Abstract
The habenula is a small epithalamic structure with widespread connections to multiple cortical, subcortical and brainstem regions. It has been identified as the central structure modulating the reward value of social interactions, behavioral adaptation, sensory integration and circadian rhythm. Autism spectrum disorder (ASD) is characterized by social communication deficits, restricted interests, repetitive behaviors, and is frequently associated with altered sensory perception and mood and sleep disorders. The habenula is implicated in all these behaviors and results of preclinical studies suggest a possible involvement of the habenula in the pathophysiology of this disorder. Using anatomical magnetic resonance imaging and automated segmentation we show that the habenula is significantly enlarged in ASD subjects compared to controls across the entire age range studied (6–30 years). No differences were observed between sexes. Furthermore, support-vector machine modeling classified ASD with 85% accuracy (model using habenula volume, age and sex) and 64% accuracy in cross validation. The Social Responsiveness Scale (SRS) significantly differed between groups, however, it was not related to individual habenula volume. The present study is the first to provide evidence in human subjects of an involvement of the habenula in the pathophysiology of ASD.
Subject terms: Autism spectrum disorders, Brain, Social behaviour
Introduction
The habenula is a small phylogenetically preserved epithalamic structure that plays a key role in the control of the monoaminergic system1,2. It is divided into lateral and medial parts based on characteristic cytoarchitectonic and connectivity patterns. Through its rich widespread connections, especially to the hypothalamus, limbic areas and brainstem nuclei (illustrated in Fig. 1) the habenula is implicated, among others, in social interaction, reward processing, behavioral adaptation, sensory integration and circadian rhythm (Fig. 1)1–8.
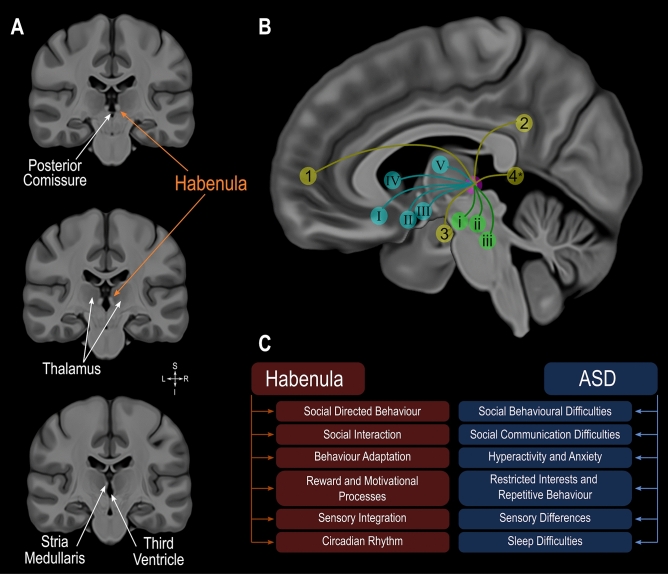
Figure 1.
Habenula anatomy, boundaries and connections displayed using a high-resolution, high contrast template by Neudorfer and colleagues50. (A) Coronal slices illustrating the location of the Habenula, a structure appearing bright (hyperintense) on T1 weighted magnetic resonance images, surrounding structures and its boundaries. (B) Diagram illustrating the connectivity of the habenula. Cortical regions in yellow: (1) medial prefrontal cortex; (2) cingulate gyrus; (3) hippocampus and parahippocampal gyrus; (4) posterior insula (*estimated location). Subcortical regions in blue: (I) basal forebrain; (II) hypothalamus; (III) nucleus basalis of Meynert; IV, basal ganglia; V, thalamus. Brainstem regions in green: (i) ventral tegmental area; (ii) substantia nigra; (iii) periaqueductal grey—raphe nuclei. (C) Functions that the Habenula is critically involved in and differences found in autism spectrum disorder. ASD: autism spectrum disorder.
Core Autism Spectrum Disorder (ASD) symptoms are social communication difficulties, restricted interests, repetitive behaviors. Furthermore, altered sensory perception as well as mood and sleep disorders are frequently identified in ASD subjects (Fig. 1)9,10. ASD is a neurodevelopmental disorder that is 3 to 4 times more frequently diagnosed in males than females11,12. Affected individuals usually show symptoms of the disorder as early as 12 to 18 months of age9,13–15 and multiple differences in functional and morphological brain phenotype have been reported in ASD15–23. In particular studies demonstrate that brain development is altered in individuals with ASD as exemplified by deviations from normal developmental trajectories in regional cortical thickness, surface area and structure volume16–18,23–25. Some of these brain changes are related to individual characteristics such as symptom severity and sex. Specific differences associated with ASD have been found in key areas of the brain that underlie processing of social cues, areas critically involved in understanding others such as the posterior superior temporal sulcus area, fusiform gyrus and amygdala26–30.
The habenula is prominently involved in the processing of social information and the regulation of social behavior5,8. It is, furthermore, a key region for reward processing and critically involved in a number of depressive behaviours1,6,31,32. In addition, studies have implicated the habenula in bipolar disorder (BD)33–35, obsessive–compulsive disorder (OCD)36,37 as well as schizophrenia38,39 and altered habenula volume has been reported in subjects diagnosed with BD and schizophrenia40,41. Previous studies have reported altered amygdala volume in subjects with ASD compared to age matched controls25,42. While subsequent research showed these volume differences might only be apparent at certain ages, these point to an altered developmental trajectory of this important social brain area in ASD21,23,25,42,43. So while neuroimaging and brain stimulation techniques have provided some insight into the role of the habenula in psychiatric disorders further research is warranted to obtain a deeper understanding of the neurocircuitry of social behaviour and relate it to the differences found in human disorders6,40,41,44–49. Similar to the amygdala findings, altered habenula volume across development might be found in subjects with ASD compared to age matched controls like it has been described in BD41.
