Abstract
Several onco-virotherapy candidates have been developed and clinically evaluated for the treatment of cancer, and several are approved for clinical use. In this systematic review we explored the clinical impact of onco-virotherapy compared to other cancer therapies by analyzing factors such as trial design, patient background, therapy design, delivery strategies, and study outcomes. For this purpose, we retrieved clinical studies from three platforms: ClinicalTrials.gov, PubMed, and EMBASE. We found that most studies were performed in patients with advanced and metastatic tumors, using a broad range of genetically engineered vectors and mainly administered intratumorally. Therapeutic safety was the most frequently assessed outcome, while relatively few studies focused on immunological antitumor responses. Moreover, only 59 out of 896 clinical studies were randomized controlled trials reporting comparative data. This systemic review thus reveals the need of more, and better controlled, clinical studies to increase our understanding on the application of onco-virotherapy either as a single treatment or in combination with other cancer immunotherapies.
Keywords: oncolytic virotherapy, cancer, clinical study, trial, outcomes, immune response, systematic review
Graphical abstract
Bhatt and colleagues demonstrate that onco-virotherapy has proven to be clinically safe due to efforts in vector design, rational choices of therapeutic dosage, and delivery strategies. However, the analysis reveals the need of more controlled trials in addition to including early stage cancer patients and evaluation of immune responses.
Introduction
In the last two decades, viral vector-based therapies are gaining increasing attention as a promising strategy for cancer treatment. Studies in the field of cancer virotherapy have explored the administration of viral vectors as agents for therapeutic vaccines,1 gene therapy,2, 3, 4 and more recently as oncolytic therapeutics.5,6 To date, several viral vectors are approved for clinical application. Safety and efficacy are the primary goals of clinical trials and, therefore, the clinical success of cancer virotherapy depends on these outcomes. Advanced genetic engineering tools have allowed researchers to improve safety by enhancing tumor targeting and tumor replication.5 Additionally, with these tools, the efficacy of viral vectors for onco-virotherapy can be enhanced by encoding transgenes that strengthen the oncolytic potential or that elicit stronger antitumoral immune responses.7 Besides vector design, factors such as clinical trial design, patient background, dose, frequency, delivery strategy, issues related to immune-mediated virus elimination, and the choice of clinical outcome measures may also influence clinical success. In this study we aimed to analyze these parameters based on a systematic review. For this purpose we retrieved articles from several platforms in the context of onco-virotherapy for antitumor responses. Previous systematic reviews on onco-virotherapy were based on a limited number of articles retrieved exclusively from PubMed,6,8 thereby overlooking studies archived by other platforms, while a recent review focused on randomized controlled trials only.9 To provide a broader overview of the global trends in clinical research related to onco-virotherapy we conducted an extensive literature survey that includes a more complete set of articles and trials retrieved from multiple platforms, including ClinicalTrials.gov, PubMed (Medline), and EMBASE. For a comprehensive analysis of the clinical research in onco-virotherapy to date, our dataset includes phase I–IV trials, along with cohort and case studies.
Results
Scenario of clinical studies evaluating onco-virotherapy
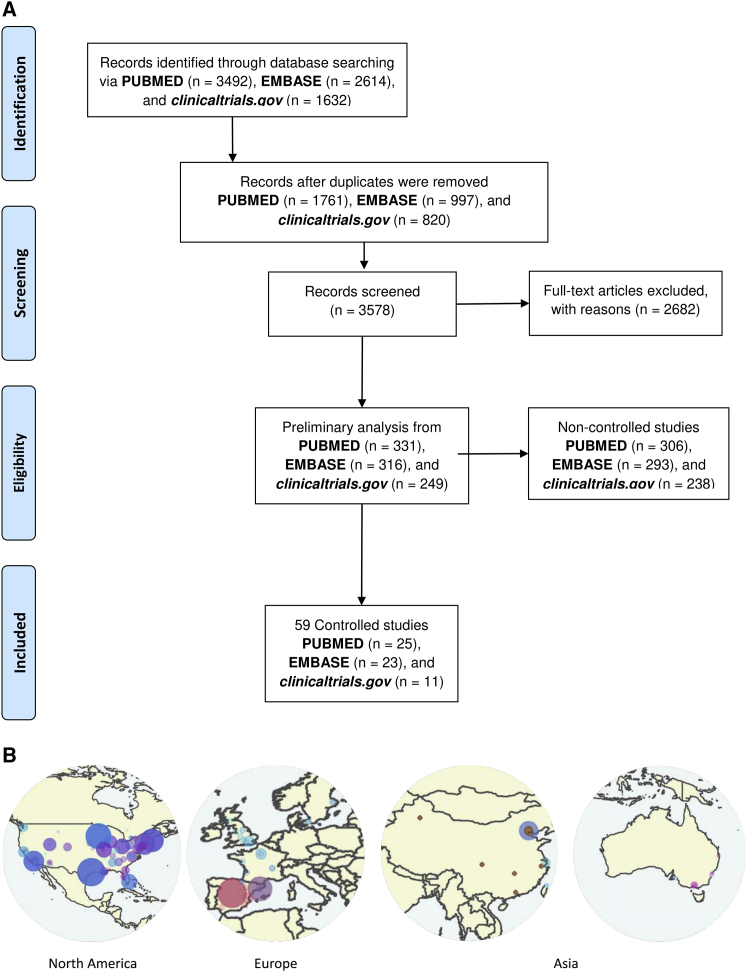
A systematic search performed on PubMed, EMBASE, and ClinicalTrials.gov, retrieved until August 2020, found 249 trials, 331 articles on PubMed, and 316 articles on EMBASE that contained relevant terms (Supplemental information) and fulfilled the necessary inclusion criteria required for the analysis on clinical data related to onco-virotherapy (Figure 1A). Of these trials and articles, 59 entries contained data from controlled clinical trials, allowing the comparison of onco-virotherapy with either placebo, standard palliative care, or conventional therapy (Figure 1A). Most of these studies were performed in North America, the Republic of China, and Europe (Figure 1B).
Figure 1.
Screening of studies focusing on clinical safety and efficacy of onco-virotherapy
(A) Systematic review process and inclusion of articles and trials based on target criteria, where excluded reports were those that did not focus on the application of onco-virotherapy for cancer patients, or were they reviews, preclinical studies, or commentaries, or articles in which the abstract was not reported in English. (B) Geographical distribution of labs and institutes assessing safety and efficacy of onco-virotherapy.
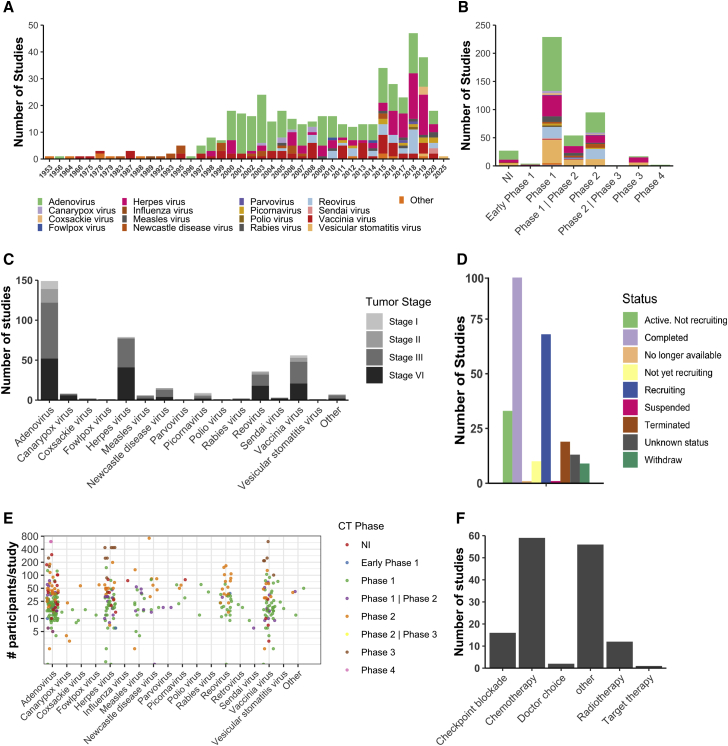
There has been an increase in the number of studies in the past two decades, which can be attributed to the widespread availability of genetic engineering platforms and molecular techniques to design and test onco-virotherapy in both pre-clinical and clinical stages (Figure 2A). Especially the approval of talimogene laherparepvec10 (T-VEC, herpes virus with infected cell protein [ICP] 34.5 and ICP47 deletion, encoding granulocyte-macrophage colony-stimulating factor [GM-CSF]) by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2015, revived the interest for clinical applications of onco-virotherapy (Figure 2A). Most clinical studies have been conducted at phase I and II stages, often to test the safety and maximum tolerated dosage of the onco-virotherapy (Figure 2B). Although a wide range of viral vectors have been tested in phase I and II trials for safety,5 few studies have progressed further to phase III trials (Figure 2B). In terms of the genetic nature, both enveloped and non-enveloped DNA and RNA vectors have been tested. Adenovirus (non-enveloped DNA virus) was the most commonly studied platform with 42.5% of studies, followed by herpes simplex virus (enveloped DNA virus) with 21.3% of studies, vaccinia virus (enveloped DNA virus) with 13.2% of studies, and reovirus (non-enveloped RNA virus) with 7.3% of studies. Patients with advanced and metastatic tumors were the most frequently recruited patients to receive onco-virotherapy (Figure 2C), likely due to the fact that cancer patients with a good prognosis generally benefit from standard care. However, this may limit our understanding of the potential efficacy of onco-virotherapy in patients with early stage cancers.
Figure 2.
