Abstract
Background:
A decline in cortical thickness during early life appears to be a normal neuromaturational process. Accelerated cortical thinning has been linked with conversion to psychosis among individuals at clinical high-risk for psychosis (CHR-P). Previous research indicates that exposure to life event stress (LES) is associated with exaggerated cortical thinning in both healthy and clinical populations, and LES is also linked with conversion to psychosis in CHR-P. To date, there are no reports on the relationship of LES with cortical thickness in CHR-P. The present study examines this relationship and whether LES is linked with cortical thinning to a greater degree in CHR-P who convert to psychosis compared to CHR-P who do not convert and healthy controls.
Methods:
Controlling for age and gender (364 male, 262 female), the present study examined associations between LES and baseline cortical thickness in 436 CHR-P (375 non-converters and 61 converters) and 190 comparison subjects in the North American Prodrome Longitudinal Study.
Results:
Findings indicate that pre-baseline cumulative LES is associated with reduced baseline cortical thickness in several regions among the CHR-P and control groups. Evidence suggests that LES is a risk factor for thinner cortex to the same extent across diagnostic groups, while CHR-P status is linked with thinner cortex in select regions after accounting for LES.
Conclusions:
This research provides additional evidence to support the role of LES in cortical thinning in both healthy youth and those at CHR-P. Potential underlying mechanisms of the findings and implications for future research are discussed.
Keywords: cortical thickness, life stress, clinical high-risk, psychosis, neuromaturation, environment
Introduction
Chronic or repeated exposure to stress is assumed to be associated with psychotic symptoms and risk for psychotic disorders (1–4). Individuals deemed at clinical high-risk for psychosis (CHR-P), based on structured measures for the assessment of psychosis-risk syndromes, report more life event stress (LES) than healthy comparison subjects (5–8). Consistent with the notion that LES plays a role in triggering psychosis, there is also evidence from prospective studies that stress exposure is linked with increased risk of subsequently developing a psychotic disorder (9–11). While the scientific study of stress has burgeoned, it has also become more apparent that its adverse effects are nonspecific; stress is associated with increased risk for a range of psychiatric disorders, declines in physical health, and decreased levels of functioning in healthy individuals (12).
Potential neurobiological mediators of the adverse effects of stress have been documented in both animal and human research. Included among these are alterations in the immune system response, gonadal hormone levels, and brain structure and function (13). Animal research has demonstrated stress-induced dendritic spine remodeling (14, 15) and hypothalamic-pituitary-adrenal (HPA) axis dysfunction in structural abnormalities in the hippocampus and medial prefrontal cortex (16, 17). Dysregulation of the HPA axis, modulated, in part, by negative feedback via glucocorticoid (e.g., cortisol in humans) receptors in the hippocampus, amygdala, and prefrontal cortex, is also associated with the emergence of psychosis (18). More broadly, studies of humans suggest that both acute and chronically high levels of stress exposure are linked to atrophy of dendrites and suppression of neurogenesis (19).
Research with humans has also revealed associations of regional brain morphology with LES, and it appears that stress can alter the anticipated trajectory of cortical development (20). In particular, cortical thickness, partially reflecting the number of cells within organizational columns in the brain, decreases linearly with age and shows accelerated reductions during adolescence and young adulthood, in conjunction with ongoing myelination, synaptic pruning, and pubertal changes (21–25). Although the neural mechanisms of aberrant cortical thinning are not fully known, prior work has demonstrated an inverse relation between persistent stress and/or trauma exposure and cortical thickness in studies of both healthy and clinical samples, including patients with PTSD (26), schizophrenia (27), adolescents exposed to maltreatment (28), and infants exposed to pain (29). Independent of stress or trauma exposure, increased cortical thinning is implicated in several psychiatric conditions, including ADHD (30), major depressive disorder (31), bipolar disorder (32), and psychosis (33).
In the case of psychotic disorders, there is evidence that atypical cortical thinning predates the onset of clinical symptoms of psychosis. Cross-sectional investigations of individuals at CHR-P have shown significantly reduced cortical thickness in CHR-P compared to healthy controls (HC) in frontal, temporal and parietal regions, with even greater reductions in thickness in these and other regions in chronic schizophrenia relative to CHR-P (34–36; but see 37, 38). In one of the largest longitudinal studies of individuals at CHR-P, the North American Prodrome Longitudinal Study (NAPLS 2), CHR-P subjects who subsequently developed psychosis showed a steeper rate of gray matter loss, especially in the right superior frontal, middle frontal, and medial orbitofrontal cortex, when compared with HC and CHR-P subjects who did not convert to psychosis (39). Although the determinants of accelerated declines in cortical thickness in the prodrome to psychoses have not been fully established (40, 41), genetic influences on cortical thickness have been demonstrated (42–44), and there is evidence that cortical thinning is sensitive to environmental exposures (45, 46). In individuals with schizophrenia and bipolar disorder, research has found that both a global index of environmental risk (e.g., adversity, cannabis use) and genetic risk for schizophrenia are associated with global cortical thinning (47).
Diathesis-stress models have dominated theorizing about the etiology of psychotic illnesses, with recent models assuming that a confluence of brain vulnerabilities, stress, and neuromaturational processes give rise to psychosis (48, 49). Thus, the accelerated declines in cortical thickness observed in CHR-P may partially reflect the effect of stress exposure. This study extends prior work from NAPLS 2 to test the hypothesis that pre-baseline cumulative LES is inversely associated with cortical thickness in HC and CHR-P. Given that this is the first study to examine the potential influence of LES on cortical thickness in individuals at CHR-P, we have no a priori hypotheses concerning specific cortical regions which may be associated with LES. Moreover, given evidence that LES is inversely associated with cortical thickness in both healthy and clinical populations (26–29), we do not anticipate an interaction between diagnostic group and LES in predicting cortical thickness.
Methods and Materials
Sample
The present sample included participants between the ages of 12 and 30 years from the second phase of the multi-site NAPLS study (50). The participants included were those who had completed a magnetic resonance imaging (MRI) scan with a T1-weighted structural image that passed quality assurance metrics and the Psychiatric Epidemiology Research Interview Life Events Scale (51) at baseline. Using the criterion of < 5% missing item responses, N = 626 had total LES scores and usable MRI data. Specifically, this sample included 190 HC, 375 non-converters (CHR-NC; those who demonstrated attenuated psychotic symptoms at baseline but did not convert to psychotic disorder by completion of the two-year follow-up) and 61 converters (CHR-C; those who had clinical symptoms of psychosis by completion of the study). The aims, recruitment methods, and inclusion criteria for NAPLS 2 have been described elsewhere (50, 52), and demographic characteristics of this sample are presented in Table 1.
Table 1.
