Abstract
Both the Rho family of low-molecular-weight GTP-binding proteins and protein kinases C (PKCs) mediate responses to a variety of extracellular and intracellular signals. They share many downstream targets, including remodeling of the actin cytoskeleton, activation of p70S6 kinase and c-jun N-terminal kinase (JNK), and regulation of transcription and cell proliferation. We therefore investigated whether Rho family GTP-binding proteins bind to PKCs. We found that Cdc42 associates with atypical PKCs (aPKCs) PKCζ and -λ in a GTP-dependent manner. The regulatory domain of the aPKCs mediates the interaction. Expression of activated Cdc42 results in the translocation of PKCλ from the nucleus into the cytosol, and Cdc42 and PKCλ colocalize at the plasma membrane and in the cytoplasm. Expression of activated Cdc42 leads to a loss of stress fibers, as does overexpression of either the wild type or an activated form of PKCλ. Kinase-dead PKCλ and -ζ constructs acted as dominant negatives and restored stress fibers in cells expressing the activated V12 Cdc42 mutant, indicating that Cdc42-dependent loss of stress fibers requires aPKCs. Kinase-dead PKCλ and -ζ and dominant-negative N17 Cdc42 also blocked Ras-induced loss of stress fibers, suggesting that this pathway may also be important for Ras-dependent cytoskeletal changes. N17 Rac did not block Ras-induced loss of stress fibers, nor did kinase-dead PKCλ block V12 Rac-stimulated loss of stress fibers. These results indicate that Cdc42 and Rac use different pathways to regulate stress fibers.
The actin cytoskeleton is dynamic, regulation of actin polymerization is important for cell motility, adherence, and division, and transformed cells have an altered actin cytoskeleton, including the loss of stress fibers. The Rho family of small GTP-binding proteins plays a central role in regulating actin polymerization, the formation of cellular structures dependent on actin, and transformation by some oncogenes (21). Experiments using both constitutively active and dominant-negative mutants of Rho family members have shown that Rac causes ruffling and is necessary for formation of lamellipodia and cell movement (35). Activated Rho leads to the formation of stress fibers, and activated Cdc42 causes filopodia to form (17, 28, 34). In addition, expression of activated forms of Rac or Cdc42 causes cells to lose stress fibers (48). Rho family GTP-binding proteins also stimulate other signaling pathways that are important in both normal cellular function and transformation, including cell cycle progression, activation of the c-jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase pathways, and regulation of transcription. Understanding the regulation of Rho family signaling is important for insight into a number of cellular functions.
Most signals transmitted by Rho family members depend on the association of effector proteins with the GTP-bound form of Rac, Rho, or Cdc42. The first step in their activation is the catalysis of GTP exchange for GDP by guanine nucleotide exchange factors (GEFs). Signaling is terminated by GTPase-activating proteins (GAPs), which stimulate GTP hydrolysis and conversion of the proteins to the GDP-bound form. Recent efforts have focused on identifying the immediate effector molecules that interact with the activated GTP-binding proteins. A number of direct targets have been identified, including protein serine/threonine and tyrosine kinases, a protein phosphatase, lipid kinases, and adapter proteins. Identification of the proteins that associate with activated Rho family members has been a fruitful approach to understanding their signaling pathways.
Protein kinases C (PKCs) regulate many of the same pathways regulated by Rho family GTP binding proteins. The mammalian PKC family is subdivided into conventional PKC members, comprising the α, βI, βII, and γ isoforms; novel PKCs comprising the δ, e, η, and θ forms; and atypical PKCs (aPKCs) ι/λ and ζ (26). The conventional PKCs are activated by calcium binding to the C2 domain and diacylglycerol binding to the C1 domain. The C2 domain of the novel PKCs does not bind calcium but they are activated by diacylglycerol. The aPKCs have only one cysteine-rich motif in the C1 domain and do not have a C2 domain.
PKCs and Rho family GTP-binding proteins both have effects on the actin cytoskeleton, the activation of p70S6 kinase, the JNK pathway, and the fos and NF-κβ promoters (44). Both PKCs and Rho family members are necessary for cell adherence and spreading and have effects on neurite outgrowth (20, 31, 40, 47). In some cell types, activation of PKCs with phorbol myristate acetate causes cells to ruffle. PKCθ is necessary for formation of filopodia and stress fibers in endothelial cells and can also phosphorylate and activate ezrin, radixin, and moesin (30, 41).
Several recent studies have directly linked PKC signaling and Rho family signaling. The Saccharomyces cerevisiae Rho family protein Rho1p binds to PKC1, the yeast PKC homolog, and stimulates PKC1 activity in the presence of phosphatidylserine (14, 29). Rho1p-activated PKC1 initiates a MAP kinase pathway, which is crucial to cell wall integrity. PKCα binds to RhoA in a GTP-dependent manner and appears to act downstream of RhoA in activation of an AP-1 promoter in Jurkat cells (7). The activation of PKCζ by interleukin 2 was blocked by Clostridium difficile toxin B, which inhibits all Rho family members, suggesting that PKCζ can be activated by or requires Rho family members (13). The aPKCs were also shown to act downstream of Ras to mediate Ras-dependent cytoskeletal reorganization, perhaps in a Rac-dependent pathway (43).
The signaling pathways and targets shared by Rho family GTP-binding proteins and PKCs led us to ask whether there is direct interaction of PKCs with Rho family members. We found that mammalian Cdc42 associates with aPKCλ and -ζ in a GTP-dependent manner, but the binding does not seem to be direct. The interaction appears to be necessary for Cdc42 and Ras to cause disassembly of stress fibers and could be necessary for aPKC activation of p70S6 kinase.
MATERIALS AND METHODS
Materials.
PKC isoform-specific antibodies were purchased from Santa Cruz Biotechnology, Inc. The aPKC-specific antibody binds to both PKCλ and PKCζ and thus is referred to as the pan-aPKC antibody. Glutathione S-transferase (GST) antibody was purchased from Upstate Biotechnology, Inc. A monoclonal antibody specific for the T7 peptide was purchased from Novagen. Secondary antibody conjugates used for immunofluorescence were obtained from Pierce and Boehringer Mannheim. Unstripped rat brains were obtained from Pel Freez Biologicals. Cells were obtained from the American Type Culture Collection, except for E1a/E1b-transformed 293 cells, which were a gift from Yang Shi (Department of Pathology, Harvard Medical School). PKCζ was purified from baculovirus-infected insect cells as described previously (27). All other reagents were obtained from Sigma Chemical Co. unless otherwise stated.
Constructs.