Scenario of clinical studies assessing onco-virotherapy
(A) Trends in clinical studies published as trials and articles assessing the role of different onco-virotherapy. (B) Frequency of studies published as trials and articles according to phase and type of onco-virotherapy studied, with the legend the same as in (A). (C) Patient tumor stage and status that received onco-virotherapy. (D) Onco-virotherapy trial status as per ClinicalTrials.gov. (E) Number of cancer patients recruited per study and treated via onco-virotherapy in different phases. (F) Frequency of therapeutic combination with onco-virotherapy.
Although more than 200 trials related to onco-virotherapy are registered on ClinicalTrials.gov, fewer than 100 trials have been completed (Figure 2D). Many studies have reported being terminated or suspended due to funding issues or a lack of participants, and some trials are still active or recruiting patients. More than 2,000 cancer patients have been recruited and treated with onco-virotherapy, with phase I/II trials mostly conducted in a relatively small group of patients, and phase III trials with more than 200 patients per group (Figure 2E). Onco-virotherapy has been given to patients as a monotherapy, while occasionally it has been combined with chemotherapy and radiotherapy, and in some cases with targeted therapy (Figure 2F). Moreover, a limited number of case studies that did not have success with conventional checkpoint therapy or radiotherapy/chemotherapy (indicated as doctor’s choice in Figure 2F) later proceeded with onco-virotherapy alone11 or in combination with cyclophosphamide12 to treat recurrent tumors in patients. Recent preclinical findings supporting the combination of immunotherapy have also led to clinical studies where checkpoint inhibitors targeting PD-1 (programmed death receptor-1) or PD-L1 (programmed death ligand-1) have been administered along with onco-virotherapy.13
Viral modifications and strategic therapy design to improve safety and efficacy
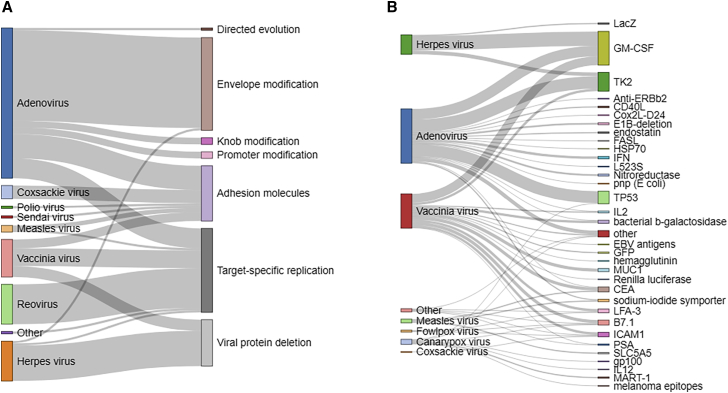
To establish the safety of onco-virotherapy for cancer patients, genetic modifications have been performed on a wide range of viral vectors to improve tumor targeting and attachment or to enhance tumor-specific replication (Figure 3A). Adenoviruses, herpes viruses, vaccinia viruses, and reoviruses have often been engineered to improve tumor specificity by such modifications. Improvement in targeting of adenovirus was for example achieved through knob modifications, and attachment was improved by adenoviral fiber protein delta-24-RGD modification or via intercellular adhesion molecules. Alternatively, viral replication was restricted to tumor cells through modification or deletion of viral proteins such as early proteins (E1–E4) in adenovirus, and deletion of ICP34.5 and ICP47 in herpes virus (Table S1). Moreover, in some cases reovirus, vaccinia virus, and adenovirus were designed to have target-specific replication in tumor cells with differentially activated pathways such as RAS GTPases (rat sarcoma GTPases) or p16-RB (retinoblastoma protein) pathways (Table S1). The 2018 Nobel prize-winning technique of directed evolution has also been implemented as a means to screen adenoviruses with improved selectivity for tumor cells and their subsequent oncolysis.
Figure 3.
Viral modifications to improve safety and efficacy
(A) Virus modifications to improve tumor specificity. (B) Introduction of transgenes to improve therapeutic efficiency. Each line represents a single study (trial or article).
Viral vectors have also been engineered to deliver and encode transgenes that act as “suicide-genetic switch” for controlled lysis of target cancer cells, for example by using ganciclovir to induce cell death of tumor cells expressing a thymidine kinase (TK2) transgene (Figure 3B). Simultaneously, viral vectors have been modified to improve efficacy by incorporating genes to enhance or direct antitumor immune responses. These are either tumor-specific antigens such as prostate-specific antigen (PSA) and mucin1 (MUC1), antitumor genes including tumor suppressor protein 53 (TP53), and genes encoding growth factors, cytokines, or ligand molecules, such as GM-CSF, interferon, interleukin-12, Fas ligand, and CD40 ligand, or marker genes encoding fluorescent proteins or enzymes that can be used for detection and quantification of transgene expression such as galactosidase and luciferase (Figure 3B). The most commonly encoded genes were found to be GM-CSF, TP53, and TK2, while adenoviruses, herpes viruses, and vaccinia viruses were the most frequently engineered vectors of choice.
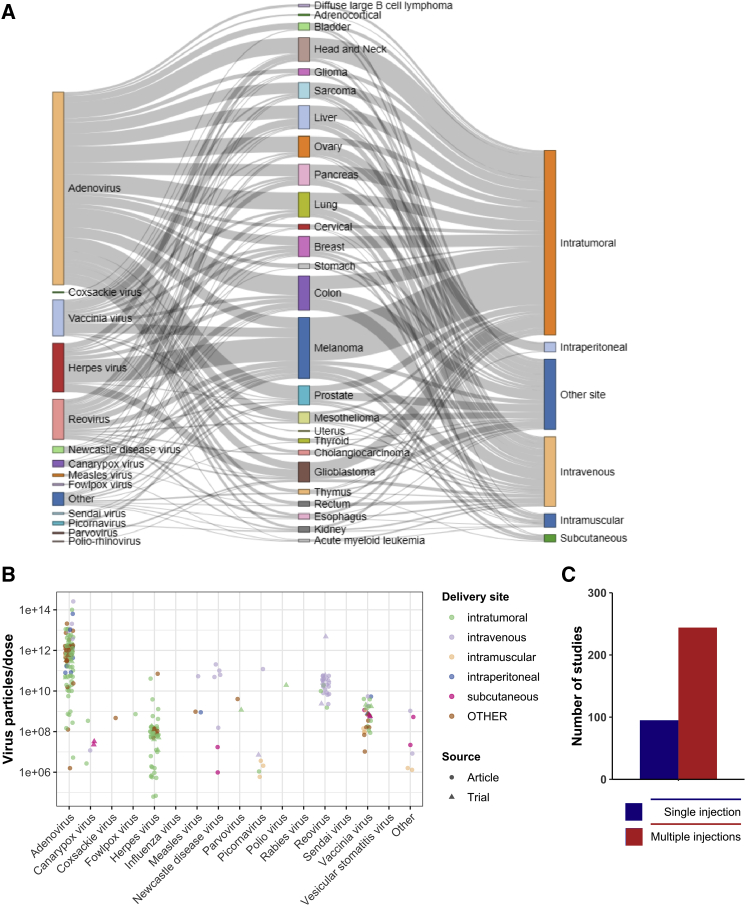
Regarding therapeutic delivery, intratumoral delivery of viral vectors has remained the preferred route of injection due to safety and efficacy concerns by restricting viral infection to tumor. Nevertheless, intravenous, subcutaneous, and intramuscular routes have also been tested to achieve better biodistribution and to target distant metastatic sites (Figure 4A). In the case of melanoma, intratumoral delivery has remained a preferred choice due to easier accessibility of tumors. A wide range of virus doses have been tested for each viral vector type, and safety has been associated with lower doses, albeit at the cost of therapeutic efficacy. For example, adenoviruses have been given to patients at doses considered safe up to 1014 particles per injection for the best efficacy, whereas herpes viruses have demonstrated to be efficient in the range of 106–108 particles per injection (Figure 4B). In terms of the number of virus injections given to cancer patients, multiple injections were preferred and the scheme varied from daily, weekly, and monthly intervals (Figure 4C; Table S1).
Figure 4.
Strategic design to improve safety and efficacy
(A) Trends in choice of viral vector and delivery site according to tumor type. Each line represents a single study (trial or article). (B) Maximum tolerated dose per virus type in patients. (C) Frequency of injections applied.