Demographic Characteristics
| Characteristic | HC (n = 190) | CHR-NC (n = 375) | CHR-C (n = 61) | Statistical Test for Significance (2-Tailed) | P value | Post Hoc Tukey Test |
|---|---|---|---|---|---|---|
| Age, mean (SD), range | 20.8 (4.6), 12 – 34 | 19.2 (3.9), 12 – 34 | 18.7 (3.6), 12 – 28 | F = 10.6 | < .001 | HC > CHR-NC, CHR-C |
| Male gender, No. (%) | 101 (53.2) | 224 (59.7) | 39 (63.9) | χ2 = 3.2 | .21 | NA |
| Education level, mean (SD) | 13.1 (3.6) | 11.5 (2.8) | 11.3 (2.6) | F = 18.6 | < .001 | HC > CHR-NC, CHR-C |
| Paternal education score, mean (SD) | 6.5 (1.6) | 6.3 (1.7) | 6.4 (1.8) | F = .97 | .38 | NA |
| Maternal education score, mean (SD) | 6.7 (1.5) | 6.3 (1.6) | 6.6 (1.7) | F = 4.2 | .01 | CHR-NC < HC, CHR-C |
| Race/ethnicity, No. (%) | ||||||
| White | 107 (56.3) | 224 (59.7) | 32 (52.5) | χ2 = 1.5 | .48 | NA |
| Hispanic or Latino | 28 (14.7) | 69 (18.4) | 9 (14.8) | χ2 = 1.4 | .49 | NA |
| Black | 35 (18.4) | 58 (15.5) | 9 (14.8) | χ2 = 93 | .63 | NA |
| Asian | 22 (11.6) | 22 (5.9) | 7 (11.5) | χ2 = 6.4 | .06 | NA |
| First Nations | 4 (2.1) | 7 (1.9) | 1 (1.6) | χ2 = .07 | .97 | NA |
| Multiracial | 14 (7.4) | 40 (10.7) | 9 (14.8) | χ2 = 3.2 | .21 | NA |
| LES, mean (SD) | 57.9 (37.1) | 104.5 (133.0) | 107.2 (100.8) | F = 12.1 | < .001 | HC < CHR-NC, CHR-C |
Briefly, all CHR-NC participants in the present study met criteria for attenuated psychotic symptom syndrome (APSS) on the Structured Interview for Psychosis Risk Syndromes (v5.6, 2014) (SIPS). All CHR-C participants in the present study met criteria for a score of “6” (i.e., severe and psychotic) on at least one positive symptom subscale of the SIPS at the two-year follow-up. All participants provided consent (or parental assent where appropriate) in accordance with relevant guidelines and regulations at the eight participating sites of the NAPLS 2 consortia.
Magnetic Resonance Imaging (MRI) Acquisition and Processing
High-resolution, T1-weighted brain images were acquired using 3T scanners at the eight participating sites of NAPLS 2. Details regarding data quality assurance and preprocessing procedures have been described in detail in previous reports (53, 54). Surface-based measures of cortical thickness are reliable across MRI scanners used in this study (53). T1-weighted MRI scans for 626 participants were processed through a structural MRI pipeline using FreeSurfer version 5.2 (55). This process involves extracting surface-based thickness measures from each scan by calculating the shortest distance from each point on the gray to white matter boundary to the pial surface of each cortical vertex (56). Participants’ thickness data was resampled to an average subject space (fsaverage5 containing 10,242 vertices per hemisphere) and a 10mm full-width half maximum (FWHM) smoothing kernel was applied, a smoothing level previously shown to balance sensitivity and specificity while minimizing false discovery proportion (57).
Statistical Analyses
To determine if—and where—a relationship between the variables of interest and cortical thickness is localized to specific cortical areas, vertex-level analyses were conducted. A hierarchical linear regression model was used to examine the additive effects of the following blocks of variables on cortical thickness: 1) age and gender, 2) LES, 3) diagnostic group, coded as an ordinal variable (0 = HC, 1 = CHR-NC, 2 = CHR-C), and 4) the interaction of LES and diagnostic group. Variables in each block were added to those already present in the model from prior blocks, and all analyses included MRI scanner as a covariate. Permutation-based linear modeling using FSL PALM software (58), a MATLAB-based package, was used to calculate associations between the variables of interest and vertex-level cortical thickness using 1,000 permutations. Threshold-free cluster enhancement (TFCE), an image enhancement and thresholding technique (59), was applied to raw statistical images to produce maps that represent spatial clustering of cortical thickness effects. All results were corrected for multiple comparisons across vertices and hemispheres using family-wise error correction built into FSL PALM.
Additional analyses were conducted to unpack the interaction between LES and diagnostic group in the single region in which the interaction term was significant (discussed below). Specifically, the mean thickness across vertices reaching significance in this analysis was calculated for each participant, and the correlation between thickness and LES was calculated separately for each diagnostic group using built-in statistical packages in R version 3.5.3.
After observing a significant main effect of gender on cortical thickness in several regions, post-hoc analyses were conducted to assess two-way and three-way interactions with gender by including the interaction of LES and gender, diagnostic group and gender, and the interaction between all three of these variables in a fifth block in the hierarchical linear regression model.
In addition, after observing significant associations between LES and cortical thickness across groups, a post-hoc mediation analysis (with scanner, age, and gender as covariates) explored whether cortical thickness in areas of the brain significantly associated with LES at baseline (M) partially mediates the association between pre-baseline cumulative LES (X) and diagnostic outcome (Y). The ordering of X, M, and Y was selected according to a robust literature regarding the influence of stress on brain structure (14–19) and psychiatric illness (9–11), as well as prior work indicating that accelerations in cortical thinning predate psychosis onset (34–36, 39). Mediation analyses were conducted in R using the mediation package (60). Significance was assessed using 95% confidence intervals based on non-parametric bootstrapping using 10,000 permutations. Sensitivity analyses were conducted to assess the assumption of sequential ignorability inherent in causal mediation and are reported in Supplemental materials.
Stress Measure
Lifetime events (e.g., end of a romantic relationship, death of loved one) were assessed via the Life Events Scale (51), modified to exclude events of lesser relevance to youth (e.g., getting divorced) (10). Participants indicated whether they had experienced each stressor and the level of distress elicited by each endorsed event (scored on a 7-point scale where 1 = “no stress” and 7 = “caused me to panic”). Participants could report multiple exposures to the same event. A cumulative LES score was calculated by summing the rankings of perceived stress across all events experienced over the lifespan. This measure of cumulative LES was used because previous analyses showed it was more predictive of CHR-P transition to psychosis than the total count of stressful events (10).
Results
Demographic characteristics for each diagnostic group are presented in Table 1, and inter-correlations among continuous variables of interest are presented in Table 2. There was no significant association between gender and diagnostic group, χ2 = 4.18, p = .12. Independent samples t-tests were conducted for continuous measures and gender; there was no significant difference in age (t = − 1.10, p = .27) or LES score (t = − 1.61, p = .30) between the genders.
