Abstract
Herbal medicines are currently being adopted as alternatives to orthodox medicines for the management of drug-resistant and emerging multidrug-resistant microbial strains of various diseases, including typhoid fever. A herbal decoction, MA 001, manufactured by the Centre for Plant Medicine Research (CPMR), has been used for the treatment of typhoid fever for at least two decades in Ghana with desirable outcomes. MA 001 is formulated from Citrus aurantifolia, Spondias mombin, Latana camara, Bidens pilosa, Trema occidentalis, Psidium guajava, Morinda lucida, Vernonia amygdalina, Persea americana, Paulina pinnatta, Momordia charantia and Cnestis ferruguinea medicinal plants. The low palatability and compliance to treatment due to the bulky nature of the decoction poses challenges in its optimum use. This study sought to design and formulate the therapeutic components of the aqueous herbal decoction of MA 001 into an optimal solid dosage form of effervescent granules to improve the delivery of MA 001 as well as increase patient compliance and convenience of product handling. The methods involved pre-formulation studies on the suitability of effervescent vehicles, formulation and evaluation of effervescent granules for drug excipient interactions using high performance liquid chromatography analysis. The findings indicate that the effervescent granules were suitable for use in the delivery of the therapeutic constituents for the treatment of typhoid fever as done with the decoction due to minimal herbal extract-excipient interaction.
Keywords: Typhoid fever, Herbal medicine, Effervescent granules, Formulation, High performance liquid chromatography
Typhoid fever; Herbal medicine; Effervescent granules; Formulation; High performance liquid chromatography
1. Introduction
Typhoid fever is a major public health concern. It is usually associated with poor sanitation, overcrowding and contamination of food and water. It is a major cause of morbidity and mortality. A global report in 2017, indicated that there were 14.7 million cases of typhoid and paratyphoid fevers with a fatality of about 135,000 [1]. There is a growing concern over the rapid emergence of antimicrobial resistance to the causative agents Salmonella enterica serovar typhi and Salmonella enterica serovar paratyphi A [2]. Key amongst several challenges in the fight aimed at reducing the incidence of Typhoid fever is the lack of access to proper water, sanitation, and vaccines, predominantly in developing countries. In addition, the increasing rate of antimicrobial resistance to the available antibiotics such as chloramphenicol and ciprofloxacin is also a major challenge [1, 2].
Natural compounds of plant origin have been documented to have effective therapeutic effects, and are useful alternatives to conventional drug treatment in human health. Plants are a renewable source for the synthesis of phytoconstituents that are useful for the treatment of diseases [3]. Recent developments have led to the investigation of plant medicine as antimicrobial and antibiofilm drugs that present suitable alternatives for antimicrobial chemotherapyagainst which microbes have a low capacity to develop resistance. This has been attributed to their potential multiple mechanisms of action that render them less susceptible to antimicrobial resistance. This indicates that plant-based medicines may be able to provide the much-needed treatment against constantly developing resistant pathogens for which orthodox antimicrobial agents are becoming increasingly ineffective [3, 4].
Previously, chloramphenicol was the treatment of choice for typhoid fever. With reports of plasmid-mediated multi-drug resistance to chloramphenicol, amoxicillin and cotrimoxazole, ciprofloxacin, a fluoroquinolone was used for treatment. Subsequently, the spread of resistant bacterial strains to ciprofloxacin has become a limitation to its use. Hence a combination of a cephalosporin and azithromycin are used against bacterial strains of Salmonella typhi with reduced susceptibility to fluoroquinolones [5, 6].
The development of microbial resistance to orthodox medicines has increased the exploitation of herbal medicines as alternatives [7]. An aqueous herbal decoction, coded MA 001, produced by the Centre for Plant Medicine, (CPMR), Mampong-Akuapem [8] has been used to treat typhoid fever in Ghana for at least two decades. The product is prepared from medicinal plants, namely Citrus aurantifolia, Spondias mombin, Latana camara, Bidens pilosa, Trema occidentalis, Psidium guajava, Morinda lucida, Vernonia amygdalina, Persea americana, Paulina pinnatta, Momordia charantia and Cnestis ferruguinea [9]
The administration of the right quantity of drugs is critical in the treatment of infectious diseases such as typhoid fever. However, MA 001, being a liquid preparation, poses challenges in terms of compliance in addition to accuracy of dosing. The dosage regimen for MA 001 is 30 mL three times daily for three weeks. A patient requires the purchase of six (6) bottles (330mL per bottle) of MA 001 to complete treatment. This may have implications for patient convenience and compliance. Bayor and colleagues [10] have reported that 95% of households preferred using spoons to take oral liquid remedies. However, many spoons do not meet the required volumes for drug administration. For example, the tablespoon is reported to have a deviation of ±1.2–1.5mL, making it less accurate and unable to meet the 15 mL volume requirement per tablespoon. Therefore, although MA 001 may be effective in treating typhoid [8], the need to formulate MA 001 into various solid dosage formulations may enhance patient convenience, compliance and accuracy in dosing.
