Abstract
Aims
This study aimed to explore the potential protective effect of α-lipoic acid on busulfan-induced pulmonary fibrosis in rats.
Main methods
Eighteen adult male rats were divided into 3 groups; control, busulfan, and busulfan plus α-lipoic acid groups. Lung index ratio, serum level of proinflammatory cytokine were assessed. The activities of antioxidant enzymes and lipid peroxidation products were estimated in the lung tissues in addition to the histopathological analyses. The deposition of the collagen in the lung tissues was evaluated by Sirius red staining. The expressions of α-smooth muscle actin (α-SMA), TNF-α, and Caspase 3 were determined immunohistochemically. The pulmonary expression of COX-2 and NOX-4 mRNA was assessed using qRT-PCR.
Key findings
Administration of ALA significantly protect the lung against BUS-induced pulmonary fibrosis, besides the upregulation of antioxidants, and downregulation of pro-inflammatory cytokines. Also, it reduced collagen deposition that associated with a decreased expression of α-SMA, TNF-α, and Caspase 3 in the lung tissues. Moreover, ALA significantly upregulated the expression of COX-2 concomitant with the downregulation of elevated NOX-4.
Significance
ALA attenuates the lung cytotoxicity of busulfan through its anti-inflammatory, anti-apoptotic, and antifibrotic effects that may be mediated by upregulation of COX-2 and downregulation of NOX-4.
Keywords: Busulfan, Pulmonary fibrosis, α-lipoic acid (ALA), Apoptosis, COX-2, NOX-4
Busulfan; Pulmonary fibrosis; α-lipoic acid (ALA); Apoptosis; COX-2; NOX-4.
1. Introduction
Pulmonary fibrosis is the commonest worldwide interstitial lung disease affecting more than five million individuals with an average survival time of about three years. Apart from inhaling mineral dust/asbestos and cigarette smoking, pulmonary fibrosis can be developed in response to the toxic effect of anti-neoplastic drugs such as busulfan [1].
Busulfan (BUS) is an alkylating agent used for the treatment of neoplastic and autoimmune diseases [2]. It works by sticking to one of the cancer cell's DNA strands and inhibits cell division [3, 4]. Busulfan causes undesirable effects on the different body organs, it is associated with pulmonary toxicity that includes acute lung injury, chronic interstitial fibrosis, and alveolar hemorrhage [3, 4]. Yet, the exact mechanism of busulfan-induced lung injury still unknown. According to Matijasic et al. [5] the purpose was that increased alveolar wall permeability results in fibrin leakage, motivating alveolar lining cells to enlarge and undergo bizarre changes. In addition, it was hypothesized that these changes in type II alveolar cells cause aberrant surfactant secretion, all leading to increased surface tension and suction pressure, again promoting pulmonary edema.21 Moreover, patients with busulfan lung frequently displays both lymphocytosis and neutrophilia results from a delayed hypersensitivity reaction in which numerous chemokines and proinflammatory cytokines mediate a sustained CD8 cytotoxic T cell response, resulting in tissue damage.
Alpha-lipoic acid (ALA, thioctic acid) is an organosulfur component present in plants, animals, and humans [6, 7]. Naturally, ALA is located in the mitochondria, where it acts as a cofactor for some enzymatic complexes involved in the Krebs cycle. Also, ALA acts as an antioxidant and can increases and repairs the intrinsic antioxidant systems and supports their production [8, 9, 10]. A healthy body makes enough ALA to supply its energy requirements; therefore, there is no daily requirement for this supplement. However, several medical conditions appear to be accompanied by low levels of ALA specifically, diabetes, liver cirrhosis, and heart disease which suggests that supplementation would be helpful [11].
The exact mode of action of ALA has not been fully elucidated. This research study is designed to examine the potential effect of ALA on a rat model of pulmonary fibrosis induced by intraperitoneal (i.p.) administration of busulfan and to evaluate its antioxidant, anti-inflammatory, antiapoptotic and antifibrotic effects. Moreover, the possible intracellular pathway of ALA was also examined concerning COX-2 activation and NOX-4 suppression.
2. Methods
2.1. Experimental design
A total of 18 adult albino male rats (200–240 g weight) were obtained from Medical Experimental Research Center (MERC)- Mansoura Faculty of Medicine- Egypt. Rats were acclimatized for one week before the initiation of the experiment, the room temperature was adjusted to 24 ± 5 and 45–55% humidity and a regular 12:12 h light-dark cycle with free access of the rats to water and food ad libitum. The protocol of this experimental study was carried out following the U.K. Animals (Scientific Procedures) Act, 1986, and associated guidelines. It was approved by Our Institutional Research Board - IRB (Code number: R.20.08.993).
After acclimatization, the animals were randomly divided into 3 groups, each contains 6 rats: the normal control group, received phosphate buffer solution (PBS) intraperitoneally, BUS induced lung injury group, the rats were received two doses of BUS by (i.p.) injection with 14 days interval. Each dose was 15 mg/kg dissolved in 0.5 mL PBS [12], BUS + ALA group, α-lipoic acid was given on top of busulfan (20 mg/kg/day for 6 weeks). Both BUS and ALA were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
After six weeks from the beginning of the experiment, all rats were weighed then deeply anesthetized with thiopental sodium. Blood samples were obtained from the heart directly, and stored at -20 °C for subsequent biochemical assessments. Animals were weighed before sacrifice then the lung was removed and weighed to determine the lung index which was calculated by dividing lung weight (g)/body weight (g) then multiplying by 100. One of the lung lobes was preserved in formaldehyde solution for histopathological and immunohistochemical examinations. The other one was weighed and divided into two halves, one half was stored at −20 °C for detection of enzyme activities and level of PGE2 while the other half was preserved in RNAlater Stabilization Solution (ThermoFisher Scientific) at 4 °C overnight then it was stored at −80 °C until total RNA extraction.
2.2. Measurement of TNF-α, IL-1β, IL-6, and IL-10 serum levels
Serum levels of TNF-α, IL-1β, IL-6, and IL-10 were determined using enzyme-linked immunosorbent assay kits (Bioscience Co., USA) in accordance with the manufacturer's instructions. The absorbance was read at 450nm using a plate reader.
2.3. Activities of enzymatic antioxidants
Superoxide dismutase (SOD) activity was assessed in homogenized lung tissues standard enzymatic kit (Cayman Chemical Company, USA). The absorbance was read at 440–460 nm. Glutathione peroxidase (GPx) activity was measured in lung tissues by using an available kit (Cayman Chemical, USA) and the absorbance was read once every minute at 340 nm.
2.4. Detection of lipid peroxidation product
Lipid peroxidation was determined in the form of malondialdehyde (MDA) according to Ohkawa et al. [13]. MDA was assessed in the rats' lung tissues with ELISA kits (R&D system), in accordance with the manufacturer's protocols. The absorbance was measured at 532 nm.
