Abstract
Background
White matter (WM) impairment is a hallmark of amyotrophic lateral sclerosis (ALS). This study evaluated the capacity of mean apparent propagator magnetic resonance imaging (MAP-MRI) for detecting ALS-related WM alterations.
Methods
Diffusion images were obtained from 52 ALS patients and 51 controls. MAP-derived indices [return-to-origin/-axis/-plane probability (RTOP/RTAP/RTPP) and non-Gaussianity (NG)/perpendicular/parallel NG (NG⊥/NG||)] were computed. Measures from diffusion tensor/kurtosis imaging (DTI/DKI) and neurite orientation dispersion and density imaging (NODDI) were also obtained. Voxel-wise analysis (VBA) was performed to determine differences in these parameters. Relationship between MAP parameters and disease severity (assessed by the revised ALS Functional Rating Scale (ALSFRS-R)) was evaluated by Pearson’s correlation analysis in a voxel-wise way. ALS patients were further divided into two subgroups: 29 with limb-only involvement and 23 with both bulbar and limb involvement. Subgroup analysis was then conducted to investigate diffusion parameter differences related to bulbar impairment.
Results
The VBA (with threshold of P < 0.05 after family-wise error correction (FWE)) showed that ALS patients had significantly decreased RTOP/RTAP/RTPP and NG/ NG⊥/NG|| in a set of WM areas, including the bilateral precentral gyrus, corona radiata, posterior limb of internal capsule, midbrain, middle corpus callosum, anterior corpus callosum, parahippocampal gyrus, and medulla. MAP-MRI had the capacity to capture WM damage in ALS, which was higher than DTI and similar to DKI/NODDI. RTOP/RTAP/NG/NG⊥/NG|| parameters, especially in the bilateral posterior limb of internal capsule and middle corpus callosum, were significantly correlated with ALSFRS-R (with threshold of FWE-corrected P < 0.05). The VBA (with FWE-corrected P < 0.05) revealed the significant RTAP reduction in subgroup with both bulbar and limb involvement, compared with those with limb-only involvement.
Conclusions
Microstructural impairments in corticospinal tract and corpus callosum represent the consistent characteristic of ALS. MAP-MRI could provide alternative measures depicting ALS-related WM alterations, complementary to the common diffusion imaging methods.
Keywords: Amyotrophic lateral sclerosis, White matter, Mean apparent propagator, Diffusion weighted imaging, Corticospinal tract
Abbreviations: WM, white matter; ALS, amyotrophic lateral sclerosis; MAP, mean apparent propagator; MRI, magnetic resonance imaging; DTI, diffusion tensor imaging; DKI, diffusion kurtosis imaging; NODDI, neurite orientation dispersion and density imaging; RTOP, return-to-origin probability; RTAP, return-to-axis probability; RTPP, return-to-plane probability; NG, non-Gaussianity; NG⊥, perpendicular NG; NG||, parallel NG; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity; AD, axial diffusivity; MK, mean kurtosis; RK, radial kurtosis; AK, axial kurtosis; NDI, neurite density index; ODI, orientation dispersion index; ISO, isotropic compartment fraction; VBA, voxel-wise analysis; ALSFRS-R, revised ALS Functional Rating Scale; CST, corticospinal tract; PDF, probability density function; HCs, healthy controls
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a rare, progressive neurodegenerative disorder with unknown etiology and devastating effects that result in the loss of upper and lower motor neurons. Clinical manifestations of ALS include motor dysfunction (e.g., muscle weakness, muscle atrophy, and dysphagia) and cognitive and behavioral impairment (Hardiman et al., 2017). ALS progresses rapidly, leading to respiratory failure and ultimately the death of ALS patients due within 3 to 5 years of symptom onset (Hardiman et al., 2011). Unfortunately, there is currently no effective treatment available for ALS. Of note, previous studies have demonstrated that the modifications of white matter (WM) microstructure and function (e.g., the increase in axonal initial segment diameter and axonal ribosome numbers and altered axonal excitability (Iwai et al., 2016, Sasaki and Maruyama, 1992, Verheijen et al., 2014)) are early events implicated in the pathogenesis of ALS. Therefore, investigating the WM alteration related to ALS might offer novel insights and therapeutic targets for ALS treatment.
The impairment of WM integrity in ALS has been well documented. Neuropathological studies have identified ALS-related WM abnormalities, including axonal loss and demyelination in the corticospinal tract (CST) and spinal cord (Matsusue et al., 2007) and the accumulation of phosphorylated 43-kDA TAR DNA-binding protein (pTDP-43) in the CST, corpus callosum, and cingulum (Fatima et al., 2015). Neuroimaging studies have also provided evidence of WM impairment in ALS. At the macroscopic level, a meta-analysis conducted on voxel-based morphometry studies found volume losses in several WM tracts in ALS, including the CST, subcortical arcuate fibers, interhemispheric fibers, and projection fibers to the striatum and cortico-ponto-cerebellar tract (Chen et al., 2018). At the microscopic level, based on voxel-based analysis (VBA), diffusion tensor imaging (DTI) studies have reported ALS-related WM damage (as reflected by decreased fractional anisotropy (FA)) in several regions, involving the CST, corpus callosum, cingulum, and superior longitudinal fasciculus (Prudlo et al., 2012, Zhang et al., 2018). Furthermore, DTI studies examining voxel-based connectivity index and FA metric have shown aberrant WM connectivity in ALS that involved the CST and motor network (Ciccarelli et al., 2006, Verstraete et al., 2011).
However, DTI assumes that water molecule diffusion in the brain follows Gaussian distribution, weakening its capability to detect diffusion heterogeneity in brain tissue (Arab et al., 2018). Several additional diffusion magnetic resonance imaging (MRI) techniques, such as diffusion kurtosis imaging (DKI) and neurite orientation and dispersion density imaging (NODDI), further elaborated ALS-related WM microstructure alterations. For instance, a prior DKI study in ALS found abnormal WM microstructures (as reflected by decreased mean kurtosis (MK) and radial kurtosis (RK)) in the CST pathway and middle corpus callosum (Huang et al., 2020). Moreover, recent NODDI studies have detected ALS-related WM damage (as reflected by decreased neurite density index (NDI)) in the bilateral CST, corpus callosum, and frontotemporal-related tracts (Broad et al., 2019, Wen et al., 2019).