Flag epitope-tagged PKCλ and -ζ were obtained from John Blenis and Angela Romanelli (Department of Cell Biology, Harvard Medical School). A kinase-active form of PKCλ was made by mutation of alanine 120 to glutamate. A primer with the mutation was used to obtain a fragment containing the mutation by PCR. This fragment was cut with EcoNI and SgrAI and ligated into the pCMV5 vector containing PKCλ, which had been cut with the same enzymes. A kinase-dead form of PKCω in pCMV5 was made by ligating an EcoRI/AflII fragment from kinase-dead PKCλ in the SRD vector (S. Ohno, Department of Molecular Biology, Yokohama City University School of Medicine, Yokohama, Japan) into pCMV5 containing PKCλ that had been cut with the same enzymes. The T7 epitope-tagged regulatory domain of PKCζ was made by removing the AccI fragment from PKCζ and pSKV3 (obtained from Kiyotaka Nishikawa, Department of Cell Biology, Harvard Medical School), which contains a 5′ T7 sequence. The L61 Ras construct has been previously described (4).
Preparation and nucleotide loading of GTP-binding proteins.
GST fusion proteins of human Ras, Rac1, Cdc42, and RhoA were expressed in bacteria and purified with glutathione-Sepharose (GSH) beads as described previously (42). The fusion proteins were stored in 10 mM HEPES (pH 7.5)–0.5 mM dithiothreitol (DTT) with 50% glycerol at −80°C. The proteins were active, as determined by their ability to bind 3H-GTP in solution. The fusion proteins were loaded with nucleotide by incubating them in a mixture of 20 mM HEPES (pH 7.5), 100 mM NaCl, 1 mM EDTA, and 1 mM DTT with a 10-fold excess of either GTPγS or GDPβS for 15 min at 30°C. MgCl2 was then added to a final concentration of 5 mM, and incubations continued for a further 10 min. Twenty-five microliters of a 50% slurry of GSH beads was added as carrier to each incubation, and unbound nucleotide was removed by washing the beads twice with 1 ml of HNM (20 mM HEPES [pH 7.5], 100 mM NaCl, 5 mM MgCl2).
Association of GST fusion proteins with PKCs.
Rat brains were finely diced and homogenized in a mixture of 50 mM HEPES (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM DTT, 0.25 M sucrose, 0.5 mM phenylmethylsulfonyl fluoride, and 1-μg/ml (each) leupeptin, antipain, and pepstatin (1 brain per 40 ml of buffer) with a Dounce homogenizer. The homogenate was centrifuged at 100,000 × g for 45 min. Triton X-100 was added to the supernatant (cytosol) to a final concentration of 0.1% (wt/vol). GST fusion proteins were loaded with nucleotide as described above and then incubated with 1 ml of cytosol for 75 min at 4°C with constant rocking. The beads were washed once with 1 ml of homogenization buffer containing 0.1% (wt/vol) Triton X-100 and twice with 1 ml of HNM. Associated proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to Immobilon-P membrane (Millipore). Membranes were blocked with 2% (wt/vol) bovine serum albumin (BSA) in Tris-buffered saline. The blots were probed with primary antibody and visualized with horseradish peroxidase-conjugated secondary antibody (Boehringer Mannheim) by enhanced chemiluminescence (DuPont NEN) according to the manufacturer's instructions.
Protein kinase assays.
Assays of PKC and p70S6 kinase activities were done as previously described (8, 27).
Cell culture and transient transfections.
Cells were grown in Dulbecco's modified Eagles' medium (DMEM) containing 10% (vol/vol) fetal calf serum (NIH 3T3 and COS-7 cells) or 10% (vol/vol) heat-inactivated fetal calf serum (293 cells). The mammalian expression vector pEBG encoding various alleles of RhoA, Rac, and Cdc42 fused to GST has been previously described (8). COS cells were transfected by the DEAE-dextran method. NIH 3T3 cells and 293 cells were transfected with Lipofectamine (Gibco BRL), except for immunofluorescence studies, in which Superfect reagent (Qiagen) was used. In each case, the manufacturer's instructions were followed. One or 5 μg of each DNA construct was used per 3.5- or 10-cm-diameter plate, respectively. Cells were harvested 48 h after transfection with the homogenization buffer described above, without sucrose, containing 1% (wt/vol) Triton X-100. Cell lysates were incubated with 25 μl of a 50% (vol/vol) slurry of glutathione-Sepharose beads for 2 h at 4°C with constant rocking. The beads were then processed as described in the section above.
Immunofluorescence.
NIH 3T3 cells growing on 25-mm-diameter coverslips were transiently transfected as described above. In some experiments, pEGFP (Clontech), which expresses green fluorescent protein (GFP), was cotransfected and the expression of GFP was used to identify transfected cells. Following transfection the cells were washed with phosphate-buffered saline (PBS) and starved overnight in serum-free DMEM. The cells were then washed with PBS before fixation for 10 min in 3% (wt/vol) paraformaldehyde. Following three washes with PBS, the cells were permeabilized for 4 min in PBS containing 0.2% (vol/vol) Triton X-100 and washed a further three times with PBS. Cells were then blocked with PBS containing 1% (wt/vol) BSA for 10 min before addition of primary antibodies diluted in blocking buffer for 1 h. The cells were washed three times with blocking buffer before the addition of secondary antibodies (conjugated to fluorophores) or rhodamine-labeled phalloidin (100 ng/ml), diluted in blocking buffer, for 1 h. The cells were washed three times in PBS and once in distilled water prior to mounting of the coverslips on Fluoromont-G mounting solution (Fisher). Images were visualized with a Nikon Diaphot 300 microscope captured on a Photometrics digital camera with Phase 3 Imaging Systems software. Stress fibers were classified as being either present or absent in cells in cells stained with rhodamine phalloidin and viewed by fluorescent microscopy.
RESULTS
GST-Cdc42 associates with aPKC in rat brain cytosol.