Table 2.
Two-tailed bivariate Pearson correlations among continuous predictors, by diagnostic group, with significant correlations flagged at p = .01*
| Age | LES | |
|---|---|---|
| Healthy Controls (n = 190) | ||
| Age | .35* | |
| LES | .35* | |
| Non-converters (n = 375) | ||
| Age | .28* | |
| LES | .28* | |
| Converters (n = 61) | ||
| Age | .36* | |
| LES | .36* |
Hierarchical linear regression was conducted using continuous (vertex-level) maps of cortical thickness, to determine if—and where—on the cortical surface there is a significant association between LES and cortical thickness at baseline. Main effects for age and gender were entered in the first block. Age was significantly inversely associated with thickness across the cortex (β = − .36 – − .03, tfce fwep < 0.05; Supplemental Figure 1a), consistent with previous reports (21, 22). Female gender was associated with greater thickness across a large area of frontal cortex including the superior frontal gyrus (β = .06 – .21, tfce fwep < 0.05; Supplemental Figure 1b), similar to prior work (61). Female gender was also associated with thinner cortex in a small portion of bilateral temporal cortex (β = −.25 – −.09, tfce fwep < 0.05), particularly the right lateral temporal lobe, as observed in prior work (62).
LES was entered in the second block of the regression model and was significantly inversely associated with thickness of superior and middle temporal cortex bilaterally, as well as additional aspects of temporal, parietal, occipital, and orbitofrontal cortex in the right hemisphere including the parahippocampal gyrus and inferior frontal gyrus (β = − .15 – − .04, tfce fwep < 0.05; Figure 1a). In order to determine whether diagnostic group contributed to prediction after accounting for stress, it was dummy-coded and entered in the third block as an ordinal variable (0 = HC, 1 = CHR-NC, 2 = CHR-C). Diagnostic group was significantly inversely associated with thickness across much of parietal, temporal, and occipital cortex bilaterally, as well as aspects of the left anterior insula (β = − .17 – − .04, fwep < 0.05; Figure 1b).
Figure 1.
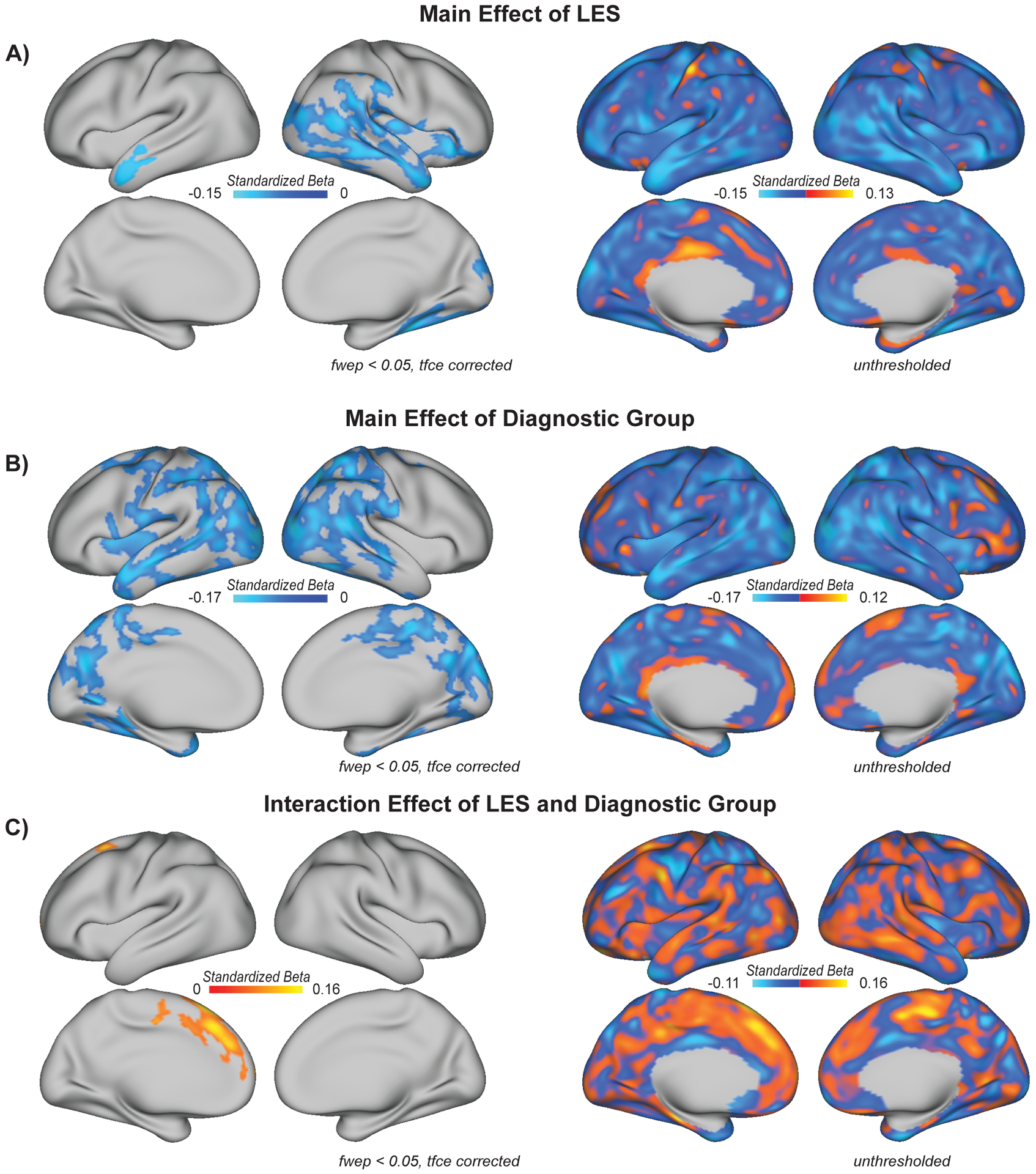
A) Life event stress (LES) is inversely correlated with cortical thickness in bilateral superior and middle temporal cortex, as well as right OFC, IFG, and parahippocampal gyrus, as discussed in the text (β = − .15 – − .04, tfce fwep < 0.05). Map indicates the effects of LES on thickness after controlling for age, gender, and MRI scanner. B) Individuals in the CHR-P group exhibit lower cortical thickness, particularly in bilateral temporal, occipital, and parietal areas (β = − .17 – − .04, tfce fwep < 0.05). Map indicates the effect of diagnostic group (0 = HC, 1 = CHR-NC, 2 = CHR-C) on thickness after controlling for age, gender, LES, and MRI scanner. C) An interaction between diagnostic group and LES reached significance in left superior frontal cortex (β = 0.06 – 0.16, tfce fwep < 0.05), as discussed in the text. Map indicates the LES and diagnostic group interaction effect on cortical thickness after controlling for main effects of each, as well as age, gender, and MRI scanner. fwep = family wise error corrected p-value; tcfe = threshold free cluster enhancement; OFC = orbitofrontal cortex; IFG = inferior frontal gyrus.