Quite recently, Özarslan et al. proposed an efficient, quantitative, and robust mathematical and physical framework, namely, mean apparent propagator (MAP) MRI, to model the three-dimensional q-space signal and transform it into diffusion propagators (Ozarslan et al., 2013). By measuring the probability density function (PDF) of spin displacement, MAP-MRI could provide useful measures in complex tissue microstructures (e.g., restrictions and multiple compartments) (Avram et al., 2016). In fact, it extends the diffusion signal analytically in the local DTI reference frame using a complete set of orthogonal basis functions (Ozarslan et al., 2013). It contributes to improve robustness to noise and immunity from signal confounds; and with scalar descriptors of the propagator, MAP-MRI can better quantify brain microstructural characteristics (Avram et al., 2016). The quantitative measures derived from MAP-MRI include zero-displacement probabilities, non-Gaussianity (NG), and propagator anisotropy, which are relevant to the tissue microstructural features such as cellularity and restrictions, diffusion heterogeneity, and the degree of anisotropy (Avram et al., 2016). Several studies have already investigated the application value of MAP-MRI. MAP-MRI provides a more comprehensive and sensitive brain microstructure characterization in healthy subjects (Avram et al., 2016, Fick et al., 2016). In addition, MAP-MRI has been demonstrated to provide sensitive detection of microstructural alterations in the brain due to multiple pathological conditions such as stroke (Brusini et al., 2016), Alzheimer’s disease (Fick et al., 2017), and epilepsy (Ma et al., 2020).
In this study, we investigated the ALS-related WM microstructure alterations using MAP-MRI for the first time. This study had the following aims: 1) to evaluate the feasibility of MAP-MRI for capturing disrupted WM microstructures in ALS patients; 2) to compare the capability of MAP-MRI with conventional DTI, DKI, and NODDI for the detection of ALS-related WM damage; and 3) to examine the potential of MAP-MRI measures for monitoring the disease severity of ALS.
2. Methods
2.1. Subjects
The subjects in this study comprised a total of 103 patients, among which 52 had sporadic ALS and 51 were healthy controls (HCs). ALS was diagnosed using the El Escorial criteria [23], and the revised ALS Functional Rating Scale (ALSFRS-R) was used to assess the severity of disease. There were no significant differences in terms of age, gender, and education level between the ALS patient and control groups. Detailed clinical and demographic data are shown in Table 1. The following exclusion criteria were used: 1) other neuropsychiatric diseases (e.g., Parkinson’s disease, Alzheimer’s disease, epilepsy, schizophrenia, and depression); 2) taking psychotropic drugs; 3) suffering from other severe disorders (e.g., respiratory failure, cardiovascular diseases, malignant tumors); and 4) contraindications of MRI examination. This study was approved by the Research Ethics Committee of Fujian Medical University Union Hospital, and written informed consent was obtained from all subjects.
Table 1.
Demographic and clinical characteristics of the study subjects.
| Healthy controls (n = 51) |
ALS patients (n = 52) |
P value* | |
|---|---|---|---|
| Age (years) | 52.8 ± 9.1 | 54.3 ± 10.7 | 0.434 |
| Sex (female/male) | 14/37 | 20/32 | 0.235 |
| Education (years) | 8.4 ± 3.1 | 7.6 ± 4.2 | 0.280 |
| Site of onset (bulbar/limb) | – | 9/43 | – |
| Disease distribution clinically (bulbar and limb involvement/limb involvement only) | – | 23/29 | – |
| Diagnostic category (definite/probable/possible) | – | 10/20/22 | – |
| ALSFRS-R score | – | 41.0 ± 6.0 | – |
| Disease duration (months) | – | 15.5 ± 14.5 | – |
| Progression rate | – | 0.58 ± 0.56 | – |
ALS, amyotrophic lateral sclerosis; ALSFRS-R, revised ALS Functional Rating Scale. The rate of disease progression was calculated using the equation: (48 - ALSFRS-R)/Disease duration. “-” denotes no data available. * indicates that the continuous and categorical variables were compared using a two-sample t test and chi-square test, respectively.
2.2. MRI data acquisition
Participants were scanned on a Siemens Prisma 3.0 T scanner with 64-channel head coil. Diffusion images were acquired with a spin-echo echo-planar imaging sequence, using a full q-space Cartesian grid sampling scheme, which has been applied in previous MAP-MRI studies (Jiang et al., 2021, Le et al., 2020, Mao et al., 2020). For each participant, 100 diffusion-weighted images were acquired at following b-values (direction number): 0 (2), 350 (6), 600 (12), 1000 (8), 1400 (6), 1650 (24), 2000 (24), 2700 (12), and 3000 (6) s/mm2. The other sampling parameters were as follows: repetition time = 4000 ms; echo time = 72 ms; diffusion time parameters (Δ = 35 ms and δ = 15.9 ms); field of view = 220 mm × 220 mm; matrix = 110 × 110; slice thickness = 2 mm; slice number = 72 axial slices (gap = 0); isotropic resolution = 2 mm3; slice acceleration factor = 2; inplane acceleration factor = 2; total scanning time = 429 s. The signal-to-noise ratio (SNR) of diffusion data, which was calculated according to reference (Kaufman et al., 1989), was 16.7 ∼ 57.2 for b-value of 0 ∼ 3000 s/mm2.
2.3. Diffusion data processing
For each subject’s data, the quantitative metrics from DTI (FA, mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD)), DKI (MK, axial kurtosis (AK), and RK), NODDI (NDI, orientation dispersion index (ODI), and isotropic component fraction (ISO)), and MAP (return-to-origin probability (RTOP), return-to-axis probability (RTAP), return-to-plane probability (RTPP), NG, perpendicular NG (NG⊥), and parallel NG (NG||)) models were simultaneously calculated using an in-house developed tool called NeuDiLab, which was based on Python 3.5 and open-resource projects previously validated (Mao et al., 2020). The processing pipeline included the following steps: 1) the skull was removed by BET from FSL (Jenkinson et al., 2012); 2) eddy current distortion and motion artifacts were corrected using the bneddy tool from DiffusionKit (Xie et al., 2016); 3) a 3D Gaussian filter was applied to diffusion data with a full width at half maximum (FWHM) at 3 mm to increase SNR and reduce potential misregistration among diffusion data. The FWHM was set at 1.5 times of voxel size according to previous studies (Tabesh et al., 2011, Yan et al., 2014); 4) metrics of DTI, DKI, and MAP were estimated by algorithms from DIPY (Garyfallidis et al., 2014), and the NODDI model metrics were estimated by AMICO (Daducci et al., 2015). DTI metrics were calculated only on data points with b-values ≤ 1000 s/mm2; whereas, other diffusion metrics were calculated with all acquired b-values.
2.4. Voxel-based analysis
The VBA was conducted to investigate the alterations in the DTI, DKI, NODDI, and MAP metrics between patients and HCs by using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/), which implemented the general linear model. The normalization process adopted a two-step strategy based on b0 data: 1) A subject-specific template was first created by nonlinearly normalizing b0 data of all HCs to EPI MNI template in SPM, followed by average and Gaussian smoothing with FWHM = 6 mm; 2) b0 data of all subjects were normalized to the subject-specific template and the resulting deformation fields were applied to their corresponding quantitative metrics of all diffusion models. Finally, Gaussian smoothing was applied with FWHM = 6 mm. Between-group differences in the diffusion metrics were then determined by a two-sample t-test with the statistical threshold set at P < 0.05 after family-wise error correction. The covariates were age, gender, and education level. The VBA processes were confined to a WM mask, which was created by selecting a threshold of 0.2 on the averaged FA map of all subjects.
