Abstract
Background
The phase 3 LIBERTY ASTHMA QUEST study (ClinicalTrials.gov: NCT02414854) in patients with uncontrolled, moderate-to-severe asthma has demonstrated the efficacy and safety of dupilumab 200 and 300 mg every 2 weeks versus placebo. This post hoc analysis assessed the effect of dupilumab on efficacy outcomes and asthma control across a range of historical exacerbation rates in patients with type 2-high asthma.
Methods
Annualised severe exacerbation rates over the 52-week treatment period, pre-bronchodilator forced expiratory volume in 1 s (FEV1) at weeks 12 and 52, and the five-item Asthma Control Questionnaire (ACQ-5) score at weeks 24 and 52 were assessed in patients with ≥1, ≥2 or ≥3 exacerbations in the previous year. Subgroups were stratified by baseline blood eosinophils ≥150 or ≥300 cells·μL−1 or baseline exhaled nitric oxide fraction ≥25 ppb and baseline inhaled corticosteroid (ICS) dose.
Results
Across all type 2-high subgroups, dupilumab versus placebo significantly reduced severe exacerbations by 54–90%, with greater improvements in patients with more exacerbations prior to study initiation. Similarly, improvements in FEV1 (least squares (LS) mean difference versus placebo: ≥1 exacerbations, 0.15–0.25 L; ≥2 exacerbations, 0.12–0.32 L; ≥3 exacerbations, 0.09–0.38 L; majority p<0.05) and ACQ-5 score (LS mean difference range: ≥1 exacerbations, −0.30 to −0.57; ≥2 exacerbations, −0.29 to −0.56; ≥3 exacerbations, −0.43 to −0.61; all p<0.05) were observed, irrespective of prior exacerbation history, across all subgroups.
Conclusions
Dupilumab significantly reduced severe exacerbations and improved FEV1 and asthma control in patients with elevated type 2 biomarkers irrespective of exacerbation history and baseline ICS dose.
Short abstract
Dupilumab reduced severe exacerbations and improved lung function and asthma control in patients with type 2-high asthma, irrespective of exacerbation history and baseline ICS dose. These data will aid clinicians managing patients with severe disease. https://bit.ly/2PjnSm6
Introduction
Asthma exacerbations, which may sometimes necessitate a change in a patient's current treatment, account for significant mortality and present a considerable economic burden due to the healthcare costs associated with their management [1, 2]. Patients with type 2 asthma and/or patients receiving higher doses of inhaled corticosteroids (ICS) have more severe disease and require higher doses of steroids to maintain asthma control. These subpopulations are therefore at greater risk of future exacerbations [3]. Moreover, asthma exacerbation history, particularly of recent events, has been shown to be a significant independent predictor of future exacerbation risk [4, 5].
Dupilumab is a fully human VelocImmune-derived monoclonal antibody that blocks the shared receptor component for interleukin (IL)-4 and IL-13, thus inhibiting signalling of both IL-4 and IL-13, which are key and central drivers of type 2 inflammation in multiple diseases [6–9]. These cytokines, together with IL-5, play important roles in the pathogenesis of asthma [9, 10]. Dupilumab is approved for patients aged ≥12 years with moderate-to-severe eosinophilic or oral corticosteroid-dependent asthma, for adult patients with inadequately controlled chronic rhinosinusitis with nasal polyps, and for the treatment of patients with inadequately controlled, moderate-to-severe atopic dermatitis aged >6 years in the USA and adults in the European Union and other countries [11–20].
In the phase 3 LIBERTY ASTHMA QUEST study, dupilumab 200 and 300 mg every 2 weeks versus matched placebo significantly reduced severe exacerbation rates and improved pre-bronchodilator forced expiratory volume in 1 s (FEV1), asthma control and quality of life measures in patients with uncontrolled, moderate-to-severe asthma [15]. Treatment effects were generally of greater magnitude in patients with elevated baseline levels of type 2 inflammatory biomarkers (blood eosinophils ≥150 cells·μL−1 or exhaled nitric oxide fraction (FENO) ≥25 ppb) [15].
Understanding the efficacy of treatments such as dupilumab in patients with high disease burden (evidenced by frequent, recent exacerbations and high-dose ICS use) is of considerable relevance to clinicians treating patients with severe disease. The aim of this post hoc analysis of the LIBERTY ASTHMA QUEST study was to assess the effect of dupilumab on severe exacerbations, lung function and asthma control in subgroups of patients with a type 2-high phenotype, who had experienced a range of exacerbations in the previous year (≥1, ≥2 or ≥3) and who were further stratified by baseline ICS dose (high versus medium).