Several preclinical studies have investigated the relationship between habenula and autism phenotypes by exploring behaviour, genetics, electrophysiology and functional neuroanatomy of wild-type and transgenic animals. A transcriptomic-anatomic analysis of the rodent habenula revealed a large collection of enriched genes associated with autism-related transcripts51. Electrophysiological recordings of habenular neurons detected transient and high frequency T-type Ca2+ channel-mediated firing, a channel implicated in ASD52, altered ion channel function, abnormal firing pattern and hypo-excitability53. The inhibition of the lateral habenula in juvenile and adult rats by microinjection of GABA-A and GABA-B receptor agonists markedly reduces social play behaviour (juvenile rats) and behavioural flexibility (adult rats), suggesting a critical role of the habenula in processing social information and selecting behavioural actions under challenging cognitive or emotional situations, differences also seen in ASD8,54. Interestingly, reduction in oxytocin innervation in the lateral habenula, a neuropeptide closely involved in social bonding, is thought to be the underlying mechanism of social impairment in the Mecp2-null mouse model of Rett syndrome55.
In line with these findings, a study using excitatory designer receptor exclusively activated by designer drugs (DREADD) showed that frontal cortex activation suppressed social behaviour via activation of lateral habenula neurons; inhibition of these neurons prevented the social behavioural deficits observed after frontal cortex activation5. Integrity of the fiber tracts connecting the habenula to the midbrain tegmentum were also described as critical for social behaviour as observed in double Tg mice designed to express alterations in neural crest-derived cells56. Furthermore, altered temporal patterns in the mesolimbic/habenular reward circuit have been described in the fmr1 knockout rat model of Fragile X syndrome and associated with the abnormal behavioural response in odor-investigation paradigms57.
Thus, an involvement of the habenula in the neurobiology of ASD is plausible but has yet to be demonstrated in humans. Here, we investigated the hypothesis that the habenula plays a role in ASD by analyzing morphometric habenula characteristics in a large cohort of ASD subjects (220 subjects; 184 males) and age matched typically developing controls (TDC; 303 subjects; 213 males) from the Autism Brain Imaging Data Exchange (ABIDE) repository58,59, with an age range spanning from early childhood to adulthood (6 to 30 years; Table 1). A complete Social Responsiveness Scale (SRS)60 score was available for all subjects, ASD and TDC. The SRS is a rating scale filled out by a next of kin or caregiver to quantitatively measure autistic traits and is a widely used reliable as a screening tool for children, adolescents and adults61–63. Possible effects of sex and individual SRS score on habenula volume were investigated.
Table 1.
Demographics.
| Site | n | ASD | TDC | Average total SRS | Average full IQ | Average total brain volume (mm3) | Average bilateral habenula volume (mm3) |
|---|---|---|---|---|---|---|---|
| n (M/F) | n (M/F) | ||||||
| Age mean ± SD (range) | Age mean ± SD (range) | ||||||
| University of Leuven | 52 | 23 (21/2) | 29 (25/4) | ASD: 82.522 ± 30.133 | ASD: 114.095 ± 12.429 | ASD: 3,035,573.715 ± 294,170.657 | ASD: 26.245 ± 4.944 |
| 17.79 y ± 4.34 y (12–29 y) | 18.32 y ± 5.11 y (12–29 y) | TDC: 30.345 ± 22.946 | TDC: 112.957 ± 12.323 | TDC: 3,051,012.325 ± 283,960.252 | TDC: 26.227 ± 4.834 | ||
| New York University | 133 | 65 (59/6) | 68 (55/13) | ASD: 91.831 ± 27.939 | ASD: 106.769 ± 18.068 | ASD: 3,000,324.962 ± 390,755.495 | ASD: 27.930 ± 5.901 |
| 14.13 y ± 6.32 y (6–30 y) | 11.37 y ± 3.04 y (6–18 y) | TDC: 22.696 ± 13.036 | TDC: 113.652 ± 15.394 | TDC: 2,955,228.120 ± 261,652.335 | TDC: 26.619 ± 5.759 | ||
| University of Utah School of Medicine | 52 | 27 (27/0) | 25 (25/0) | ASD: 84.148 ± 32.884 | ASD: 100.852 ± 14.114 | ASD: 3,060,374.104 ± 285,066.478 | ASD: 27.922 ± 5.279 |
| 20.48 y ± 3.98 y (14–29 y) | 20.75 y ± 5.25 y (10–30 y) | TDC: 16.160 y ± 12.733 | TDC: 114.280 ± 14.513 | TDC: 3,172,042.086 y ± 344,710.406 | TDC: 25.596 y ± 6.764 | ||
| Yale Child Study Center | 24 | 12 (8/4) | 12 (7/5) | ASD: 95.500 ± 29.998 | ASD: 94.750 ± 19.377 | ASD: 2,902,912.449 ± 310,118.915 | ASD: 27.083 ± 4.144 |
