Abstract
The biosynthesis pathway for riboflavin (vitamin B2), the precursor of the essential cofactors flavin mononucleotide and flavin adenine dinucleotide, is present in bacteria and plants but is absent in vertebrates. Due to their conservation in bacterial species and their absence in humans, the riboflavin synthesis genes should be well suited either for detection of bacterial DNA in human specimens or for the differentiation of pathogenic bacteria by molecular techniques. A DNA fragment carrying the genes ribD, ribC, and ribE, which encode homologues of riboflavin deaminase (RibD) and subunits of riboflavin synthetase (RibC and RibE), respectively, was isolated from a plasmid-based DNA library of the human pathogen Bartonella henselae by complementation of a ribC mutation in Escherichia coli. Sequence analysis of the ribC gene region in strains of B. henselae, which were previously shown to be genetically different, revealed that the ribC gene is highly conserved at the species level. PCR amplification with primers derived from the ribC locus of B. henselae was used to isolate the corresponding DNA regions in B. bacilliformis, B. clarridgeiae, and B. quintana. Sequence analysis indicated that the riboflavin synthesis genes are conserved and show the same operon-like genetic organization in all four Bartonella species. Primer oligonucleotides designed on the basis of localized differences within the ribC DNA region were successfully used to develop species-specific PCR assays for the differentiation of B. henselae, B. clarridgeiae, B. quintana, and B. bacilliformis. The results obtained indicate that the riboflavin synthesis genes are excellent targets for PCR-directed differentiation of these emerging pathogens. The PCR assays developed should increase our diagnostic potential to differentiate Bartonella species, especially B. henselae and the newly recognized species B. clarridgeiae.
Bacteria of the genus Bartonella are fastidious, gram-negative, slow-growing microorganisms. During recent years, the number of Bartonella species isolated increased remarkably (7, 20), and the number of recognized diseases caused by Bartonella species increased as well (2, 40). Five species are known to cause human diseases. Bartonella bacilliformis is the causative agent of bartonellosis, a biphasic disease which is endemic in regions of the South American Andes. Up to now, B. elizabethae has been isolated only once, from the blood of a patient with endocarditis (12). The two species most often involved in human infections worldwide are B. henselae and B. quintana. The latter species is the causative agent of trench fever and of bacillary angiomatosis in human immunodeficiency virus (HIV)-infected patients (46). A large number of clinical manifestations, especially cases of endocarditis in homeless people, have also been related to this agent (31). B. henselae, which was first isolated in 1992 from the blood of an HIV-infected patient (36), is the main causative agent of cat scratch disease (CSD) and is known to be involved in different clinical disorders in immunocompromised as well as in immunocompetent patients.
The newly recognized species B. clarridgeiae was first isolated from the cat of a patient with B. henselae septicemia (25) and was later detected in cat populations in India, the United States, and France (17, 19, 23, 29). Recently, two cases of CSD caused by B. clarridgeiae were described, although both cases were confirmed only serologically (24, 28).
The Bartonella species B. henselae, B. quintana, and B. clarridgeiae are phenotypically and genotypically very similar, and differentiation of these species usually requires molecular techniques. The serologic cross-reactivity between B. henselae and B. quintana in patients with CSD is very high (95%), and the seroprevalence of B. henselae in healthy people is up to 30% (33, 41). Therefore, the development of species-specific molecular techniques, especially for the detection and differentiation of infections possibly caused by the newly recognized species B. clarridgeiae, seems to be urgent.
Genes encoding enzymes of the riboflavin biosynthetic pathway (3) are evolutionarily conserved in bacteria and plants and absent in humans. They are, therefore, excellent target candidates for the detection and differentiation of invasive pathogenic bacteria. Riboflavin (vitamin B2) is the precursor of flavin mononucleotide and flavin adenine dinucleotide, which are both essential cofactors for electron transport functions of proteins involved in the basic energy metabolism of the cell.
Riboflavin is synthesized from GTP, and the corresponding biosynthetic pathway is present in bacteria, fungi, and plants but absent in vertebrates, including humans. In Escherichia coli, five enzymes, designated RibA (GTP-cyclohydrolase II), RibB (DHBP synthetase), RibC (riboflavin synthase), RibD (riboflavin deaminase/reductase), and RibE (ribityl-lumazine synthetase), are involved in riboflavin synthesis. The coding genes, designated ribA to ribE, have been most extensively investigated in E. coli (3, 13, 38) and Bacillus subtilis (35).
Homologues of the riboflavin synthesis genes were isolated from many microorganisms, including Actinobacillus pleuropneumoniae (15), Azospirillum brasilense (47), Haemophilus influenzae (14), Helicobacter pylori (5), Photobacterium spp. (26), Pichia guilliermondii (27), and Saccharomyces cerevisiae (45), and from the plant Arabidopsis thaliana (22).
The functional importance of riboflavin synthesis genes has led to their conservation during evolution, and homology among different genera is significant, as shown, e.g., for the RibA protein of H. pylori, which is 40 to 60% similar to homologues in nonrelated bacterial species (5).
This study reports the characterization of the genes ribC, ribD, and ribE from B. henselae, B. quintana, B. bacilliformis, and B. clarridgeiae. The function of the B. henselae ribC gene has been confirmed, and the sequence of the ribC DNA region could be used as a target for the molecular differentiation of Bartonella species by PCR analysis.