Methods
Study design and patients
LIBERTY ASTHMA QUEST (ClinicalTrials.gov: NCT02414854) was a phase 3 multinational, multicentre, randomised, double-blind, placebo-controlled study that assessed the effect of dupilumab in patients with uncontrolled, moderate-to-severe asthma [15]. Patients aged ≥12 years with physician-diagnosed asthma for 12 months (based on the Global Initiative for Asthma 2014 guidelines) were eligible to participate [21]. The study was open to all patients, irrespective of eosinophilic status or any other biomarker requirement. Patients were randomised in a 2:2:1:1 ratio to add-on subcutaneously administered dupilumab 200 mg (loading dose 400 mg) or 300 mg (loading dose 600 mg) every 2 weeks or matched-volume placebos for 52 weeks. Full details of the study's design and methodology have been reported previously [15, 22]; complete patient eligibility criteria, including the requirement for all patients to have had ≥1 exacerbations within the year before enrolment, can be found in the supplementary appendix of the primary manuscript [15].
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. An independent data and safety monitoring committee conducted blinded monitoring of patient safety data. Study conduct and documentation were monitored by local institutional review boards or ethics committees and all patients provided written informed consent before participating in the trial.
Study end-points and populations analysed
The end-points analysed in this post hoc analysis of LIBERTY ASTHMA QUEST were: annualised rate of severe exacerbations during the 52-week treatment period, least squares (LS) mean change from baseline in pre-bronchodilator FEV1 (L) at weeks 12 and 52, and LS mean change from baseline in the five-item Asthma Control Questionnaire (ACQ-5) score at weeks 24 and 52. A severe asthma exacerbation was defined as a deterioration of asthma requiring treatment with systemic corticosteroids for >3 days, or hospitalisation or emergency room visit because of asthma requiring systemic corticosteroids. ACQ-5 is a patient-reported measure of the adequacy of asthma control and of change in asthma control; higher scores indicate less asthma control [23].
Subgroup analyses were performed for patients stratified by number of exacerbations in the previous year (≥1, ≥2 or ≥3), baseline blood eosinophils (≥150 or ≥300 cells·μL−1), baseline FENO (≥25 ppb) and baseline ICS dose (medium or high). Analyses were also performed in the subgroup of patients without elevated levels of type 2 biomarkers at baseline (eosinophils <150 cells·μL−1 and FENO <25 ppb).
Statistical analysis
The estimates for adjusted annualised severe exacerbation rates were derived using a negative binomial regression model, with total number of events occurring during the 52-week treatment period as the response variable, and the four intervention groups, age, geographic region, baseline eosinophil strata (<300 or ≥300 cells·µL−1), baseline ICS dose level and number of exacerbations in the previous year as covariates. Change from baseline in pre-bronchodilator FEV1 and ACQ-5 scores was analysed using mixed effects models with repeated measures, including assigned intervention, age, baseline eosinophil strata, visit, intervention-by-visit interaction, region (pooled country), corresponding baseline pre-bronchodilator FEV1 or ACQ-5 value and baseline-by-visit interaction as covariates. Sex and baseline height were included as additional covariates in the models for FEV1. For patients who discontinued the assigned intervention and remained in the trial, measurements after the intervention were discontinued and were included in the analysis.
A p-value of <0.05 for the comparison between each dupilumab dose and placebo (within each subgroup) was considered statistically significant.
Results
Patient characteristics
1902 patients were enrolled in the LIBERTY ASTHMA QUEST trial (1264 assigned to dupilumab and 638 assigned to placebo), all of whom had experienced ≥1 severe exacerbation events in the previous year [15]. There were no major differences in baseline characteristics across the subgroups, although patients who had experienced ≥3 exacerbations in the year prior to study entry had relatively lower pre-bronchodilator FEV1 and a higher requirement for ICS use at baseline than did those who had experienced fewer exacerbations. Patients who had experienced ≥3 exacerbations also had higher mean baseline levels of blood eosinophils and FENO (table 1).
TABLE 1.
