Abstract
In this study, we investigated whether eriodictyol exerts an effect on the production and gene expression of MUC5AC mucin in human pulmonary epithelial NCI-H292 cells. The cells were pretreated with eriodictyol for 30 min and then stimulated with phorbol 12-myristate 13-acetate (PMA) for 24 h. The effect of eriodictyol on PMA-induced nuclear factor kappa B (NF-κB) signaling pathway was also investigated. Eriodictyol suppressed the MUC5AC mucin production and gene expression induced by PMA via suppression of inhibitory kappa Bα degradation and NF-κB p65 nuclear translocation. These results suggest that eriodictyol inhibits mucin gene expression and production in human airway epithelial cells via regulation of the NF-κB signaling pathway.
Keywords: MUC5AC, Pulmonary mucin, Eriodictyol
INTRODUCTION
Airway mucus is a thin gel-like layer lining the airway luminal surface that consists of water, ions, and various molecules that show antimicrobial and anti-oxidative activities (Adler and Li, 2001). Mucus glycoproteins (mucins) that confer mucus viscoelasticity form a major biological macromolecular component of mucus. In normal lung physiology, airway mucus exerts its function as a physical defensive barrier against airway epithelial damage induced by inhaled dust, irritating gases, and microbes, such as bacteria and viruses, thereby protecting the pulmonary system (Lillehoj and Kim, 2002). However, under the pathological state of the pulmonary system, such as chronic obstructive pulmonary diseases, cystic fibrosis, and asthma, the hyperproduction and/or hypersecretion of airway mucus, indicating the changes in mucin quality or quantity, have been reported to compromise the host defense system, ultimately leading to increased morbidity and mortality (Rose and Voynow, 2006). Although mucolytics, expectorants, and glucocorticoids have been clinically used for the management of abnormal airway mucus secretion, several drawbacks are associated with their use, including rebound mucus hypersecretion due to airway luminal wall irritation and various adverse side effects (Li et al., 2020). Thus, it is necessary to develop novel agents that regulate the production and/or secretion of mucins by controlling their biosynthesis and/or degradation.
Achieving this may require investigating natural compounds (derived from diverse medicinal plants that are used empirically for alleviating inflammatory pulmonary diseases), with respect to the activity to regulate abnormal secretion and/or production of mucins. We have reported various natural products that modulate the gene expression, production, and secretion of airway mucins (Kim et al., 2012; Seo et al, 2014; Choi et al., 2019; Li et al., 2020).
According to a multitude of reports, eriodictyol, a flavanone, is present in numerous vegetables, fruits, and medicinal plants; moreover, it shows a broad range of biological activities, including antioxidative, antineoplastic, anti-inflammatory, antidiabetic, neuroprotective, antiobesity, cardioprotective, and hepatoprotective activities (Islam et al., 2020). Eriodictyol exerted anticancer effects on human lung cancer cells (Zhang et al., 2019). Eniafe et al. (2018) suggested that eriodictyol may be used for the long-term regulation of asthmatic contraction of bronchial smooth muscle. In addition, Deshpande et al. (2020) suggested that eriodictyol can be used as a potential regulator for coronavirus disease 2019 via in silico analysis using molecular docking technology.
However, there are no studies about the potential effect of eriodictyol on mucin production and mucin gene expression in airway epithelial cells. Among the many subtypes of human mucins, mucin 5AC (MUC5AC) is known as a major type of airway mucin (Lillehoj and Kim, 2002). In this study, we investigated the effect of eriodictyol on MUC5AC mucin production and gene expression induced by phorbol ester in NCI-H292 cells. The human pulmonary mucoepidermoid cell line NCI-H292 is often used for identifying the signaling pathways involved in airway mucin production and gene expression (Li et al., 1997; Takeyama et al., 1999; Shao et al., 2003). In addition, phorbol ester induces airway MUC5AC gene expression and mucin production, and nuclear factor kappa B (NF-κB) signaling is involved in this activity of phorbol ester in airway epithelial cells (Ishinaga et al., 2005; Laos et al., 2006; Wu et al., 2007; Kim et al., 2012; Choi et al., 2019). Thus, to elucidate the mechanism of eriodictyol, we examined whether it exerts any effect on phorbol ester-induced activation of the NF-κB signaling pathway in NCI-H292 cells.