MATERIALS AND METHODS
Culture conditions.
Bacterial strains are listed in Table 1. B. clarridgeiae was cultivated on Columbia blood agar plates. Cultures of B. henselae and B. quintana were propagated on chocolate agar, and B. bacilliformis was grown on hemin cysteine blood agar. B. henselae, B. quintana, and B. clarridgeiae were grown at 37°C. B. bacilliformis was cultured at 30°C. Cultures were propagated in a humid atmosphere containing 5% carbon dioxide.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| B. bacilliformis ATCC 35685 | American Type Culture Collection | |
| B. clarridgeiae | ||
| 73 | From cat | 19 |
| R13762 | From cat | 29 |
| R8051 | From cat | 29 |
| B. henselae | ||
| Houston-1 | Variant IV, from HIV patient | 36 |
| FR96 strains | Variants I to IV, From cat | 42 |
| R8015 | From cat | E. Marston (29) |
| R8016 | From cat | E. Marston (29) |
| R8017 | From cat | E. Marston (29) |
| R8018 | From cat | E. Marston (29) |
| B. quintana | ||
| CIP103739 | CIP | |
| München | A. Sander (44) | |
| E. coli | ||
| BSV23 | thi hsd (r− m−) ribC::Tn5 Km | A. Bacher |
| RR28 | thi leu pro lac gal ara xyl endA recA hsd (r− m−) pheS12 supE44 | 38 |
| Rib2 | RR28 with a point mutation in ribD | A. Bacher |
| TOP10 | F−mcrAΔ(mrr-hsdRMS-mcrBC) f80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL endA1 nupG | Invitrogen |
| Plasmids | ||
| pZERO-2 | Cloning vector; ccdB neo oriColEI Km | Invitrogen |
| pBH-RIBC1 | pZERO-2 with a 2.3-kb PstI DNA fragment carrying the ribC gene from B. henselae; Km | This study |
Km, resistant to kanamycin.
E. coli was grown in Luria-Bertani (LB) medium (32) at 37°C. When necessary, the medium was supplemented with kanamycin at a concentration of 20 mg/liter.
The riboflavin-deficient mutant strains of E. coli, which are unable to grow on rich media without addition of riboflavin, were propagated on LB agar supplemented with riboflavin (400 mg/liter).
Isolation and manipulation of bacterial DNA.
Isolation, cloning, and manipulation of DNA were performed with E. coli TOP10 according to standard protocols (39). Plasmid pBH-RIBC1 (Table 1) was isolated from E. coli, previously grown in 100 ml of LB medium with kanamycin (20 mg/liter), by anion-exchange chromatography with a commercial kit (Qiagen).
The genomic DNA library of B. henselae Houston-1 (Table 1) was constructed by cloning PstI-fragmented DNA into plasmid pZERO-2 with the Zero Background cloning kit from Invitrogen according to the manufacturer’s recommendations. The library was propagated in E. coli TOP10. For the isolation of the riboflavin synthesis genes, the DNA library was transferred into the riboflavin-deficient mutant strain E. coli BSV23. Clones harboring plasmids which restored riboflavin synthesis were selected by the ability to grow on LB agar without added riboflavin.
PCR amplification.
The DNA sequences of the primer oligonucleotides used for the PCR analysis of the Bartonella species and the sizes of the corresponding PCR products are listed in Table 2. PCR analysis was performed with 50 μl of a PCR mixture described earlier (4) which contained 1 U of Taq DNA polymerase, 25 pmol of each primer, and 100 ng of target DNA. Amplification under standard conditions was performed in a Techne thermocycler with 30 cycles each of 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C, followed by a terminal extension step of 10 min at 72°C.
TABLE 2.
Primers and conditions used for the differentiation of Bartonella species by PCR
| Species is specific for PCR assay | Primers | Nucleotide sequence | Annealing temp (°C) | PCR product size (bp)a |
|---|---|---|---|---|
| B. clarridgeiae | PBC5 | TACATAACGAGCCAATT | ||
| PBC15 | TAGCTTTAGAACAATATGGT | 50 | 895 | |
| B. henselae | PBH-L1 | GATATCGGTTGTGTTGAAGA | ||
| PBH-R1 | AATAAAAGGTATAAAACGCT | 55 | 393 | |
| B. bacilliformis | PBH-L1 | Same as for B. henselae | ||
| PBB-R1b | AAAGGCGCTAACTGTTC | 62 | 386 | |
| B. quintana | PBH-L1 | Same as for B. henselae | ||
| PBQ-R1b | AAAGGGCGTGAATTTTG | 60 | 390 | |
| Various species | PBH3 | CCAAGTGCTACATAACCATC | ||
| PBH4 | CGGGTTGTTATTGCTCTTAC | 55 | 1,723b |
The sizes of the PCR products were calculated from the riboflavin synthesis gene cluster of each species.
The size of a fragment from B. henselae was calculated.
Optimized annealing temperatures for the species-specific PCR analyses are given in Table 2. The PCR products were electrophoretically separated on 1.2 or 1.6% agarose gels and stained with ethidium bromide.
The sizes of the PCR products were determined by comparison to a 1-kb marker DNA ladder (Gibco-BRL).
DNA sequence analysis.