Baseline demographic and clinical characteristics of LIBERTY ASTHMA QUEST patients by exacerbation history and type 2 biomarkers
| Eosinophils ≥150 cells·μL−1 | Eosinophils ≥300 cells·μL−1 | FENO ≥25 ppb | ||||
| Combined placebo | Combined dupilumab | Combined placebo | Combined dupilumab | Combined placebo | Combined dupilumab | |
| ≥1 exacerbations in the past year | ||||||
| Subjects n | 469 | 889 | 290 | 541 | 334 | 609 |
| Age years | 47.8±15.0 | 47.4±15.4 | 47.5±15.3 | 47.0±15.2 | 47.2±15.8 | 47.2±15.3 |
| Female | 63.3 | 60.1 | 60.7 | 61.2 | 59.6 | 59.3 |
| Severe asthma exacerbations experienced in the past year n | 2.24±1.87 | 2.09±2.47 | 2.34±1.99 | 2.19±2.03 | 2.27±1.94 | 2.11±2.02 |
| High-dose ICS use | 53.9 | 50.7 | 54.8 | 50.3 | 50.0 | 49.9 |
| Medium-dose ICS use | 45.2 | 48.1 | 44.5 | 48.6 | 49.4 | 49.3 |
| Pre-BD FEV1 L | 1.76±0.59 | 1.80±0.61 | 1.76±0.61 | 1.78±0.62 | 1.79±0.61 | 1.80±0.62 |
| Post-BD FEV1 L | 2.16±0.71 | 2.19±0.72 | 2.17±0.71 | 2.17±0.72 | 2.21±0.73 | 2.20±0.75 |
| ACQ-5 score | 2.79±0.77 | 2.78±0.80 | 2.82±0.73 | 2.79±0.82 | 2.71±0.76 | 2.74±0.80 |
| Baseline blood eosinophils cells·μL−1 | 487.93±391.22 | 462.31±371.85 | 654.86±416.61 | 623.66±399.43 | 485.38±442.92 | 457.11±427.30 |
| Baseline FENO ppb | 39.28±32.79 | 38.73±35.01 | 45.85±36.48 | 45.46±39.37 | 55.27±36.73 | 54.74±36.19 |
| ≥2 exacerbations in the past year | ||||||
| Subjects n | 260 | 425 | 159 | 286 | 183 | 303 |
| Age years | 47.7±14.4 | 47.7±14.7 | 47.8±14.6 | 48.0±14.5 | 47.0±15.5 | 47.2±14.6 |
| Female | 64.6 | 61.9 | 62.3 | 63.6 | 59.0 | 61.4 |
| Severe asthma exacerbations experienced in the past year n | 3.23±2.02 | 3.28±3.17 | 3.45±2.12 | 3.26±2.32 | 3.32±2.11 | 3.24±2.38 |
| High-dose ICS use | 59.6 | 60.2 | 61.0 | 58.4 | 51.9 | 58.1 |
| Medium-dose ICS use | 39.2 | 39.3 | 38.4 | 41.3 | 47.0 | 41.6 |
| Pre-BD FEV1 L | 1.73±0.54 | 1.76±0.61 | 1.71±0.55 | 1.71±0.60 | 1.78±0.57 | 1.76±0.64 |
| Post-BD FEV1 L | 2.13±0.65 | 2.13±0.72 | 2.10±0.66 | 2.08±0.70 | 2.19±0.69 | 2.14±0.74 |
| ACQ-5 score | 2.88±0.79 | 2.84±0.86 | 2.87±0.75 | 2.84±0.86 | 2.76±0.80 | 2.79±0.81 |
| Baseline blood eosinophils cells·μL−1 | 520.15±446.84 | 525.25±451.58 | 712.26±480.34 | 676.89±481.57 | 544.12±509.07 | 522.28±514.99 |
| Baseline FENO ppb | 42.09±36.44 | 41.42±38.47 | 49.35±41.01 | 47.25±42.30 | 58.74±43.19 | 57.29±40.23 |
| ≥3 exacerbations in the past year | ||||||
| Subjects n | 119 | 200 | 83 | 140 | 87 | 141 |
| Age years | 47.4±13.6 | 49.0±14.6 | 47.2±13.6 | 49.0±14.3 | 47.0±14.2 | 47.7±14.7 |
| Female | 65.5 | 65.5 | 62.7 | 65.7 | 60.9 | 64.5 |
| Severe asthma exacerbations experienced in the past year n | 4.69±2.23 | 4.72±4.18 | 4.78±2.21 | 4.56±2.76 | 4.77±2.32 | 4.67±2.90 |
| High-dose ICS use | 60.5 | 63.5 | 63.9 | 62.9 | 55.2 | 62.4 |
| Medium-dose ICS use | 38.7 | 36.0 | 34.9 | 37.1 | 43.7 | 37.6 |
| Pre-BD FEV1 L | 1.69±0.50 | 1.67±0.61 | 1.68±0.48 | 1.63±0.62 | 1.73±0.50 | 1.67±0.62 |
| Post-BD FEV1 L | 2.05±0.56 | 2.04±0.75 | 2.04±0.55 | 2.00±0.75 | 2.11±0.58 | 2.03±0.72 |
| ACQ-5 score | 2.99±0.88 | 2.96±0.94 | 2.93±0.86 | 2.93±0.96 | 2.84±0.89 | 2.96±0.90 |
| Baseline blood eosinophils cells·μL−1 | 608.74±499.18 | 562.35±547.84 | 779.88±509.71 | 713.36±593.56 | 610.69±554.79 | 579.29±632.32 |
| Baseline FENO ppb | 40.09±28.04 | 44.34±42.95 | 44.75±28.67 | 50.15±47.19 | 52.82±27.01 | 60.55±45.33 |
Data are presented as mean±sd or %, unless otherwise stated. FENO: exhaled nitric oxide fraction; ICS: inhaled corticosteroid; BD: bronchodilator; FEV1: forced expiratory volume in 1 s; ACQ-5: five-item Asthma Control Questionnaire.
