Abstract
Background
Cyclophosphamide (CPA) is a cytotoxic prodrug that needs to be metabolized by cytochrome P450 enzymes, like CYP2B6. Unfortunately, CYP2B6 is a very polymorphic enzyme and can cause a change in 3-hydroxypropyl mercapturic acid (3-HPMA), the most found CYP metabolite in urine levels. Change in 3-HPMA levels can also indicate the level change in its precursor, acrolein, which is responsible for the hematuria incidence after CPA administration.
This review's purpose is to obtain a conclusion about the optimal 3-HPMA analysis method in urine after the administration of cyclophosphamide using liquid chromatography-tandem mass spectrometry (LC-MS/MS) through literature review from previous studies. Also, this review was written to examine the relationship between levels of 3-HPMA in urine, polymorphisms of CYP2B6 enzymes, and the incidence of hematuria after cyclophosphamide administration in cancer patients.
Methods
Major databases, such as Universitas Indonesia's library database ScienceDirect, PubMed/Medline, Frontiers Media, and Google Scholar database, were used to find both published and unpublished studies without a time limit until 2020. Studies on pharmacokinetics, pharmacodynamics, drug therapy monitoring of cyclophosphamide, bioanalysis, and polymerase chain reaction (PCR) published in Indonesian and English were included. Meanwhile, non-related studies or studies written in other languages besides Indonesian and English were excluded. Two independent reviewers screened the titles, abstracts, and full-text manuscripts. Data obtained from eligible sources were used to answer the purpose of this review in a narrative form.
Results
The authors found 436 related studies from various databases and websites. Then, the authors narrowed it down into 62 pieces of literature by removing the duplicates and reviewing the abstracts and full-text manuscripts. Out of 62 sources, the authors found 30 studies that explained 3-HPMA analysis using LC/MS-MS, CYP2B6 polymorphisms, and hematuria occurrences. The authors used those 30 studies to build a conclusion regarding the purpose of this study. We strengthened the results with some additional information from the other 32 eligible sources.
Conclusions
The authors conclude that according to literature searches from previous studies, the optimal 3-HPMA analysis method in urine after cyclophosphamide administration using LC-MS/MS is using triple quadrupole LC-MS/MS; source of positive ion electrospray ionization (ESI); mobile phase combination of 0.1% formic acid in water (A) - 0.1% formic acid in acetonitrile (90:10 v/v) (B); the Acquity® BEH C18 column (2.1 × 100 mm; 1.7 μm); injection volume of 10 μl; flow rate of 0.2 ml/minute; gradient elution method. Detection was carried out using mass spectrometry with m/z ratio of 222.10 > 90 for 3-HPMA and m/z 164.10 > 122 for n-acetylcysteine (NAC). The optimum sample preparation method is acidification and dilution ratio of 1:5 v/v. Also, there is a relationship between 3-HPMA levels, CYP2B6 polymorphisms, and the occurrences of hematuria after the administration of cyclophosphamide, which is a type of CYP2B6 polymorph, namely CYP2B6∗6, can increase cyclophosphamide hydroxylation so that it can increase the levels of acrolein and 3-HPMA, as its metabolites, and risk of hematuria.
Ethics and dissemination
This research does not use human participants, human data, or human tissue for being directly studied for the review. Therefore, ethics approval and consent to participate are not applicable.
Registration
This research has not been registered yet.
Keywords: Cyclophosphamide (CPA), 3-hydroxypropyl mercapturic acid (3-HPMA), CYP2B6, Hemorrhagic cystitis, Hematuria
Cyclophosphamide (CPA), 3-hydroxypropyl mercapturic acid (3-HPMA), CYP2B6, Hemorrhagic cystitis, Hematuria.
1. Introduction
Cancer is a condition of abnormal cell growth and causes a disruption in body's homeostasis. This condition is very dangerous and has caused many deaths. There are almost 10.0 million deaths caused by cancer occurred in 2020 worldwide [1]. The death rate from cancer in Indonesia is also high with the total of 396,914 new cases in 2020. The most common type of cancer found in men is lung cancer with the total of 25,943 new cases, while the most common type of cancer found in women is breast cancer with the total of 65,858 new cases [2].
Various attempts have been done to treat cancer, one of which is using chemotherapy. Cyclophosphamide is one of the alkylating agents that is often used in chemotherapy because it has a broad spectrum of activity against human tumors [3]. According to the Food and Drug Administration or FDA (2013), cyclophosphamide is generally indicated for treating stage 3 and 4 malignant lymphomas according to the Ann Arbor classification [4]. This also applies to Hodgkin's and non-Hodgkin's lymphoma, lymphocytic lymphoma, small lymphocytic lymphoma, Burkitt lymphoma, and multiple myeloma. Cyclophosphamide is also used in other cancer chemotherapy, such as breast cancer and retinoblastoma [5]. Even though it has a therapeutic effect, cyclophosphamide can also cause various dangerous side effects, one of which is hemorrhagic cystitis [6]. Hemorrhagic cystitis is an inflammatory condition of the bladder that can cause hematuria. Hematuria is a term that states the presence of blood in the urine. The presence of blood in the urine causes the color of the urine to turn red-brown.
Cyclophosphamide is a prodrug, so it needs to be converted to its active metabolite to have pharmacological effects. The enzymes that play a role in cyclophosphamide metabolism are cytochrome P450 enzymes, such as CYP2B6, which is a very polymorphic enzyme [7]. Active metabolites of cyclophosphamide are phosphoramide mustard and acrolein. Then, acrolein will be metabolized into mercapturic acids by glutathione transferase (GST) and excreted in urine [8]. Among the various metabolites formed, the metabolite with the highest concentration in urine is 3-hydroxypropyl mercapturic acid (3-HPMA: 52.5–73.8%) [9].
Of the two active metabolites of cyclophosphamide, acrolein is the one that can cause a dangerous side effect. Acrolein has toxic properties in the kidneys and bladder because it has an electrophilic structure [8]. The activity of acrolein must be monitored, even though the acrolein itself will be metabolized and excreted in urine. Since 3-HPMA is the metabolite of acrolein with the highest concentration found in urine, it can be used as a biomarker to monitor acrolein's activity in the body [9].
Analysis of 3-HPMA levels in urine can be done by various methods. One method of analysis that is often applied in bioanalysis and provides excellent results is using liquid chromatography-tandem mass spectrometry (LC-MS/MS). LC-MS/MS has various advantages compared to other analytical methods, such as being able to analyze in a short time with a small number of analytes. For the analysis of CYP2B6 polymorphisms, analysis can be done using the polymerase chain reaction (PCR) technique. The PCR method can be used to select one or more sequences in the DNA matrix and multiply it into ten to billions of copies [10].
There is a variety of literature that has explained the incidences of hematuria after cyclophosphamide administration, such as analysis of 3-HPMA levels in breast cancer patients after giving cyclophosphamide using ultra-high-performance liquid chromatography-tandem mass spectrometry and analysis of the incidence of hemorrhagic cystitis after administration of low-dose cyclophosphamide [11, 12]. However, there is still no research stating the correlation between the hematuria incidences, 3-HPMA levels, and CYP2B6 polymorphism in a single comprehensive manuscript. In this study, we tried to find and write down the connections between these three things.
2. Methods
2.1. Study protocol
The Preferred Reporting Items for Systematical Reviews and Meta-Analysis (PRISMA) statement guidelines guided this review article [13]. We searched full-text manuscripts written in English and Indonesian in Universitas Indonesia's library database, ScienceDirect, PubMed/Medline, Frontiers Media, and Google Scholar. Most of the literature used were research journals from the last five years. Also, we used some compendiums and guidelines (from EMA, FDA, and QIAGEN) to strengthen our argumentations. We screened potential studies according to inclusion criteria by following the guidelines. We screened all titles and abstracts by reading and assessing to obtain relevant data.
2.2. Inclusion and exclusion criteria
The inclusion criteria for this article review were: (1) Cross-sectional, experiment, review, and case report studies written in Indonesian or English (especially studies which were published in the last five years), (2) Studies, reviews, and guidelines that explained pharmacokinetics, pharmacodynamics, monitoring of drug therapy from cyclophosphamide, bioanalysis, and polymerase chain reaction, and (3) Studies that used LC-MS/MS as its analysis method. Meanwhile, the exclusion criteria were: (1) Cohort studies, (2) Opinion studies, (3) Studies written in other languages besides Indonesian and English, (4) Non-related studies, and (5) Duplicate publications.
2.3. Search strategy
We searched studies, both published and unpublished, at four international databases of Science Direct, PubMed/Medline, Frontiers Media, and Google Scholar search engine, as well as Universitas Indonesia's library database (http://lib.ui.ac.id/) using keywords including “cyclophosphamide”, “cancer”, “breast cancer”, “acrolein”, “3-Hydroxypropyl mercapturic acid (3-HPMA)”, “hematuria”, “CYP2B6 polymorphisms”, “bioanalysis”, “LC-MS/MS”, “urine samples”, “blood samples”, “polymerase chain reaction (PCR)”, and “DNA extraction” without a time limit until 2020. The manual and guideline search was also done using the list of references in the review articles.
2.4. Selection of studies
All search results were collected and screened. After removing the duplicates, we assessed all identified studies based on title and abstract according to the inclusion criteria without using any specific data extraction form. If there were doubts about the article based on the title and abstract, the full-text article were assessed to check whether they met the inclusion criteria of this study. If there were disagreements between the reviewers, we solved them through discussion.
2.5. Data collection and items
We gathered the data independently according to the year of publication, study subjects, study design, and methods of study. Then, we set the variables, which were as follows:
-
A.
Dependent variables: The 3-HPMA levels in urine, CYP2B6 polymorphisms, and hematuria incidences.