2.5. Measurement of PGE2 levels in lung tissues
The levels of PGE2 were measured in the lung tissues by a competitive enzyme immunoassay kit (Abcam, ab133021, Cambridge, UK) following the manufacturer's protocol. The absorbance of PEG2 was measured at 405 nm with a microplate reader.
2.6. Measurement of hydroxyproline in the lung tissues
Hydroxyproline content of the lung tissue assay was performed using an ELISA kit (Hangzhou Eastbiopharm Co., Ltd., China). The absorbance was read at 450nm using a plate reader.
2.7. Histopathological techniques
The lung was dissected and fixed in 10% (neutral) formalin, cleansed through running water, dehydrated gradually by increasing concentrations of alcohol, cleared by xylene, and then embedded in paraffin blocks. Sections were cut at 3–5 um. and stained with hematoxylin-eosin (H&E) as described by Bancroft and Christopher [14]. Lung sections were examined microscopically and photomicrographed.
2.7.1. Immunohistochemical staining
Immunohistochemistry was done on 3–4μm thick sections which were mounted on charged slides and then analysis was done through the immunoperoxidase method. Concisely, deparaffinization of the sections was performed, then they were treated with H2O2 (0.3 %)/methanol for 10 min at room temperature to block endogenous peroxidase activity. Antigen retrieval was achieved by heating sections in 10 mM citrate buffer for 10 min at 95–100C (PH 6) then allowed to cool at room temperature for 1 h. Sections were left with the primary antibodies for Caspase3 as a marker for apoptotic activity, TNF-α as a marker for inflammation and macrophage activity, and Alpha smooth muscle actin (α-SMA) as a fibrogenic marker (Servicebio; Cat. No. GB11532 (rabbit polyclonal antibody), Santa Cruz; sc-52746 (mouse monoclonal antibody) and Thermoscientific; MS-113-RQ (mouse monoclonal antibody), respectively) overnight at 4 °C (1:800, 1:50 and 1:800 dilutions, respectively). Then, sections were incubated in Diaminobenzidine (DAB) reagent to demonstrate peroxidase activity. The sections were left in PBS at 4 °C overnight. Incubation of the slides with universal mouse/rabbit polydetector plus (BSB 0257, Bio SB) for half an hour was done then washed by PBS to identify the primary antibody binding. Finally, DAB was added for 4 min then the sections were counterstained with hematoxylin. For the negative control, we replaced the primary antibody with PBS. The sections were then washed, dehydrated, and examined by a light microscope. Dark brown areas in the cytoplasm or nucleus demonstrate positive staining and the background is blue [15].
2.8. Quantification of PCR products
Lung tissue samples were homogenized by five strokes of liquid nitrogen. Total cellular RNA was isolated using the QIAzol reagent (Qiagen, Germany), in accordance with the manufacturer's protocols. The 260/280 and 260/230 ratios of absorbcance values were measured to assess the purity and concentration of RNA samples using Thermo Scientific NanoDrop 2000 (USA). For gene expression detection, 1 ug of RNA samples were reverse transcribed using SensiFAST™ cDNA Synthesis Kit (Bioline, UK). The cDNA templates were amplified using a real-time PCR machine (Pikoreal 96, ThermoScientific). Quantitative real-time PCR (qRT-PCR) was done using SYBR green PCR Master Mix (Bioline, UK) in a total volume of 20 μl using a real-time PCR instrument (Applied Biosystems 7500). Relative gene expression levels was represented as ΔCt = Ct target gene – Ct control gene; fold change of gene expression was calculated according to the 2−ΔΔCT method. Primers for NOX-4 and COX-2 are listed in Table 1.
Table 1.
The sequence of rat primers used in the qRT-PCR analysis.
| Gene | Sequence | Product size |
|---|---|---|
| Cyclo-oxygenase 2 (COX-2) |
Forward primer: GGAGCAACCGATGTGGAATTG Reverse primer: GCCGGTATCTGCCTTCATGT |
104 bp |
| NADPH oxidase 4 (NOX-4) |
Forward primer: TGTTGGGCCTAGGATTGTGT Reverse primer: CTTCTGTGATCCGCGAAGGT |
119 bp |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) |
Forward primer: TGCCACTCAGAAGACTGTGG Reverse primer: GGATGCAGGGATGATGTTCT |
85 bp |
3. Theory/calculation
This study aims to examine the potential protective effect of ALA on a rat model of pulmonary fibrosis induced by intraperitoneal (i.p.) administration of busulfan and to evaluate its antioxidant, anti-inflammatory, anti-apoptotic and antifibrotic effects. Moreover, the possible intracellular pathway of ALA was also examined concerning COX-2 activation and NOX-4 suppression.
Data were analyzed using the SPSS program (version 21). First, the normality of data was tested using the Shapiro test. Descriptive data (means ± SDM) were calculated for each dependent variable. Differences between groups were analyzed using one-way ANOVA followed by a post-hoc Tuckey's HSD test. P ≤ 0.05 was used as the decision rule to test the significance.
4. Results
4.1. Effect of ALA on the body weight and lung indices in BUS-induced lung fibrosis rat model
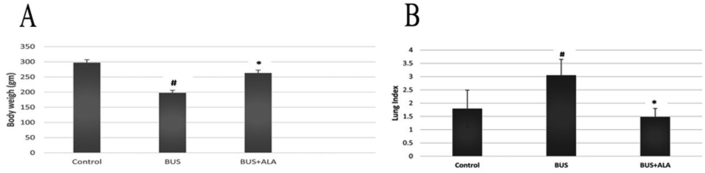
BUS administration induces a significantly lower body weight associated with a significantly higher lung index in comparison to those of normal ones (p < 0.01, p < 0.01, respectively). However, the lower body weight and the lung index in the BUS group were significantly changed by the administration of ALA treatment (P < 0.01), Figure 1.
Figure 1.
Effects of ALA on the final body weight (A) and lung index (B) in all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD. # Represent the significance between the BUS group and the control normal group. ∗: represent the significant changes between the BUS + ALA group and the BUS group.