Annualised rate of severe asthma exacerbations
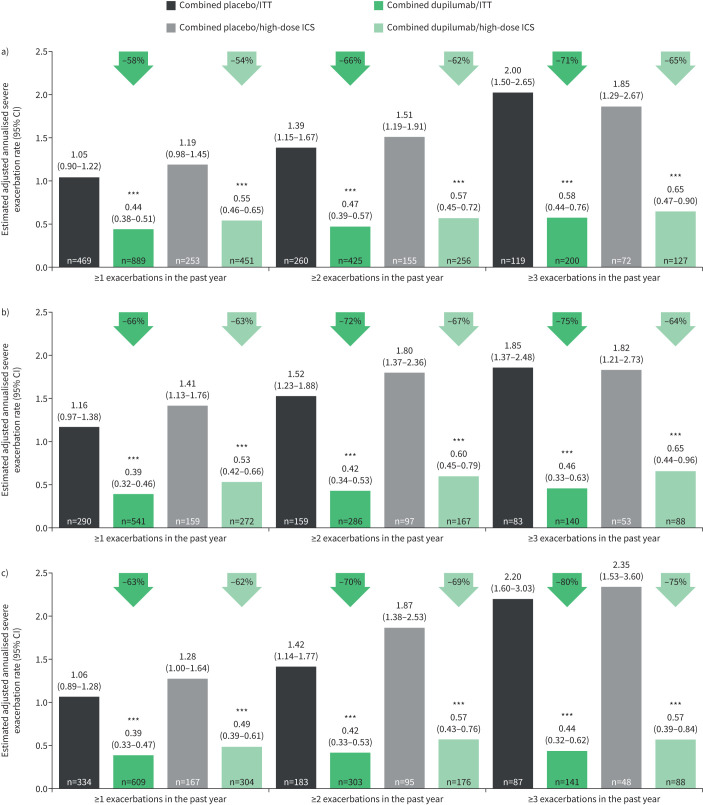
Dupilumab every 2 weeks (combined treatment groups) significantly reduced severe exacerbation rates versus placebo in patients with elevated baseline blood eosinophils irrespective of exacerbation history or ICS dose at baseline, with a greater magnitude of improvement observed in patients with ≥2 or ≥3 prior exacerbations (figure 1). Reductions in exacerbation rates versus placebo ranged from 58% to 71% in the intention-to-treat (ITT) and 54% to 65% in the ICS high-dose subgroup of patients with baseline eosinophils ≥150 cells·µL−1, 66% to 75% (ITT) and 63% to 67% (high-dose ICS) in patients with baseline eosinophils ≥300 cells·µL−1, and 63% to 80% (ITT) and 62% to 75% (high-dose ICS) in patients with baseline FENO ≥25 ppb (all p<0.001) (figure 1a–c). Similar trends were observed in patients on medium-dose ICS at baseline (supplementary figure S1a). Across all high type 2 biomarker subgroups, adjusted annualised rates of exacerbation following dupilumab treatment ranged from 0.16 (in patients with ≥300 cells·µL−1 and medium-dose ICS treated with combined dupilumab 200 and 300 mg) to 0.65 (patients with ≥150 cells·µL−1 and high-dose ICS treated with combined dupilumab 200 and 300 mg) compared with rates ranging from 0.86 to 2.35 in placebo-treated patients.
FIGURE 1.
Reduction of annualised rate (95% CI) of severe exacerbations during the 52-week intervention period in subgroups of LIBERTY ASTHMA QUEST patients categorised by a) baseline blood eosinophils ≥150 cells·μL−1, b) baseline blood eosinophils ≥300 cells·μL−1 and c) baseline exhaled nitric oxide fraction ≥25 ppb (intention-to-treat (ITT) or high-dose inhaled corticosteroid (ICS)). ***: p<0.001 versus placebo.
In the subgroup of patients with baseline eosinophils <150 cells·µL−1 and baseline FENO <25 ppb, numerical improvements versus placebo in annualised exacerbation rates were observed, although these were not statistically significant (supplementary figure S1b).