| 13.12 y ± 3.28 y (7–18 y) | 14.75 y ± 1.91 y (11–18 y) | TDC: 27.833 ± 24.439 | TDC: 98.667 ± 13.780 | TDC: 2,912,640.938 ± 298,063.917 | TDC: 26.333 ± 4.355 | ||
| George Town University | 21 | 7 (6/1) | 14 (9/5) | ASD: 85.571 ± 42.308 | ASD: 114.857 ± 9.737 | ASD: 2,925,413.917 ± 161,283.395 | ASD: 29.857 ± 5.113 |
| 11.55 y ± 0.74 y (10–13 y) | 10.95 y ± 1.83 y (8–14 y) | TDC: 19.571 ± 19.575 | TDC: 117.214 ± 13.227 | TDC: 2,904,958.613 ± 336,317.471 | TDC: 21.071 ± 3.772 | ||
| Indiana University | 16 | 10 (9/1) | 6 (4/2) | ASD: 88.700 ± 38.006 | ASD: 114.000 ± 16.083 | ASD: 3,217,222.049 ± 279,441.641 | ASD: 27.817 ± 5.060 |
| 21.80 y ± 4.02 y (17–28 y) | 22.17 y ± 2.32 y (20–25 y) | TDC: 48.500 ± 12.373 | TDC: 114.500 ± 5.541 | TDC: 3,106,244.547 ± 253,565.846 | TDC: 23.896 ± 2.144 | ||
| Kennedy Krieger Institute | 107 | 18 (9/9) | 89 (52/37) | ASD: 95.222 ± 25.915 | ASD: 108.722 ± 13.702 | ASD: 2,896,036.193 ± 293,025.042 | ASD: 25.782 ± 4.218 |
| 10.75 y ± 1.83 y (8–13 y) | 10.53 y ± 1.31 y (9–13 y) | TDC: 16.531 ± 10.583 | TDC: 119.313 ± 10.322 | TDC: 2,823,588.809 ± 266,549.718 | TDC: 25.806 ± 3.906 | ||
| Oregon Health and Science University | 65 | 27 (22/5) | 38 (18/20) | ASD: 91.778 ± 26.536 | ASD: 106.111 ± 17.190 | ASD: 3,101,614.595 ± 389,037.345 | ASD: 25.652 ± 5.700 |
| 12.07 y ± 2.02 y (8–15 y) | 10.34 y ± 1.65 y (8–14 y) | TDC: 24.474 ± 17.573 | TDC: 116.816 ± 11.613 | TDC: 2,980,955.747 ± 300,032.667 | TDC: 26.087 ± 3.959 | ||
| San Diego State University | 37 | 21 (15/6) | 16 (14/2) | ASD: 100.571 ± 23.756 | ASD: 97.429 ± 14.678 | ASD: 3,000,965.011 ± 291,295.616 | ASD: 27.143 ± 6.142 |
| 13.71 y ± 3.06 y (8–18 y) | 14.15 y ± 2.79 y (10–18 y) | TDC: 17.938 ± 10.951 | TDC: 103.313 ± 9.286 | TDC: 3,046,140.478 ± 270,841.463 | TDC: 25.250 ± 4.553 | ||
| University of California Davis | 16 | 10 (8/2) | 6 (4/2) | ASD: 75.800 ± 34.428 | ASD: 104.800 ± 11.243 | ASD: 3,308,879.166 ± 373,647.297 | ASD: 28.600 ± 6.569 |
| 15.23 y ± 2.05 y (12–18 y) | 15.88 y ± 1.14 y (14–17 y) | TDC: 10.667 ± 6.593 | TDC: 114.167 ± 12.416 | TDC: 114.167 ± 12.416 | TDC: 28.500 ± 6.058 | ||
| Total | 523 | 220 (184/36) | 303 (213/90) | ASD: 90.150 ± 29.551 | ASD: 105.316 ± 16.464 | ASD: 3,031,526.311 ± 344,653.350 | ASD: 27.263 ± 5.490 |
| 14.99 y ± 5.34 y (6–30 y) | 12.94 y ± 4.64 y (6–30 y) | TDC: 21.868 ± 16.026 | TDC: 113.652 ± 12.977 | TDC: 2,976,081.507 ± 302,749.991 | TDC: 25.657 ± 4.849 |
ASD autism spectrum disorder, TDC typically developing controls, IQ intelligence quotient, SRS social responsiveness scale.
This investigation was performed using the fully automated segmentation of the habenula tool in MAGeTbrain41. This tool has been shown to be reliable to evaluate habenula volume in large datasets that includes subjects with a wide age range spanning childhood to late adulthood and using distinct MRI acquisition parameters41. MAGeTbrain is a well established methodology that produces a segmentation for each subject using a multi-atlas voting procedure via image registration. Segmentations from each atlas are propagated to create a large number of candidate segmentations that are fused using majority vote, a process that reduces bias and averages registration errors while allowing for the neuroanatomical variability of the subjects to refine each individual subject’s final segmentation64–66.
Results
The morphometry (volume) of the left and right habenula of each subject was obtained through automatic segmentation (Fig. 2A)41,64. To ensure that the automated segmentation process can be reliably applied in autistic subjects, in particular focusing on the pediatric sample, the left and right habenula of 24 randomly selected brains (8 from the childhood sample (< 11 years); 8 from the adolescence sample (12–17 years); 8 from the young adults sample (18–25 years); 48 habenula altogether) were manually segmented. The Dice similarity coefficients (DSC) are: childhood sample: DSC left habenula 0.82, DSC right habenula 0.82 (range 0.73–0.88); adolescence sample: DSC left habenula 0.82, DSC right habenula 0.83 (range 0.70–0.91); young adult sample: DSC left habenula 0.81, DSC right habenula 0.80 (range 0.72–0.89) overall: DSC left habenula 0.82, DSC right habenula 0.82 (range 0.70–0.91). These results are similar to those reported in the previous validation41 and confirm that the habenula can be reliably segmented in this patient population using this method.
