Abstract
Objective
Patients with bone metastasis (BM) of small cell lung cancer (SCLC) have a poor prognosis. We aimed to identify predictors and prognostic factors in patients with BM of SCLC and construct nomograms to predict BM.
Methods
We retrospectively analyzed 18,187 cases from the Surveillance, Epidemiology, and End Results database reported between 2010 and 2016. Differences in overall survival (OS) and cancer-specific survival (CSS) were evaluated after propensity score matching. Independent predictors for BM and prognostic factors for patients with BM of SCLC were determined using univariate and multivariate regression analyses. Two nomograms were constructed and evaluated using C-statistics.
Results
BM was observed in 4014 (22.07%) patients. Kaplan–Meier survival analysis revealed significant differences between BM and non-BM groups. The median OS for patients with and without BM was 6 and 7 months, respectively. The median CSS for patients with and without BM was 9 and 13 months, respectively. Age, sex, tumor size, N stage, chemotherapy, surgery, radiotherapy, and liver/brain/lung metastases were related to BM and independent prognostic factors for OS and CSS. Diagnostic and prognostic nomograms were generated.
Conclusion
Our nomograms predicted the incidence of BM and the 5-month survival rate of patients with SCLC and BM.
Keywords: Small cell lung cancer, bone metastasis, Surveillance, Epidemiology, and End Results database, prognosis, predictor, nomogram
Introduction
Cancer of the lung and bronchus ranks second among all malignancies. Although the mortality associated with this cancer has declined in recent years, it remains the leading cause of death among all cancer types. According to the 2020 Cancer Statistics, the American Cancer Society estimated that the number of lung and bronchus cancer cases in the United States in 2020 increased by 116,300 in men and 112,520 in women. 1 Previous reports indicated that lung cancer is the most commonly diagnosed malignancy in both sexes (combined), accounting for 11.6% of all cancer cases. It is also the leading cause of cancer death (18.4% of the total cancer deaths) worldwide. 2 Among the various subtypes of lung cancer, small cell lung cancer (SCLC) is an aggressive pathological type. Although it is highly sensitive to initial chemotherapy and radiotherapy treatment, most patients have poor outcomes due to recurrence and disease progression. 3
Bone metastasis (BM) is a common disease progression outcome and occurs in a variety of malignant tumors, including lung cancer, prostate cancer, breast cancer, and others. It has been previously reported that the deterioration of BM results in hypercalcemia, severe bone pain, pathological fracture, spinal cord compression, and other skeletal-related events (SREs). 4 Severe SREs further lead to loss of bone function, thereby reducing the quality of life of patients. Additionally, BM significantly reduces the survival time of patients and is almost always incurable. 5
Early diagnosis and treatment of BM can significantly reduce the occurrence and development of SREs, subsequently improving the survival rate of patients.6,7 Approximately 40% of patients with lung cancer develop BM at an advanced stage of the disease. 8 However, in accordance with the screening guidelines of the National Comprehensive Cancer Network, invasive examination or skeletal imaging is not routinely recommended for asymptomatic patients. Therefore, it is particularly important to identify predictors of BM for lung cancer and construct an early screening model. Thus, the present study analyzed epidemiological data for BM in patients with SCLC from the Surveillance, Epidemiology, and End Results (SEER) database, the largest publicly available cancer database, to identify factors affecting the occurrence of BM and prognosis of patients with BM of SCLC. An epidemiological prediction score model and survival model of BM were generated to predict the incidence of BM in patients with SCLC and the 5-month survival rate of patients with BM of SCLC.
Materials and methods
Patient selection
The data from 1973 to 2016 included in the present study were downloaded from SEER*Stat software (Version 8.3.6.1 https: //seer.cancer.gov/data/access.html). Because this was a retrospective analysis of data from the SEER database, medical ethics review and patient consent were not applicable. All procedures performed in the studies were in line with the Helsinki Declaration and its later amendments and other comparable ethical standards. The reporting of the present study conforms to the TRIPOD guidelines. 9
We defined the inclusion criteria of patients with lung cancer as follows: (1) patients who were histologically diagnosed with SCLC (The International Classification of Diseases for Oncology third edition (ICD-O-3) was used to identify SCLC by site codes [8002, 8041, 8043, 8144, 8145]) from 2010 to 2016; (2) the staging of lymph nodes followed the 7th edition of the American Joint Committee on Cancer; (3) all relevant data were available, such as age at the initial diagnosis, race, sex, N-stage, primary site, histological type (ICD-O-3), bone/liver/lung metastasis, chemotherapy recode, radiation recode, operation information, survival duration (months), cause-specific death classification, and survival status. The following conditions were used as the exclusion criteria: (1) diagnosed with autopsies or death certificates and (2) patients with incomplete information. Finally, only eligible patients were included in this cohort study. Patients with SCLC and BM were defined as the BM group, and those without BM were defined as the non-BM group.
Statistical analyses and nomogram generation
All statistical analyses were conducted with IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA) and R (www.r-project.org). Categorical variables in the BM and non-BM groups were compared by the chi-square test. Univariate logistic analysis was applied to determine the factors related to BM. Significant variables were included in the multivariate logistic regression analysis to determine the independent risk factors of BM in patients with SCLC, and the results of multivariate logistic regression were presented in forest plots using R software. Regarding prognostic factors, the significant variables in the univariate Cox proportion hazard regression analysis were applied to construct a multivariate Cox proportional hazards model to determine the independent prognostic factors of patients with BM of SCLC. In this study, a two-sided p < 0.05 was considered to indicate statistical significance.
