Highlights
-
•
Spontaneous fasting and/or postprandial hypoglycemia is common in people with CF.
-
•
The clinical significance of OGTT related hypoglycemia in CF is not clear.
-
•
Dietary modification is the first line treatment for postprandial hypoglycemia.
-
•
Patients with CFRD are at risk of hypoglycemia related to insulin therapy.
-
•
CGMs and closed loop insulin pump systems may reduce hypoglycemia risk in CFRD.
Keywords: Hypoglycemia, Cystic fibrosis, Cystic fibrosis related diabetes, Reactive hypoglycemia, Oral glucose tolerance test, Continuous glucose monitor
Abstract
Spontaneous episodes of hypoglycemia can occur in people with cystic fibrosis (CF) without diabetes, who are not on glucose lowering medications. Spontaneous hypoglycemia in CF could occur both in the fasting or postprandial state (reactive hypoglycemia). The pathophysiology of fasting hypoglycemia is thought to be related to malnutrition and increased energy expenditure in the setting of inflammation and acute infections. Reactive hypoglycemia is thought to be due to impaired first phase insulin release in response to a glucose load, followed by a delayed and extended second phase insulin secretion; ineffective counterregulatory response to dropping glucose levels may also play a role. The overall prevalence of spontaneous hypoglycemia varies from 7 to 69% as examined with oral glucose tolerance test (OGTT) or with continuous glucose monitoring (CGM) under free living conditions. Spontaneous hypoglycemia in CF is associated with worse lung function, higher hospitalization rates, and worse clinical status. In addition, patients with CF related diabetes on glucose-lowering therapies are at risk for iatrogenic hypoglycemia. In this article, we will review the pathophysiology, prevalence, risk factors, clinical implications, and management of spontaneous and iatrogenic hypoglycemia in patients with CF.
Background
People with cystic fibrosis (CF) are at risk for both spontaneous and iatrogenic hypoglycemia. Impaired glucose tolerance and cystic fibrosis related diabetes (CFRD) are common complications of CF. Individuals with CFRD on insulin and other glucose lowering therapies are at risk for iatrogenic hypoglycemia, similar to patients with other types of diabetes mellitus. In addition, children and adults with CF without diabetes who are not on glucose-lowering therapy are also at risk for spontaneous hypoglycemic episodes. In this article, we will review the definition and normal physiologic response to hypoglycemia [1]. We will then review the prevalence, pathophysiology, risk factors and management of both iatrogenic and spontaneous hypoglycemia in people with CF. We will utilize clinical cases to illustrate these hypoglycemic disorders in CF.
Defining hypoglycemia
Spontaneous hypoglycemia
Plasma glucose concentrations under normal physiologic conditions are maintained within a narrow range [2]. Hypoglycemia is uncommon in the absence of diabetes and glucose-lowering therapies due to the presence of effective and redundant counterregulatory mechanisms to declining glucose levels [2], [3]. Plasma glucose levels below the normal range can lead to development of symptoms and signs of hypoglycemia and puts the person at risk of serious harm [4], [5]. It is challenging to establish a single biochemical threshold of plasma glucose level to define spontaneous hypoglycemia in people without diabetes, and spurious low plasma glucose results can be misleading [3]. Healthy individuals start experiencing typical symptoms of hypoglycemia once the plasma glucose level is below 60 mg/dl [6]. It is recommended to diagnose hypoglycemia in this setting when Whipple’s triad is documented [4]. Establishing Whipple’s triad requires 1. Presence of typical symptoms of Hypoglycemia, 2. Low plasma glucose concurrent with symptoms, and 3. Relief of symptoms when glucose level increases [3].
Classification of hypoglycemia in diabetes
Hypoglycemia is a common adverse effect of insulin and insulin secretagogue (sulfonylureas, meglitinides) therapy and is associated with both acute and long-term negative health consequences [7], [8]. Current recommendations classify hypoglycemia in diabetes into 3 levels, which are listed in Table 1 [9], [10]. This classification was proposed by The International Hypoglycemia Study Group and has been endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Level 1 hypoglycemia (i.e., plasma glucose < 70 mg/dL but ≥ 54 mg/dL) is not true hypoglycemia but considered an alert level. In people without diabetes, plasma glucose below 70 mg/dL could be normal; but in people with diabetes on glucose lowering therapies, this level should alert the patient to take intervention to prevent the glucose level from dropping further into the level 2 hypoglycemia range [9], [10]. Level 2 (glucose < 54 mg/dL) is considered clinically important as the patient can develop neuroglycopenic symptoms at this threshold and requires immediate intervention to prevent severe (Level 3) hypoglycemia. Neuroglycopenic symptoms include diaphoresis, feeling dizzy or confused, fatigue, and blurry vision [6], [11]. This threshold of hypoglycemia is also associated with development of impaired awareness of hypoglycemia, cardiac arrhythmia, and mortality [9], [10]. Lastly, there is no specific glycemic threshold for Level 3 hypoglycemia, but it is defined as a severe event leading to altered mental status that requires external assistance to recover from hypoglycemia.
