Abstract
Objectives: To identify the lowest dose of Depo-Provera that, when administered off-label subcutaneously, suppressed ovulation and had a pharmacokinetic profile consistent with a 4-month contraceptive effect.
Study Design: We conducted a randomized, multicenter, parallel-group study to evaluate the pharmacokinetics (PK) and pharmacodynamics (PD) of medroxyprogesterone acetate (MPA) after subcutaneous injection of three different doses of Depo-Provera. We randomized sixty women between 18 and 40 years of age at low risk of pregnancy with confirmed ovulation and body mass index of 18 to 35 kg/m2 to receive a single injection of 45, 75 or 105 mg of Depo-Provera, or a single injection of Depo-subQ provera 104 as a reference drug (15 women per group) and followed them for 7.5 months. We evaluated suppression of ovulation as the primary outcome, and MPA concentrations, pharmacokinetic parameters, safety, and local tolerability as secondary outcomes.
Results: Five women ovulated within four months of treatment initiation (three in the 45 mg group and two in the 75 mg group). MPA levels associated with ovulation were in general low, largely ≤ 0.2 ng/mL (the presumed contraceptive threshold). No women in either the 105 mg group or the Depo-subQ provera 104 group ovulated within four months. The PK parameters including Cmax, C119, and AUC0−119 for these 2 groups were similar but not equivalent.
Conclusion: A dose of 105 mg of Depo-Provera injected subcutaneously was the lowest tested dose that consistently suppressed ovulation and maintained serum MPA levels consistent with contraceptive effect for at least 4 months. The PK and PD results for the 105 mg dose were similar to Depo-subQ provera 104 over this period.
Key words: Contraception, Depot medroxyprogesterone acetate, Depo-provera, Ovulation, Pharmacokinetics, Subcutaneous
Implications
Depo-subQ provera 104 and Sayana Press may provide effective contraception for 4 months. A direct comparison of the PK and PD profiles of Depo-subQ provera 104 and 105 mg of Depo-Provera administered subcutaneously opens the possibility of developing an alternative lower-cost subcutaneous MPA injectable.
1. Introduction
Three-month Depo-Provera (medroxyprogesterone acetate (MPA) injectable suspension, 150 mg/mL) for intramuscular use (hereinafter referred to as Depo-Provera) and its generic alternatives have been used by millions of women worldwide for decades [1]. More recently, Pfizer, Inc. (New York City, NY, USA) developed a lower-dose MPA injectable suspension (104 mg/0.65 mL) for subcutaneous administration. This product is available in a pre-filled glass syringe (depo-subQ provera 104, hereinafter referred to as Depo-subQ 104) and in a pre-filled, single-dose, non-reusable subcutaneous injection system (Sayana Press). Sayana Press is approved in the European Union and approximately 24 countries worldwide, and is currently undergoing global introduction in low- and middle-income countries supported by multiple donors [2,3]. Currently, there are no generic alternatives to the 104 mg subcutaneous formulation.
Despite the 30% lower drug dose load compared to intramuscular Depo-Provera, the subcutaneous 104 mg formulation is still associated with side effects some of which are dose-dependent (e.g., weight change, reduced bone mineral density (BMD), amenorrhea, delayed return to ovulation) [4,5]. Therefore, further lowering the dose of MPA is a desirable goal supported by ample clinical evidence. Phase 3 trials observed zero pregnancies among over 2000 women using Depo-SubQ 104 for up to 1 year [6,7]. This high efficacy is consistent with the existing pharmacokinetic (PK) and pharmacodynamic (PD) data. Among 68 subjects who received a single injection of Depo-subQ 104 or Sayana Press, none showed ovarian activity during Days 93 to 150 [8]. In the dose-finding study of Depo-Provera administered subcutaneously leading to the development of Depo-subQ 104 [9], both the 50 and 75 mg doses suppressed ovulation and maintained MPA concentrations at or above 0.2 ng/mL, the level presumed necessary to exert a consistent contraceptive effect [10], for at least three months. Based on these findings, we hypothesized that subcutaneous administration of Depo-Provera at a dose lower than 104 mg may be effective for three months, which could noticeably lower MPA exposure compared to current subcutaneous and intramuscular regimens.
We conducted a PK/PD study of 45, 75, and 105 mg of Depo-Provera injected subcutaneously (off-label) to determine whether a lower dose would suppress ovulation and have a PK profile consistent with a contraceptive effect for four months (three months of use followed by a 1-month grace period for re-injection).
2. Materials and methods
2.1. Study design and participants
We conducted a four-arm randomized partially blinded parallel-group study (clinicaltrials.gov no: NCT02732418) at three investigational centers: Biomedical Research Department at PROFAMILIA (Santo Domingo, Dominican Republic), Family Planning clinic, Department of Obstetrics and Gynecology, School of Medicine, University of Campinas (Campinas, Brazil), and Instituto Chileno de Medicina Reproductiva (ICMER) (Santiago, Chile) between November 2016 and May 2018. FHI 360′s Protection of Human Subjects Committee and Institutional Review Boards of participating centers approved the study. All participants gave written informed consent before entering the study.