Pre-bronchodilator FEV1
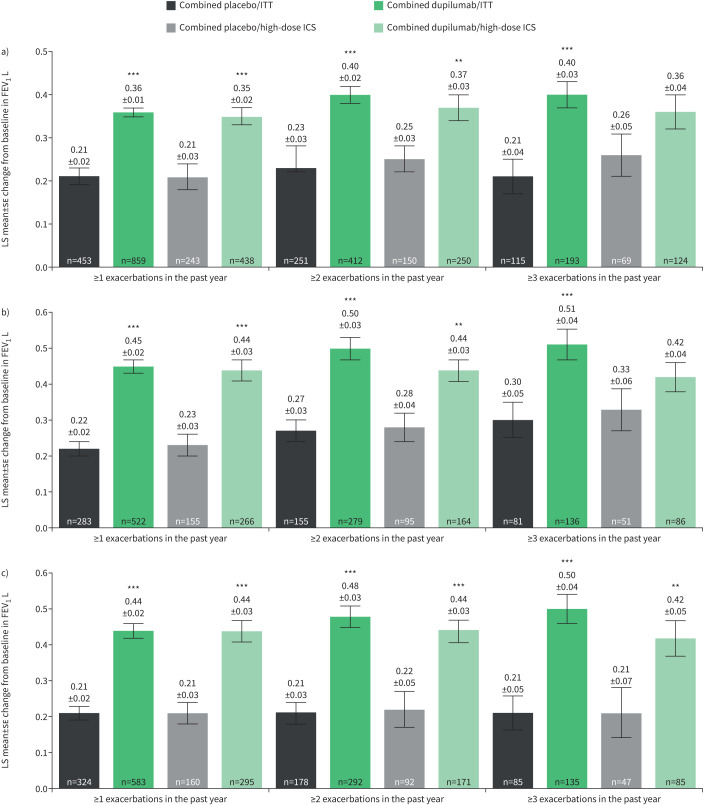
Dupilumab 200 and 300 mg every 2 weeks (combined treatment groups) versus matched placebo improved pre-bronchodilator FEV1 at week 12, irrespective of exacerbation history across all subgroups, stratified by baseline eosinophils, FENO or ICS dose (figure 2 and supplementary figure S2); these improvements were statistically significant in a majority of cases and the level of improvement increased with the number of prior exacerbations. In the subpopulation of patients who had elevated baseline biomarker levels (blood eosinophils ≥150 or ≥300 cells·μL−1 or baseline FENO ≥25 ppb), further stratified by baseline ICS dose groups, and who had experienced ≥1 exacerbations in the previous year, LS mean change from baseline at week 12 in pre-bronchodilator FEV1 ranged from 0.35 to 0.46 L with dupilumab and 0.20 to 0.23 L with placebo (LS mean difference versus placebo ranged from 0.15 to 0.25 L; all p<0.001) (figure 2a–c, and supplementary figure S2a and b). Improvements with dupilumab were also evident in the elevated biomarker subgroups with ≥2 and ≥3 prior exacerbations. In the former group, dupilumab treatment increased pre-bronchodilator FEV1 by a range of 0.37 to 0.56 L compared with changes of 0.19 to 0.28 L with placebo (LS mean difference versus placebo ranged from 0.12 to 0.31 L; all p<0.01) (figure 2a–c and supplementary figure S2a). In the subgroups of patients with elevated biomarker levels and ≥3 prior exacerbations, improvements with dupilumab and placebo at week 12 were between 0.36 and 0.66 L and 0.19 and 0.33 L, respectively (LS mean difference versus placebo ranged from 0.09 to 0.38 L; all p<0.01 versus placebo except in patients with baseline blood eosinophils ≥150 cells·μL−1 and high ICS dose (p=0.0679) (figure 2a) and patients with baseline blood eosinophils ≥300 cells·μL−1 and high ICS dose (p=0.1717) (figure 2b). Improvements were sustained at week 52 (supplementary figure S2a and b).
FIGURE 2.
Least squares (LS) mean±se change from baseline at week 12 in pre-bronchodilator forced expiratory volume in 1 s (FEV1) in patients categorised by a) baseline blood eosinophils ≥150 cells·μL−1, b) baseline blood eosinophils ≥300 cells·μL−1 and c) baseline exhaled nitric oxide fraction ≥25 ppb (intention-to-treat (ITT) or high-dose inhaled corticosteroid (ICS)). **: p<0.01; ***: p<0.001 versus placebo.
In the subgroup of patients without elevated baseline biomarkers (i.e. baseline blood eosinophils <150 cells·μL−1 and baseline FENO <25 ppb), numerical improvements with dupilumab treatment versus placebo were observed at weeks 12 and 52, although these were largely not statistically significant (supplementary figure S2c).