An interaction term for diagnostic group by LES was tested and entered in a fourth block of the regression. The interaction term was significantly associated with thickness in the left superior frontal cortex (β = 0.06 – 0.16, tfce fwep < 0.05; Figure 1c). Inter-correlations between LES and left superior frontal thickness, conducted separately by group, revealed significant inverse correlations between LES and left superior frontal thickness in HC (r = − .30, p < .01) and CHR-NC (r = − .18, p < .01); there was a positive relation in CHR-C (r = .31, p < 0.05). The group by LES interaction term did not reach significance in any other cortical area.
Areas of cortex associated with LES overlapped partially with those associated with diagnostic group, particularly in the right lateral temporal and occipital cortex and left lateral anterior temporal lobe (Figure 2). One possible explanation for this finding is that LES contributes to cortical thinning in brain areas associated with diagnostic outcomes. To begin to assess this possibility, a mediation analysis was conducted to determine if cortical thickness in areas significantly associated with LES partially mediates associations between LES and diagnostic group. The mean thickness across vertices shown to be associated with LES in both hemispheres (Figure 1a) was calculated for each participant. Cortical thickness among vertices significantly associated with LES were shown to partially mediate associations between LES and diagnostic group (c/c’ = 0.15/ 0.13, indirect effect = 0.014, 95% CI = [0.005, 0.03], all p-values < 0.001), indicating that cortical thickness may be a biomarker through which LES is associated with diagnostic group. See Supplemental Figure 2 for a sensitivity analysis of causal mediation effects.
Figure 2.
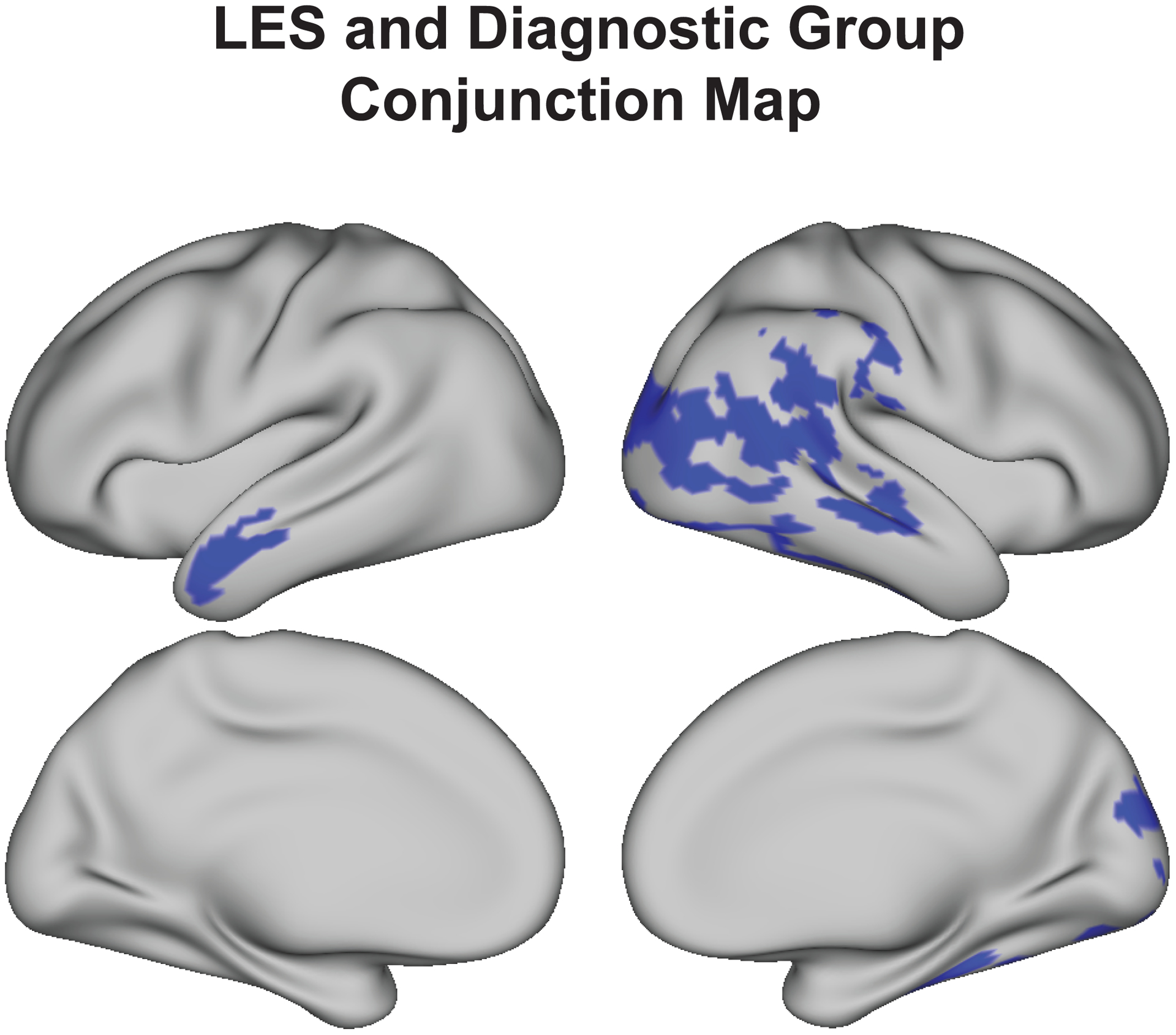
LES and diagnostic group are both associated with thinner cortex in several of the same cortical areas, including right lateral occipital and temporal cortex and the left lateral anterior temporal lobe. Conjunction map indicates vertices (shown in blue) which are sensitive to both LES (Figure 1a) and diagnostic group (Figure 1b), separately.
In addition, after observing a significant main effect of gender, and given known differences in stress reactivity (63, 64), cortical thinning (65, 66), and outcomes related to CHR-P (67, 68) between the genders, two-way and three-way interactions between gender, LES, and diagnostic group were entered and tested in a fifth block of the regression. All relationships between interaction terms and cortical thickness were insignificant (Supplemental Figure 3).
Discussion
Abnormalities in cortical thickness have been observed in a number of psychiatric conditions (39–41), and a great deal of research has focused on variables that moderate deviations in normative trajectories of neurodevelopment (4, 25, 69, 70). The present research extends prior work by demonstrating that cortical thickness at baseline is related to pre-baseline cumulative LES in both healthy and CHR-P youth. These findings are consistent with previous reports indicating adverse effects of stress on brain morphology in both healthy and clinical populations (13, 71, 72).