We enrolled 60 non-pregnant women between 18 and 40 years of age, with regular menstrual cycles, at low risk of pregnancy (using permanent contraception or a copper intrauterine device (IUD)) and with a body mass index (BMI) of 18 to 35 kg/m2. We confirmed ovulation in all women prior to enrollment by two consecutive progesterone (P) measurements ≥ 4.7 ng/mL obtained within five days during the two to three weeks preceding menses [11]. We excluded women who had medical contraindications or were allergic to MPA, were recently pregnant (within 3 months), had used any hormonal contraceptives in a month prior to enrollment, had used MPA injectables in the last 12 months or monthly injectables in the last 6 months.
We randomized participants using a 1:1:1:1 allocation ratio to receive a single injection of one of three doses of Depo-Provera (150 mg/mL): 45 mg/0.3 mL (45 mg group), 75 mg/0.5 mL (75 mg group) or 105 mg/0.7 mL (105 mg group), or of Depo-subQ 104 (104 mg/0.65 mL; reference group). We generated the randomization sequence, stratified by investigational site with a block size of 4, using SAS/STAT software, and concealed treatment allocation using sequentially numbered opaque randomization envelopes.
The study was not fully blinded due to differences in volume and packaging of the study drugs. To minimize potential bias, clinical staff prepared the syringes and administered injections outside the view of the participant. Staff involved in randomization and administering injections were not involved in assessment of study outcomes. All other research staff and laboratory personnel remained blinded to treatment assignments throughout the study.
2.2. Procedures
We administered all injections in the abdomen within the first 5 days of the menstrual cycle. We followed the subcutaneous injection technique recommended by Pfizer [4]. To maintain the same depth of injection in all study groups, we administered Depo-Provera through a 26-gage 3/8-inch needle, the needle size provided with the prefilled Depo-subQ 104 syringe. We measured blood pressure (BP), height and weight, and collected blood samples for MPA, P, and estradiol (E2) prior to injection.
We monitored participants for 7.5 months (224 days) after the injection. We defined the “Month 4″ timepoint as 119 days after treatment initiation. We measured serum P and E2 on Days 3 and 7, and then weekly through Week 9. Starting in Week 10, we performed transvaginal sonography (TVS), and measured P and E2 until ovulation or Month 7.5, whichever was earlier, following an algorithm designed to increase the likelihood of detecting follicle rupture (supplemental Figure S2). We measured serum MPA levels on Days 1, 3, 7, 10, 14, 21, 28, 35, 42; then at Weeks 8, 10, 12, 13, 15, 17, 19, 21, 23, 25, 26, 28, 30, and 32. We evaluated study participants for weight, BP, adverse events (AEs), concomitant medication use, bleeding patterns, and acceptability throughout the study.
2.3. Outcomes
Our primary PD endpoint was time to ovulation where ovulation was defined as rupture of the lead follicle followed by a single elevated P ≥ 4.7 ng/mL, or a single elevated P of ≥ 4.7 ng/mL in the absence of an ultrasound result. We formulated our definition of ovulation based on the approach previously used in PK/PD studies of Depo-subQ 104 based on a single elevated P of ≥ 4.7 ng/mL combined with Hoogland Scores, a commonly used classification of ovarian function [10,12,13].
To reduce inter-observer variability, a single technician at each investigational site performed TVS using a 6.5-MHz vaginal transducer with a real-time scanner (Toshiba Ultrasound System Nemio MX, or similar equipment). We defined a lead (or dominant) follicle as a follicle with the mean diameter of 12 mm or more, and follicle rupture as abrupt disappearance or a reduction in size of at least 50% of the echo image of the lead follicle compared to the previous exam. Transvaginal sonography was scheduled weekly starting at Week 10, and then biweekly after a follicle ≥15 mm was detected (supplemental Figure S2). Previous research demonstrated that the biweekly schedule was sufficient to reliably detect the follicle rupture [14,15]. We assessed various supportive PD parameters, including duration of the luteal phase, maximum ovulatory P concentration, confirmatory measurement of P within 5 days of the initial elevated result, and quality of cervical mucus in women with follicular development. The supportive data will be described in a separate publication.
Secondary PK outcomes included the maximum serum MPA concentration (Cmax), time to Cmax (Tmax), drug concentrations on Days 91 (C91) and 119 (C119), area under the concentration-time profile (AUC) through day 91 (AUC0–91), day 119 (AUC0–119), and extrapolated to infinity (AUC0-∞), and the apparent terminal half-life.