4.2. Effects of ALA on the inflammatory markers in BUS-induced lung fibrosis in a rat model
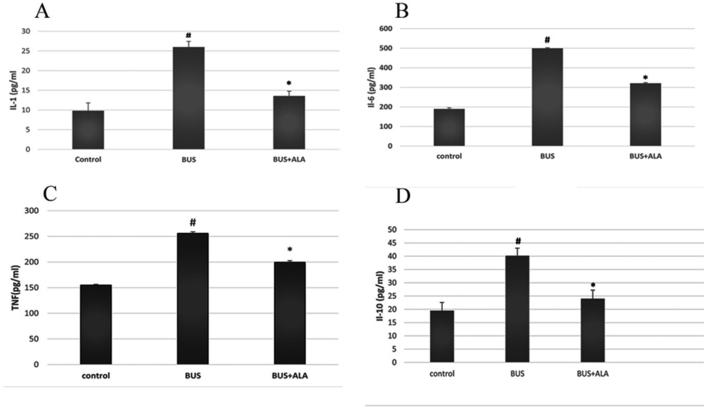
BUS induced a significantly higher serum levels of TNF-α (p < 0.001), IL-1β (p < 0.01), IL-6 (p < 0.001) and IL-10 (p < 0.001) in comparison to those of the normal rats. However, ALA treatment significantly mitigated the elevated levels of TNF-α (p < 0.001), IL-1β (p < 0.01), IL-6 level (p < 0.001) and IL-10 level (p < 0.001) in comparison to those of BUS group, Figure 2.
Figure 2.
Effects of ALA on the serum levels of proinflammatory cytokines in all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD. #: represent the significant changes between the BUS group and the control normal group. ∗: represent the significant changes between the BUS + ALA group and the BUS group.
4.3. Effects of ALA on the antioxidant enzymes and oxidative stress markers in the lung tissues of BUS-induced lung fibrosis rat model
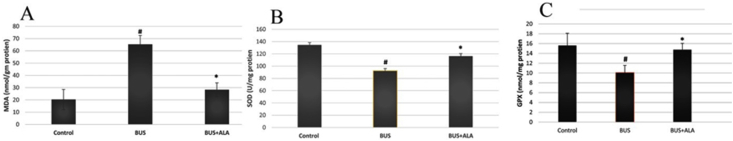
A significantly higher MDA level was noticed in the lung tissues of the BUS group compared to those of the normal group (p < 0.001). Moreover, significantly lower activities of GPx and SOD enzyme were observed in BUS-induced lung fibrotic rats in comparison to those of the control ones (p < 0.01). On contrary, treatment of the fibrotic rats with ALA produced a significantly lower level of MDA (p < 0.001) and restored both GPx and SOD enzyme activities (p < 0.01) in comparison to those of the BUS group, Figure 3.
Figure 3.
Effects of ALA on the oxidant state in the lung tissues of all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD. #: represent the significant changes between the BUS group and the control normal group. ∗: represent the significant changes between the BUS + ALA group and the BUS group.
4.4. Effect of ALA on the level of PGE2 in BUS-induced lung fibrosis
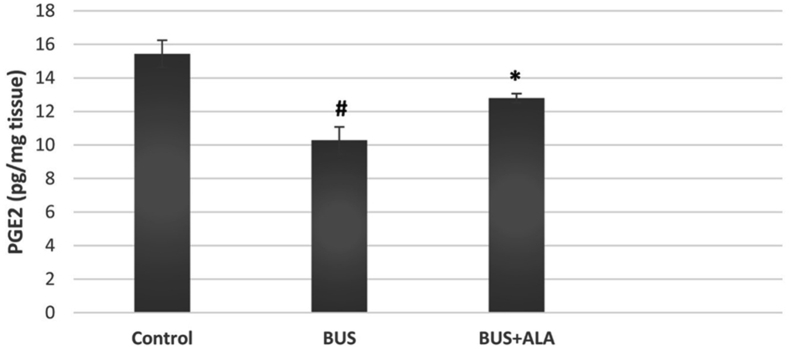
BUS induced a significantly lower level of PGE2 in the lung tissues relative to that of normal ones (p < 0.001). However, the treatment of the BUS rats with ALA exhibited a significant increase in the level of PGE2 compared to the BUS group (p < 0.001), Figure 4.
Figure 4.
Effect of ALA on the level of PGE2 in the lung tissues of different groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD. #: represent the significant changes between the BUS group and the control normal group. ∗: represent the significant changes between the BUS + ALA group and the BUS group.
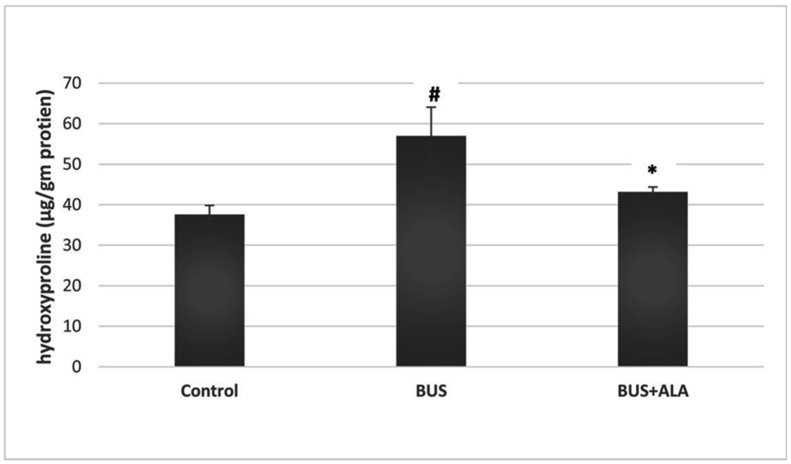
4.5. Effect of ALA on hydroxyproline and collagen deposition in the lung tissue of BUS-induced lung fibrosis rat model
Administration of BUS induced significantly more hydroxyproline content in the lung tissues relative to its level in the normal group (p < 0.05). On the other hand, the treatment of the fibrotic rats with ALA significantly reduced the hydroxyproline content in ALA treated rats relative to the untreated BUS group (p < 0.05), Figure 5.
Figure 5.
Effect of ALA on the hydroxyproline content in the lung tissues of all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD. #: represent the significant changes between the BUS group and the control normal group. ∗: represent the significant changes between the BUS + ALA group and the BUS group.