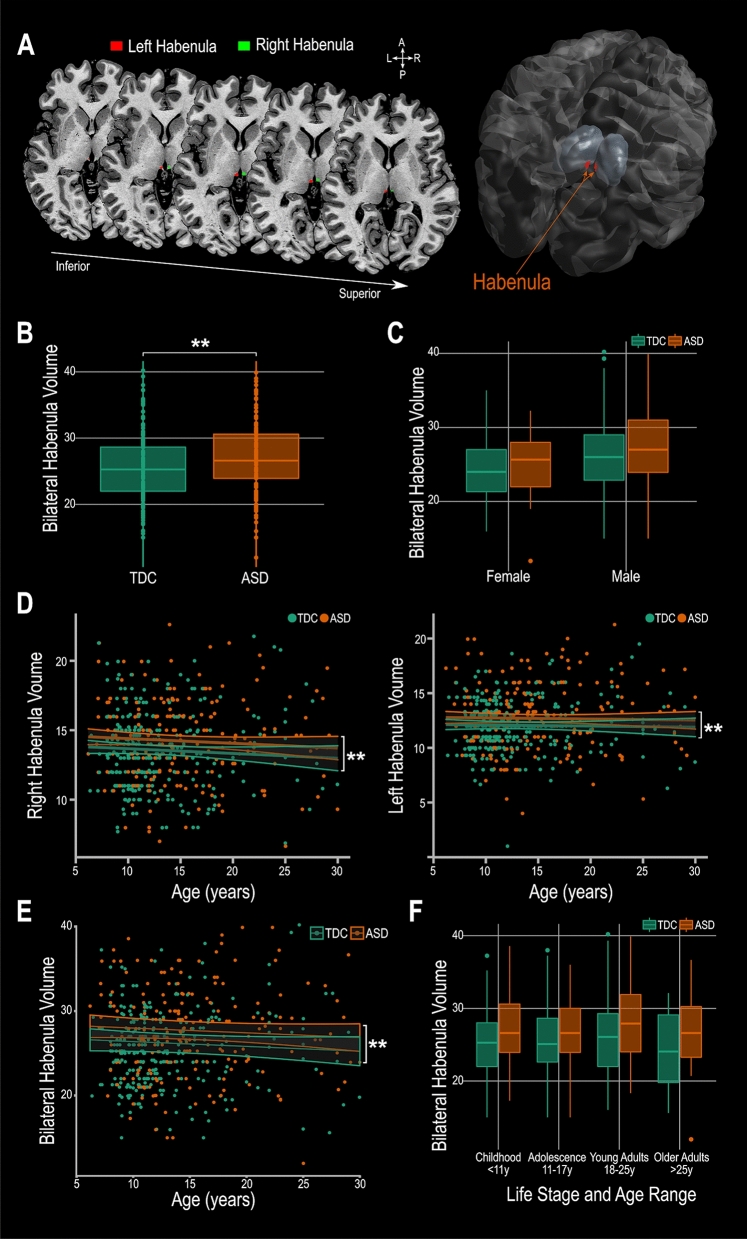
Figure 2.
Habenula volume differences found in ASD. (A) Example MAGeTBrain habenula segmentation on a T1 weighted magnetic resonance image illustrated on the axial plane and 3D reconstruction of the habenula. (B) Bilateral habenula volume is greater in ASD compared to TDC. (C) The group effect (bilateral habenula larger in ASD vs TDC) is independent of sex. (D) There is no effect or laterality; the habenula is larger in ASD compared to TDC in the right and in the left hemisphere. This effect is apparent across the entire age range tested (E) within all age groups (F). ** indicates p ≤ 0.01. ASD: autism spectrum disorder subjects; TDC: typically developing controls.
Comparing total bilateral habenula volume (co-varied with age, sex and total brain volume with the study site used as a random intercept effect) we found that ASD subjects have significantly larger bilateral habenula volumes compared to TDC (ASD subjects: 27.1 mm3 ± 5.3; TDC: 25.5 mm3 ± 4.5; t = 3.28, p = 0.001; Fig. 2B). Habenula volume did not differ between males and females (Fig. 2C). This significant volume difference is apparent in both the right (t = 2.89, p = 0.004) and left (t = 2.76, p = 0.006) habenula (Fig. 2D) and across the entire age range studied (6 to 30 years; Fig. 2E,F).
The SRS scores are significantly different between groups (t = 32.23, p < 2e−16) (Fig. 3A), as expected as it is used as a screening tool, individual SRS scores, however, are not related to individual habenula volumes beyond diagnosis (larger in ASD) (Fig. 3B). Thus individual autistic traits as quantified by the SRS were not found to be related to individual bilateral habenula volume within either group (ASD or TDC).
Figure 3.
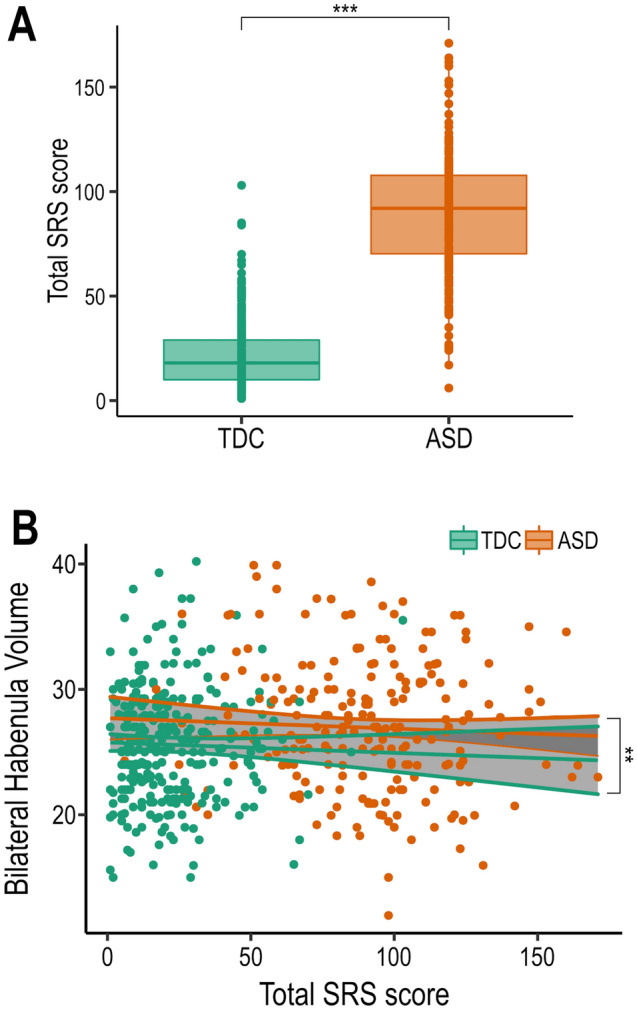
(A) Main effect of diagnosis on SRS score. (B) No significant interaction of Habenula volume and SRS score in either group. ** indicates p ≤ 0.01; *** indicates p ≤ 0.001. SD: autism spectrum disorder subjects; TDC: Typically developing controls.
Support-vector machine modelling demonstrates that habenula volume differentiates between groups (model using age, sex and bilateral habenula volume) and can claissfy ASD as compared to TDC with 85% accuracy. The accuracy dropped to 64% in the cross validation (Table 2).