2.5. Correlation analysis
A Pearson’s correlation analysis was conducted among ALS patients to evaluate the relationship between disease severity as assessed by an ALSFRS-R score and MAP metrics in a voxel-wise way. The covariates were age, gender, and education level. The correlation analysis was confined to the WM areas in which the significant between-group differences in diffusion metrics were found during VBA. The statistical threshold was set at a P value < 0.05 with family-wise error correction. Using the same procedures, the Pearson’s correlation analyses were also conducted to investigate the relationship between disease severity and other diffusion metrics (see Supplementary Fig. 1).
2.6. Subgroup analysis
The ALS patients were further divided into two subgroups according to the disease clinical distribution: 29 with limb-only involvement and 23 with both bulbar and limb involvement. The scores on the three bulbar items in the ALSFRS-R (i.e. speech, sialorrhoea, and swallowing; a score of four per item indicating normal function) were used to define the presence of bulbar involvement. The VBA of DTI, DKI, NODDI, and MAP metrics between two subgroups were performed using the general linear model framework implemented in SPM12. The statistical threshold was set at P < 0.05 after family-wise error correction. The covariates were age, gender, and education level.
3. Results
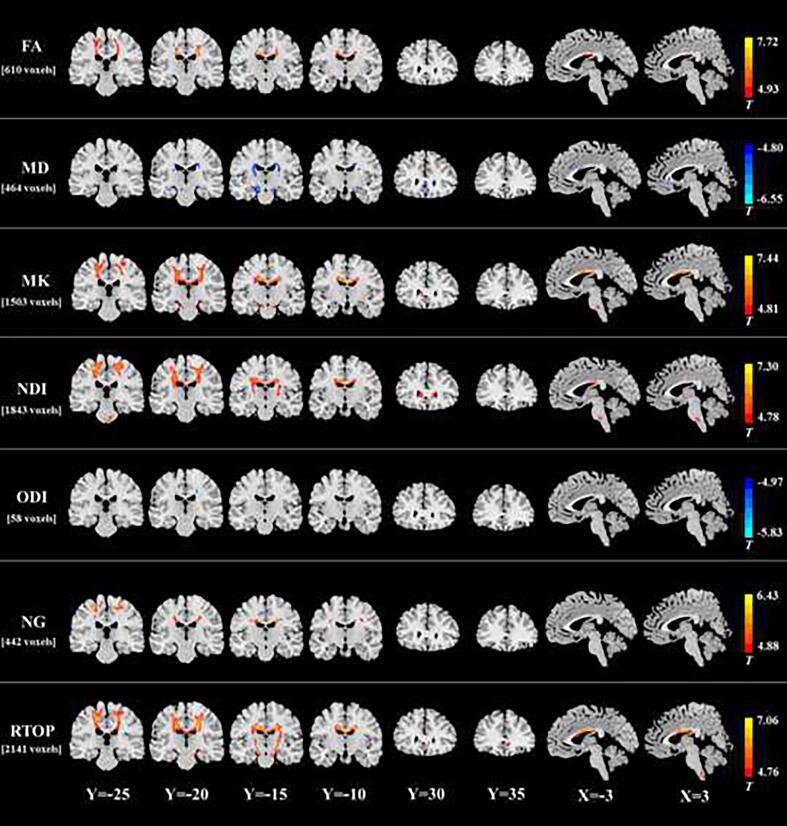
Compared with HCs, ALS patients showed an extensive reduction of MAP metrics in a set of WM regions. The patients with ALS had decreased RTOP in the bilateral precentral gyrus, corona radiata, posterior limb of internal capsule, midbrain, middle corpus callosum, anterior corpus callosum, parahippocampal gyrus, and medulla (Fig. 1 and Table 2). In these WM areas, the MD/ODI increment or FA/MK/NDI/NG reduction was also observed in the ALS group. In fact, VBA revealed the greatest differences in RTOP (2141 voxels), followed in order by NDI (1843 voxels), MK (1503 voxels), FA (610 voxels), MD (464 voxels), NG (442 voxels), and ODI (58 voxels). No significant ISO difference was found between two groups.
Fig. 1.
White matter regions in the ALS group with significant decreases in FA, MK, NDI, NG, and RTOP and increases in MD and ODI. The spatial extent of WM area with altered diffusion measures is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
Table 2.
White matter regions with altered non-directional diffusion metrics in the ALS group.
| Regions | Voxels | MNI coordinates |
Peak T-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| FA reduction | |||||
| Bilateral precentral gyrus, corona radiata, and middle corpus callosum | 600 | −16 | −16 | 34 | 7.72 |
| Right middle occipital gyrus | 10 | 32 | −74 | 0 | 5.68 |
| MD increment | |||||
| Left midbrain and parahippocampal gyrus | 137 | −10 | −18 | −16 | −6.55 |
| Right corona radiata and posterior limb of internal capsule | 116 | 22 | −18 | 32 | −5.45 |
| Left corona radiata and posterior limb of internal capsule | 90 | −24 | −16 | 22 | −5.61 |
| Bilateral anterior corpus callosum | 31 | 0 | 28 | 2 | −5.53 |
| Right midbrain | 27 | 14 | −14 | −16 | −5.38 |
| Bilateral middle corpus callosum | 20 | −2 | 8 | 22 | −5.24 |
| Right parahippocampal gyrus | 18 | 22 | –32 | −6 | −5.04 |
| Right anterior cingulate gyrus | 15 | 6 | 34 | −10 | −5.57 |
| Left anterior corpus callosum | 10 | −8 | 24 | 16 | −5.21 |
| MK reduction | |||||
| Bilateral precentral gyrus, corona radiata, and middle corpus callosum | 1356 | −2 | −10 | 26 | 7.44 |
| Left midbrain and pons | 57 | −12 | −18 | −20 | 6.49 |
| Right midbrain and parahippocampal gyrus | 52 | 12 | −16 | −20 | 6.00 |
| Left anterior corpus callosum | 21 | −10 | 30 | 0 | 5.19 |
| Pons | 17 | −4 | −28 | −42 | 5.68 |
| NDI reduction | |||||
| Bilateral precentral gyrus, corona radiata, posterior limb of internal capsule, and middle corpus callosum, and right anterior corpus callosum | 1678 | −26 | –22 | 54 | 7.30 |
| Pons and medulla | 98 | 4 | −26 | −44 | 6.33 |
| Left anterior corpus callosum | 67 | −12 | 30 | 4 | 5.33 |
| ODI increment | |||||
| Right corona radiata | 24 | 20 | −18 | 36 | −5.83 |
| Left corona radiata | 22 | −18 | −18 | 34 | −5.76 |
| Left middle corpus callosum | 12 | −6 | −12 | 28 | −5.66 |
| NG reduction | |||||
| Left precentral gyrus and corona radiata | 249 | −10 | −26 | 66 | 6.43 |
| Right corona radiata | 112 | 18 | −16 | 32 | 6.02 |
| Right precentral gyrus | 81 | 26 | −24 | 62 | 6.05 |
| RTOP reduction | |||||
| Bilateral precentral gyrus, corona radiata, and middle corpus callosum, and right posterior limb of internal capsule, midbrain, and parahippocampal gyrus | 1949 | 4 | −4 | 24 | 7.06 |
| Left posterior limb of internal capsule, midbrain, and parahippocampal gyrus | 113 | −14 | −18 | −18 | 6.06 |
| Medulla | 35 | 0 | −42 | −64 | 6.39 |
| Right anterior corpus callosum | 31 | 10 | 32 | −2 | 5.12 |
| Left anterior corpus callosum | 13 | −10 | 32 | 0 | 5.01 |
In the between-group comparison on radially directional measurements (Fig. 2 and Table 3), we found that RTAP reduction was the most extensive (1957 voxels), followed in order by NG⊥ decrease (1684 voxels), RK decrease (1405 voxels), and RD increase (762 voxels) in ALS patients. The spatial distribution of WM areas with altered radially directional metrics was highly similar to those of areas with RTOP/NDI/MK/FA/MD/NG/ODI changes.