Table 1.
| Classification of hypoglycemia | ||
|---|---|---|
| Level | Name | Glycemic criteria/description |
| Level 1 | Hypoglycemia alert | Glucose < 70 mg/dL and ≥ 54 mg/dL |
| Level 2 | Clinically important | Glucose < 54 mg/dL |
| Level 3 | Severe | A severe event characterized by altered mental and/or physical status requiring assistance |
Normal physiologic counterregulatory response to hypoglycemia and alterations in diabetes
The normal physiological response to declining plasma glucose levels in humans is to first decrease endogenous pancreatic islet β-cell insulin secretion followed by increase in islet α-cell glucagon secretion [2]. When plasma glucose levels decrease below ∼ 80 mg/dL (4.4 mmol/L) [12], insulin levels begin to decline. Once plasma glucose levels drop below ∼68 mg/dL (3.8 mmol/L), glucagon and epinephrine levels increase, with the former playing the primary role and the latter a secondary role [6], [13], [14], [15]. Growth hormone (GH) levels rise when glucose is ∼ 67 mg/dL and cortisol when glucose is ∼ 55 mg/dL (3.1 mmol/L), however they likely do not play a critical role in acutely restoring euglycemia since their effects are more protracted over several hours [1], [6], [16]. Patients with diabetes who are treated with exogenous insulin cannot reduce circulating insulin levels in response to lowering glucose. In insulin-deficient diabetes, the glucagon response to declining glucose is also lost and these patients are therefore dependent on the sympathoadrenal response as the first line of defense to prevent progression and recovery from hypoglycemia [2]. Repeated exposure to iatrogenic hypoglycemia can further impair the sympathoadrenal response and lead to development of impaired awareness of hypoglycemia.
Spontaneous hypoglycemia in CF
Case 1: 27 year-old female with CF and pancreatic insufficiency (PI), without prior history of CFRD. The patient complains of frequent symptoms of shakiness, tremors, dizziness and occasionally sweating 2–3 h after meals. She underwent monitoring of glucose trends utilizing a diagnostic continuous glucose monitor (CGM) and kept a diary of symptoms during this period (Fig. 1). Average sensor glucose was 112 mg/dL over 14 days with variability of 31%. 91% of the readings were between 70 and 180 mg/dL, 4% were between 181 and 200 mg/dL and 4% of readings were below 54 mg/dL. There was a pattern of hypoglycemia after post meal spikes in glucose readings and the low glucose readings corresponded with her symptoms, thereby supporting the diagnosis of reactive hypoglycemia.
Fig. 1.
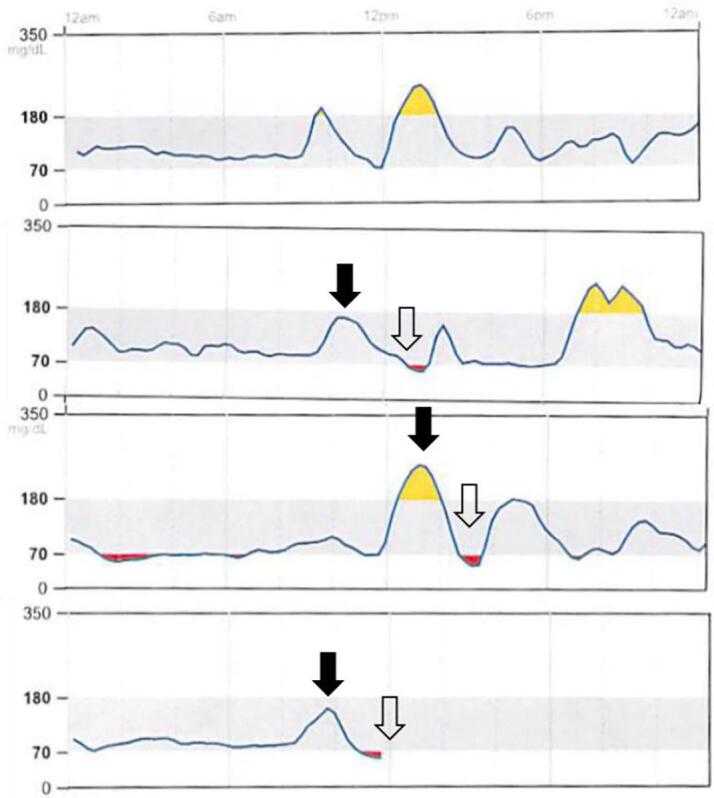
Continuous glucose monitor (CGM) trendline in a person with CF without diabetes with spontaneous hypoglycemia. CGM tracing for 4 days show post prandial hyperglycemia ( ) followed by reactive hypoglycemia (
) followed by reactive hypoglycemia ( ).
).
Case 2: 19 year-old male with CF and PI. Growth percentiles are as follows: height 88%, weight 61%, and BMI 32%. FEV is 104% of predicted. No known history of CFRD. He was previously on lumacaftor/ivacaftor, switched to tezacaftor/ivacaftor, and then later switched to elexacaftor/tezacaftor/ivacaftor. His three most recent 2-hour OGTTs were notable for low 2-hour glucose levels of 56 mg/dL, 31 mg/dL, and 57 mg/dL. The patient says that he felt completely normal before, during, and after the 2-hour OGTT. He denies any symptoms suggestive of hypoglycemia in free-living conditions.
Frequency of spontaneous hypoglycemia in CF
People with CF without diabetes are recommended to undergo annual oral glucose tolerance test (OGTT) to screen for CFRD starting at age 10 years. Spontaneous hypoglycemia is most commonly reported in people with CF in the setting of an OGTT. Additionally, the prevalence of hypoglycemia has been examined by utilizing CGM in free-living conditions. Case 1 and 2 illustrate two common clinical scenarios of spontaneous hypoglycemia in people with CF. A systematic review that included 11 studies reported that the prevalence of hypoglycemia during or after an OGTT ranged from 7 to 59% and hypoglycemia detected using CGM ranged from 13 to 69% [17]. The prevalence of fasting hypoglycemia in CF patients prior to an OGTT has been reported to range between 5 and 13% [17]. This wide range in prevalence of hypoglycemia is likely due to lack of a unifying definition for hypoglycemia, as well as differences in study design. Biochemical cutoffs to define hypoglycemia in the CF studies vary from to < 72 mg/dL (4 mmol/L) to < 50 mg/dL (2.8 mmol/L)) [17].