Propensity score matching (PSM) analyses were used to balance differences in the clinical baseline characteristics between the BM and non-BM groups. PSM was performed using IBM SPSS Statistics for Windows, Version 26.0. The two groups were matched based on a ratio of 1:1. The overall survival (OS) and cancer-specific survival (CSS) were analyzed using Kaplan–Meier curves, and the differences were compared with the log-rank test.
A nomogram was used to predict the incidence of BM and the OS of patients with BM of SCLC. Meaningful and commonly used indicators in multivariate analyses were incorporated into the construction of nomograms using R. The bootstrap method was used for internal validation, and the c-index for each nomogram was calculated.
Results
Characteristics of patients with SCLC
A total of 268,251 patients were newly diagnosed with SCLC between 2010 and 2016. Based on the selection criteria, a total of 18,187 patients were included in this study. The characteristics of the patients before PSM are presented in Table 1. Regarding treatment methods, only 4.27% of all patients underwent surgery, whereas 72.58% and 49.82% received chemotherapy and radiotherapy, respectively. In terms of metastatic sites, BM was reported in 22.07% of patients, whereas 15.70% exhibited brain metastasis.
Table 1.
Characteristics of patients with SCLC before PSM.
| Characteristic | BM |
Non-BM |
χ2 | p | ||
|---|---|---|---|---|---|---|
| N = 4014 | N = 14,173 | |||||
| Age (years) | ||||||
| ≤54 | 386 | 9.62% | 1246 | 8.79% | 39.209 | <0.001 |
| 55–64 | 1130 | 28.15% | 3704 | 26.13% | ||
| 65–74 | 1539 | 38.34% | 5164 | 36.44% | ||
| 75–84 | 818 | 20.38% | 3356 | 23.68% | ||
| ≥85 | 141 | 3.51% | 703 | 4.96% | ||
| Race | ||||||
| White | 3575 | 89.06% | 12,217 | 86.20% | 22.459 | <0.001 |
| Black | 1431 | 35.65% | 1170 | 8.26% | ||
| Other (American Indian/ Alaska Native, Asian/Pacific Islander) | 964 | 24.02% | 786 | 5.55% | ||
| Sex | ||||||
| Women | 1820 | 45.34% | 7424 | 52.38% | 62.030 | <0.001 |
| Men | 2194 | 54.66% | 6749 | 47.62% | ||
| Primary site | ||||||
| Main bronchus | 521 | 12.98% | 1583 | 11.17% | 12.598 | 0.013 |
| Upper lobe | 2273 | 56.63% | 8108 | 57.21% | ||
| Middle lobe | 167 | 4.16% | 666 | 4.70% | ||
| Lower lobe | 990 | 24.66% | 3618 | 25.53% | ||
| Overlapping lesion of lung | 63 | 1.57% | 198 | 1.40% | ||
| Tumor size (cm) | ||||||
| 0 ≤ × ≤ 3 | 1050 | 26.16% | 4687 | 33.07% | 71.151 | <0.001 |
| 3 < × ≤ 5 | 1137 | 28.33% | 3688 | 26.02% | ||
| 5 < × ≤ 7 | 851 | 21.20% | 2603 | 18.37% | ||
| >7 | 976 | 24.31% | 3195 | 22.54% | ||
| N stage | ||||||
| N0 | 342 | 8.52% | 2883 | 20.34% | 467.013 | <0.001 |
| N1 | 237 | 5.90% | 1266 | 8.93% | ||
| N2 | 2302 | 57.35% | 7523 | 53.08% | ||
| N3 | 1133 | 28.23% | 2501 | 17.65% | ||
| Surgery | ||||||
| No | 3987 | 99.33% | 13,423 | 94.71% | 163.191 | <0.001 |
| Yes | 27 | 0.67% | 750 | 5.29% | ||
| Chemotherapy | ||||||
| No | 1035 | 25.78% | 3951 | 27.88% | 6.881 | 0.009 |
| Yes | 2979 | 74.22% | 10,222 | 72.12% | ||
| Radiation therapy | ||||||
| No | 2389 | 59.52% | 6738 | 47.54% | 179.448 | <0.001 |
| Yes | 1625 | 40.48% | 7435 | 52.46% | ||
| Brain metastasis | ||||||
| No | 3216 | 80.12% | 12,116 | 85.49% | 68.084 | <0.001 |
| Yes | 798 | 19.88% | 2057 | 14.51% | ||
| Liver metastasis | ||||||
| No | 1752 | 43.65% | 11,203 | 79.04% | 1912.663 | <0.001 |
| Yes | 2262 | 56.35% | 2970 | 20.96% | ||
| Lung metastasis | ||||||
| No | 3153 | 78.55% | 12,595 | 88.87% | 286.677 | <0.001 |
| Yes | 861 | 21.45% | 1578 | 11.13% | ||
BM, bone metastasis; SCLC, small cell lung cancer; PSM, propensity score matching.