-
B.
Independent variables: Bioanalytical method, DNA extraction, and PCR method.
-
C.
Confounding: The difference of hydration in subjects, MESNA efficacy, subjects' physiology, and foods intake.
If there were disagreements between the reviewers, we solved them through discussion. There was no data synthesis using statistical tools because this study is not a quantitative study. We also didn't perform a meta-analysis. Data that matched with the inclusion criteria were further processed to make a conclusion.
2.6. Study risk of bias assessment
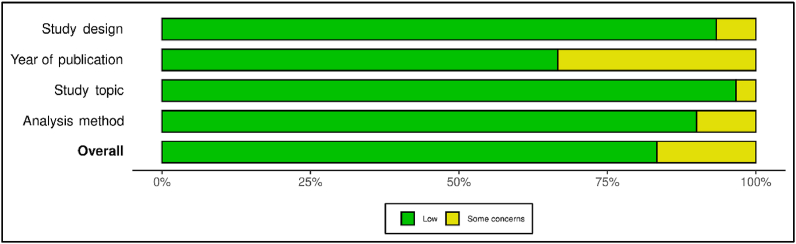
Quality evaluation from all eligible sources was accomplished using the risk of bias assessment QUADAS-2 tool for diagnostic test accuracy studies [14]. Study risk of bias assessment carried out independently by two reviewers. Robvis application was used for making the risk of bias plots and graph (Figure 1 & Figure 2) [15]. The criteria of this assessment were four domains: study design, year of publication, study topic, and analysis method. They were answered as “yes,” “no,” or “unclear”. “Yes” indicates a low risk of bias. The risk of bias was judged as “low,” “high,” or “some concern.”
Figure 1.
Distribution of the risk of bias judgments within each domain.
Figure 2.
The plots of the domain-level risk of bias judgments for each individual study.
3. Results
3.1. Study selection
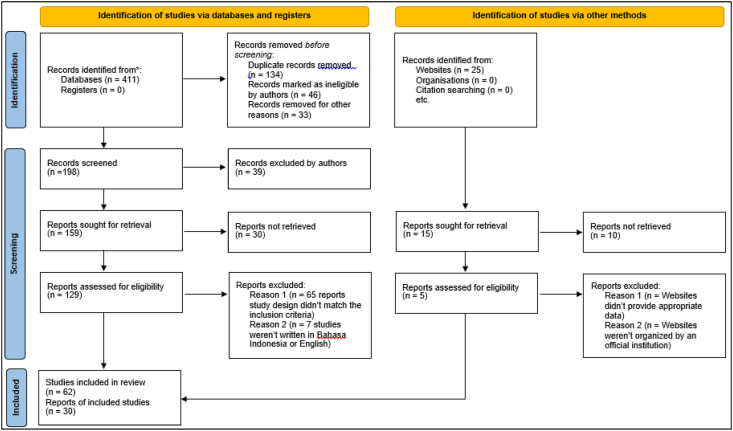
A total of 436 potentially relevant studies were identified through database and website searching. Among those articles, 213 pieces of literature were removed because they were duplicates and ineligible. Of the remaining 223 articles, 39 were excluded after the title and abstract screening. Of the 184 remaining articles, 40 were excluded because they didn't provide appropriate data. Of the 144 remaining articles, 82 were excluded after we found that they did not meet the inclusion criteria by doing full-text analysis. Of the 62 eligible sources, 30 articles that explained 3-HPMA analysis using LC/MS-MS, CYP2B6 polymorphisms, and hematuria occurrences were used to build a conclusion regarding the purpose of this study. The rest of the eligible sources were used to strengthen the argument of the review results. The illustration of this process is shown in Figure 3.
Figure 3.
PRISMA flowchart.
3.2. Quality appraisal
In the study risk of bias assessment, there was a high percentage of “low” over “some concern” and there was no “high” risk of bias. There were 5 out of 30 studies that had been considered as sources with “some concerns”. However, we have reviewed and used the data from those sources carefully to prevent bias. Overall, we concluded that all 30 assessed studies were considered as high-quality literature because they met the inclusion criteria and have low risks of bias. All studies we used as sources to make this review article were assessed according to the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool.
3.3. Findings of the review
We made an article review in a narrative form from 62 sources. Of 62 eligible sources, 30 were used to make a conclusion regarding to the optimum 3-HPMA bioanalytical method and the relationship between 3-HPMA levels in urine, polymorphisms of CYP2B6 enzymes, and the incidence of hematuria after cyclophosphamide administration in cancer patients. Full details of the used studies reported by the authors are presented in Table 1.
Table 1.
Main findings of each study.
| Author (Year) | Study Design | Study Topic | Main Findings |
|---|---|---|---|
| Harahap et al. (2020) [11] | Cross-sectional | Analysis of 3-HPMA using LC-MS/MS |
|
| Moein et al. (2017) [32] | Review | Bioanalytical method development |
|
| Zhao et al. (2017) [35] | Experimental | Urine as a biological sample for bioanalysis |
|
| Drouin et al. (2020) [36] | Review | Urine sample preparation for bioanalysis |
|
| Xu et al. (2012) [37] | Review | Urine sample preparation for bioanalysis |
|
| Novák et al. (2016) [38] | Review | Urine sample preparation for bioanalysis |
|
| Tully et al. (2018) [39] | Animal research study | 3-HPMA analysis in urine samples |
|
| Harahap et al. (2020) [40] | Experimental | Bioanalytical method for analyzing the 3-HPMA concentration in urine samples |
|
| Carmella et al. (2007) [41] | Cross-sectional | Bioanalytical method for analyzing the 3-HPMA concentration in urine samples |
|
| Yan et al. (2010) [42] | Experimental | Bioanalytical method for analyzing the 3-HPMA concentration in urine samples |
|
| Gao et al. (2020) [43] | Case report | Single nucleotide polymorphism (SNP) study |
|
| Bruijins et al. (2018) [45] | Experimental | DNA extraction methods |
|
| Ng et al. (2018) [46] | Experimental | DNA extraction methods |
|
| Martins et al. (2015) [47] | Experimental | DNA extraction methods from biological samples |
|
| Guha et al. (2017) [48] | Experimental | DNA extraction methods from biological samples |
|
| Kaewkhao et al. (2019) [49] | Experimental | DNA extraction methods from biological samples |
|
| Nakajima et al. (2007) [51] | Cross-sectional | CYP2B6 polymorphisms method analysis |
|
| Kumar et al. (2019) [12] | Case report | Cyclophosphamide side effect |
|
| Saito et al. (2018) [52] | Cross-sectional | Cyclophosphamide side effect |
|
| Teles et al. (2017) [53] | Review | Cyclophosphamide side effect |
|
| Doshi et al. (2019) [54] | Review | Cyclophosphamide side effect |
|
| Shu et al. (2016) [55] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Hedrich et al. (2016) [56] | Review | CYP2B6 polymorphisms |
|
| Zanger et al. (2013) [57] | Review | CYP2B6 polymorphisms |
|
| Lang et al. (2001) [58] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Lamba et al. (2003) [59] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Lang et al. (2004) [22] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Xie et al. (2003) [60] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Xie et al. (2006) [61] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
| Kirchheiner et al. (2003) [62] | Cross-sectional | CYP2B6 polymorphisms on cyclophosphamide metabolism |
|
We used those collected data to conclude our review article. We performed a literature review from 10 studies that explained the analysis of 3-HPMA, bioanalytical method development, and urine as a biological sample to find the most optimal 3-HPMA analysis method. According to those 10 studies, we conclude that the best 3-HPMA analysis method in urine after the administration of cyclophosphamide using liquid chromatography-tandem mass spectrometry (LC-MS/MS) is using triple quadrupole LC-MS/MS; source of positive ion ESI; mobile phase combination of 0.1% formic acid in water (A) - 0.1% formic acid in acetonitrile (90:10 v/v) (B); the Acquity® BEH C18 column (2.1 × 100 mm; 1.7 μm); injection volume of 10 μl; flow rate of 0.2 ml/minute; gradient elution method. The samples should be prepared by acidification and dilution ratio of 1:5 v/v. Detection should be carried out using mass spectrometry with the m/z ratio of 222.10 > 90 for 3-HPMA and m/z 164.10 > 122 for NAC. Also, we performed a literature review from another 20 studies that explained single nucleotide polymorphism (SNP), CYP2B6 variants, and cyclophosphamide's side effect to find the correlation between the 3-HPMA levels, polymorphism of CYP2B6 enzyme, and the incidence of hematuria after cyclophosphamide administration. According to those 20 studies, we conclude that a type of CYP2B6 polymorph, which is CYP2B6∗6, can increase cyclophosphamide hydroxylation so that it can increase levels of acrolein and 3-HPMA, as its metabolites, and the risk of hematuria. The reasons for our conclusions will be explained thoroughly in the next section of this review article.
4. Discussion
4.1. Anticancer agents
4.1.1. Cancer
Cancer is a condition that disrupts the body's homeostasis due to abnormal cell growth. Cancer cells are less specialized so they will continue to divide and multiply. These cells can interfere with normal cells and form tumor masses that can spread to other organs through the blood and lymph. If it doesn't being treated properly, this condition can lead to death [16].
The cause of a cancer development in a person's body still can't be ascertained precisely, but there are internal and external factors that can increase a person's risk for developing cancer. One of the internal factors that can cause the development of cancer cells is genetic factor. Genetic cancer has a prevalence of 5–10% of all cancers [16]. Various external factors can increase the risk of cancer development, one of which is chemical exposure. Exposure to uncontrolled chemicals can cause mutations in cells, causing these cells to grow abnormally. Besides, there are some other external factors, such as poor lifestyle habits (i.e. smoking, alcoholic beverages, and lack of exercise), consumption of certain drugs, viruses, and bacteria.
