target-alone wells) / (100 – % spontaneous lysis in target-alone wells). Each point represents mean ± SEM of technical triplicates from one representative experiment.
(B) anti-canine CAR T cell specificity was assessed as detailed in A against GL-1 canine lymphoma cells, that do or do not express the CD20 CAR target. Data are represented as mean ± SEM of technical triplicates from one representative experiment.
Ancillary considerations for the use of canine CAR T cells in pet dogs: Dose and QC tests
The use of autologous, genetically re-directed T cells has shown unprecedented success in the treatment of hematological malignancies. However, further implementation is needed to improve their efficacy particularly in solid cancers. Pet dogs share a close phylogenetic relationship with man and spontaneously develop tumors with similar clinical, immunological, biological, and genetic features as their human counterparts. Dogs and humans share the same environment that may influence tumor development and progression. Further, the size, longevity and physiology of the canine immune system resemble those of man. Lastly, comparable barriers to effective immunotherapy exist between dogs and humans. As many canine cancer patients are treated with the same therapies as humans, dogs are being increasingly recognized as a much needed, clinically relevant, parallel patient population in which to evaluate next generation cell-based immunotherapeutic strategies and to identify correlative biomarkers of response.
The Beau Biden National Cancer Moonshot Initiative provides funding mechanisms to support a network for canine cancer immunotherapy clinical trials (PRECINCT) aimed at catalyzing research productivity within a collaborative network and accelerating human translation through comparative oncology (www.precinctnetwork.org). This, together with a standardized protocol for efficient transduction and expansion of canine CAR T cell, can greatly facilitate the use of the dog “model” by human and comparative oncology researchers, and promote the performance of multicenter clinical trials, with data harmonization across institutions.
The protocol described here enables effective engineering of primary canine T cells and their expansion up to clinical scale (see expected results). Our recommended target cell dose for infusion in canine patients is 0.2 to 5.0 × 106 CAR positive T cells/kg body weight. In addition, a series of quality control (QC) tests are recommended on end-of-expansion CAR T cell products. Table 3 summarizes a list of potential QC tests.
Table 3.
Proposed QC tests and cutoffs for end-of-expansion, clinical canine CAR T cellular products
| Assessment | Method | Cutoff |
|---|---|---|
| Identity | ||
|
Flow cytometrya | Positive |
| Purity | ||
|
Flow cytometryb | > 3% |
|
Trypan Blue, flow cytometryb | > 90% viable |
|
Flow cytometryb | None |
|
Microscopy | None |
| Safety | ||
|
Gram stainingc | Negative |
|
MycoAlert (biochemical) testd | Negative |
|
RD114 qPCRe | Decreased to undetectable |
| Potency | ||
|
ELISAf | Positive |
CAR expression could be also confirmed by PCR.
The same flow cytometry panel used for day-4 and end-of-expansion assessment may be sufficient to determine the proportion of CAR+ cells, T cells and viable cells.
Gram staining can be performed in-house using commercially available kits, e.g., Remel™ Gram Staining Kit, Thermo Scientific™, cat# R40080, or at commercial microbiology laboratories, i.e., the microbiology laboratory at the University of Pennsylvania’s School of Veterinary Medicine.
Mycoplasma contamination can be quickly detected using a biochemical, luciferase-based assay that selectively detects the activity of mycoplasmal enzymes, e.g., MycoAlert Mycoplasma Detection Kit (Lonza).
Strategies to monitor for the emergence of Replication-Competent Retrovirus (RCR), including RD114 pseudotyped retrovirus in canines, have been previously described (Narushima et al., 2011). These may entail qPCR assays and should be performed on CAR T cell samples during manufacturing and after infusion on patient samples obtained at defined time points.
To assess IFNy release, we use the Canine IFN-gamma DuoSet ELISA kit, RnDSystems Cat# DY781B, according to the manufacture’s recommendations Canine-IFN-gamma-Duoset-ELISA. For an example of assay setup, please refer to (Panjwani et al., 2020).