In addition, several of the brain areas in which lower thickness was significantly associated with higher LES in this report have previously been identified as sensitive to the effects of cumulative LES. Longitudinal work examining relations between regional brain morphology and stress has found reduced orbitofrontal thickness (a brain region involved in decision-making) in adulthood to be associated with greater self-reported LES from infancy to adolescence (73). Other work has found an association between exposure to chronic stress (i.e., child abuse) and reduced thickness in right orbitofrontal cortex and inferior frontal gyrus, as well as bilateral parahippocampal cortex and temporal areas involved in learning and memory, auditory and visual function, in adolescents (28). Additionally, associations between allostatic load (i.e., a quantified index of multiple stress indicators) and reduced inferior parietal thickness (involved in somatosensory and visual-spatial functions) have been observed in both control subjects and patients with schizophrenia (4).
This study is the first to examine an association between LES and cortical thickness in CHR-P, and to test if LES and diagnostic group interact to predict cortical thickness. The lack of a significant interaction term (with exception of an unexpected interaction in left superior frontal cortex, discussed below) supports our hypothesis that LES is inversely associated with cortical thickness proportionately across groups. This is consistent with evidence suggesting that LES is a nonspecific risk factor for aberrant cortical thinning (4, 26–29, 73).
Indeed, in the present study, higher LES was significantly associated with reduced right orbitofrontal thickness, a region which showed accelerated reductions in thickness in CHR-C subjects in prior work from NAPLS 2 (39). However, diagnostic group did not predict orbitofrontal thickness after accounting for LES in the present study. Relatedly, thickness reductions in the parahippocampal gyrus have previously been demonstrated in CHR-P (74); however, in the present study, the association between parahippocampal thickness and diagnostic group was insignificant after accounting for LES (Figure 1b). These findings suggest that lower thickness in these areas may be better explained by stress exposure, although further research is needed to replicate these findings and elucidate where reductions in cortical thickness are better accounted for by LES versus diagnostic group.
Results of this study extend prior work indicating greater thickness reductions in parietal and temporal areas in CHR-P individuals than in HC (34, 36). Cortical thickness in brain areas significantly associated with LES partially mediated the association between LES and diagnostic group, and while our mediation analysis was speculative, results may suggest that LES impacts clinical outcomes in part through changing underlying brain morphology. Especially given the high sensitivity of mediation effects (Supplemental Figure 2), longitudinal work testing temporal precedence among LES, brain morphological changes, and clinical outcomes is needed.
Indeed, while it cannot be ruled out that reduced cortical thickness reflects a pre-baseline vulnerability that increases the likelihood of stress exposure or reactivity to stress (75), animal research indicates that stress exposure does have the potential to alter brain structure (76, 77), and studies of humans have identified associations between previous stress exposure and alterations in brain morphology (19, 20). Stress-induced HPA activation figures prominently in these processes. Glucocorticoids act as anti-inflammatory hormones under homeostatic conditions, but under conditions of prolonged stress, pro-inflammatory mechanisms and pathological processes may be triggered (13, 63, 78). In particular, it has been suggested that levels of brain-derived neurotrophic factor, important for the growth and differentiation of neurons, are suppressed when the inflammatory system is activated, leading to cognitive deficits and brain abnormalities (19, 49, 79). Given that cortical thickness partially reflects cell density (22, 80), and prolonged exposure to glucocorticoids may suppress neurogenesis (49), it is possible that prolonged stress conditions and cortisol release amplify normative levels of endogenous cortisol (81, 82) and suppress neurogenesis. Cellular processes including reduced neurogenesis are believed to present as exaggerated thinning of the cortex (22, 80) as observed at baseline in the present study.
The finding of a significant interaction between diagnostic group and LES in thickness of the left superior frontal cortex should be interpreted with caution in the present study. Examination of this interaction revealed significant, inverse associations, as anticipated, between LES and left superior frontal thickness in HC and CHR-NC, and a significant positive association in CHR-C. One possible explanation for this finding is that the CHR-C group was more likely to be on antipsychotic medication at baseline (39, 83). There is evidence that cumulative doses of second-generation antipsychotics increase cortical thickness in frontal regions; however, first-generation antipsychotics have been shown to reduce cortical thickness (84, 85). It is possible that second-generation antipsychotic medication reduces declines in cortical thickness which could contribute to the association between LES and left superior frontal thickness in CHR-C. We are underpowered to examine medication effects on cortical thickness (n = 6 CHR-C subjects were on antipsychotic medication at baseline), though supplemental analyses indicated that excluding participants who were prescribed antipsychotic medications did not change the observed interaction results (Supplemental Figure 4). Though interaction results in this report do not appear to be driven by antipsychotic medication use, further research is needed to examine whether first- and second-generation antipsychotics have discrete effects on cortical thickness in CHR-P.
In addition, while we observed a main effect of gender on thickness in frontal and temporal regions, consistent with prior work (62, 63), we did not observe an interaction between gender and LES, gender and diagnostic group, or all three of these variables. Future research examining the complex interplay among these variables should consider the potential roles of gonadal hormones (25, 66) and endogenous glucocorticoids (14, 64), given their putative roles in cortical thinning and differences between the genders.
The present study has several limitations. First, this is a cross-sectional examination of a dynamic phenomenon (i.e., cortical thinning) which precludes firm conclusions about causal direction; i.e., whether LES precipitates reduced cortical thickness or reduced cortical thickness is a pre-existing characteristic influencing how individuals respond to stress. It is likely that the relation is bidirectional. Recent cross-sectional work has demonstrated that greater thickness in cortical regions associated with stress and emotional processing is associated with greater psychological resilience (75), and reduced prefrontal cortical thickness is associated with greater emotional (e.g., amygdalar) reactivity (86). In addition, given evidence that cortical thickness relates to both genetic and environmental variables (47, 87), elucidating the respective contributions of such variables to aberrant cortical thinning is an important area for future research. Prior research also suggests that psychosis risk prediction is optimized when other factors, such as neurocognitive function, symptom severity, and genetic risk, are considered (10, 88). Longitudinal examination of the relations among these factors and other indications of psychological health in youth at CHR-P and comparison subjects (e.g., stress, trauma, medication use, brain morphology) are warranted. For example, it is possible that stressful experiences are associated with greater impulsivity among CHR-P, as has been found among individuals with a trauma history (89), potentially leading to greater substance use or other behaviors that influence brain structure. In addition, it cannot be ruled out that the CHR-C group in the present study included individuals with a relatively favorable prognosis given that CHR-P subjects were recruited from a help-seeking population (50, 52). As such, CHR-P participants in the present study may have higher insight, greater social support, etc., than the general population of CHR-P. This may have dampened our capacity to detect differential effects of LES on cortical thickness between CHR-NC and CHR-C. Finally, research in this area should examine interactions between developmental stage and brain abnormalities. In particular, normative HPA axis activation and heightened levels of endogenous glucocorticoids render adolescence a developmental period particularly vulnerable to environmental stress (90, 91). There is evidence that the adverse effects of stress exposure are more pronounced in adolescence than in adulthood (92–94); relatedly, some research suggests that genetic influences on cortical thickness are dependent on age (42, 95).