MPA was assayed centrally by PPD Development (PPD; Richmond, VA, USA). After collection, sites stored serum samples at −20°C and sent them to PPD in batches. PPD confirmed long-term stability of MPA samples at −20°C (proprietary data on file). The PPD MPA assay is a proprietary validated method that uses sensitive and selective high-performance liquid chromatography coupled with mass spectrometry (HPLC/MS). The nominal range of MPA was 0.02 to 5.00 ng/mL. Correlation coefficients were all >0.997, and inter-assay accuracy was 92.3% to 99.2%, with intra-assay accuracy between 97.8% to 100%. Local accredited laboratories measured serum E2 and P concentrations. The laboratory in the Dominican Republic used an electrochemiluminescence immunoassay (Roche Elecsys 2010) with sensitivities of 12 pg/mL and 0.15 ng/mL, respectively, and interassay coefficients of variation (CVs) of 4.3%-9.9% for E2 and 3.7%-5.5% for P. The laboratory in Chile measured E2 and P concentrations by an electrochemiluminescence immunoassay (Roche Cobas) with sensitivities of 5.0 pg/mL and 0.05 ng/mL, respectively, and interassay CVs of 3.9% to 5.6% for E2 and 3.3% to 8.2% for P. The laboratory in Brazil measured E2 and P concentrations by electrochemiluminescence immunoassay (Siemens Advia Centaur) with sensitivities of 18 pmol/L and 0.1 nmol/L, respectively, and interassay CVs of 2.3% to 6.2% for E2 and 0.9% to 2.0% for P.
We assessed safety throughout the study by monitoring BP, pulse, weight, circulating levels of E2, use of concomitant medications, bleeding pattern, occurrence of AEs, and injections site reactions (ISRs). We asked questions about bleeding and spotting at Months 3 and 7.5 including but not limited to the date of last bleeding and a description of bleeding/spotting pattern since last visit, how acceptable bleeding patterns were and whether participants would use this method outside this study.
2.4. Statistical analysis
Based on similar PK/PD studies [9,13], we considered the target sample size of 15 women in each group sufficient to provide meaningful insights into the distributions of PK and PD outcomes, and to inform the selection of a dose for potential efficacy studies. The PD and PK analyses were performed on the primary evaluable population that excluded participants with detectable MPA at baseline (> 5% of a participant's Cmax) or with major protocol violations at enrollment that could interfere with interpretation of the PK and PD data. These analyses also excluded time after a participant initiated use of any medications known to impact PK or PD of MPA.
We summarized demographic and baseline characteristics descriptively by treatment group and site. We explored overall differences across sites using analysis of variance for continuous variables and Fisher's Exact tests for categorical variables. We estimated the cumulative probability of return to ovulation in each treatment group through Month 7.5 from treatment initiation based on the Kaplan-Meier product-limit method, with 95% confidence intervals (CI) derived using the complementary log-log transformation.
We estimated PK parameters for each participant based on non-compartmental analysis implemented using a validated SAS macro. We calculated AUC values using the linear/log trapezoidal rule and imputed any missing concentrations for target times within the range of data using the two specimens collected closest in time to the target date. If the missing concentration fell after a participant's discontinuation or after the first value below the limit of quantification, we imputed the value using the estimated terminal phase rate constant. The calculation for terminal half-life excluded any specimens obtained on or after the first occurrence of a value below the limit of quantification. We calculated geometric means (GMs) and percent coefficients of variation by treatment group for each PK parameter. We used GM ratios with 90% CI to compare the Depo-Provera and reference groups, but we did no formal bioequivalence hypothesis tests. We summarized GM MPA concentrations graphically. We also summarized select PK parameters by treatment group, site, BMI, and age and exploratorily assessed for significance using analysis of covariance. All analyses were conducted using SAS/STAT software, Version 9.4 of the SAS System for Windows.
3. Results
3.1. Demographic and baseline characteristics
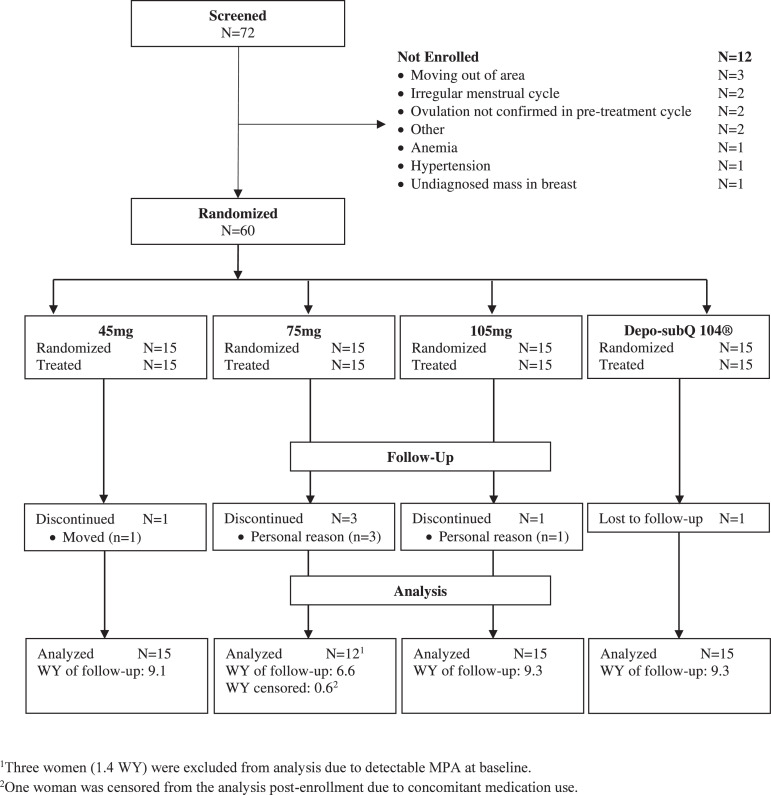
We screened 72 healthy women and randomized 60 (83.3%) to one of four study groups (15 women per group). Most participants (n = 54, 90.0%) completed the study (Fig. 1). We censored three women in the 75 mg group due to detectable MPA at baseline and one woman (0.6 women-years) from the primary analysis due to the use of prohibited medication.