The development of cancer is divided into 3 stages, which are initiation, promotion, and progression. Initiation is the initial stage when cells are exposed to carcinogenic agents. Carcinogens can cause irreversible mutations in DNA thereby increasing the risk of developing cancer. Then, the promotion phase takes place when the exposed cells mutate to a greater number of mutated cells than normal cells. This stage takes place supported by external risk factors. Then, the stage of progression takes place. At progression stage, cancer cells are further increased. At this stage, the treatment given is palliative.
Chemotherapy is a pharmacological therapy to fight cancer cells. However, most chemotherapy agents can damage normal cells as well because they work non-selectively, so the dose must be determined precisely. Based on its mechanism of action, chemotherapy agents are divided into several types, such as the nucleic acids structure changer, deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) synthesis inhibitor, and cell division inhibitor. Based on its type, chemotherapy agents are divided into several types as well, such as alkylating agents, mitotic inhibitors, antitumor antibiotics, corticosteroids, topoisomerase inhibitors, and other antineoplastics [17].
Each chemotherapy agent works differently. The alkylating agent works by giving a methyl group to the nitrogen base (N7 guanine) to damage the DNA. Because it has a broad spectrum it also can affect the production of blood cells in the bone marrow. Antimetabolites work by acting as a building block so that DNA can't make cell walls and cells can't be produced. The examples of antimetabolites are 5-fluorouracil, 6-mercaptopurine, methotrexate, and capecitabine. Antitumor antibiotics can disrupt the cycle that inhibits enzymes who play a role in copying DNA in the cell cycle and binds to DNA. However, this group of agents can attack the heart. The examples of antitumor antibiotics are doxorubicin, daunorubicin, epirubicin, bleomycin, dactinomycin, mitomycin-C, mitoxantrone. Topoisomerase inhibitors (plant alkaloids) have a therapeutic effect that blocks the action of topoisomerases, enzymes that are used to open DNA strands. The examples of topoisomerase I (camptothecin) class are irinotecan and topotecan that can be extracted from Camptotheca acuminate, whereas examples of topoisomerase II (epipodophyllotoxin) class are etoposide, teniposide, and mitoxantrone that can be extracted from Asteriscus graveolens. Also, there is another plant alkaloids class, the mitotic inhibitors class, which inhibits cell division and enzymes to make the required proteins. These types of drugs can damage nerves. The examples of the mitotic inhibitor class are cabazitaxel, docetaxel, paclitaxel (taxane class), and vincristine & vinorelbine (vinca class). There are also other anticancer classes, such as all-trans-retinoic acid, arsenic trioxide, asparaginase, eribulin, hydroxyurea, ixabepilone, mitotane, omacetaxine, pegaspargase, procarbazine, romidepsin, and vorinostat. Apart from antineoplastic agents, corticosteroid drugs, such as prednisone, methylprednisolone, and dexamethasone, are also often used to prevent chemotherapy-induced nausea, vomiting, and allergy reaction [17].
The explanation regarding chemotherapy agents can still be elaborated further. However, this journal will only discuss the alkylating agent class more.
4.1.2. Alkylating agents
Alkylating agents are chemotherapeutic agents who can attack the DNA by forming covalent bonds in the nucleophilic group, usually in the N7 position of guanine, of cells. This alkylation of N7 guanine causes the guanine residue to become more acidic, decreases the stability of the imidazole ring, and opens the chance for a crosslinking between the two nucleic acids. This DNA adduct will cause DNA replication to be inhibited. These agents work throughout the cell cycle but are most active during the resting phase of the cell. Several types of alkylating agents, such as nitrogen mustard, methylhydrazine derivatives, alkyl sulfonates, nitrosourea, triazine, and platinum coordinating complexes, are often used in chemotherapy [18].
4.1.3. Nitrogen mustard
Nitrogen mustard is an alkylating agent that can form bonds with DNA, link two strands, and inhibit DNA replication. Nitrogen mustard makes aziridinium ions which are very reactive to the DNA of tumor cells and also normal cells. The formation of aziridinium ions will make bis (2-chloroethyl) amines undergo first-order nucleophilic substitution cyclization. Then, the aziridinium ion will undergo the addition of nucleophiles from DNA to form monoalkylation through the nucleophilic substitution mechanism to crosslink the two strands of DNA. This principle is used for the development of antineoplastic agents, such as cyclophosphamide, melphalan, chlorambucil, mechlorethamine, and estramustine [19].
4.2. Cyclophosphamide
Cyclophosphamide considered effective for inhibiting DNA replication. Phosphoramide mustard, the active metabolite of cyclophosphamide, can induce a crosslinking of DNA in the N7 position of guanine which leads to cell apoptosis. This mechanism of action makes cyclophosphamide suitable to be given to cancer patients with malignant lymphoma, leukemia, neuroblastoma, retinoblastoma, ovarian, breast, endometrial, and lung carcinomas. Also, cyclophosphamide can be used in organ transplantation procedure as an immunosuppressant. The dosage used varies depending on the treatment regimen. According to the FDA (2013), the intravenous dose of cyclophosphamide for malignant disease without hematologic deficiency is 40–50 mg per kg in divided doses for 2–5 days. Other regimens are 10–15 mg per kg for 7–10 days or 3–5 mg per kg twice a week [4].
Cyclophosphamide orally can be well absorbed in the digestive tract (the value of bioavailability reaches ≥75%). Cyclophosphamide is also widely distributed in tissues and can penetrate the blood-brain barrier with a distribution volume of around 30–50 L. Because it is still a prodrug, cyclophosphamide needs to be metabolized first in the liver by CYP450 enzymes, such as CYP2B6, CYP3A4, and CYP3A5. Then, cyclophosphamide and its metabolites (if administered intravenously) will be excreted in urine with half-lives varying from 3 to 12 hours with a total clearance value of 4–5.6 L/hour. [4].
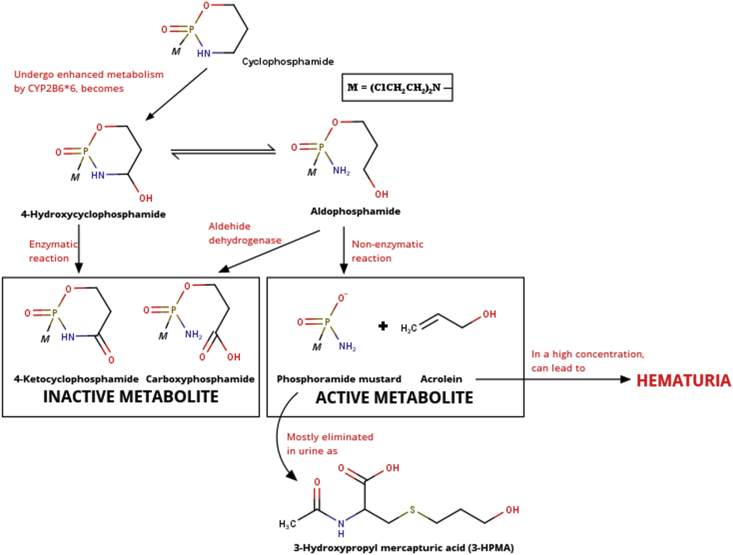
Cyclophosphamide metabolism starts from hydroxylation by the enzymes CYP2B6 and CYP3A4 to 4-hydroxycyclophosphamide. Then, alcohol dehydrogenase oxidizes it to 4-ketocyclophosphamide, whereas aldehyde oxidase oxidizes aldophosphamide to carboxyphosphamide. Both of these compounds are inactive metabolites. Active cyclophosphamide metabolites, namely phosphoramide mustard and acrolein, are formed from non-enzymatic reactions originating from aldophosphamide. The use of cyclophosphamide can have varied effects on each individual. One cause of these variations is the polymorphism of the CYP2B6 enzyme. This CYP2B6 enzyme has a proportion of 1–5% of the total cytochrome P450 content. CYP2B6 enzyme has an important role in the biotransformation of drugs and other xenobiotics [20]. Genotypes CYP2B6 and CYP2C19 have been shown to influence the pharmacokinetics and activation of cyclophosphamide (seen from its half-life) in breast cancer patients [3]. Therefore, polymorphisms of genes that encode the CYP2B6 enzyme need to be evaluated to monitor cyclophosphamide toxicity profile [21].
CYP2B6 polymorphisms, both genotypic and phenotypic, are also associated with the side effects that can occur. There are at least 9 Single-Nucleotide Polymorphism (SNP) with 5 variations of amino acids, which are CYP2B6∗2 (R22C), CYP2B6∗3 (S259R), CYP2B6∗4 (K262R), CYP2B6∗6 (Q172H and K262R), and CYP2B6∗7 (Q172H, K262R, and R487C). In the Caucasians, variants of CYP2B6 that are often found are CYP2B6∗4 (K262R) by 33%, CYP2B6∗6 (Q172H and K262R) by 28%, and CYP2B6∗5 (R487C) by 14%. There are also some other genotypic polymorphisms of the CYP2B6 enzyme, such as CYP2B6∗8, CYP2B6∗11, CYP2B6∗12, CYP2B6∗14, and CYP2B6∗15 which cause decreased expression and function of cytochrome P450 enzymes [22].