Table 2.
SVM classifier and cross validation.
| Support vector machine (SVM) classifier | |||
|---|---|---|---|
| ASD | Control | Total | |
| Predicted ASD | 256 | 43 | 299 |
| Predicted control | 47 | 260 | 307 |
| Total | 303 | 303 | 606 |
| Accuracy: 85%; Sensitivity: 85%; Specificity: 86% | |||
| Four fold cross validation of SVM classifier | |||
|---|---|---|---|
| ASD | Control | Total | |
| Predicted ASD | 191 | 108 | 299 |
| Predicted control | 112 | 195 | 307 |
| Total | 303 | 303 | 606 |
| Accuracy: 64%; Sensitivity: 63%; Specificity:64% | |||
A support vector machine classifier using individual age, sex and bilateral habenula volume as input is able to distinguish between ASD and TDC control subjects with 85% accuracy using a balanced dataset created by adding ASD subjects randomly picked from the original 220. The accuracy drops to 64% in a fourfold cross validation where for every quarter of the original dataset, the SVM is trained on the remaining three quarters and applied to the unseen data.
ASD, autism spectrum disorder; TDC, typically developing controls; SVM support vector machine.
Discussion
Investigating habenula volume in a large cohort of ASD and TDC subjects spanning in age from childhood to young adulthood we found that habenula volume is larger in subjects diagnosed with ASD across all ages. While studies using preclinical models have provided evidence of an involvement of the habenula in autism phenotypes8,51–57 these findings in human subjects provide further evidence of the involvement of the habenula in the pathophysiology of ASD. The habenula volume difference did not show any evidence of being the product of an altered developmental trajectory. Similarly, there was no evidence of an effect of sex or symptom severity as measured by the individual SRS score. These findings are distinctly different compared to other morphological differences associated with ASD and in particular are unlike the abnormal developmental time course, sex differences and effects of symptom severity described in alterations of amygdala volume associated with ASD20,23,42,43. Likewise, a previous study investigating habenula volume differences associated with schizophrenia and BD found that habenula volume differences were only apparent in certain age ranges41.
While T1w contrast does not allow one to discern the underlying cause of these volume differences (e.g. increased dendrites, microglia, angiogenesis, neuroglia) previous work in animals showed that social deficits are associated with increased habenula activity and that experimentally inhibiting habenula activity improves social behaviour5. The habenula has been identified as the central structure mediating the reward value of social interactions and shown to be a key region for adapting behavioural strategies, integrating both internal (previous reward experience) and external (sensory input) information to initiate necessary behavioural adjustments2,4. Furthermore, an optimal habenula function is necessary for flexible behavioural adjustments2,4, sensory processing2,6, motivational processes1,2 and regulation of circadian rhythms3 all domains where differences are found in ASD9,10 (e.g. social communication difficulties, restricted interests and repetitive behaviors, altered sensory perception and mood and sleep disorders; Fig. 1).
This study has a number of limitations. While the rigorous quality control of the individual MRI scans is necessary to ensure that the findings are reliable, subjects excluded due to quality control issues were more likely to be from the ASD group, significantly younger, had lower full IQ and higher symptom severity scores and were more likely to be male (Bedford et al. Supplementary Table S516). Future tools might allow correction of motion artefacts and will eliminate potential biases in the data exclusion. The study covered a large age range, but did not include subjects from early childhood. ASD is typically diagnosed in that period and further studies are needed to investigate the early trajectory of habenula volume and a possible relationship of habenula volume with autistic symptoms and diagnosis. Furthermore, while the study included female ASD subjects, the group was relatively small.
Despite these limitations, the robust finding of increased habenula volume in ASD compared to TDC subjects provides the first evidence in human subjects of an involvement of the habenula in some aspect of the pathophysiology of ASD. The fact that there is a strong effect of diagnosis indepedent of age, sex or symptom severity as assessed by the SRS score might point to the habenula being implied in a broader range of behavioral symptoms, beyond the classic deficits of social behaviour and social interaction. Further studies investigating neurotransmitters, metabolites, connectivity patterns and neurochemical binding are necessary to unravel the neurobiological mechanisms underlying the involvement of the habenula in ASD.
Materials and methods
Subjects
Habenula volumes were estimated using T1-weighted magnetic resonance images (MRI) scans of subjects from the Autism Brain Imaging Data Exchange (ABIDE) repository58,59. ABIDE is a large-scale, publicly available multi-site database providing MRI scans as well as behavioral data from individuals with autism spectrum disorder and typically developing controls. For detailed demographics, imaging acquisition parameters, local institutional review boards and written informed consent, please visit (https://fcon_1000.projects.nitrc.org/indi/abide/). Inclusion criteria were motion and scan quality as previously described16, complete Social Responsiveness Scale scores60, sufficient data to model the developmental trajectories in ASD subjects and TDC. The SRS is a rating scale filled out by a next of kin or caregiver to quantitatively measure autistic traits and is a widely used reliable screening tool for children, adolescents and adults61–63. This resulted in a final dataset of 220 ASD subjects (184 males) and 303 TDC (213 males) ages 6 to 30 years (Table 1).
