Abstract
Clostridium butyricum has been widely considered an antibiotic substitute in recent years. It can promote growth performance, improve the immune response and enhance the intestinal barrier function of the host. In the present study, 1-d-old Arbor Acres (AA) broilers were fed C. butyricum (1 × 109 cfu/kg) for 28 d. The transcriptomic characteristics of epithelial cells of the cecal mucosa were determined by RNA-sequence, and the cecal microbiota composition was explored by 16S ribosomal RNA gene sequencing. The changes in the intestinal mucosa of broilers were then analyzed by tissue staining. Gene Ontology (GO) annotations identified substance transport and processes and pathways that might participate in intestinal development and cell viability. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that the differentially expressed genes are involved in numerous pathways related to amino acid and vitamin metabolism and antioxidant and defensive functions, among others. The relative expression of some genes associated with intestinal barrier function (claudins 2, 15, 19, and 23, tight junction proteins 1, 2, and 3 and mucin 1) was significantly increased in the treatment group (P < 0.05 or P < 0.01). Moreover, the proportion of Firmicutes was higher in the C. butyricum-treated group, whereas the proportion of Proteobacteria was higher in the control group. At the genus level, the relative abundances of Butyricicoccus and Lactobacillus, among other bacteria, were increased after C. butyricum supplementation. The tissue staining analysis showed that the cecal mucosa of broilers was significantly ameliorated after the addition of C. butyricum (P < 0.05 or P < 0.01). These results showed that dietary supplementation with C. butyricum can enhance the antioxidant capacity, mucosal barrier function, and stabilize the cecal microbiota, resulting in improving the growth performance.
Keywords: Clostridium butyricum, RNA-sequence, 16S ribosomal RNA, Intestinal barrier, Broiler
1. Introduction
The irrational use of antibiotics usually leads to animal-derived food safety risks, ecological hazards and the development of antibacterial resistance (Ang et al., 2004; Levy and Marshall, 2004). Probiotics, prebiotics, feed enzymes, herbs, acidifiers and essential oils, which work by interacting with gut microbes and the host intestinal mucosa, have been the focus of published research on antibiotic substitutes (Liu et al., 2020; Saadatmand et al., 2019; Yan et al., 2019). The intestinal mucosa, which harbors a very large microbial community that not only nourishes the host but also enhances immunity and disease resistance, serves as a defensive frontier (Chambers and Gong, 2011; Kau et al., 2011; Torok et al., 2011). However, the mechanism underlying the effect of antibiotic substitutes on gut microbes and the intestinal mucosa remains unclear.
Clostridium butyricum (C. butyricum) colonizes mainly in the hindgut and thrives and reproduces primarily through the residual chyme digested and absorbed by the host. The digestive enzyme system of C. butyricum can provide metabolites, particularly short-chain fatty acids (SCFA), as an energy source, which can improve the host's body weight and feed conversion efficiency (Kong et al., 2006; Takahashi et al., 2018; Zhao et al., 2014). Metabolites (enzymes, vitamins and SCFA) can also promote the proliferation of beneficial bacteria, including Bifidobacterium and Lactobacillus, and inhibit the growth of harmful bacteria, such as Escherichia coli and Salmonella enteritis, thus improving the composition of the intestinal microbiota (Zhao et al., 2020; Miao et al., 2018; Romick-Rosendale et al., 2014; Takahashi et al., 2004; Zhang et al., 2016). Butyric acid, a nutrient favored by intestinal epithelial cells, promotes the growth and development of intestinal organs and improves the integrity of intestinal barriers (Leeson et al., 2005; Peng et al., 2009; Wang et al., 2012; Yan and Ajuwon, 2017). Studies have demonstrated that some probiotics can promote restoration of the intestinal barrier integrity damaged by pathogens and toxins (Gadde et al., 2017; Park et al., 2020). C. butyricum decreases the production of interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α) in the liver and cecal tissue and alleviates inflammation through the downregulation of toll-like receptor 4 (TLR4)-, myeloid differentiation factor 88 (MyD88)-, and nuclear factor kappa B (NF-κB)-dependent pathways (Zhao et al., 2017b). Gao et al. (2012) demonstrated that C. butyricum could prevent enterohemorrhagic E. coli (EHEC)-induced apoptosis through the inhibition of EHEC viability and the induction of EHEC-mediated apoptosis. Chickens fed diets supplemented with C. butyricum exhibit high activities of superoxide dismutase and glutathione S-transferase, and low malondialdehyde concentrations in the liver and intestinal mucosa, which indicates that C. butyricum has antioxidant properties (Liao et al., 2015a, 2015c). The immunoglobulin level in serum and the volatile fatty acid level in the cecal digesta are increased in broilers fed a diet supplemented with C. butyricum (Han et al., 2018). Previous studies have shown that C. butyricum supplementation increases the intramuscular fat levels and improves the flavor of cooked breast muscle (Liu et al., 2018; Zhao et al., 2017a).
In recent years, C. butyricum has been widely used as an alternative to antibiotics to promote growth performance and nutrient utilization efficiency and thus improve the intestinal morphology (Abdel-Latif et al., 2018; Liao et al., 2015c; Song et al., 2006), but the underlying mechanisms, particularly the mechanism through which C. butyricum improves host disease resistance and production performance, are less well understood. The composition of the intestinal microbiota is multifarious, and the metabolic functions of intestinal epithelial cells might show differences due to a variety of influencing factors, including the feed, drinking water, breed, and rearing model. In-depth research on supplementation with C. butyricum by combining 16S ribosomal RNA (16S rRNA) gene sequencing and RNA-sequence (RNA-seq) technology can help us better understand the precise mechanism and guide the exploration of valuable probiotics as alternatives to antibiotics. Therefore, the purpose of this study was to investigate the effects of dietary supplementation with C. butyricum on the cecal mucosa in broilers using high-throughput sequencing techniques and histological staining (Lindner et al., 2013; Wu et al., 2017).
2. Materials and methods
2.1. Ethics approval and consent to participate
To ensure the welfare of the animals, all experiments and animal procedures were conducted strictly according to the protocols recommended by the Institutional Animal Care and Use Committee (IACUC) of Henan Agricultural University (permit number: 19-0220) and the protocols supported by the regulations for animal experiments established by the Ministry of Science and Technology in China (2014). All the experiments and methods were designed with the aim of minimizing animal suffering.