The use of cyclophosphamide can cause various undesirable drug reactions. Alkylating agents, in general, attack normal cells so that it can cause myelosuppression, immunosuppression, bone marrow infection, and alopecia. Cyclophosphamide can also damage the cells of other organs and cause some side effects, such as myocarditis in the heart and pneumonitis in the lungs. There are also some mild side effects that can be occurred, such as fever, nausea, and vomiting. Cyclophosphamide also should not be consumed by cancer patients who have hypersensitivity to cyclophosphamide and urinary tract obstruction [4]. The specific side effect of using cyclophosphamide is hemorrhagic cystitis. Hemorrhagic cystitis is an inflammation of the bladder that can cause hematuria. Hematuria itself is the presence of blood in the urine. This is caused by one of the active metabolites of cyclophosphamide, which is acrolein.
Acrolein, or also called acryl aldehyde, allyl aldehyde, ethylene aldehyde, propenal, and prop-2-enal, is an unsaturated aldehyde compound with a rancid odor. This compound can arise from the dehydration of carbohydrates, vegetable oils, fats, and amino acids. One of the biggest contributors to acrolein is smoking. This compound is also a metabolite of various compounds, one of which is cyclophosphamide [23].
Acrolein can break down proteins and damage DNA strands because it has unsaturated aldehyde residues, causing cells to die. Besides, acrolein also increases the levels of reactive oxygen species in the urothelium by catalyzing the reaction of glutathione to glutathionyl propionaldehyde (GTPD). GTPD activates the NF-κB apoptotic pathway and interacts with several enzymes, such as xanthine oxidase and aldehyde dehydrogenase, to form superoxide radicals, such as peroxynitrite. Peroxynitrite can damage the crosslinking of DNA thereby stimulating increased regulation of genes that repair DNA damage and depletion of nicotinamide adenine dinucleotide (NAD) and adenosine triphosphate (ATP). This cycle continues until the energy source of the cell is depleted and protein synthesis cannot be carried out anymore so that the cell dies. This inflammatory condition is called hemorrhagic cystitis. This condition makes the urothelial wall decay and mixed with urine [24]. This condition is called hematuria, which is the presence of blood in the urine.
Acrolein is one of the active metabolites of cyclophosphamide. After this compound is formed, acrolein will be conjugated by glutathione and metabolized into several polar mercapturic acids so that it can be excreted in urine. In fact, the compound N-acetyl-S-2-carboxy-2-hydroxyethyl silcysteine (5.4–10.4%), N-acetyl-S-2-carboxyethyl cysteine (2.6–18.3%), N-acetyl -S-3-hydroxypropylcysteine (3-HPMA: 52.5–73.8%), and 3-hydroxy propionic acid (5.2–33.2%) were detected in urine from mice 4–24 hours after intravenous administration of acrolein. Of the various mercapturic acids, 3-HPMA is considered as the main metabolite of acrolein in the urine [9].
3-HPMA, which also called N-acetyl-S-(3-hydroxypropyl) cysteine, S-(3-hydroxypropyl) cysteine N-acetate, and 2-acetamide-3-(3′-hydroxypropylthio) propanoate acid, is one of the main metabolites of acrolein in the urine. The formation of 3-HPMA is one of the detoxification pathways of acrolein. This metabolite is a stable metabolite and a high abundance of acrolein. In addition, 3-HPMA is also known as a non-invasive biomarker [9]. Therefore, 3-HPMA can be a biomarker of several diseases caused by acrolein [9].
4.3. Liquid chromatography tandem mass spectrometry (LC-MS/MS)
LC-MS/MS is a combination consisting of an ultra-high performance liquid chromatography (UPLC) pumping system with columns and injectors from mass spectrometry. This method is commonly used for pharmacokinetic, proteomic, and drug development testing. This method utilizes a mixture of the two principles, which are physicochemical separation in liquid chromatography and mass detection so that the ability of the two analytical methods can be improved. The use of this instrument is also superior when compared to gas chromatography-tandem mass spectrometry because it is not limited to volatile molecules, but can also be used to measure highly polar analyte without derivatization techniques in sample preparation [25].
4.3.1. Ultra-high performance liquid chromatography (UPLC)
Chromatography is a compound separation technique based on the affinity of the analyte for other compounds. The substances dissolved in the analyte will experience different flow rates because they are influenced by differences in adsorption, solubility, partitioning, molecular size, ionic charge density, or vapor pressure. This difference causes each solute in the analyte to be identified properly.
UPLC is an instrument that can separate compounds with high speed, resolution, and sensitivity with shorter analysis time than conventional high-performance liquid chromatography (HPLC). This is caused by the difference of pressure used. UPLC is a combination of 1.7 μm column packing and chromatographic systems that can operate at pressures of 6,000–15,000 psi, whereas conventional HPLC uses column packing of 3–5 μm and operate at pressures of 2,000–4,000 psi. UPLC that is standardized with mass spectrometry is often used in the pharmaceutical analysis in various discoveries and developments, such as in the discovery of new drugs, product characterization, metabolic studies (in vitro and in vivo), and identification of pollutants and degradation products [26].
The instrumentation on UPLC does not differ much from conventional HPLC. The UPLC system consists of a solvent reservoir, pump, injector, column, detector, and computer. In short, the mobile phase will be pumped by the reservoir through the injector to be able to flow into the column and come out through the detector. Then, the mobile phase is injected into the column so that it experiences separation. The peak of the chromatogram is produced by an elution that is detected by a detector, then sent to the data processing system [27, 28].
4.3.2. Mass spectrometry
Mass spectrometry is a quantitative and qualitative analysis method by measuring the mass to charge ratio (m/z). This technique utilizes the principle of the ion formation reaction of organic or inorganic compounds to separate the ions so that it is good for measuring the molecular weight of the sample. Ions that have been sorted will be detected by a detector on the instrument. The produced electrical signal is used to form a mass spectrum that can be seen as a histogram. The resulting spectrum contains information about the number of ions at different m/z. The detected ion can be related to the original molecule, its fragments, or other species formed during the ionization process. Therefore, mass spectrometry provides the identification of molecules with high selectivity [29]. Mass spectrometry consists of inlet systems, ionizing sources, mass analyzers, and detector [30, 31].
4.4. Bioanalytical method for determination of 3-HPMA levels in urine samples
Bioanalysis is a method used for the identification, qualification, and quantification of compounds from biological samples. The examples of biological samples are blood, plasma, serum, urine, skin, saliva, feces, organ tissue, and hair. The size of molecules measured in bioanalysis can vary, from small ones, such as drugs, metabolites, and biomarkers, to large ones, such as peptides. Therefore, bioanalysis is an important part of drug discovery and development [32].
There are some steps that should be done before carrying out a bioanalysis. The first step is collecting samples from preclinical or clinical studies which are then sent to the laboratory. The second step is sample preparation to reduce interference from the other compounds in biological samples. Sample preparation makes the results of the analysis valid. The final step is the process validation.
The validation of the bioanalysis is a stage of meeting the requirements of the design, condition, limitations, and suitability of the bioanalysis system. This has to be done because each analytes has different properties that requires different methods as well. Method validation is done as an effort to verify a method to produce accurate analysis results [33]. Validation is performed on various parameters as follows:
-
A.
Selectivity
Selectivity is the ability of a method to analyze compounds that are suitable for the purpose of the study. Selectivity testing is done by analyzing blanks regularly. The matrix and ion suppression effects also need to be determined when validating selectivity in the LC-MS/MS tool. Selectivity testing also requires internal standards to avoid interference of the other compounds with analytes. The researcher must confirm that the analysis conditions are free of potential interference when validation is performed. The acceptance criteria for selectivity are blank response responses from LLOQ analyte less than 20% and from internal standards less than 5% [33].
-
B.
Carry-over
Carry-over must be minimized in sample analysis. Carry-over done by injecting the blank after a high concentration sample or calibration standard at the upper limit of quantification (ULOQ). The acceptance criteria for carry-over are the percentage of carry-over on the blank after the high concentration sample should not exceed 20% of LLOQ. If carry-over cannot be avoided, the sample should not be chosen randomly and the influence of carry-over must be analyzed [33].
-
C.
Lower Limit of Quantitation (LLOQ)
The lower limit of quantification (LLOQ) is the lowest concentration value of the analyte that can still be calculated accurately and precisely. LLOQ is considered as the lowest calibration standard. The LLOQ value of the sample must be 5 times greater than the blank and not greater than 5% Cmax [34].
-
D.
Calibration Curve
The calibration curve is the range of concentrations needed to analyze a sample according to the response of the instrument. Calibration standards must be prepared on the same matrix as the sample. The calibration curve must contain the highest and lowest values of the concentration that can be analyzed. The lowest concentration value is defined as LLOQ, while the highest is ULOQ. To find out the concentration range, a blank, a zero, and a minimum of 6 calibration standards, must be used. The concentration measured in the instrument must be ±15% of the actual concentration, except for LLOQ (must be ±20%). At least 75% of calibration standards must meet these criteria [33].
-
E.
Accuracy and Precision
Accuracy states the degree of closeness of the concentration value of the analysis results with the actual concentration value and is displayed in the form of a percentage, while precision states the degree of repeatability of the analyte measurement and is expressed as a coefficient of variation (CV). This validation involves a quality control (QC) sample which is self-injected at 4 different concentrations that fall into the calibration curve range, namely LLOQ, QCL (three times LLOQ), QCM (30–50% of the range of calibration curves), and QCH (75% of ULOQ). Validation of accuracy and precision can be done on a daily and inter-day basis. The condition for accepting validation accuracy is that the average concentration must be ±15% of the actual QC sample value, except for the LLOQ concentration, it must be ±20%. Meanwhile, the requirement for precision validation is the precision value of the analysis results must not exceed 15% CV for the QC sample, and must not exceed 20% CV for the LLOQ [33].
-
F.
Dilution Integrity
Dilution must not influence accuracy and precision. Validation of dilution integrity is done by spiking the matrix with the concentration of the analyte above the upper limit of quantification (ULOQ), then diluted by the blank matrix. The acceptance criteria is that the accuracy and precision value must remain by the specified conditions, which is ±15% CV [34].