Solid oral dosage forms include tablets, capsules, granules and powders for solution or suspension. Kumadoh and colleagues formulated [9] capsules of the product MA 001 by using various adsorbents. These solid dosage forms are comparatively more stable due to the decreased rate of degradation and microbial contamination as a result of the absence of an aqueous environment. Some geriatric patients have difficulty in swallowing solid forms such as tablets and capsules. Solid dosage forms of such preparations may be formulated as granules in sachets to be reconstituted into solution for their use when required [11]. Under proper storage conditions, it is expected that the granular dosage form will have an improved shelf life and longer lasting effect of the active ingredients. Also, this dosage form will provide additional alternatives for patients who would rather prefer herbal formulations over orthodox medicine [12]. The granular form of the drug was selected since it would have no aqueous content and would thus be less prone to microbial attack and hence decrease the degradation time of the product. In addition, geriatric patients with difficulty in swallowing will have a better alternative to the bulkier liquid product.
Effervescent salts are granules, or coarse to very coarse powders, containing the medicinal agent in a dry mixture usually composed of sodium bicarbonate, citric acid, and tartaric acid [13]. When added to water, the acids and the base react to liberate carbon dioxide, resulting in effervescence. The resulting carbonated solution normally masks the undesirable taste of the medicinal agent. Effervescent granules can be prepared via the wet granulation method, the dry granulation method, or the direct mixing method [14]. Since the decoction product is not palatable enough, the addition of an effervescent vehicle to the granule formulation may help to improve the taste and palatability of the solution formed due to its property of rapidly dissolving granules in solution [15].
The aim of this project was to develop effervescent granules of MA 001 herbal extract into fixed dose sachets and conduct an evaluation for any potential interactions between excipients used and active principles of the effervescent granules formed using High Performance Liquid Chromatography (HPLC).
2. Materials and methods
2.1. Materials
MA 001 Extract (obtained from CPMR), Light Magnesium Carbonate, Anhydrous Maize Starch, Anhydrous Citric Acid, Anhydrous Sodium Bicarbonate, glucose and Sucrose. All reagents used were of analytical grade, HPLC grade (water, acetonitrile, and 0.1 percent phosphoric acid).
2.1.1. Equipment
Gallen Kamp Hot Air Oven (Agilent), HPLC 1200 system (Agilent, Palo Alto, CA, USA), RP18 column (4.6 × 250 mm, 5.0 μm), Retsch laboratory Sieves (850 μm & 425 μm), Vortex Machine, Centrifuge, HPLC vials, Volumetric flask, Pipette, Wash bottle, Measuring Cylinder, White Flat tile, Desiccator, Magnetic Stirrer, Unscrambler® X software (CAMO Scientific, Norway) and Origin 8 (OriginLab cooperation USA).
2.2. Methods
2.2.1. Plant material collection and authentication
All the plant materials used in the formulation of MA 001 were obtained from the CPMR plant materials store after authentication by the Plant Development Department. They were further dried and pulverized at the plant material processing unit of the CPMR.
2.2.2. Preparation of MA 001 extract
The MA 001 Extract, the active ingredient for the formulation, consisting of a combination of plant materials obtained from plants, namely: Spondias mombin (leaves), Persea americana (leaves), Psidium guajava (leaves), Trema orientalis (leaves), Cnestis ferruguinea (leaves), Momordica charantia (aerial part), Vernonia amygdalina(leaves), Latana carnara (leaves), Paullinia pinnata (leaves), Citrus aurantifolia (leaves), Morinda lucida (leaves) and Bidens Pilosa (aerial part) was produced according to a classified formula at the production unit of the CPMR and made available as a dark hygroscopic extract after freeze drying. No excipients were added.
2.2.3. Determination of the total solid residue of MA 001 decoction and the equivalent hygroscopic weight of MA 001 extract
Ten (10 mL) of the sample (decoction before freeze drying without excipients) was measured and poured into a weighed clean crucible. The preparation was dried over a water bath and subsequently in an oven at 105 °C for about 2 h to a constant weight. The dried sample after cooling in a desiccator was weighed and the weight of the extract determined. The weight of the dried sample only was determined as % w/v [16]. The same procedure was repeated for 0.5 g of the freeze-dried extract to determine the equivalent weight to be used in product formulation.