PSM analysis
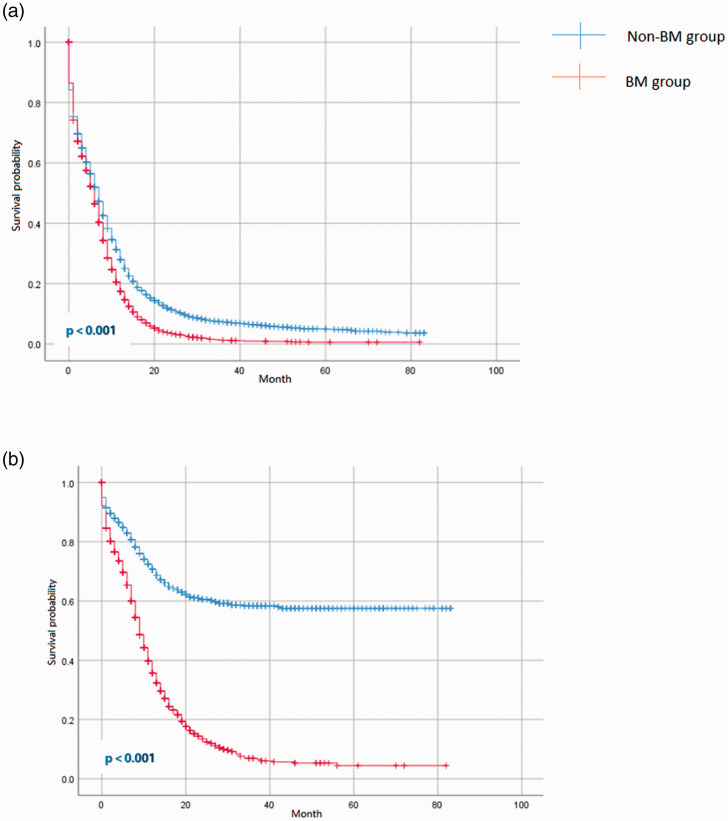
To avoid any bias from other factors, such as age and sex, PSM was used to balance 12 pairs of clinical and histopathological characteristics during the evaluation of prognosis. After 1:1 matching, a total of 7920 cases were matched in the BM and non-BM groups. As shown in Table 2, the differences in the clinical and histopathological characteristics between the two groups after PSM were balanced (p > 0.05). OS and CSS were calculated using the data collected before and after PSM. Before PSM, the median OS for patients with and without BM was 6 months [95% confidence interval (CI): 5.7–6.3] and 9 months (95% CI: 8.8–9.2), respectively. The median CSS for patients with and without BM was 6 months (95% CI: 5.7–6.3) and 9 months (95% CI: 8.7–9.3), respectively. Importantly, the OS and CSS of patients with BM were significantly different from patients without BM after PSM (p < 0.001). The median OS for patients with BM and without BM was 6 (95% CI: 5.7–6.3) and 7 (95% CI: 6.7–7.3) months, respectively. After PSM, the median CSS for patients with BM and without BM was 9 (95% CI: 8.6–9.4) and 13 (95% CI: 12.3–13.6) months, respectively. No, statistically significant differences were observed in OS between the patients with BM before and after PSM. The Kaplan–Meier curves for OS and CSS after PSM are shown in Figure 1.
Table 2.
Characteristics of patients with SCLC after PSM.
| Characteristic | BM |
Non-BM |
χ2 | p |
|---|---|---|---|---|
| N = 3960 | N = 3960 | |||
| Age (years) | ||||
| ≤54 | 380 | 379 | 5.358 | 0.252 |
| 55–64 | 1108 | 1043 | ||
| 65–74 | 1518 | 1424 | ||
| 75–84 | 814 | 909 | ||
| ≥85 | 140 | 205 | ||
| Race | ||||
| White | 3523 | 3376 | 5.012 | 0.0816 |
| Black | 260 | 353 | ||
| Other (American Indian/ Alaska Native, Asian/ Pacific Islander) | 177 | 231 | ||
| Sex | ||||
| Women | 1812 | 1837 | 0.318 | 0.537 |
| Men | 2148 | 2123 | ||
| Primary site | ||||
| Main bronchus | 507 | 474 | 4.582 | 0.333 |
| Upper lobe | 2241 | 2190 | ||
| Middle lobe | 166 | 182 | ||
| Lower lobe | 984 | 1049 | ||
| Overlapping lesion of lung | 62 | 65 | ||
| Tumor size (cm) | ||||
| 0 ≤ × ≤ 3 | 1038 | 1096 | 3.474 | 0.324 |
| 3 < × ≤ 5 | 1126 | 1074 | ||
| 5 < × ≤ 7 | 842 | 815 | ||
| >7 | 954 | 975 | ||
| N stage | ||||
| N0 | 342 | 388 | 7.460 | 0.059 |
| N1 | 237 | 248 | ||
| N2 | 2289 | 2324 | ||
| N3 | 1092 | 1000 | ||
| Surgery | ||||
| No | 3933 | 3932 | 0.018 | 0.892 |
| Yes | 27 | 28 | ||
| Chemotherapy | ||||
| No | 1030 | 1070 | 1.037 | 0.309 |
| Yes | 2930 | 2890 | ||
| Radiation therapy | ||||
| No | 2351 | 2308 | 0.964 | 0.326 |
| Yes | 1609 | 1652 | ||
| Brain metastasis | ||||
| No | 3189 | 3182 | 0.039 | 0.843 |
| Yes | 771 | 778 | ||
| Liver metastasis | ||||
| No | 1752 | 1816 | 2.089 | 0.148 |
| Yes | 2208 | 2144 | ||
| Lung metastasis | ||||
| No | 3146 | 3187 | 1.325 | 0.25 |
| Yes | 814 | 773 |
BM, bone metastasis; SCLC, small cell lung cancer; PSM, propensity score matching.