-
G.
Matrix Effects
Matrix effects are tested with a minimum of 6 blank matrixes obtained from 6 different individuals. Matrix factor of each analyte and the internal standard must be calculated from the peak area in the presence of a matrix with the peak area in the absence of a matrix. The validation test for matrix effects must be carried out at low and high concentrations (maximum 3 times LLOQ and close to ULOQ). The acceptance requirement is the normalized matrix effect does not exceed 15% CV [34].
-
H.
Stability
- Validation of stability needs to be done to ensure that there is no influence of the container during the preparation, analysis, and storage of samples on the concentration of the analyte. Stability must be ensured at every stage of the analysis method. This validation must be tested with proper dilution. We can test the stability of the analyte by using QCL and QCH, which are analyzed immediately after preparation. QCL and QCH are also must be re-tested after storage. A calibration curve is used for the stability test of QC samples by comparing the obtained concentration with the actual analyte concentration. The acceptance criteria is that the %diff for each concentration level must be less than 15%. Kinds of stability that need to be tested are as follows:
-
a.stability of the stock solution and internal standard analytes and work solutions,
-
b.freeze and thaw stability of the analyte on the matrix under frozen storage conditions transferred to room temperature or analysis temperature,
-
c.short-term stability of the analyte at room temperature or analysis temperature, and
-
d.long-term stability of the analyte under storage conditions in the freezer.
-
a.
Some tests are better done if the conditions allow, namely the stability of the sample during the analysis process running at room temperature or storage conditions during analysis, bench-top stability, extract stability, and autosampler stability [33].
After the validation step has been done, the detection and analysis of samples can be carried out. For this stage, LC-MS/MS is the right choice because of its very high selectivity and sensitivity [32]. Results of the analysis can be produced from a variety of biological samples, one of which is urine.
Urine is a waste product of the body's metabolism that must be removed. Urine is formed in the kidney, collected in the bladder, and excreted through the urethra. Urine consists of water (91–96%), inorganic salts, and metabolites. Because it can contain metabolites, urine can be a valuable biological sample for bioanalysis. Also, this fluid can reflect the changes that occur in the body so that urine can be said to be an important source for the discovery of biomarkers of disease. Urinary proteomics studies have identified various biomarkers for urogenital diseases, such as acute kidney injury, bladder cancer, and diabetic nephropathy [35]. For bioanalysis purpose, urine can be prepared by dilute and shoot method, solid phase extraction, or protein precipitation [36, 37, 37].
Some previous researchers have carried out an analysis of 3-HPMA levels in urine. The research subjects also came from various groups, such as multiple sclerosis patients, smokers, and breast cancer patients [39, 40, 41, 42]. This can be done because acrolein can come from many sources, such as fried foods and the environment [8]. These studies use different bioanalytical methods and conditions and all of them produce valid analysis results. However, for this article, the authors are more focused on discussing the methods that have been developed by Harahap et al. (2020) [40].
Harahap et al. (2020) has developed a bioanalytical method of 3-HPMA levels analysis in urine using LC-MS/MS. Her research used several different preparation methods and analysis conditions of LC-MS/MS. Then, a valid method, according to European Medicines Agency (EMA) (2011) and FDA (2018) guidelines, which produced the most optimal result was chosen. The most optimal preparation and analysis conditions were done by dilution and acidification method. An urine sample taken 4 hours after the administration of cyclophosphamide in the volume of ±30 ml. Then, the sample was filtered with a 0.22 μm filter. Filtrate with the volume of 100 μl was added with 10 μl of formic acid, 50 μl of internal standard N-Acetyl-L-cysteine (NAC) at a concentration of 10 ppm, and 390 μl of a mobile phase mixture solution. The sample was mixed in a vortex mixer for 35 seconds. Then, the sample was centrifuged at 10,000 rpm for 5 minutes. For urinalysis, prepared samples will be entered into the "Cobas U411 Urine Analyzer" [40]. Then, samples and standards was being injected into the LC-MS/MS instrument under selected analytical conditions with an optimal phase gradient elution (Table 2).
Table 2.
Gradient elution profile of mobile phases in LC-MS/MS. Retrieved from Harahap et al. (2020) [40] has been reprocessed.
| Minutes | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0,00 | 90 | 10 |
| 2,00 | 10 | 90 |
| 4,00 | 10 | 90 |
| 4,10 | 90 | 10 |
| 7,00 | 90 | 10 |
That analysis method and condition produced a good result with a LLOQ value of 40 ng/ml and a linear calibration curve of 40–10,000 ng/ml (r = 0.999). Accuracy and precision were also obtained for LLOQ, QCL, QCM, and QCH (40, 1,500, 5,000, and 8,000 ng/ml) and met the acceptance requirements, which is <20% CV. Other parameters, such as carry-over, the integrity of dilution, and matrix effect, also meet the requirements. Stock solution and working solution were stable for 1 month and have passed the criteria of freeze-thaw stability and autosampler stability tests [40].
There are differences in the Harahap et al. (2020) analysis method with the predecessor researcher analysis methods, such as from Carmella et al. (2007) and Yan et al. (2010). The differences of analysis methods are shown in Table 3. These different methods produced different results. The authors examine the differences in the results of the LLOQ scores. The LLOQ value obtained, which is 40.0 ng/ml, is quite large when compared to the LLOQ value from previous studies, which is 0.9 ng/ml (Carmella et al., 2007) and 22.0 ng/ml (Yan et al., 2010) [40, 41, 42].
Table 3.
Bioanalytical methods and condition's differences between previous studies.
| No. | Analysis Condition | Harahap et al. (2020) [40] | Carmella, Chen, Zhang, Zhang, Hatsukami, & Hecht, (2007) [41] | Yan, Byrd, Brown, & Borgerding, (2010) [42] |
|---|---|---|---|---|
| 1 | Subject | 17 breast cancer patients with cyclophosphamide chemotherapy regimens | 35 smokers and 21 orang non-smokers | 1 smoker and 2 non-smokers |
| 2 | Instrument type | Triple quadrupole LC-MS/MS, positive ion ESI | Triple quadrupole LC-APCI-MS/MS-SRM, negative APCI | LC-MS/MS, negative ion ESI |
| 3 | Mobile phase | 0,1% formic acid in water (A) - 0,1% formic acid in acetonitrile (90:10 v/v) (B) | 15 mM Acetic ammonium (NH4OAc) (A) – methanol (MeOH) (B) | 10 mM Ammonium formate in water (A) – acetonitrile (B) |
| 4 | Column | Acquity® BEH C18 (2,1 × 100 mm; 1,7 μm) | Synergi Max-RP C12 (4,6 mm × 25 cm; 4 μm) | Phenomenex HILIC (100 × 2 mm; 3 μm) |
| 5 | Flow rate | 0,2 ml/min | 0,8 ml/min | 1,0–1,2 ml/min |
| 6 | Elution method | Gradient | Gradient | Gradient |
| 7 | Detection method | m/z 222,10 > 90,97 and 164,10 > 122,02 for 3-HPMA and NAC | m/z 220 > 91 for 3-HPMA, m/z 223 > 94 for [13C3]3-HPMA, and m/z 237 > 105 for injection standard | m/z 220,2 > 91,1 and 223,2 > 91,1 for 3-HPMA and 3-HPMA d3 |
| 8 | Sample preparation method | Dilution and acidification with formic acid | Solid phase extraction with 2% ammonium hydroxide (NH4OH) and methanol | Dilution with ammonium formate |
| 9 | Obtained LLOQ | 40,0 ng/ml | 0,9 ng/ml | 22,0 ng/ml |
List of abbreviations: LC-MS/MS: Liquid chromatography-tandem mass spectrometry; ESI: Electrospray ionization; LC-APCI-MS/MS-SRM: Liquid chromatography coupled with atmospheric pressure chemical ionization and selected reaction monitoring mass spectrometry; 3-HPMA: 3-Hydroxypropyl mercapturic acid; LLOQ: Lower limit of quantification.
The Harahap et al. (2020) analysis method has several advantages over the other methods. The level of 3-HPMA abundance is quite high in the urine so the method of dilution is considered better because it is easier and cheaper to do compared to SPE. Then, the methods of Yan et al. (2010) also easily produce optimal results but have never been tested directly on cancer patients receiving cyclophosphamide therapy regimens. LLOQ values obtained from the analysis method of Harahap et al. (2020) were also still relatively small. This method has also been applied directly to 17 breast cancer patients who received a cyclophosphamide therapy regimen by Harahap et al. (2020) and to 40 other breast cancer patients who received a cyclophosphamide therapy regimen by Harahap et al. (2020) [11, 40]. Hence, after comparing the analysis methods from Harahap et al. (2020), Carmella et al. (2007), and Yan et al. (2010), and also obtaining some additional information from 7 literature, the authors conclude that the Harahap et al. (2020) analysis method is the best method for analyzing 3-HPMA levels in the urine of cancer patients receiving cyclophosphamide.
4.5. The CYP2B6 polymorphism analysis method
Polymerase chain reaction (PCR) is a method of enzymatic DNA replication without the use of living organisms. This method can be used to produce large amounts of DNA in a short time. The advantages of this method make PCR can be used for various things, one of which is to find out the gene polymorphism that experiences SNP. High-density SNP structures are used to collect large amounts of genotype data [43].
One PCR process cycle consists of 3 working steps. The first stage is denaturing stage, which is the separation of DNA fragments into a single chain. Then, proceed to the second stage, annealing stage. After that, the third stage, the extension/elongation stage, will take place. This cycle continues to repeat until the amount of DNA is sufficient to be analyzed [44].