Fig. 2.
White matter regions in the ALS group with significant increase in RD and decreases in RK, NG⊥, and RTAP. The spatial extent of WM area with altered diffusion measures is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
Table 3.
White matter regions with altered radially directional diffusion metrics in the ALS group.
| Regions | Voxels | MNI coordinates |
Peak T-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| RD increment | |||||
| Right precentral gyrus and corona radiata | 279 | 20 | −18 | 34 | −7.07 |
| Left precentral gyrus and corona radiata | 195 | −16 | −16 | 36 | −6.77 |
| Left midbrain and parahippocampal gyrus | 88 | −14 | −16 | −14 | −6.19 |
| Bilateral anterior cingulate gyrus and anterior corpus callosum | 78 | 8 | 36 | −6 | −5.49 |
| Right midbrain | 42 | 16 | −14 | −14 | −5.73 |
| Right posterior limb of internal capsule | 26 | 22 | −12 | 2 | −5.82 |
| Bilateral middle corpus callosum | 26 | −4 | 6 | 24 | −5.36 |
| Left parahippocampal gyrus | 18 | –22 | –32 | −6 | −5.27 |
| Left posterior limb of internal capsule | 10 | –22 | −16 | 2 | −5.39 |
| RK reduction | |||||
| Bilateral precentral gyrus, corona radiata, and middle corpus callosum | 1328 | −4 | −10 | 26 | 7.73 |
| Medulla | 61 | 0 | −40 | −62 | 7.39 |
| Pons | 16 | −4 | −28 | −42 | 5.57 |
| NG⊥ reduction | |||||
| Left precentral gyrus, and bilateral corona radiata, posterior limb of internal capsule, and middle corpus callosum | 1440 | −2 | −10 | 26 | 6.98 |
| Right precentral gyrus and corona radiata | 140 | 28 | −20 | 54 | 6.20 |
| Right anterior corpus callosum | 43 | 10 | 32 | −4 | 5.71 |
| Left anterior corpus callosum | 36 | −8 | 30 | 2 | 5.53 |
| Right anterior corpus callosum | 15 | 12 | 22 | 14 | 5.21 |
| Right midbrain | 10 | 20 | −16 | −10 | 5.67 |
| RTAP reduction | |||||
| Bilateral precentral gyrus, corona radiata, and middle corpus callosum | 1711 | −16 | −16 | 34 | 7.65 |
| Right midbrain, parahippocampal gyrus, and posterior limb of internal capsule | 121 | 24 | −14 | 6 | 6.32 |
| Left midbrain and posterior limb of internal capsule | 68 | –22 | −16 | 4 | 5.97 |
| Medulla | 39 | 0 | −40 | −62 | 6.75 |
| Right anterior corpus callosum | 18 | 10 | 32 | −2 | 5.12 |
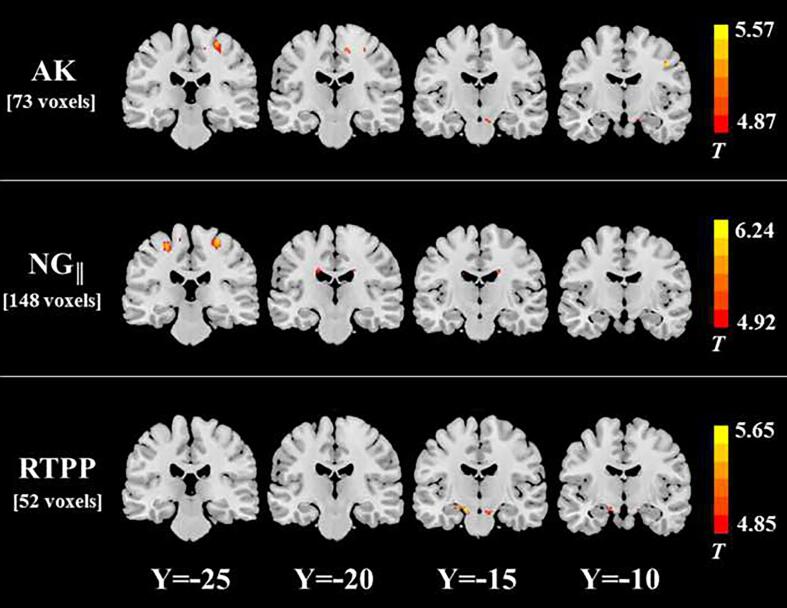
In the between-group comparison on the axially directional measurements (Fig. 3 and Table 4), we found that WM areas with NG|| reduction (148 voxels) were more extensive than those with AK decrease (73 voxels) or RTPP decrease (52 voxels) in the ALS group. The spatial distribution of WM areas with altered axially directional metrics was similar to those of areas with RTOP/NDI/MK/FA/MD/NG/ODI changes, but their spatial extent was dramatically reduced. No significant AD difference was found between two groups.
Fig. 3.
White matter regions in the ALS group with significant decreases in AK, NG||, and RTPP. The spatial extent of WM area with altered diffusion measures is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
Table 4.