Expected outcomes
We observed a greater proliferation when culturing canine T cells that were subjected to freezing at 38.8°C (range 38.3°C–39.2°C), which is the canine physiological body temperature (24-fold change at 38.8°C vs 11-fold change at 37°C over 5 days, n=7, P=0.0156, Figure 7A). This is consistent with observations reported by others in T cells that were cryopreserved (Szopa et al., 2021). Further, cells activated at 38.8°C showed higher transduction efficiencies than their paired controls at 37°C upon retroviral infection (66% vs 50%, Figure 7B), which is known to be more effective in highly proliferative cells. On this basis, we routinely activate canine T cells at 38.8°C, achieving in excess of 70% transduction efficiency in primary canine T cells (mean 71.53% ±4.233, range 57.0–84.9, Figure 7C).
Figure 7.
Effect of optimal temperature on primary canine T cell proliferation and transduction
(A) 5-day fold-change of bead stimulated canine T cells kept at 37˚ or 38.8°C.
(B) TE upon 48h activation at 37°C or 38.8°C (37°C: 49.88 ± 1.069, range 47.40%–51.90% vs 38.8°C: 65.75% ± 2.366, range 60.40%–70.90%).
(C) Representative transduction efficiencies of T cells from healthy dogs transduced with RD114 pseudotyped retrovirus. Bars show mean ± SEM. ∗ indicates p<0.05 as determined based on non-parametric, Wilcoxon matched pairs signed rank test.
We anticipate 4 to 6 doublings at the end-of expansion, although anticipate donor-to-donor variability (see Figures 8B and 8C and Limitations).
Figure 8.
Canine CAR T cell expandability
(A) Representative flow cytometry plots of CAR expression in end-of-expansion CAR transduced T cells relative to UTD control cells.
(B) Doublings of CAR T and UTD cell populations shown in (A). Bars show mean ± SEM of technical triplicates.
(C) Doublings of CAR T cell populations from 6 independent experiments using cryopreserved T cells isolated from healthy dogs. Mean 5.41, range 4.84–6.57.
(D) Total T cell absolute numbers. From 1.8 ×106 cells, total T cells reached 8.68×107 cells at the end of expansion (range 5.17 ×107-1.71×108).
(E) With a mean TE 71.53% (shown in Figure 7C), the mean CAR+ cell number was 6.15 ×107 in end-of-expansion CAR T products (range 2.96 ×107 - 1.06 ×108). Same symbols correspond to same donor/experiment cells.
In vitro functional assessment of canine CAR T cells generated and expanded using our implemented protocol confirmed that they are functional and have the potential to specifically kill canine targets expressing the CAR antigen compared to parent, unmodified T cells (Figure 9).
Limitations
Our protocol enables transduction of primary canine T cells isolated from healthy donor as well as canine cancer patients. However, transduction efficiencies and T cell expansion may vary among donors. This has been also described for primary human T cells and has been attributed to inter- and intra-donor T cells functional heterogeneity, i.e., (1) different expression of receptors that determine susceptibility to virus infection and (2) different proliferative potential in distinct T cell subsets, which may also exhibit variable frequency across individuals (Milone and O’Doherty 2018; Noaks et al., 2021). Furthermore, transduction efficiencies and T cell expansion may be lower in patient derived T cells as active disease and/or recent/multiple lines of chemotherapy may affect their proliferative capacity (Fraietta et al., 2018), a key determinant of successful infection by retroviruses.
The viral production workflow described in this protocol has been optimized for manufacturing of anti-CD20 CAR retrovirus using MSGV1 transfer vector in combination with our Gag/Pol and RD114 plasmids. Different plasmids may require some optimization to achieve similar TE.
While primary canine T cells grow best at 38.8°C, not all research environments may have the opportunity to dedicate an incubator to canine cell culture. Primary canine T cells can be cultured at 37°C, although their infectability and proliferation are reduced.
Troubleshooting
Problem 1
Retroviral titers are low (step 6f).
Potential solution 1
Poor HEK 293T health/maintenance and plasmid quality might be the cause. Healthy HEK 293T cells are the first determinant of successful and efficient virus production. Cells should be passaged when they are 70–80% confluent and split down to 10–15%, e.g., 1:6 - 1:8. Using this approach, cells require regular passaging every other day or every 2 days. Overgrowth prior to passaging can compromise their subsequent transfection efficiency. In this case, consider discarding and thawing a fresh vial.