The polymorphism analysis method is divided into 2 stages, which are DNA extraction from biological samples and PCR. DNA extraction can be carried out from various biological samples that contain DNA, such as blood, urine, buccal swab, and hair. DNA extracted from buccal swab samples is not good because research from Bruijns et al. (2018) shows that there are parts of DNA that cannot be extracted from swabs and some types of swabs bind DNA effectively. The efficiency of various types of swabs never exceeds a value of 50% [45]. Urine samples are considered not good because they only contain a few nucleated cells and are not ideal for being used as a source of DNA [46]. It has been reported that hair has a low concentration of noncoding DNA and/or a high level of degradation [47]. Blood sample is better from the other biological samples because it contains nucleated white blood cells and there are many protocols regarding DNA isolation from blood that have been published [48]. Therefore, we conclude that it is best to use blood samples to carry out a DNA extraction process.
Blood is the body's tissue that functions in the body's circulation system. This suspension tissue is circulated through the heart and blood vessels to carry various substances, such as nutrients, oxygen, carbon dioxide, drugs, metabolites, protein, and others. Of various biological samples, blood got the most important attention in biological research [48]. However, blood is the most complex biological sample because many components in it can interfere with the process of bioanalysis. Handling of blood samples also needs to be careful because too much water can cause blood cells to undergo hemolysis. Besides, blood samples are also not good to be applied to children if large volume samples are needed [49].
DNA extraction from the blood can be done quickly and easily using the available kit. The authors took the genomic DNA extraction procedure from the QIAamp DNA Mini and Blood Mini Handbook by QIAGEN (2016) [50]. Genomic DNA was extracted using the QIAamp DNA Mini Kit. Before the extraction process begins, make sure the water bath has been heated to 56 °C and the buffer solution has been prepared. Also, we have to make sure that all centrifugation steps are carried out at room temperature (15–25 °C) so that the centrifugation process can produce an accurate and precise results. After that, genomic DNA extraction can begin. A total of 20 μl QIAGEN Protease is mixed with 200 μl blood samples in a microcentrifuge tube 1.5 ml. Then, 200 μl of AL is added and mixed by pulse-vortexing for 15 seconds, and incubated for 10 minutes at 56 °C. Microcentrifuge tubes are centrifuged to release droplets from the lid. Then, 200 μl of ethanol (96–100%) is added to the sample and mixed by pulse-vortexing for 15 seconds. Microcentrifuge tubes are centrifuged to release droplets from inside the lid. The mixture is put into a QIAamp Mini rotary column, closed, and centrifuged at 6,000 x g for 1 minute. The QIAamp Mini rotary column is placed in a 2 ml collection tube and the tube containing the filtrate is removed. The column is opened and 500 μl added to the AW1 buffer, centrifugation is carried out at 6,000 x g for 1 minute. The QIAamp Mini rotary column is placed in a 2 ml collection tube and the tube containing the filtrate is removed. The column is opened and 500 μl of AW2 buffer added to the solution, centrifugation is carried out at a speed of 20,000 x g for 3 minutes. The column is placed in a new 2 ml collection tube, the old tube removed for another centrifugation for 1 minute. The QIAamp Mini rotary column is placed on a microcentrifuge tube clean 1.5 ml and the tube containing the filtrate is removed. The column is opened and added 200 μl of AE or distilled water. Finally, the solution was incubated at 15–25 °C for 1 minute, then centrifuged for 1 minute at 6,000 x g. The filtrate was refrigerated at -30 °C to -15 °C. After that, the PCR can be performed.
PCR is carried out on a mixture of DNA extract, Taq polymerase enzyme, primer, and four deoxyribonucleotide triphosphates in buffer solution [10]. The addition of magnesium chloride compounds is also needed as a cofactor to stimulate the DNA polymerase reaction. Various PCR methods have been used to analyze CYP2B6 polymorphisms. However, the authors chose the PCR method from Nakajima et al. (2007) because it has been tested to produce valid analysis results for various genotypes of the CYP2B6 gene polymorphism, whereas other studies only analyze the polymorphisms produced by mutations in specific exons [51].
Genotyping CYP2B6 was focused on exons 4 and 5. The selected primers were 2B6∗9S and 2B6∗9-AS for c.516G > T and primers 2B6∗4-S and 2B6∗4-AS for c.785A > G. The PCR procedure was carried out on 25 μl samples containing genomic DNA (100 ng), 1 x PCR buffer [Tris-HCl buffer 67 mmol/l (pH 8.8), (NH4)2SO4 16.6 mmol/l, Triton X-100 0.45%, gelatin 0.02%], MgCl2 1.0–3.0 mmol/l, primers 0.4 mmol/l, dNTPs 250 mmol/l, and Taq DNA polymerase 1 U. Denaturation is done at 94 °C for 3 minutes. Then, the amplification is done by denaturation at 94 °C for 30 seconds, annealing for 30 seconds, and extension/elongation at 72 °C for 30–90 seconds for 30 cycles. The final extension was carried out at 72 °C for 5 minutes. [51].
4.6. Relationship between the concentration of 3-HPMA, hematuria, and CYP2B6 polymorphisms
Data about the concentration of 3-HPMA is important to be known because cyclophosphamide is one of the drugs commonly given in chemotherapy. Not only at high doses, but cyclophosphamide can also cause hemorrhagic cystitis at low doses. There is a reported case of hemorrhagic cystitis in 24 hours after the first cycle of therapy regimen of cyclophosphamide and docetaxel at a dose of 600 mg/m2 and 75 mg/m2 on a 63 years old woman with stage IIA breast cancer [12].
The toxicity of acrolein against the bladder can be prevented. MESNA drugs, or 2-mercaptoethanesulfonate, and strong hydration to increase urination are considered effective in preventing CPA-induced hemorrhagic cystitis [52]. Usually, MESNA is given before the administration of cyclophosphamide. MESNA's sulfhydryl group can bind to the vinyl acrolein group and make it an inert product [24]. However, MESNA also has side effects, such as high-frequency nausea and vomiting [53]. Besides, despite its high effectiveness, forced use of MESNA and hydration cannot prevent hemorrhagic cystitis and hematuria in patients receiving high doses of cyclophosphamide [54].
Research from Harahap et al. (2020) helped to prove that fact. The study from Harahap et al. (2020) showed that the levels of 3-HPMA in the urine of breast cancer patients receiving cyclophosphamide treatment regimens were highly varied, ranging from 650–5,596 ng/mg creatinine with an average level of 1,383 ng/mg creatinine. The %CV obtained is also quite large, which is 48.71%. In addition, from 40 subjects who received cyclophosphamide therapy regimens and had been given MESNA before, 7 subjects were tested positive for hematuria with levels of 3-HPMA in urine as follows: 4,924; 4,464; 5,128; 4,891; 4,818; 4,200; 5,448 ng/mg creatinine. All subjects who were positive for hematuria had high levels of 3-HPMA, which is above 4,000 ng/mg creatinine. However, 4 subjects had 3-HPMA levels above 4,000 ng/mg creatinine which were not positive for hematuria [11]. This is probably due to other factors that can be confounding in this study, such as differences in patient hydration, MESNA efficacy, patient physiology, food consumed, and CYP2B6 polymorphism.
Approximately, 75–80% of the CPA dose will be metabolized to 4-hydroxycyclophosphamide (4-OH-CPA) after being administered into the body [51]. The CPA hydroxylation process is catalyzed by the liver CYP450 enzyme [55]. Then, 4-OH-CPA and its tautomer, aldophosphamide, will be metabolized back to its metabolites, one of which is acrolein. However, the liver CYP450 enzyme is polymorphic, especially CYP2B6. Also, it has been reported that CYP2B6 plays a role in the metabolism of 2–10% of anticancer drugs that are often used, such as CPA [56]. CYP2B6 expression in each individual is very varied, depending on non-genetic factors, genetic polymorphism, ability to be induced, and inhibition by various compounds [57]. The polymorphic nature of the CYP gene can affect an individual's response to a drug and its side effects.
Various allele variants are formed by the CYP2B6 gene polymorphism. This polymorphism occurs because of mutations in the nitrogen base which can produce amino acid changes. Lang et al. (2001) identified 9 SNPs that produced 5 amino acid substitutions, which are CYP2B6∗2 (R22C), CYP2B6∗3 (S259R), CYP2B6∗4 (K262R), CYP2B6∗5 (R487C), CYP2B6∗6 (Q172H and K262R), and CYP2B6∗7 (Q172H, K262, R762) [58]. Then, Lamba et al. (2003) reported the presence of variants of CYP2B6∗8 (K139E) and CYP2B6∗9 (Q172H) [59]. Furthermore, Lang et al. (2004) reported back alleles variants of CYP2B6∗10 (Q21L and R22C), CYP2B6∗11 (M46V), CYP2B6∗12 (G99E), CYP2B6∗13 (K139E, Q172H, and K262R), CYP2B6∗14 (R140Q), and CYP2B6∗15 (I391N) [22].
Among all of the polymorphism variants, the CYP2B6∗6 (Q172H & K262R) allele has been the most common allele, happening in 15–60% of different populations [57]. This polymorph form cause changes in the work of CYP2B6 to metabolize some drugs, one of which is CPA. It has been reported that CPA clearance in the subject who has a homozygous CYP2B6∗6 is higher than the subject with homozygous CYP2B6∗1 or heterozygous CYP2B6∗1/∗6 significantly [61]. CYP2B6∗6 allele can increase CPA hydroxylation [60, 61, 62]. Increased CPA hydroxylation can increase level of 4-OH-CPA while increasing levels of acrolein and 3-HPMA at a later stage. The increasing level of acrolein can lead to the toxicity of CPA, which is hematuria.