Figure 1.
Kaplan–Meier curves of overall survival (a) and cancer-specific survival (b) for patients stratified by the presence (N = 4014) or absence (N = 14,173) of BM.
BM, bone metastasis.
Predictors of BM in patients with SCLC
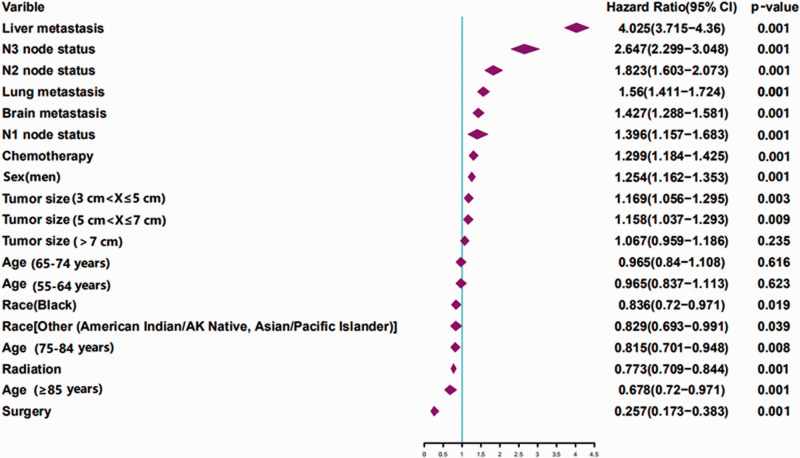
For univariate logistic regression analysis, age, race, sex, tumor size, N stage, surgery, chemotherapy, radiation, brain metastasis, liver metastasis, and lung metastasis were identified to be closely related to the risk of BM. The related factors were further used for multivariate analysis, which indicated no differences between the two groups in patients 55 to 74 years old (Table 3). Importantly, age ≥85 years (p < 0.001), Black race (p = 0.018), other races (p = 0.041), men (p < 0.001), larger tumor size (p < 0.001), higher N stage (p < 0.001), surgery (p < 0.001), chemotherapy (p < 0.001), radiation (p < 0.001), brain metastasis (p < 0.001), liver metastasis (p < 0.001), and lung metastasis (p < 0.001) were identified as independent predictors of BM in newly diagnosed patients with SCLC. For better presentation of the results, forest plots were used (Figure 2).
Table 3.
Univariate logistic regression analyses of factors associated with BM.
| Characteristic | HR (95%CI) | p |
|---|---|---|
| Age (years) | ||
| ≤54 | 1 | |
| 55–64 | 0.966 (0.838–1.115) | 0.639 |
| 65–74 | 0.967 (0.842–1.111) | 0.639 |
| 75–84 | 0.818 (0.704–0.952) | 0.009 |
| ≥85 | 0.683 (0.54–0.863) | <0.001 |
| Race | ||
| White | 1 | |
| Black | 0.836 (0.72–0.97) | 0.018 |
| Other* | 0.83 (0.694–0.992) | 0.041 |
| Sex | ||
| Women | 1 | |
| Men | 1.254 (1.162–1.353) | <0.001 |
| Primary site | ||
| Main bronchus | 1 | |
| Upper lobe | 0.976 (0.866–1.1) | 0.692 |
| Middle lobe | 0.937 (0.758–1.158) | 0.548 |
| Lower lobe | 0.945 (0.827–1.08) | 0.409 |
| Overlapping lesion | 1.016 (0.736–1.403) | 0.922 |
| Tumor size (cm) | ||
| 0 ≤ × ≤ 3 | 1 | |
| 3 < × ≤ 5 | 1.167 (1.053–1.293) | 0.003 |
| 5 < × ≤ 7 | 1.152 (1.03–1.289) | 0.013 |
| >7 | 1.058 (0.949–1.179) | <0.001 |
| N stage | ||
| N0 | 1 | |
| N1 | 1.396 (1.158–1.684) | <0.001 |
| N2 | 1.82 (1.6-2.069) | <0.001 |
| N3 | 2.642 (2.295–3.042) | <0.001 |
| Surgery | ||
| No | 1 | |
| Yes | 0.258 (0.173–0.383) | <0.001 |
| Chemotherapy | ||
| No | 1 | |
| Yes | 1.3 (1.184–1.426) | <0.001 |
| Radiation therapy | ||
| No | 1 | |
| Yes | 0.773 (0.708–0.843) | <0.001 |
| Brain metastasis | ||
| No | 1 | |
| Yes | 1.429 (1.29–1.582) | <0.001 |
| Liver metastasis | ||
| No | 1 | |
| Yes | 4.025 (3.716–4.361) | <0.001 |
| Lung metastasis | ||
| No | 1 | |
| Yes | 1.559 (1.411–1.724) | <0.001 |
*Other: American Indian/Alaska Native, Asian/Pacific Islander.
BM, bone metastasis; HR, hazard ratio; CI, confidence interval.
Figure 2.
Multivariable logistic regression analyses of factors associated with BM.
BM, bone metastasis; AK, Alaska; CI, confidence interval.