Further tips that may help improve titers include using low-passage HEK 293T (e.g., subcultured <25 times from the first use, including previous freeze-thaw cycles), passaging HEK 293T 3 times after thawing before transfection and handling ≤ 3 dishes at a time, as HEK293T are particularly sensitive to the levels of CO2 in the atmosphere and pH changes. Last, check that plasmid preparations are endotoxin-free, and DNA is mostly supercoiled. Of note, low titers based on infection of K562 cells with RD114 pseudotyped viral supernatants may still enable effective transduction of activated canine T cells upon concentration on RetroNectin. The virus binding step 28f-g can be repeated if titers are very low and higher volumes of supernatant are required.
Problem 2
Activation of fresh cells with anti-CD3/anti-CD28 coated beads is poor (step 27).
Potential solution 2
The activating beads might be insufficient or nonfunctional. Check the concentration of the beads in the plate and in the vial to exclude that the bead-to-cell ratio is lower than 3-to-1 and that the original concentration is lower than 4 ×107/mL. Inaccurate handling, such as poor resuspension before removing a bead aliquot from the vial, may alter the bead concentration. Nonfunctional beads may be due to poor manufacturing and/or storage. Confirm that the amounts of anti-CD3 and anti-CD28 used at the time of coating was correct. Buffers should always be fresh, as lower pH and/or poor BSA blocking may also reduce the efficiency of the coating. A trial test could be performed using increasing bead-to-cell ratios. If higher bead numbers do not lead to greater T cell activation and higher T cell counts, the beads might be nonfunctional, and a new batch should be manufactured.
Problem 3
Activation of cryopreserved cells with anti-CD3/anti-CD28 coated beads is poor (step 27).
Potential solution 3
It is vital to cryopreserve and thaw cells correctly to preserve the viability. A higher seeding density, i.e., 4–5 ×106 cells / mL and a few hours rest (4–12h) in the presence of cytokines and without beads, may help cells recover faster and more completely. As cells may continue to die during the first hours after thawing and a variable number of cells may also be lost after washing, it is important to confirm cell number by counting immediately after thawing and before addition of the beads.
Problem 4
T cells are well activated, and virus titers are adequate, but T cell transduction efficiency is low (step 37).
Potential solution 4
Check the RetroNectin coating and virus binding steps. The ability of RetroNectin to concentrate virus may be affected by incorrect handling, e.g., filtering before use, too low dilutions (up to 1-in-10 can be used) or excess of freeze-thaw cycles. Further, different subjects exhibit different proliferative potential and/or susceptibility to infection, which leads to heterogeneous transduction efficiencies across individuals. Where possible, small-scale trial infections using different amounts of virus (e.g., 0.125 mL, 0.25 mL, 0.5 mL and 1 mL virus supernatant) may help choose the best volume of virus/MOIs for a specific T cell preparation.
Problem 5
UTD cells expand more than CAR T cells (step 39).
Potential solution 5
This could be relevant particularly in the case of low transduction efficiencies and could be minimized by reducing the quantity of cytokines and rapidly weaning them. If necessary, CAR T cells can be purified by FACS or MACS. For example, CAR T cells could be labeled as described in Figure 6 using Biotinylated anti-CAR antibody, followed by immune magnetic selection using Anti-Biotin MicroBeads as per manufacturer’s recommendations (Anti-Biotin MicroBeads). Alternatively, in the case of co-expressed marker genes, a biotinylated antibody specifically recognizing the marker gene could be used.
Problem 6
Functionality of cryopreserved CAR T cells is poor (step 41).
Potential solution 6
Cryopreservation and thawing techniques are critical to preserve canine CAR T viability and function. It may be beneficial to plate the cells at higher density than usually recommended for fresh cells (4–5 × 106 vs 1–2 × 106). Similarly, extra supplementation of IL-15 (10–30 ng/mL) could be employed to exploit the well-established, growth factor-like properties of IL-15.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, N.J. Mason (nmason@vet.upenn.edu).