2.2.4. Pre-formulation studies
Preliminary studies were done, for optimal formulation properties, with the use of two adsorbents, light magnesium carbonate and anhydrous maize starch mixed with the MA 001 extract was mixed in the proportions, 2:1, 1:1, 1:2, 1:3 of adsorbent, after which granulation was done using both wet and dry granulation methods. The mass formed after granulation was dried in a hot air oven at 40 °C for 24 h. The ease of scraping of the dried mass was taken into consideration, as it was critical for this type of formulation [9]. The dried mass of the mixture of extract and adsorbent was then sieved with 850 μm and 425 μm sieves [9]. In the determination of the appropriate acid or base saccharates, 1.42 g of citric acid and 0.85 g of sugar (acid saccharate); 1.7 g of sodium bicarbonate and 0.56 g of sugar (base saccharate) were weighed respectively and ground in mortars with the help of pestles, and placed in porcelain dishes.
One hundred milligrams (100 mg) of the Acid-Sugar mix and 100 mg of the Base-Sugar mix were weighed respectively and placed together into one beaker. 200 mL of distilled water was then added to the mixture in the beaker at 25 °C. The effervescence reaction was observed and recorded. After the preliminary selection of the suitable saccharate, the suitable extract-adsorbent mix was also chosen. The 1:2 Extract-Starch mix, with a particle size 850μm, exhibited desirable properties for mixing with optimal properties for granulation. In addition, the saccharate produced had the required properties for the formulation. This combination of extract, starch and effervescence vehicle ingredients were used for the final formulation of the product [16]. The formula used in the preparation of the effervescent granules has been indicated in Table 1: Master formula for preparation of effervescent granules (240 mg MA 001 extract per dose/sachet).
Table 1.
Master formula for preparation of effervescent granules (240 mg MA 001 extract per dose/sachet).
| Ingredient | Quantity (mg) |
|---|---|
| MA 001 Extract | 240 |
| Citric Acid | 265 |
| Sodium Bicarbonate | 265 |
| Saccharate | 250 |
| Maize Starch | 480 |
2.2.5. Formulation of effervescent granules
The required quantity of maize starch was weighed and added geometrically to the hygroscopic extract in a mortar and triturated till a homogeneous mixture was formed. Drying of the mixture was done in a hot air oven at 40 °C for 24 h. The mass formed was sieved with a sieve size of 850 μm and the other excipients, including the effervescent vehicle ingredients, were then added to complete the granule formulation process. The required tests on granules were then performed. The formulated granules were sealed in sachets and labelled appropriately.
2.2.6. Evaluation of granules
2.2.6.1. Determination of flow properties of granules
The bulk and tapped densities, Hausner ratio (HR) and Carr's compressibility index (CI) of the formulated granules were determined using the method described by [17] and the British Pharmacopoeia, 2016. The reference values have been indicated in Table 2: Reference Values for Hausner ratio and Compressibility index.
Table 2.
Reference values for Hausner ratio and compressibility index.
| Hausner ratio | Flow character | Compressibility index (%) |
|---|---|---|
| 1–1.11 | Excellent | 1–10 |
| 1.12–1.18 | Good | 11–15 |
| 1.19–1.25 | Fair | 16–20 |
| 1.26–1.34 | Passable | 21–25 |
| 1.35–1.45 | Poor | 26–31 |
| 1.26–1.59 | Very poor | 32–37 |
| >1.60 | Very, very poor | >38 |
[16].
2.2.6.2. Effervescence test
The granules were tested for effervescence in accordance with the BP 2016 specification. One dose (1.5 g) of the effervescent granules was placed in a beaker containing 200 mL of distilled water at 25 °C. The mixture was observed for the evolution of bubbles of gas. The time it took for the evolution of bubbles, if any, to cease, was recorded. The determination was done in triplicate and the effervescence time recorded.
2.2.7. Determination of herbal extract-excipient interactions using HPLC fingerprinting of extract, excipients and granules
HPLC analyses were carried out on the samples as outlined in Table 3; Key for HPLC Sample Labels.
Table 3.
HPLC sample label key.
| SA | Sample A | Extract + Starch + Effervescent Vehicle |
|---|---|---|
| SB | Sample B | Extract + Starch + Effervescent Vehicle |
| SC | Sample C | Extract + Starch + Effervescent Vehicle |
| SD | Sample D | Extract + Starch (Granules) |
| SE | Sample E | Extract + Starch (Granules) |
| SF | Sample F | Extract + Starch (Granules) |
| SG | Sample G | Extract Only |
| SH | Sample H | Extract Only |
| SI | Sample I | Extract Only |
| SS | Sample SS | Starch |
| SCA | Sample SCA | Saccharate of Citric Acid |
| SSB | Sample SSB | Saccharate of Sodium Bicarbonate |
The method employed by Wagner and colleagues [18] and Butnariu and colleagues [19] was slightly modified. Ten milligrams (10 mg) of each sample were weighed and dissolved in 1 mL of distilled water. The mixture was vortexed and then centrifuged for 10 min and filtered. Five hundred microliters (500 μL) of the supernatant was pipetted into a clean empty HPLC vial and labelled appropriately. The vial was then inserted into the injection chamber. Isocratic elution was employed in the chromatographic process.