2.2. Experimental design, animals and management
A total of 120 one-day-old Arbor Acres (AA) broilers of similar weight were provided by the hennery at the Henan Research Center of Germplasm Resources for Poultry. All broilers were randomly divided into 2 groups with 6 replicates each, and each replicate included 10 birds. The broilers in the C. butyricum group (group B, B stands for butyricum) were fed a basal diet supplemented with C. butyricum F06 (1 × 109 cfu/kg). C. butyricum F06 has been deposited in the China Center for Type Culture Collection, and the deposition number is CCTCC M 2019962. The C. butyricum, which was prepared in liquid, was sprayed into the basal diet while stirring. The broilers in the control group (group C, C stands for control) were fed only the basal diet. The feeding stages of AA broilers were divided into 0 to 14 d and 15 to 28 d, which corresponds to the nutritional requirements of chickens (NRC, 1994). The composition and nutritional level of the basal diet, which are based on NRC (1994) and China's feed composition and nutritional value, are shown in Appendix Table 1. Supplementation with C. butyricum was started on the 1st day. The birds were raised in cages and provided free access to feed and water.
Previous studies have shown that the intestinal microbiota of 3- to 4-week-old AA broilers had developed to a generally stable state (Huang et al., 2018). Therefore, in this study, C. butyricum was added to the feed for 4 weeks, and samples were obtained at an age of 28 d. One broiler was randomly selected from each replicate; in other words, 6 broilers (6 from the replicates of the treatment group and 6 from replicates of the control group) were randomly selected from each replicate for the follow-up test, and both the treatment and control groups included 6 independent biological replicates to avoid the influence of extreme individual differences on the experimental data. By using the same broiler breed (with male chickens at the same age) as well as the same basal dietary components, feeding methods and environment, a sampling rate of 10% would ensure high data reliability. All broilers were maintained in accordance with appropriate guidelines for raising broilers. Every chicken was fed in a single cage, and one chicken was randomly selected from each cage.
2.3. Sample collection
2.3.1. Collection of cecal epithelial cells for RNA-seq
One broiler was randomly selected from each replicate, anesthetized with pentobarbital sodium, and euthanized by intravenous bloodletting. From each chicken, a section of the fresh cecum was collected, washed with freshly prepared diethyl pyrocarbonate (DEPC) water, collected in a sterile and RNase-/DNase-free EP tube and stored at −80 °C for RNA-seq.
2.3.2. Collection of cecal contents for 16S rRNA gene sequencing
Another segment of the cecum was used for 16S rRNA gene sequencing as soon as possible by transferring the fresh contents into sterile enzyme-treated EP tubes. A total of 12 samples were used in the 16S rRNA gene sequencing study.
2.3.3. Collection of cecal tissue for Hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) staining
The intestinal tissue was fixed with 4% formaldehyde for 15 min and stored at room temperature for tissue section staining.
2.4. Extraction and quality assessment of total RNA and DNA from cecal tissue and library construction
Total RNA was extracted from cecal epithelial cells using the TRIzol reagent (Invitrogen Life Technologies), and the concentration, quality and integrity were then determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). The quality assessment results of RNA are shown in Appendix Table 2. Three micrograms of RNA were used as the input material for RNA sample preparation. Sequencing libraries were generated using the TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). The sequencing library was then sequenced using a HiSeq platform (Illumina) (Shanghai Personal Biotechnology Co., Ltd).
Total bacterial genomic DNA samples were extracted using the Fast DNA Extraction Kit (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer's instructions and stored at −20 °C prior to further analysis. The quantity and quality of the extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and by agarose gel electrophoresis, respectively. PCR amplification of the bacterial 16S rRNA gene V3–V4 region was performed using the forward primer (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR conditions were as described by Wang et al. (2020) (Shanghai Personal Biotechnology Co., Ltd). Illumina's TruSeq Nano DNA LT Library Prep Kit was used to prepare the sequencing library.
2.5. Bioinformatics analysis of transcriptome sequencing data
After obtaining the transcriptome sequencing data of 12 chicken cecal epithelial cells, we used the chicken reference genome (GRCg6a) as the reference sequence for routine transcriptome analysis. First, raw data were filtered, and the filtered reads were compared to the reference genome by using HISAT2 software. Based on the comparison results, HTseq (0.11.3) software was used to calculate the expression of each gene. On this basis, expression difference analysis, enrichment analysis and cluster analysis were carried out for the samples, and the relevant pictures were drawn with the R programming language (3.6.1).
2.6. Illumina MiSeq high-throughput sequencing technology
The 16S rRNA library sequencing process was as follows: 1) before sequencing on the computer, the library was tested using an Agilent Bioanalyzer with an Agilent High-Sensitivity DNA Kit; 2) the library was quantified using a Quant-iT PicoGreen dsDNA Array Kit and a Promega QuantiFluor fluorescence quantitation system; 3) after diluting the qualified online sequencing libraries (the index sequence was not repeatable), the samples were mixed according to the required sequencing amounts at corresponding proportions and transformed into a single chain by NaOH for online sequencing; and 4) MiSeq Reagent Kit V3 (600 cycles) was used for 2 × 300 bp sequencing. Due to the short read length of MiSeq sequencing and to ensure the sequencing quality, the suggested optimal sequencing length of the target fragment was 200 to 450 bp.
2.7. Bioinformatics analysis of 16S rRNA gene sequencing data
The analyses of sequencing data were mainly performed using QIIME and R packages (v3.2.0). Operational taxonomic unit (OTU)-level alpha diversity indexes, such as the Chao1 richness estimator, abundance-based coverage estimator (the ACE metric), Shannon diversity index, and Simpson index, were calculated using the OTU table in QIIME. Abundance curves ranked based on the OTU level were generated to compare the richness and evenness of the OTU among the samples. Beta diversity analysis was performed to investigate the structural variations in the microbial communities across samples using UniFrac distance metrics. The main purpose of the beta diversity analysis was to investigate the similarity in the community structure among different samples. Principal component analysis (PCA) was performed for the natural decomposition of the community data structure and for sorting the samples (ordination) to observe the differences between samples. The classification units and samples were sorted according to the clustering results and are presented as a thermal map. Taxa at high and low abundances could be distinguished by clustering, and the similarity in the community composition between samples can be depicted as a color gradient.