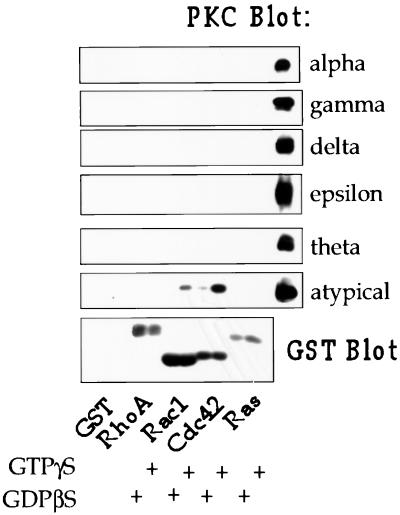
GST fusions of the Rho family GTP-binding proteins RhoA, Rac1, and Cdc42 and a GST fusion protein of H-Ras were expressed in bacteria, purified with GSH beads, and tested for their ability to associate with PKCs in rat brain cytosol. The proteins were first loaded with GTPγS or GDPβS and then incubated with rat brain homogenate. The beads were washed and the proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membrane for Western blotting. As shown in Fig. 1, Cdc42 and, to a lesser extent, Rac associated with aPKC in a GTP-dependent manner. Since the available antibodies do not distinguish between PKCλ and -ζ (data not shown), we were not able to determine in these experiments which aPKC(s) associated with Cdc42. Neither GST alone, RhoA, or Ras associated with any PKC (the PKCα antibody also detects PKCβI and PKCβII; data not shown). Ras has previously been reported to associate with aPKCζ (10). An explanation for our failure to detect PKCζ binding to Ras may be because full-length PKCζ was reported to associate poorly with Ras in vitro compared with the regulatory region of PKCζ. The interaction of RhoA with PKCα requires prenylation of RhoA and so would not have been detected in this experiment (7).
FIG. 1.
Cdc42 associates with aPKCs in rat brain cytosol. GST alone or GST fusion proteins of RhoA, Rac1, Cdc42, or Ras, bound to glutathione-Sepharose beads, were loaded with GTPγS or GDPβS and then incubated with rat brain cytosol as described in Materials and Methods. G protein-associated PKCs were detected by Western blotting with PKC isoform-specific antibodies. These antibodies were also used to immunoprecipitate PKC from rat brain cytosol, and these immunoprecipitates are included as positive controls on the right side of each blot. The GST fusion proteins were visualized by Western blotting with a GST antibody.
Cdc42 associates with PKCλ and -ζ in vivo.
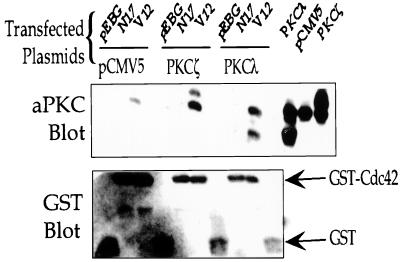
To determine whether Cdc42 associates with aPKCs in vivo, we transfected NIH 3T3 cells with GST-tagged Cdc42 mutants and PKCλ or -ζ. We compared the binding of aPKCs to the dominant-negative N17 mutant of Cdc42, which is bound to GDP; the GTPase-deficient V12 mutant of Cdc42, which is bound to GTP; and GST alone. Cell lysates were incubated with GSH beads, and the associated proteins were analyzed by Western blotting with the aPKC antibody. Endogenous aPKC associated with V12 Cdc42, but not with N17 Cdc42 or GST alone, in cells transfected only with Cdc42 constructs (Fig. 2, lanes 1 to 3). There was a marked increase in the amount of aPKC associated with V12 Cdc42 in cells cotransfected with either PKCλ or -ζ and Cdc42, indicating that Cdc42 associates with both PKCλ and -ζ in a GTP-dependent manner (Fig. 2, lanes 4 to 9). The higher band in lanes 4 to 6 is full-length PKCζ, and the lower band is a proteolytic product. In lanes 7 to 9, the upper band is full-length PKCλ, and the lower band is a proteolytic product.
FIG. 2.
V12 Cdc42 associates with PKCλ and -ζ in vivo. NIH 3T3 cells were transfected with GST-tagged V12 or N17 Cdc42 and either vector alone (pCMV5), PKCζ, or PKCλ, as described in Materials and Methods. Forty-eight hours after transfection, the cells were lysed, and Cdc42 was isolated by incubating the cell lysates with glutathione-Sepharose beads. The beads were washed, and the associated proteins were detected by Western blotting with the pan-aPKC antibody (upper panel). Lysates were blotted to determine PKC expression (three right-hand lanes in the upper panel). The amount of GST or GST-Cdc42 associated with the beads was determined by Western blotting with a GST antibody (lower panel).
The association between Cdc42 and aPKCs is likely indirect.
To investigate whether Cdc42 associates directly with aPKCs, we incubated bacterially expressed GST-Cdc42, bound to GSH beads and loaded with GTPγS, with purified PKCζ. PKCζ was purified from insect cells infected with a baculovirus expressing PKCζ. We detected no binding, as assessed by both kinase assays and Western blotting (data not shown). Similarly, bacterially expressed GST fusions of either full-length PKCζ or of the regulatory domain of PKCζ did not bind to Cdc42, which had been expressed in bacteria, cleaved from GST, and loaded with 3H-GTP (data not shown). We also detected no signal when using Cdc42 loaded with [γ-32P]GTP in a far-Western blot for purified PKCζ which had been separated by SDS-PAGE and transferred to nitrocellulose paper (23). A control using the Wiskott-Aldridge syndrome protein gave a positive signal in this assay. Since we were unable to detect a direct interaction between Cdc42 and aPKCs by using multiple approaches, we think it is likely that a third protein mediates the interaction of aPKCs and Cdc42. We cannot rule out the possibility, however, that aPKCs undergo a covalent modification in mammalian cells, which does not occur in insect cells, that allows them to interact directly with Cdc42. Since Cdc42 expressed in bacteria binds to aPKCs in cell lysates, we do not think prenylation of Cdc42 is necessary for the interaction.
The effector region of Cdc42 and the regulatory domain of aPKC mediate their association.
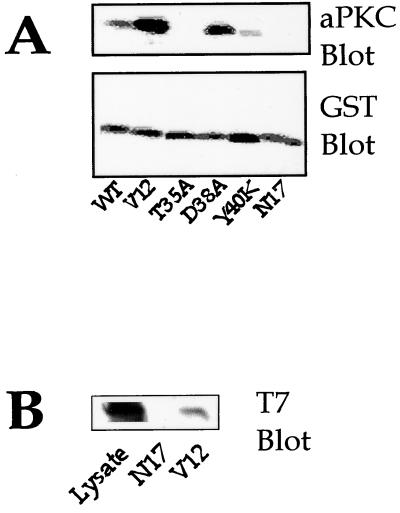
An effector region in the N terminus of Rho family proteins mediates GTP-dependent binding to effector molecules. Mutations in this region diminish or abolish the GTP-dependent association with effector proteins (8, 18, 45). To confirm that the effector region of Cdc42 is necessary for the interaction with aPKCs and to determine which region of the effector region is necessary for the interaction, we transfected COS-7 cells with GST V12 Cdc42 containing additional mutations in the effector region. Cell lysates were incubated with GSH beads, the beads were washed, and the association of endogenous aPKCs was determined by Western blotting (Fig. 3A). As expected, V12 Cdc42 bound well to aPKCs, wild-type Cdc42 bound less well, and little binding was detected with N17 Cdc42. The V12/T35A mutant of V12 Cdc42 did not associate with aPKCs, whereas the V12/D38A mutant and, to a lesser extent, the V12/Y40K mutant retained their ability to associate with aPKC (Fig. 3A). The T35A mutant also fails to activate p70S6 kinase (8).