White matter regions with altered axially directional diffusion metrics in the ALS group.
| Regions | Voxels | MNI coordinates |
Peak T-value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| AK reduction | |||||
| Right precentral gyrus | 34 | 26 | −24 | 62 | 5.39 |
| Right precentral gyrus | 14 | 10 | –22 | 56 | 5.19 |
| Right precentral gyrus | 13 | 44 | −10 | 40 | 5.57 |
| Right midbrain | 12 | 8 | −14 | −20 | 5.15 |
| NG||reduction | |||||
| Left precentral gyrus | 46 | −12 | −26 | 66 | 6.24 |
| Right precentral gyrus | 35 | 28 | −24 | 62 | 5.97 |
| Left precentral gyrus | 34 | −26 | −24 | 58 | 5.90 |
| Left middle corpus callosum | 17 | −18 | −20 | 32 | 5.43 |
| Right middle corpus callosum | 16 | 18 | −16 | 32 | 5.51 |
| RTPP reduction | |||||
| Left midbrain and parahippocampal gyrus | 39 | −14 | −12 | −16 | 5.65 |
| Right midbrain | 13 | 8 | −14 | −18 | 5.19 |
Figure 4 shows the results of voxel-wise correlation analysis among the patients. The areas in which RTOP was positively correlated with ALSFRS-R score included 509 voxels in WM and involved the left precentral gyrus, left corona radiata, and bilateral posterior limb of internal capsule and middle corpus callosum. The WM areas in which RTAP was correlated with ALSFRS-R score showed highly similar spatial distribution and were less extensive (384 voxels). There was no WM area whose RTPP value was found to be significantly correlated with ALSFRS-R score. The NG⊥ in the bilateral posterior limb of internal capsule and middle corpus callosum (513 voxels) was positively correlated with ALSFRS-R score, whereas the WM areas in which NG/NG|| (91 and 31 voxels, respectively) was correlated with ALSFRS-R score were much less extensive. In addition, in several WM areas, FA, MK, RK, and NDI were found to be positively correlated with ALSFRS-R score, while MD and RD were observed to be negatively correlated with ALSFRS-R score (Supplementary Fig. 1). These WM areas primarily involved bilateral corona radiata, posterior limb of internal capsule, and midbrain (Supplementary Fig. 1).
Fig. 4.
The correlation between mean apparent propagator metrics and ALS disease severity indicated by ALSFRS-R score. The spatial extent of WM area in which MAP metrics are correlated with ALSFRS-R score is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
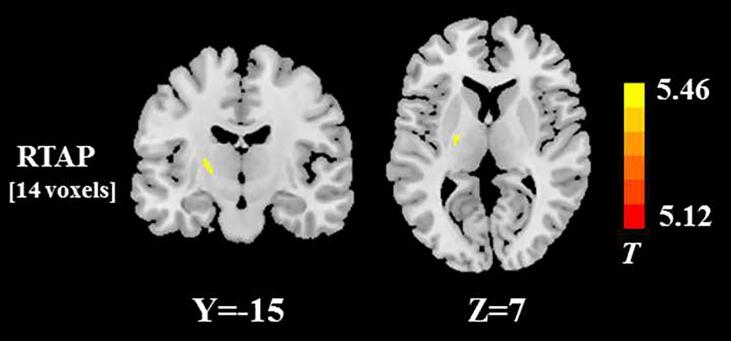
In the subgroup analysis using the VBA, the significant RTAP reduction was found in left posterior limb of internal capsule in the ALS patients with both bulbar and limb involvement, compared with those with limb-only involvement (Fig. 5). No significant differences in the other DTI, DKI, NODDI, and MAP metrics were found between the two ALS subgroups, after family-wise error correction for multiple comparisons.
Fig. 5.
White matter regions with the significant RTAP difference between two ALS subgroups stratified according to the presence or absence of bulbar involvement. The spatial extent of WM area is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
4. Discussion
In this study, we explored the performance of MAP-MRI in detecting ALS-related WM alterations and compared it with other diffusion models such as conventional DTI, DKI, and NODDI. Our main findings can be summarized as follows. First, ALS patients showed extensive alteration of non-directional MAP metrics (as reflected by decreased RTOP and NG) in a set of WM areas involving several motor-related brain regions (including the bilateral precentral gyrus, corona radiata, posterior limb of internal capsule, midbrain, medulla, and middle corpus callosum) and cognition-related regions (including the anterior corpus callosum and parahippocampal gyrus). Second, WM regions with decreased radially directional MAP metrics showed a similar distribution to that with RTOP and NG changes; the spatial distribution of WM regions with decreased axially directional MAP metrics was also similar to the distribution of RTOP and NG changes, but the spatial extent was dramatically reduced. Third, MAP-MRI showed the robust capacity to capture WM damage, which was higher than that of the conventional DTI and similar to that of other advanced diffusion models (such as DKI and NODDI), regardless of the directional or non-directional metrics used. Fourth, voxel-wise correlation analysis showed that MAP metrics were correlated with disease severity (as represented by ALSFRS-R score) with various degrees. Fifth, the significant WM abnormality related to bulbar impairment was only detected by MAP measure (i.e. RTAP). Taken together, MAP-MRI can offer complementary information for ALS-related WM damage to other diffusion models, which might provide novel insights on the pathogenesis of ALS.
The specific neuropathological substrate that underlies ALS-related WM damage is linked to multiple factors such as axon and myelin. As previous pathological studies have found, ALS is a progressive axonopathy. Distal axonal degeneration is found at the very early stage of ALS, prior to symptom onset (Fischer et al., 2004, Moloney et al., 2014). Several ALS genes (e.g., dynactin 1 and tublin 4A) encode cytoskeletal proteins important for the maintenance of axonal transport and integrity (Pensato et al., 2015, Puls et al., 2003); mutations in these genes might contribute to the axonal disruption. On the other hand, glial cells (e.g., astrocytes and oligodendrocytes) are critical for preserving axonal integrity (Edgar and Nave, 2009). For instance, astrocytes participate in the differentiation and maturation of oligodendrocytes (Li et al., 2016); oligodendrocytes generate and maintain the myelin sheath around the axon, ensuring the conduction of action potentials (Philips and Rothstein, 2014). Astrocyte and oligodendrocyte dysfunction in ALS has been well documented (Philips and Rothstein, 2014), which might lead to the axonal demyelination that has been described in ALS (Hayashi et al., 1986, Smith, 1960). Our study determined that ALS patients had abnormalities in directional MAP metrics, which was in accordance with neuropathological findings. Directional diffusion metrics, including parallel indices such as AD, AK, RTPP, and NG|| and perpendicular metrics such as RD, RK, RTAP, and NG⊥, respectively reflected the presence of restrictive barriers or heterogeneous diffusion in the axial and radial orientation, which are associated with axonal integrity and myelination (Andica et al., 2019, Avram et al., 2016). Changes in directional MAP metrics induced by ALS could therefore provide distinctive WM biomarkers for axonal loss and demyelination.