2.8. Verification of RNA-seq results by reverse-transcription quantitative PCR (RT-qPCR)
To verify the accuracy of the transcriptome data, 6 genes, including 3 significantly upregulated genes, 1 significantly downregulated gene and 2 genes with no significant difference in expression, were randomly selected. Primers were designed for quantitative reverse transcription analysis using RNA as the material for transcriptome sequencing. The primer sequences are shown in Appendix Table 3. The RT-qPCR protocol was as follows: One microgram of RNA was reverse transcribed to cDNA, and qPCR were performed in a 10-μL volume containing 1.0 μL of cDNA, 5 μL of SYBR Premix Ex Taq II (TaKaRa, Dalian, China), 0.5 μL of each primer (10 μmol/L), and 3 μL of RNase-free water. A LightCycler96 real time PCR system was used (Roche Applied Science, Indianapolis, IN, USA), and the amplification conditions were as follows: 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 30 s; and a final 10-min extension at 72 °C... All the reactions were performed in triplicate. The gene expression data were normalized to the reference gene using the ΔΔCT method, and the 2ˆ-ΔCT values were statistically analyzed.
2.9. HE and PAS staining
Fresh tissues were fixed with polyformaldehyde solution (4%) for more than 24 h, and paraffin sections were then prepared and used for HE and PAS staining. The specific methods were performed according to the previous descriptions (Bialkowska et al., 2016; Osho et al., 2017; Serafini et al., 2017). Microscopic examination and image acquisition and analysis were then performed. The software program used for the histological analysis was ImageJ (1.82u).
2.10. Statistical analysis
A completely randomized test design was used in the study. The significance of the difference between the means of the groups was determined by Student's t-test. Differences with P < 0.05 (∗) and P < 0.01 (∗∗) were considered to be significant and highly significant, respectively. The statistical calculations used in this study were performed using IBM SPSS Statistics (version 24.0).
3. Results
3.1. Alpha and beta diversity in groups B and C
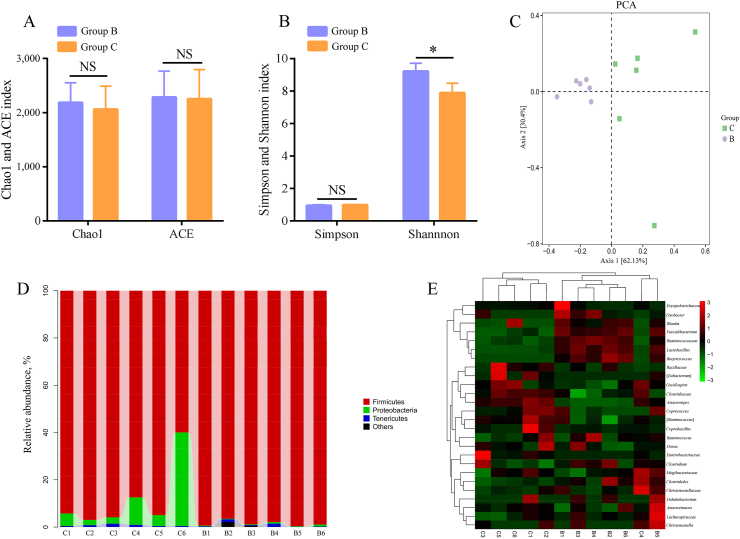
Many indexes (Simpson, Chao1, ACE, and Shannon) reflect the alpha diversity of microbial communities, and an analysis of the Simpson, Chao1, ACE, Shannon and other alpha diversity indexes of groups B and C (Fig. 1A, B) showed that the Shannon index of group B was significantly higher than that of group C (P = 0.02). The PCA chart (Fig. 1C) showed distinct separation between groups B and C, and the degree of dispersion among the group B samples was less than that among the group C samples.
Fig. 1.
Microbial diversity of the chicken cecum. (A) Chao1 and the observed abundance-based coverage estimator (ACE) indexes. (B) Simpson and Shannon indexes. (C) Principal component analysis (PCA) plot of the cecal microbiota in groups B and C. (D) Composition and distribution of the microbiota at the phylum level. (E) The heat map showing the composition of the genus-level microbiota combined with the results from the cluster analysis. B1 to B6 refer to group B, the Clostridium butyricum group; C1 to C6 refer to group C, the control group. ∗, P < 0.05; NS, no significant difference.
3.2. Differences at the phylum and genus levels between groups B and C
Distribution tables of the composition and abundance of each sample at 5 levels, namely, phylum, class, order, family and genus, were obtained using QIIME software, and the analysis results are presented as histograms. The phylum-level analysis showed that Firmicutes was the main phylum in both groups. The Proteobacteria content in group C was higher than that in group B (Fig. 1D). The community composition data at the generic level were clustered according to the abundance distribution of the taxa or the similarity between samples. The 50 most abundant genera were clustered and analyzed using R software, and a heat map was drawn (Fig. 1E). The analysis at the genus level revealed that the relative abundances of Butyricicoccus and Lactobacillus, among other bacteria, increased in response to C. butyricum supplementation.
3.3. Differentially expressed genes
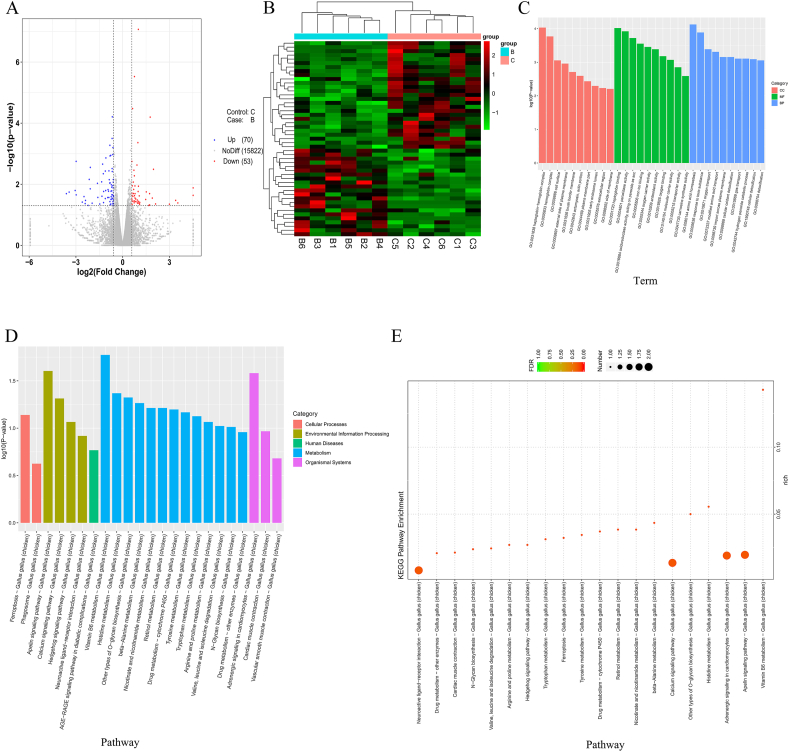
The comparison of all the genes in groups B and C identified 123 differentially expressed genes (DEG), which included 70 upregulated and 53 downregulated genes (Fig. 2A, Appendix Table 4). A cluster pattern analysis of the DEG between groups B and C is shown in Fig. 2B, and the results indicated that DEG from each of 6 samples selected from group B or C presented a similar expression pattern.