Hypoglycemia on OGTT has been well described in healthy individuals without diabetes. In 1983, Lev-Ran reported that the median nadir glucose on OGTT in people without diabetes (N = 400) was 63 mg/dL; the 10th percentile was a nadir of 48 mg/dL, and the 2.5th percentile was a nadir of 41 mg/dL. The nadir typically occurred at 3–4 hr. The authors concluded that in the absence of symptoms, these low plasma glucose episodes on OGTT screening should not be considered abnormal [18]. In 2014, Parekh et al. completed OGTTs on 6,477 subjects for diabetes screening and found that 5.5% had low plasma glucose < 60 mg/dl (3.3 mmol/L) [19].
Hypoglycemia rates are higher during extended (3 or 4 hr) compared to standard (2 hr) OGTT. Postprandial hypoglycemia in CF patients has been reported as 6–15% on 2-hr OGTT [17], 45–79% on 3-hr OGTT [17], [20], [21], and 26% on mixed meal tolerance test (MMTT) [22]. In a study of youth with CF (mean age ∼ 14 years), Chan et al. reported that rates of hypoglycemia on CGM, defined as time < 70 mg/dL and < 60 mg/dL, were not significantly different in subjects with CF (stratified according to glucose tolerance) compared to healthy controls [23]. In another study, Haliloglu et al. sequentially performed OGTT and CGM in children and adolescents with CF (N = 45, mean age 13 years) [24]. Authors found higher rates of hypoglycemia on CGM compared to OGTT. The rate of hypoglycemia [≥3% time < 60 mg/dL (3.3 mmol/L)] on CGM was 27.5% compared to 13.3% [plasma < 60 mg/dL (3.3 mmol/L)] on OGTT. Data from CGM studies have suggested that CGM may be more sensitive than other methodologies for identifying episodes of spontaneous hypoglycemia in patients with CF [1], [25].
Pathophysiology of spontaneous hypoglycemia in CF
Fasting hypoglycemia
Hypoglycemia following prolonged fasts is most often attributed to a combination of malnutrition and increased energy expenditure due to the metabolic and respiratory demands of CF or acute infection. Other contributing factors that have been suggested include dysregulated basal insulin secretion and depleted liver glycogen stores leading to impaired gluconeogenesis. In CF-related liver disease, structural and cellular liver damage could also increase the risk of hypoglycemia [17], [26]. As with people without CF, it is important to rule out other etiologies of spontaneous hypoglycemia such as other drugs which have been associated with hypoglycemia (i.e., in addition to antihyperglycemic agents) [3], and cortisol deficiency due to use of exogenous glucocorticoids [1]. Conditions that lead to endogenous hyperinsulinism including islet (insulinoma) or non-islet cell tumors can present with both fasting or postprandial hypoglycemia [3].
Postprandial or OGTT related hypoglycemia
Mechanisms underlying development of postprandial or OGTT-related hypoglycemia are not fully understood. MMTT may better mimic the pathophysiology of reactive hypoglycemia in free-living conditions [3]. In two recent publications, Kilberg and colleagues examined the mechanisms of postprandial hypoglycemia in CF. In the first study, Kilberg [22] et al. reported that non-diabetic adolescents and young adults with CF and PI who experienced hypoglycemia (defined as glucose < 70 mg/dL) during a 4-hour MMTT had higher peak glucose, impaired early insulin secretion and higher late insulin levels. In this study, glucagon concentration was not different between the CF subjects who experienced hypoglycemia compared to no hypoglycemia. Of note, there was no healthy control group for comparison. In the second study [21], the authors report data from frequently sampled 3-hr OGTT. CF subjects with PI who experienced hypoglycemia (defined as glucose < 65 mg/dL) on OGTT had higher 1 hr glucose, lower early insulin and inappropriately higher late insulin compared to a historical healthy control group with hypoglycemia. The CF hypoglycemia group also had blunted glucagon and free fatty acids (FFA) response compared to the healthy control group with hypoglycemia. Authors interpreted the blunted increase in FFA to suggest impaired catecholamine response to hypoglycemia. In this study, there was no difference in early or late insulin, glucagon or FFA between CF subjects with hypoglycemia compared to CF without hypoglycemia.
In another recent study, Armaghanian et al. reported similar results in subjects with CF without diabetes who underwent 3-hour OGTT. Participants who experienced hypoglycemia [≤60 mg/dL (3.3 mmol/L)] had higher glucose peak at 60 min with delayed insulin response compared to subjects without hypoglycemia. There was no difference in glucagon, glucagon-like peptide-1 (GLP-1) or gastric inhibitory polypeptide (GIP) levels between the groups. A previous study by the same group found lower insulin levels in subjects who experienced hypoglycemia [ ≤70 mg/dL (3.9 mmol/L)] during 2-hr OGTT compared to the non-hypoglycemia control group. Aitken et al. also reported lower insulin levels in non-diabetic adults who experienced hypoglycemia during 3-hr OGTT with no significant difference in GLP-1, GIP, glucagon, noradrenaline, cortisol, and GH in the hypoglycemia vs. no hypoglycemia group. Adrenaline was higher in the hypoglycemia group. Authors concluded that late hypoglycemia on OGTT was likely due to insufficient counterregulatory response rather than increased or delayed insulin secretion [27]. Overall these data suggest that both dysregulation in insulin secretion and impaired counterregulatory response to lowering glucose may contribute to postprandial hypoglycemia in CF.