In conclusion, this is the first study to examine the relation between LES and cortical thickness in CHR-P. With exception of an interaction between diagnostic group and LES in left superior frontal thickness, the findings indicate that the adverse effects of LES are nonspecific; LES was associated with reduced cortical thickness in both HC and CHR-P groups in bilateral superior and middle temporal cortex, as well as additional aspects of temporal, parietal, occipital, and orbitofrontal cortex in the right hemisphere. Moreover, LES accounted for significant thickness reductions in temporal, parietal, orbitofrontal, and parahippocampal regions previously associated with CHR-P in studies that did not test for effects of LES (34–38). Given the considerable overlap between brain regions demonstrating thickness reductions associated with LES and diagnostic group in this and prior work, further research is needed to elucidate the relationships among these variables in contributing to deviations in brain morphology.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation (NSF) (No. DGE-1752134) to Ms. Collins, by National Institutes of Health (NIH) grants U01 MH081902 to Dr. Cannon, P50 MH066286 to Dr. Bearden, U01 MH081857 to Dr. Cornblatt, U01 MH82022 to Dr. Woods, U01 MH066134 to Dr. Addington, U01 MH081984 to Dr. Cadenhead, R01 U01 MH066069 to Dr. Perkins, U01 MH082004 to Dr. Mathalon, U01 MH081928 to Dr. Seidman, and U01 MH081988 to Dr. Walker.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Norman RM, Malla AK. (1993): Stressful life events and schizophrenia: A review of the research. British Journal of Psychiatry 162: 161–166. [DOI] [PubMed] [Google Scholar]
- 2.Walker EF, Diforio D. (1997): Schizophrenia: A neural diathesis-stress model. Psychological Review 104(4): 667. [DOI] [PubMed] [Google Scholar]
- 3.Cotter D, Pariante CM. (2002): Stress and the progression of the developmental hypothesis of schizophrenia. British Journal of Psychiatry 181: 363–365. [DOI] [PubMed] [Google Scholar]
- 4.Chiappelli J, Kochunov P, Savransky A, Fisseha F, Wisner K, Du X, et al. (2017): Allostatic load and reduced cortical thickness in schizophrenia. Psychoneuroendocrinology 77: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lysaker PH, LaRocco VA. (2008): The prevalence and correlates of trauma-related symptoms in schizophrenia spectrum disorder. Comprehensive Psychiatry 49: 330–334. [DOI] [PubMed] [Google Scholar]
- 6.Sahin S, Yuksel C, Guler J, Karadayi G, Akturan E, Gode E, et al. (2013): The history of childhood trauma among individuals with ultra high risk for psychosis is as common as among patients with first-episode schizophrenia. Early Interventions in Psychiatry 7: 414–420. [DOI] [PubMed] [Google Scholar]
- 7.Velthorst E, Nelson B, O’Connor K, Mossalheb N, de Haan L, Bruxner A, et al. (2013): History of trauma and the association with baseline symptoms in an ultra-high risk for psychosis cohort. Psychiatry Research 210: 75–81. [DOI] [PubMed] [Google Scholar]
- 8.Popovic D, Schmitt A, Kaurani L, Senner F, Papiol S, Malchow B, Fischer A, et al. (2019): Childhood trauma in schizophrenia: Current findings and research perspectives. Frontiers in Neuroscience 13(274): 10.3389/fnins.2019.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. (2012): Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophrenia Bulletin 38: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trotman HD, Holtzman CW, Walker EF, Addington JM, Bearden CE, Cadenhead KS, et al. (2014): Stress exposure and sensitivity in the clinical high-risk syndrome: Initial findings from the North American Prodrome Longitudinal Study (NAPLS). Schizophrenia Research 160: 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayo D, Corey S, Kelly LH, Yohannes S, Youngquist AL, Stuart BK, et al. (2017): The role of trauma and stressful life events among individuals at clinical high risk for psychosis: A review. Frontiers in Psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen S, Murphy ML, Prather AA. (2019): Ten surprising facts about stressful life events and disease risk. Annual Review of Psychology 70: 577–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS, Akil H. (2020): Revisiting the stress concept: Implications for affective disorders. Journal of Neuroscience 40(1): 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eiland L, Romeo RD. (2013): Stress and the developing adolescent brain. Neuroscience 249: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colyn L, Venzala E, Marco S, Perez-Otano I, Tordera RM. (2019): Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behavioral Brain Research 373: 10.1016/j.bbr.2019.112079. [DOI] [PubMed] [Google Scholar]
- 16.Hermann JP, Ostrander MM, Mueller NK, Figueiredo H. (2005): Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuropsychopharmacology 29: 1201–1213. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RM, Johnson SB, Lingg RT, Hinz DC, Romig-Martin SA, Radley JJ. (2019): Evidence for similar prefrontal structural and functional alterations in male and female rats following chronic stress or glucocorticoid exposure. Cerebral Cortex 00: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI…Walker EF. (2013): Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience 249: 172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf OT. (2003): HPA axis and memory. Best Practices in Research in Clinical Endocrinology 17: 287–299. [DOI] [PubMed] [Google Scholar]
- 20.Merz EC, Desai PM, Maskus EA, Melvin SA, Rehman R, Torres SD, et al. (2019): Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biological Psychiatry 86(12): 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, et al. (2011): How does your cortex grow? Journal of Neuroscience 31: 7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wierenga LM, Langen M, Oranje B, Durston S. (2014): Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87: 120–126. [DOI] [PubMed] [Google Scholar]
- 23.Koelkebeck K, Miyata J, Kubota M, Kohl W, Son S, Fukuyama H, et al. (2014): The contribution of cortical thickness and surface area to gray matter asymmetries in the healthy human brain. Human Brain Mapping 35: 6011–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gennatas ED, Avants BB, Wolf DH, Satterthwaite TD, Ruparel K, Ciric R, et al. (2017): Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. The Journal of Neuroscience 37(20): 5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. (2015): A longitudinal study: Changes in cortical thickness and surface area during pubertal maturation. PloS One: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, Vermetten E. (2008): Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage 41(3): 675–681. [DOI] [PubMed] [Google Scholar]
- 27.Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J. (2011): Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biological Psychiatry 69(5): 487–494. [DOI] [PubMed] [Google Scholar]
- 28.Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, et al. (2016): Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry 57(10): 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranger M, Chau CM, Garg A, Woodward TS, Beg MF, Bjornson B, et al. (2013): Neonatal pain-related stress predicts cortical thickness at age 7 years in children born very preterm. PLoS One 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar U, Arya A, Agarwal V. (2017): Neural alterations in ADHD children as indicated by voxel-based cortical thickness and morphometry analysis. Brain and Development 39(5): 403–410. [DOI] [PubMed] [Google Scholar]