Fig. 2.
The RNA-seq of chicken cecal epithelial cells. (A) Volcano plot of differentially expressed genes. (B) Clustering heatmap of differentially expressed genes. Each row represents a gene, and each column is a sample. The red color indicates highly expressed genes, and the green color indicates genes showing with low expression. B1 to B6 refer to Group B, the Clostridium butyricum group; C1 to C6 refer to Group C, control group. (C) Histogram of the Gene Ontology (GO) enrichment analysis results. The horizontal coordinate is the term of the GO level 2 term, the vertical coordinate is the -log10(P-value) showing the enrichment of each term, and the number on the column shows the number of differential genes enriched in each term. In each GO classification, the top 10 GO terms with the lowest P-value, i.e., the most significant enrichment, were selected for display. (D and E) The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis results. According to the KEGG enrichment analysis of the differentially expressed genes, the first 20 pathways with the smallest P-value, i.e., the most significant enrichment, were selected for display in D. The abscissa is the name of the pathway, and the ordinate is the -log10(P-value) showing the enrichment of each pathway; the number of genes in the column shows the number of differentially expressed genes enriched in the pathway (E).
3.4. Verification of transcriptome sequencing results by RT-qPCR
The transcriptome data and RT-qPCR results are shown in Appendix Fig. 1. Six genes identified by RT-qPCR were consistent with the transcriptome data, and the fold changes were basically the same, which indicated the consistency between the RT-qPCR and transcriptome data.
3.5. Gene ontology enrichment analysis of the differentially expressed genes
The 123 DEG were classified by GO annotation into 3 main categories: biological processes, cell components and molecular functions. The most significant GO terms obtained from the annotation of the DEG were amino acid homeostasis (GO:0080144), haptoglobin-hemoglobin complex (GO:0031838), and haptoglobin binding (GO:0031720) (P < 0.01) (Fig. 2C). Further analysis revealed that up to 30 entries related to transport function were found among 336 GO terms showing significant enrichment, and some functions that related to transport function, cell viability and defense response were also significantly enriched (P < 0.05 or P < 0.01) (Appendix Table 5).
3.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differentially expressed genes
KEGG taxonomic annotation of the DEG was performed. As shown in Fig. 2D and Appendix Table 6, 123 DEG were annotated in 5 one-level KEGG hierarchies: metabolism, environmental information processing, organismal systems, cellular processes and human diseases. Further KEGG enrichment analysis identified the first 20 KEGG pathways, as shown in the scatter plots in Fig. 2E. According to the graph, the most prominent KEGG pathways were vitamin B6 metabolism, the apelin signaling pathway, adrenergic signaling in cardiomyocytes, histidine metabolism, other types of O-glycan biosynthesis, the calcium signaling pathway, amino acid metabolism (alanine, tyrosine, tryptophan, arginine, proline, valine, leucine and isoleucine) and metabolism of other vitamins (nicotinate, nicotinamide, and retinol).
3.7. Expression level of tight junction protein (TJP)-related genes in cecal epithelium cells
In this study, the relative expression levels of claudins 2, 15, 19, and 23, TJP1, TJP2, and TJP3 and mucin 1 in the chickens fed C. butyricum were significantly higher than those in the control group (P < 0.05 or P < 0.01); in contrast, claudin 20 expression was significantly lower in the C. butyricum-fed chickens than that in the control group (P = 0.013). No significant difference in the relative expression of other genes associated with tight junctions (TJ), occludin, cingulin, vinculin, hepatocyte nuclear factor 4 alpha (HNF4A, a transcription regulator that acts on occludin) (P = 0.176) and vascular endothelial growth factor A (VEGFA, a cytokine that indirectly regulates TJ) (P = 0.652) was found (Appendix Table 7).
3.8. Results of HE and PAS staining
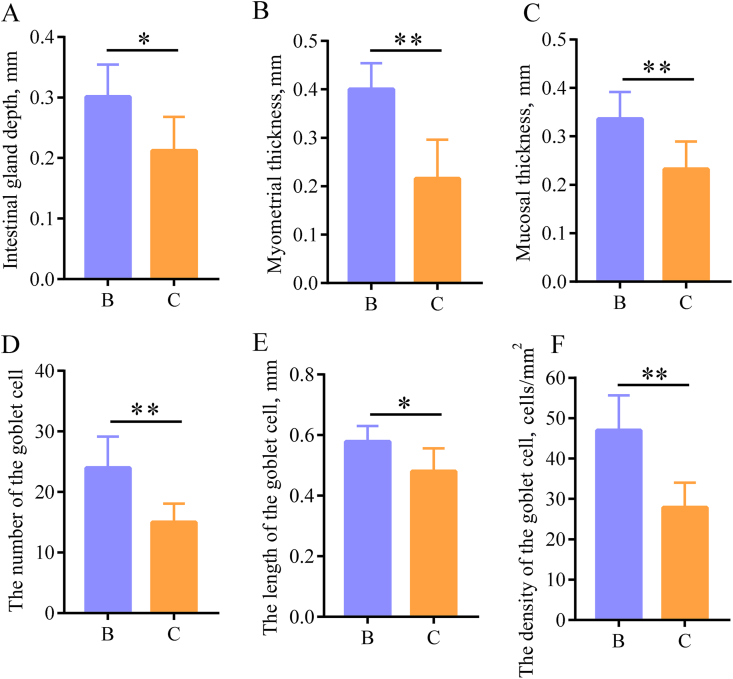
The HE staining results showed that the intestinal gland depth, mucosal layer thickness and muscle layer thickness in group B were significantly higher than those in group C (P < 0.05 or P < 0.01) (Fig. 3A, B, C and Fig. 4). The PAS staining results revealed a significantly higher number of goblet cells in group B than in group C (P < 0.05 or P < 0.01) (Fig. 3D, E, F and Fig. 4).