Clinical implications of spontaneous hypoglycemia in CF
The clinical implications of OGTT-related hypoglycemia in CF are not clear. Biochemical hypoglycemia is common during OGTT, and it is not clear if symptomatic or asymptomatic episodes of low glucose during OGTT are associated with hypoglycemia during free living situations or other clinically relevant outcomes. OGTT-related hypoglycemia in the general population has been linked to potential increased risk of type 2 diabetes [28]. It has been postulated that reactive hypoglycemia seen during OGTT may be a precursor or predictor of CFRD. Progression of β-cell dysfunction that may underlie reactive hypoglycemia could eventually lead to development of CFRD. However, recent studies in patients with CF have not demonstrated that OGTT related hypoglycemia increases the risk of progression to CFRD [29], [30]. Hypoglycemia during OGTT has not consistently been shown to be associated with poor clinical outcomes; on the contrary in some studies hypoglycemia is associated with higher FEV1 and BMI. In one study, hypoglycemia during OGTT did not predict hypoglycemia in the free-living state as measured by CGM [31]. In contrast, low fasting plasma glucose (<60 mg/dl) was shown to be associated with higher rates of hospitalization and worse clinical status [32]. In children and adolescents with CF without diabetes, spontaneous hypoglycemia and higher glucose variability on CGM was associated with lower lung function independent of BMI [24]. Future larger longitudinal studies are needed to understand the prognostic implications of reactive hypoglycemia in free-living conditions and OGTT-related hypoglycemia in people with CF. Table 2 summarizes the potential mechanisms and clinical implications of spontaneous hypoglycemia in CF.
Table 2.
Potential mechanisms and clinical implications of spontaneous hypoglycemia in CF.
| Subcategories | Fasting | Postprandial/Reactive |
|---|---|---|
| Potential mechanisms |
|
|
| Prognosis/ implications |
|
|
Management of spontaneous hypoglycemia in patients who are not on glucose-lowering therapy
Patients diagnosed with spontaneous hypoglycemia in free-living conditions should be evaluated for potential underlying causes. Acute management of postprandial hypoglycemia is similar to the management of iatrogenic hypoglycemia and is discussed later in this paper. Dietary interventions are the first line of management for postprandial hypoglycemia. Dietary strategies that may help prevent postprandial/ reactive hypoglycemia include eating a balanced diet – ensuring protein, healthy fats and high-fiber foods are included. Sugary foods, sweetened beverages and processed simple carbohydrates should be avoided, especially on an empty stomach. Carbohydrate counting has been suggested as a useful tool to manage postprandial hypoglycemia [33]. Patients are counseled to avoid meals with very high carbohydrate amounts, and instead to distribute total carbohydrate intake over meals and snacks throughout day. One suggested approach for people with CF with reactive hypoglycemia is consuming 45–60 g of carbohydrate with breakfast, lunch, and supper, alternating with 30 g of carbohydrates midmorning, midafternoon and at bedtime [33]. These carbohydrate goals can be applied in most adolescent and adult patients with reactive hypoglycemia. The precise carbohydrate intake should be customized with the support of a dietitian for individuals who are younger or have unique nutritional needs (i.e., malnutrition or overweight).
Iatrogenic Hypoglycemia in CF
Case 3: 39-year-old male with CF and PI. He was diagnosed with CFRD at age 19 and is currently managed with a basal bolus insulin regimen. His diabetes is complicated by mild nonproliferative diabetic retinopathy and impaired awareness of hypoglycemia. He does not feel typical symptoms of hypoglycemia until the glucose reading is in the 40′s. Over his lifetime, he has experienced 3 episodes of severe hypoglycemia. The most recent episode occurred about 3 months ago while he was at a grocery store and required assistance by emergency medical services (EMS) personnel for treatment of hypoglycemia. Recent review of his glucose meter download showed average glucose 181 mg/dL (range 42–438 mg/dL). He frequently under-doses or misses meal insulin boluses at work due to concern of hypoglycemia.
Frequency of iatrogenic hypoglycemia in CFRD
Iatrogenic hypoglycemia is extremely common in both type 1 diabetes (T1D) and type 2 diabetes (T2D). In a large global observational study, 83% of individuals with T1D and 46.5% with insulin-treated T2D reported at least one episode of any hypoglycemia over a 4-week period [34]. During this period, 14% with T1D and 8.9% with T2D reported a severe hypoglycemic event. There are limited studies examining the prevalence of hypoglycemia in CFRD. In a study by Tierney et al. (N = 20), 58% of CFRD patients who kept a 1-week diary self-reported at least 2 episodes of hypoglycemia. During the first 3 months of the CFRDT trial, 16% of patients on pre-meal aspart insulin and 23% of those on repaglinide had episodes of non-severe hypoglycemia [35]. In a 12-week trial comparing glargine insulin vs. neutral protamine Hagedorn (NPH) for basal insulin (N = 20), the frequency of mild hypoglycemia events was 6 ± 1 times per subject with glargine insulin and 5 ± 1 times per subject with NPH insulin (P = 0.3) [36]. Fortunately, these studies did not report any severe hypoglycemic episodes. Severe hypoglycemia is considered a rare occurrence in CFRD, although more research is needed to examine the prevalence of both non-severe and severe hypoglycemia in CFRD [1].