- 31.Suh JS, Schneider MA, Minuzzi L, MacQueen GM, Strother SC, Kennedy SH, et al. (2019): Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Progress in Neuropsychopharmacology and Biological Psychiatry 88: 287–302. [DOI] [PubMed] [Google Scholar]
- 32.Hanford LC, Nazarov A, Hall GB, Sassi RB. (2016): Cortical thickness in bipolar disorder: A systematic review. Bipolar Disorders 18(1): 4–18. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Nuechterlein KH, Phillips O, Hamilton LS, Subotnik KL, Asarnow RF, et al. (2010): The contributions of disease and genetic factors toward cortical thinning in schizophrenia: The UCLA family study. Schizophrenia Research (2–3): 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, et al. (2011): Cortical thickness reductions in individuals at ultra-high-risk for psychosis. Schizophrenia Bulletin 37: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak YB, Kim M, Cho KIK, Lee J, Lee TY, Kwon JS. (2019): Reduced cortical thickness in subjects at clinical high risk for psychosis and clinical attributes. Aust NZ J Psychiatry 53(3): 219–227. [DOI] [PubMed] [Google Scholar]
- 36.Tomyshev AS, Lebedeva IS, Akhadov TA, Omelchenko MA, Rumyantsev AO, Kaleda VG. (2019): Alterations in white matter microstructure and cortical thickness in individuals at ultra-high risk of psychosis: A multimodal tractography and surface-based morphometry study. Psychiatry Res Neuroimaging 289: 26–36. [DOI] [PubMed] [Google Scholar]
- 37.Thormodsen R, Rimol LM, Tamnes CK, Juuhl-Langseth M, Holmen A, Emblem KE, et al. (2013): Age-related cortical thickness differences in adolescents with early-onset schizophrenia compared with healthy adolescents. Psychiatry Research: Neuroimaging 214: 190–196. [DOI] [PubMed] [Google Scholar]
- 38.Klauser P, Zhou J, Lim JK, Poh JS, Zheng H, Tng HY, et al. (2015): Lack of evidence for regional brain volume or cortical thickness abnormalities in youths at clinical high risk for psychosis: Findings from the longitudinal youth at risk study. Schizophrenia Bulletin 41: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon TD, Chung Y, He G, Sun D, Jacobsen A, van Erp TG, et al. (2015): Progressive reduction in cortical thickness as psychosis develops: A multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biological Psychiatry 77(2): 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagstyl K, Ronan L, Goodyer IM, Fletcher PC. (2015): Cortical thickness gradients in structural hierarchies. Neuroimage 111: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez DL, Matin N, Williams B, Tanev K, Makris N, LaFrance WC Jr, et al. (2018): Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Human Brain Mapping 39: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, et al. (2009): Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping 30:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neilson E, Shen X, Cos SR, Clarke TK, Wigmore EM, Gibson J, et al. (2019): Impact of polygenic risk for schizophrenia on cortical structure in UK Biobank. Biological Psychiatry 86(7): 536–544. [DOI] [PubMed] [Google Scholar]
- 44.Alnæs D, Kaufmann T, van der Meer D, Córdova-Palomera A, Rokicki J, Moberget T, et al. (2019): Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry 76(7): 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccolo LR, Merz EC, He X, Sowell ER, Noble KG. (2016): Age-related differences in cortical thickness vary by socioeconomic status. PLoS One 11(9): e0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shakoor S, Zavos HMS, Haworth CMA, McGuire P, Cardno AG, Freeman D, et al. (2016): Association between stressful life events and psychotic experiences in adolescence: Evidence for gene-environment correlations. The British Journal of Psychiatry 208: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neilson E, Bois C, Gibson J, Duff B, Watson A, Roberts N, et al. (2017): Effects of environmental risks and polygenic loading for schizophrenia on cortical thickness. Schizophrenia Research 184: 128–136. [DOI] [PubMed] [Google Scholar]
- 48.Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. (2010): Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. Journal of Abnormal Psychology 119: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pruessner M, Cullen AE, Aas M, Walker EF. (2017): The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neuroscience and Biobehavioral Reviews 73: 191–218. [DOI] [PubMed] [Google Scholar]
- 50.Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, et al. (2012): North American Prodrome Longitudinal Study (NAPLS 2): Overview and recruitment. Schizophrenia Research 142: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. (1978): Exemplification of a method for scaling life events: The Peri Life Events Scale. Journal of Health and Social Behavior 19: 205–229. [PubMed] [Google Scholar]
- 52.Addington J, Piskulic D, Liu L, Lockwood J, Cadenhead KS, Cannon TD, et al. (2017): Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophrenia Research 190: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon TD, Sun F, McEwen SJ, Papademetris X, He G, van Erp TG, et al. (2014): Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Human Brain Mapping 35: 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung Y, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, et al. (2018): Use of machine learning to determine deviance in neuroanatomical maturity associated with future psychosis in youths at clinically high risk. JAMA Psychiatry 75(9): 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischl B (2012): FreeSurfer. Neuroimage 62(2): 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischl B, Dale AM. (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20): 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernal-Rusiel JL, Atienza M, Cantero JL. (2010): Determining the optimal level of smoothing in cortical thickness analysis: a hierarchical approach based on sequential statistical thresholding. Neuroimage, 52(1): 158–171. [DOI] [PubMed] [Google Scholar]
- 58.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. (2014): Permutation inference for the general linear model. Neuroimage 92: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SM, Nichols TE. (2009): Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage 44(1): 83–98. [DOI] [PubMed] [Google Scholar]
- 60.Tingley D, Yamamoto T, Hirose K, Keele L, & Imai K. (2014): Mediation: R package for causal mediation analysis. Journal of Statistical Software. [Google Scholar]
- 61.Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, Kim SI. (2006): Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage, 31(1): 31–38. [DOI] [PubMed] [Google Scholar]
- 62.Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, DeLuca H, et al. (2006): Gender effects on cortical thickness and the influence of scaling. Human Brain Mapping, 27(4): 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudolph KD, Hammen C. (1999): Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development, 70(3): 660–677. [DOI] [PubMed] [Google Scholar]