Fig. 3.
Indexes for epithelial cells of the cecal mucosa. (A) Intestinal gland depth of the cecum. (B) Myometrial thickness of the cecum. (C) Mucosal thickness of the cecum. (D) Number of goblet cells. (E) Length of goblet cells. (F) Density of goblet cells. B refers to group B, the Clostridium butyricum group; C refers to group C, control group. ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 4.
Hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) staining of epithelial cells of the cecal mucosa. Group B means the C. butyricum group. Group C means the control group.
3.9. Analysis of the performance of broilers
The analysis of broiler performance (Table 1) showed that the body weight of group B was significantly higher than that of group C on d 14 and 28 (P < 0.05 or P < 0.01), the total feed consumption of group B (total 28 d) was not significantly different to that of group C (P = 0.204), and the FCR (28th day) of group B was 1.57, which was lower than that of group C (1.72).
Table 1.
Body weight, feed consumption and feed conversion ratio of the Clostridium butyricum group and control group.
| Item | Group B1 | Group C2 | P-value3 |
|---|---|---|---|
| Body weight, g | |||
| d 1 | 56.57 ± 1.04 | 57.09 ± 1.00 | 0.516 |
| d 14 | 657.11 ± 12.21 | 596.47 ± 18.62 | 0.016 |
| d 28 | 2,004.99 ± 43.75 | 1,808.37 ± 22.73 | <0.01 |
| Feed consumption, g | |||
| Total 14 d | 769.21 ± 15.32 | 765.67 ± 13.65 | 0.725 |
| Total 28 d | 3,061.60 ± 35.47 | 3,017.28 ± 37.29 | 0.204 |
| FCR | |||
| d 28 | 1.57 | 1.72 | – |
FCR = feed conversion ratio.
Group B means the C. butyricum group.
Group C means the control group.
P > 0.05 indicates a non-significant difference; P < 0.05 indicates a significant difference; P < 0.01 indicates a highly significant difference.
4. Discussion
This study aimed to investigate the probiotic effects of C. butyricum on the cecal mucosa in broilers. The abundance of transport function-related terms indicates that cecal epithelial cells underwent marked improvements in metabolite transport and thus in growth performance. GO:0031720 (haptoglobin binding), GO:004601 (peroxidase activity), GO:0016684 (oxidoreductase activity), GO:0016209 (antioxidant activity) and GO:0047730 (carnosine synthase activity) are GO terms related to antioxidant capacity, and the GO terms GO:0042742 (defense response to bacterium) and GO:0050832 (defense response to fungus) are related to defense functions through the upregulation of avian beta-defensin 4 (AvBD4). The significant enrichment of antioxidant activity-, metabolism- and transport-related terms demonstrates that probiotics can improve the antioxidant capacity, disease resistance and growth performance of the host (Liao et al., 2015b; Yang et al., 2012; Zhang et al., 2014).
The analysis of the KEGG categories revealed that vitamin, amino acid and O-glycan biosynthesis were the most enriched metabolic pathways. C. butyricum ferments in the cecum and thus provides a large number of SCFA, amino acids, vitamins and other metabolites to the host (Kuroiwa et al., 1990; Yang et al., 2012). These abundant nutrients are transported into epithelial cells of the cecal mucosa through the upregulation of carrier genes, such as solute carrier family 7 member 11 (SLC7A11), low-density lipoprotein-related protein 2 (LRP2), translocator protein 2 (TSPO2), hemoglobin subunit mu (HBM), hemoglobin subunit alpha 1 (HBA1) and ENSGALG00000050921 (very-long-chain fatty acid-CoA ligase activity), which might shed light on the mechanisms through which dietary supplementation with C. butyricum can improve growth performance (Table 1). C. butyricum and its metabolites enhance the antioxidant and antistress capacity of broilers through the upregulation of ENSGALG00000043254 (eosinophil peroxidase, EPX) and ENSGALG00000044760 (dioxygenase), as well as ENSGALG00000043435 (carnosine synthase 1, CARNS1), which catalyzes the conversion of beta-alanine, arginine, proline and histidine to carnosine and anserine. Enhanced immune function and reduced inflammation were obtained by upregulation of the genes ENSGALG00000043254 (EPX), ENSGALG00000044631 (B cell activation) and ENSGALG00000048617 (T cell activation) and downregulation of the gene ENSGALG00000027887 (C1q and tumor necrosis factor-related protein 3), which are related to the production of IL-6 and TNF-α (Kopp et al., 2010). However, consistent with other reports (Chen et al., 2018; Lee et al., 2014), the mRNA levels of proinflammatory cytokines (IFN-γ) showed no clear differences between the 2 groups under normal physiological conditions. According to previous reports (Hamer et al., 2008; Takahashi et al., 2018), C. butyricum produces beneficial products, such as butyric acid, in the intestine, and the use of these products as vital energy sources promotes the turnover and viability or repair of intestinal epithelial tissue. The cecum experiences high levels of stress and uses various strategies, including controlled apoptosis, for maintenance of an optimal barrier, particularly by enrichment of the Hedgehog signaling pathway, which is important for the control of cecum homeostasis (Chen et al., 2018). Apelin is an endogenous peptide capable of binding the apelin receptor (APJ), which is widely expressed in various tissues and organ systems. Apelin signaling pathways have been implicated in different key physiological processes, such as angiogenesis, cardiovascular function, cell proliferation and energy metabolism regulation. The KEGG pathways associated with the apelin signaling pathway were significantly enriched by C. butyricum (P = 0.024).