FIG. 3.
Characterization of the domains of Cdc42 and aPKC involved in their association. (A) COS cells were transiently transfected with the indicated GST-tagged Cdc42 mutants. Forty-eight hours after transfection, the cells were lysed, and Cdc42 was isolated by incubating the lysates with glutathione-Sepharose beads. Associated endogenous aPKC was detected by Western blotting with the pan-aPKC antibody (upper panel), and Cdc42 bound to the beads was detected by Western blotting with GST antibody (lower panel). (B) 293 cells were transfected with V12 or N17 GST-Cdc42 and the regulatory domain of PKCζ with a T7 epitope tag. Forty-eight hours after transfection, Cdc42 was isolated as described above. Association of the regulatory domain of PKCζ with V12 or N17 GST-Cdc42 was determined by Western blotting with a T7-specific monoclonal antibody. Expression of the regulatory domain was determined by Western blotting of the lysate with T7 antibody.
A number of proteins that bind directly to Rac and/or Cdc42 contain a conserved sequence termed the Cdc42/Rac interactive binding (CRIB) motif (6). The aPKCs neither contain a CRIB motif, nor do they exhibit sequence homology with other non-CRIB, Cdc42/Rac effector proteins. The regulatory domain of aPKCs seems to be a protein-protein interaction domain and mediates the association of aPKCs with other proteins (10–12, 32). We therefore thought the regulatory domain of aPKCs might also bind to Cdc42. To determine whether the regulatory domain of aPKCs is sufficient for association with Cdc42, we transfected 293 cells with a T7-tagged construct containing just the regulatory domain of PKCζ and constructs expressing GST fusions of N17 or V12 Cdc42. Cell lysates were incubated with GSH beads, and the associated proteins were analyzed by Western blotting with the T7 antibody. As shown in Fig. 3B, the regulatory domain of PKCζ associated with V12 Cdc42, but not N17 Cdc42, indicating that this domain is sufficient for association with Cdc42. The regulatory domains of PKCζ and -λ are highly homologous, so it is likely that the regulatory domain of PKCλ also mediates binding to Cdc42.
Cdc42 alone is not sufficient to activate aPKC.
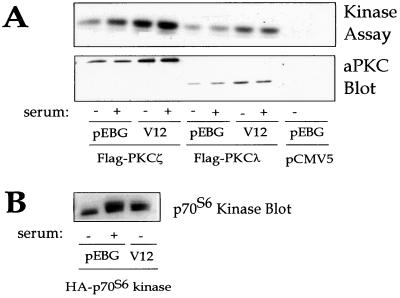
Cdc42 activates other protein kinases with which it associates, and Rho1p activates PKC1, so we determined whether Cdc42 activates aPKCs (8, 14, 48). NIH 3T3 cells were transfected with constructs expressing Flag epitope-tagged PKCζ and V12 Cdc42 or GST alone. The activity of Flag-PKCζ was assessed by in vitro kinase assay on Flag immunoprecipitates with myelin basic protein as a substrate. Cotransfection of V12 Cdc42 with Flag-PKCζ failed to stimulate the kinase activity of PKCζ (Fig. 4A). The slight increase in aPKC kinase activity in Flag immunoprecipitates from cells coexpressing V12 Cdc42 compared to those coexpressing the vector is accounted for by the increased expression of the tagged aPKC in the former cells (Fig. 4B). Similar results were obtained with PKCλ (data not shown). As a control, V12 Cdc42 stimulation of p70S6 kinase activity was assayed with S6 as a substrate. We also determined whether Cdc42 affected the kinase activity in the presence of phosphatidylserine, as reported for Rho1p and PKC1. Phosphatidylserine increased PKCζ activity, as previously reported, but there was no additional activation by Cdc42 (data not shown). Thus, in contrast to the activation of other protein kinases with which it associates, Cdc42 alone does not activate aPKCs.
FIG. 4.
Cdc42 does not activate aPKC. (A) 293 cells were transfected with Flag epitope-tagged PKCζ or -λ and V12 Cdc42 or vector alone (pEBG). Cells were serum starved for 24 h and then stimulated with or without 10% fetal calf serum for 10 min. Lysates were immunoprecipitated with the Flag antibody. Half of the immunoprecipitate was used to assay PKC activity with myelin basic protein as the substrate (upper panel), and the other half was used for Western blotting with the aPKC antibody (lower panel). (B) 293 cells were transfected with hemagglutinin (HA)-p70S6 kinase and V12 Cdc42 or pEBG. Cells were stimulated with serum as described above. p70S6 kinase was immunoprecipitated with HA antibody and visualized by Western blotting with HA antibody.
Cdc42 induces translocation of aPKCλ from the nucleus to the cytoplasm.
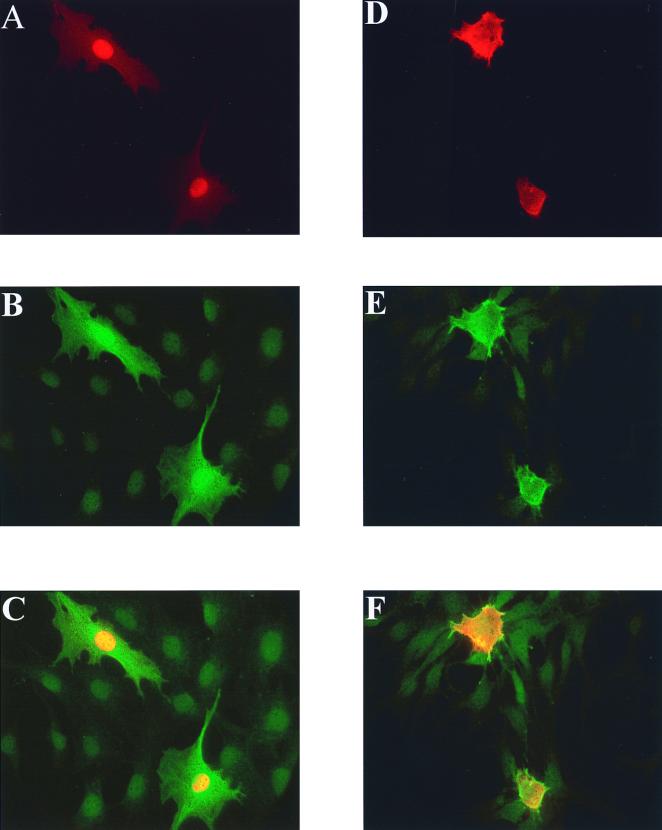
Since PKCλ is primarily localized to the nucleus and Cdc42 is a cytoplasmic protein, we thought that the interaction of Cdc42 and PKCλ might change the localization of one or both proteins (2). We transfected NIH 3T3 cells with epitope-tagged PKCλ with either GST alone or with GST V12 Cdc42 and determined the localization of PKCλ by indirect immunofluorescence. As previously reported, we found that PKCλ was localized within the nuclei of transfected cells (Fig. 5A to C). However, in cells cotransfected with V12 Cdc42, PKCζ was found primarily in the cytoplasm, where it colocalized with Cdc42 both in the cytoplasm and at the cell periphery (Fig. 5D to F). PKCζ is normally cytoplasmic, and we did not detect a change in the location of PKCζ when it was coexpressed with V12 Cdc42. The ability of the effector region mutants of Cdc42 to induce nuclear to cytoplasmic translocation correlated with their ability to bind Cdc42 (data not shown). Relocalization of PKCλ to the cytosol by Cdc42 provides further evidence of the interaction in vivo and suggests that the function of the complex is to localize PKCλ. PKCλ has been reported to move from the nucleus to the cytosol in response to stimulation of cells with EGF or PDGE, and our data suggest Cdc42 could function in this pathway (2).