Risk factors and clinical significance of hypoglycemia in diabetes
Risk factors of hypoglycemia are well studied in people with T1D and T2D (Table 3). Risk of hypoglycemia increases with advanced age, longer duration of diabetes and intensive insulin therapy. Behavioral factors like miscalculating carbohydrate or meal insulin boluses, alcohol consumption, exercise and weight loss are also associated with hypoglycemia. Insulin regimens may need to be adjusted in people with medical comorbidities like renal/liver insufficiency or cognitive impairment to avoid hypoglycemia [1], [4], [37], [38]. Recurrent episodes of iatrogenic hypoglycemia lead to development of impaired awareness of hypoglycemia (IAH) which in turn further increases the risk of hypoglycemia. The prevalence of IAH is reported to be around 25% and 10% in people with T1D and advanced T2D, respectively [39]. As illustrated in case 3, patients with CFRD on insulin therapy are also at risk of IAH. Future studies are needed to examine the prevalence of IAH in patients with CFRD.
Table 3.
Risk factors of hypoglycemia in diabetes
|
Hypoglycemia in hospitalized patients with CFRD
As in T1D and T2D, hypoglycemia is also reported in the inpatient setting in patients with CFRD. In a study of 66 hospitalized adults with insulin treated CFRD, at least one episode of hypoglycemia was noted in half of the subjects. In this study, capillary glucose values were typically measured four times daily, and hypoglycemia was noted on 6.3% of capillary blood glucose readings [40]. Hypoglycemia was more common in female patients and the majority of events were captured between the hours of 24:00-06:00, consistent with patterns in T1D and T2D [41]. Furthermore, one or more episodes of inpatient hypoglycemia was associated with an increased risk of readmission and death. The authors concluded that 7-point glucose monitoring, rather than the more typical 4-point glucose monitoring, should be considered in hospitalized individuals with insulin-treated CFRD.
Management and prevention of hypoglycemia in people with CFRD on glucose-lowering therapy
Acute management of hypoglycemia
For blood glucose < 70 mg/dL (3.9 mmol/L), the patient should consume 15–20 g of fast-acting (glucose only or glucose-containing) carbohydrates in adults (or 0.3 g/kg in pediatric patients), then repeat the glucose level in 15 min [9], [42], [43]. If the glucose level is again < 70 mg/dL (3.9 mmol/L), then the patient should consume the same amount of fast-acting carbohydrates and repeat the blood glucose level in 15 min. These steps may be repeated several times. Once the glucose level reaches ≥ 70 mg/dL (3.9 mmol/L), a meal or snack should be consumed to help prevent hypoglycemia recurrence. If the patient develops altered mental status and requires assistance of another person (severe hypoglycemia), then emergency glucagon should be administered (intramuscular, subcutaneous, or intranasal) [9]. Emergency glucagon should be prescribed to patients with diabetes who are at risk for level 2 and 3 hypoglycemia. In the hospital setting, 25 g of 50% dextrose may be given by intravenous (IV) bolus to treat hypoglycemia [1].
Prevention of iatrogenic hypoglycemia
Among patients with diabetes treated with insulin, hypoglycemia is most commonly attributed to mismatches between exogenous insulin dosing and carbohydrate intake. While there are limited data on prevention of hypoglycemia among people with CFRD, data from people with other types of diabetes have shown reduced hypoglycemia with the use of diabetes self-management education, personalized glycemic targets with relative permissive hyperglycemia among those with recurrent hypoglycemia and/ or impaired awareness of hypoglycemia [44], frequent blood glucose monitoring [45], CGM use [46], [47], insulin regimens combining long- and rapid-acting insulins (as compared to NPH-based regimens) and new ultra-rapid acting insulin analogs [48]. The use of continuous subcutaneous insulin infusion (CSII), predictive low glucose suspend technology, and hybrid closed loop systems have been shown to be effective strategies for hypoglycemia prevention among people with T1D and T2D; they may be useful for preventing hypoglycemia in CFRD patients as well but would be more optimal if CF-specific algorithms are developed [38], [47], [49], [50]. Although not specific to CFRD, administering rapid –acting insulin analogues 15–20 min before a meal (as compared to immediately before the meal) has been associated with a 30% decrease in postprandial glucose values and a decreased risk for hypoglycemia [51].
Potential impact of CFTR modulators on hypoglycemia risk
The advent of CFTR modulator therapy in 2012 marked a new era of cystic fibrosis care. CFTR modulator drugs target the malfunctioning CFTR protein and are specific to gene [52]. Ivacaftor was the first approved modulator and is a highly effective potentiator for gating mutation G551D as well as multiple other gating and conductance mutations [53], [54]. Additional modulators followed, including Orkambi (Lumacaftor-Ivacaftor) in 2015 and Symdeko (Tezacaftor-Ivacaftor) in 2018 with expanded genotype eligibility to homozygous and heterozygous f508del but less impactful therapeutic effect on respiratory outcomes [55], [56], [57], [58]. Most recently in 2019, Trikafta, a highly effective triple modulator therapy (Elexacaftor-Tezacaftor-Ivacaftor) was released for homozygous and heterozygous f508del individuals [59], [60], [61].
There have been case reports of individuals with G551D mutations as well as homozygous f508del with comorbid insulin-requiring CFRD who weaned off insulin therapy; and some developed persistent hypoglycemia following Ivacaftor treatment [62], [63], [64]. The findings of these case reports have been bolstered by a recent retrospective cohort study series of 14 CFRD patients on Ivacaftor therapy (alone or in combination), which showed that 4 successfully stopped insulin and a fifth lowered insulin needs significantly. Three participants had ongoing recurrent hypoglycemic events off insulin therapy [65]. Hypoglycemia was documented as an adverse reaction in clinical trials of Trikafta [59]. More research is needed to understand the impact of highly effective modulators on insulin secretion; and their possible impact on CFRD management and risk of iatrogenic and spontaneous hypoglycemia.