The monolayer intestinal epithelium is a physical barrier against invading intraluminal pathogens and toxins. The tight junctional complexes that span the extracellular space to interact with adjacent epithelial cells consist of TJ, gap junctions, adherens junctions, and desmosomes, which maintain the integrity of the epithelial barrier by regulating the paracellular permeability (Takuya and Suzuki, 2013). Tight junctions include occludin, claudins, junctional adhesion molecules, and tricellulin, which are transmembrane proteins (Chiba et al., 2008). The intracellular domains of these proteins interact with cytosolic scaffold proteins such as zonula occludens-1 (ZO-1), zonula occludens-2 (ZO-2), zonula occludens-3 (ZO-3), and cingulin, which in turn bind to the actomyosin cytoskeleton (Cordenonsi et al., 1999). The extracellular loop domains interact with similar adjacent cells, which can be grouped into 2 categories: “seal” and “pore” functions (Westphal et al., 2010). The effect of C. butyricum on TJP in a healthy cecal barrier has not been reported. TJ form a multimolecular complex that regulates the paracellular permeability of intestinal epithelial cells. The interactions among TJP are essential for the assembly and maintenance of the TJ barrier integrity. The TJ complex exhibits a dynamic response to stimuli such as proinflammatory cytokines, LPS or pathogen infection and undergoes constitutive injury and remodeling (Wageha et al., 2017). Probiotics reportedly alleviate the downregulation of intestinal TJP caused by a variety of factors (Anderson et al., 2010; Chang et al., 2019; Song et al., 2014). The relative expression of TJP (claudins 2, 15, 19, and 23, TJP1, 2, and 3 and mucin 1) in the cecal mucosa was significantly increased in chickens fed a diet supplemented with C. butyricum (P < 0.05 or P < 0.01), but the expression of claudin 20 was significantly lower in the C. butyricum-fed chickens than in the control group (P = 0.013). In addition, no significant difference in the relative expression of other genes, occludin, cingulin, vinculin and hepatocyte nuclear factor 4 alpha (HNF4A, a transcription regulator that acts on occludin) or vascular endothelial growth factor A (VEGFA, a cytokine that indirectly regulates TJ) associated with TJ was found (P = 0.625). The role of each TJP in intestinal barrier formation is not entirely clear. The causal relationship between dynamic properties and the molecular mechanisms that underlie the functional properties of TJ need to be explored.
The HE and PAS staining analysis showed that the mucosal layer acts as a protective barrier of the intestine by blocking pathogen invasion and preventing the entry of harmful substances into the intestinal mucosa, which indicates the complex interplay among the mucus composition, microbiota and intestinal health (Elderman et al., 2017). Mucin secreted by goblet cells plays a significant role in protection against irritating substances and harmful bacteria. Moreover, mucin, as a nutrient source, provides a colonization “niche” for growing probiotics to adhere to mucous membranes (Duritis and Mugurevics, 2016; Montagne et al., 2004; Ventura et al., 2013; Wang and Peng, 2008). Mature mucin needs large amounts of sulfur-containing amino acids and sugar chains. Transcriptome sequencing showed significant enrichment of O-glycan biosynthesis (ST6GAL2) and the sulfur amino acid metabolic process (SLC7A11), which was consistent with a previous result (Struwe et al., 2015). The HE and PAS staining analysis showed that C. butyricum can increase the number and density of goblet cells, which further reveals the vital role of C. butyricum in disease resistance.
In addition to its effects on the intestinal mucosa, some studies have indicated that dietary supplementation with C. butyricum also exerts an effect on the intestinal microbiota (Liu et al., 2018; Sun et al., 2016). In this study, the 16S rRNA gene sequencing of the cecal contents of 28-d-old broilers showed that the Shannon index of the C. butyricum-treated group was significantly higher than that of the control group (P = 0.02). The diversity of the intestinal microbiota serves as the foundation for digestion, nutrient uptake, health maintenance, intestinal physiological functions, and promotion of intestinal immune system development in animals (Xu et al., 2016). Moreover, the beta diversity results showed a distinct separation, and the degree of dispersion among the broilers fed C. butyricum was lower than that among the control broilers, which indicates that the similarity of the microbial communities among the group B samples was high and showed little difference. These results suggest that broilers fed C. butyricum exhibit high intestinal microbial diversity and stable microbial community structures (Choi et al., 2018; Huang et al., 2018). The phylum-level classification revealed that Firmicutes was the dominant phylum in both groups. The Proteobacteria content in group C was higher than that in group B. Further analysis of the microbiota at the genus level revealed that the relative abundances of the probiotics Butyricicoccus, Lactobacillus, and Blautia, among others, in group B were higher than those in group C. This finding might have been obtained because the metabolites of C. butyricum can promote the proliferation of Bifidobacterium and Lactobacillus (Kong et al., 2011; Miao et al., 2018; Zhan et al., 2019), and C. butyricum can produce butyric acid, which can reduce the intestinal pH and inhibit the proliferation of harmful bacteria (Gao et al., 2013; Kong et al., 2011; Zhao et al., 2020).
5. Conclusions
Overall, the results of the present study indicated that dietary supplementation with C. butyricum can be beneficial for intestinal health (gut microbiota and intestinal mucosal function) and can be used to enhance disease resistance and antioxidant stress in animal production.
Author contributions
Keke Li, Fuchun Jian and Ruirui Jiang: Data curation, Xianhua Wan, Xiangli Sun, Hong Li and Yanbin Wang: Methodology, Wenting Li, Ruirui Jiang, Ruili Han and Xiangtao Kang: Visualization, Laipeng Xu and YanBin Wang: Writing – original draft, YanBin Wang and Xiangli Sun: Writing-Reviewing and Editing, Yanbin Wang and Xiangtao Kang: Supervision, Resources, Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This research was funded by the National Key Research and Development Program of China (No. 2018YFD0501904), the Program for Innovation Research Team of Ministry of Education (No. IRT16R23) and the Scientific Studio of Zhongyuan Scholars (No. 30601985). Sequencing services were provided by Personal Biotechnology Co., Ltd. (Shanghai, China).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.01.009.
Contributor Information
Xiangtao Kang, Email: xtkang2001@263.net.
Yanbin Wang, Email: ybwang2008@henau.edu.cn.
Appendix.