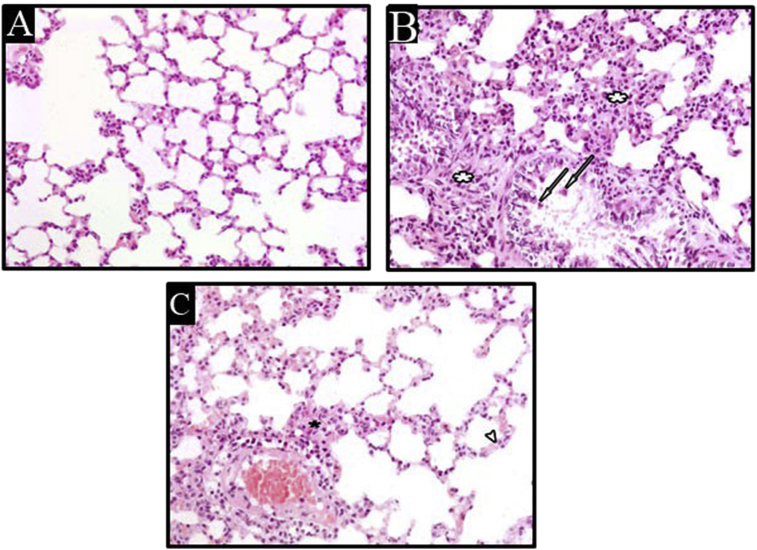
4.6. Histopathological evaluation
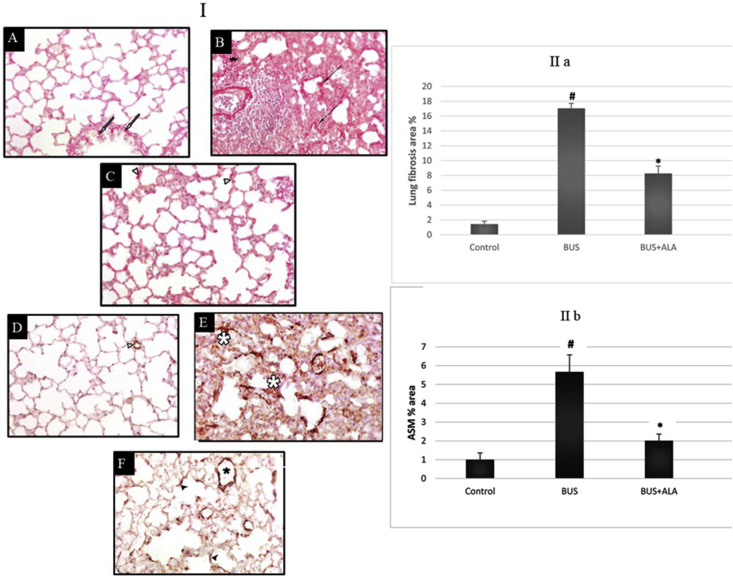
In H&E-stained sections, the lung of the rats received normal saline revealed normally organized architecture with thin interalveolar septa Figure 6A. While, the lung tissues of the untreated BUS group revealed the disorganization of lung architecture, cellular infiltration in the interalveolar septa associated with the presence of intraluminal cellular debris in pulmonary bronchiole, Figure 6B. Rats treated with ALA showed almost normal architecture of pulmonary tissues associated with an obvious reduction in cellular infiltration, Figure 6C. The percentage area stained with Sirius red in the BUS group showed a significant increase (p < 0.05) compared to the control group, Figure 7B. On the other hand, the percentage area stained with Sirius red in the BUS + ALA group was markedly reduced relative to the untreated BUS group (p < 0.05) Figures 7C and 7(IIa).
Figure 6.
Photomicrographs of H and E stained lung sections of different groups, lung sections of the control group (A) show normal lung architecture. The lung section of BUS group (B) shows a disorganized architecture of the lung, increased cellular infiltration (white asterisks), and pulmonary bronchiole contains intraluminal cellular debris (arrows). The lung section of the BUS + ALA group (C) shows more or less organized lung architecture and mild cellular infiltration (black asterisk) (H&E stain x 400).
Figure 7.
I: Photomicrographs of Sirus red-stained lung sections (I; A-C) and α-smooth muscle actin (α-SMA) immuno-stained lung sections (I; D-F) of different groups. II: The percentage area of fibrosis (IIa) and the percentage area of α-SMA (IIb) in all experimental groups. I; A-C: Photomicrographs of Sirus red-stained lung sections of different groups: lung sections of the control group (A) show normal collagen distribution in both pulmonary interstitium and the bronchiolar wall (white arrows). The lung section of BUS group (B) shows excessive collagen deposition in the pulmonary interstitium (black asterisk) and the surrounding alveolar wall (black arrows). The lung section of the BUS + ALA group (C) shows less marked interstitial collagen deposition (arrowheads) (Sirus red stain x 400). I; D-F: Photomicrographs of α-smooth muscle actin (α-SMA) immuno-stained lung sections of different groups: lung sections of the control group (D) show normally immuno-positive reaction around blood vessels (white arrowheads). The lung section of the BUS group (E) shows markedly increased immuno-positive reaction in the pulmonary interstitium (asterisk). The lung section of the BUS + ALA group (F) shows mild immuno-positive reaction (black arrowheads) (α-SMA immune stain x 400). IIa: The percentage area of fibrosis in all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD, # represents significance compared to the control group; ∗ represents significance compared to the BUS group. IIb: The percentage area of α-SMA in all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD, # represents significance compared with the control group; ∗ represents significance compared with the BUS group.
4.7. Effect of ALA on the immunohistochemical expression of α-SMA in BUS-induced lung fibrosis
A significantly higher expression of α-SMA was noticed in the untreated BUS rats’ lungs in comparison to that of the normal rats (p < 0.001). On the other hand, rats who received ALA on top of BUS showed a lower expression of α-SMA in comparison to that of the non-treated BUS rats (p < 0.001), Figure 7 (1D-F) and 7 (IIb).
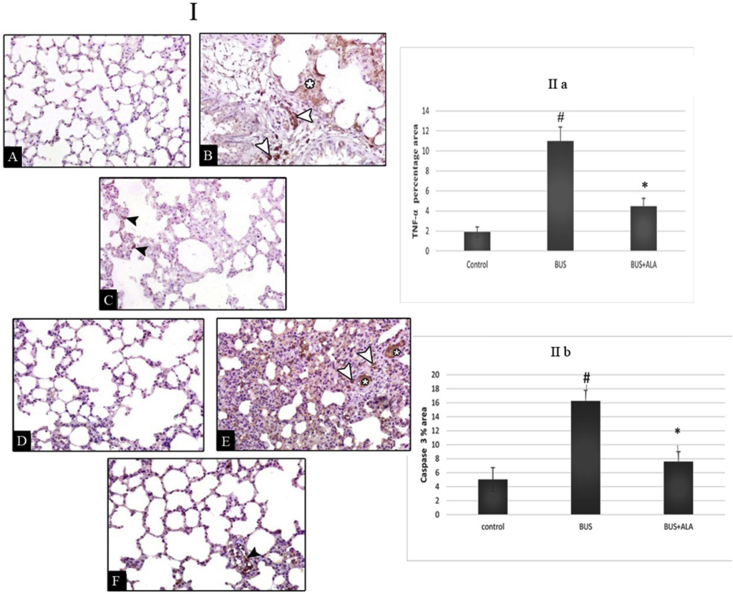
4.8. Effect of ALA on the immunohistochemical expression of TNF-α in BUS-induced lung fibrosis
A significantly higher expression of TNF-α was recorded in the untreated BUS rats’ lungs in comparison to that of the normal rats (p < 0.01). Meanwhile, diseased rats that received ALA showed a markedly lower expression of TNF-α in comparison to that of the non-treated BUS rats (p < 0.01), Figure 8 (I A-C) &IIa.
Figure 8.