The sequences of the riboflavin synthesis genes were determined on both strands by the dideoxynucleotide chain termination method with the PRISM ready reaction dye cycle sequencing kit (ABI) with fluorescence-labeled deoxynucleoside triphosphates. Products of the sequencing reactions were separated on a polyacrylamide gel under denaturing conditions and analyzed in an ABI sequencing apparatus. Database searches were performed with the BLAST search engines provided via the Internet by the National Center for Biotechnology Information (33a). Nucleotide and protein sequence comparisons were performed with the BESTFIT and PILEUP algorithms of the University of Wisconsin Genetics Computer Group software.
Nucleotide sequence accession numbers.
The DNA sequences of the ribC DNA regions of B. henselae, B. clarridgeiae, B. quintana, and B. bacilliformis have been assigned EMBL database accession no. AJ132928, AJ236916, AJ236917, and AJ236918, respectively. The sequences of the RibD, RibC, and RibE proteins from E. coli were obtained from the SWISSPROT database (accession no. P25539, P29015, and 1786617, respectively).
RESULTS
Cloning of the ribC gene from B. henselae.
A plasmid-based DNA library consisting of PstI-fragmented DNA from B. henselae Houston-1 cloned into plasmid pZERO-2 was transferred into the ribC mutant E. coli BSV23. Plasmids which restored riboflavin synthesis in the mutant were selected by growth on LB agar without addition of riboflavin. Three clones that were able to grow normally on LB agar with kanamycin were obtained, and the corresponding plasmids were isolated and restricted with the enzymes PstI, HindIII, and EcoRV. This analysis revealed that all three plasmids carried an identical B. henselae PstI fragment of 2.3 kb (Fig. 1). One plasmid, designated pBH-RIBC1, was chosen for further analysis. After E. coli BSV23 was retransformed and reproducible growth of the transformants on LB agar had confirmed that the restoration of riboflavin synthesis was plasmid mediated, B. henselae DNA was completely sequenced on both strands.
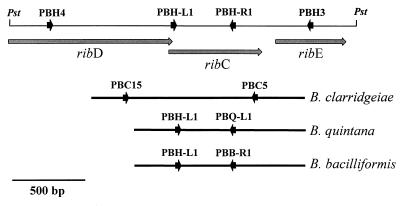
FIG. 1.
Schematic representations of the DNA regions comprising the genes ribC, ribD, and ribE in B. henselae and other Bartonella species. The upper line represents the DNA region of B. henselae Houston-1 cloned into plasmid pBH-RIBC1. The binding sites of the oligonucleotide primers used for the differentiation of Bartonella species by PCR are marked by the arrowheads. The bars indicate the sequenced DNA regions amplified with primers PBH3 and PBH4 from B. quintana, B. bacilliformis, and B. clarridgeiae. Sequences of primer oligonucleotides are given in Table 2.
Analysis of the DNA sequence indicated that the 2,307 bp contained three open reading frames which are transcribed in identical directions (Fig. 1). Comparison of the deduced amino acid sequences with protein sequences in databases showed that the open reading frames encode homologues of the riboflavin synthesis proteins RibD (riboflavin deaminase/reductase, EC 3.5.4.-), RibC (riboflavin synthase [alpha chain], EC 2.5.1.9), and RibE (ribityl-lumazine synthase, EC 2.5.1.9); these proteins were previously isolated from E. coli, and their functions were characterized (3). Consequently, the B. henselae genes were designated ribD, ribC, and ribE.
The ribD gene, located at the left end of the cloned fragment (Fig. 1), consists of 1,089 bp. A comparison of the 363 amino acids encoded by ribD to corresponding proteins from E. coli and B. subtilis indicated that the part of the gene encoding the first 8 to 10 amino acids of the N terminus, including the start codon, was not on the cloned DNA fragment. The fact that plasmid pBH-RIBC1 did not restore riboflavin synthesis in the ribD mutant E. coli Rib2 (data not shown) further indicated that the ribD gene is partial and not functional in E. coli. The deduced B. henselae RibD protein has significant homology with RibD from E. coli (33% identity and 48% similarity) (Fig. 2A). Among the species for which molecular data of RibD are available, the riboflavin deaminase/reductase from B. subtilis (designated RibG) showed the highest degree of similarity (37% identity and 49% similarity).
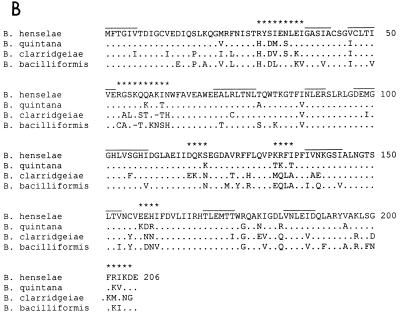
FIG. 2.
Alignments of the RibD, RibC, and RibE proteins from B. henselae and E. coli. The amino acid sequences of RibD (A), RibC (B), and RibE (C) from B. henselae were deduced from the DNA sequence cloned into pBH-RIBC1 and aligned with the sequences of the same proteins from E. coli. For the RibD protein, only the N-terminal region is shown because the homology of the middle and C-terminal regions of the proteins is very low (//). Amino acids that were found to be identical or conservatively exchanged are marked by double or single dots, respectively. Stretches of more than two amino acids conserved between proteins from both species are overlined. These regions are also conserved in the corresponding proteins from other bacterial species (listed in the introduction).