Future directions
Future studies are needed to better understand the pathophysiology, clinical implications, and optimal management of spontaneous hypoglycemia in CF. Future studies will elucidate whether highly effective CFTR modulator therapies modify the risk of hypoglycemia in CF patients with and without diabetes. More research is needed to better understand the epidemiology of treatment related hypoglycemia and its complications in CFRD. Future research is also needed to understand the impact of diabetes technologies including CGM and hybrid closed loop insulin systems on the risk of hypoglycemia.
Summary
Hypoglycemia is common in the lives of people with CF, both with and without diabetes. There are significant knowledge gaps in our understanding of underlying mechanisms and optimal management of spontaneous and iatrogenic hypoglycemia in CF. Hypoglycemia can adversely affect quality of life and can have serious short and long-term consequences. It is crucial for clinicians to screen patients with CF for hypoglycemia; and educate patients and their caregivers regarding symptoms, management, and prevention of hypoglycemia. More research in CF related hypoglycemia and advancement in diabetes related therapies and technology can significantly reduce the burden of hypoglycemia in people with CF.
Funding sources
This work was supported by the Cystic Fibrosis Foundation EnVision II CF: Emerging Leaders in CF Endocrinology. The sponsor provided support and national faculty mentorship to develop expertise in CF Endocrinology but was not involved in the development of this manuscript.
CRediT authorship contribution statement
Rebecca Hicks: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Brynn E. Marks: Writing – original draft, Writing – review & editing. Rachael Oxman: Writing – original draft, Writing – review & editing. Amir Moheet: Conceptualization, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: BEM has received investigator initiated research funding from Dexcom, Inc and Tandem Diabetes Care.
References
- 1.Moheet A., Chan C.L., Granados A., Ode K.L., Moran A., Battezzati A. Hypoglycemia in cystic fibrosis: prevalence, impact and treatment. J Cyst Fibros. 2019;18(Suppl 2):S19–S24. doi: 10.1016/j.jcf.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye N., Seaquist E.R. Neuroendocrine responses to hypoglycemia. Ann N Y Acad Sci. 2010;1212:12–28. doi: 10.1111/j.1749-6632.2010.05820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer P.E., Axelrod L., Grossman A.B., Heller S.R., Montori V.M., Seaquist E.R. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 4.Seaquist E.R.A.J., Childs B., Cryer P., Dagogo-Jack S., Fish L. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Assocation and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryer P.E. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia. 2009;52(1):35–37. doi: 10.1007/s00125-008-1205-7. [DOI] [PubMed] [Google Scholar]
- 6.Mitrakou A., Ryan C., Veneman T., Mokan M., Jenssen T., Kiss I. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol. 1991;260(1 Pt 1):E67–E74. doi: 10.1152/ajpendo.1991.260.1.E67. [DOI] [PubMed] [Google Scholar]
- 7.Amiel S.A. The consequences of hypoglycaemia. Diabetologia. 2021;64(5):963–970. doi: 10.1007/s00125-020-05366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moheet A., Seaquist E. Hypoglycemia as a driver of cardiovascular risk in diabetes. Curr Atherosc Rep. 2013;15(9):1–6. doi: 10.1007/s11883-013-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American D.A. 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 10.Group IHS. Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40(1):155-7. [DOI] [PubMed]
- 11.Towler D.A., Havlin C.E., Craft S., Cryer P. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes. 1993;42(12):1791–1798. doi: 10.2337/diab.42.12.1791. [DOI] [PubMed] [Google Scholar]
- 12.Fanelli C., Pampanelli S., Epifano L., Rambotti A.M., Ciofetta M., Modarelli F. Relative roles of insulin and hypoglycaemia on induction of neuroendocrine responses to, symptoms of, and deterioration of cognitive function in hypoglycaemia in male and female humans. Diabetologia. 1994;37(8):797–807. doi: 10.1007/BF00404337. [DOI] [PubMed] [Google Scholar]
- 13.Stanley S., Moheet A., Seaquist E.R. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr Rev. 2019;40(3):768–788. doi: 10.1210/er.2018-00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman R.P. Antecedent hypoglycemia does not alter increased epinephrine-induced lipolysis in type 1 diabetes mellitus. Metabolism. 2006;55(3):371–380. doi: 10.1016/j.metabol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Gerich J., Davis J., Lorenzi M., Rizza R., Bohannon N., Karam J. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. 1979;236(4):E380–E385. doi: 10.1152/ajpendo.1979.236.4.E380. [DOI] [PubMed] [Google Scholar]
- 16.Rizza R.A., Cryer P.E., Gerich J.E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armaghanian N., Brand-Miller J.C., Markovic T.P., Steinbeck K.S. Hypoglycaemia in cystic fibrosis in the absence of diabetes: a systematic review. J Cyst Fibros. 2016;15(3):274–284. doi: 10.1016/j.jcf.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Lev-Ran A. Nadirs of oral glucose tolerance tests are independent of age and sex. Diabetes Care. 1983;6(4):405–408. doi: 10.2337/diacare.6.4.405. [DOI] [PubMed] [Google Scholar]