Prognosis of patients with BM of SCLC
The prognostic factors of OS and CSS were predicted using univariate and multivariate Cox proportional hazard regression analyses (Table 4). Importantly, sex, tumor size, N stage, surgery performed, chemotherapy, radiation therapy, and bone/brain/liver/lung metastases were found to be statistically significant in the univariate analysis for both OS and CSS (p < 0.05). As expected, age >65 years was statistically significant in the univariate analysis of OS. In the case of CSS, patients aged 65 to 74 years and those older than 85 years exhibited a statistically significant difference (p < 0.05). In contrast, race was identified as a risk factor for CSS but not OS.
Table 4.
Cox-regression of univariate and multivariate analyses associated with the OS and CSS of patients with BM of SCLC.
| OS |
CSS |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | ||||||||
| ≤ 54 | 1 | 1 | 1 | 1 | ||||
| 55–64 | 1.023 (0.935–1.119) | 0.623 | 1.024 (0.936–1.119) | 0.61 | 1.049 (0.923–1.192) | 0.466 | ||
| 65–74 | 1.148 (1.053–1.252) | 0.002 | 1.149 (1.054–1.253) | 0.002 | 1.144 (1.011–1.296) | 0.033 | ||
| 75–84 | 1.294 (1.178–1.421) | 0.001 | 1.292 (1.177–1.418) | 0.001 | 1.143 (0.996–1.311) | 0.057 | ||
| ≥ 85 | 1.259 (1.095–1.447) | 0.001 | 1.257 (1.095–1.444) | 0.001 | 1.354 (1.084–1.692) | 0.008 | ||
| Race | ||||||||
| White | 1 | 1 | 1 | |||||
| Black | 0.922 (0.842–1.009) | 0.077 | 0.859 (0.743–0.993) | 0.04 | 0.86 (0.744–0.994) | 0.041 | ||
| Other* | 0.942 (0.845–1.05) | 0.281 | 0.815 (0.68–0.977) | 0.027 | 0.81 (0.676–0.970) | 0.022 | ||
| Sex | ||||||||
| Women | 1 | 1 | 1 | 1 | ||||
| Men | 1.081 (1.012–1.155) | 0.001 | 1.094 (1.043–1.147) | 0.001 | 1.221 (1.136–1.312) | 0.001 | 1.202 (1.118–1.293) | 0.001 |
| Primary site | ||||||||
| Main bronchus | 1 | 1 | 1 | |||||
| Upper lobe | 1.034 (0.959–1.115) | 0.379 | 0.983 (0.88–1.097) | 0.756 | ||||
| Middle lobe | 0.976 (0.854–1.115) | 0.724 | 0.852 (0.69–1.051) | 0.134 | ||||
| Lower lobe | 1.028 (0.946–1.117) | 0.517 | 1.021 (0.903–1.154) | 0.738 | ||||
| Overlapping lesion | 0.887 (0.726–1.084) | 0.242 | 0.972 (0.731–1.292) | 0.846 | ||||
| Tumor size (cm) | ||||||||
| 0 ≤ × ≤ 3 | 1 | 1 | 1 | 1 | ||||
| 3 < × ≤ 5 | 1.169 (1.096–1.247) | 0.001 | 1.162 (1.09–1.239) | 0.001 | 1.285 (1.164–1.420) | 0.001 | 1.247 (1.129–1.378) | 0.001 |
| 5 < × ≤ 7 | 1.159 (1.08–1.244) | 0.001 | 1.146 (1.069–1.228) | 0.001 | 1.277 (1.148–1.421) | 0.001 | 1.233 (1.108–1.372) | 0.001 |
| >7 | 1.259 (1.176–1.348) | 0.001 | 1.244 (1.163–1.33) | 0.001 | 1.386 (1.254–1.533) | 0.001 | 1.456 (1.316–1.612) | 0.001 |
| N stage | ||||||||
| N0 | 1 | 1 | 1 | 1 | ||||
| N1 | 1.149 (1.014-1.301) | 0.030 | 1.156 (1.02–1.309) | 0.023 | 1.316 (1.081–1.603) | 0.006 | 1.321 (1.086–1.608) | 0.005 |
| N2 | 1.313 (1.203-1.432) | 0.001 | 1.319 (1.209–1.438) | 0.001 | 1.411 (1.224–1.627) | 0.001 | 1.418 (1.23–1.634) | 0.001 |
| N3 | 1.332 (1.213-1.464) | 0.001 | 1.338 (1.218–1.47) | 0.001 | 1.425 (1.224–1.658) | 0.001 | 1.432 (1.231–1.666) | 0.001 |
| Surgery | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.536 (0.391–0.736) | 0.001 | 0.531 (0.387–0.729) | 0.001 | 0.531 (0.334–0.844) | 0.007 | 0.61 (0.382–0.973) | 0.038 |
| Chemotherapy | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.285 (0.268–0.302) | 0.001 | 0.285 (0.269–0.302) | 0.001 | 0.273 (0.251-0.296) | 0.001 | 0.26 (0.238–0.284) | 0.001 |
| Radiation therapy | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 0.637 (0.603–0.672) | 0.001 | 0.637 (0.603–0.673) | 0.001 | 0.479 (0.444–0.517) | 0.001 | 0.634 (0.584–0.688) | 0.001 |
| Bone metastasis | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.254 (1.195–1.316) | 0.001 | 1.256 (1.198–1.318) | 0.001 | 2.884 (2.665–3.120) | 0.001 | 2.689 (2.486–2.91) | 0.001 |
| Brain metastasis | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.533 (1.44–1.633) | 0.001 | 1.531 (1.438–1.63) | 0.001 | 1.273 (1.165–1.392) | 0.001 | 1.513 (0.376–1.633) | 0.001 |
| Liver metastasis | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.559 (1.481–1.64) | 0.001 | 1.565 (1.487–1.646) | 0.001 | 2.328 (2.157–2.512) | 0.001 | 1.941 (1.796–2.099) | 0.001 |
| Lung metastasis | ||||||||
| No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.149 (1.083–1.218) | 0.001 | 1.146 (1.081–1.215) | 0.001 | 1.409 (1.293–1.535) | 0.001 | 1.184 (1.086–1.291) | 0.001 |
*Other: American Indian/Alaska Native, Asian/Pacific Islander.