Image processing, MAGeTbrain segmentation and validation
The fully automated segmentation of the Habenula has been previously validated and shown to be a reliable toll for the assessment of habenula volume in large datasets, across a wide age range spanning from childhood to late adulthood, and using distinct MRI acquisition parameters41. The dataset used to establish validity and reliability included 103 scans (out of a total 356) of children and adolescents aged 4 to 17 years of age. All automatic segmentations were rigorously visually inspected to ensure successful segmentation41. The validation included comparing the automatic segmentations to manually segmented habenulas (n = 30 (2 × 15 subjects)). These were randomly sampled and thus included approximately 10 manually segmented habenulas (~ 1/3 of scans) of subjects younger than 18 years. Reliable segmentation (as compared to manual segmentation for two raters) was demonstrated across individual habenulas ranging from in volume from 7 to over 20 mm3 and ages from 4 to 50 years demonstrating that reliable automatic segmentation is independent of habenula size and subject age. Image processing and quality control of the images was performed similar to previously described16. The images were pre-processed using iterative non-uniformity correction and skull-stripped (https://github.com/CoBrALab/minc-bpipe-library). In-scanner subject motion may bias neuroanatomical measures derived from the anatomical images67–69, and might lead to erroneous morphological group differences being found. All images were therefore rigorously quality controlled by two independent raters (SAB, and either ST or MMC). The detailed QC method and examples can be found in the main text and supplement of Bedford and colleagues16. Only scans of high quality were included in the study (see exclusion criteria above) to ensure the reliability of the morphological measurements. The Multiple Automatically Generated Templates (MAGeT) brain segmentation algorithm was used to segment the habenula (https://github.com/CoBrALab/MAGeTbrain)41,64. MAGeTbrain was designed, from the onset, to improve the segmentation accuracy and robustness of atlas-based segmentation techniques. It has been shown to provide reliable and accurate segmentations of subcortical structures as well as hippocampal subfields and cerebellar lobules using only T1w image65,70–72. MAGeTbrain employs label propagation to produce individual segmentations using five segmented high-resolution atlases. It employs image registration and habenula segmentation is aided by the high local contrast provided by the third ventricle and the thalamus (Fig. 1)41. These atlases are then, again using image registration, propagated to 21 template images selected from the input dataset. In doing so a large number (5 × 21 = 105) of candidate segmentations is created, which are fused using majority vote to derive a final segmentation for each subject. Using this template library has two advantages: it helps reduce atlas bias and it reduces registration errors by averaging65. To ensure accurate segmentation, the resulting individual habenula segmentation of each subject was visually inspected independently by two raters (JG and FVG) in 3D overlaid onto the individual T1-weighted MRI image using DISPLAY (https://www.mcgill.ca/bic/software/minc/minctoolkit). Segmentation of the habenula was inspected in continuous slices in all three planes and rated as successful (correct location and extend in all dimensions) or failed segmentations (over- or under segmentation). Both raters agreed on the quality of segmentation in all cases.
Furthermore, to confirm that the habenula can be reliably segmented using the automated method in this autistic patients population the left and right habenula of 24 randomly selected brains were manually segmented. The 24 brains were taken from the childhood sample (< 11 years), the adolescence sample (12–17 years) and the young adults sample (18–25 years) (8 each, 48 habenula altogether). The Dice similarity coefficients (DSC) were calculated and used for validation. Previous work demonstrated the reliability with average DSC scores between 0.78 and 0.8441.
Statistical analysis
The lme4 (version 1.1-21), e1071 (version 1.7-3) and lmerTest (version 3.1.1) packages in R (version 3.6.1; https://www.r-project.org) were used to perform the statistical analysis. Bilateral habenula volumes (left habenula + right habenula) were calculated and used for subsequent analysis. A linear mixed effect model corrected for age, sex and total brain volume with the site used as a random intercept effect was used to test for a possible effect of diagnosis on individual habenula volume: linear mixed effect model = “bilateral habenula volume” ~ “age” + “sex” + “total brain volume” + “group (ASD or TDC)” + (1|”study site”).
Individual total brain volume was derived from the brain mask created during preprocessing and describes the volume of the cerebrum, cerebellum, brainstem and ventricles. As similar linear mixed effect model corrected for age, sex and total brain volume with the site used as a random intercept effect was used to test for a possible effect of individual SRS score on individual habenula volume within each group (ASD or TDC) separately.
Support-vector machine (SVM) learning was used to interrogate the value of habenula volume in predicting diagnosis. To this end age, sex and bilateral habenula volume were used as predictors using a balanced dataset of 303 observations for both AD and TDC; additional observations for the ASD group were created by random sampling with replacement. A fourfold cross validation was used to validate the model.
Acknowledgements
GAD acknowledges that this work was supported in part by funding provided by Brain Canada, in partnership with Health Canada, for the Canadian Open Neuroscience Platform initiative. This research was enabled in part by support provided by SciNet (http://www.scinethpc.ca) and Compute Canada (http://www.computecanada.ca).
Author contributions
J.G. analyzed and interpreted the data and contributed to all drafts of the manuscript; F.V.G. wrote the draft manuscript and its revisions; S.A.B., S.T. and M.M.C. performed data quality control and contributed to all drafts of the manuscript; H.B. and G.A.D. contributed to all drafts of the manuscript.
Data availability
The dataset analysed during the current study (Autism Brain Imaging Data Exchange, ABIDE I & ABIDE II) is publicly available at https://fcon_1000.projects.nitrc.org/indi/abide/. The tool used in this study for automatic segmentation of the habenula is freely available at https://github.com/CoBrALab/MAGeTbrain. The processed data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jürgen Germann and Flavia Venetucci Gouveia.