Evaluation of clinical outcomes related to safety and efficacy
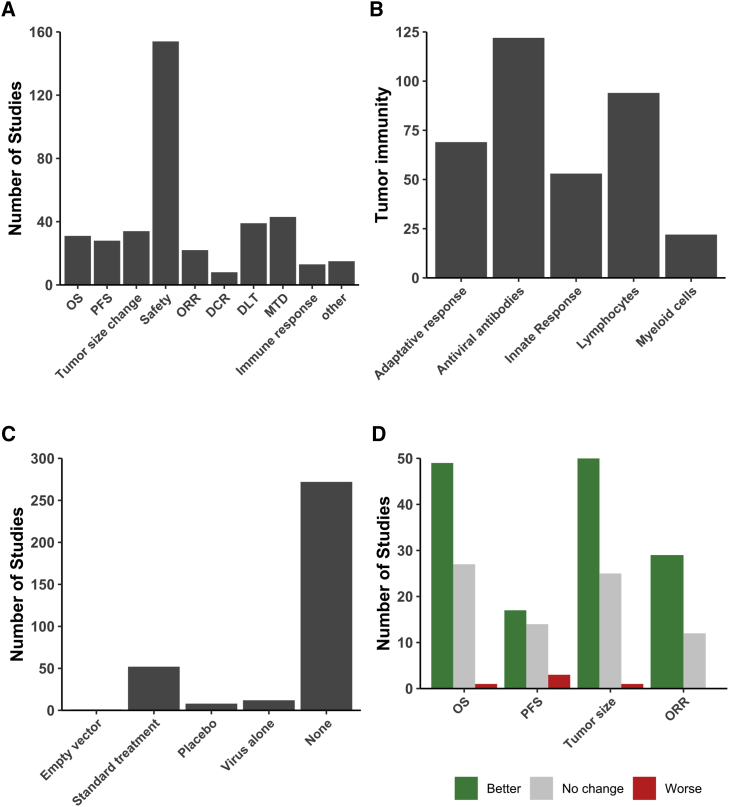
To test the therapeutic efficacy of viruses, studies have assessed different clinical outcomes such as overall survival, tumor size change, and overall response rate. However, as most of the trials comprised phase I/II stages, the most common study outcome was safety (Figure 5A). Although onco-virotherapy is nowadays also considered to induce antitumor immune responses, there have been relatively few (or have been fewer) studies assessing immunological outcomes (Figure 5A). Interestingly, the most commonly studied immunological features were antibody responses to viral vectors and antitumor adaptive responses mediated via lymphocytes (Figure 5B). Immune responses related to myeloid cells have been rarely assessed in clinical studies,6,14, 15, 16, 17, 18, 19 which might be due to difficulties in obtaining and processing clinical tissue samples from the patients as compared to the easier accessibility of peripheral blood to study lymphocytes, antibodies, and cytokine-based innate responses (Figure 5B).
Figure 5.
Clinical outcomes studied related to efficacy
(A) Frequent clinical outcomes studied after onco-virotherapy. (B) Type of immunological outcomes studied. (C) Commonly assessed control groups in comparison to onco-virotherapy. (D) Significant improvement or not in clinical outcomes after onco-virotherapy as compared to respective control groups. OS, overall survival; PFS, progression-free survival; ORR, objective response rate; DCR, disease control rate; DLT, dose limiting toxicity; MTD, maximum tolerated dose.
Considering the controlled clinical trials, onco-virotherapy has often been compared with standard palliative care and/or treatment in addition to placebo groups of patients (Figure 5C). In the case of combinatorial therapeutic approaches, the control group was treated with virus alone. Onco-virotherapy, as compared to these standard treatments, either resulted in a better or similar outcome but rarely worsened the outcome as based on overall survival, progression-free survival, and decrease in tumor size (Figure 5D). The factors related to each of the controlled trials are summarized in Table 1. Of note, most trials did not involve control groups. However, also many of the trials with control groups had major limitations, as onco-virotherapy had to be compared to standard treatment or onco-virotherapy plus standard treatment. For example, in some cases the standard treatment was palliative care, placebo therapy, or observational data from tumor type-matched patients, which does not provide an indication of the improvement due to onco-virotherapy in comparison to conventional chemotherapy and radiotherapy. Differences in individual trial design and the multitude of outcome measures make it difficult to compare studies performed by independent-unrelated institutes. Also, many articles and clinical trials have incomplete descriptions of the methods employed, increasing the difficulty of making comparisons and meta-analysis even more.
Table 1.
Summary of controlled clinical trials exploring safety and efficacy of onco-virotherapy
| Study | Virus type | Dose | Tumor type and stage | Mean age and sex | Control group | Transgene encoded | Tumor Specificity | Follow-up (months) | Endpoint | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1975, Everall et al.20 | vaccinia virus | 18 | melanoma, stage 1 | 49.5 years, both | wide local excision | N/A | N/A | 48 | progression-free survival | no change |
| 1989, Freedman et al.21 | influenza A virus | N/A | uterine cervix carcinoma | 46 years, female | radiotherapy | N/A | N/A | 95 | progression-free survival | no change |
| 1992, Schlag et al.22 | Newcastle disease virus | 17 | colorectal cancer with metastasis to the liver, stage 4 | 55 years, both | surgery | N/A | N/A | N/A | N/A | N/A |
| 1993, Csatary et al.23 | Newcastle disease virus | N/A | various cancers, stage 3 | N/A, both | placebo | N/A | N/A | 24 | overall survival | better |
| 1996, Ockert et al.24 | Newcastle disease virus | N/A | colorectal carcinoma | N/A, both | surgery | N/A | N/A | 22 | safety | safe |
| 1995, Hinkel et al.25 | Newcastle disease virus | 26 | renal cell carcinoma, stage 3–4 | N/A, both | uninfected irradiated renal carcinoma cells, virus alone, interleukin (IL)-2 alone | N/A | N/A | N/A | N/A | N/A |
| 1995, Wallack et al.26 | vaccinia virus | N/A | melanoma, stage 2 | N/A, both | vaccinia virus alone | N/A | N/A | 48 | progression-free survival | no change |
| 1997, Wallack et al.27 | vaccinia virus | N/A | surgically resected melanoma, stage 2 | N/A, both | vaccinia virus alone | N/A | N/A | 42.28 | overall survival | no change |
| 1998, Wallack et al.28 | vaccinia virus | N/A | melanoma, stage 3 | N/A, both | vaccinia virus alone | N/A | N/A | 46.3 | N/A | no change |
| 2000, Sandmair et al.29 | adenovirus | 310 | glioma, stage 3–4 | 51 years, both | LacZ galactosidase | N/A | N/A | 15 | safety | safe |
| 2000, Rainov et al.3 | herpes virus | N/A | newly diagnosed, previously untreated glioblastoma multiforme | 59.3 years, both | surgical resection and radiotherapy | thymidine kinase 2 | deletion ICP34.5 and ICP47 | N/A | Progression-free survival | no change |
| 2002, Habib et al.30 | adenovirus | 311 | hepatocellular carcinoma, stage 2 | 59 years, both | percutaneous ethanol injection | N/A | E1B deletion | 1 | safety | safe |
| 2003, Voit et al.31 | Newcastle disease virus | N/A | melanoma, stage 3 | 53.5 years, both | placebo | N/A | N/A | 18 | safety | safe |
| 2003, Zhang et al.32 | adenovirus | 112 | head and neck squamous cell carcinoma | N/A | radiotherapy | TP53 | N/A | N/A | tumor size | better |
| 2003, Chen C.33 | adenovirus | N/A | nasopharyngeal carcinoma | N/A, both | radiotherapy | TP53 | N/A | 3 | safety | safe |
| 2004, Xia et al.34 | adenovirus | 1.512 | squamous cell cancer of head and neck or esophagus | N/A, both | cisplatin with 5-fluorouracil or adriamycin with 5-fluorouracil | N/A | E1B-55-kDa gene deletion | N/A | objective response rate | better |
| 2006, Spaner et al.35 | canarypox | 56 | melanoma, stage 3–4 | 50 years, both | antigen peptides alone | gp100 antigen | N/A | 8 | N/A | N/A |
| 2006, Lindsey et al.36 | vaccinia virus | 29 | melanoma, stage 3–4 | 47 years, both | virus alone | tyrosinase | N/A | 4 | N/A | no change |
| 2008, ClinicalTrials.gov: NCT00613509 | canarypox | N/A | melanoma, stage 3–4 | 52.8 years, both | interferon alpha-2b | multiple melanoma antigens | N/A | 88 | progression-free survival | better |
| 2008, Dong et al.37 | adenovirus | 212 | lung, ovarian, liver, breast, celiothelioma, stage 3–4 | 59 years, both | cisplatin | TP53 | N/A | 2 | objective response rate | better |
| 2009, Guan et al.38 | adenovirus | 112 | non-small cell lung cancer, stage 3–4 | 58 years, both | bronchial arterial infusion | TP53 | N/A | 12 | safety | safe |
| 2009, Pan et al.39 | adenovirus | 112 | nasopharyngeal carcinoma, stage 2–4 | 48.5 years, both | radiotherapy | TP53 | N/A | 72 | objective response rate | better |
| 2009, Tian et al.40 | adenovirus | 112 | hepatocellular carcinoma | 55.5 years, both | transcatheter arterial chemoembolization | TP53 | N/A | 128 | safety | safe |
| 2010, Yang et al.41 | adenovirus | 312 | hepatocellular carcinoma | 55 years, both | fractionated stereotactic radiotherapy | TP53 | N/A | 35 | safety | safe |
| 2010, ClinicalTrials.gov: NCT01280058 | reovirus | N/A | pancreatic cancer, stage 4 | 64 years, both | carboplatin with paclitaxel | N/A | RAS proto-oncogene dependency | 48 | progression-free survival | worse |
| 2011, Heo et al.42 | vaccinia virus | 19 | liver cancer | 47.6 years, male | historical data of control patients or sorafenib alone | GM-CSF | EGFR-Ras dependency | 2 | tumor size | better |
| 2011, Cerullo et al.43 | adenovirus | 112 | advanced metastatic solid tumors, stage 3–4 | 61 years, both | cyclophosphamide in combination with virotherapy | GM-CSF | RGD-D24 targeting | 12 | overall survival | better |