Optimized conditions developed for the HPLC analyses were as shown below:
Mobile Phase – 30 % Acetonitrile: 70 % water with 0.1% Phosphoric Acid.
Flow rate – 1 mL/min.
Injection volume – 50.0 μl.
Temperature (Column Oven) – 40 °C.
Run time – 30 min.
Wavelength (UV detection) – 300 nm.
2.2.8. HPLC analyses
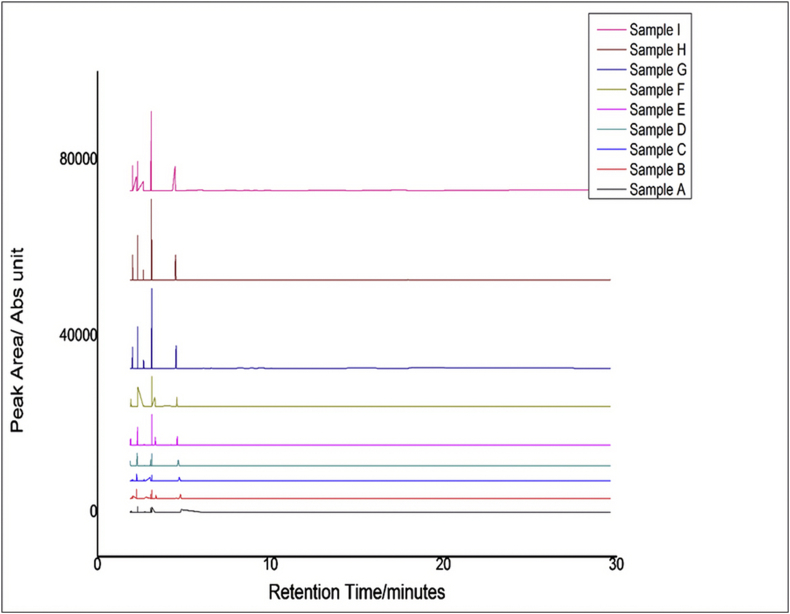
Chemometric analysis of the HPLC data was conducted with the use of Unscrambler® X software (CAMO Scientific, Norway) and Origin 8 (OriginLab cooperation USA). Principal Component Analysis and Cluster Analysis were carried out on the HPLC data. The obtained results have been presented in the results section as Figure 1: HPLC fingerprint of samples of extract and extract-excipient combinations.
Figure 1.
HPLC fingerprint of samples of extract and extract-excipient combinations.
2.2.9. Correlated optimized wapping (COW)
It was essential to remove variation in HPLC data resulting from instrumental noise and concentration, correlated optimized wapping and area normalizations. Data pretreatment was used to align peaks where the signals exhibited shifts in their position along the x (or retention time) axis and concentration variation respectively. Correlated Optimized Wapping was conducted using 13 segments and 6 slacks with Sample I as the reference sample to align the principal peaks in the various chromatograms. Figure 2: HPLC chromatogram of samples after peak alignment by COW indicates the results of this analysis.
Figure 2.
HPLC chromatogram of samples after peak alignment by COW.
2.3. Principal component analysis (PCA)
The principal component analysis was performed with 22 data points against 12 samples, making a data matrix consisting of 22 columns and 12 rows. The analysis was focused on the part of the chromatogram where prominent peaks (from 2.050 to 4.529 min) in the chromatogram were observed. PCA was performed on pre-treated Correlated Optimized Wapping data using three PCs and cross-validation, with Hoteling T2 outlier determination at a 95% confidence interval. Results have been indicated in Figure 3: PCA of pure extract, formulations and excipient of HPLC chromatogram data.
Figure 3.
PCA of pure extract, formulations and excipient of HPLC chromatogram data.
2.4. Cluster analysis (CA)
Hierarchical complete linkage cluster analysis with squared Euclidean distance was also conducted on pre-treated Correlated Optimized Wapping data. The results of this analysis have been presented in Figure 4: Cluster Analysis of HPLC data.
Figure 4.
Cluster Analysis of HPLC data.
2.5. Statistical analysis
Microsoft excel (version 2016, Microsoft, USA), was employed in the determination of the mean and standard deviation of values obtained for replicate methods.
3. Results
3.1. Determination of the total solid residue and equivalent hygroscopic weight of MA 001 decoction
The mean total solid residue per dose of 30 mL was determined to be 240 mg ± 10 mg.
The mean total solid residue per 240 mg dose, equivalent to the hygroscopic extract was determined to be 293.83 mg–294 mg.
3.2. Preformulation studies
It was generally easy to mix extract and maize starch in the various proportions in both the dry and wet granulation methods. However, it was generally difficult to mix extract and light magnesium carbonate in the various proportions in the dry granulation but easy in the wet granulation method. The properties of the granules formed after drying in the various proportions are presented in Table 4; Preformulation properties of Extract-Starch mixtures using dry and wet granulation. The flow properties of the formulated granules have been shown in Table 5: Results of the determination of the Flow Properties, effervescence time of formulated granules.