FIG. 5.
Cdc42 induces translocation of aPKCλ from the nucleus to the cytosol in NIH 3T3 cells. NIH 3T3 cells were transfected with Flag epitope-tagged PKCλ (A to F) and either pEBG vector (A to C) or V12 Cdc42 (panels D to F) as described in Materials and Methods. Fixed cells were stained for PKCλ by using Flag primary antibody and a rhodamine-conjugated secondary antibody (A and D). GST and GST-V12 Cdc42 were stained with GST primary antibody and Cy2-conjugated secondary antibody (B and E). Panels A and B and D and E were merged to give the images shown in panels C and F, respectively.
Cdc42 and PKCλ induce the loss of stress fibers in NIH 3T3 cells.
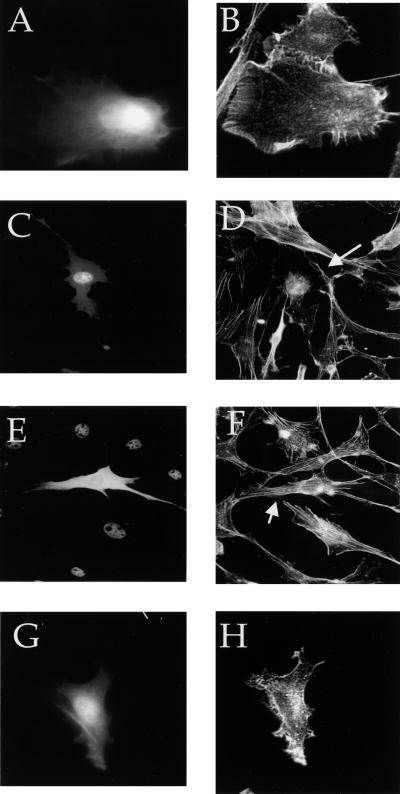
To investigate the Cdc42 signaling pathway or pathways that require aPKCs, we overexpressed the aPKCs to determine whether they would activate Cdc42-dependent events. Since Cdc42 causes changes in the actin cytoskeleton that lead to the formation of filopodia and the loss of stress fibers, as well as ruffling due to Rac activation, we determined whether overexpression of aPKCs similarly affects the actin cytoskeleton. V12 Cdc42-induced loss of stress fibers in NIH 3T3 cells (Fig. 6A and B and 7E), as has been previously reported (17). Transient overexpression of PKCλ, but not of PKCζ, induced the loss of stress fibers in NIH 3T3 cells (Fig. 6C to F). Cells expressing an activated form of PKCλ also lacked stress fibers (Fig. 6G and H). Although PKCλ is primarily nuclear, the absolute amount of protein in the cytoplasm is likely to be much higher in transfected cells due to overexpression. We propose that it is this cytoplasmic fraction that mediates stress fiber loss. A similar appearance of cells expressing activated PKCλ was recently reported (43). These results indicate that PKCλ causes the loss of stress fibers, but do not prove that it is necessary for Cdc42-dependent loss of stress fibers.
FIG. 6.
Cdc42 and PKCλ expression in NIH 3T3 fibroblasts reduces the stress fiber content. NIH 3T3 cells were transfected with hemagglutinin (HA)-tagged V12 Cdc42 alone (A and B) or Flag epitope-tagged PKCλ (C and D), T7 epitope-tagged PKCζ (E and F), or Flag epitope-tagged constitutively active PKCλ (G and H) as described in Materials and Methods. The cells were serum starved overnight and then fixed. Actin was stained with rhodamine-conjugated phalloidin (B, D, F, and H). The epitope-tagged aPKCs were stained with the appropriate epitope-directed antibodies followed by fluorescein isothiocyanate-conjugated secondary antibody (C and E), or cells were cotransfected with pEGFP, and the fluorescence of GFP was used to identify the transfected cells (A and G). The arrows indicate the transfected cells.
Kinase-dead PKCλ and -ζ block Cdc42-dependent loss of stress fibers.