ACQ-5 scores
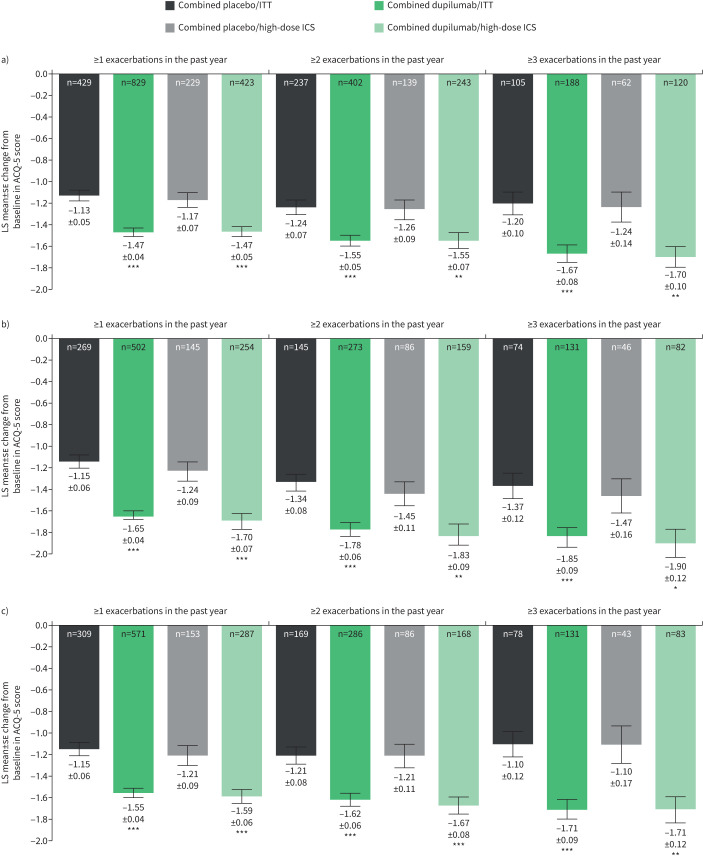
Dupilumab every 2 weeks (combined treatment groups) versus matched placebo improved asthma control (ACQ-5 score) at week 24 irrespective of exacerbation history across all subgroups stratified by baseline eosinophils, FENO or ICS dose (figure 3 and supplementary figure S3). As with the FEV1 end-point, these improvements versus placebo were statistically significant in a majority of cases, with greater improvements observed in the patients who had elevated biomarker levels at baseline and who had ≥3 prior exacerbations. In the subgroups of these patients who had experienced ≥1 exacerbations in the previous year, LS mean change in ACQ-5 score at week 24 ranged from −1.47 to −1.70 for dupilumab and −1.05 to −1.24 for placebo (LS mean difference versus placebo ranged from −0.30 to −0.56; all p<0.001) (figure 3a–c, and supplementary figure S3a and b). In those who had ≥2 and ≥3 exacerbations in the previous year, improvements with dupilumab ranged from −1.51 to −1.83 and −1.58 to −1.90, respectively; patients receiving placebo reported lower improvements in asthma control, ranging from −1.12 to −1.45 and −1.07 to −1.47, respectively (LS mean difference versus placebo ranged from −0.29 to −0.56 and −0.43 to −0.61 in patients with ≥2 and ≥3 prior exacerbations, respectively; all p<0.05). Improvements were sustained at week 52 (supplementary figure S3a and b).
FIGURE 3.
Least squares (LS) mean±se change from baseline at week 24 in five-item Asthma Control Questionnaire (ACQ-5) score in patients categorised by a) baseline blood eosinophils ≥150 cells·μL−1, b) baseline blood eosinophils ≥300 cells·μL−1 and c) baseline exhaled nitric oxide fraction ≥25 ppb (intention-to-treat (ITT) or high-dose inhaled corticosteroid (ICS)). *: p<0.05; **: p<0.01; ***: p<0.001 versus placebo.
As with exacerbation rate and FEV1 end-points, in the subgroup of patients without elevated baseline biomarkers, numerical improvements with dupilumab versus placebo were observed at weeks 24 and 52 (supplementary figure S3c), although these were largely not statistically significant.
Discussion
This post hoc analysis of the LIBERTY ASTHMA QUEST phase 3, randomised, placebo-controlled trial assessed the effect of add-on dupilumab treatment on annualised rates of severe exacerbations, lung function (pre-bronchodilator FEV1) and asthma control (ACQ-5 score) in subgroups of patients categorised by exacerbation history, i.e. ≥1, ≥2 or ≥3 exacerbations in the year prior to study enrolment. These subpopulations were further stratified by elevated baseline levels of blood eosinophils (≥150 or ≥300 cells·μL−1) and FENO (≥25 ppb), i.e. two biomarkers of type 2 inflammation, and ICS dose at entry (medium or high). The rationale for selecting these strata was based on the knowledge that recent exacerbations independently predict future exacerbation risk [4, 5], and that patients who have elevated type 2 biomarkers and/or are receiving high-dose ICS have more severe disease and are therefore at greater risk of asthma exacerbations [3, 24]. Indeed, these observations were corroborated by the baseline characteristics of the patients, with the subgroup of patients who had experienced ≥3 exacerbations in the year prior to study entry displaying relatively worse lung function (pre-bronchodilator FEV1) and a higher requirement for ICS use. Moreover, there also appeared to be a strong link between the number of previous exacerbations experienced and baseline type 2 biomarker levels, indicating that patients with a stronger type 2 signature have higher exacerbation risk.