We observed impaired WM microstructures (as reflected by decreased RTOP and NG) in the ALS group in several brain areas, including motor as well as cognitive regions. WM damage in motor regions in ALS primarily involved the CST pathway (including the bilateral precentral gyrus, corona radiata, posterior limb of internal capsule, midbrain, and medulla) and middle corpus callosum, which was in line with previous diffusion and structural MRI studies (Huang et al., 2020, Ishaque et al., 2019, Wen et al., 2019, Yamauchi et al., 1995, Zhang et al., 2018). The CST transmits motor impulses from the motor and premotor cortices to the spinal cord, thereby mediating voluntary distal movements (Welniarz et al., 2017); and the middle corpus callosum connects homologous cortical regions relevant for motor function such as precentral frontal regions and parietal lobes (Catani and Thiebaut de Schotten, 2008), which are involved in the control and coordination of bilateral movements (Richmond and Fling, 2019). WM damage in these motor regions might cause the delay or disruption of motor impulse conduction, which is a potential mechanism of motor dysfunction in ALS. Additionally, in accordance with previous studies (Abrahams et al., 2005, Chapman et al., 2014), we also observed WM microstructural alterations in several cognition-related regions such as the anterior corpus callosum and parahippocampal gyrus. The anterior corpus callosum aids in the inter-hemispheric integration of cognitive information by connecting the prefrontal and orbitofrontal areas (Catani and Thiebaut de Schotten, 2008); the parahippocampal gyrus is part of a large network connecting frontal, parietal, and temporal cortices, which is closely linked to many cognitive processes (e.g., visuospatial processing and episodic memory) (Aminoff et al., 2013). Thus, it is speculated that WM abnormalities in these cognition-related regions might be specifically associated with the presence of well-described cognitive impairments in ALS (Hardiman et al., 2017). A future study which directly examine the association between ALS-related WM impairment and cognitive dysfunction is recommended to verify this speculation.
In keeping with previous studies (Bao et al., 2018, Schuster et al., 2016), our findings revealed that DTI could detect ALS-related WM microstructure alterations, particularly RD (which is considered as the most sensitive DTI metric and reflects WM demyelination). Furthermore, our results agreed with preceding DKI research in ALS (Huang et al., 2020), which has demonstrated that DKI (as a mathematical extension of DTI quantifying non-Gaussian diffusion) provides a better characterization of WM abnormalities in ALS, relative to DTI. Consistent with prior NODDI studies in ALS (Broad et al., 2019, Wen et al., 2019), our results also suggested that NODDI can offer higher sensitivity and greater tissue specificity than DTI in detecting WM abnormalities. More importantly, compared with conventional DTI and other advanced diffusion models (DKI/NODDI), our findings revealed that MAP-MRI could provide additional indicators to effectively depict ALS-related WM alterations. Owing to the inherent limitation of DTI on the assumption of Gaussian spin displacement distribution, its ability to describe intricate tissue microanatomy is weakened, while MAP-MRI efficiently measures the PDF of spin displacements and quantifies useful metrics of this PDF, thereby facilitating the indication of multiple-compartmental and restricted diffusion in complex tissue microstructures (Avram et al., 2016). Thus, it was not unexpected that the sensitivity of MAP was higher than that of DTI. Furthermore, in comparison with DKI, MAP-MRI might offer sensitive indicators to comprehensively characterize WM pathological alterations such as axonal loss and demyelination. For instance, axonal loss in ALS has been validated, but that was rarely captured by the parallel diffusion metric (AK) derived from DKI; while ALS patients showed a more extensive distribution of WM regions with altered axial MAP metrics (i.e., NG|| and RTPP), indicating that axial MAP metrics may detect axonal damage with higher capability. In addition, it seemed that MAP-MRI detected a broader range of ALS-related WM damage than NODDI, suggesting that at least MAP-MRI might have the potential similar to the NODDI in clinical application.
In consistence with previous report that the extent of WM impairment is associated with clinical involvement in ALS (Broad et al., 2019), we found the more widespread RTAP reduction in the subgroup with both limb and bulbar involvement compared to those with limb involvement alone. Thus, our findings also suggested the potential of MAP measurements for assessing the effect of ALS disease heterogeneity on WM microstructures. On the other hand, no significant difference related to bulbar impairment was observed in other diffusion parameters (e.g. NODDI metrics), which didn’t keep in line with previous studies (Broad et al., 2019). This discrepancy may be due to the differences in the sample collection and MRI data acquisition and processing between the current and previous diffusion studies.
This study had several limitations. First, due to the limited sample size, further research with a larger set of subjects is necessary to validate our results. Second, the cognitive function in ALS patients was not evaluated, hindering identification of the relationship between distinctive cognitive dysfunctions and the impaired integrity of specific WM regions. Third, in the acquisition protocol for MAP-MRI, the future studies could evaluate a better distribution of the gradient sampling, since the higher b-values are likely to indicate lower SNR which was not compensated by the denser sampling in this study. Fourth, in consideration of clinical feasibility (e.g. the relatively short period for MRI scanning), the diffusion acquisition protocol employed in this study was primarily designed towards the application of MAP-MRI, but was not the most optimal for other diffusion models (e.g. NODDI (Zhang et al., 2012)). The risk for performance underestimation of these diffusion models should be evaluated in the future study. Fifth, VBA method was applied in this study. Whereas, another method, called tract-based spatial statistics (TBSS), could be considered for voxel-wise group comparison of diffusion parametric maps especially when only white matter is included in the analysis, since its improved sensitivity and objectivity of the comparison and the obviation of spatial smoothing for diffusion data (Smith et al., 2006). Sixth, in data processing step, we applied Gaussian filter to improve fitting result of diffusion model, but it may also introduce slight smoothing effect. The advanced filters that can preserve more image details could be recommended in the future study (Glasser et al., 2013). Finally, as a limitation of cross-sectional study design, we did not perform the longitudinal investigation about how MAP-MRI metrics change over time.
In conclusion, our findings suggested that WM microstructural impairment, especially in the CST pathway and corpus callosum, represents the consistent characteristic of ALS and may be responsible for the disease progression. MAP-MRI could provide alternative measures depicting ALS-related WM alterations, complementary to the common diffusion imaging methods.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82071900, 81671271 and 81974199), Fujian Province Natural Science Foundation (No. 2021J01754), and Fujian Province Joint Funds for the Innovation of Science and Technology (No. 2019Y9067).
CRediT authorship contribution statement
Hua-Jun Chen: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. Chuanyin Zhan: Data curation, Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing. Li-Min Cai: Data curation, Formal analysis, Investigation, Writing – review & editing. Jia-Hui Lin: Data curation, Formal analysis, Investigation, Writing – review & editing. Min-Xiong Zhou: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Zhang-Yu Zou: Data curation, Formal analysis, Writing – review & editing. Xu-Feng Yao: Data curation, Formal analysis. Yan-Juan Lin: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the participants who contributed to this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102863.
Contributor Information
Hua-Jun Chen, Email: chj0075@126.com.
Yan-Juan Lin, Email: fjxhlyj@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
The correlation between DTI, DKI, and NODDI metrics and ALS disease severity indicated by ALSFRS-R score. The spatial extent of WM area in which diffusion metrics are correlated with ALSFRS-R score is indicated by total voxel number. Left and right sides of the image respectively indicate the left and right hemisphere of brain.