According to the literature review from 20 studies, we found a relationship between 3-HPMA concentration, hematuria, and CYP2B6 polymorphisms. CYP2B6 polymorphism, in a form of the CYP2B6∗6 allele, could increase the 3-HPMA levels in urine. The concentration of 3-HPMA is directly proportional to the level of acrolein. A high level of acrolein can increase the risk of hematuria occurrences. Therefore, CYP2B6 polymorphisms should be considered as an important variable to see the elevation of the 3-HPMA concentration and the risk of hematuria occurrences after cyclophosphamide administration. The scheme of our conclusion regarding the relation between 3-HPMA concentration, hematuria, and CYP2B6 polymorphisms is shown in Figure 4.
Figure 4.
The scheme of the correlation between 3-HPMA level, polymorphism of CYP2B6 enzyme, and the incidence of hematuria after cyclophosphamide administration.
4.7. Ideas
Based on the literature review and analysis written from 62 eligible sources, the idea proposed by the authors are the need for research that combines the three things that are the focus of this review article, which are levels of 3-HPMA, CYP2B6 polymorphism, and the incidence of hematuria after the administration of cyclophosphamide. Research can be done by combining the two methods mentioned above, namely the bioanalytical method for the analysis of 3-HPMA levels in urine samples and PCR to determine the CYP2B6 polymorphism from blood samples. However, the chosen subjects must meet the inclusion criteria because high levels of 3-HPMA and hematuria can be caused by other things, such as smoking and macronutrient metabolism. The inclusion criteria proposed by the authors are cancer patients aged 18–70 years, getting cyclophosphamide in their chemotherapy regimen, not smoking, not be suffering from other serious illnesses other than cancer, not having menstruation, and are willing to follow the research by signing informed consent. Other than that, CYP2B6 polymorphism research can take a very long time due to its many polymorphic forms. To overcome this, the authors have the idea to focus more on exons 4 and 5 first because it has been mentioned in the literature that amino acid substitution in the 2 exons can increase the hydroxylation of CPA [60, 61, 62]. The results of this proposed study can be applied to improve the effectiveness of therapy in patients receiving cyclophosphamide in their treatment regimens.
Also, the authors see that each branch of pharmaceutical science has made rapid progress. Progress in these fields needs to be put to good use. Therefore, the authors have the idea that various branches of health science should be integrated into the handling of patients, especially patients with severe diseases like cancer. The integration of biotechnology, clinical pharmacy, and bioanalysis is very useful for increasing the effectiveness of the use of cancer drugs. Biotechnology is used to discuss the genetic condition of the patient, clinical pharmacy is applied to discuss the physiological state of the patient, and bioanalysis is applied to qualify and quantify the metabolites or biomarkers in the patient's body. This idea is proposed to improve the assurance of drug compatibility with patients so that the side effects that could occur can be avoided. This effort is expected to increase the efficacy and efficiency of each patient's therapy.
5. Conclusion
The authors conclude that according to a literature review from 3 compared studies and 7 supporting literature, the most optimal bioanalytical method of 3-HPMA levels analysis in urine after cyclophosphamide administration using LC-MS/MS is using triple quadrupole LC-MS/MS; source of positive ion ESI; mobile phase combination of 0.1% formic acid in water (A) - 0.1% formic acid in acetonitrile (90:10 v/v) (B); the Acquity® BEH C18 column (2.1 × 100 mm; 1.7 μm); injection volume of 10 μl; flow rate of 0.2 ml/minute; gradient elution method. Detection should be carried out using mass spectrometry with the m/z ratio of 222.10 > 90 for 3-HPMA and m/z 164.10 > 122 for NAC. The optimal sample preparation method is acidification and a dilution ratio of 1:5 v/v. Also, according to a literature review from 20 studies, we found that there was a relationship between 3-HPMA levels, CYP2B6 polymorphisms, and the occurrences of hematuria after the administration of cyclophosphamide, which is a type of CYP2B6 polymorph, namely CYP2B6∗6, can increase cyclophosphamide hydroxylation so that it can increase levels of acrolein and 3-HPMA, as its metabolites, and the risk of hematuria. There's no bias in this study, but the 3-HPMA levels and hematuria cases may be varied in different subjects with different additional treatments. Also, coronavirus disease pandemic made author could not get a primary data from actual subjects. Preferably, research on the relationship between 3-HPMA levels, CYP2B6 polymorphisms, and hematuria occurrences in breast cancer patients after cyclophosphamide administration must be conducted in a large number of subjects (>100 subjects) that met the inclusion criteria in the future to prove the theory that has been explained.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the National Research and Innovation Agency, Republic of Indonesia.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to say thank you to Universitas Indonesia and Dharmais Cancer Hospital for giving us a lot of information and support needed.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer STATISTICS 2020: GLOBOCAN estimates of incidence and MORTALITY worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . The Global Cancer Observatory; 2021. 360 Indonesia Fact Sheets.https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf [Google Scholar]
- 3.Veal G.J., Cole M., Chinnaswamy G., Sludden J., Jamieson D., Errington J., Malik G., Hill C.R., Chamberlain T., Boddy A.V. Cyclophosphamide pharmacokinetics and pharmacogenetics in children with B-cell non-Hodgkin's lymphoma. Eur. J. Cancer. 2016;55:56–64. doi: 10.1016/j.ejca.2015.12.007. (Oxford, England: 1990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration . U.S Department of Health and Human Services; 2013. Prescribing Information for Cyclophosphamide. [Google Scholar]
- 5.Ogino M.H., Tadi P. StatPearls; 2021. Cyclophosphamide.https://www.ncbi.nlm.nih.gov/books/NBK553087/ [PubMed] [Google Scholar]
- 6.Tanaka T., Nakashima Y., Sasaki H., Masaki M., Mogi A., Tamura K., Takamatsu Y. Severe hemorrhagic cystitis caused by cyclophosphamide and capecitabine therapy in breast cancer patients: two case reports and literature review. Case Rep. Oncol. 2019;12:69–75. doi: 10.1159/000496331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding Y., Li Q., Feng Q., Xu D., Wu C., Zhao J., Zhou X., Yang Y., Niu H., He P., Xing L. CYP2B6 genetic polymorphisms influence chronic obstructive pulmonary disease susceptibility in the Hainan population. Int. J. Chronic Obstr. Pulm. Dis. 2019;14:2103–2115. doi: 10.2147/COPD.S214961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens J.F., Maier C.S. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi K., Igarashi K., Toida T. Recent progress in analytical methods for determination of urinary 3-hydroxypropylmercapturic acid, a major metabolite of acrolein. Biol. Pharm. Bull. 2016;39(6):915–919. doi: 10.1248/bpb.b15-01022. [DOI] [PubMed] [Google Scholar]
- 10.Kadri Karim. IntechOpen; 2020. Synthetic Biology - New Interdisciplinary Science. [eBook edition] [Google Scholar]
- 11.Harahap Y., Yanuar A., Muhammad C., Melhan M., Purwanto D. Quantification of 3-hydroxypropyl mercapturic acid in the urine of patients with breast cancer to monitor cyclophosphamide toxicity. Ther. Drug Monit. 2020;42(4):548–553. doi: 10.1097/FTD.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P.A., Paulraj S., Sivapiragasam A. Hemorrhagic cystitis with low-dose cyclophosphamide therapy for breast cancer: a rare occurrence. J. Med. Cases. 2019;10(6):179–182. [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-wilson E., McDonald S., McGuinness L., Stewart L.A., Thomas J.…Moher J. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J., Reitsma J.B., Leeflang M.M., Sterne J.A., Bossuyt P.M., QUADAS-2 Group Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 15.McGuinness L.A. 2019. Robvis: an R Package and Web Application for Visualising Risk-Of-Bias Assessments.https://github.com/mcguinlu/robvis [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute . National Cancer Institute Website; 2017. The Genetics of Cancer.https://www.cancer.gov/about-cancer/causes-prevention/genetics [Google Scholar]
- 17.American Cancer Society . American Cancer Society Website; 2019. How Chemotherapy Drugs Work.https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html [Google Scholar]
- 18.Brunton L.L., Chabner B., Knollmann B.C., editors. Goodman & Gilman's Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2011. [Google Scholar]