Fig. 1.
Participant Disposition.
There were no meaningful differences between the treatment groups in the demographic and baseline characteristics (Table 1). Median age was 33 (range: 22 – 40). Median weight and BMI were 68.1 kg and 26.9 kg/m2, respectively. Approximately half of women (53%) used permanent contraception, and the rest used a copper IUD.
Table 1.
Demographic and baseline characteristics by treatment group.
| 45 mg (n = 15) | 75 mg (n = 12)a | 105 mg (n = 15) | Depo-subQ 104 (n = 15) | Overall (N = 57) | |
| Age at Baseline (years) | |||||
| Median (Min to Max) | 30.0 (22 – 40) | 34.5 (27 – 40) | 33.0 (24 – 39) | 36.0 (22 – 39) | 33.0 (22 – 40) |
| Cohabitation Status: N (%) | |||||
| Married/cohabitating | 9 (60.0) | 10 (83.3) | 13 (86.7) | 12 (80.0) | 44 (77.2) |
| Single | 6 (40.0) | 1 (8.3) | 1 (6.7) | 3 (20.0) | 11 (19.3) |
| Divorced | 0 (0.0) | 1 (8.3) | 1 (6.7) | 0 (0.0) | 2 (3.5) |
| Education (years) | |||||
| Median (Min to Max) | 11.0 (4 – 16) | 12.0 (1 – 18) | 12.0 (8 – 16) | 12.0 (3 – 17) | 12.0 (1 – 18) |
| Race: N (%) | |||||
| White | 8 (53.3) | 6 (50.0) | 8 (53.3) | 8 (53.3) | 30 (52.6) |
| Black or African American | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) | 1 (1.8) |
| Biracial | 7 (46.7) | 6 (50.0) | 6 (40.0) | 7 (46.7) | 26 (45.6) |
| Weight (kg) | |||||
| Median (Min to Max) | 71.9 (48 – 100) | 68.6 (46 – 84) | 68.7 (56 – 86) | 65.0 (46 – 95) | 68.1 (46 – 100) |
| BMI (kg/m2): N (%) | |||||
| < 25 | 5 (33.3) | 3 (25.0) | 2 (13.3) | 9 (60.0) | 19 (33.3) |
| 25–29 | 6 (40.0) | 5 (41.7) | 10 (66.7) | 3 (20.0) | 24 (42.1) |
| ≥ 30 | 4 (26.7) | 4 (33.3) | 3 (20.0) | 3 (20.0) | 14 (24.6) |
| Median (Min to Max) | 26.8 (19 – 34) | 27.1 (20 – 33) | 27.2 (23 – 33) | 24.8 (19 – 34) | 26.9 (19 – 34) |
| Ever pregnant: N (%) | |||||
| No | 2 (13.3) | 0 (0.0) | 0 (0.0) | 2 (13.3) | 4 (7.0) |
| Yes | 13 (86.7) | 12 (100) | 15 (100) | 13 (86.7) | 53 (93.0) |
| Contraceptive methods | |||||
| used in the pastb: N (%) | |||||
| Oral contraceptives | 11 (73.3) | 5 (41.7) | 10 (66.7) | 11 (73.3) | 37 (64.9) |
| Non-hormonal IUD | 4 (26.7) | 4 (33.3) | 4 (26.7) | 4 (26.7) | 16 (28.1) |
| Depo-Povera | 6 (40.0) | 5 (41.7) | 3 (20.0) | 4 (26.7) | 18 (31.6) |
| Combined injectable | 3 (20.0) | 4 (33.3) | 2 (13.3) | 3 (20.0) | 12 (21.1) |
| Condoms | 9 (60.0) | 5 (41.7) | 6 (40.0) | 9 (60.0) | 29 (50.9) |
| Contraceptive method | |||||
| using currently: N (%) | |||||
| Permanent contraception | 7 (46.7) | 8 (66.7) | 8 (53.3) | 8 (53.3) | 31 (54.4) |
| Copper IUD | 8 (53.3) | 4 (33.3) | 7 (46.7) | 7 (46.7) | 26 (45.6) |
Excludes data from three women due to elevated MPA levels at baseline.
Only methods reported by at least 20% of participants. Multiple responses may apply.