I; Photomicrographs of Tumor necrosis factor-α (TNF-α) (I; A-C) and caspase 3 (II; D-F) immuno-stained lung sections of different groups. II; The percentage area of TNF-α (IIa) and caspase 3 (IIb)in all experimental groups. I (A-C): Photomicrographs of Tumor necrosis factor-α (TNF-α) immuno-stained lung sections of different groups: lung sections of the control group (A) show a negative immune reaction. The lung section of BUS group (B) shows markedly increased immuno-positive reaction in the pulmonary interstitium (asterisk) and mononuclear cells (white arrowheads). The lung section of BUS + ALA group IV (C) shows the mild immuno-positive reaction in the pulmonary interstitium (black arrowheads) (TNF-α immune stain x 400). I (D-F): Photomicrographs of caspase 3 immuno-stained lung sections of different groups: lung sections of the control group (A) show a negative immune reaction. The lung section of BUS group (B) shows markedly increased immuno-positive reaction in the pulmonary interstitium (asterisks) and mononuclear cells (white arrowheads). The lung section of the BUS + ALA group (C) shows the mild immuno-positive reaction in mononuclear cells (black arrowhead) (caspase 3 immune stain x 400). II: The percentage area of TNF-α (IIa) and caspase (IIb) in all experimental groups. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD, # represents significance compared with the control group; ∗ represents significance compared with the BUS group.
4.9. Effect of ALA on the immunohistochemical expression of Caspase-3 in BUS-induced lung fibrosis
The results revealed significantly more Caspase-3 immunoreactive cells in the BUS group than that of the normal ones (p < 0.05). In contrast, relative to the BUS group, the ALA-treated fibrotic groups showed a lower number of Caspase-3 immunoreactive cells (p < 0.05) Figure (I D-F) &IIb.
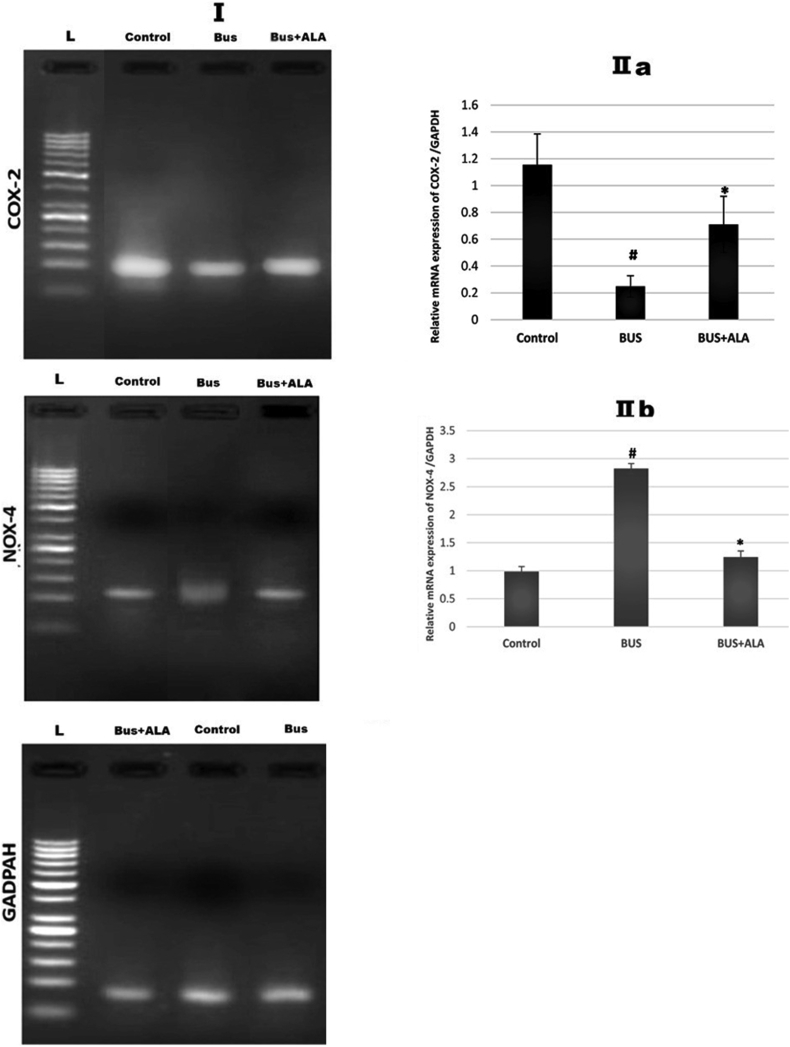
4.10. Effect of ALA on the expression of COX-2 in BUS-induced lung fibrosis
The results showed significantly less COX-2 expression at the mRNA level in the non-treated BUS group relative to that of the control ones (p < 0.004). In contrast, when the comparison was made to the BUS group, the ALA-treated BUS groups showed markedly more COX-2 expression (p < 0.05), Figure 9.
Figure 9.
Effect of ALA on the expression of COX-2 and NOX-4 in BUS-induced lung fibrosis; I: Represent mRNA expression of COX2 and NOX4 in different experimental groups. IIa and IIb represent the relative expression of COX2 and NOX4 mRNA in different groups, respectively. The test used: One-way ANOVA followed by posthoc Tukey's test. Data are expressed as mean ± SD, # represents significance compared to the control group; ∗ represents significance compared to the BUS group. Gel images are provided in supplementary files.
4.11. Effect of ALA on the expression of NOX-4 in BUS-induced lung fibrosis
The results presented in Figure 9 showed a significantly higher expression of NOX-4 mRNA in the untreated BUS rats’ lungs relative to the normal results (p < 0.001). On the other hand, the fibrotic rats that received ALA supplementation showed markedly lower expression of NOX-4 mRNA relative to that of the non-treated BUS rats (p < 0.001).
5. Discussion
In the current work, we investigated the potential protective effect of ALA against pulmonary injury induced by BUS in rats. Our data revealed a significant decrease in the rats’ body weights associated with a marked increase in the lung index in the BUS group. The increase in the weight of the lung indicates the occurrence of pulmonary fibrosis. It occurs by transferring the alkyl group(s) that present in BUS to different body cells to induce lung injury in the form of fibrosis [16]. On the other hand, the administration of ALA to the diseased rats exhibited a significant elevation in the bodyweight with a marked decline in the lung index.
The obtained data from the serum analysis revealed a significant elevation in the levels of TNF-α, IL-1β and, IL-6 in the BUS group. Chitra et al. [17] reported that increased proinflammatory cytokines during the inflammatory response could be responsible for the activation of the fibroblasts and the release of profibrogenic cytokine (TGF-β) and subsequent deposition of collagen. On contrary, the administration of ALA significantly reduced the elevated levels of these proinflammatory cytokines in BUS administrated rats. These results indicate the beneficial effect of ALA against BUS-induced lung fibrosis that may be mediated by the reduction of proinflammatory cytokine production. ALA treatment attenuates the upregulation of cytokines and displays powerful anti-inflammatory effects.