Across all subgroups, dupilumab 200 and 300 mg every 2 weeks (combined) versus matched placebo significantly reduced severe annualised exacerbation rates, irrespective of the number of exacerbations the patients had experienced in the year prior to the study start. Additionally, there was a trend showing that the magnitude of the improvements compared with placebo increased along with the number of severe asthma exacerbation events experienced in the previous year. Because the risk of exacerbations tends to increase proportionally to the number of recent exacerbations [4, 5], this finding suggests that dupilumab suppresses this increased exacerbation risk, a phenomenon that has also been reported for other asthma biologics [22, 25]. Across all high type 2 biomarker subgroups, even in those considered to be the most severe and difficult to treat due to high rates of prior exacerbations coupled with a high type 2 signature, dupilumab treatment exhibited considerable efficacy in improving lung function, with improvements of up to 0.38 L compared with placebo. Clinically meaningful improvements were also observed in ACQ-5 score [23], indicating better asthma control, regardless of the number of severe asthma exacerbations patients had experienced in the previous year, or their baseline level of type 2 biomarkers or ICS dose. Even in the most severe patients with a high type 2 signature who had experienced ≥3 exacerbations in the previous year, clinically meaningful and statistically significant improvements in ACQ-5 score were observed at week 24 with dupilumab compared with placebo.
For all end-points, numerical improvements with dupilumab compared with placebo treatment were observed in the subgroup of patients who did not display a type 2 phenotype (i.e. who had baseline biomarker concentrations of eosinophils <150 cells·µL−1 and FENO <25 ppb). However, most of these improvements were not statistically significant and the magnitude of the treatment effects was minimal compared with those observed in type 2-high subpopulations. That greater treatment effects were observed in patients with elevated type 2 biomarkers is consistent with the mechanism of action of dupilumab, which by binding to IL-4 receptor α inhibits the type 2 cytokines IL-4 and IL-13, and thus the type 2 inflammation exhibited by these patients [9]. Similar findings have been reported in other dupilumab asthma studies [14–16].
Although the data analysed in this study were collected in a large, randomised, stringently controlled clinical trial, a limitation of the analysis presented is its post hoc nature, as the parent study was not powered to specifically investigate differences in the subpopulations described. Accordingly, sample sizes for many of the subgroups analysed and described were low. Additionally, the aetiology of many of the exacerbations assessed in this study, whether in the year prior to study start or during the study itself, were unknown. Exacerbations can be triggered by multiple factors, including viral or bacterial respiratory infection, environmental allergens, pollutants and occupational exposures [26]. With hindsight, better characterisation of the causes of the exacerbations studied could have facilitated a comparative examination of dupilumab efficacy in each of these phenotypes.
In conclusion, this post hoc analysis confirms that the significant reduction in severe exacerbation rates and improvements in asthma control and lung function observed with dupilumab in the overall ITT population of LIBERTY ASTHMA QUEST extend to patients with elevated type 2 inflammation biomarkers, irrespective of exacerbation history and ICS dose at baseline. These findings suggest that prior history of exacerbations adds no further value to prognostication for the treating clinician but is of considerable value to clinicians in order to understand the efficacy of dupilumab, particularly among patients with a very severe disease burden.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04498-2020.SUPPLEMENT (1.5MB, pdf)
Shareable PDF
Acknowledgements
We thank Nora Crikelair (Regeneron Pharmaceuticals, Inc., USA) and Colin Mitchell (Sanofi, USA) for their contributions. Medical writing/editorial assistance was provided by Sinéad Holland (Excerpta Medica, UK).
Footnotes
This article has supplementary material available from erj.ersjournals.com
LIBERTY ASTHMA QUEST is registered at ClinicalTrials.gov with identifier number NCT02414854. Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi's data sharing criteria, eligible studies and process for requesting access can be found at: http://www.clinicalstudydatarequest.com.
Conflict of interest: J. Corren reports nonfinancial support for research from Sanofi, outside the submitted work.
Conflict of interest: C.H. Katelaris reports nonfinancial support (advisory board member and principal study investigator) from Sanofi, outside the submitted work.
Conflict of interest: M. Castro reports nonfinancial support for research from the American Lung Association, Gossamer, NIH, PCORI and Shionogi, personal fees for lectures and nonfinancial support for research from AstraZeneca, personal fees for lectures and consultancy, and nonfinancial support for research from Boehringer Ingelheim and Sanofi, personal fees for consultancy and nonfinancial support for research from Novartis, personal fees for consultancy from Boston Scientific and Vida Pharma, personal fees for lectures and consultancy from Teva, personal fees for lectures from Genentech and Regeneron Pharmaceuticals, Inc., personal fees (royalties) from Elsevier, outside the submitted work.