Table 4.
Preformulation properties of extract-starch mixtures using dry and wet granulation.
| Granulation method | Extract: Adsorbent mix ratio | Nature of granules after drying | Ease of scraping after drying | Inference for granulation | |
|---|---|---|---|---|---|
| Maize starch | Dry | 2:1 | Sticky and gummy | Easy | Undesirable |
| 1:1 | Sticky and gummy | Easy | Undesirable | ||
| 1:2 | Dry | Easy | Desirable | ||
| 1:3 | Dry | Easy | Desirable | ||
| Wet | 2:1 | Sticky and gummy | Easy | Undesirable | |
| 1:1 | Sticky and gummy | Difficult | Undesirable | ||
| 1:2 | Dry | Easy | Desirable | ||
| 1:3 | Dry | Easy | Desirable | ||
| Light Magnesium Carbonate | Dry | 2:1 | Sticky and gummy | Easy | Undesirable |
| 1:1 | Dry | Easy | Desirable | ||
| 1:2 | Dry | Easy | Desirable | ||
| 1:3 | Dry | Easy | Desirable | ||
| Wet | 2:1 | Sticky and gummy | Easy | Undesirable | |
| 1:1 | Sticky and gummy | Difficult | Undesirable | ||
| 1:2 | Dry | Easy | Desirable | ||
| 1:3 | Dry | Easy | Desirable |
Table 5.
Results of the determination of the flow properties and effervescence time of formulated granules.
| Granules | Vo (mL) | Vf (mL) | Hausner Ratio | Carr's Index | Inference of flow property | Effervescence time (seconds) n = 3 |
|---|---|---|---|---|---|---|
| Fine (425 μm) | 3.0 | 2.0 | 1.50 | 33.33 % | Very poor | 252 ± 20 |
| Coarse (850 μm) | 1.6 | 1.4 | 1.12 | 12.50 % | Good | 229 ± 18 |
Vo represents initial volume, Vf: final volume, Carr's index = (100 x [(Vo - Vf)/Vo]), Hausner ratio = (Vo/Vf).
4. Discussion
The formulation of effervescent granules of MA 001 into fixed dose sachets was undertaken to address issues such as inaccurate dosing resulting from differences in volumes of household spoons used in drug administration [10, 20, 21], patient convenience and compliance. Patient convenience and compliance are critical for effective treatment outcomes in the management of microbial infections [22, 23, 24].
The results show that the formulation of effervescent granules of MA 001 achieved the desired purpose. Additionally, the stability of the solid dosage form was enhanced as the potential degradation of active therapeutic principles in aqueous media, microbial contamination, and the associated disruption of its shelf life were prevented [25, 26]. Effervescent granules of MA 001, which form a solution on contact with water, will provide an improvement in taste, solubility, and bioavailability of the herbal extract [27, 28].
Several herbal products, despite having exceptional therapeutic potential, have limited or reduced therapeutic action due to their poor aqueous solubility [29, 30]. MA 001's formulation is intended to improve the efficiency of therapeutically active constituent delivery [31, 32]. Dissolution is enhanced with the subsequent solution formed [15].
For the purpose of producing dry effervescent granules devoid of an aqueous component, the dry granulation process was selected as the method of choice for all subsequent granulation processes required for testing and formulation of the final product. Drying as a technique plays a useful role in enhancing the stability and extending the shelf life of pharmaceutical products and it is known that dry powder extracts facilitate the ease of formulation of liquid-based products into solid dosage forms [33, 34]. The 1:2 Extract-Starch Mix demonstrated great ease of mixing and scraping before and after drying in a hot air oven. It was the most cost effective and feasible mix, producing desirable results, including the demonstration of good flow properties of the coarse granules. These reasons account for its selection over the Extract-LMC Mix [15]. The determination of flow properties of the granules is important in ensuring reproducibility of filling and dose consistency and weight uniformity in relation to the physical properties of effervescent granules of MA 001. The Angle of repose and Hausner's ratio are indicative of the inter particle friction in a powder and determine the extent of thecohesiveness and free-flowing properties of the powder. The values obtained indicate that the granules to be used are of good flow properties and optimum filling will be achieved during manufacturing into fixed dose sachets [13, 15].
Table sugar (sucrose) was selected as the saccharate of choice due to the observed production of favorable tests of effervescence that produced no film or foam on the walls of the vessel during and after effervescence. From the effervescence test, numerous bubbles of gas evolved under observation. The evolution of gas around the individual granules ceased within 5 min, indicating the suitability of the product.