References
- Abrahams S., Goldstein L.H., Suckling J., Ng V., Simmons A., Chitnis X., Atkins L., Williams S.C.R., Leigh P.N. Frontotemporal white matter changes in amyotrophic lateral sclerosis. J. Neurol. 2005;252(3):321–331. doi: 10.1007/s00415-005-0646-x. [DOI] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andica C., Kamagata K., Hatano T., Saito Y., Ogaki K., Hattori N., Aoki S. MR biomarkers of degenerative brain disorders derived from diffusion imaging. J. Magn. Reson. Imaging. 2019;52(6):1620–1636. doi: 10.1002/jmri.27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab A., Wojna-Pelczar A., Khairnar A., Szabó N., Ruda-Kucerova J. Principles of diffusion kurtosis imaging and its role in early diagnosis of neurodegenerative disorders. Brain Res. Bull. 2018;139:91–98. doi: 10.1016/j.brainresbull.2018.01.015. [DOI] [PubMed] [Google Scholar]
- Avram A.V., Sarlls J.E., Barnett A.S., Ozarslan E., Thomas C., Irfanoglu M.O., Hutchinson E., Pierpaoli C., Basser P.J. Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. NeuroImage. 2016;127:422–434. doi: 10.1016/j.neuroimage.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Yang L., Chen Y., Zhang B., Li H., Tang W., Geng D., Li Y. Radial diffusivity as an imaging biomarker for early diagnosis of non-demented amyotrophic lateral sclerosis. Eur. Radiol. 2018;28(12):4940–4948. doi: 10.1007/s00330-018-5506-z. [DOI] [PubMed] [Google Scholar]
- Broad R.J., Gabel M.C., Dowell N.G., Schwartzman D.J., Seth A.K., Zhang H., Alexander D.C., Cercignani M., Leigh P.N. Neurite orientation and dispersion density imaging (NODDI) detects cortical and corticospinal tract degeneration in ALS. J. Neurol. Neurosurg. Psychiatry. 2019;90(4):404–411. doi: 10.1136/jnnp-2018-318830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusini L., Obertino S., Galazzo I.B., Zucchelli M., Krueger G., Granziera C., Menegaz G. Ensemble average propagator-based detection of microstructural alterations after stroke. Int. J. Comput. Assist. Radiol. Surg. 2016;11(9):1585–1597. doi: 10.1007/s11548-016-1442-z. [DOI] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chapman M.C., Jelsone-Swain L., Johnson T.D., Gruis K.L., Welsh R.C. Diffusion tensor MRI of the corpus callosum in amyotrophic lateral sclerosis. J. Magn. Reson. Imaging. 2014;39(3):641–647. doi: 10.1002/jmri.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Zhou B., Zhu H., Kuang W., Bi F., Ai H., Gu Z., Huang X., Lui S.u., Gong Q. White matter volume loss in amyotrophic lateral sclerosis: a meta-analysis of voxel-based morphometry studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;83:110–117. doi: 10.1016/j.pnpbp.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O., Behrens T.E., Altmann D.R., Orrell R.W., Howard R.S., Johansen-Berg H., Miller D.H., Matthews P.M., Thompson A.J. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129:1859–1871. doi: 10.1093/brain/awl100. [DOI] [PubMed] [Google Scholar]
- Daducci A., Canales-Rodríguez E.J., Zhang H., Dyrby T.B., Alexander D.C., Thiran J.-P. Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. Neuroimage. 2015;105:32–44. doi: 10.1016/j.neuroimage.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Edgar J.M., Nave K.-A. The role of CNS glia in preserving axon function. Curr. Opin. Neurobiol. 2009;19(5):498–504. doi: 10.1016/j.conb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Fatima M., Tan R., Halliday G.M., Kril J.J. Spread of pathology in amyotrophic lateral sclerosis: assessment of phosphorylated TDP-43 along axonal pathways. Acta Neuropathol. Commun. 2015;3(1) doi: 10.1186/s40478-015-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R.H.J., Daianu M., Pizzolato M., Wassermann D., Jacobs R.E., Thompson P.M., Town T., Deriche R. In: Computational Diffusion MRI. Fuster A., Ghosh A., Kaden E., Rathi Y., Reisert M., editors. Springer International Publishing; Cham: 2017. Comparison of Biomarkers in Transgenic Alzheimer Rats Using Multi-Shell Diffusion MRI; pp. 187–199. [Google Scholar]
- Fick R.H.J., Wassermann D., Caruyer E., Deriche R. MAPL: Tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. NeuroImage. 2016;134:365–385. doi: 10.1016/j.neuroimage.2016.03.046. [DOI] [PubMed] [Google Scholar]
- Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A., Khan J., Polak M.A., Glass J.D. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 2004;185(2):232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Garyfallidis E., Brett M., Amirbekian B., Rokem A., van der Walt S., Descoteaux M., Nimmo-Smith I., Dipy C. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 2014;8:8. doi: 10.3389/fninf.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman O., Al-Chalabi A., Chio A., Corr E.M., Logroscino G., Robberecht W., Shaw P.J., Simmons Z., van den Berg L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- Hardiman O., van den Berg L.H., Kiernan M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011;7(11):639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Nagashima K., Urano Y., Iwata M. Spinocerebellar degeneration with prominent involvement of the motor neuron system: autopsy report of a sporadic case. Acta Neuropathol. 1986;70(1):82–85. doi: 10.1007/BF00689519. [DOI] [PubMed] [Google Scholar]
- Huang N.-X., Zou Z.-Y., Xue Y.-J., Chen H.-J. Abnormal cerebral microstructures revealed by diffusion kurtosis imaging in amyotrophic lateral sclerosis. J. Magn. Reson. Imaging. 2020;51(2):554–562. doi: 10.1002/jmri.26843. [DOI] [PubMed] [Google Scholar]
- Ishaque A., Mah D., Seres P., Luk C., Johnston W., Chenji S., Beaulieu C., Yang Y.-H., Kalra S. Corticospinal tract degeneration in ALS unmasked in T1-weighted images using texture analysis. Hum. Brain Mapp. 2019;40(4):1174–1183. doi: 10.1002/hbm.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y., Shibuya K., Misawa S., Sekiguchi Y., Watanabe K., Amino H., Kuwabara S., Phillips W.D. Axonal dysfunction precedes motor neuronal death in amyotrophic lateral sclerosis. PLoS One. 2016;11(7):e0158596. doi: 10.1371/journal.pone.0158596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jiang R., Jiang S., Song S., Wei X., Deng K., Zhang Z., Xue Y. Laplacian-regularized mean apparent propagator-MRI in evaluating Corticospinal tract injury in patients with brain Glioma. Korean J Radiol. 2021;22(5):759. doi: 10.3348/kjr.2020.0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Kramer D.M., Crooks L.E., Ortendahl D.A. Measuring signal-to-noise ratios in MR imaging. Radiology. 1989;173(1):265–267. doi: 10.1148/radiology.173.1.2781018. [DOI] [PubMed] [Google Scholar]