IL-10 levels have been reported to be markedly increased in multiple experimental models of pulmonary inflammation and fibrosis [18, 19]. Consistent with these previous studies, our results revealed a significant elevation in the serum level of IL-10 of BUS-administered rats. Doughty et al. [20] explained this increase in the level of IL-10 to be a compensatory anti-inflammatory response and to be proportionate with the already released pro-inflammatory cytokines. On the other hand, the ALA administration significantly reduced the serum level of IL-10. The down-regulation of IL-10 may be an additional pathway involved in its beneficial anti-inflammatory effects. It is associated with a decrease in the level of pro-inflammatory cytokines.
Daniil et al. [21] demonstrated that oxidative stress plays a chief role in pulmonary fibrosis. The generation of reactive oxygen species (ROS) and reactive nitrogen species has been reported to be involved in the pathogenesis of fibrosis [22, 23]. Moreover, the markers of oxidative stress have been identified in the patients’ lungs suffering from pulmonary fibrosis and also in animal models of lung fibrosis [17]. In the present study, the administration of BUS significantly compromised oxidants/antioxidants hemostasis as manifested by a significant elevation of MDA content in the lung tissues concomitant with a reduction in the activities of GPx and SOD. Meanwhile, treatment of BUS administrated rats with ALA restores the cellular redox activity, these results indicate the ability of ALA to maintain oxidant-antioxidant balance.
In context to all aforementioned alterations, the histopathological changes manifested by disorganization of the lung architecture increased cellular infiltration, and the presence of intraluminal cellular debris in the pulmonary bronchiole was observed following BUS administration. These findings were following the study of Ida et al. [24]. These histological alterations were markedly corrected upon the ALA administration. Overall, our results indicate that ALA could be beneficial against BUS-induced lung fibrosis. This was supported by the finding conducted by Azmoonfar et.al [25]. who reported that ALA was able to mitigate fibrosis and pneumonitis markers in mice lung tissues following lung irradiation.
Excessive collagen deposition is a vital phenomenon in pulmonary fibrosis. As previously known, BUS induces the production of free radicals, upregulating the synthesis of collagen in the lungs. Furthermore, the administration of BUS encourages cytokine dysregulation and the development of inflammation that activates the fibroblasts leading to an imbalance between collagen deposition and reabsorption [26]. In agreement with these previous data, the present study, revealed an obvious increase in the hydroxyproline content in the BUS group while it was reduced markedly with ALA treatment. Control normal group shows normal collagen distribution in both pulmonary interstitium and bronchiolar wall, whereas in the BUS group; the lung section shows excessive collagen deposition in the pulmonary interstitium and surrounding alveolar wall. On the other hand, ALA treated rats revealed less marked interstitial collagen deposition. This effect of ALA could be explained by inhibiting lung inflammatory cell accumulation and thus reducing ROS production, removal of the free radicals from the lung tissues, detoxifying free radicals generated by BUS, and eventually inhibition of fibroblast activation and proliferation.
Several researchers found that apoptosis may contribute to the deposition of collagen and the progression of pulmonary fibrosis through modulation of the immune response and paracrine signaling [27, 28, 29]. A previous study showed that direct administration of apoptotic cells to the rats’ lungs induced pulmonary inflammation and fibrosis indicating a direct relationship between apoptosis and the detected lung pathologies [28]. In agreement with these previous studies, our results demonstrated that the expression of apoptotic cells, Capase3 immunoreactive cells, in the lung of the BUS group was significantly higher than the control ones and this finding indicating the involvement of Caspase-dependent apoptotic signaling pathways in the BUS-induced lung injury. On the other hand, we noticed a significant decrease in the expression of Caspase-3 immunoreactive cells in ALA-treated rats which may suggest its powerful anti-apoptotic effect.
Furthermore, our findings illustrate a remarkable increase in the number of α-SMA -positive cells in the areas of active fibrosis, which become prominent with increased collagen deposition in the BUS group. TGF-β1 is considered the most powerful profibrogenic cytokine [14]. TGF-β1 can encourage the secretion of collagens from the pulmonary fibroblasts and/or induce transformation of fibroblasts to myofibroblasts that express α-SMA; both are critical steps in the initiation and the progression of pulmonary fibrosis [30, 31]. And this could be explained in our results, as there was a remarkable increase in the serum level of pro-inflammatory cytokines that are responsible for the formation of the fibroblasts and the release of TGF-β. Meanwhile, diseased rats treated with ALA exhibited a marked decrease in the number of α-SMA -immunoreactive cells associated with a decline in collagen deposition and hydroxyproline content in the lung tissue. It was concomitant with a significant decrease in the serum level of pro-inflammatory cytokines. All these results indicate the protective effect of ALA against BUS-induced pulmonary fibrosis which may be related to its anti-inflammatory, antioxidant, and anti-apoptotic effects.
Many pieces of evidence suggest that PGE2 may play a role in limiting fibrotic responses in the lung and this pathway may be attenuated in patients with pulmonary fibrosis. Previous reports observed that patients with idiopathic pulmonary fibrosis exhibited a marked decrease in the expression of COX-2 and subsequently reduced PGE2 production in bronchoalveolar fluid and fibroblasts [32, 33, 34]. Moreover, Maher et al. [35] demonstrated a decline in the expression of both COX-2 and PGE2 in the fibroblasts from the patients suffering from pulmonary fibrosis. They suggested that these low levels of COX-2 and PGE2 promote survival of fibroblast and prevent its apoptosis in the fibrotic lung. Moreover, Ex-vivo studies have shown that PGE2 can decrease proliferation, activation, and collagen synthesis from pulmonary fibroblasts [36, 37, 38, 39, 40]. Besides, several early studies using mice lacking COX-2 suggested that COX-2-derived prostaglandins could limit some aspects of the histological changes and collagen production in the bleomycin and vanadium pentoxide models of lung fibrosis [41, 42]. A decreased expression of COX-2 was observed and was associated with a marked decrease in the level of PGE2 in the non-treated BUS group in this study. The administration of ALA significantly elevated COX-2 mRNA levels in the fibrotic lung and subsequently increased the level of PGE2 in the lung tissue. According to Al-Matubsi et al. [43], administration of ALA increases the Activities of COX2 in the Offspring of Rats with Streptozotocin-Induced Diabetes. Also, administration of ALA (20 mg) increases the expression of COX2 in brain tissue suffered from acute ischemic stroke [44]. Our results proposed that the protective effects of ALA against BUS-induced lung damage may be mediated by restoring the normal level of COX-2 and PGE2.