PCA was performed to visualize and reduce the dimensions of the data sets. The decrease in peak intensity in samples A-F may be due to low concentration of extract in these samples because the equivalent weight of extract used in the HPLC analysis was not employed in the various samples prepared, making the HPLC data concentration dependent. This effect was minimized to some extent by performing data pre-treatment such as area normalization to align shifted peaks using correlated optimized wapping. PCA is a multivariate statistical technique used to reduce the dimensionality in the data set. It calculates and uses the covariance matrix to determine the variables responsible for maximum variation within a data [35]. Here, PCA was performed on a pretreated chromatogram of the various herbal formulations for the evaluation of batch consistency and excipient-extract compatibility.
Principal component (PC) 1 accounted for 93% of the variation in the data set, whereas PC 2 accounted for 5% of the variation, making a total of 98% of the variation in the data sets. Clustering of the formulations (samples A-D, F) and clustering of the pure extract (sample G-I) may be attributed to the occurrence of low concentrations of extract and high concentrations of excipients in these samples which seem to mask the peaks of the extract. Although the pure extracts did not form a close cluster with the various formulations, the relative distance between these two clusters was very small. The clustering of all formulations suggests a high degree of batch consistency. Hoteling T2 outlier determination [36] was used to further determine if these samples are similar and possibly contain the same principal components. The occurrence of all samples within the outlier ellipsoid (Figure 3) implies the presence of no outliers, indicating that the samples contained similar components [37].
To substantiate the batch consistency and extract-excipient compatibility, a hierarchical complete linkage cluster analysis was performed on the pretreated chromatogram. Clusters analysis uses the data points from the various chromatograms to determine the similarities between various samples by calculating the ecludian distance between them, representing how close the samples resemble each other [38]. Similar to the PCA analysis, two main clusters; clustering of formulation and clustering of pure extracts, were observed. The squared ecludian distance observed between the two main clusters is very minimal (<1), hence it confirms the presence of the same principal component within the samples [38]. All evaluation tests confirmed that there was little or no herbal extract-excipient interaction indicative that the granules formed were stable [39, 40, 41]. The formulation technology employed is beneficial for industrial applications, in the potential ease of scaling up, ease of formulation, enhanced stability of therapeutic principles and reproducibility coupled with the use of unsophisticated equipment, A simple, convenient, cost effective, and environmentally friendly method is a beneficial approach. The use of natural antimicrobials for treatment provides a holistic approach to typhoid fever management [42, 43].
Strict controls over the use of antibiotics in livestock, coupled with strict sanitary standards in the food supply chain, have not been able to adequately address concerns about increasing drug resistant Salmonella strains [3, 4, 44, 45, 46]. The formulation design of effervescent granules of MA 001, with improved palatability and stability, is a good alternative for the effective treatment of emerging multidrug-resistant strains of Salmonella [3, 4]. This can significantly help to reduce the spread of typhoid fever, and the potential public health threat of the spread of zoonotic Salmonellosis. The strategies adopted for the formulation development of effervescent granules of MA 001 also provide useful information for scientists and practitioners with the mindset of integrating herbal remedies into the regular health care delivery systems [47].
5. Conclusion
The effervescent granules of MA 001 for the treatment of typhoid fever were successfully formulated using the 1:2 extract: starch ratio with sucrose as the saccharate of choice. All evaluation tests, including the flow properties and herbal extract-excipient interactions, indicated that the final product was stable and devoid of herbal extract-excipient interactions, which could likely cause product instability. The formulated granules may have improved taste and stability and enhance compliance with treatment, which is essential in the treatment of typhoid fever infections.
Declarations
Author contribution statement
Ofosua Adi-Dako, Papa Yaw Addo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Doris Kumadoh: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Godfred Egbi: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Samuel Okyem: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Alexander Nyarko: Conceived and designed the experiments.