There were differences in demographic and baseline characteristics across the sites, some of them significant (supplemental Table S1). Participants in Brazil were on average older and heavier. All participants in the Dominican Republic were either black or biracial, with 9.5 median years of education while all participants in Chile were white, with 13 median years of education. All participants in the Dominican Republic used permanent contraception while majority in Chile and Brazil were using a copper IUD.
3.2. Pharmacodynamics
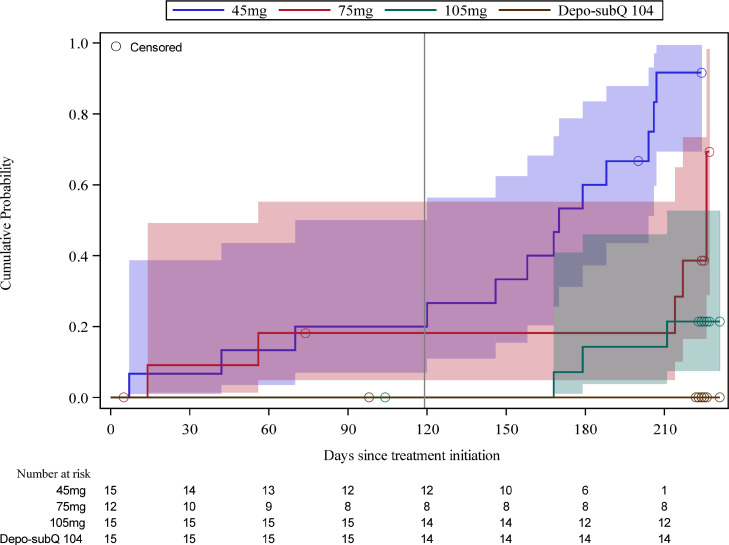
During the first four months post-injection, 3/15 (20%), 2/12 (16.7%), 0/15 (0%) women ovulated in the 45 mg, 75 mg, and 105 mg groups, respectively. Two women in the 45 mg group ovulated once (on Days 42 and 70, respectively), and one ovulated twice (on Days 7 and 36). The latter participant's injection may have been mistimed (administered too late in the cycle to suppress ovulation) as her E2 level on the day of injection of 163.7 pg/mL was inconsistent with the early follicular phase. Three of the 4 ovulations in the 45 mg group (on Days 7, 36 and 42) were characterized by elevated P only (i.e., no rupture of the lead follicle) because TVS was not routinely performed until Week 10 (supplemental Figure S2). In the 75 mg group, one woman ovulated on Days 56 and 81, and the second one on Days 14 and 104. Only the second ovulation in each woman was characterized by both the rupture of the lead follicle and elevated P, again because of the timing of initiation of routine TVS. The cumulative probability of ovulation through Month 4 was 0.2 (95% CI 0.07, 0.50) and 0.18 (95% CI 0.05, 0.55) in the 45 mg and 75 mg groups, respectively (Fig. 2).
Fig. 2.
Kaplan-Meier estimates of cumulative probabilities of return to ovulation, with 95% confidence intervals and numbers at risk below the x-axis. Median times to ovulation for the 45 mg and 75 mg groups were 170 and 226 days, respectively. Medians for the 105 mg and Depo-subQ 104 groups were not estimable due to the small number of ovulations in those groups. Reference line indicates Month 4 of follow-up.
Ten women in the 45 mg group, 3 in the 75 mg group and 3 in the 105 mg group ovulated between Month 4 and the end of the study. No participants in the reference group ovulated during the study.
3.3. Pharmacokinetics
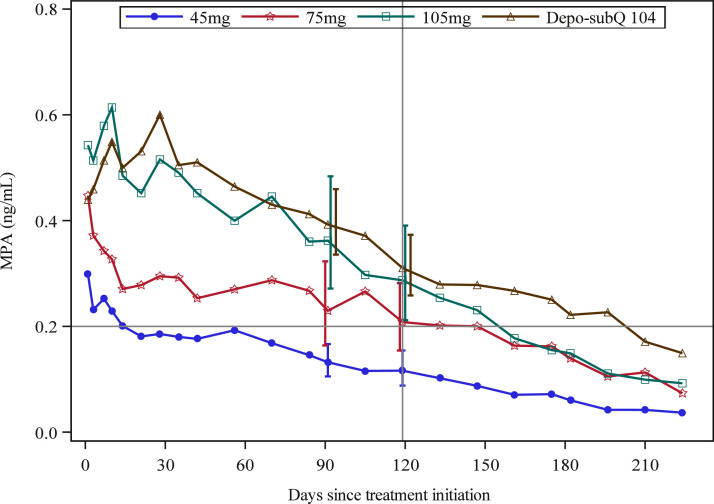
MPA remained largely ≤ 0.2 ng/mL for most of follow-up period in three women who ovulated during the first 4 months post-injection in the 45 mg group. The MPA concentration never reached 0.2 ng/mL in one woman, and barely crossed that level in the other two. In both women in the 75 mg group who ovulated during the first four months post-injection, MPA was consistently < 0.2 ng/mL after Month 2, and in one of the 2 women MPA did not reach the contraceptive threshold of 0.2 ng/mL until Day 27. At Day 119 (4 months), 12 (85.7%) and 4 (40%) participants had MPA concentrations below 0.2 ng/mL in the 45 mg and 75 mg groups, respectively.