The following is the Supplementary data to this article:
References
- Abdel-Latif M.A., Abd El-Hack M.E., Swelum A.A., Saadeldin I.M., Elbestawy A.R., Shewita R.S. Single and combined effects of Clostridium butyricum and Saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals. 2018;8(10):184. doi: 10.3390/ani8100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.C., Cookson A.L., McNabb W.C., Park Z., McCann M.J., Kelly W.J. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang J.Y., Ezike E., Asmar B.I. Antibacterial resistance. Indian J Pediatr. 2004;71(3):229–239. doi: 10.1007/BF02724275. [DOI] [PubMed] [Google Scholar]
- Bialkowska A.B., Ghaleb A.M., Nandan M.O., Yang V.W. Improved Swiss-rolling technique for intestinal tissue preparation for immunohistochemical and immunofluorescent analyses. J Vis Exp. 2016;113:e54161. doi: 10.3791/54161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J.R., Gong J.S. The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res Int. 2011;44(10):3149–3159. [Google Scholar]
- Chang C.H., Teng P.Y., Lee T.T., Yu B. Effects of multi-strain probiotic supplementation on intestinal microbiota, tight junctions, and inflammation in young broiler chickens challenged with Salmonella enterica subsp. enterica. Asian-Australas J Anim Sci. 2019;33(11):1797–1808. doi: 10.5713/ajas.19.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wen C., Zhou Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J Sci Food Agric. 2018;98(9):3343–3350. doi: 10.1002/jsfa.8838. [DOI] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. Transmembrane proteins of tight junctions. Biochim Biophys Acta. 2008;1778(3):588–600. doi: 10.1016/j.bbamem.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Lee K., Kim D.W., Kil D.Y., Kim G.B., Cha C.J. Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poultry Sci. 2018;97(3):970–979. doi: 10.3382/ps/pex360. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., D'Atri F., Hammar E., Parry D.A., Kendrick-Jones Cingulin contains globular and coiled-coil domains and interacts with Zo-1, Zo-2, Zo-3, and myosin. J Cell Biol. 1999;147(7):1569–1581. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duritis I., Mugurevics A. Distribution and characterisation of goblet cells in the large intestine of ostriches during the pre- and post-hatch period. Anat Histol Embryol. 2016;45(6):457–462. doi: 10.1111/ahe.12221. [DOI] [PubMed] [Google Scholar]
- Elderman M., Sovran B., Hugenholtz F., Graversen K., Huijskes M., Houtsma E. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PloS One. 2017;12(9):e0184274. doi: 10.1371/journal.pone.0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadde U.D., Oh S., Lee Y., Davis E., Zimmerman N., Rehberger T. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immunological stress and improve intestinal barrier gene expression in commercial broiler chickens. Res Vet Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Gao Q., Qi L., Wu T., Wang J. Ability of Clostridium butyricum to inhibit Escherichia coli-induced apoptosis in chicken embryo intestinal cells. Vet Microbiol. 2012;160(3–4):395–402. doi: 10.1016/j.vetmic.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Gao Q., Xiao Y., Sun P., Peng S., Yin F., Ma X. Vitro protective efficacy of Clostridium butyricum against fish pathogen infections. Indian J Microbiol. 2013;53(4):453–459. doi: 10.1007/s12088-013-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Brummer R.J. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27(2):104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Han J., Wang Y., Song D., Lu Z., Dong Z., Miao H. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric Immunol. 2018;29(1):797–807. [Google Scholar]
- Huang P., Zhang Y., Xiao K., Jiang F., Wang H., Tang D. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6(1):211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau A.L., Ahern P.P., Griffin N.W., Goodman A.L., Gordon J.I. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., He G.-Q., Jia J.-L., Zhu Q.-L., Ruan H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbiol. 2011;62(2):512–517. doi: 10.1007/s00284-010-9737-8. [DOI] [PubMed] [Google Scholar]
- Kong Q., He G.Q., Chen F., Ruan H. Studies on a kinetic model for butyric acid bioproduction by Clostridium butyricum. Lett Appl Microbiol. 2006;43(1):71–77. doi: 10.1111/j.1472-765X.2006.01902.x. [DOI] [PubMed] [Google Scholar]
- Kopp A., Bala M., Buechler C., Falk W., Gross P., Neumeier M. C1q/TNF-Related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 2010;151(11):5267–5278. doi: 10.1210/en.2010-0571. [DOI] [PubMed] [Google Scholar]
- Kuroiwa T., Iwanaga M., Kobari K., Higashionna A., Kinjyo F., Saito A. Preventive effect of Clostridium butyricum M588 against the proliferation of Clostridium difficile during antimicrobial therapy. Kansenshogaku Zasshi. 1990;64(11):1425–1432. doi: 10.11150/kansenshogakuzasshi1970.64.1425. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Lillehoj H.S., Jang S.I., Lee S.H. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res Vet Sci. 2014;97(2):304–308. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Leeson S., Namkung H., Antongiovanni M., Lee E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poultry Sci. 2005;84(9):1418–1422. doi: 10.1093/ps/84.9.1418. [DOI] [PubMed] [Google Scholar]
- Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12):S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- Liao X., Wu R., Ma G., Zhao L., Zheng Z., Zhang R. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids Health Dis. 2015;14(1):36. doi: 10.1186/s12944-015-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X.D., Ma G., Cai J., Fu Y., Yan X.Y., Wei X.B. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poultry Sci. 2015;94(4):662–667. doi: 10.3382/ps/pev038. [DOI] [PubMed] [Google Scholar]
- Lindner S.E., Mikolajczak S.A., Vaughan A.M., Moon W., Joyce B.R., Sullivan W.J. Perturbations of PlasmodiumPuf2 expression and RNA-seq of Puf2-deficient sporozoites reveal a critical role in maintaining RNA homeostasis and parasite transmissibility. Cell Microbiol. 2013;15(7):1266–1283. doi: 10.1111/cmi.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhou J., Li W., Rong X., Gao Y., Zhao L. Effects of sporoderm-broken spores of Ganoderma lucidum on growth performance, antioxidant function and immune response of broilers. Anim Nutr. 2020;6(1):39–46. doi: 10.1016/j.aninu.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li S., Wang Z., Zhang B. Effects of dietary lipids and Clostridium butyricum on chicken volatile flavour compounds. Indian J Anim Res. 2018;52(8):1167–1173. [Google Scholar]
- Miao R., Zhu X., Wan C., Wang Z., Wen Y., Li Y. Effect of Clostridium butyricum supplementation on the development of intestinal flora and the immune system of neonatal mice. Exp Ther Med. 2018;15(1):1081–1086. doi: 10.3892/etm.2017.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Piel C., Lalles J.P. Effect of diet on mucin kinetics and composition: nutrition and health implications. Nutr Rev. 2004;62(3):105–114. doi: 10.1111/j.1753-4887.2004.tb00031.x. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
- Osho S.O., Wang T., Horn N.L., Adeola O. Comparison of goblet cell staining methods in jejunal mucosa of Turkey poults. Poultry Sci. 2017;96(3):556–559. doi: 10.3382/ps/pew324. [DOI] [PubMed] [Google Scholar]