BM, bone metastasis; SCLC, small cell lung cancer; HR, hazard ratio; CI, confidence interval; CSS, cancer-specific survival; OS, overall survival.
The multivariate Cox proportional hazard regression analysis revealed that factors, including men, Black, other races, N2 stage, N3 stage, N4 stage, surgery, chemotherapy, radiation, brain metastasis, liver metastasis, and lung metastasis were independent prognostic factors for both OS and CSS. However, age >65 years was identified as an independent prognostic factor in the OS group, whereas Black and other races were independent prognostic factors in the CSS group.
Diagnostic and prognostic nomograms for patients with BM of SCLC
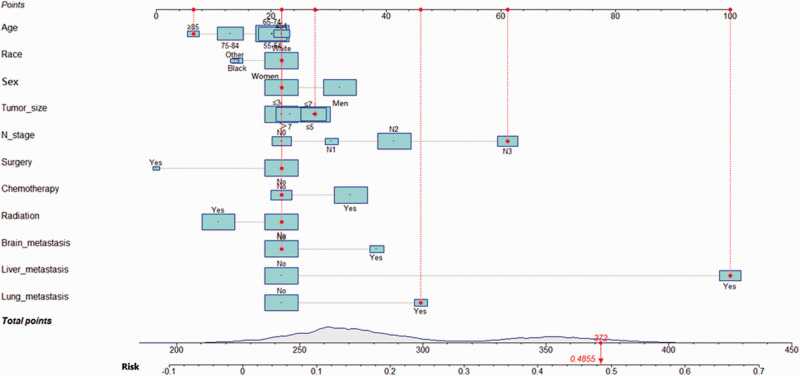
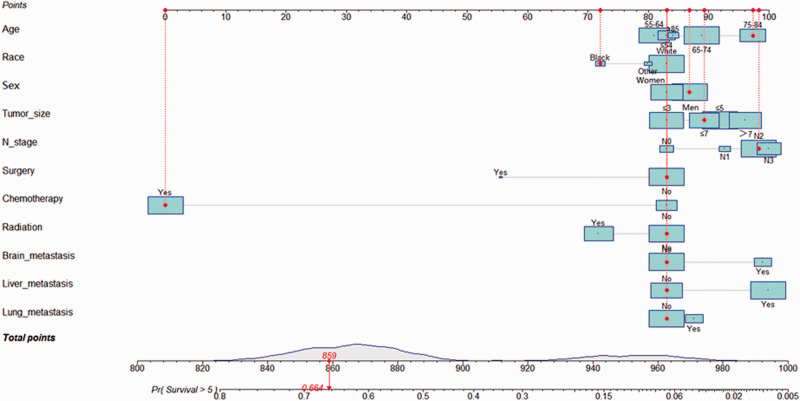
The nomograms for diagnosis and prognosis were established based on the results of the multivariate logistic regression analysis and Cox proportional hazards regression model (Figure 3 and Figure 4). These included multiple factors, such as age, race, sex, tumor size, N stage, surgery, chemotherapy, radiation, brain metastasis, liver metastasis, and lung metastasis. In the internal validation set, the c-index of the diagnosis nomogram was 0.745, and the c-index of the prognosis nomogram was 0.565.
Figure 3.
A nomogram for predicting the risk of BM in patients with SCLC. Other includes American Indians/Alaska Natives and Asian/Pacific Islanders. The size of the cyan square and grey area under the curve on the total points axis represents the sample size. The population distribution of patients with SCLC are shown.
BM, bone metastasis; SCLC, small cell lung cancer.
Figure 4.
A nomogram for predicting the prognosis of patients with BM of SCLC. Other includes American Indians/Alaska Natives and Asian/Pacific Islanders. The size of the cyan square and grey area under the curve on the total points axis represents the sample size. The population distribution of patients with SCLC are shown.
BM, bone metastasis; SCLC, small cell lung cancer.
Using the diagnosis nomogram, we included an example of an 86-year-old white woman who presented with a 4-cm tumor and a positive supraclavicular lymph node. The patient had not received radiotherapy, chemotherapy, or surgical treatment but exhibited liver metastasis and bilateral lung lesions. The total points were 372, and the probability of BM was approximately 48.55%. Similarly, using the prognosis nomogram, we included an example of a 76-year-old Black man with BM of SCLC who exhibited a 6-cm tumor and stage N2. The patient presented with no previous history of surgical treatment and radiotherapy. However, chemotherapy was previously administered. He showed no signs of liver, brain, or lung metastases. Here, the total score was ∼859, and the probability that the patient would survive for >5 months (median OS) was calculated to be 66.4%.