| 2012, Koski et al.44 | adenovirus | 111 | colorectal, sarcoma, pancreatic, lung, breast, mesothelioma | 57.5 years, both | verapamil | GM-CSF | integrin-targeted Ad5-D34-RGD | N/A | overall survival | no change |
| 2013, Suriano et al.45 | vaccinia virus | N/A | melanoma, stage 3 | 50 years, both | N/A | N/A | N/A | N/A | overall survival | no change |
| 2013, Westphal et al.46 | adenovirus | 112 | high-grade glioma, stage 3 | 55.4 years, both | resection and standard care | thymidine kinase 2 | N/A | 152 | overall survival | no change |
| 2014, Dong et al.47 | adenovirus | 112 | unresectable hepatocellular carcinoma, stage 3-4 | 54 years, both | transarterial chemoembolization alone | N/A | N/A | N/A | progression-free survival | better |
| 2014, Freytag et al.48 | adenovirus | 112 | intermediate risk prostate cancer, stage 2 | 61 years, male | radiotherapy | thymidine kinase 2 | N/A | 48 | safety | safe |
| 2015 and 2018, ClincialTrials.gov: NCT00769704 | herpes virus | 48 | melanoma, stage 3–4 | 64 years, both | GM-CSF therapy | GM-CSF | deletion of ICP34.5 and ICP47 | 44.4 | other | N/A |
| 2015, Donina et al.49 | picornavirus | 16 | melanoma, stage 1–2 | 62.3 years, both | untreated observational group | N/A | N/A | 47.8 | overall survival | better |
| 2015, ClincialTrials.gov: NCT00769703 | herpes virus | 18 | melanoma, stage 3–4 | GM-CSF therapy | GM-CSF | deletion of ICP34.5 and ICP47 | 44 | disease control rate | better | |
| 2015, Kanerva et al.50 | adenovirus | N/A | various cancers | 60.5 years, both | matched controls, cancer type, and disease phase | GM-CSF | integrin-targeted | 46 | safety | safe |
| 2015, Lin et al.51 | adenovirus | 112 | hepatocellular carcinoma | 55 years, both | transarterial chemoembolization of carboplatin | N/A | E1B deletion | 12 | overall survival | better |
| 2015, ClinicalTrials.gov: NCT00179309 | vaccinia virus | 19 | breast cancer, stage 4 | 54.3 years, both | docetaxel | CEA, MUC1, and TRICOM | CEA and MUC1 | 197 | progression-free survival | better |
| 2015, ClinicalTrials.gov: NCT00634595 | adenovirus | 112 | head and neck squamous cell carcinoma, stage 3–4 | 52 years, both | cisplatin and paclitaxel | endostatin | N/A | 10 | N/A | no change |
| 2016, ClinicalTrials.gov: NCT02705196 | adenovirus | N/A | pancreatic cancer, stage 3 | >18 years, both | gemcitabine and paclitaxel with and without anti-PD-L1 antibody | N/A | N/A | N/A | N/A | N/A |
| 2016, Andtbacka et al.10,52 | herpes virus | N/A | melanoma, stage 3–4 | 63 years, both | subcutaneous injection of GM-CSF | GM-CSF | deletion of ICP34.5 and ICP47 | N/A | tumor size | better |
| 2016, Gao et al.53 | adenovirus | 510 | malignant and solid tumors, stage 3–4 | 35 years, both | adriamycin alone | GM-CSF | N/A | N/A | N/A | better |
| 2016, ClinicalTrials.gov: NCT00870181 | adenovirus | 112 | high-grade gliomas, stage 3–4 | 52.5 years, both | surgery, systemic chemotherapy, or palliative care | thymidine kinase 2 | N/A | 71 | progression-free survival | better |
| 2016, Andtbacka et al.10,52 | herpes virus | 18 | unresected melanoma, stage 3–4 | 63 years, both | GM-CSF therapy | GM-CSF | deletion of ICP34.5 and ICP47 | 30 | disease control rate | N/A |
| 2016, et al.54 | adenovirus | 112 | cervical cancer, stage 2–3 | 52 years, female | radiotherapy in combination with brachytherapy | TP53 | N/A | 605 | safety | safe |
| 2016, Harrington et al.55 | herpes virus | 18 | melanoma, stage 3–4 | 63 years, both | GM-CSF therapy | GM-CSF | deletion of ICP34.5 and ICP47 | 18 | overall survival | better |
| 2017, Ma et al.56 | adenovirus | 112 | nasopharyngeal carcinoma, stage 2 | N/A, both | radiation, cisplatin or 5-fluorouracil | TP53 | N/A | 36 | overall survival | better |
| 2017, Cohn et al.57 | reovirus | 310 | ovarian, tubal, or peritoneal cancer, stage 2–3 | 60 years, female | paclitaxel | N/A | N/A | 128 | overall survival | no change |
| 2018, Bradbury et al.58 | reovirus | 4.512 | lung adenocarcinoma, stage 3–4 | 64 years, both | chemotherapy | N/A | N/A | 180 | overall survival | no change |
| 2018, Xiao et al.4 | adenovirus | 112 | advanced unresectable soft-tissue sarcomas, stage 3 | 49 years, both | hyperthermia alone or in combination with radiotherapy | TP53 | N/A | N/A | disease control rate | better |
| 2018, Liu et al.59 | adenovirus | 19 | hypopharyngeal squamous cell carcinoma | 57.9 years, both | surgery alone or in combination with chemo-radiotherapy | TP53 | N/A | 36 | overall survival | better |
| 2018, ClinicalTrials.gov: NCT01622543 | reovirus | 310 | colorectal cancer, stage 4 | 50 years, both | leucovorin, 5-fluorouracil, oxaliplatin, or bevacizumab | N/A | N/A | 13 | progression free survival | worse |
| 2018, ClinicalTrials.gov: NCT01619813 | reovirus | 310 | metastatic prostate adeno-carcinoma, stage 4 | 69 years, male | docetaxel and prednisone | N/A | N/A | 20 | progression-free survival | better |
| 2018, ClinicalTrials.gov: NCT01656538 | reovirus | 310 | metastatic breast cancer, stage 4 | 44 years, female | paclitaxel | N/A | N/A | 295 | progression-free survival | no change |
| 2018, ClinicalTrials.gov: NCT01708993 | reovirus | 4.510 | on-small cell lung cancer, stage 3-4 | 63 years, both | pemetrexed or docetaxel | N/A | N/A | 27 | safety | safe |
| 2018, ClinicalTrials.gov: NCT01740297 | herpes virus | 48 | unresectable melanoma, stage 3-4 | 64.5 years, both | ipilimumab | GM-CSF | deletion of ICP34.5 and ICP47 | 156 | safety | safe |
| 2018, He et al.60 | adenovirus | 112 | hepatocellular carcinoma, stage 1–4 | 55 years, both | transarterial chemo-embolization | N/A | N/A | 13 | overall survival | better |
| 2019, NCT01387555 | vaccinia virus | 19 | hepatocellular carcinoma, stage 3 | 57 years, both | supportive care | GM-CSF | N/A | 4.3 | overall survival | no change |
| 2020, Schenk et al.61 | picornavirus | 111 | small cell carcinoma | 63 years, both | saline | N/A | natural | 17 | progression-free survival | worse |
N/A, not available; GM-CSF, granulocyte-macrophage colony-stimulating factor; TP53, tumor protein 53; ICP, infected cell protein.
Published articles and clinical trials
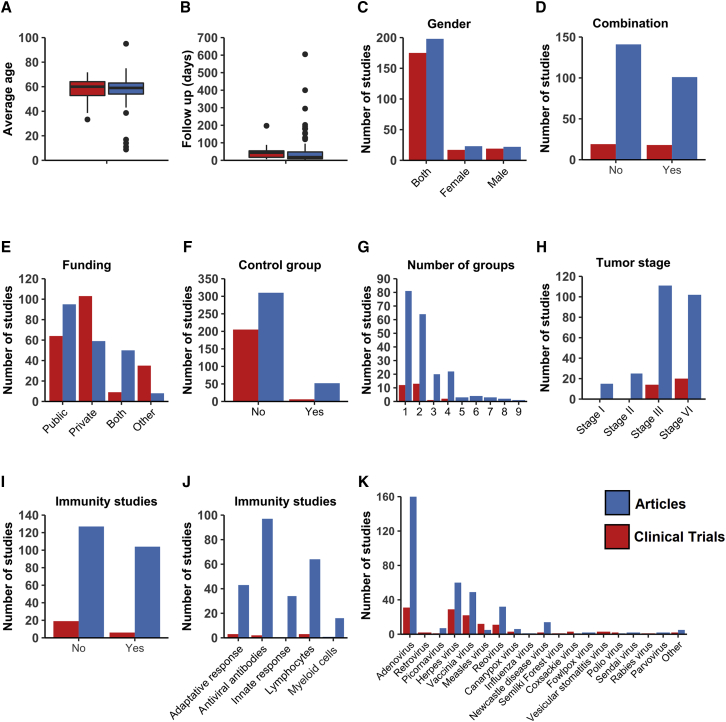
Finally, as a reference to the design and conduct of future systematic analysis on clinical trials, we demonstrate the importance of including multiple databases to retrieve information. Through this study, we found that there were only a few variables showing similarity between the information collected via articles (obtained from PubMed and EMBASE) and trials (from ClinicalTrials.gov). Patient background-related information, such as age (Figure 6A), follow-up period (Figure 6B), and sex (Figure 6C), were equal between clinical trial and articles. However, other variables showed high disparity. Information related to trial design, such as the type of therapeutic combination, was found to be different between the data obtained from trials and articles (Figure 6D). Clinical trials were more often funded by private institutions, while articles more often received public funding (Figure 6E). Furthermore, the number of control groups was lower for the clinical trials compared to the articles (Figure 6F), and the number of study groups was smaller (Figure 6G). Clinical trials mainly focused on treating patients with advanced and metastatic cancer types, whereas articles also treated stage I and II cancer patients (Figure 6H) and investigated immune responses (Figures 6I and 6J). Although adenovirus was the most frequently studied viral vector by both clinical trials and articles, clinical trials studied herpes and vaccinia virus almost equally (Figure 6K). The described disparity is probably caused by the fact that many trials are not registered on ClinicalTrials.gov, and that articles on PubMed and EMBASE, in contrast to trials on ClinicalTrials.gov, are peer reviewed (Figure 1A).
Figure 6.