NOX-4 has been found to modulate the TGF-β/SMAD-signaling through intracellular production of ROS [45]. It has been recently shown that NOX-4 is expressed in the pulmonary arterial adventitial fibroblasts and contributes to ROS generation under hypoxic conditions. It subsequently stimulates proliferation, activation, and inhibiting apoptosis of fibroblasts [46]. Furthermore, NOX-4 is involved in the differentiation of human cardiac fibroblasts into myofibroblasts through TGF-β1 [47]. The current work demonstrates a marked elevation in the expression of NOX-4 mRNA in the BUS rats’ lung tissue. However, the administration of ALA mitigates the increased levels of NOX-4 that is associated with attenuating lung fibrosis and decreases the expression of α-SMA. This finding is suggesting that the anti-fibrotic effect of ALA is mainly mediated through inhibition of myofibroblast differentiation.
Liu et al. [48] reported that ALA can alleviate bleomycin-induced pulmonary fibrosis by suppressing oxidative stress. It showed a significant reduction of hydroxyproline and total proteins and ameliorated the MMP-1/TIMP-1 ratio. Further molecular mechanisms regarding the activity of ALA against BUS-induced pulmonary injury were studied in this work as the expression of α-SMA (indicator for fibrosis), and the expression of PGE-2, COX-2, and NOX-4 that mediate myofibroblast activity. Inflammatory mediators and apoptotic markers were also investigated in this study to roll out the protective effect of ALA against lung fibrosis.
6. Conclusions
According to our results, ALA may be considered as a potential protector against BUS-induced pulmonary fibrosis through its antioxidant, anti-inflammatory, antiapoptotic and antifibrotic effects as determined by biochemical and semiquantitative morphological indices of lung injury. Also, it exerts a powerful reduction in the deposition of the collagen with the upregulation of COX-2 expression in lung tissue associated with an increase in the level of PGE2 and downregulation of NOX-4 mRNA expression. Thus, ALA may be a promising protective agent against BUS-induced lung damage.
Declarations
Author contribution statement
Ahlam Elmasry; Mahmoud M. Elalfy: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mona G. Elhadidy: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hassan Reda Hassan Elsayed; Shereen Hamed:Performed the experiments.
Mohammad El-Nablaway; Shereen Hamed: Performed the experiments; Analyzed and interpreted the data.
Mohammed R Rabei: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Physiology, Histology Departments, and MERC, Faculty of Medicine, Mansoura University, Egypt, are sincerely acknowledged for facilitating the experimental and histopathological assessment parts of this study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
COX2.
GADPAH.
NOX4.
References
- 1.Leger P., Limper A.H., Maldonado F. Pulmonary toxicities from conventional chemotherapy. Clin. Chest Med. 2017;38:209–222. doi: 10.1016/j.ccm.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Oliner H., Schwartz R., Rubio F., Dameshek W. Interstitial pulmonary fibrosis following busulfan therapy. Am. J. Med. 1961;31:134–139. doi: 10.1016/0002-9343(61)90229-7. [DOI] [PubMed] [Google Scholar]
- 3.Dun M.D., Aitken R.J., Nixon B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update. 2012;18:420–435. doi: 10.1093/humupd/dms009. [DOI] [PubMed] [Google Scholar]
- 4.Iwamoto T., Hiraku Y., Oikawa S., Mizutani H., Kojima M., Kawanishi S. DNA intrastrand cross-link at the 5’-GA-3’ sequence formed by busulfan and its role in the cytotoxic effect. Cancer Sci. 2004;95:454–458. doi: 10.1111/j.1349-7006.2004.tb03231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matijasic N., Bonevski A., Pivac V.T., Pavic I. Vol. 32. 2019. Busulfan-Induced Lung Injury in Pediatric Oncology; pp. 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szeląg M., Mikulski D., Molski M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J. Mol. Model. 2012;18:2907–2916. doi: 10.1007/s00894-011-1306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moura F.A., de Andrade K.Q., dos Santos J.C.F., Goulart M.O.F. Lipoic Acid: its antioxidant and anti-inflammatory role and clinical applications. Curr. Top. Med. Chem. 2015;15:458–483. doi: 10.2174/1568026615666150114161358. [DOI] [PubMed] [Google Scholar]
- 8.Gorąca A., Huk-Kolega H., Piechota A., Kleniewska P., Ciejka E., Skibska B. Lipoic acid - biological activity and therapeutic potential. Pharmacol. Rep. 2011;63:849–858. doi: 10.1016/s1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 9.Han D., Handelman G., Marcocci L., Sen C.K., Roy S., Kobuchi H., Tritschler H.J., Flohé L., Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 10.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kagan V.E., Shvedova A., Serbinova E., Khan S., Swanson C., Powell R., Packer L. Dihydrolipoic acid--a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992;44:1637–1649. doi: 10.1016/0006-2952(92)90482-x. [DOI] [PubMed] [Google Scholar]
- 12.Aboul Fotouh G.I., Abdel-Dayem M.M., Ismail D.I., Mohamed H.H. Histological study on the protective effect of endogenous stem cell mobilization in busulfan-induced testicular injury in albino rats. J. Microsc. Ultrastruct. 2018;6:197–204. doi: 10.4103/JMAU.JMAU_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Bancroft L., Christopher J.D. In: Bancroft’S Theory and Practice of Histological Techniques. Suvarna Kim S., Layton Christopher, Bancroft John D., editors. Vol. 2019. 8thChurchill Livingstone Elsevier; Oxford, England: 2019. The hematoxylins and eosin. And connective and other mesenchymal tissues with their stains; p. 126. 138&153–175. [Google Scholar]
- 15.Ramos-Vara J.A., Miller M.A. When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry--the red, brown, and blue technique. Vet. Pathol. 2014;51:42–87. doi: 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- 16.Ahar N.H., Khaki A., Akbari G., Novin M.G. The effect of Busulfan on body weight, Testis weight and MDA enzymes in male rats. Int. J. Women’s Heal. Reprod. Sci. 2014;2:316–319. [Google Scholar]