The ribC gene, located in the center of the 2.3-kb PstI fragment, consists of 621 bp (Fig. 1). This gene is complete and functionally active in E. coli, as demonstrated by its complementation of the ribC mutant strain BSV23. This confirmed the function of the protein as riboflavin synthase (alpha chain). Riboflavin synthase of E. coli catalyzes the terminal step of riboflavin synthesis. The protein is a heteropolymer in which RibC is the alpha subunit and RibE is the beta subunit. The 206 amino acids of the deduced B. henselae RibC protein are 36% identical and 50% similar to RibC from E. coli. A similar degree of homology was found for RibC proteins from B. subtilis (34% identity and 55% similarity) and other bacterial species (data not shown).
The ribE gene of B. henselae, which in other bacterial species encodes the beta subunit of riboflavin synthase, is 468 bp in length. The 155-amino-acid sequence encoded by ribE is homologous to RibE from E. coli (39% identity and 52% similarity). Similar degrees of homology were found for RibE proteins from B. subtilis (35% identity and 50% similarity) and other bacterial species.
Stretches of amino acids conserved in RibC, RibD, and RibE from B. henselae and E. coli (Fig. 2) are also present in the corresponding proteins from other bacterial species. The corresponding amino acids might be involved in the enzymatic functions of the proteins, as shown for substrate binding sites in the RibC protein (13). The motif MFTGIV, which is conserved in the N terminus of RibC from all species analyzed so far, is also present in RibC from B. henselae (Fig. 2B).
Analysis of the ribC gene in isolates of B. henselae.
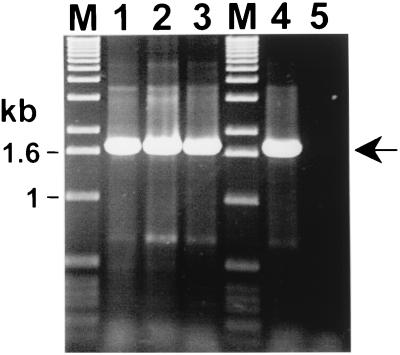
In order to investigate whether the ribC gene region is a constant part of the B. henselae population, DNA from strain Houston-1 and from 17 B. henselae strains isolated from cats (42) was analyzed by PCR with primers PBH3 and PBH4, which were designed with the DNA cloned into plasmid pBH-RIBC1 (Fig. 1). The analysis showed that the expected 1.7-kb PCR product could be amplified from all strains (Fig. 3, lanes 1 to 4).
FIG. 3.
PCR analysis for the detection of the ribC DNA region in B. henselae and in other Bartonella species. PCR with primers PBH3 and PBH4 (Fig. 1) were used to amplify a 1.7-kb DNA fragment which carries the ribC gene (arrow). PCR analysis was performed with 100 ng (lane 1), 10 ng (lane 2), and 1 ng (lane 3) of isolated total DNA from B. henselae FR96/K4 as the target. Total DNA from B. henselae Houston-1 (100 ng) served as a positive control (lane 4). PCR analysis with primers PBH3 and PBH4 generated products with sizes similar to those of DNA of other B. henselae isolates, B. quintana, B. bacilliformis, and B. clarridgeiae, listed in Table 1 (not shown). Lanes M, marker DNA fragments. The PCR products were separated on a 1.2% agarose gel and stained with ethidium bromide.
In order to confirm the identity of the amplified DNA and to investigate whether the ribC DNA region is conserved in the B. henselae population, the PCR products amplified from strains FR96/BK3, FR96/BK8, FR96/BK75II, FR96/BK77, FR96/BK78, FR96/BK79, FR96/BK26II, FR96/BK36, FR96/BK38, FR96/BK75, FR96/K4, and FR96/K7 were sequenced. These strains represent B. henselae variants I, II, and III (Table 1), which can be differentiated by various molecular methods, as described earlier (42). Furthermore, strains FR96/K7 and Houston-1 contain a 16S rRNA gene of Bergmans type 1, whereas all other strains contain 16S ribosomal DNA (rDNA) of Bergmans type 2 (6, 42).
Interstrain comparisons of the DNA sequences revealed that the ribC DNA region is highly conserved among B. henselae strains (99% identity). Nucleotide substitutions were detected only in strains of variant II. The positions and the substituted nucleotides were identical in all variant II isolates analyzed (Table 3). The sequences of variants III and I were identical to the sequence of strain Houston-1, which represents variant IV as determined earlier (42). No substitutions were found in the ribC DNA regions of strains comprising different 16S rDNA gene types.
TABLE 3.
Nucleotide differences in B. henselae strains
| Position in sequencea | Nucleotide in:
|
|
|---|---|---|
| Variants I, III, and IV | Variant II | |
| 384 | G | A |
| 407 | C | T |
| 479 | G | A |
| 634 | A | G |
| 754 | G | A |
| 758 | A | G |
| 1043 | A | T |
| 1310 | A | G |
| 1360 | G | A |
| 1494 | A | G |
| 1499 | G | A |
| 1741 | T | C |
| 1907 | G | A |
Position of the nucleotide in the sequence of the riboflavin synthesis gene cluster in B. henselae Houston-1.
Isolation and analysis of the ribC genes from other Bartonella species.