Disparity of data obtained from clinicaltrials.gov and articles
Disparities are reported in terms of (A) patient age, (B) follow-up days, (C) sex, (D) combinatorial strategy with onco-virotherapy, (E) funding, (F) inclusion of control group, (G) number of groups per study, (H) patient tumor stage, (I) immunological outcomes studied, (J) type of immunological outcomes studied, and (K) type of onco-virotherapy studied. Bars in red represent clinical trials and in blue represent articles (retrieved from PubMed and EMBASE).
Discussion
Onco-virotherapy is a promising form of immunotherapy for the treatment of cancer. In this review we evaluated the clinical impact of onco-virotherapy for cancer patients who received virotherapy in comparison with cancer patients who received other therapies by means of a systematic analysis. Overall, our results indicate that onco-virotherapy has proven to be safe due to efforts in vector design, rational choices of therapeutic dosage, and delivery strategies. Simultaneously, various viral vectors have shown clinical efficacy in terms of better therapeutic outcomes as compared to standard care. Moreover, combinational strategies such as checkpoint blockade, chemotherapy, radiotherapy, and even introduction of immunogenic transgenes has improved clinical efficacy. With this analysis, we aim to provide a reference for clinicians and researchers in the onco-virotherapy field.
Our analysis identified 18 viral vectors that were used as therapeutic platform to treat 26 cancer types. These studies used the following virus types: adenovirus (42.5% of studies), canarypox virus (1.3% of studies), coxsackie virus (0.6% of studies), fowlpox virus (0.3% of studies), herpes virus (21.3% of studies), influenza virus (0.1% of studies), measles virus (3% of studies), Newcastle disease virus (3.6% of studies), parvovirus (0.4% of studies), picornavirus (1.5% of studies), polio virus (0.4% of studies), rabies virus (0.1% of studies), retrovirus (0.3% of studies), reovirus (7.3% of studies), Semliki Forest virus (0.1% of studies), Sendai virus (0.3% of studies), vaccinia virus (13.2% of studies), and vesicular stomatitis virus (0.9% of studies). A wide range of cancers were treated in the clinics such as melanoma (17.1% of studies), colon cancer (9.7% of studies), lung cancer (7% of studies), head and neck cancer (6.8% of studies), liver cancer (6.5% of studies), ovarian cancer (5.9% of studies), pancreatic cancer (5.9% of studies), breast cancer (5.6% of studies), glioblastoma (5.6% of studies), prostate cancer (5.4% of studies), sarcoma (4.5% of studies), mesothelioma (3.2% of studies), bladder cancer (2% of studies), glioma (1.8% of studies), esophageal cancer (1.6% of studies), renal cancer (1.6% of studies), cervical cancer (1.4% of studies), cholangiocarcinoma (1.4% of studies), rectal cancer (1.4% of studies), stomach cancer (1.4% of studies), thymus cancer (1.4% of studies), thyroid cancer (1.1% of studies), diffuse large B cell lymphoma (0.7% of studies), acute myeloid leukemia (0.7% of studies), adrenocortical cancer (0.5% of studies), and uterine cancer (0.2% of studies). The clinical studies have been successful in recruiting patient irrespective of sex, age, and diversity in tumor types and stages. However, very few studies (<5% of studies) have evaluated the potential of onco-virotherapy on pediatric patients, whereas most (>80%) studies were focused on patients of 35–70 years in age.
Adenoviruses and herpesviruses were the most utilized virus types in clinical studies. Since 2015, the oncolytic herpes virus T-VEC is globally approved for the treatment of advanced melanoma.62,63 However, the genetically modified adenovirus H101 (E1B-55K/E3B deletion), also known as Oncorine, was the very first oncolytic virus to be approved in 2005 in China for the treatment of head and neck cancer.64,65 Moreover, adenoviruses have been extensively tested as gene therapy vectors, vaccine platforms, and synthetic biology tools, so for engineering, adenoviral vectors are more often chosen over other viruses as recently reviewed by Peter and Kühnel.66 Similarly, viral vectors with an acceptable safety profile, such as the vaccinia virus and measles virus, have also been preferred choices for clinical testing.1,67 Overall, this suggests that the development and easier accessibility of genetic engineering kits for vector modification has the potential to support the demand for novel viral therapeutics and their assessment in clinical research.
Strikingly, many viruses were not subjected to any genetic modification during the earlier years of onco-virotherapy development, although this approach has the potential to enhance the immunogenicity of viral vectors by the introduction of immunogenic genes. Nonetheless, onco-virotherapy modification gained popularity since 2000, and a large fraction of our analyzed trials were initiated in the years thereafter. Considering such native (non-modified) viruses, reovirus has been the most commonly used viral vector in onco-virotherapy that has not undergone genetic modifications.68 Similarly, canarypox and fowlpox viruses have been used in their native form to deliver prostate tumor antigens, as they exhibit a weaker tropism to human cells and preferentially infect tumor cells.69 Also, vesicular stomatitis virus is being exploited as therapeutic (ClinicalTrials.gov: NCT01628640 and NCT03120624) due to its sensitivity to interferon-mediated antiviral responses exhibited by normal cells, which allows preferential infection and lysis of tumor cells devoid of active interferon responses.70
Many of the utilized viral vectors were administered in combination with another form of therapy such as chemotherapy,34,40,42,43,57,71, immune checkpoint inhibitors,13,72,73 or radiotherapy.3,4,32,59 Furthermore, most viruses were injected intratumorally and were administered multiple times, which is not surprising, as intratumoral administration of viruses has been shown to be effective. Additionally, multiple injections increase the possibility of inducing stronger antitumor effects and related immune responses.74 However, multiple intratumoral injections also face the limitations of an invasive approach, for example in patients with glioblastoma,75 or in pediatric patients,76,77 thus indicating room for improvement.78 Furthermore, immune responses such as virus neutralization by antiviral antibodies,79 neutralization mediated by complement activation,80,81 and cellular-antiviral responses mediated by natural killer (NK) cells82 and T cells also prove to be a challenge to onco-virotherapy. This has led to an increased requirement of dosage during subsequent rounds of treatment to counterbalance virus elimination in patients.
In addition to improving the safety and efficacy of viral vectors via genetic modifications and combinatorial approaches, efforts have also been made in overcoming the challenges related to manufacturing a clinical-grade stock of these viruses. Factors such as ensuring sterility and proper handling during production, improvement of virus yields, appropriate purification strategies, and formulation for long-term stability and storage have been discussed in detail in existing literature.83,84 Moreover, regulatory aspects ranging from virus design and production up to therapeutic utilization in clinics have remained of immense importance for safe application of onco-virotherapy.85 Taken together, these factors can potentially influence the feasibility of producing the maximum dose required for patient treatment, especially in the case of multiple injections and for virus types that require high dose for effective therapy. Manufacturing challenges can also impact the cost of therapy where the economic evaluation of onco-virotherapy has yet to demonstrate itself as a less expensive alternative to existing therapies.86,87
Non-randomized cohort studies and non-controlled trials have primarily focused on assessing the safety profile of the viral vector, and therefore determinations of dose limiting toxicity and maximum tolerated dose have remained important. Additionally, side effects such as fever, fatigue, flu-like symptoms, nausea, and pain at the site of injection, among others, have also been reported to occur after onco-virotherapy, although rarely in severe form.6
In most controlled trials, onco-virotherapy treatment resulted in better outcomes for individual variables (>70% of controlled trials) or no change (>40% of controlled trials), although some trials reported worse outcomes (Table 1). This indicates that further improvements in onco-virotherapy are still needed. We found that most of the studies did not include the immune response in their outcome measures, which was unexpected and remarkable, as immunogenic effects are characteristic for onco-virotherapy. Instead, most trials focused on general outcome measures such as progression-free survival, overall survival, and tumor size change. Hence, it would be important that in the future onco-virotherapy clinical trials also assess the immune response as an outcome measure. Moreover, most of the trials have chosen clinical criteria of assessment based on the published guidelines such as RECIST.6 However, recent literature88 and our review indicate the need to establish new parameters to evaluate tumor response to virotherapy in terms of immune response, reduction of metastasis, and alteration in tumor metabolism and growth.
Only a limited number of trials compared the efficacy of onco-virotherapy with conventional treatments such as chemotherapy or radiotherapy (Table 1). For example, the FDA approved therapeutic T-VEC, which is administered intratumorally, has only been studied in comparison with intravenous GM-CSF injections, where T-VEC showed significantly better outcomes.63 So far the performance of T-VEC in comparison with chemotherapy and/or radiotherapy has not been investigated. Interestingly, recent trials in which T-VEC was combined with checkpoint immunotherapy resulted in better outcomes as compared to monotherapy of either T-VEC or checkpoint immunotherapy.13 Again, this emphasizes the importance of assessing immune responses after onco-virotherapy.
From the more than 20 different solid tumor types evaluated, skin cutaneous melanoma was most commonly studied. This can likely be ascribed to the fact that this tumor type is accessible for intratumoral injection without the need of surgical interventions. Also, most melanoma cells contain a high mutational burden,89 and this increases the likelihood of tumor-specific antigen release into the tumor microenvironment upon oncolysis, thereby improving the potency of the onco-virotherapy. Additionally, most tumors studied were in an advanced or metastatic state, likely due to the fact that cancer patients with a good prognosis generally benefit from standard care. This is probably a confounding factor during the assessment of onco-virotherapy, as advanced patients are generally less likely to respond to any therapeutic intervention. Therefore, including patients who suffer from early and localized cancer, as well as high-risk or early refractory patients, may be a strategy to further explore the effectiveness of onco-virotherapy, as these patients would be more likely to benefit.