Christina Osei-Asare, Esther Eshun Oppong: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Emmanuel Adase: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the University of Ghana, School of Pharmacy and the Centre for Plant Medicine Research, Mampong-Akuapem, Ghana who provided the facilities and equipment used for this research.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Stanaway J.D., Reiner R.C., Blacker B.F. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study. Lancet Infect. Dis. 2017;19:369–381. doi: 10.1016/S1473-3099(18)30685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saporito L., Colomba C., Titone L. In: International Encyclopedia of Public Health. second ed. QUAH S.R., editor. Academic Press; Oxford: 2017. Typhoid fever. [Google Scholar]
- 3.Beoletto V.G., De Las Mercedes Oliva M., Marioli J.M., Carezzano M.E., Demo M.S. 2016. Antimicrobial Natural Products against Bacterial Biofilms; pp. 14–291. [Google Scholar]
- 4.Upadhyay A., Karumathil D.P., Upadhyaya I., Bhattaram V., Venkitanarayanan K. In: Antibiotic Resistance. Mechanisms and New Antimicrobial Approaches. Kon K., Rai M., editors. Academic Press; 2016. Controlling bacterial antibiotic resistance using plant-derived antimicrobials; pp. 205–215. [Google Scholar]
- 5.Veeraraghavan B., Pragasam A.K., Bakthavatchalam Y.D., Ralph R. Vol. 4. 2018. Typhoid fever: issues in laboratory detection, treatment options & concerns in management in developing countries. (6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P., Dahiya S., Manral N. Vol. 36. 2018. Changing Trends of Culture-Positive Typhoid Fever and Antimicrobial Susceptibility in a Tertiary Care North Indian Hospital over the Last Decade; p. 70. [DOI] [PubMed] [Google Scholar]
- 7.Vadhana P., Bhoj R.S., Bharadwaj M., Varan S.S. Emergence of herbal antimicrobial drug resisrance in clinical bacterial isolates. Pharm. Anal. Acta. 2015;6(10):434. [Google Scholar]
- 8.Mills-Robertson F. Thesis submited to the University of Ghana; 2004. Characterization of Wild-type Salmonella and Their Susceptibility to “Mist Enterica” an Anti-typhoid Herbal Preparation. [Google Scholar]
- 9.Kumadoh D., Adotey J., Ofori-Kwakye K., Kipo S.L., Prah T., Patterson S. Development of oral capsules from Enterica herbal decoction-a traditional remedy for typhoid fever in Ghana. J. Appl. Pharmaceut. Sci. 2015;5(4):83–88. [Google Scholar]
- 10.Bayor M.T., Kipo L.S., Kwakye K.O. The accuracy and quality of household spoons and enclosed dosing devices used in the administration of oral liquid medications in Ghana. 2010;2:150–153. (1) [Google Scholar]
- 11.Ipci K., Birdane L., Altintoprak N., Muluk N.B., Passali D. Effervescent tablets: a safe and practical delivery system for drug administration. ENT Updates. 2016;6:46. [Google Scholar]
- 12.Fakeye T.O., Adisa R., Musa I.E. Attitude and use of herbal medicines among pregnant women in Nigeria. BMC Compl. Altern. Med. 2009;9:53. doi: 10.1186/1472-6882-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen L., Ansel H.C. Lippincott Williams & Wilkins; 2013. Ansel's Pharmaceutical Dosage Forms and Drug Delivery Systems; p. 794. [Google Scholar]
- 14.Iyer V.S., Srinivas S.C. 2007. Effervescent Granular Formulations of Antiretroviral Drugs. Google Patent WO2007060682. [Google Scholar]
- 15.Aulton M.E., Kevin T. fourth ed. Churchill Livingstone; Edinburgh: 2013. Powder Flow. Pharmaceutics. The Design and Manufacture of Medicines; pp. 187–199. [Google Scholar]
- 16.Pharmacopoeia B. 2016. British Pharmacopoeia. [Google Scholar]
- 17.Aulton M.E., Kelvin T. fifth ed. Churchill Livingston Elsevier; Chine: 2018. Power Flow. The Deisgn and Manufacture of Medicine; pp. 189–200. [Google Scholar]
- 18.Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A., editors. Thin- Layer and High Performance Liquid Chromatography of Chinese Drugs. Vol. 3. Springer; 2011. Chromatographic fingerprint analysis of herbal medicines. [Google Scholar]
- 19.Butnariu M., Caunii A., Putnoky S. Reverse phase chromatographic behaviour of major components in Capsicum Annuum extract. 2012;6:146. doi: 10.1186/1752-153X-6-146. (1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannan L., Blocher N. Cultural impact on medication instructions: the case of the Turkish teaspoon. J. Patient Saf. 2018;14(2) doi: 10.1097/PTS.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 21.Joshi P., Bavdekar S.B. Vol. 86. 2019. Liquid drug dosage measurement errors with different dosing devices; pp. 382–385. (4) [DOI] [PubMed] [Google Scholar]
- 22.Hambly J.L., Haywood A., Hattingh L., Nair R.G., Dentistry C. Vol. 8. 2017. Comparison between Self-formulation and Compounded-formulation Dexamethasone Mouth Rinse for Oral Lichen Planus: a Pilot, Randomized, Cross-over Trial. [DOI] [PubMed] [Google Scholar]
- 23.Patadia J., Tripathi R., Joshi A. Vol. 17. 2016. Melt-in-mouth multi-particulate system for the treatment of ADHD: a convenient platform for pediatric use; pp. 878–890. (4) [DOI] [PubMed] [Google Scholar]