- 64.Goel N, Workman JL, Lee TT, Innala L, Viau V. (2014): Sex differences in the HPA axis. Comprehensive Physiology 4(3): 1121–1155. [DOI] [PubMed] [Google Scholar]
- 65.Luders E, Toga AW. (2010): Sex differences in brain anatomy. Progress in Brain Research 186. [DOI] [PubMed] [Google Scholar]
- 66.Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, et al. (2012): Sex matters during adolescence: Testosterone-related cortical thickness maturation differs between boys and girls. PLos One 7(3): e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rietschel L, Lambert M, Karow A, Zink M, Muller H, Heinz A, et al. (2017): Clinical high risk for psychosis: Gender differences in symptoms and social functioning. Early Intervention in Psychiatry 11(4): 306–313. [DOI] [PubMed] [Google Scholar]
- 68.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. (2012): Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophrenia Research Treatment. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Baram TZ. (2016): Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 41: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shalev G, Admon R, Berman Z, Joel D. (2020): A mosaic of sex-related structural changes in the human brain following exposure to real-life stress. Brain Structure and Function 225: 461–466. [DOI] [PubMed] [Google Scholar]
- 71.Lim L, Hart H, Mehta M, Worker A, Simmons A, Mirza K, Rubia K. (2018): Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychological Medicine 48(6): 1034–1046. [DOI] [PubMed] [Google Scholar]
- 72.Agorastos A, Pervanidou P, Chrousos GP, Baker DG. (2019): Developmental trajectories of early life stress and trauma: A narrative review on neurobiological aspects beyond stress system dysregulation. Frontiers in Psychiatry 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monninger M, Kraaijvenvanger EJ, Pollok TM, Boecker-Schlier R, Jennen-Streinmetz C, Baumeister S, et al. (2020): The long-term impact of early life stress on orbitofrontal cortical thickness. Cerebral Cortex 30: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 74.Tognin S, Riecher-Rossler A, Meisenzahl EM, Wood SJ, Hutton C, Borgwardt SJ, et al. (2014): Reduced parahippocampal cortical thickness in subjects at ultra-high risk for psychosis. Psychological Medicine 44(3): 10.1017/S0033291713000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kahl M, Wagner G, de la Cruz F, Kohler S, Schultz CC. (2020): Resilience and cortical thickness: A MRI study. European Archives of Psychiatry and Clinical Neuroscience 270(5): 533–539. [DOI] [PubMed] [Google Scholar]
- 76.Sarabdjitsingh RA, Loi M, Joels M, Dijkhuizen RM, van der Toorn A. (2017): Early life stress-induced alterations in rat brain structures measured with high resolution MRI. PLoS One 12(9): e0185061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magalhães R, Barrière DA, Novais A, Marques F, Marques P, Cerqueira J, et al. (2018): The dynamics of stress: A longitudinal MRI study of rat brain structure and connectome. Molecular Psychiatry 23: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 78.Vinson GP. (2009): The adrenal cortex and life. Molecular and Cellular Endocrinology 300: 2–6. [DOI] [PubMed] [Google Scholar]
- 79.McEwen BS, Gianaros PJ. (2011): Stress- and allostasis-induced brain plasticity. Annual Reviews of Medicine 62: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clausen AN, Clarke E, Phillips RD, Haswell C, VA Mid-Atlantic MIRECC Workgroup, Morey RA. (2020): Combat exposure, posttraumatic stress disorder, and head injuries differentially relate to alterations in cortical thickness in military Veterans. Neuropsychopharmacology 45(3): 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pryce CR. (2008): Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: Inter-species and intra-species differences. Brain Research Reviews 57: 596–605. [DOI] [PubMed] [Google Scholar]
- 82.Dziedzic N, Ho A, Adabi B, Foilb AR, Romeo RD. (2014): Shifts in hormonal stress reactivity during adolescence are not associated with changes in glucocorticoid receptor levels in the brain and pituitary of male rats. Developmental Neuroscience 36: 261–268. [DOI] [PubMed] [Google Scholar]
- 83.Cornblatt BA, Carrión RE, Addington J, Seidman L, Walker EF, Cannon TD, et al. (2012): Risk factors for psychosis: Impaired social and role functioning. Schizophrenia Bulletin 38(6): 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, et al. (2011): Changes in cortical thickness during the course of illness in schizophrenia. Archives of General Psychiatry 68(9): 871–880. [DOI] [PubMed] [Google Scholar]
- 85.Ansell BRE, Dwyer DB, Wood SJ, Bora E, Brewer WJ, Proffitt TM, et al. (2015): Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychological Medicine 45(3): 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albaugh MD, Hudziak JJ, Orr C, Spechler PA, Chaarani B, Mackey S, et al. (2019): Amygdalar reactivity is associated with prefrontal cortical thickness in a large population-based sample of adolescents. PLoS One 14(5): e0216152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin Jin M, Jeon H, Ho Hyun M, Lee SH. (2019): Influence of childhood trauma and brain-derived neurotrophic factor Val66Met polymorphism on posttraumatic stress symptoms and cortical thickness. Nature 9: 6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Perkins DO, Olde Loohuis L, Barbee J, Ford J, Jeffries CD, Addington J, et al. (2020): Polygenic risk score contribution to psychosis prediction in a target population of persons at clinical high risk. American Journal of Psychiatry 177(2): 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris VL, Huffman LG, Naish KR, Holshausen K, Oshri A, McKinnon M, et al. (2020): Impulsivity as a mediating factor in the association between posttraumatic stress disorder symptoms and substance use. Psychological Trauma 12(6): 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lupien SJ, Ouellet-Morin I, Herba CM, Juster R, McEwen BS. (2016): From Vulnerability to Neurotoxicity: A Developmental Approach to the Effects of Stress on the Brain and Behavior. In Spengler D, Binder E (Eds.). Epigenetics and Neuroendocrinology: Clinical Focus on Psychiatry, Vol 1. Cham: Springer. [Google Scholar]
- 91.Romeo RD. (2017): The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. Brain Research 1654: 185–191. [DOI] [PubMed] [Google Scholar]
- 92.Lupien SJ, McEwen BS, Gunnar MR, Heim C. (2009): Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience 10: 434–445. [DOI] [PubMed] [Google Scholar]
- 93.Pechtel P, Pizzagalli DA. (2011): Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 214: 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yahfoufi N, Matar C, Ismail N. (2020): Adolescence and aging: Impact of adolescence inflammatory stress and microbiota alterations on brain development, aging, and neurodegeneration. Journals of Gerontology Series A: Biological and Medical Sciences 75(7): 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pui-Yee Wong A, French L, Leonard G, Perron M, Pike GB, Richer L, et al. (2018): Inter-regional variations in gene expression and age-related cortical thinning in the adolescent brain. Cerebral Cortex 28(4): 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