Finally, we aspire that the information gathered here can be used as a starting point to construct an interactive database that provides information to clinicians and researchers who are interested in the therapeutic potential of onco-virotherapy. Moreover, our search strategies can be used to regularly update such a database by collecting and screening trial-related data from ClinicalTrials.gov and articles from PubMed and EMBASE. Furthermore, we encourage clinicians and researchers to continue reviewing literature associated with clinical research by assessing multiple platforms, as it increases the possibilities of finding trial results and articles that are exclusive to a particular platform.
Materials and methods
Protocol and eligibility
We used the preferred reporting items for systematic review and meta-analysis protocol (PRISMA-P) statement as a guide for our analysis. To define our research question, we utilized the PICOS (patient, intervention, comparator, outcome, study type) framework based on the accepted PRISMA guidelines. Accordingly, we focused on cancer patients (P) who receive onco-virotherapy (I) compared with patients who receive other therapies (including placebo, chemotherapy, immunotherapy, radiotherapy) (C), the clinical impact with respect to response rate (O1) or tumor size change (O2) or safety (O3), and such (O4-to-n), and in a clinical setup (S) for therapeutic purposes.
Search strategy and screening of articles
We retrieved clinical trials from the ClinicalTrials.gov registry (https://clinicaltrials.gov) and PubMed (https://PubMed.ncbi.nlm.nih.gov) and EMBASE (https://www.embase.com) databases until August 2020. For each medium, we used a different search strategy, as specified in the Supplemental information. Through the search strategy, we found 3,492 articles from PubMed, 2,614 articles from EMBASE, and 1,632 trials from ClinicalTrials.gov. After the removal of duplicates, at least two authors manually screened the retrieved articles for inclusion, where we excluded articles or trials that did not focus on the application of onco-virotherapy for cancer patients, articles that were reviews, preclinical studies or commentaries, and articles in which the abstract was not reported in English (Figure 1). Any conflicts were resolved through discussion. This allowed us to include 331 articles from PubMed, 316 articles from EMBASE, and 249 trials from ClinicalTrials.gov. Subsequently, we added all articles in our database using browser-based REDCap software (Vanderbilt University, Nashville, TN, USA). All of the data and results are provided in Table S1 and are intended to serve as a resource for future studies.
Preliminary qualitative analysis and screening of controlled clinical studies
To observe the trends in clinical studies exploring the potential of onco-virotherapy for cancer treatment, we did a preliminary analysis of studies including retrieved data from articles and trials (Figures 1, 2, 3, 4, and 5). This preliminary analysis was based on the literature found via PubMed (331 articles), EMBASE (316 articles), and ClinicalTrials.gov (249 trials) as described earlier. Finally, we identified and summarized 59 controlled clinical studies reporting comparative data from respective articles and trials (Table 1). All figures and tables were made using ggplot2, or networkD3 R packages,90 and the graphical abstract was made using BioRender.
Acknowledgments
This work was supported by CNPq (grant award 305700/2017-0 to R.C.), CAPES (finance code 001, to D.K.B.), and by an ATTP-GSMS scholarship (Abel Tasman Talent Program to D.K.B.). The authors would like to thank Dr. Peter G. Braun and Sjoukje van der Werf from the university library at the University of Groningen for support in formulation of the search strategy for the systematic review. Moreover, the authors thank the reviewers for their valuable feedback regarding the manuscript.
Author contributions
All authors made substantial contributions to the manuscript. Conception and design, D.K.B., L.W., and T.D.; supervision and funding acquisition, T.D. and R.C.; collection and assembly of data, D.K.B., L.W., and L.R.C.B.; data analysis and interpretation, D.K.B., L.W., L.R.C.B., T.D., and R.C.; manuscript writing, D.K.B., L.W., L.R.C.B., R.C., and T.D. All authors reviewed the manuscript and approved the final version for submission.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omto.2021.09.008.
Supplemental information
Each article or trial is listed in a row, while the respective variable of interest is organized in columns
References
- 1.Guo Z.S., Lu B., Guo Z., Giehl E., Feist M., Dai E., Liu W., Storkus W.J., He Y., Liu Z., Bartlett D.L. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J. Immunother. Cancer. 2019;7:6. doi: 10.1186/s40425-018-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wold W.S.M., Toth K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013;13:421–433. doi: 10.2174/1566523213666131125095046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rainov N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 4.Xiao S.W., Xu Y.-Z., Xiao B.-F., Jiang J., Liu C.-Q., Fang Z.W., Li D.-M., Li X.F., Cai Y., Li Y.H. Recombinant adenovirus-p53 gene therapy for advanced unresectable soft-tissue sarcomas. Hum. Gene Ther. 2018;29:699–707. doi: 10.1089/hum.2017.103. [DOI] [PubMed] [Google Scholar]
- 5.Buijs P.R.A., Verhagen J.H.E., van Eijck C.H.J., van den Hoogen B.G. Oncolytic viruses: From bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 2015;11:1573–1584. doi: 10.1080/21645515.2015.1037058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macedo N., Miller D.M., Haq R., Kaufman H.L. Clinical landscape of oncolytic virus research in 2020. J. Immunother. Cancer. 2020;8:e001486. doi: 10.1136/jitc-2020-001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin N.T., Bell J.C. Oncolytic virus combination therapy: Killing one bird with two stones. Mol. Ther. 2018;26:1414–1422. doi: 10.1016/j.ymthe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook M., Chauhan A. Clinical application of oncolytic viruses: A systematic review. Int. J. Mol. Sci. 2020;21:7505. doi: 10.3390/ijms21207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z., Jiang Z., Zhang Y., Huang X., Liu Q. Efficacy and safety of oncolytic viruses in randomized controlled trials: A systematic review and meta-analysis. Cancers (Basel) 2020;12:1416. doi: 10.3390/cancers12061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andtbacka R.H.I., Agarwala S.S., Ollila D.W., Hallmeyer S., Milhem M., Amatruda T., Nemunaitis J.J., Harrington K.J., Chen L., Shilkrut M. Cutaneous head and neck melanoma in OPTiM, a randomized phase 3 trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor for the treatment of unresected stage IIIB/IIIC/IV melanoma. Head Neck. 2016;38:1752–1758. doi: 10.1002/hed.24522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westbrook B.C., Norwood T.G., Terry N.L.J., McKee S.B., Conry R.M. Talimogene laherparepvec induces durable response of regionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019;5:782–786. doi: 10.1016/j.jdcr.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassilev L., Ranki T., Joensuu T., Jäger E., Karbach J., Wahle C., Partanen K., Kairemo K., Alanko T., Turkki R. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8+ T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. OncoImmunology. 2015;4:e1017702. doi: 10.1080/2162402X.2015.1017702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taipale K., Liikanen I., Koski A., Heiskanen R., Kanerva A., Hemminki O., Oksanen M., Grönberg-Vähä-Koskela S., Hemminki K., Joensuu T., Hemminki A. Predictive and prognostic clinical variables in cancer patients treated with adenoviral oncolytic immunotherapy. Mol. Ther. 2016;24:1323–1332. doi: 10.1038/mt.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett A.S., Pavlidakey P.G., Contreras C.M., De Los Santos J.F., Kim J.Y., McKee S.B., Kaufman H.L., Conry R.M. Chronic granulomatous dermatitis induced by talimogene laherparepvec therapy of melanoma metastases. J. Cutan. Pathol. 2018;45:48–53. doi: 10.1111/cup.13048. [DOI] [PubMed] [Google Scholar]
- 16.Hemminki O., Oksanen M., Taipale K., Liikanen I., Koski A., Joensuu T., Kanerva A., Hemminki A. Oncograms visualize factors influencing long-term survival of cancer patients treated with adenoviral oncolytic immunotherapy. Mol. Ther. Oncolytics. 2018;9:41–50. doi: 10.1016/j.omto.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J.C.C., Coffin R.S., Davis C.J., Graham N.J., Groves N., Guest P.J., Harrington K.J., James N.D., Love C.A., McNeish I. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 19.Hwang T.-H., Moon A., Burke J., Ribas A., Stephenson J., Breitbach C.J., Daneshmand M., De Silva N., Parato K., Diallo J.-S. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. 2011;19:1913–1922. doi: 10.1038/mt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everall J.D., O’Doherty C.J., Wand J., Dowd P.M. Treatment of primary melanoma by intralesional vaccinia before excision. Lancet. 1975;2:583–586. doi: 10.1016/s0140-6736(75)90172-5. [DOI] [PubMed] [Google Scholar]