The same primer pair, PBH3 and PBH4, which flanks the ribC gene in B. henselae, could be used to amplify a 1.7-kb DNA fragment (Fig. 3) from B. bacilliformis, B. clarridgeiae, and B. quintana. The PCR product amplified from each species was sequenced, and interspecies comparisons revealed that the ribC gene region is conserved among Bartonella species. Detailed alignments of the ribC coding sequences from different species (Fig. 4A) revealed that homology was most pronounced for ribC from B. quintana, which is 91% homologous to ribC from B. henselae. The ribC genes from B. bacilliformis and B. clarridgeiae were found to be significantly less homologous to ribC from B. henselae (both had 82% identity). This was confirmed at the protein level, since alignments of the deduced sequences of the proteins (Fig. 4B) showed that the RibC proteins from B. quintana, B. bacilliformis, and B. clarridgeiae are 89, 74, and 77% identical and 93, 84, and 83% similar to the RibC protein from B. henselae, respectively.
FIG. 4.
Alignments of the ribC genes and of the RibC proteins from Bartonella species. (A) The sequences of the oligonucleotide primers used for the differentiation of Bartonella species are underlined and marked by arrows. Asterisks indicate ribC DNA regions variable among Bartonella species. (B) Stretches of amino acids conserved in RibC homologues from unrelated microorganisms are overlined. Amino acids highly variable in the RibC proteins from different Bartonella species are marked with asterisks.
The parts of ribD and ribE corresponding to the C and N termini, respectively, are also conserved among Bartonella species, but the sequences are too short for detailed alignments. Stretches of amino acids conserved in the RibC proteins from other bacteria are also conserved in the RibC proteins from different Bartonella species.
Differentiation of Bartonella species with sequence information for the riboflavin synthesis genes.
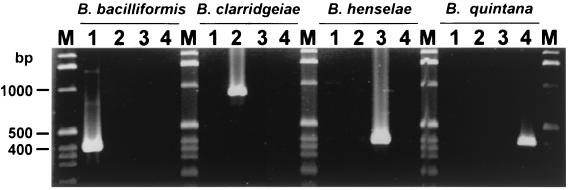
In order to develop PCR assays for the differentiation of Bartonella species, primer oligonucleotides PBH-L1, PBH-R1, PBC5, PBC15, PBQ-R1, and PBB-R1 were designed from local species-specific DNA polymorphisms of the ribC locus (Fig. 1 and 4A and Table 2). After the optimization of the annealing temperatures, PCR analysis of DNA from B. henselae, B. bacilliformis, B. clarridgeiae, and B. quintana, with appropriate primer combinations (Table 2), generated products of the expected sizes (Fig. 5 and Table 2). The fact that the PCR products were amplified exclusively from DNA of the species from which the primers were designed indicates that each assay is specific for one species and can be used for differentiation (Fig. 5).
FIG. 5.
Species-specific differentiation of Bartonella species by PCR analysis with primers designed from the ribC DNA region. Primers designed from the ribC DNA regions of B. bacilliformis (PBH-L1 and PBB-R1), B. clarridgeiae (PBH5 and PBH15), B. henselae (PBH-L1 and PBH-R1), and B. quintana (PBH-L1 and PBQ-R1) (as indicated at the top) were used for PCR analysis of Bartonella species under stringent species-specific conditions (Table 2). DNA isolated (100 ng) from B. bacilliformis (lanes 1), B. clarridgeiae (lanes 2), B. henselae (lanes 3), and B. quintana (lanes 4) was analyzed. Sizes of PCR products are listed in Table 2. Lanes M, marker DNA fragments. The PCR products were separated on a 1.6% agarose gel and stained with ethidium bromide.
To further examine the specificity and reproducibility of the PCR-directed differentiation system, DNA isolated from all the strains of each Bartonella species listed in Table 1 was subjected to PCR analysis with the species-specific primer combinations (results not shown). The fact that the species-specific PCR products of the expected sizes (Fig. 5) were generated exclusively from strains of the species from which the primers were designed indicates that the PCR assays based on the ribC DNA region are reproducible, are not strain dependent, and show no interspecies cross-reactions.
The specific signals were also obtained from dilution series of whole cells analyzed by each PCR assay under stringent conditions (Table 2), indicating that the isolation of DNA can be omitted.
DISCUSSION
Species-specific diagnosis of infections caused by bacteria of the genus Bartonella is difficult even now. Distinguishing these pathogens from other bacteria in routine cultures is more a fortunate coincidence than a reliable method for identifying these organisms. Serological methods for the detection of Bartonella antibodies may be useful for immunocompetent patients with clinical manifestations like CSD, but differentiation between the species B. henselae and B. quintana is not possible (11, 41). Additionally, no serological tests for the detection of B. clarridgeiae antibodies are commercially available, and no serological data concerning infections of immunocompromised and HIV-infected patients exist.
Therefore, the differentiation of Bartonella species involved in human infections requires molecular diagnostic procedures. However, most primers used for the differentiation of Bartonella species by PCR are only genus specific; identification at the species level requires sequencing of amplified DNA or hybridization with a species-specific probe (1, 37, 43). Restriction fragment length polymorphisms of the 16S rRNA gene and sequence polymorphisms of the citrate synthase gene have been used for the differentiation of Bartonella species (8, 9). Recently, the ftsZ gene of Bartonella species has been successfully used to differentiate B. henselae, B. quintana, and B. bacilliformis, but testing for specificity, strain dependence, or detection in clinical specimens is still in progress (21). However, as the rRNA genes are highly conserved within the genus Bartonella, the usage of PCR assays based on chromosomal genes, like ftsZ and gltA, or the riboflavin synthesis genes analyzed in this study, should improve species-specific differentiation.