- Park I., Lee Y., Goo D., Zimmerman N.P., Smith A.H., Rehberger T. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poultry Sci. 2020;99(2):725–733. doi: 10.1016/j.psj.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L.Y., Li Z.R., Green R.S., Holzman I.R., Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romick-Rosendale L.E., Legomarcino A., Patel N.B., Morrow A.L., Kennedy M.A. Prolonged antibiotic use induces intestinal injury in mice that is repaired after removing antibiotic pressure: implications for empiric antibiotic therapy. Metabolomics. 2014;10(1):8–20. doi: 10.1007/s11306-013-0546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadatmand N., Toghyani M., Gheisari A. Effects of dietary fiber and threonine on performance, intestinal morphology and immune responses in broiler chickens. Anim Nutr. 2019;5(3):248–255. doi: 10.1016/j.aninu.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini S., Santos M.M., Tannuri A.C.A., Zerbini M.C.N., Coelho M.C.D., Goncalves J.D. Is hematoxylin-eosin staining in rectal mucosal and submucosal biopsies still useful for the diagnosis of Hirschsprung disease? Diagn Pathol. 2017;12(1):5. doi: 10.1186/s13000-017-0673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y.L., Jiao L.F., Zou X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poultry Sci. 2014;93(3):581–588. doi: 10.3382/ps.2013-03455. [DOI] [PubMed] [Google Scholar]
- Song Z.F., Wu T.X., Cai L.S., Zhang L.J., Zheng X.D. Effects of dietary supplementation with clostridium butyricum on the growth performance and humoral immune response in Miichthys miiuy. J Zhejiang Univ - Sci B. 2006;7(7):596–602. doi: 10.1631/jzus.2006.B0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struwe W.B., Gough R., Gallagher M.E., Kenny D.T., Carrington S.D., Karlsson N.G. Identification of O-glycan structures from chicken intestinal mucins provides insight into campylobactor jejuni pathogenicity. Mol Cell Proteomics. 2015;14(6):1464–1477. doi: 10.1074/mcp.M114.044867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Wang F., Ling Z., Yu X., Chen W., Li H. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. doi: 10.1016/j.brainres.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Takahashi M., McCartney E., Knox A., Francesch M., Oka K., Wada K. Effects of the butyric acid-producing strain Clostridium butyricum MIYAIRI 588 on broiler and piglet zootechnical performance and prevention of necrotic enteritis. Anim Sci J. 2018;89(6):895–905. doi: 10.1111/asj.13006. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Taguchi H., Yamaguchi H., Osaki T., Komatsu A., Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157 : H7 infection in mice. FEMS Immunol Med Microbiol. 2004;41(3):219–226. doi: 10.1016/j.femsim.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Takuya, Suzuki Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok V.A., Hughes R.J., Mikkelsen L.L., Perez-Maldonado R., Balding K., MacAlpine R. Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials. Appl Environ Microbiol. 2011;77(17):5868–5878. doi: 10.1128/AEM.00165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A., do Nascimento A.A., dos Santos M.A.J., Vieira-Lopes D.A., Sales A., Pinheiro N.L. Histological description of morphogenesis of the gastroesophageal mucosa of Gallus gallus domesticus (linnaeus, 1758) Int J Morphol. 2013;31(4):1331–1339. [Google Scholar]
- Wageha A., Claudia H., Michael H. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 2017;9(2):60. doi: 10.3390/toxins9020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.-B., Wang P.-Y., Wang X., Wan Y.-L., Liu Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- Wang J.X., Peng K.M. Developmental morphology of the small intestine of African ostrich chicks. Poultry Sci. 2008;87(12):2629–2635. doi: 10.3382/ps.2008-00163. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu L., Sun X., Wan X., Sun G., Jiang R. Characteristics of the fecal microbiota of high- and low-yield hens and effects of fecal microbiota transplantation on egg production performance. Res Vet Sci. 2020;129:164–173. doi: 10.1016/j.rvsc.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Westphal J.K., DöRfel M.J., Krug S.M., Cording J.D., Piontek J.R., Blasig I.E. Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell Mol Life Sci. 2010;67(12):2057–2068. doi: 10.1007/s00018-010-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Xiong W., Li C., Zhai M., Li Y., Ma F. MicroRNA-dependent regulation of metamorphosis and identification of microRNAs in the red flour beetle, Tribolium castaneum. Genomics. 2017;109(5–6):362–373. doi: 10.1016/j.ygeno.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Xu Y., Yang H., Zhang L., Su Y., Shi D., Xiao H. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016;16(1):259. doi: 10.1186/s12866-016-0877-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Ajuwon K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PloS One. 2017;12(6):e0179586. doi: 10.1371/journal.pone.0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., An S., Lv Z., Wang Z., Wu Y., Zhu Y. Effects of phytonutrients on growth performance, antioxidative status, and energy utilization of broilers fed low energy diets. Anim Nutr. 2019;5(3):270–277. doi: 10.1016/j.aninu.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.M., Cao G.T., Ferket P.R., Liu T.T., Chen A.G. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poultry Sci. 2012;91(9):2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]
- Zhan H.Q., Dong X.Y., Li L.L., Zheng Y.X., Gong Y.J., Zou X.T. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poultry Sci. 2019;98(2):896–903. doi: 10.3382/ps/pey436. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao G.T., Zeng X.F., Zhou L., Ferket P.R., Xiao Y.P. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Sci. 2014;93(1):46–53. doi: 10.3382/ps.2013-03412. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang L., Zhan X., Zeng X., Zhou L., Cao G. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. 2016;7:3. doi: 10.1186/s40104-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Yang J., Ju Z., Wu J., Wang L., Lin H. Clostridium butyricum ameliorates Salmonella enteritis induced inflammation by enhancing and improving immunity of the intestinal epithelial barrier at the intestinal mucosal level. Front Microbiol. 2020;11:299. doi: 10.3389/fmicb.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Ding X., Yang Z.B., Shen Y., Shao D., Shi S.R. Effects of Clostridium butyricum on growth performance, lipid metabolism and the caecal microecological environment of broilers. Eur Poult Sci. 2017;81 doi: 10.1399/eps.2017.187. [DOI] [Google Scholar]
- Zhao X., Guo Y.M., Liu H.B., Gao J., Nie W. Clostridium butyricum reduce lipogenesis through bacterial wall components and butyrate. Appl Microbiol Biotechnol. 2014;98(17):7549–7557. doi: 10.1007/s00253-014-5829-x. [DOI] [PubMed] [Google Scholar]
- Zhao X., Yang J., Wang L., Lin H., Sun S. Protection mechanism of Clostridium butyricum against Salmonella enteritidis infection in broilers. Front Microbiol. 2017;8:1523. doi: 10.3389/fmicb.2017.01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.