- 21.Freedman R.S., Bowen J.M., Atkinson E.N., Wallace S., Lotzová E., Silva E., Edwards C.L., Delclos L., Scott W., Patenia B. Randomized comparison of viral oncolysate plus radiation and radiation alone in uterine cervix carcinoma. Am. J. Clin. Oncol. 1989;12:244–250. doi: 10.1097/00000421-198906000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Schlag P., Manasterski M., Gerneth T., Hohenberger P., Dueck M., Herfarth C., Liebrich W., Schirrmacher V. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. First evaluation of clinical response of a phase II-trial. Cancer Immunol. Immunother. 1992;35:325–330. doi: 10.1007/BF01741145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csatary L.K., Eckhardt S., Bukosza I., Czegledi F., Fenyvesi C., Gergely P., Bodey B., Csatary C.M. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 1993;17:619–627. [PubMed] [Google Scholar]
- 24.Ockert D., Schirrmacher V., Beck N., Stoelben E., Ahlert T., Flechtenmacher J., Hagmüller E., Buchcik R., Nagel M., Saeger H.D. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin. Cancer Res. 1996;2:21–28. [PubMed] [Google Scholar]
- 25.Hinkel A., de Riese W., Alefelder J., Laumann D., Falkenberg F.W., Senge T., Hertle L. Active specific immunotherapy with an autologous virus-modified tumour cell vaccine in human renal cell carcinoma (RCC) Eur. J. Cancer. 1995;31A:1719–1720. doi: 10.1016/0959-8049(95)00308-6. [DOI] [PubMed] [Google Scholar]
- 26.Wallack M.K., Sivanandham M., Balch C.M., Urist M.M., Bland K.I., Murray D., Robinson W.A., Flaherty L.E., Richards J.M., Bartolucci A.A. A phase III randomized, double-blind multiinstitutional trial of vaccinia melanoma oncolysate-active specific immunotherapy for patients with stage II melanoma. Cancer. 1995;75:34–42. doi: 10.1002/1097-0142(19950101)75:1<34::aid-cncr2820750108>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Wallack M.K., Sivanandham M., Ditaranto K., Shaw P., Balch C.M., Urist M.M., Bland K.I., Murray D., Robinson W.A., Flaherty L. Increased survival of patients treated with a vaccinia melanoma oncolysate vaccine: Second interim analysis of data from a phase III, multi-institutional trial. Ann. Surg. 1997;226:198–206. doi: 10.1097/00000658-199708000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallack M.K., Sivanandham M., Balch C.M., Urist M.M., Bland K.I., Murray D., Robinson W.A., Flaherty L., Richards J.M., Bartolucci A.A., Rosen L. Surgical adjuvant active specific immunotherapy for patients with stage III melanoma: The final analysis of data from a phase III, randomized, double-blind, multicenter vaccinia melanoma oncolysate trial. J. Am. Coll. Surg. 1998;187:69–77, discussion 77–79. doi: 10.1016/s1072-7515(98)00097-0. [DOI] [PubMed] [Google Scholar]
- 29.Sandmair A.-M., Loimas S., Puranen P., Immonen A., Kossila M., Puranen M., Hurskainen H., Tyynelä K., Turunen M., Vanninen R. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 30.Habib N., Salama H., Abd El Latif Abu Median A., Isac Anis I., Abd Al Aziz R.A., Sarraf C., Mitry R., Havlik R., Seth P., Hartwigsen J. Clinical trial of E1B-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 2002;9:254–259. doi: 10.1038/sj.cgt.7700431. [DOI] [PubMed] [Google Scholar]
- 31.Voit C., Kron M., Schwurzer-Voit M., Sterry W. Intradermal injection of Newcastle disease virus-modified autologous melanoma cell lysate and interleukin-2 for adjuvant treatment of melanoma patients with resectable stage III disease. J. Dtsch Dermatol. Ges. 2003;1:120–125. doi: 10.1046/j.1610-0387.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S.W., Xiao S.W., Liu C.Q., Sun Y., Su X., Li D.M., Xu G., Cai Y., Zhu G.Y., Xu B., Lü Y.Y. [Treatment of head and neck squamous cell carcinoma by recombinant adenovirus-p53 combined with radiotherapy: A phase II clinical trial of 42 cases] Zhonghua Yi Xue Za Zhi. 2003;83:2023–2028. [PubMed] [Google Scholar]
- 33.Chen C.B., Pan J.J., Xu L.Y. [Recombinant adenovirus p53 agent injection combined with radiotherapy in treatment of nasopharyngeal carcinoma: a phase II clinical trial] Zhonghua Yi Xue Za Zhi. 2003;83:2033–2035. [PubMed] [Google Scholar]
- 34.Xia Z.-J., Chang J.-H., Zhang L., Jiang W.-Q., Guan Z.-Z., Liu J.-W., Zhang Y., Hu X.-H., Wu G.-H., Wang H.-Q. Vol. 23. 2004. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus]. Ai Zheng; pp. 1666–1670. [PubMed] [Google Scholar]
- 35.Spaner D.E., Astsaturov I., Vogel T., Petrella T., Elias I., Burdett-Radoux S., Verma S., Iscoe N., Hamilton P., Berinstein N.L. Enhanced viral and tumor immunity with intranodal injection of canary pox viruses expressing the melanoma antigen, gp100. Cancer. 2006;106:890–899. doi: 10.1002/cncr.21669. [DOI] [PubMed] [Google Scholar]
- 36.Lindsey K.R., Gritz L., Sherry R., Abati A., Fetsch P.A., Goldfeder L.C., Gonzales M.I., Zinnack K.A., Rogers-Freezer L., Haworth L. Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma. Clin. Cancer Res. 2006;12:2526–2537. doi: 10.1158/1078-0432.CCR-05-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong M., Li X., Hong L.-J., Xie R., Zhao H.-L., Li K., Wang H.-H., Shin W.-D., Shen H.-J. Advanced malignant pleural or peritoneal effusion in patients treated with recombinant adenovirus p53 injection plus cisplatin. J. Int. Med. Res. 2008;36:1273–1278. doi: 10.1177/147323000803600614. [DOI] [PubMed] [Google Scholar]
- 38.Guan Y.S., Liu Y., Zou Q., He Q., La Z., Yang L., Hu Y. Adenovirus-mediated wild-type p53 gene transfer in combination with bronchial arterial infusion for treatment of advanced non-small-cell lung cancer, one year follow-up. J. Zhejiang Univ. Sci. B. 2009;10:331–340. doi: 10.1631/jzus.B0820248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan J.J., Zhang S.W., Chen C.B., Xiao S.W., Sun Y., Liu C.Q., Su X., Li D.M., Xu G., Xu B., Lu Y.Y. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009;27:799–804. doi: 10.1200/JCO.2008.18.9670. [DOI] [PubMed] [Google Scholar]
- 40.Tian G., Liu J., Zhou J.S.R., Chen W. Multiple hepatic arterial injections of recombinant adenovirus p53 and 5-fluorouracil after transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a pilot phase II trial. Anticancer Drugs. 2009;20:389–395. doi: 10.1097/CAD.0b013e32832a2df9. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z.X., Wang D., Wang G., Zhang Q.H., Liu J.M., Peng P., Liu X.H. Clinical study of recombinant adenovirus-p53 combined with fractionated stereotactic radiotherapy for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2010;136:625–630. doi: 10.1007/s00432-009-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heo J., Breitbach C.J., Moon A., Kim C.W., Patt R., Kim M.K., Lee Y.K., Oh S.Y., Woo H.Y., Parato K. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: Preclinical and clinical demonstration of combination efficacy. Mol. Ther. 2011;19:1170–1179. doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cerullo V., Diaconu I., Kangasniemi L., Rajecki M., Escutenaire S., Koski A., Romano V., Rouvinen N., Tuuminen T., Laasonen L. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol. Ther. 2011;19:1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koski A., Raki M., Nokisalmi P., Liikanen I., Kangasniemi L., Joensuu T., Kanerva A., Pesonen S., Alemany R., Hemminki A. Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer. Mol. Ther. 2012;20:221–229. doi: 10.1038/mt.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suriano R., Rajoria S., George A.L., Geliebter J., Tiwari R.K., Wallack M. Follow-up analysis of a randomized phase III immunotherapeutic clinical trial on melanoma. Mol. Clin. Oncol. 2013;1:466–472. doi: 10.3892/mco.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westphal M., Ylä-Herttuala S., Martin J., Warnke P., Menei P., Eckland D., Kinley J., Kay R., Ram Z., ASPECT Study Group Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 47.Dong J., Li W., Dong A., Mao S., Shen L., Li S., Gong X., Wu P. Gene therapy for unresectable hepatocellular carcinoma using recombinant human adenovirus type 5. Med. Oncol. 2014;31:95. doi: 10.1007/s12032-014-0095-4. [DOI] [PubMed] [Google Scholar]
- 48.Freytag S.O., Stricker H., Lu M., Elshaikh M., Aref I., Pradhan D., Levin K., Kim J.H., Peabody J., Siddiqui F. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:268–276. doi: 10.1016/j.ijrobp.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doniņa S., Strēle I., Proboka G., Auziņš J., Alberts P., Jonsson B., Venskus D., Muceniece A. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res. 2015;25:421–426. doi: 10.1097/CMR.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanerva A., Koski A., Liikanen I., Oksanen M., Joensuu T., Hemminki O., Palmgren J., Hemminki K., Hemminki A. Case-control estimation of the impact of oncolytic adenovirus on the survival of patients with refractory solid tumors. Mol. Ther. 2015;23:321–329. doi: 10.1038/mt.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin X.J., Li Q.J., Lao X.M., Yang H., Li S.P. Transarterial injection of recombinant human type-5 adenovirus H101 in combination with transarterial chemoembolization (TACE) improves overall and progressive-free survival in unresectable hepatocellular carcinoma (HCC) BMC Cancer. 2015;15:707. doi: 10.1186/s12885-015-1715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andtbacka R.H.I., Ross M., Puzanov I., Milhem M., Collichio F., Delman K.A., Amatruda T., Zager J.S., Cranmer L., Hsueh E. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 2016;23:4169–4177. doi: 10.1245/s10434-016-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y.D., Chen G.L., Guo P.D., Yang X.L. [Study on the application effect of Once VexGM-CSF and adriamycin in the interventional therapy of malignant tumor in clinical curative effect] Zhonghua Yi Xue Za Zhi. 2016;96:2173–2175. doi: 10.3760/cma.j.issn.0376-2491.2016.27.013. [DOI] [PubMed] [Google Scholar]