This study reports the development of species-specific PCR assays for the differentiation of B. bacilliformis, B. clarridgeiae, B. henselae, and B. quintana based on sequence information for genes encoding enzymes involved in riboflavin synthesis. The riboflavin synthesis genes were chosen because they are, due to their evolutionary conservation and their absence in humans (3), excellent targets for the diagnosis of invasive pathogens. Their usefulness is further supported by the fact that the genetic organization of riboflavin synthesis genes differs remarkably among bacterial species, which increases the specificity of PCR-based techniques. The ribC gene was isolated from B. henselae, and the functional complementation of a ribC-deficient mutant of E. coli confirmed that the encoded protein has the activity of riboflavin synthase (alpha chain), which is involved in the catalysis of the terminal step of riboflavin biosynthesis (13). The ribC gene of B. henselae is flanked by the genes ribD and ribE, which encode homologues of the riboflavin synthesis proteins RibD and RibE. In E. coli, the RibE and RibC proteins form the multienzyme complex riboflavin synthase, which catalyzes the terminal step in riboflavin synthesis (3). The gene order of ribD, ribC, and ribE is conserved in B. henselae, B. quintana, B. clarridgeiae, and B. bacilliformis. The clustering suggests that the genes are organized as an operon, which is also the case for riboflavin synthesis genes in the gram-positive bacterium B. subtilis and in gram-negative bacteria, like Actinobacillus spp. and Photobacterium spp. (15, 35). In the latter species, the rib genes are part of the lux operon (26). Within these operons, the gene order of ribD, ribC, and ribE homologues is different from that in Bartonella species. In other gram-negative organisms, e.g., E. coli, H. pylori, and H. influenzae, the rib genes are randomly distributed in the chromosome (3, 5, 14).
The ribC gene and the parts of the flanking ribD and ribE genes corresponding to the C and N termini, respectively, are conserved in the four Bartonella species investigated. The amino acid identity of the RibC proteins from B. henselae and B. quintana (90%) is significantly higher than that of the RibC proteins from B. bacilliformis and B. clarridgeiae (80%), which are more distantly related. For B. henselae, B. quintana, and B. bacilliformis, this degree of homology is consistent with the relatedness of the Bartonella species investigated on the basis of the citrate synthase (gltA) and ftsZ genes in earlier studies (9, 21, 34). Taken together, these findings indicate that B. henselae and B. quintana are more related to each other than to other Bartonella species.
For B. clarridgeiae, not much sequence data besides those for the riboflavin synthesis genes investigated in this study are available in databases. The gene for 16S rRNA (19, 23), the citrate synthase gene (gltA) (10, 34), and the gene for a 60-kDa heat shock protein (30), which is also conserved in other Bartonella species (18), have been investigated, but the sequence data have not been used for species-specific PCR assays which allow direct identification of B. clarridgeiae without sequencing or restriction fragment length polymorphism analysis. The intermediate level of homology for the ribC gene, in the range of 80%, did not indicate a closer evolutionary relationship between B. clarridgeiae and any of the other Bartonella species investigated in this study.
The comparative analysis of the riboflavin synthesis proteins does not allow us to state any evolutionary relationships between B. henselae and another bacterial species for which molecular data on riboflavin synthesis genes are available. The constant degree of homology of the riboflavin synthesis proteins, even to those of unrelated bacterial species, supports the separate evolutionary position of the genus Bartonella.
The genetic analysis of the riboflavin synthesis genes ribD, ribC, and ribE in strains of B. henselae showed that the rib genes are a constant part of the B. henselae genome and are highly conserved with respect to the nucleotide sequence. Single nucleotide substitutions were detected exclusively in strains which were characterized earlier as variant II by various other molecular techniques (42). The fact that these substitutions were located at identical positions and concerned identical nucleotides is further evidence for genetic variations within the B. henselae population, which supports the assumption that stable subtypes exist within the population. On the other hand, strains harboring 16S rDNA of Bergmans type 1 and type 2 did not show any differences in the rib gene DNA sequence, indicating that mutations in these genetic loci are not linked to each other.
PCR analysis with oligonucleotide primers designed from the ribC DNA region allowed species-specific differentiation of the Bartonella species, as shown by the amplification of DNA from the species from which the primers were designed, but not from the others. The fact that the analysis was not strain dependent might indicate that the approach could be of use for the detection of Bartonella species in clinical specimens, which is currently under investigation.
The presence of riboflavin synthesis genes in Bartonella species is strong evidence for their ability to produce this essential vitamin, which could be of relevance to their establishment and survival in the host, as shown for the swine pathogen A. pleuropneumoniae (16).
In summary, the results indicate that the riboflavin synthesis genes ribD, ribC, and ribE are excellent targets for the differentiation of Bartonella species. The species-specific PCR assays developed should increase our diagnostic potential to differentiate among Bartonella species of clinical relevance. The PCR assay specific for B. clarridgeiae is one of the first systems available for molecular differentiation. It facilitates discrimination of B. clarridgeiae and B. henselae, which should help to clarify the role of this putative pathogen in human diseases.