- 24.Gupta A.K., Foley K.A., Versteeg S.G. Vol. 182. 2017. New Antifungal Agents and New Formulations against Dermatophytes; pp. 127–141. (1-2) [DOI] [PubMed] [Google Scholar]
- 25.Mwesigwa E., Basit A.W. Vol. 497. 2016. An investigation into moisture barrier film coating efficacy and its relevance to drug stability in solid dosage forms; pp. 70–77. (1-2) [DOI] [PubMed] [Google Scholar]
- 26.Dao H., Lakhani P., Police A. Vol. 19. 2018. Microbial Stability of Pharmaceutical and Cosmetic Products; pp. 60–78. [DOI] [PubMed] [Google Scholar]
- 27.Devi J.R., Das B. Preparation and evaluation of ibuprofen effervescent granules. J. Asian Pharm. Clin. Res. 2019;12:52–55. [Google Scholar]
- 28.Wati S., Saryanti D. Vol. 2. 2019. Effervescent granule formulation of bitter melon extract (Momordica charantia L.) with gelatin as a wet granulation binder; pp. 20–28. (1) [Google Scholar]
- 29.Ansari M.J., Parveen R. Therapeutics. Solubility and stability enhancement of curcumin. Improving drug properties of natural pigment. 2016;7(2):113–116. [Google Scholar]
- 30.Byeon J.C., Ahn J.B., Jang W.S. Vol. 49. 2019. Recent formulation approaches to oral delivery of herbal medicines; pp. 17–26. [Google Scholar]
- 31.Mukherjee P.K., Harwansh R.K., Bhattacharyya S. Evidence-Based Validation of Herbal Medicine. Elsevier; 2015. Bioavailability of herbal products: approach toward improved pharmacokinetics. [Google Scholar]
- 32.De Vries K., Strydom M., Steenkamp V. Vol. 11. 2018. Bioavailability of resveratrol: possibilities for enhancement; pp. 71–77. [Google Scholar]
- 33.Zhou J., Gong Y., Ma H. Vol. 114. 2015. Effect of drying methods on the free and conjugated bufadienolide content in toad venom determined by ultra-performance liquid chromatography-triple quadrupole mass spectrometry coupled with a pattern recognition approach; pp. 482–487. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira C.C.C., De Freitas Cabral T.P., Tacon L.A., Villardi I.L., Lanchote A.D., De Freitas L.A. Vol. 319. 2017. Solid state stability of polyphenols from a plant extract after fluid bed atmospheric spray-freeze-drying; pp. 494–504. [Google Scholar]
- 35.David C.C., Jacobs D.J. Principal component analysis: a method for determining the essential dynamics of proteins. Methods Mol. Biol. 2014;1084:193–226. doi: 10.1007/978-1-62703-658-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha P., Roy N., Mukherjee D., Sarkar A.K. Application of principal component analysis for outlier detection in heterogeneous traffic data. Proc. Comp. Sci. 2016;83:107–114. [Google Scholar]
- 37.Serneels S., Verdonck T. Vol. 52. 2008. Principal component analysis for data containing outliers and missing elements; pp. 1712–1727. [Google Scholar]
- 38.Fávero L., Belfiore P. Academic Press; 2019. Data Science for Business and Decision Making. [Google Scholar]
- 39.Govindaraghavan S., Sucher N.J. Quality assessment of medicinal herbs and their extracts: criteria and prerequisites for consistent safety and efficacy of herbal medicines. Epilepsy Behav. 2015;52:363–371. doi: 10.1016/j.yebeh.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Peng L., Wang Y.Y., Zhu H., Chen Q. Fingerprint profile of active components for Artemisia selengensis Turcz by HPLC–PAD combined with chemometrics. Food Chem. 2011;125:1064–1071. [Google Scholar]
- 41.Lu H., Ju M., Chu S. Vol. 23. 2018. Quantitative and chemical fingerprint analysis for the quality evaluation of Platycodi Radix collected from various regions in China by HPLC coupled with chemometrics; p. 1823. (7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiremath P.S., Saha R.N. Controlled release hydrophilic matrix tablet formulations of isoniazid: design and in vitro studies. AAPS PharmSciTech. 2008;9:1171–1178. doi: 10.1208/s12249-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohajeri E., Ansari M., Pardakhty A.J.P.S. Vol. 21. 2015. Controlled release imatinib mesylate tablet formulation: using hydrophilic matrix system; pp. 157–166. [Google Scholar]
- 44.Manyi-Loh C., Mamphweli S., Meyer E., Okoh A. Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules. 2018;23(4):795. doi: 10.3390/molecules23040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van T.T.H., Yidana Z., Smooker P.M., Coloe P.J. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J. Global Antimicrob. Res. 2020;20:170–177. doi: 10.1016/j.jgar.2019.07.031. [DOI] [PubMed] [Google Scholar]
- 46.Nair D.V.T., Venkitanarayanan K., Kollanoor J.A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods. 2018;7(10):167. doi: 10.3390/foods7100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C., Guo D.A., Liu L.J.P. Vol. 44. 2018. Quality Transitivity and Traceability System of Herbal Medicine Products Based on Quality Markers; pp. 247–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.