- 54.Su X., Chen W.J., Xiao S.W., Li X.F., Xu G., Pan J.J., Zhang S.W. Effect and safety of recombinant adenovirus-p53 transfer combined with radiotherapy on long-term survival of locally advanced cervical cancer. Hum. Gene Ther. 2016;27:1008–1014. doi: 10.1089/hum.2016.043. [DOI] [PubMed] [Google Scholar]
- 55.Harrington K.J., Andtbacka R.H., Collichio F., Downey G., Chen L., Szabo Z., Kaufman H.L. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: Subanalysis of the phase III OPTiM trial. OncoTargets Ther. 2016;9:7081–7093. doi: 10.2147/OTT.S115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma W.-S., Ma J.-G., Xing L.-N. Efficacy and safety of recombinant human adenovirus p53 combined with chemoradiotherapy in the treatment of recurrent nasopharyngeal carcinoma. Anticancer Drugs. 2017;28:230–236. doi: 10.1097/CAD.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 57.Cohn D.E., Sill M.W., Walker J.L., O’Malley D., Nagel C.I., Rutledge T.L., Bradley W., Richardson D.L., Moxley K.M., Aghajanian C. Randomized phase IIB evaluation of weekly paclitaxel versus weekly paclitaxel with oncolytic reovirus (Reolysin®) in recurrent ovarian, tubal, or peritoneal cancer: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;146:477–483. doi: 10.1016/j.ygyno.2017.07.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradbury P.A., Morris D.G., Nicholas G., Tu D., Tehfe M., Goffin J.R., Shepherd F.A., Gregg R.W., Rothenstein J., Lee C. Canadian Cancer Trials Group (CCTG) IND211: A randomized trial of pelareorep (Reolysin) in patients with previously treated advanced or metastatic non-small cell lung cancer receiving standard salvage therapy. Lung Cancer. 2018;120:142–148. doi: 10.1016/j.lungcan.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Liu J., Lv D., Wang H., Zou J., Chen F., Yang H. Recombinant adenovirus-p53 enhances the therapeutic effect of surgery and chemoradiotherapy combination in hypopharyngeal squamous cell carcinomas patients. Medicine (Baltimore) 2018;97:e12193. doi: 10.1097/MD.0000000000012193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He C., Zhang Y., Lin X. Increased overall survival and decreased cancer-specific mortality in patients with hepatocellular carcinoma treated by transarterial chemoembolization and human adenovirus type-5 combination therapy: A competing risk analysis. J. Gastrointest. Surg. 2018;22:989–997. doi: 10.1007/s11605-018-3703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schenk E.L., Mandrekar S.J., Dy G.K., Aubry M.C., Tan A.D., Dakhil S.R., Sachs B.A., Nieva J.J., Bertino E., Lee Hann C. A randomized double-blind phase II study of the Seneca Valley virus (NTX-010) versus placebo for patients with extensive-stage SCLC (ES SCLC) who were stable or responding after at least four cycles of platinum-based chemotherapy: North Central Cancer Treatment Group (Alliance) N0923 Study. J. Thorac. Oncol. 2020;15:110–119. doi: 10.1016/j.jtho.2019.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q.-N., Li Y., Zhao Q., Tian M., Chen L.-L., Miao L.-Y., Zhou Y.-J. Recombinant human adenovirus type 5 (Oncorine) reverses resistance to immune checkpoint inhibitor in a patient with recurrent non-small cell lung cancer: A case report. Thorac. Cancer. 2021;12:1617–1619. doi: 10.1111/1759-7714.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 66.Peter M., Kühnel F. Oncolytic adenovirus in cancer immunotherapy. Cancers (Basel) 2020;12:3354. doi: 10.3390/cancers12113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aref S., Bailey K., Fielding A. Measles to the rescue: A review of oncolytic measles virus. Viruses. 2016;8:294. doi: 10.3390/v8100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Müller L., Berkeley R., Barr T., Ilett E., Errington-Mais F. Past, present and future of oncolytic reovirus. Cancers (Basel) 2020;12:3219. doi: 10.3390/cancers12113219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basler M., Groettrup M. Advances in prostate cancer immunotherapies. Drugs Aging. 2007;24:197–221. doi: 10.2165/00002512-200724030-00003. [DOI] [PubMed] [Google Scholar]
- 70.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 71.Mahalingam D., Fountzilas C., Moseley J., Noronha N., Tran H., Chakrabarty R., Selvaggi G., Coffey M., Thompson B., Sarantopoulos J. A phase II study of REOLYSIN® (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother. Pharmacol. 2017;79:697–703. doi: 10.1007/s00280-017-3260-6. [DOI] [PubMed] [Google Scholar]
- 72.Mahalingam D., Wilkinson G.A., Eng K.H., Fields P., Raber P., Moseley J.L., Cheetham K., Coffey M., Nuovo G., Kalinski P. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase Ib study. Clin. Cancer Res. 2020;26:71–81. doi: 10.1158/1078-0432.CCR-19-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB–IV melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rommelfanger D.M., Compte M., Diaz R.M., Ilett E., Alvarez-Vallina L., Thompson J.M., Kottke T.J., Melcher A., Vile R.G. The efficacy versus toxicity profile of combination virotherapy and TLR immunotherapy highlights the danger of administering TLR agonists to oncolytic virus-treated mice. Mol. Ther. 2013;21:348–357. doi: 10.1038/mt.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Packer R.J., Raffel C., Villablanca J.G., Tonn J.C., Burdach S.E., Burger K., LaFond D., McComb J.G., Cogen P.H., Vezina G., Kapcala L.P. Treatment of progressive or recurrent pediatric malignant supratentorial brain tumors with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration. J. Neurosurg. 2000;92:249–254. doi: 10.3171/jns.2000.92.2.0249. [DOI] [PubMed] [Google Scholar]
- 76.Burke M.J., Ahern C., Weigel B.J., Poirier J.T., Rudin C.M., Chen Y., Cripe T.P., Bernhardt M.B., Blaney S.M. Phase I trial of Seneca Valley virus (NTX-010) in children with relapsed/refractory solid tumors: a report of the Children’s Oncology Group. Pediatr. Blood Cancer. 2015;62:743–750. doi: 10.1002/pbc.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolb E.A., Sampson V., Stabley D., Walter A., Sol-Church K., Cripe T., Hingorani P., Ahern C.H., Weigel B.J., Zwiebel J., Blaney S.M. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr. Blood Cancer. 2015;62:751–758. doi: 10.1002/pbc.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li L., Liu S., Han D., Tang B., Ma J. Delivery and biosafety of oncolytic virotherapy. Front. Oncol. 2020;10:475. doi: 10.3389/fonc.2020.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Power A.T., Wang J., Falls T.J., Paterson J.M., Parato K.A., Lichty B.D., Stojdl D.F., Forsyth P.A.J., Atkins H., Bell J.C. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 80.Evgin L., Acuna S.A., Tanese de Souza C., Marguerie M., Lemay C.G., Ilkow C.S., Findlay C.S., Falls T., Parato K.A., Hanwell D. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol. Ther. 2015;23:1066–1076. doi: 10.1038/mt.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evgin L., Ilkow C.S., Bourgeois-Daigneault M.-C., de Souza C.T., Stubbert L., Huh M.S., Jennings V.A., Marguerie M., Acuna S.A., Keller B.A. Complement inhibition enables tumor delivery of LCMV glycoprotein pseudotyped viruses in the presence of antiviral antibodies. Mol. Ther. Oncolytics. 2016;3:16027. doi: 10.1038/mto.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marotel M., Hasim M.S., Hagerman A., Ardolino M. The two-faces of NK cells in oncolytic virotherapy. Cytokine Growth Factor Rev. 2020;56:59–68. doi: 10.1016/j.cytogfr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 83.Ungerechts G., Bossow S., Leuchs B., Holm P.S., Rommelaere J., Coffey M., Coffin R., Bell J., Nettelbeck D.M. Moving oncolytic viruses into the clinic: Clinical-grade production, purification, and characterization of diverse oncolytic viruses. Mol. Ther. Methods Clin. Dev. 2016;3:16018. doi: 10.1038/mtm.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Working P.K., Lin A., Borellini F. Meeting product development challenges in manufacturing clinical grade oncolytic adenoviruses. Oncogene. 2005;24:7792–7801. doi: 10.1038/sj.onc.1209045. [DOI] [PubMed] [Google Scholar]
- 85.Yamaguchi T., Uchida E. Oncolytic virus: Regulatory aspects from quality control to clinical studies. Curr. Cancer Drug Targets. 2018;18:202–208. doi: 10.2174/1568009617666170222142650. [DOI] [PubMed] [Google Scholar]
- 86.Almutairi A.R., Alkhatib N.S., Oh M., Curiel-Lewandrowski C., Babiker H.M., Cranmer L.D., McBride A., Abraham I. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22–28. doi: 10.1001/jamadermatol.2018.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Corrigan P.A., Beaulieu C., Patel R.B., Lowe D.K. Talimogene laherparepvec: An oncolytic virus therapy for melanoma. Ann. Pharmacother. 2017;51:675–681. doi: 10.1177/1060028017702654. [DOI] [PubMed] [Google Scholar]
- 88.Inno A., Lo Russo G., Salgarello M., Corrao G., Casolino R., Galli G., Modena A., Romano L., Pusceddu S., Greco F.G. The evolving landscape of criteria for evaluating tumor response in the era of cancer immunotherapy: From Karnofsky to iRECIST. Tumori. 2018;104:88–95. doi: 10.1177/0300891618766173. [DOI] [PubMed] [Google Scholar]
- 89.McNamara M.G., Jacobs T., Lamarca A., Hubner R.A., Valle J.W., Amir E. Impact of high tumor mutational burden in solid tumors and challenges for biomarker application. Cancer Treat. Rev. 2020;89:102084. doi: 10.1016/j.ctrv.2020.102084. [DOI] [PubMed] [Google Scholar]
- 90.Wickham H. Springer; 2009. ggplot2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each article or trial is listed in a row, while the respective variable of interest is organized in columns