We calculated GM MPA concentrations for all four study groups through 7.5 months of treatment (Fig. 3 and Table 2). The GM Cmax increased with dose, from 0.40 ng/mL in the 45 mg group to 0.87 ng/mL in the 105 mg group. Similarly, the GM C119 and AUC0–119 increased with dose, from 0.12 ng/mL and 21.2 days*ng/mL in the 45 mg group to 0.29 ng/mL and 52.0 days*ng/mL in the 105 mg group (Table 2).
Fig. 3.
Geometric Mean MPA Concentrations with 95% CIs (shifted slightly for visibility). Horizontal reference line at 0.2 ng/mL is the presumed MPA threshold required to exert consistent contraceptive effect, and vertical reference line indicates Month 4 of follow-up.
Table 2.
Geometric mean (GM) and percent coefficient of variation (%CV) of MPA PK parameter estimates by treatment group.
| 45mg | 75mg | 105mg | Depo-subQ 104 | |||||
| N | GM (%CV) | n | GM (%CV) | N | GM (%CV) | n | GM (%CV) | |
| Cmax | 15 | 0.40 (73.5) | 12 | 0.60 (68.1) | 15 | 0.87 (44.0) | 15 | 0.81 (48.0) |
| Tmax | 15 | 4.2 (152.2) | 12 | 4.9 (214.5) | 15 | 14.2 (128.2) | 15 | 16.7 (84.6) |
| C91 | 15 | 0.13 (41.9) | 10 | 0.23 (45.1) | 15 | 0.36 (50.9) | 15 | 0.39 (29.7) |
| C119 | 14 | 0.12 (48.8) | 10 | 0.21 (38.5) | 14 | 0.29 (43.7) | 13 | 0.31 (27.7) |
| AUC0–91 | 15 | 17.8 (37.1) | 11 | 26.0 (44.2) | 15 | 43.2 (29.3) | 15 | 44.6 (28.8) |
| AUC0–119 | 15 | 21.2 (35.2) | 11 | 32.6 (44.1) | 15 | 52.0 (29.1) | 15 | 54.5 (26.5) |
| AUC0-inf | 15 | 36.0 (26.8) | 10 | 59.8 (31.3) | 14 | 81.3 (30.4) | 15 | 99.6 (24.0) |
| Half-life | 15 | 72.9 (56.6) | 10 | 67.4 (75.6) | 14 | 47.1 (74.6) | 15 | 69.2 (71.3) |
AUC0-x , area under the concentration-time curve (days*ng/mL) from day 0 to day x; half-life (days); Cmax , maximum concentration; Tmax , time (days) of maximum concentration, Cx = concentration (ng/mL) at day x.
The PK profiles of 105 mg of Depo-Provera and Depo-subQ 104 were similar albeit not equivalent during the first four months after the injection, with GM ratios (90% CI) for Cmax, C119, and AUC0–119 of 1.07 (0.83, 1.39), 0.93 (0.69, 1.24), and 0.95 (0.79, 1.16), respectively. At Day 119, 14.3% (2/14) and 15.4% (2/13) participants had MPA concentrations below 0.2 ng/mL in the 105 mg and reference groups, respectively. After Day 119, however, MPA concentrations generally declined more rapidly in the 105 mg group compared to Depo-SubQ 104, resulting in a GM ratio AUC0-inf of 0.82 (0.68, 0.99).
The observed individual MPA levels when ovulation returned across participants in all treatment groups ranged between 0.020 (below the limit of detection) and 0.188 ng/mL, with a median value of 0.060. Study site and BMI had significant impact on key PK parameters (all p-values ≤ 0.02) when assessed in a model adjusting for treatment group, site, BMI, and age, with participants at the Chile site and participants with lower BMI generally having larger estimated PK values (supplemental Table S2). Age did not have a significant impact on PK (all p-values ≥ 0.09).
3.4. Safety and acceptability
Injection site reactions were infrequent. Five participants reported minor ISRs (e.g., pain, redness, itching) that all resolved within 7 days after occurrence. The mean highest E2 concentrations measured in 30-day intervals increased from ∼ 50 pg/mL at baseline (Day 0) to 300 pg/mL in the first month post-injection in the 45 mg and 75 mg groups and remained in the 200–400 pg/mL range for the duration of the study. Estradiol levels in the 105 mg and Depo-subQ 104 groups remained below 200 pg/mL for at least three months post-injection but increased in the subsequent months (supplemental Figure S1).
At least 75% of the participants in all treatment groups experienced disrupted bleeding patterns (mainly spotting and irregular bleeding) at Month 3 with no substantial difference between groups. More participants in the 45 mg and 75 mg groups reported having regular bleeding at Month 7.5 (at least 40%) compared to the higher dose groups (less than 30%; supplemental Table S3). Notwithstanding bleeding irregularities, at the end of the study at least 50% of participants in each group found their bleeding pattern acceptable and would use this method for contraception outside the study.