- 19.Parekh S., Bodicoat D.H., Brady E., Webb D., Mani H., Mostafa S. Clinical characteristics of people experiencing biochemical hypoglycaemia during an oral glucose tolerance test: cross-sectional analyses from a UK multi-ethnic population. Diabetes Res Clin Pract. 2014;104(3):427–434. doi: 10.1016/j.diabres.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Armaghanian N., Hetherington J., Parameswaran V., Chua E.L., Markovic T.P., Brand-Miller J. Hypoglycemia in cystic fibrosis during an extended oral glucose tolerance test. Pediatr Pulmonol. 2020;55(12):3391–3399. doi: 10.1002/ppul.25081. [DOI] [PubMed] [Google Scholar]
- 21.Kilberg M.J., Harris C., Sheikh S., Stefanovski D., Cuchel M., Kubrak C. Hypoglycemia and islet dysfunction following oral glucose tolerance testing in pancreatic-insufficient cystic fibrosis. J Clin Endocrinol Metab. 2020;105(10):3179–3189. doi: 10.1210/clinem/dgaa448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilberg M.J., Sheikh S., Stefanovski D., Kubrak C., De Leon D.D., Hadjiliadis D. Dysregulated insulin in pancreatic insufficient cystic fibrosis with post-prandial hypoglycemia. J Cyst Fibros. 2020;19(2):310–315. doi: 10.1016/j.jcf.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan C.L., Vigers T., Pyle L., Zeitler P.S., Sagel S.D., Nadeau K.J. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros. 2018;17(6):783–790. doi: 10.1016/j.jcf.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haliloglu B., Gokdemir Y., Atay Z., Abali S., Guran T., Karakoc F. Hypoglycemia is common in children with cystic fibrosis and seen predominantly in females. Pediatr Diabet. 2017;18(7):607–613. doi: 10.1111/pedi.12470. [DOI] [PubMed] [Google Scholar]
- 25.Chan C.L., Hope E., Thurston J., Vigers T., Pyle L., Zeitler P.S. Hemoglobin A(1c) accurately predicts continuous glucose monitoring-derived average glucose in youth and young adults with cystic fibrosis. Diabetes Care. 2018;41(7):1406–1413. doi: 10.2337/dc17-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armaghanian N., Markovic T.P., Brand-Miller J.C., Bye P.T.P., Moriarty C.P., Steinbeck K.S. Hypoglycaemia in cystic fibrosis: an analysis of a single centre adult cystic fibrosis clinic. J Cyst Fibros. 2018;17(4):542–547. doi: 10.1016/j.jcf.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Aitken M.L., Szkudlinska M.A., Boyko E.J., Ng D., Utzschneider K.M., Kahn S.E. Impaired counterregulatory responses to hypoglycaemia following oral glucose in adults with cystic fibrosis. Diabetologia. 2020;63(5):1055–1065. doi: 10.1007/s00125-020-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conn J.W., Fajans S.S., Seltzer H.S. Spontaneous hypoglycemia as an early manifestation of diabetes mellitus. Diabetes. 1956;5(6):437–442. doi: 10.2337/diab.5.6.437. [DOI] [PubMed] [Google Scholar]
- 29.Radike K., Molz K., Holl R.W., Poeter B., Hebestreit H., Ballmann M. Prognostic relevance of hypoglycemia following an oral glucose challenge for cystic fibrosis-related diabetes. Diabetes Care. 2011;34(4) doi: 10.2337/dc10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannik L.A., Chang K.A., Annoh P.Q.K., Sykes J., Gilmour J., Robert R. Prevalence of hypoglycemia during oral glucose tolerance testing in adults with cystic fibrosis and risk of developing cystic fibrosis-related diabetes. J Cyst Fibros. 2018;17(4):536–541. doi: 10.1016/j.jcf.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Khare S., Harindhanavudhi T., Wang Q., Espinosa R., Griffin T.B., Downs E.M. Factors associated with weight gain and improvement in FEV1 in people with cystic fibrosis on elexacaftor-tezacaftor-ivacaftor. Pediatr Pulmonol. 2020;55(S2):S38–S361. [Google Scholar]
- 32.Battezzati A., Battezzati P.M., Costantini D., Seia M., Zazzeron L., Russo M.C. Spontaneous hypoglycemia in patients with cystic fibrosis. Eur J Endocrinol. 2007;156(3):369–376. doi: 10.1530/eje.1.02344. [DOI] [PubMed] [Google Scholar]
- 33.Kaminski B.A., Goldsweig B.K., Sidhaye A., Blackman S.M., Schindler T., Moran A. Cystic fibrosis related diabetes: nutrition and growth considerations. J Cyst Fibros. 2019;18(Suppl 2):S32–S37. doi: 10.1016/j.jcf.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Khunti K., Alsifri S., Aronson R., Cigrovski Berković M., Enters-Weijnen C., Forsén T. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907–915. doi: 10.1111/dom.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran A., Pekow P., Grover P., Zorn M., Slovis B., Pilewski J. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care. 2009;32(10):1783–1788. doi: 10.2337/dc09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grover P., Thomas W., Moran A. Glargine versus NPH insulin in cystic fibrosis related diabetes. J Cyst Fibros. 2008;7(2):134–136. doi: 10.1016/j.jcf.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Group UHS Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140–1147. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 38.International Hypoglycaemia Study G. Minimizing Hypoglycemia in Diabetes. Diabetes Care. 2015;38(8):1583-91. [DOI] [PubMed]
- 39.Graveling A.J., Frier B.M. Impaired awareness of hypoglycaemia: a review. Diabetes Metab. 2010;36(Suppl 3):S64–S74. doi: 10.1016/S1262-3636(10)70470-5. [DOI] [PubMed] [Google Scholar]