Materials availability
This manuscript entails implementation and validation of a reproducible and highly effective protocol to CAR engineer canine T cells. No new unique reagents were generated. Requests pertaining to cell lines and other reagents used in this study should be directed to the lead contact N.J. Mason (nmason@vet.upenn.edu).
Acknowledgments
This work was supported through NIH/NCI U24-CA224122, the Parker Institute for Cancer Immunotherapy, the Richard Lichter Animal Charity, and the PETCO love Foundation. M.J.A. is supported through NIH/NCI K08CA252619. The Gag-Pol plasmid was kindly donated by Dr. Andrei Thomas-Tikhonenko (University of Pennsylvania), while the RD114 plasmid was a kind gift of Dr. Daniel J. Powell, Jr. (University of Pennsylvania).
Author contributions
Conceptualization, A.R. and N.J.M.; methodology, A.R., M.J.A., K.P.H., B.T.K., and N.J.M.; validation, A.R., K.P.H., and B.K.; formal analysis, A.R., K.P.H., and B.T.K.; investigation, A.R., M.J.A., K.P.H., and B.T.K.; data curation, A.R., K.P.H., B.T.K., and N.J.M; writing – original draft, review & editing, A.R., M.J.A., B.T.K., and N.J.M.; visualization, A.R. and M.J.A; supervision and funding acquisition N.J.M.
Declaration of interests
N.J.M. is a co-founder of Vetigenics LLC. All authors declare no competing interests.
Contributor Information
Antonia Rotolo, Email: antonia.rotolo@pennmedicine.upenn.edu.
Nicola J. Mason, Email: nmason@vet.upenn.edu.
Data and code availability
This study did not generate data sets code.
References
- Fraietta J.A., Lacey S.F., Orlando E.J., Pruteanu-Malinici I., Gohil M., Lundh S., Boesteanu A.C., Wang Y., O'Connor R.S., Hwang W.T. Determinants of response and resistance to cd19 chimeric antigen receptor (car) t cell therapy of chronic lymphocytic leukemia. Nat. Med. 2018;24:563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giry-Laterrière M., Verhoeyen E., Salmon P. Lentiviral vectors. Methods Mol. Biol. 2011;737:183–209. doi: 10.1007/978-1-61779-095-9_8. [DOI] [PubMed] [Google Scholar]
- Milone M.C., O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narushima R., Shimazaki T., Takahashi T. Development of a real-time reverse-transcription-pcr method for detection of rd114 virus in canine vaccines. Biologicals. 2011;39:89–93. doi: 10.1016/j.biologicals.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noaks E., Peticone C., Kotsopoulou E., Bracewell D.G. Enriching leukapheresis improves t cell activation and transduction efficiency during car t processing. Mol. Ther. 2021;20:675–687. doi: 10.1016/j.omtm.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panjwani M.K., Atherton M.J., MaloneyHuss M.A., Haran K.P., Xiong A., Gupta M., Kulikovsaya I., Lacey S.F., Mason N.J. Establishing a model system for evaluating car t cell therapy using dogs with spontaneous diffuse large b cell lymphoma. OncoImmunology. 2020;9:1676615. doi: 10.1080/2162402X.2019.1676615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotolo A., Caputo V.S., Holubova M., Baxan N., Dubois O., Chaudhry M.S., Xiao X., Goudevenou K., Pitcher D.S., Petevi K. Enhanced anti-lymphoma activity of car19-inkt cells underpinned by dual cd19 and cd1d targeting. Cancer Cell. 2018;34:596–610 e511. doi: 10.1016/j.ccell.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai O., Igase M., Mizuno T. Optimization of canine cd20 chimeric antigen receptor t cell manufacturing and in vitro cytotoxic activity against b-cell lymphoma. Vet. Comp. Oncol. 2020;18:739–752. doi: 10.1111/vco.12602. [DOI] [PubMed] [Google Scholar]
- Szopa I.M., Granica M., Bujak J.K., Łabędź A., Błaszczyk M., Paulos C.M., Majchrzak-Kuligowska K. Effective activation and expansion of canine lymphocytes using a novel nano-sized magnetic beads approach. Front. Immunol. 2021;12:604066. doi: 10.3389/fimmu.2021.604066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate data sets code.