References
- 1.Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nat. Rev. Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H, Cui Y, Yang Y. Circuits and functions of the lateral habenula in health and in disease. Nat. Rev. Neurosci. 2020;21:277–295. doi: 10.1038/s41583-020-0292-4. [DOI] [PubMed] [Google Scholar]
- 3.Mizumori SJY, Baker PM. The lateral habenula and adaptive behaviors. Trends Neurosci. 2017;40:481–493. doi: 10.1016/j.tins.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Baker PM, et al. The lateral habenula circuitry: Reward processing and cognitive control. J. Neurosci. 2016;36:11482–11488. doi: 10.1523/JNEUROSCI.2350-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benekareddy M, et al. Identification of a corticohabenular circuit regulating socially directed behavior. Biol. Psychiatry. 2018;83:607–617. doi: 10.1016/j.biopsych.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Boulos L-J, Darcq E, Kieffer BL. Translating the habenula-from rodents to humans. Biol. Psychiatry. 2017;81:296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torrisi S, et al. Resting state connectivity of the human habenula at ultra-high field. Neuroimage. 2017;147:872–879. doi: 10.1016/j.neuroimage.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Kerkhof LWM, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ. Functional integrity of the habenula is necessary for social play behaviour in rats. Eur. J. Neurosci. 2013;38:3465–3475. doi: 10.1111/ejn.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentani H, et al. Autism spectrum disorders: An overview on diagnosis and treatment. Braz. J. Psychiatry. 2013;35(Suppl 1):S62–72. doi: 10.1590/1516-4446-2013-S104. [DOI] [PubMed] [Google Scholar]
- 10.Lord C, et al. Autism spectrum disorder. Nat. Rev. Dis. Primers. 2020;6:5. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maenner MJ, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020;69:1–12. doi: 10.15585/mmwr.ss6904a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvekot J, et al. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism. 2017;21:646–658. doi: 10.1177/1362361316672178. [DOI] [PubMed] [Google Scholar]
- 13.Filipek PA, et al. Practice parameter: Screening and diagnosis of autism: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/WNL.55.4.468. [DOI] [PubMed] [Google Scholar]
- 14.Chawarska K, Klin A, Volkmar FR. Autism Spectrum Disorders in Infants and Toddlers: Diagnosis, Assessment, and Treatment. Guilford Press; 2010. [Google Scholar]
- 15.Pagnozzi AM, Conti E, Calderoni S, Fripp J, Rose SE. A systematic review of structural MRI biomarkers in autism spectrum disorder: A machine learning perspective. Int. J. Dev. Neurosci. 2018;71:68–82. doi: 10.1016/j.ijdevneu.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Bedford SA, et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol. Psychiatry. 2020;25:614–628. doi: 10.1038/s41380-019-0420-6. [DOI] [PubMed] [Google Scholar]
- 17.Hazlett HC, et al. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khundrakpam BS, Lewis JD, Kostopoulos P, Carbonell F, Evans AC. Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: A large-scale MRI study. Cereb. Cortex. 2017;27(3):1721–1731. doi: 10.1093/cercor/bhx038. [DOI] [PubMed] [Google Scholar]
- 19.Emerson RW, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci. Transl. Med. 2017;9(393):eaag2882. doi: 10.1126/scitranslmed.aag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Haar S, Berman S, Behrmann M, Dinstein I. Anatomical abnormalities in autism? Cereb. Cortex. 2016;26:1440–1452. doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser MD, et al. Neural signatures of autism. Proc. Natl. Acad. Sci. USA. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rooij D, et al. Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: Results from the ENIGMA ASD working group. Am. J. Psychiatry. 2017;175:359–369. doi: 10.1176/appi.ajp.2017.17010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moradi E, Khundrakpam B, Lewis JD, Evans AC, Tohka J. Predicting symptom severity in autism spectrum disorder based on cortical thickness measures in agglomerative data. Neuroimage. 2017;144:128–141. doi: 10.1016/j.neuroimage.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Xu Q, Zuo C, Liao S, Long Y, Wang Y. Abnormal development pattern of the amygdala and hippocampus from childhood to adulthood with autism. J. Clin. Neurosci. 2020;78:327–332. doi: 10.1016/j.jocn.2020.03.049. [DOI] [PubMed] [Google Scholar]
- 26.Sato W, Uono S. The atypical social brain network in autism: Advances in structural and functional MRI studies. Curr. Opin. Neurol. 2019;32:617–621. doi: 10.1097/WCO.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 27.Volkmar FR. Understanding the social brain in autism. Dev. Psychobiol. 2011;53:428–434. doi: 10.1002/dev.20556. [DOI] [PubMed] [Google Scholar]
- 28.Misra V. The social brain network and autism. Ann. Neurosci. 2014;21:69–73. doi: 10.5214/ans.0972.7531.210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blakemore S-J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 30.Foulkes L, Blakemore SJ. Studying individual differences in human adolescent brain development. Nat. Neurosci. 2018;21(3):315–323. doi: 10.1038/s41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- 31.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 2009;12(1):77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loonen AJM, Kupka RW, Ivanova SA. Circuits regulating pleasure and happiness in bipolar disorder. Front. Neural Circuits. 2017;11:35. doi: 10.3389/fncir.2017.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakhoury M. The habenula in psychiatric disorders: More than three decades of translational investigation. Neurosci. Biobehav. Rev. 2017;83:721–735. doi: 10.1016/j.neubiorev.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Schafer M, et al. Imaging habenula volume in schizophrenia and bipolar disorder. Front. Psychiatry. 2018;9:456. doi: 10.3389/fpsyt.2018.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loonen AJM, Ivanova SA. Consider role of glutamatergic habenula-projecting globus pallidus in OCD. Pharmacopsychiatry. 2019;52:203–204. doi: 10.1055/a-0835-6447. [DOI] [PubMed] [Google Scholar]
- 37.Stein DJ, et al. Obsessive-compulsive disorder. Nat. Rev. Dis. Primers. 2019;5:52. doi: 10.1038/s41572-019-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stopper CM, Floresco SB. Dopaminergic circuitry and risk/reward decision making: Implications for schizophrenia. Schizophr. Bull. 2015;41:9–14. doi: 10.1093/schbul/sbu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepard PD, Holcomb HH, Gold JM. Schizophrenia in translation: The presence of absence: Habenular regulation of dopamine neurons and the encoding of negative outcomes. Schizophr. Bull. 2006;32:417–421. doi: 10.1093/schbul/sbj083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savitz JB, et al. Habenula volume in bipolar disorder and major depressive disorder: A high-resolution magnetic resonance imaging study. Biol. Psychiatry. 2011;69:336–343. doi: 10.1016/j.biopsych.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Germann J, et al. Fully automated habenula segmentation provides robust and reliable volume estimation across large magnetic resonance imaging datasets, suggesting intriguing developmental trajectories in psychiatric disease. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:923–929. doi: 10.1016/j.bpsc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Schumann CM, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernhardt BC, Di Martino A, Valk SL, Wallace GL. Neuroimaging-based phenotyping of the autism spectrum. In: Wöhr M, Krach S, editors. Social Behavior from Rodents to Humans: Neural Foundations and Clinical Implications. Springer International Publishing; 2017. pp. 341–355. [Google Scholar]