Discussion
The present study focused on the malignant progression and prognosis of lung cancer. Although several studies have been conducted on non-small cell lung cancer (NSCLC) in recent years, and the treatment efficacy of patients with NSCLC has improved significantly, the prognosis of patients with SCLC remains poor, mainly due to limited treatment options. SCLC accounts for ∼15% of total lung cancer cases 10 and is a particularly aggressive cancer type with a high propensity to metastasize. Generally, patients are diagnosed at an advanced stage, which is a negative independent prognostic factor for patients with SCLC. 11
Bone is the third most common metastatic site for a variety of solid tumors. 4 Bone metastases are further categorized as simultaneous bone metastases (SBM) and metachronous bone metastases (MBM). In lung cancer, SBM and MBM might exhibit different clinicopathological features, treatment sensitivities, and prognoses. 12 Because the criteria for synchronous and heterochronic bone metastases of cancer remain unclear, the survival differences in patients with SCLC have not been clearly elucidated, but patients with SBM usually exhibit a significant tumor burden and widespread organ destruction. Additionally, these patients often suffer from emotional and financial stress and poorer prognoses.13,14 However, there are limited studies on the predictive factors and prognosis of BM in patients with SCLC. Therefore, the identification of accurate prognostic factors and development of a convenient and practical prediction model is important to facilitate personalized treatment.
The incidence of BM from SCLC and its associated mortality rate are high. According to a previous report, BM was observed in 27% to 41% of patients with SCLC. 12 In the present study, 22.7% of patients with SCLC exhibited BM, which was similar to previous studies. The mortality rates associated with BM were 90.49%, 90.3%, and 91.04% in 4, 6, and 12 months, respectively, in patients with SCLC. The selection of related risk factors was based on the study conducted by Zhang et al., in which the relationship between different pathological types of lung cancer and BM was explored. In particular, the study included several factors, including population characteristics (sex and race), tumor characteristics (stage and metastatic site), and information regarding treatment methods. 15 Similar to the results reported by Li et al., 16 older age, sex, race, and N stage were found to be related to the occurrence of BM. Compared with patients with a tumor size ≤3 cm, patients with tumors 3 to 7 cm in size exhibited a higher risk of BM. Interestingly, both studies from our laboratory reported no correlation between a larger tumor (tumor size ≥7 cm) and a higher risk of metastasis. Previous studies showed that BM occurs in three stages. Specifically, metastases escape from the primary tumor, enter the circulation, and finally colonize the bone. 17 Li et al. hypothesized that under the regulation of cell adhesion factors, tumors ≥ 7 cm in size would not easily detach from the primary tumor to begin subsequent metastasis. 16 However, based on the discussion of the present study, we predict that the adhesion between tumors might have an impact. Nevertheless, other factors might also affect this particular result. Wang et al. demonstrated that a larger tumor size and multiple lymph node metastases were associated with the development of SCLC. 18 Thus, during the analysis of the effect of tumor size on the occurrence of BM, the status of lymph nodes must be considered. In particular, more precise analyses of several factors might better explain this result. Thus, clinicians should pay more attention to larger tumors and re-examine emission computed tomography or positron emission tomography-computed tomography scans in a timely manner. As shown in the forest plot (Figure 3), the hazard ratio of liver metastasis reached 4.025. This indicated that the presence of liver metastasis would substantially increase the risk of BM. The liver was previously reported to be the most prevalent site of metastasis (61.9%), and liver metastasis was the most common type of hematogenous metastasis in extensive-stage SCLC. 19 Therefore, in patients with liver metastases, closer attention should be paid to the condition of their bones.
Because diverse factors affect the survival time of patients, PSM is used to balance factors that might affect survival analysis. PSM is a commonly used method to eliminate the effect of bias during the statistical analysis of observational data. 20 To the best of our knowledge, the present study is the first to analyze the CSS and OS of patients with BM of SCLC using PSM. This further highlights the importance of determining the influence of BM on the prognosis of SCLC. After PSM, a significant difference was observed in OS and CSS between the BM group and non-BM group. Similarly, the median survival time was different (OS: 6 versus 7 months; CSS: 9 versus 13 months). The results of OS were consistent with a previous study conducted in 2016. 21 This is important as the data included in the present study were collected up to 2016. Furthermore, the survival times of patients with BM before and after PSM were compared. Importantly, no significant difference was detected in the survival time before and after PSM, which further demonstrated that this method did not affect the raw data. For prognostic factors, the results of multivariate Cox proportional hazard regression analysis identified men, larger tumor size, higher N stage, and brain/liver/lung metastases as risk factors for both OS and CSS in patients with BM. In addition, age >65 years was identified as a risk factor for OS but not CSS. One possible explanation is an age-related increase in the probability of death from other causes, whereas age might not be as closely related to CSS. Importantly, Black or other races were identified as risk factors for CSS but not OS. This result is not surprising because African Americans in the United States bear a disproportionate share of the cancer burden, with the highest death rate and shortest survival compared with any other racial or ethnic group for most cancers. 22 Therefore, it is necessary to further the progress aimed at the elimination of these racial disparities. Finally, two novel nomograms were generated for the diagnosis and prognosis of SCLC. Compared with previous studies with a broad scope, the nomogram developed in the present study was found to be more accurate, which might increase the convenience and provide important clues for clinical diagnosis and treatment.