4. Discussion
A single subcutaneous injection of 45 mg and 75 mg of Depo-Provera in our study did not achieve consistent suppression of ovulation over the first 4 months following injection. The MPA levels in all women that ovulated during this period were in general low, remaining largely ≤0.2 ng/mL. We observed somewhat different PK and PD results than reported in the dose-range finding study conducted by Pfizer [9]. While this could be due to chance, several factors could have contributed to actual effects. Pfizer used saline-diluted Depo-Provera to obtain the 50 mg dose in 0.5 mL, whereas we used 0.3 mL of undiluted 150 mg/mL Depo-Provera to obtain the 45 mg dose. Higher concentration formulations and smaller injection volumes are known to reduce maximum plasma concentrations and delay absorption for some drugs [16]. Different length of the needle, injection technique, or site of injection could have played a role as well. Regardless, subcutaneous administration of 45 and 75 mg Depo-Provera proved insufficient to yield a PK/PD response consistent with a contraceptive effect for 4 months (3 months of use plus a 1-month grace period for re-injection).
A subcutaneous dose of 105 mg of Depo-Provera was the lowest dose in our study that effectively suppressed ovulation for 4 months. From a PK perspective, only 4 participants in this group had MPA concentrations < 0.2 ng/mL at any point in the first 4 months of treatment and only 2 participants had MPA concentrations < 0.2 ng/mL on Day 119 (Month 4).
The PK profile associated with a single injection of 105 mg Depo-Provera administered subcutaneously was similar to that of Depo-subQ 104 for 4 months. However, between Month 4 and the end of the study, MPA levels in the 105 mg group were generally lower than in the reference group. Consistent with this finding, 3 participants ovulated in the 105 mg group over this period, compared to none in the Depo-subQ 104 group. In response to these findings, we assessed expelled volume from 6 pre-filled Depo-subQ 104 syringes and found volumes 5% to 10% higher in a laboratory setting than the nominal 0.65 mL (data on file). If this held true in a clinical site setting, the slightly larger dose expelled could potentially explain the observed differences in the PK and PD responses of the 105 mg and reference groups beyond Month 4.
Many side effects of MPA-containing injectables are dose dependent, including the effect on BMD that is related to hypoestrogenism due to the suppressed ovarian E2 production [17]. In our study lower doses of MPA led to higher circulating levels of E2 that could potentially lead to decreased adverse impact on BMD. Higher doses of MPA, as expected, led to a more profound suppression of the ovarian activity in the first 3 months. However, the subsequent increase of E2 levels could result in a fuller recovery of the ovarian function between re-injections and lower the risk of hypoestrogenism if re-injection interval is extended. These findings provide support for alternative strategies to lower overall MPA exposure and potentially improve the safety profile of MPA injectables, such as administering Sayana Press 3 times a year instead of 4 (clinicaltrials.gov no: NCT03154125) and administering 150 mg of Depo-Provera subcutaneously twice a year for a 6-month protection [18].
Our study has limitations. To reduce variability of MPA measures, we limited BMI to 35 kg/m2 and used the abdomen as a single injection site. Whether our results are generalizable to a broader population and for other sites of injection is unclear. In addition, the modest sample size was not designed to make definitive conclusions regarding efficacy or comparative PK without additional studies. Finally, the unblinded nature of the study introduced the possibility of bias. But the objective nature of the main study outcomes and the fact that the laboratory personnel conducting assessments were blinded mitigated potential impact of bias on validity of the study results. Strengths of this study include a randomized design, a robust PD algorithm for the ovulation detection based on both hormonal and ultrasound measurements, and excellent retention rates at all 3 sites.
Our study has important potential programmatic and public health implications. These results support previous conclusions that Depo-subQ 104 may be effective for at least 4 months [9], and lend further justification for the clinical trial evaluating contraceptive effectiveness of Sayana Press administered 3 times a year (clinicaltrials.gov no: NCT03154125). Extending the re-injection interval from 3 to 4 months would reduce MPA exposure by 25%, possibly improve dose-dependent side effects, and decrease programmatic costs, thereby facilitating access to Sayana Press in resource-constrained settings. While the value and feasibility of seeking to change the duration of action from 3 to 4 months requires careful consideration, extending the grace period for re-injection from 1 to 4 weeks in the label would be a valuable programmatic attribute [19].
Lastly, our data demonstrating similar PK and PD profiles of Depo-subQ 104 and 105 mg of Depo-Provera during the first 4 months of treatment suggest a product development path for an alternative lower-cost subcutaneous MPA injectable.
Acknowledgments
Acknowledgments
We are grateful to the study participants and research staff at PROFAMILIA in the Dominican Republic, UNICAMP in Brazil, and ICMER in Chile for their hard work, dedication to our research and common vision of improving life of women around the world by increasing access to safe and effective contraception.