- Le H., Zeng W., Zhang H., Li J., Wu X., Xie M., Yan X., Zhou M., Zhang H., Wang M., Hong G., Shen J. Mean apparent propagator MRI is better than conventional diffusion tensor imaging for the evaluation of Parkinson's disease: a prospective pilot study. Front. Aging Neurosci. 2020;12 doi: 10.3389/fnagi.2020.563595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang L., Chu Y., Namaka M., Deng B., Kong J., Bi X. Astrocytes in oligodendrocyte lineage development and white matter pathology. Front. Cell. Neurosci. 2016;10:119. doi: 10.3389/fncel.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Zhang X., Zhang H., Yan X., Gao A., Song C., Wang S., Lian Y., Cheng J. Mean apparent propagator-MRI: a new diffusion model which improves temporal lobe epilepsy lateralization. Eur. J. Radiol. 2020;126:108914. doi: 10.1016/j.ejrad.2020.108914. [DOI] [PubMed] [Google Scholar]
- Mao J., Zeng W., Zhang Q., Yang Z., Yan X., Zhang H., Wang M., Yang G., Zhou M., Shen J. Differentiation between high-grade gliomas and solitary brain metastases: a comparison of five diffusion-weighted MRI models. BMC Med. Imaging. 2020;20:124. doi: 10.1186/s12880-020-00524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusue E., Sugihara S., Fujii S., Kinoshita T., Nakano T., Ohama E., Ogawa T. Cerebral cortical and white matter lesions in amyotrophic lateral sclerosis with dementia: correlation with MR and pathologic examinations. Am. J. Neuroradiol. 2007;28(8):1505–1510. doi: 10.3174/ajnr.A0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney E.B., de Winter F., Verhaagen J. ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease. Front. Neurosci. 2014;8:252. doi: 10.3389/fnins.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozarslan E., Koay C.G., Shepherd T.M., Komlosh M.E., İrfanoğlu M.O., Pierpaoli C., Basser P.J. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. NeuroImage. 2013;78:16–32. doi: 10.1016/j.neuroimage.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensato V., Tiloca C., Corrado L., Bertolin C., Sardone V., Del Bo R., Calini D., Mandrioli J., Lauria G., Mazzini L., Querin G., Ceroni M., Cantello R., Corti S., Castellotti B., Soldà G., Duga S., Comi G.P., Cereda C., Sorarù G., D’Alfonso S., Taroni F., Shaw C.E., Landers J.E., Ticozzi N., Ratti A., Gellera C., Silani V. TUBA4A gene analysis in sporadic amyotrophic lateral sclerosis: identification of novel mutations. J. Neurol. 2015;262(5):1376–1378. doi: 10.1007/s00415-015-7739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips T., Rothstein J.D. Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2014;262:111–120. doi: 10.1016/j.expneurol.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudlo J., Bißbort C., Glass A., Grossmann A., Hauenstein K., Benecke R., Teipel S.J. White matter pathology in ALS and lower motor neuron ALS variants: a diffusion tensor imaging study using tract-based spatial statistics. J. Neurol. 2012;259(9):1848–1859. doi: 10.1007/s00415-012-6420-y. [DOI] [PubMed] [Google Scholar]
- Puls I., Jonnakuty C., LaMonte B.H., Holzbaur E.L.F., Tokito M., Mann E., Floeter M.K., Bidus K., Drayna D., Oh S.J., Brown R.H., Ludlow C.L., Fischbeck K.H. Mutant dynactin in motor neuron disease. Nat. Genet. 2003;33(4):455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Richmond S.B., Fling B.W. Transcallosal control of bilateral actions. Exerc. Sport Sci. Rev. 2019;47:251–257. doi: 10.1249/JES.0000000000000202. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Maruyama S. Increase in diameter of the axonal initial segment is an early change in amyotrophic lateral sclerosis. J. Neurol. Sci. 1992;110(1-2):114–120. doi: 10.1016/0022-510x(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Schuster C., Elamin M., Hardiman O., Bede P. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur. J. Neurol. 2016;23(8):1361–1371. doi: 10.1111/ene.13038. [DOI] [PubMed] [Google Scholar]
- Smith M.C. Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 1960;23(4):269–282. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Tabesh A., Jensen J.H., Ardekani B.A., Helpern J.A. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn. Reson. Med. 2011;65(3):823–836. doi: 10.1002/mrm.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen M.H.G., Peviani M., Hendricusdottir R., Bell E.M., Lammens M., Smit A.B., Bendotti C., van Minnen J., Gillingwater T.H. Increased axonal ribosome numbers is an early event in the pathogenesis of amyotrophic lateral sclerosis. PLoS One. 2014;9(1):e87255. doi: 10.1371/journal.pone.0087255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete E., Veldink J.H., Mandl R.C.W., van den Berg L.H., van den Heuvel M.P., He Y. Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS One. 2011;6(9):e24239. doi: 10.1371/journal.pone.0024239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welniarz Q., Dusart I., Roze E. The corticospinal tract: evolution, development, and human disorders. Dev. Neurobiol. 2017;77:810–829. doi: 10.1002/dneu.22455. [DOI] [PubMed] [Google Scholar]
- Wen J., Zhang H., Alexander D.C., Durrleman S., Routier A., Rinaldi D., Houot M., Couratier P., Hannequin D., Pasquier F., Zhang J., Colliot O., Le Ber I., Bertrand A. Neurite density is reduced in the presymptomatic phase of C9orf72 disease. J. Neurol. Neurosurg. Psychiatry. 2019;90(4):387–394. doi: 10.1136/jnnp-2018-318994. [DOI] [PubMed] [Google Scholar]
- Xie S., Chen L., Zuo N., Jiang T. DiffusionKit: a light one-stop solution for diffusion MRI data analysis. J. Neurosci. Methods. 2016;273:107–119. doi: 10.1016/j.jneumeth.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Yamauchi H., Fukuyama H., Ouchi Y., Nagahama Y., Kimura J., Asato R., Konishi J. Corpus callosum atrophy in amyotrophic lateral sclerosis. J. Neurol. Sci. 1995;134(1-2):189–196. doi: 10.1016/0022-510x(95)00220-6. [DOI] [PubMed] [Google Scholar]
- Yan X., Zhou M., Ying L., Liu W., Yang G., Wu D., Zhou Y., Peterson B.S., Xu D. A fast schema for parameter estimation in diffusion kurtosis imaging. Comput. Med. Imaging Graph. 2014;38(6):469–480. doi: 10.1016/j.compmedimag.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Chen G., He M., Dai J., Shang H., Gong Q., Jia Z. Altered white matter microarchitecture in amyotrophic lateral sclerosis: a voxel-based meta-analysis of diffusion tensor imaging. Neuroimage Clin. 2018;19:122–129. doi: 10.1016/j.nicl.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61(4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]