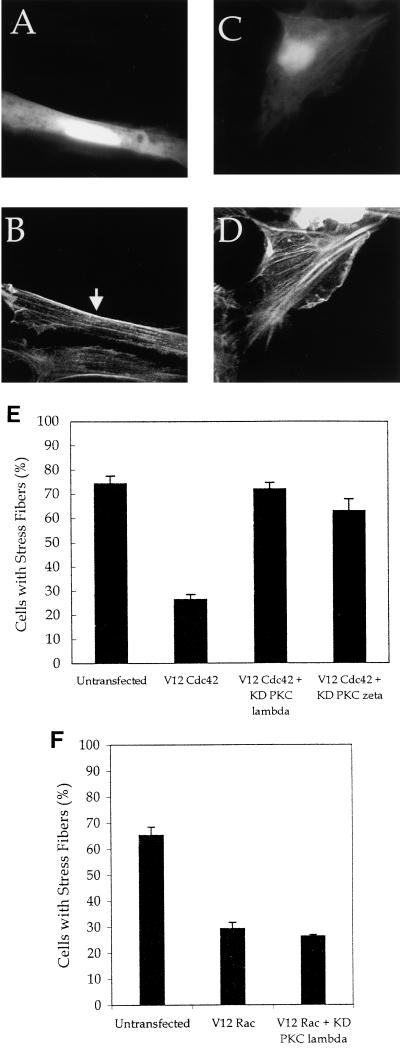
To determine if the effect of Cdc42 on stress fibers requires aPKCs, we investigated whether kinase-dead forms of PKCλ and -ζ would act as dominant negatives and inhibit Cdc42-induced changes in the actin cytoskeleton. NIH 3T3 cells were transfected with V12 Cdc42, and kinase-dead PKCλ or -ζ and actin structures were examined. Stress fibers were present in about 75% of untransfected cells (Fig. 7E). Expression of V12 Cdc42 resulted in the loss of stress fibers in most cells (Fig. 6A and 7E), and expression of kinase-dead PKCλ or -ζ blocked Cdc42-induced loss of stress fibers (Fig. 7A to E). Expression of kinase-dead PKCλ or -ζ in the absence of V12 Cdc42 had no detectable effect on the actin cytoskeleton (data not shown). Expression of kinase-dead PKCλ or -ζ did not affect the presence of filopodia or ruffles in cells also expressing V12 Cdc42. We also investigated whether expression of kinase-dead PKCλ blocked the loss of stress fibers in cells expressing V12 Rac and found that it did not (Fig. 7F). These data suggest that the effect of the kinase-dead aPKCs is specific to the Cdc42 pathway regulating stress fibers and that they do not block all Cdc42-dependent signaling. We also tested the ability of kinase-dead forms of PKCλ and -ζ to block Cdc42-dependent activation of JNK and the FOS promoter and found no effect (data not shown), further supporting the specificity of aPKCs for regulating Cdc42-dependent effects on stress fibers.
FIG. 7.
Kinase-dead PKCλ and -ζ block Cdc42-induced loss of stress fibers. NIH 3T3 cells were transfected with 0.25 μg of pEGFP, 0.5 μg of hemagglutinin (HA)-tagged V12 Cdc42 (A to E) or 0.5 μg of myc V12 Rac (F) and 2.5 μg of kinase-dead PKCλ (A, B, and F) or kinase-dead PKCζ (C and D). Twenty-four hours after transfection, the cells were serum starved. Forty-eight hours after transfection, the cells were fixed and stained with rhodamine-phalloidin (B and D). Transfected cells were identified by GFP fluorescence (A and C). The arrows indicate the transfected cells. (E) The numbers of cells with stress fibers were counted in the experiments described above. (F) The numbers of cells with stress fibers were counted in untransfected cells, V12 Rac-expressing cells, and cells expressing V12 Rac and kinase-dead (KD) PKCλ. Data are the means of three to four experiments in which at least 100 cells were counted per experiment. The error bars represent the standard error of the mean.
Ras-dependent loss of stress fibers requires Cdc42.
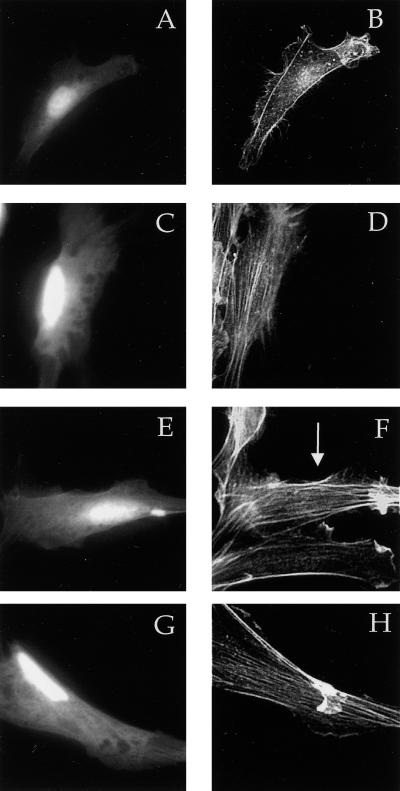
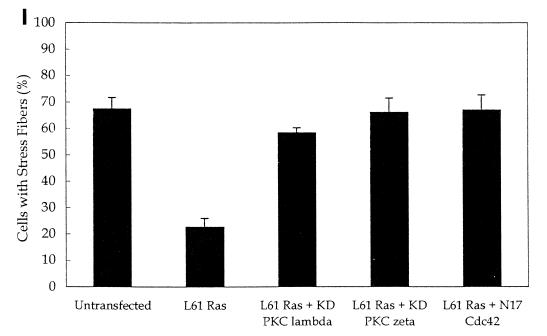
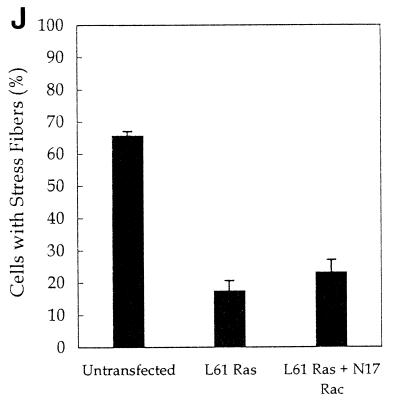
It was recently reported that kinase-dead forms of PKCλ and -ζ block Ras-induced loss of stress fibers via a pathway requiring Rac (43). Earlier work showed that dominant-negative N17 Cdc42, but not dominant-negative Rac, blocked Ras-induced cytoskeletal changes (33). Our observations favor the model that Ras-induced loss of stress fibers requires Cdc42 and its interaction with aPKCs. To test this possibility, we first confirmed that transfection of activated L61 Ras results in the loss of stress fibers (Fig. 8B and I). We also found that kinase-dead forms of PKCλ and -ζ blocked Ras-induced loss of stress fibers (Fig. 8C to F and I). To determine whether Ras-induced loss of stress fibers was mediated by Cdc42, we cotransfected L61 Ras with dominant-negative N17 Cdc42 and found that Ras-induced stress fiber loss was blocked (Fig. 8G to I). We did not find an effect of dominant-negative Rac on Ras-induced loss of stress fibers (Fig. 8J). These observations suggest that the effect of Ras on stress fibers requires Cdc42 and is likely mediated by the interaction of Cdc42 with aPKCs.
FIG. 8.
Cdc42, but not Rac, is necessary for Ras-induced loss of stress fibers. NIH 3T3 cells were transfected by using Superfect with 0.25 μg of pEGFP, 0.5 μg of L61 Ras, and either vector only (A and B), 2.5 μg of kinase-dead (KD) PKCλ (C and D), kinase-dead PKCζ (E and F), N17 Cdc42 (G and H), or N17 Rac (J). Twenty-four hours after transfection, the cells were serum starved. Forty-eight hours after transfection, the cells were fixed and stained with rhodamine-phalloidin (B, D, F, and H). Transfected cells were identified by GFP fluorescence (A, C, E, and G). The arrows indicate the transfected cells (I and J). The number of cells with stress fibers was counted in the experiments described above. Data are the means of three to four experiments in which at least 100 cells were counted per experiment. The error bars represent the standard error of the mean.