Data sharing
Individual participant data, after de-identification, will be submitted to the Development Data Library (DDL), USAID's publicly available repository for Agency-funded data-on-demand.
Footnotes
✰ Declaration of Competing Interest: We declare no conflict of interest.
✰✰ Funding: This work was made possible by the generous support of the American people through the U.S. Agency for International Development (USAID), provided to FHI 360 through Cooperative Agreement AID-OAA-A-15–00045. The contents are the responsibility of FHI 360 and do not necessarily reflect the views of the USAID or the United States Government. The funder was not involved in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.conx.2021.100070.
Appendix. Supplementary materials
References
- 1.United Nations Department of Economics and Social Affairs. Contraceptive Use by Method. 2019 https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Jan/un_2019_contraceptiveusebymethod_databooklet.pdf Available at: Accessed December 2, 2020. [Google Scholar]
- 2.Polis C.B., Achilles S.L., Hel Z., Hapgood J.P. Is a lower-dose, subcutaneous contraceptive injectable containing depot medroxyprogesterone acetate likely to impact women's risk of HIV? Contraception. 2018;97:191–197. doi: 10.1016/j.contraception.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PATH. How to Introduce and Scale Up Subcutaneous DMPA (Sayana Press): practical Guidance from PATH Based on Lessons Learned During Pilot Introduction. 2018.
- 4.Pharmacia & Upjohn Company LLC DEPO-SUBQ PROVERA 104 (medroxyprogesterone acetate) injectable suspension, for subcutaneous use. Prescribing Information. 2020 Available at: http://labeling.pfizer.com/ShowLabeling.aspx?id=549. Accessed June 10, 2021. [Google Scholar]
- 5.Pantoja M., Medeiros T., Baccarin M.C., Morais S.S., Bahamondes L., Fernandes A.M. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81:107–111. doi: 10.1016/j.contraception.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Jain J., Jakimiuk A.J., Bode F.R., Ross D., Kaunitz A.M. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269–275. doi: 10.1016/j.contraception.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Kaunitz A.M., Darney P.D., Ross D., Wolter K.D., Speroff L. Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80:7–17. doi: 10.1016/j.contraception.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 8.MHRA Public Assessment Report: sayana Press 104 mg/0.65 mL suspension for injection. MHRA, United Kingdom. 2015 [Google Scholar]
- 9.Shelton J.D., Halpern V. Subcutaneous DMPA: a better lower dose approach. Contraception. 2014;89:341–343. doi: 10.1016/j.contraception.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Jain J., Dutton C., Nicosia A., Wajszczuk C., Bode F.R., Mishell D.R.J. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception. 2004;70:11–18. doi: 10.1016/j.contraception.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Landgren B.M., Unden A.L., Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol. 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 12.Westhoff C.L., Torgal A.H., Mayeda E.R., Stanczyk F.Z., Lerner J.P., Benn E.K. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116:275–283. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 13.Toh Y.C., Jain J., Rahimy M.H., Bode F.R., Ross D. Suppression of ovulation by a new subcutaneous depot medroxyprogesterone acetate (104 mg/0.65 mL) contraceptive formulation in Asian women. Clin Ther. 2004;26:1845–1854. doi: 10.1016/j.clinthera.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Brache V., Sitruk-Ware R., Williams A., Blithe D., Croxatto H., Kumar N. Effects of a novel estrogen-free, progesterone receptor modulator contraceptive vaginal ring on inhibition of ovulation, bleeding patterns and endometrium in normal women. Contraception. 2012;85:480–488. doi: 10.1016/j.contraception.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y., Jensen J.T., Brache V., Cochon L., Williams A., Miranda M.J. A randomized study on pharmacodynamic effects of vaginal rings delivering the progesterone receptor modulator ulipristal acetate: research for a novel estrogen-free, method of contraception. Contraception. 2014;90:565–574. doi: 10.1016/j.contraception.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gradel A.K.J., Porsgaard T., Lykkesfeldt J., Seested T., Gram-Nielsen S., Kristensen N.R. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J Diabetes Res. 2018 doi: 10.1155/2018/1205121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark M.K., Sowers M., Levy B.T., Tenhundfeld P. Magnitude and variability of sequential estradiol and progesterone concentrations in women using depot medroxyprogesterone acetate for contraception. Fertil Steril. 2001;75:871–877. doi: 10.1016/s0015-0282(01)01748-4. [DOI] [PubMed] [Google Scholar]
- 18.Halpern V., Brache V., Taylor D., Lendvay A., Cochon L., Jensen J.T. Clinical trial to evaluate pharmacokinetics and pharmacodynamics of medroxyprogesterone acetate after subcutaneous administration of Depo-Provera. Fertil Steril. 2021;115:1035–1043. doi: 10.1016/j.fertnstert.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner M.J., Kwok C., Stanback J., Byamugisha J.K., Chipato T., Magwali T. Injectable contraception: what should the longest interval be for reinjections? Contraception. 2008;77:410–414. doi: 10.1016/j.contraception.2008.01.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.