- 17.Chitra P., Saiprasad G., Manikandan R., Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-κB dependant TGF-β activation: a biphasic experimental study. Toxicol. Lett. 2013;219:178–193. doi: 10.1016/j.toxlet.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Park E.-J., Roh J., Kim S.-N., Kang M.-S., Han Y.-A., Kim Y., Hong J.T., Choi K. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Arch. Toxicol. 2011;85:1121–1131. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- 19.Kim B.G., Lee P.H., Lee S.H., Kim Y.E., Shin M.Y., Kang Y., Bae S.H., Kim M.J., Rhim T., Park C.S., Jang A.S. Long-term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse model. Allergy. Asthma Immunol. Res. 2016;8:246–256. doi: 10.4168/aair.2016.8.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doughty L., Carcillo J.A., Kaplan S., Janosky J. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113:1625–1631. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 21.Daniil Z.D., Papageorgiou E., Koutsokera A., Kostikas K., Kiropoulos T., Papaioannou A.I., Gourgoulianis K.I. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm. Pharmacol. Therapeut. 2008;21:26–31. doi: 10.1016/j.pupt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Fubini B., Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003;34:1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 23.Bargagli E., Olivieri C., Bennett D., Prasse A., Muller-Quernheim J., Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir. Med. 2009;103:1245–1256. doi: 10.1016/j.rmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Ida T., Hashimoto S., Suzuki N., Ebe Y., Yano T., Sato N., Koike T. [Multiple organ failure presumably due to alkylating agents used as preconditioning drugs for autologous peripheral blood stem cell transplantation in an acute promyelocytic leukemia] Rinsho Ketsueki. 2016;57:41–46. doi: 10.11406/rinketsu.57.41. [DOI] [PubMed] [Google Scholar]
- 25.Azmoonfar R., Amini P., Yahyapour R., Rezaeyan A., Tavassoli A., Motevaseli E., Khodamoradi E., Shabeeb D., Musa A.E., Najafi M. Mitigation of radiation-induced pneumonitis and lung fibrosis using alpha-lipoic acid and resveratrol. Antiinflamm. Antiallergy. Agents Med. Chem. 2019;19:149–157. doi: 10.2174/1871523018666190319144020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinnula V.L., Myllärniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxidants Redox Signal. 2008;10:727–738. doi: 10.1089/ars.2007.1942. [DOI] [PubMed] [Google Scholar]
- 27.V Barbas-Filho J., Ferreira M.A., Sesso A., Kairalla R.A., Carvalho C.R., Capelozzi V.L. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP) J. Clin. Pathol. 2001;54:132–138. doi: 10.1136/jcp.54.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L., Scabilloni J.F., Antonini J.M., Rojanasakul Y., Castranova V., Mercer R.R. Induction of secondary apoptosis, inflammation, and lung fibrosis after intratracheal instillation of apoptotic cells in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L695–L702. doi: 10.1152/ajplung.00245.2005. [DOI] [PubMed] [Google Scholar]
- 29.Wang R., Alam G., Zagariya A., Gidea C., Pinillos H., Lalude O., Choudhary G., Oezatalay D., Uhal B.D. Apoptosis of lung epithelial cells in response to TNF-alpha requires angiotensin II generation de novo. J. Cell. Physiol. 2000;185:253–259. doi: 10.1002/1097-4652(200011)185:2<253::AID-JCP10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz J.C., Rogers D.S., Sharma V., Vittal R., White E.S., Cui Z., Thannickal V.J. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell. Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng C.M., Hsiao Y.H., Su V.Y.F., Su K.C., Wu Y.C., Chang K.T., Perng D.W. The suppression effects of thalidomide on human lung fibroblasts: cell proliferation, vascular endothelial growth factor release, and collagen production. Lung. 2013;191:361–368. doi: 10.1007/s00408-013-9477-1. [DOI] [PubMed] [Google Scholar]
- 32.Vancheri C., Sortino M.A., Tomaselli V., Mastruzzo C., Condorelli F., Bellistrí G., Pistorio M.P., Canonico P.L., Crimi N. Different expression of TNF-alpha receptors and prostaglandin E(2 )Production in normal and fibrotic lung fibroblasts: potential implications for the evolution of the inflammatory process. Am. J. Respir. Cell Mol. Biol. 2000;22:628–634. doi: 10.1165/ajrcmb.22.5.3948. [DOI] [PubMed] [Google Scholar]
- 33.Bozyk P.D., Moore B.B. Prostaglandin E2 and the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011;45:445–452. doi: 10.1165/rcmb.2011-0025RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilborn J., Crofford L.J., Burdick M.D., Kunkel S.L., Strieter R.M., Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J. Clin. Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maher T.M., Evans I.C., Bottoms S.E., Mercer P.F., Thorley A.J., Nicholson A.G., Laurent G.J., Tetley T.D., Chambers R.C., McAnulty R.J. Diminished prostaglandin E2 contributes to the apoptosis paradox in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;182:73–82. doi: 10.1164/rccm.200905-0674OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S.K., Fisher A.S., Scruggs A.M., White E.S., Hogaboam C.M., Richardson B.C., Peters-Golden M. Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am. J. Pathol. 2010;177:2245–2255. doi: 10.2353/ajpath.2010.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lovgren A.K., Jania L.A., Hartney J.M., Parsons K.K., Audoly L.P., Fitzgerald G.A., Tilley S.L., Koller B.H. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L144–L156. doi: 10.1152/ajplung.00492.2005. [DOI] [PubMed] [Google Scholar]
- 38.McAnulty R.J., Hernández-Rodriguez N.A., Mutsaers S.E., Coker R.K., Laurent G.J. Indomethacin suppresses the anti-proliferative effects of transforming growth factor-beta isoforms on fibroblast cell cultures. Biochem. J. 1997;321(Pt 3):639–643. doi: 10.1042/bj3210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohyama T., Ertl R.F., Valenti V., Spurzem J., Kawamoto M., Nakamura Y., Veys T., Allegra L., Romberger D., Rennard S.I. Prostaglandin E(2) inhibits fibroblast chemotaxis. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1257–L1263. doi: 10.1152/ajplung.2001.281.5.L1257. [DOI] [PubMed] [Google Scholar]
- 40.Kolodsick J.E., Peters-Golden M., Larios J., Toews G.B., Thannickal V.J., Moore B.B. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am. J. Respir. Cell Mol. Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 41.Keerthisingam C.B., Jenkins R.G., Harrison N.K., Hernandez-Rodriguez N.A., Booth H., Laurent G.J., Hart S.L., Foster M.L., McAnulty R.J. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 2001;158:1411–1422. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner J.C., Rice A.B., Ingram J.L., Moomaw C.R., Nyska A., Bradbury A., Sessoms A.R., Chulada P.C., Morgan D.L., Zeldin D.C., Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am. J. Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-matubsi H.Y., Oriquat G.A., Abu-samak M., Al Hanbali O.A., Salim M.D. The Offspring of Rats with Streptozotocin-Induced Diabetes. 2016. Effects of lipoic acid supplementation on activities of cyclooxygenases and levels of prostaglandins E 2 and F 2 α metabolites; p. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q., Lv C., Zhang B., Wang S. 2018. The Role of Alpha-Lipoic Acid in the Pathomechanism of Acute Ischemic Stroke; pp. 42–53. [DOI] [PubMed] [Google Scholar]
- 45.Jiang F., Liu G.-S., Dusting G.J., Chan E.C. NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T.R., Horowitz J.C., Pennathur S., Martinez F.J., Thannickal V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amara N., Goven D., Prost F., Muloway R., Crestani B., Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu R., Ahmed K.M., Nantajit D., Rosenthal F.S., Hai C.X., Li J.J. Therapeutic effects of α-lipoic acid on bleomycin-induced pulmonary fibrosis in rats. Int. J. Mol. Med. 2007;19:865–873. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.