DISCUSSION
The identification of proteins that associate with activated forms of Rho family GTP-binding proteins has led to the discovery of many new signaling pathways. We found that PKCλ and -ζ associate with Cdc42 in a GTP-dependent manner and regulate the loss of stress fibers induced by both Cdc42 and Ras. The regulatory domain of aPKCs is sufficient to associate directly with Cdc42, and the effector region of Cdc42 is required. aPKCs do not seem to associate directly with Cdc42, indicating either that a covalent modification of aPKCs regulates binding or that a third protein bridges the interaction. Expression of activated Cdc42 results in the translocation of PKCλ from the nucleus to the cytoplasm and colocalization with Cdc42, but Cdc42 does not appear to stimulate the kinase activity of aPKCs. These findings suggest that the function of the association is to localize PKCλ.
PKCλ moves from the nucleus to the cytoplasm in response to stimulation by platelet-derived growth factor and epidermal growth factor. This translocation requires phosphoinositide 3-kinase (PI 3-kinase) activity (2). Our data suggest a model in which growth factors activate PI 3-kinase, which in turn stimulates a Cdc42 GEF. Active Cdc42 associates with PKCλ in the cytoplasm and localizes it. Cdc42 may send signals that result in the translocation of PKCλ, or PKCλ may move freely out of the nucleus, but association with Cdc42 traps it in the cytoplasm and prevents nuclear import.
Fibroblasts expressing either the wild type or an activated mutant of PKCλ lack stress fibers, a phenotype previously described for activated Cdc42 (17). Uberall et al. (43) found that expression of constitutively active forms of either PKCλ or ζ resulted in the absence of stress fibers, but we have not tested the effects of an activated form of PKCζ. There are several explanations for the inability of overexpression of PKCζ to cause loss of stress fibers. It is possible that only PKCλ regulates stress fibers and that an activated PKCζ mimics the effects of PKCλ or that both PKCλ and -ζ regulate stress fibers, but that wild-type PKCζ is less active than PKCλ when overexpressed in vivo.
Kinase-dead forms of PKCλ or -ζ block Cdc42-induced, but not Rac-induced, loss of stress fibers, indicating that PKCλ is downstream of Cdc42 in regulating stress fibers. Kinase-dead forms of PKCλ and -ζ also block the loss of stress fibers in cells expressing activated Ras (43). We confirmed that kinase-dead forms of PKCλ and -ζ blocked the effect of Ras on stress fibers and found that a dominant-negative form of Cdc42, but not Rac, also blocked Ras-induced loss of stress fibers. These data indicate that Ras-stimulated loss of stress fibers depends on Cdc42 and its association with PKCλ.
Stress fibers are composed of bundled actin filaments that end in focal adhesions and are regulated by both contractility and inhibition of depolymerization of the pointed end of actin filaments. It is possible that the lack of stress fibers in cells expressing activated Cdc42 or Ras results from inhibition of RhoA activation, inhibition downstream of Rho, or activation of a separate pathway that blocks stress fiber formation. RhoA is necessary for Ras transformation, indicating that Ras likely activates RhoA. Thus, the inhibition of stress fiber formation by Ras, Cdc42, and aPKCs probably affects targets downstream of Rho that are not required for transformation by RhoA or Ras or stimulates a separate pathway (49).
Activation of RhoA and ROK leads to phosphorylation and activation of myosin light-chain kinase and inhibition of myosin light-chain phosphatase, resulting in an increase in myosin light-chain phosphorylation and contractility (16, 19). Both Rac and Cdc42 activate p21-associated kinase 1 (PAK1), which can inhibit myosin light-chain kinase and should lead to a reduction in contractility and stress fibers (39). Actin depolymerization is also regulated in part by cofilin. Phosphorylation of cofilin by LIM kinase inhibits its ability to depolymerize actin from the pointed end, leading to more prominent actin structures (3). One group found that ROK phosphorylates and activates LIM kinase, promoting stress fiber formation (22). However, other groups have found that Rac, but not Rho, activates LIM kinase (3, 46). Proteins in the pathways that regulate contractility and actin depolymerization downstream of Rho and ROK, such as myosin light-chain kinase and phosphatase and cofilin, are candidate targets for aPKCs, but the precise mechanism by which Cdc42 and aPKCs regulate stress fibers is not yet clear.
The interaction of Cdc42 with aPKCs could also be important in transformation by Cdc42 and Ras. We have not yet investigated whether kinase-dead forms of aPKCs block Cdc42-dependent transformation, but it seems likely that they will, since Cdc42 is necessary for transformation by Ras and kinase-dead PKCζ blocks Ras-dependent transformation (5, 49). Although Cdc42 itself can activate Rac, Cdc42 and Rac seem to function in separate pathways downstream of Ras (17, 28, 33). Dominant-negative Cdc42 blocks Ras-induced changes in the actin cytoskeleton, but dominant-negative Rac does not. Dominant-negative Rac, but not Cdc42, inhibits the growth of Ras-transformed cells in low serum. Our data suggest that aPKCs function downstream of Cdc42 in Ras transformation to regulate stress fibers. Uberall et al. (43) found that dominant-negative Rac blocked Ras-induced cytoskeletal changes and placed PKCλ upstream of Rac, while we found no effect of dominant-negative Rac on Ras-stimulated loss of stress fibers. Our findings are consistent with the results of Qiu et al. (33), who found that dominant-negative Cdc42, but not Rac, blocked Ras effects on the cytoskeleton.
The regulation of p70S6 kinase is complex and involves both Rho family members and PKCs. p70S6 kinase is activated by Cdc42 and associates with, and is activated by, PKCζ and λ (1, 8, 36). The A35 mutant of Cdc42 that fails to bind aPKCs also fails to stimulate p70S6 kinase activity, and kinase-dead PKCζ partially blocks Cdc42-dependent activation of p70S6 kinase (36). Although it is not yet clear how a Cdc42-aPKC complex might function to activate p70S6 kinase, there is correlative evidence suggesting that the complex could be important. Cdc42 could function similarly to Ras in the activation of the extracellular signal-regulated kinase pathway, as a component of a multiprotein complex, which may localize and or activate protein kinases.