ACKNOWLEDGMENTS
We thank Wolfgang Bredt for continuous support and encouragement. Karin Oberle and Tanja Vey provided excellent technical assistance. We are also grateful to Yves Piemont (Strasbourg, France) and Erik Marston (CDC, Atlanta, Ga.) for providing strains of B. clarridgeiae and B. henselae. The riboflavin-deficient mutant strains of E. coli were kindly provided by Sabine Eberhardt and Adelbert Bacher (Munich, Germany).
REFERENCES
- 1.Anderson B, Sims K, Regnery R, Robinson L, Schmidt M J, Goral S, Hager C, Edwards K. Detection of Rochalimaea henselae DNA in specimens from cat scratch disease patients by PCR. J Clin Microbiol. 1994;32:942–948. doi: 10.1128/jcm.32.4.942-948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacher A, Eberhardt S, Richter G. Biosynthesis of riboflavin. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 657–664. [Google Scholar]
- 4.Bereswill S, Pahl A, Bellemann P, Zeller W, Geider K. Sensitive and species-specific detection of Erwinia amylovora by polymerase chain reaction analysis. Appl Environ Microbiol. 1992;58:3522–3526. doi: 10.1128/aem.58.11.3522-3526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bereswill S, Fassbinder F, Voelzing C, Covacci A, Haas R, Kist M. Hemolytic properties and riboflavin synthesis of Helicobacter pylori: cloning and functional characterization of the ribA gene encoding GTP-cyclohydrolase II that confers hemolytic activity to Escherichia coli. Med Microbiol Immunol. 1998;186:177–187. doi: 10.1007/s004300050062. [DOI] [PubMed] [Google Scholar]
- 6.Bergmans A M C, Schellekens J F P, van Embden J D A, Schouls L M. Predominance of two Bartonella henselae variants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birtles R J, Harrison T G, Saunders N A, Molyneux D H. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995;45:1–8. doi: 10.1099/00207713-45-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Birtles R J. Differentiation of Bartonella species using restriction endonuclease analysis of PCR-amplified 16S rRNA genes. FEMS Microbiol Lett. 1995;129:261–265. doi: 10.1111/j.1574-6968.1995.tb07590.x. [DOI] [PubMed] [Google Scholar]
- 9.Birtles R J, Raoult D. Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol. 1996;46:891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- 10.Clarridge J E, III, Raich T J, Pirwani D, Simon B, Tsai L, Rodriguez-Barradas M C, Regnery R, Zollo A, Jones D C, Rambo C. Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J Clin Microbiol. 1995;33:2107–2113. doi: 10.1128/jcm.33.8.2107-2113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton M J, Robinson L E, Cooper J, Regnery R L, Olsen J G, Childs J E. Use of Bartonella antigen for serologic diagnosis of cat-scratch diseases at a national referral center. Arch Intern Med. 1995;155:1670–1676. [PubMed] [Google Scholar]
- 12.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, Steigerwalt A G, Weaver R E, Daneshvar M I, O’Connor S P. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhardt S, Richter G, Gimbel W, Werner T, Bacher A. Cloning, sequencing, mapping and hyperexpression of the ribC gene coding for riboflavin synthase of Escherichia coli. Eur J Biochem. 1996;242:712–719. doi: 10.1111/j.1432-1033.1996.0712r.x. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 15.Fuller T E, Mulks M H. Characterization of Actinobacillus pleuropneumoniae riboflavin biosynthesis genes. J Bacteriol. 1995;177:7265–7270. doi: 10.1128/jb.177.24.7265-7270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller T E, Thacker B J, Mulks M H. A riboflavin auxotroph of Actinobacillus pleuropneumoniae is attenuated in swine. Infect Immun. 1996;64:4659–4664. doi: 10.1128/iai.64.11.4659-4664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurfield A N, Boulouis H-J, Chomel B B, Heller R, Kasten R W, Yamamoto K, Piemont Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselae strains in domestic cats. J Clin Microbiol. 1997;35:2120–2123. doi: 10.1128/jcm.35.8.2120-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haake D A, Summers T A, McCoy A M, Schwartzman W. Heat shock response and groEL sequence of Bartonella henselae and Bartonella quintana. Microbiology. 1997;143:2807–2815. doi: 10.1099/00221287-143-8-2807. [DOI] [PubMed] [Google Scholar]
- 19.Heller R, Artois M, Xemar V, De Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller R, Riegel P, Hansmann Y, Delacour G, Bermond D, Dehio C, Lamarque F, Monteil H, Chomel B, Piemont Y. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol. 1998;48:1333–1339. doi: 10.1099/00207713-48-4-1333. [DOI] [PubMed] [Google Scholar]
- 21.Kelly T M, Padmalayam I, Baumstark B R. Use of the cell division protein FtsZ as a means of differentiating among Bartonella species. Clin Diagn Lab Immunol. 1998;5:766–772. doi: 10.1128/cdli.5.6.766-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Sugiyama M, Yamamoto K. Isolation of cDNAs encoding GTP-cyclohydrolase II from Arabidopsis thaliana. Gene. 1995;160:303–304. doi: 10.1016/0378-1119(95)00246-3. [DOI] [PubMed] [Google Scholar]