The present study also had certain limitations. First, the study did not include an independent external cohort to validate the model, which is an important focus of a future study. This study presented a retrospective analysis that might have led to bias. Thus, prospective research is required to verify the conclusions of this study. Additionally, some indicators were not provided by the SEER database, such as driver genes, programmed death-ligand 1 expression levels, Eastern Cooperative Oncology Group scores, type of operation, radiotherapy dose, and chemotherapy regimen. Including this information may increase the accuracy of the diagnosis and prognosis model.
For the diagnosis and treatment of patients with cancer, a multidisciplinary team approach is necessary. In fact, oncology, orthopedics, pathology, imaging, and nuclear medicine departments are indispensable. The multidisciplinary diagnosis and treatment of lung cancer have been reported to shorten the time interval between diagnosis and oncology assessment and treatment, further ensuring that patients receive individualized and effective treatment at an earlier stage. This may help further improve the prognosis of patients to a certain extent. 23
Conclusions
The present study showed that multiple factors, such as sex, tumor size, N-stage, surgery, radiotherapy, and liver/brain/lung metastases, might be related to the occurrence of BM in patients with SCLC. Additionally, these factors were identified as independent prognostic factors in patients with BM of SCLC. The OS and CSS of these patients were determined to be poor. The two generated nomograms may be conveniently applied in clinical work to predict the incidence and survival rate for BM from SCLC. In conclusion, the BM of SCLC remains a significant challenge, and additional studies are required to explore and develop novel treatment methods to improve the survival for these patients.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Chenan Liu https://orcid.org/0000-0001-6089-2686
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3.Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e400S–e419S. [DOI] [PubMed] [Google Scholar]
- 4.Turpin A, Duterque-Coquillaud M, Vieillard MH. Bone metastasis: current state of play. Transl Oncol 2020; 13: 308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fornetti J, Welm AL, Stewart SA. Understanding the Bone in Cancer Metastasis. J Bone Miner Res 2018; 33: 2099–2113. [DOI] [PubMed] [Google Scholar]
- 6.Sun XS, Liang YJ, Liu SL, et al. , Subdivision of Nasopharyngeal Carcinoma Patients with Bone-Only Metastasis at Diagnosis for Prediction of Survival and Treatment Guidance. Cancer Res Treat 2019; 51: 1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia R, Ravulapati S, Befeler A, et al. Hepatocellular carcinoma with bone metastases: incidence, prognostic significance, and management-single-center experience. J Gastrointest Cancer 2017; 48: 321–325. [DOI] [PubMed] [Google Scholar]
- 8.Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 2004; 100: 2613–2621. [DOI] [PubMed] [Google Scholar]
- 9.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med 2015; 162: 55–63. [DOI] [PubMed] [Google Scholar]
- 10.Pavan A, Attili I, Pasello G, et al. Immunotherapy in small-cell lung cancer: from molecular promises to clinical challenges. J Immunother Cancer 2019; 7: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei L, Jiang ZM, Li CH, et al. KRAS Exon 3 and PTEN Exon 7 Mutations in Small-cell Lung Cancer. Curr Med Sci 2019; 39: 379–384. [DOI] [PubMed] [Google Scholar]
- 12.Van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet 2011; 378: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 13.Fleckenstein J, Petroff A, Schafers HJ, et al. Long-term outcomes in radically treated synchronous vs metachronous oligometastatic non-small-cell lung cancer. BMC Cancer 2016; 16: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo X, Ma W, Wu H, et al. Synchronous bone metastasis in lung cancer: retrospective study of a single center of 15,716 patients from Tianjin, China. BMC Cancer 2021; 21: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Mao M, Guo X, et al. Nomogram based on homogeneous and heterogeneous associated factors for predicting bone metastases in patients with different histological types of lung cancer. BMC Cancer 2019; 19: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Liu F, Yu H, et al. Different distant metastasis patterns based on tumor size could be found in extensive-stage small cell lung cancer patients: a large, population-based SEER study. PeerJ 2019; 7: e8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Q, Xu Z, Wang L, et al. Progress in the research on the mechanism of bone metastasis in lung cancer. Mol Clin Oncol 2016; 5: 227–235. doi: 10.3892/mco.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Dou X, Liu T, et al. Tumor size and lymph node metastasis are prognostic markers of small cell lung cancer in a Chinese population. Medicine (Baltimore) 2018; 97: e11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren Y, Dai C, Zheng H, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 2016; 7: 53245–53253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han L, Dai W, Mo S, et al . Nomogram to predict the risk and survival of synchronous bone metastasis in colorectal cancer: a population-based real-world analysis. Int J Colorectal Dis 2020; 35: 1575–1585. [DOI] [PubMed] [Google Scholar]
- 21.Bernhardt EB, Jalal SI. Small Cell Lung Cancer. Cancer Treat Res 2016; 170: 301–322. [DOI] [PubMed] [Google Scholar]
- 22.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016; 66: 290–308. [DOI] [PubMed] [Google Scholar]
- 23.Stone CJL, Robinson A, Brown E, et al. Improving Timeliness of Oncology Assessment and Cancer Treatment Through Implementation of a Multidisciplinary Lung Cancer Clinic. J Oncol Pract 2019; 15: e169–e177. [DOI] [PubMed] [Google Scholar]