Conflict of interest: J.F. Maspero reports being a speaker for AstraZeneca, GlaxoSmithKline, Menarini, Novartis, Sanofi, Teva and Uriach; and has received research grants from Novartis, outside the submitted work.
Conflict of interest: L.B. Ford reports nonfinancial support for research from AstraZeneca, DBV, Genentech, Gossamer, Novartis and Teva, personal fees for consultancy from Sanofi, outside the submitted work.
Conflict of interest: D.M.G. Halpin reports personal fees for lectures and advisory board work from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Novartis, Pfizer, Sandoz and Sanofi, outside the submitted work.
Conflict of interest: M.S. Rice is an employee of Sanofi and may hold stock and/or stock options in the company.
Conflict of interest: A. Radwan is an employee and shareholder of Regeneron Pharmaceuticals, Inc.
Conflict of interest: Y. Deniz is an employee and shareholder of Regeneron Pharmaceuticals, Inc.
Conflict of interest: P.J. Rowe is an employee of Sanofi and may hold stock and/or stock options in the company.
Conflict of interest: A. Teper is a former employee of Sanofi.
Conflict of interest: M. Djandji is an employee of Sanofi and may hold stock and/or stock options in the company.
Support statement: Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing/editorial assistance was funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Krishnan V, Diette GB, Rand CS, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med 2006; 174: 633–638. doi: 10.1164/rccm.200601-007OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lane S, Molina J, Plusa T. An international observational prospective study to determine the cost of asthma exacerbations (COAX). Respir Med 2006; 100: 434–450. doi: 10.1016/j.rmed.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 3.Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. doi: 10.1038/nri3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MK, Lee JH, Miller DP, et al. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med 2007; 101: 481–489. doi: 10.1016/j.rmed.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka A, Uno T, Sato H, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: a prospective 1-year follow-up study. Allergol Int 2017; 66: 568–573. doi: 10.1016/j.alit.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 6.Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci USA 2014; 111: 5147–5152. doi: 10.1073/pnas.1323896111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanisation of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci USA 2014; 111: 5153–5158. doi: 10.1073/pnas.1324022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol 2017; 13: 425–437. doi: 10.1080/1744666X.2017.1298443 [DOI] [PubMed] [Google Scholar]
- 9.Le Floc'h A, Allinne J, Nagashima K, et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020; 75: 1188–1204. doi: 10.1111/all.14151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locksley RM. Asthma and allergic inflammation. Cell 2010; 140: 777–783. doi: 10.1016/j.cell.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regeneron Pharmaceuticals, Inc . DUPIXENT (dupilumab) injection, for subcutaneous use. Prescribing information. 2017. www.regeneron.com/sites/default/files/Dupixent_FPI.pdf Date last accessed: 1 October 2020.
- 12.Sanofi-Aventis Groupe . DUPIXENT (dupilumab) 300 mg solution for injection in pre-filled syringe. Summary of product characteristics. 2017. https://ec.europa.eu/health/documents/community-register/2019/20190506144541/anx_144541_en.pdf Date last accessed: 1 October 2020.
- 13.Pharmaceuticals and Medical Devices Agency . DUPIXENT (dupilumab). 2017. www.pmda.go.jp/files/000235351.pdf Date last accessed: 1 September 2020.
- 14.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31–44. doi: 10.1016/S0140-6736(16)30307-5 [DOI] [PubMed] [Google Scholar]
- 15.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 16.Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 17.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 18.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. doi: 10.1016/S0140-6736(17)31191-1 [DOI] [PubMed] [Google Scholar]
- 19.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335–2348. doi: 10.1056/NEJMoa1610020 [DOI] [PubMed] [Google Scholar]
- 20.Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet 2016; 387: 40–52. doi: 10.1016/S0140-6736(15)00388-8 [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2015. Available from: http://ginasthma.org/
- 22.Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: phase 3 randomised, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther 2018; 35: 737–748. doi: 10.1007/s12325-018-0702-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juniper EF, Svensson K, Mörk AC, et al. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005; 99: 553–558. doi: 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 24.O'Byrne P, Fabbri LM, Pavord ID, et al. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J 2019; 54: 1900491. doi: 10.1183/13993003.00491-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edris A, De Feyter S, Maes T, et al. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respir Res 2019; 20: 179. doi: 10.1186/s12931-019-1138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AM, Busse WW. Asthma exacerbations. 2: aetiology. Thorax 2006; 61: 809–816. doi: 10.1136/thx.2005.045179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-04498-2020.SUPPLEMENT (1.5MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-04498-2020.Shareable (240.1KB, pdf)