- 19.Singh R., Kumar S., Prasad D.N., Bhardwaj T.R. Therapeutic journery of nitrogen mustard as alkylating anticancer agents: historic to future perspectives. Eur. J. Med. Chem. 2018;151:401–433. doi: 10.1016/j.ejmech.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Hananta L., Astuti I., Sadewa A.H., Alice J., Hutagalung J., Mustofa The prevalence of CYP2B6 gene polymorphisms in malaria-endemic population of timor in East Nusa Tenggara Indonesia. Osong Publ. Health Res. Perspect. 2018;9(4):192–196. doi: 10.24171/j.phrp.2018.9.4.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tecza K., Pamula-Pilat J., Lanuszewska J., Butkiewicz D., Grzybowska E. Pharmacogenetics of toxicity of 5-fluorouracil, doxorubicin and cyclophosphamide chemotherapy in breast cancer patients. Oncotarget. 2018;9:9114–9136. doi: 10.18632/oncotarget.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang T., Klein K., Richter T., Zibat A., Kerb R., Eichelbaum M., Schwab M., Zanger U.M. Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J. Pharmacol. Exp. Therapeut. 2004;311:34–43. doi: 10.1124/jpet.104.068973. [DOI] [PubMed] [Google Scholar]
- 23.Aizenbud D., Aizenbud I., Reznick A.Z., Avezov K. Acrolein—an α,ß-unsaturated aldehyde: a review of oral cavity exposure and oral pathology effects. Rambam Maimonides Med. J. 2016;7(3) doi: 10.5041/RMMJ.10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldar S., Dru C., Bhowmick N.A. Mechanisms of hemorrhagic cystitis. Am. J. Clin. Expe. Urology. 2014;2(3):199–208. [PMC free article] [PubMed] [Google Scholar]
- 25.Harmita K., Harahap Y., Supandi . ISFI Penerbitan; 2019. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). DKI Jakarta: PT. [Google Scholar]
- 26.Beccaria M., Cabooter D. Analyst. RSC Publishing; Cambridge: 2020. Current developments in LC-MS for pharmaceutical analysis. [DOI] [PubMed] [Google Scholar]
- 27.Samatha Y., Srividiya A., Ajitha A., Rao V.U.M. Ultra performance liquid chromatography (UPLC) World J. Pharm. Pharmaceut. Sci. 2015;4(8):356–365. [Google Scholar]
- 28.Shaikh T., Hussain S. Ultra high performance liquid chromatography (UPLC): a new Trend in analysis. World J. Pharmaceut. Res. 2016;5(3):387–394. [Google Scholar]
- 29.Urban P.L. Quantitative mass spectrometry: an overview. Phil. Transact. Ser A, Math. Phy. Eng. Sci. 2016;374(2079):20150382. doi: 10.1098/rsta.2015.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoog D., Holler J., Crouch S. sixth ed. Thomson Brooks/Cole; Belmont: 2007. Principles of Instrumental Analysis. [Google Scholar]
- 31.Medhe Sharad. Mass spectrometry: detectors review. Ann. Rev. Chem. Biomol. Eng. 2018;3:51–58. [Google Scholar]
- 32.Moein M.M., El-Beqqali A., Abdel-Rehim M. Bioanalytical method development and validation: critical concepts and strategies. J. Chromatogr. B. 2017;1043:3–11. doi: 10.1016/j.jchromb.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Food and Drug Administration . U.S. Department of Health and Human Services; 2018. Guidance for Industry: Bioanalytical Method Validation. [Google Scholar]
- 34.European Medicines Agency . Committee for Medicinal Products for Human Use (CHMP); London: 2011. Guideline on Bioanalytical Method Validation. [Google Scholar]
- 35.Zhao M., Li M., Yang Y., Guo Z., Sun Y., Shao C., Li M., Sun W., Gao Y. A comprehensive analysis and annotation of human normal urinary proteome. Sci. Rep. 2017;7(1):3024. doi: 10.1038/s41598-017-03226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drouin N., Rudaz S., Schappler J. American Pharmaceutical Review; 2016. New Trends in Sample Preparation for Bioanalysis.https://www.americanpharmaceuticalreview.com/Featured-Articles/182917-New-Trends-in-Sample-Preparation-for-Bioanalysis/ [Google Scholar]
- 37.Xu Q.A., Madden T.L., editors. LC-MS in Drug Bioanalysis. Springer Publishing; New York: 2012. [Google Scholar]
- 38.Novák P., Havlíček V. Proteomic Profiling and Analytical Chemistry. 2016. Protein extraction and precipitation; pp. 51–62. [Google Scholar]
- 39.Tully M., Tang J., Zheng L., Acosta G., Tian R., Hayward L., Race N., Mattson D., Shi R. Systemic acrolein elevations in mice with experimental autoimmune Encephalomyelitis and patients with multiple sclerosis. Front. Neurol. 2018;9:420. doi: 10.3389/fneur.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harahap Y., Yanuar A., Muhammad C., Melhan M., Purwanto D., Joko . Therap. Drug Monit. Oncol.; 2020. Quantification of 3-Hydroxypropyl Mercapturic Acid in the Urine of Patients with Breast Cancer to Monitor Cyclophosphamide Toxicity; pp. 548–553. [DOI] [PubMed] [Google Scholar]
- 41.Carmella S.G., Chen M., Zhang Y., Zhang S., Hatsukami D.K., Hecht S.S. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid Chromatography−Atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem. Res. Toxicol. 2007;20(7):986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan W., Byrd G., Brown B., Borgerding M. Development and validation of a Direct LC-MS-MS method to determine the acrolein metabolite 3-HPMA in urine. J. Chromatogr. Sci. 2010;48:194–199. doi: 10.1093/chromsci/48.3.194. [DOI] [PubMed] [Google Scholar]
- 43.Gao G., Pietrak M.R., Burr G.S., Rexroad C.E., III, Peterson B.C., Palti Y. A new single nucleotide polymorphism database for North American Atlantic salmon generated through whole genome resequencing. Front. Genet. 2020;11:85. doi: 10.3389/fgene.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahern K., Rajagopal I., Tan T. 2019. Biochemistry Free for All. Oregon: LibreTexts. [Google Scholar]
- 45.Bruijns B.B., Tiggelaar R.M., Gardeniers H. The extraction and recovery efficiency of pure DNA for different types of swabs. J. Forensic Sci. 2018;63(5):1492–1499. doi: 10.1111/1556-4029.13837. [DOI] [PubMed] [Google Scholar]
- 46.Ng H.H., Ang H.C., Hoe S.Y., Lim M.-L., Tai H.E., Soh R.C.H., Syn C.K.–C. Simple DNA extraction of urine samples: effects of storage temperature and storage time. Forensic Sci. Int. 2018;287:36–39. doi: 10.1016/j.forsciint.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 47.Martins C., Lima G., Carvalho M.R., Cainé L., Porto M.J. DNA quantification by real-time PCR in different forensic samples. Forensic Sci. Int.: Genetics Suppl. Ser. 2015;5 [Google Scholar]
- 48.Guha P., Das A., Dutta S., Chaudhuri T.K. A rapid and efficient DNA extraction protocol from fresh and frozen human blood samples. J. Clin. Lab. Anal. 2017;32(1) doi: 10.1002/jcla.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaewkhao K., Chotivanich K., Winterberg M., Day N.P., Tarning J., Blessborn D. High sensitivity methods to quantify chloroquine and its metabolite in human blood samples using LC-MS/MS. Bioanalysis. 2019;11(5):333–347. doi: 10.4155/bio-2018-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.QIAGEN . Forest Stewardship Council; Hilden: 2016. QIAamp DNA Mini and Blood Mini Handbook. [Google Scholar]
- 51.Nakajima M., Komagata S., Fujiki Y., Kanada Y., Ebi H., Itoh K., Mukai H., Yokoi T., Minami H. Genetic polymorphisms of CYP2B6 affect the pharmacokinetics/pharmacodynamics of cyclophosphamide in Japanese cancer patients. Pharmacogenetics Genom. 2007;17(6):431–445. doi: 10.1097/FPC.0b013e328045c4fb. [DOI] [PubMed] [Google Scholar]
- 52.Saito Y., Kumamoto T., Shiraiwa M., Sonoda T., Arakawa A., Hashimoto H., Tamai I., Ogawa C., Terakado H. Cyclophosphamide-induced hemorrhagic cystitis in young patients with solid tumors: a single institution study. Asia Pac. J. Clin. Oncol. 2018;14(5):e460–e464. doi: 10.1111/ajco.13048. [DOI] [PubMed] [Google Scholar]
- 53.Teles K.A., Medeiros-Souza P., Lima F.A., Araújo B.G., Lima R.A. Cyclophosphamide administration routine in autoimmune rheumatic diseases: a review. Rev. Bras. Reumatol. 2017;57(6):596–604. doi: 10.1016/j.rbre.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Doshi B.R., Sajjan V.V., Manjunathswamy B.S. Managing a side effect: cyclophosphamide-induced hemorrhagic cystitis. Indian J. Drugs Dermatol. 2019;5:66–71. [Google Scholar]
- 55.Shu W., Guan S., Yang X., Liang L., Li J., Chen Z., Zhang Y., Chen L., Wang X., Huang M. Genetic markers in CYP2C19 and CYP2B6 for prediction of cyclophosphamide's 4-hydroxylation, efficacy and side effects in Chinese patients with systemic lupus erythematosus. Br. J. Clin. Pharmacol. 2016;81(2):327–340. doi: 10.1111/bcp.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hedrich W.D., Hassan H.E., Wang H. Insights into CYP2B6-mediated drug–drug interactions. Acta Pharm. Sin. B. 2016;6(5):413–425. doi: 10.1016/j.apsb.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zanger U.M., Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013;4:24. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang T., Klein K., Fischer J., Nussler A.K., Neuhaus P., Hofmann U., Eichelbaum M., Schwab M., Zanger U.M. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Lamba V., Lamba J., Yasuda K., Strom S., Davila J., Hancock M.L., Fackenthal J.D., Rogan P.K., Ring B., Wrighton S.A., Schuetz E.G. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J. Pharmacol. Exp. Therapeut. 2003;307(3):906–922. doi: 10.1124/jpet.103.054866. [DOI] [PubMed] [Google Scholar]
- 60.Xie H.-J., Yasar U., Lundgren S., Griskevicius L., Terelius Y., Hassan M., Rane A. Role of polymorphic human CYP2B6 in cyclophosphamide bioactivation. Pharmacogenomics J. 2003;3:53–61. doi: 10.1038/sj.tpj.6500157. [DOI] [PubMed] [Google Scholar]
- 61.Xie H., Griskevicius L., Ståhle L., Hassan Z., Yasar U., Rane A., Broberg U., Kimby E., Hassan M. Pharmacogenetics of cyclophosphamide in patients with hematological malignancies. Eur. J. Pharmaceut. Sci. 2006;27:54–61. doi: 10.1016/j.ejps.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Kirchheiner J., Klein C., Meineke I., Sasse J., Zanger U.M., Mürdter T.E., Roots I., Brockmöller J. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.