Kinase-dead forms of the aPKCs do not block other pathways that are regulated by both Rho family proteins and PKCs, however. Both the JNK pathway and the FOS promoter are activated by both Cdc42 and PKCs, but we found no effect of kinase-dead PKCλ or -ζ on Cdc42-induced JNK activation or stimulation of the FOS promoter (7, 9, 15, 25). These results show that the kinase-dead forms of PKCλ and -ζ are specific and do not block all Cdc42-dependent signaling by binding to the effector region. This specificity could be the result of an indirect association of PKCλ and -ζ with Cdc42, mediated by a linker protein. If only a subset of active endogenous Cdc42 associates with the linker protein, then dominant-negative aPKCs would inhibit signaling only of this fraction of Cdc42.
There are several candidate proteins that could mediate the interaction of Cdc42 with the aPKCs, if the interaction is indirect. Both PAR-4 and ζ-interacting protein (ZIP) bind to the aPKC regulatory domain like Cdc42 (11, 32). Interestingly, ZIP contains a short region of homology with the Cdc42 GEFs and Scd1. λ-interacting protein also binds to the regulatory domain, but interacts only with PKCλ and therefore is unlikely to mediate the interaction, since Cdc42 also binds to PKCζ (12). Since none of these proteins has a CRIB domain, it is possible that an unidentified protein bridges the interaction between aPKCs and Cdc42. Identification of the factors that regulate the association of aPKCs with Cdc42 and targets of the complex will help contribute to our understanding of Cdc42 and aPKC signaling.
ACKNOWLEDGMENTS
We thank Anthony Couvillon, Judith Glaven, Paola Marignani, Kimberley Tolias, Alex Toker, and Andrew Van Vugt for useful discussions and critical reading of the manuscript. Additionally, we are grateful to Alex Toker for purified PKCζ and Angela Romanelli, John Blenis, Shigeo Ohno, and Kiyotaka Nishikawa for PKC constructs.
This work was supported by NIH grant GM 54389 (C.L.C.).
REFERENCES
- 1.Akimoto K, Nakaya M, Yamanaka T, Tanaka J, Matsuda S, Weng Q P, Avruch J, Ohno S. Atypical protein kinase Clambda binds and regulates p70 S6 kinase. Biochem J. 1998;335:417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKClambda through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 3.Arber S, Barbayannis F A, Hanser H, Schneider C, Stanyon C A, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 4.Behre G, Whitmarsh A J, Coghlan M P, Hoang T, Carpenter C L, Zhang D E, Davis R J, Tenen D G. c-Jun is a JNK-independent coactivator of the PU.1 transcription factor. J Biol Chem. 1999;274:4939–4946. doi: 10.1074/jbc.274.8.4939. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkoy G, Perander M, Overvatn A, Johansen T. Reversion of Ras- and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of protein kinase C lambda is accompanied by the loss of constitutive nuclear mitogen-activated protein kinase/extracellular signal-regulated kinase activity. J Biol Chem. 1997;272:11557–11565. doi: 10.1074/jbc.272.17.11557. [DOI] [PubMed] [Google Scholar]
- 6.Burbelo P D, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 7.Chang J-H, Pratt J C, Sawasdikosol S, Kapeller R, Burakoff S J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol Cell Biol. 1998;18:4986–4993. doi: 10.1128/mcb.18.9.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou M M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 9.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C zeta. J Biol Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- 11.Diaz-Meco M T, Municio M M, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez J, Garcia A, L. R-B, Bonay P, Martinez A C, Silva A, Fresno M, Carrera A C, Eicher-Streiber C, Rebollo A. IL-2 signaling controls actin organization through Rho-like protein family, phosphatidylinositol 3-kinase, and protein kinase C-zeta. J Immunol. 1997;158:1516–1522. [PubMed] [Google Scholar]
- 14.Kamada Y, Qadota H, Python C P, Anraku Y, Ohya Y, Levin D E. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- 15.Kaneki M, Kharbanda S, Pandey P, Yoshida K, Takekawa M, Liou J-R, Stone R, Kufe D. Functional role for protein kinase Cβ as a regulator of stress-activated protein kinase activation and monocytic differentiation of myeloid leukemia cells. Mol Cell Biol. 1999;19:461–470. doi: 10.1128/mcb.19.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 17.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 19.Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis J M, Cheresh D A, Schwartz M A. Protein kinase C regulates alpha v beta 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J Cell Biol. 1996;134:1323–1332. doi: 10.1083/jcb.134.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay D J, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 22.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 23.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 24.Manser E, Leung T, Salihuddin H, Zhao Z S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 25.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 26.Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa K, Toker A, Johannes F J, Songyang Z, Cantley L C. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 28.Nobes C D, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 29.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14:5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietromonaco S F, Simons P C, Altman A, Elias L. Protein kinase C-theta phosphorylation of moesin in the actin-binding sequence. J Biol Chem. 1998;273:7594–7603. doi: 10.1074/jbc.273.13.7594. [DOI] [PubMed] [Google Scholar]
- 31.Price L S, Leng J, Schwartz M A, Bokoch G M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Chem. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puls A, Schmidt S, Grawe F, Stabel S. Interaction of protein kinase C zeta with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu R-G, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–3458. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 35.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 36.Romanelli A, Martin K A, Toker A, Blenis J. p70 S6 kinase is regulated by protein kinase Cζ and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol Cell Biol. 1999;19:2921–2928. doi: 10.1128/mcb.19.4.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahai E, Alberts A S, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol. 1999;9:136–145. doi: 10.1016/s0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- 39.Sanders L C, Matsumura F, Bokoch G M, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 40.Schlaepfer D D, Jones K C, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang S, Morgan K G, Parker C, Ware J A. Requirement for protein kinase C theta for cell cycle progression and formation of actin stress fibers and filopodia in vascular endothelial cells. J Biol Chem. 1997;272:28704–28711. doi: 10.1074/jbc.272.45.28704. [DOI] [PubMed] [Google Scholar]
- 42.Tolias K F, Cantley L C, Carpenter C L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 43.Uberall F, Hellbert K, Kampfer S, Maly K, Villunger A, Spitaler M, Mwanjewe J, Baier-Bitterlich G, Baier G, Grunicke H H. Evidence that atypical protein kinase C-lambda and atypical protein kinase C-zeta participate in Ras-mediated reorganization of the F-actin cytoskeleton. J Cell Biol. 1999;144:413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestell R G, Der C J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 47.Zeidman R, Lofgren B, Pahlman S, Larsson C. PKCepsilon, via its regulatory domain and independently of its catalytic domain, induces neurite-like processes in neuroblastoma cells. J Cell Biol. 1999;145:713–726. doi: 10.1083/jcb.145.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Z-S, Manser E, Chen X-Q, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zohn I M, Campbell S L, Khosravi-Far R, Rossman K L, Der C J. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]