- 23.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordick D L, Breitschwerdt E B. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis. 1998;4:325–328. doi: 10.3201/eid0402.980225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson P A, Artois M. Description of Bartonella clarridgeiae sp. nov. isolated from the cat of a patient with Bartonella henselae septicaemia. Med Microbiol Lett. 1996;5:64–73. [Google Scholar]
- 26.Lee C Y, O’Kane D J, Meighen E A. Riboflavin synthesis genes are linked with the lux operon of Photobacterium phosphoreum. J Bacteriol. 1994;176:2100–2104. doi: 10.1128/jb.176.7.2100-2104.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liauta-Teglivets O, Hasslacher M, Boretskii I R, Kohlwein S D, Shavlovlovskii G M. Molecular cloning of the GTP-cyclohydrolase structural gene RIB1 of Pichia guilliermondii involved in riboflavin biosynthesis. Yeast. 1995;11:945–952. doi: 10.1002/yea.320111005. [DOI] [PubMed] [Google Scholar]
- 28.Margileth A M, Baehren D F. Chest-wall abscess due to cat-scratch disease (CSD) in an adult with antibodies to Bartonella clarridgeiae: case report and review of the thoracopulmonary manifestations of CSD. Clin Infect Dis. 1998;27:353–357. doi: 10.1086/514671. [DOI] [PubMed] [Google Scholar]
- 29.Marston E L, Finkel B, Regnery R L, Winoto I L, Graham R R, Wignal S, Simanjuntak G, Olson J G. Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin Diagn Lab Immunol. 1999;6:41–44. doi: 10.1128/cdli.6.1.41-44.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marston, E. L., J. W. Sumner, and R. L. Regnery. Evaluation of intraspecies genetic variation within the 60 kDa heatshock protein (groEL) gene of Bartonella species. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 31.Maurin M, Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/cmr.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 33.Nadal D, Zbinden R. Serology to Bartonella (Rochalimaea) henselae may replace traditional diagnostic criteria for cat-scratch disease. Eur J Pediatr. 1995;154:906–908. doi: 10.1007/BF01957503. [DOI] [PubMed] [Google Scholar]
- 33a.National Center for Biotechnology Information. 21 July 1999, revision date. [Online.] http://www.ncbi.nlm.nih.gov/. [5 August 1999, last date accessed.]
- 34.Norman A F, Regnery R, Jameson P, Greene C, Krause D C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perumov D A, Glazunov E A, Gorinchuk G F. Riboflavin operon in Bacillus subtilis XVII. Studies of regulatory functions of biochemical intermediates and their derivatives. Genetika. 1986;22:748–754. [PubMed] [Google Scholar]
- 36.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 38.Richter G, Ritz H, Katzenmeier G, Volk R, Kohnle A, Lottspeich F, Allendorf D, Bacher A. Biosynthesis of riboflavin: cloning, sequencing, mapping, and expression of the gene coding for GTP cyclohydrolase II in Escherichia coli. J Bacteriol. 1993;175:4045–4051. doi: 10.1128/jb.175.13.4045-4051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Sander A, Frank B. Paronychia caused by Bartonella henselae. Lancet. 1997;350:1078. doi: 10.1016/S0140-6736(05)70459-1. [DOI] [PubMed] [Google Scholar]
- 41.Sander A, Posselt M, Oberle K, Bredt W. Seroprevalence of antibodies to Bartonella henselae in patients with cat scratch disease and in healthy controls: evaluation and comparison of two commercial serological tests. Clin Diagn Lab Immunol. 1998;5:486–490. doi: 10.1128/cdli.5.4.486-490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sander A, Ruess M, Bereswill S, Schuppler M, Steinbrueckner B. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J Clin Microbiol. 1998;36:2973–2981. doi: 10.1128/jcm.36.10.2973-2981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sander A, Posselt M, Böhm N, Ruess M, Altwegg M. Detection of Bartonella henselae DNA by two different PCR assays and determination of the genotypes of strains involved in histologically defined cat scratch disease. J Clin Microbiol. 1999;37:993–997. doi: 10.1128/jcm.37.4.993-997.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H U, Kaliebe T, Poppinger J, Bühler C, Sander A. Isolation of Bartonella quintana from an HIV-positive patient with bacillary angiomatosis. Eur J Clin Microbiol Infect Dis. 1996;15:736–741. doi: 10.1007/BF01691961. [DOI] [PubMed] [Google Scholar]
- 45.Skala J, Van Dyck L, Purnelle B, Goffeau A. The sequence of an 8.8 kb segment on the left arm of chromosome II from Saccharomyces cerevisiae reveals four new open reading frames including homologs of animal DNA polymerase alpha-primases and bacterial GTP cyclohydrolase II. Yeast. 1994;10:S13–S24. doi: 10.1002/yea.320100003. [DOI] [PubMed] [Google Scholar]
- 46.Stoler M H, Bonfiglio T A, Steigbigel R T, Peireira M. An atypical subcutaneous infection associated with the acquired immune deficiency syndrome. Am J Clin Pathol. 1983;80:714–718. doi: 10.1093/ajcp/80.5.714. [DOI] [PubMed] [Google Scholar]
- 47.van Bastelaere E, Keijers V, Vanderleyden J. Cloning and sequencing of the Azospirillum brasilense gene encoding GTP cyclohydrolase II. Gene. 1995;160:141–142. doi: 10.1016/0378-1119(94)00826-e. [DOI] [PubMed] [Google Scholar]