- 40.Jones G.C., Chong Z.M., Gilmour J., Matheson C., MacGregor G., Sainsbury C.A. Patterns and Impact of hypoglycemia, hyperglycemia, and glucose variability on inpatients with insulin-treated cystic fibrosis-related diabetes. Diabetes Ther. 2016;7(3):575–582. doi: 10.1007/s13300-016-0194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones G.C., Casey H., Perry C.G., Kennon B., Sainsbury C.A. Trends in recorded capillary blood glucose and hypoglycaemia in hospitalised patients with diabetes. Diabetes Res Clin Pract. 2014;104(1):79–83. doi: 10.1016/j.diabres.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Abraham M.B., Jones T.W., Naranjo D., Karges B., Oduwole A., Tauschmann M. ISPAD clinical practice consensus guidelines 2018: assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):178–192. doi: 10.1111/pedi.12698. [DOI] [PubMed] [Google Scholar]
- 43.Carlson J.N., Schunder-Tatzber S., Neilson C.J., Hood N. Dietary sugars versus glucose tablets for first-aid treatment of symptomatic hypoglycaemia in awake patients with diabetes: a systematic review and meta-analysis. Emerg Med J. 2017;34(2):100–106. doi: 10.1136/emermed-2015-205637. [DOI] [PubMed] [Google Scholar]
- 44.Fritsche A.S.N., Haring H., Gerich J., Stumvoll M. Avoidance of hypoglycemia restores hypoglycemia awareness by increasing β-adrenergic sensitivity in type 1 diabetes. Ann Intern Med. 1995;124(9):729–736. doi: 10.7326/0003-4819-134-9_part_1-200105010-00009. [DOI] [PubMed] [Google Scholar]
- 45.Miller K.M., Beck R.W., Bergenstal R.M., Goland R.S., Haller M.J., McGill J.B. Evidence of a strong association between frequency of self-monitoringof blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009–2014. doi: 10.2337/dc12-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickup J.C., Freeman S.C., Sutton A.J. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: Meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343(7815):1–14. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan C.L., Ode K.L., Granados A., Moheet A., Moran A., Hameed S. Continuous glucose monitoring in cystic fibrosis – A practical guide. J Cyst Fibros. 2019;18(Suppl 2):S25–S31. doi: 10.1016/j.jcf.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 48.Bue-Valleskey J., Klaff L., Cho J.I., Dellva M.A., Schloot N.C., Tobian J. Long-term efficacy and safety of ultra rapid lispro (URLi) in adults with type 1 diabetes: the PRONTO-T1D extension. Diabetes Ther. 2021;12(2):569–580. doi: 10.1007/s13300-020-00987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karges B., Schwandt A., Heidtmann B., Kordonouri O., Binder E., Schierloh U. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358–1366. doi: 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Battelino T., Nimri R., Dovc K., Phillip M., Bratina N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care. 2017;40(6):764–770. doi: 10.2337/dc16-2584. [DOI] [PubMed] [Google Scholar]
- 51.Slattery D., Amiel S.A., Choudhary P. Optimal prandial timing of bolus insulin in diabetes management: a review. Diabet Med. 2018;35(3):306–316. doi: 10.1111/dme.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foundation CF. Cystic Fibrosis Foundation: CFTR Modulator Therapies [Available from: https://www.cff.org/Life-With-CF/Treatments-and-Therapies/Medications/CFTR-Modulator-Therapies/.
- 53.Insert KFp. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203188s019lbl.pdf.
- 54.Ramsey B.W., Davies J., McElvaney N.G., Tullis E., Bell S.C., Dřevínek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Insert OFp. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211358s000lbl.pdf.
- 56.Wainwright C.E., Elborn J.S., Ramsey B.W., Marigowda G., Huang X., Cipolli M. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(3):220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Insert SFP. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf [.
- 58.Taylor-Cousar J.L., Munck A., McKone E.F., van der Ent C.K., Moeller A., Simard C. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013–2023. doi: 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- 59.Insert TFp. [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211358s000lbl.pdf.
- 60.Middleton P.G., Mall M.A., Dřevínek P., Lands L.C., McKone E.F., Polineni D. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single phe508del allele. N Engl J Med. 2019;381(19):1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heijerman H.G.M., McKone E.F., Downey D.G., Van Braeckel E., Rowe S.M., Tullis E. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christian F, Thierman A, Shirley E, Allen K, Cross C, Jones K. Sustained Glycemic Control With Ivacaftor in Cystic Fibrosis-Related Diabetes. J Investig Med High Impact Case Rep. 2019;7:2324709619842898. [DOI] [PMC free article] [PubMed]
- 63.Hayes D., Jr., McCoy K.S., Sheikh S.I. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190(5):590–591. doi: 10.1164/rccm.201405-0882LE. [DOI] [PubMed] [Google Scholar]
- 64.Mehfooz A.Z.M., Morey-Vargas O. Do CFTR modulators treat cystic fibrosis-related diabetes? J Endocr Soc. 2019;3(SUN-LB026) [Google Scholar]
- 65.Gaines H., Jones K.R., Lim J., Medhi N.F., Chen S., Scofield R.H. Effect of CFTR modulator therapy on cystic fibrosis-related diabetes. J Diabetes Complications. 2021;35(6) doi: 10.1016/j.jdiacomp.2020.107845. [DOI] [PMC free article] [PubMed] [Google Scholar]