- 44.Germann J, et al. Deep brain stimulation of the habenula: Systematic review of the literature and clinical trial registries. Front. Psychiatry. 2021;12:1410. doi: 10.3389/fpsyt.2021.730931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakobs M, Pitzer C, Sartorius A, Unterberg A, Kiening K. Acute 5 Hz deep brain stimulation of the lateral habenula is associated with depressive-like behavior in male wild-type Wistar rats. Brain Res. 2019;1721:146283. doi: 10.1016/j.brainres.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Sartorius A, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Ely BA, et al. Resting-state functional connectivity of the human habenula in healthy individuals: Associations with subclinical depression. Hum. Brain Mapp. 2016;37:2369–2384. doi: 10.1002/hbm.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt FM, et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267:107–115. doi: 10.1007/s00406-016-0675-8. [DOI] [PubMed] [Google Scholar]
- 49.Soutschek A. Neural circuits regulating social behavior: Highlighting the causal contribution of the lateral habenula. Biol. Psychiatr. 2018;83:546–547. doi: 10.1016/j.biopsych.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Neudorfer C, et al. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci. Data. 2020;7:305. doi: 10.1038/s41597-020-00644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner F, French L, Veh RW. Transcriptomic-anatomic analysis of the mouse habenula uncovers a high molecular heterogeneity among neurons in the lateral complex, while gene expression in the medial complex largely obeys subnuclear boundaries. Brain Struct. Funct. 2016;221:39–58. doi: 10.1007/s00429-014-0891-9. [DOI] [PubMed] [Google Scholar]
- 52.Vickstrom, C. R. et al. T-type calcium channels contribute to burst firing in a subpopulation of medial habenula neurons. eNeuro 7 (2020). [DOI] [PMC free article] [PubMed]
- 53.Murru L, et al. Lateral habenula dysfunctions in Tm4sf2 mice model for neurodevelopmental disorder. Neurobiol. Dis. 2021;148:105189. doi: 10.1016/j.nbd.2020.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baker PM, Oh SE, Kidder KS, Mizumori SJY. Ongoing behavioral state information signaled in the lateral habenula guides choice flexibility in freely moving rats. Front. Behav. Neurosci. 2015;9:295. doi: 10.3389/fnbeh.2015.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martínez-Rodríguez E, et al. Male-specific features are reduced in Mecp2-null mice: Analyses of vasopressinergic innervation, pheromone production and social behaviour. Brain Struct. Funct. 2020;225:2219–2238. doi: 10.1007/s00429-020-02122-6. [DOI] [PubMed] [Google Scholar]
- 56.Nakajima M, et al. Psychiatric disorder-related abnormal behavior and habenulointerpeduncular pathway defects in Wnt1-cre and Wnt1-GAL4 double transgenic mice. J. Neurochem. 2013;124:241–249. doi: 10.1111/jnc.12085. [DOI] [PubMed] [Google Scholar]
- 57.Kenkel WM, et al. Functional magnetic resonance imaging in awake transgenic fragile X rats: Evidence of dysregulation in reward processing in the mesolimbic/habenular neural circuit. Transl. Psychiatry. 2016;6:e763. doi: 10.1038/tp.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Martino A, et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data. 2017;4:170010. doi: 10.1038/sdata.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Martino A, et al. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry. 2014;19:659–667. doi: 10.1038/mp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Constantino, J. N. & Gruber, C. P. Social Responsiveness Scale (SRS) (2005).
- 61.Bölte S. Brief report: The Social Responsiveness Scale for Adults (SRS-A): Initial results in a German cohort. J. Autism Dev. Disord. 2012;42:1998–1999. doi: 10.1007/s10803-011-1424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cholemkery H, Kitzerow J, Rohrmann S, Freitag CM. Validity of the social responsiveness scale to differentiate between autism spectrum disorders and disruptive behaviour disorders. Eur. Child Adolesc. Psychiatry. 2014;23:81–93. doi: 10.1007/s00787-013-0427-5. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen PH, et al. The reliability and validity of the social responsiveness scale to measure autism symptomology in Vietnamese children. Autism Res. 2019;12:1706–1718. doi: 10.1002/aur.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chakravarty MM, et al. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum. Brain Mapp. 2013;34:2635–2654. doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pipitone J, et al. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage. 2014;101:494–512. doi: 10.1016/j.neuroimage.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 66.Winterburn JL, et al. A novel in vivo atlas of human hippocampal subfields using high-resolution 3 T magnetic resonance imaging. Neuroimage. 2013;74:254–265. doi: 10.1016/j.neuroimage.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Alexander-Bloch A, et al. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum. Brain Mapp. 2016;37:2385–2397. doi: 10.1002/hbm.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pardoe HR, Kucharsky Hiess R, Kuzniecky R. Motion and morphometry in clinical and nonclinical populations. Neuroimage. 2016;135:177–185. doi: 10.1016/j.neuroimage.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Ducharme S, et al. Trajectories of cortical thickness maturation in normal brain development—The importance of quality control procedures. Neuroimage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tullo S, et al. Warping an atlas derived from serial histology to 5 high-resolution MRIs. Sci. Data. 2018;5:180107. doi: 10.1038/sdata.2018.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makowski C, et al. Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. Neuroimage. 2018;170:182–198. doi: 10.1016/j.neuroimage.2017.02.069. [DOI] [PubMed] [Google Scholar]
- 72.Park MTM, et al. Derivation of high-resolution MRI atlases of the human cerebellum at 3T and segmentation using multiple automatically generated templates. Neuroimage. 2014;95:217–231. doi: 10.1016/j.neuroimage.2014.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analysed during the current study (Autism Brain Imaging Data Exchange, ABIDE I & ABIDE II) is publicly available at https://fcon_1000.projects.nitrc.org/indi/abide/. The tool used in this study for automatic segmentation of the habenula is freely available at https://github.com/CoBrALab/MAGeTbrain. The processed data are available from the corresponding author upon reasonable request.