MATERIALS AND METHODS
Materials
All chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise stated. Eriodictyol (AV-H19076; purity: 98.0%) was purchased from Avention (Incheon, Korea). Anti-NF-κB p65 (sc-8008), anti-β-actin (sc-8432), and anti-inhibitory kappa Bα (IκBα; sc-371) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-phospho-inhibitory kappa B kinase (IKK) α/β (Ser176/180; #2687) and phospho-specific anti-IκBα (serine 32/36; #9246) antibodies were purchased from Cell Signaling Technology Inc (Danvers, MA, USA). Anti-nuclear matrix protein p84 (ab-487) antibody was purchased from Abcam (Cambridge, MA, USA). Goat anti-mouse IgG (#401215) and goat anti-rabbit IgG (#401315) were purchased from Calbiochem (Carlsbad, CA, USA) and used as the secondary antibody.
NCI-H292 cell culture
NCI-H292 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin-streptomycin mixture (100 units/mL and 100 μg/mL, respectively), and hydroxyethyl piperazineethanesulfonic acid (25 mM) at 37°C in a water-jacketed humidified incubator (5% CO2, 95% air). For serum deprivation, confluent cells were washed twice with phosphate-buffered saline (PBS) and then cultured in RPMI 1640 with 0.2% FBS for 24 h.
Eriodictyol treatment of cells
After serum deprivation, the cells were pretreated with varying concentrations of eriodictyol for 30 min and treated with phorbol 12-myristate 13-acetate (PMA) at 10 ng/mL for 24 h in serum-free RPMI 1640. Eriodictyol was dissolved in dimethyl sulfoxide and treated in culture medium (final concentration of dimethyl sulfoxide was 0.5%). The final pH values of these solutions were between 7.0 and 7.4. The culture medium and 0.5% dimethyl sulfoxide did not affect mucin gene expression, production, and expression and activity of molecules involved in NF-κB signaling pathway in NCI-H292 cells. After 24 h, the cells were lysed with buffer solution containing 20 mM Tris, 0.5% NP-40, 250 mM NaCl, 3 mM ethylenediaminetetraacetic acid (EDTA), 3 mM ethylene glycol tetraacetic acid (EGTA), and protease inhibitor cocktail (Roche Diagnostics, IN, USA) and were collected to measure the production of MUC5AC glycoproteins in a 24-well culture plate. Total RNA was extracted to determine MUC5AC gene expression in a six-well culture plate via reverse transcription polymerase chain reaction (RT-PCR). For western blot analysis, the cells were treated with eriodictyol for 24 h and PMA for 30 min.
Quantitative analysis of MUC5AC mucin
Airway MUC5AC mucin production was measured using enzyme-linked immunosorbent assay. Cell lysates were prepared with PBS at a 1:10 dilution, and 100 μL of each sample was incubated at 42°C in a 96-well plate until dried. The plates were washed three times with PBS, blocked with 2% bovine serum albumin (fraction V) for 1 h at room temperature, and then washed three times with PBS. The plates were then incubated with 100 µL 45M1, a mouse monoclonal MUC5AC antibody (NeoMarkers, CA, USA), which was diluted at 1:200 with PBS containing 0.05% Tween 20. After 1 h, the wells were washed three times with PBS, and 100 µL of horseradish peroxidase-goat anti-mouse IgG conjugate (1:3,000) was dispensed into each well and left to incubate for 1 h, after which the plates were washed three times with PBS. Color reaction was developed with 3,3ʹ,5,5ʹ-tetramethylbenzidine peroxide solution and stopped with 1 N H2SO4. The absorbance was measured at 450 nm.
Isolation of total RNA and RT-PCR
Total RNA was isolated using the easy-BLUE Extraction Kit (iNtRON Biotechnology, Inc., Gyeonggi, Korea) and reverse transcribed using AccuPower RT Premix (BIONEER Corporation, Daejeon, Korea) according to the manufacturer’s instructions. Two micrograms of total RNA were primed with 1 µg of oligo (dT) in a final volume of 50 µL (RT reaction). Two microliters of the RT reaction product were PCR-amplified in a 25 µL using Thermorprime Plus DNA Polymerase (ABgene, Rochester, NY, USA). Rig/S15 rRNA, which encodes a small ribosomal subunit protein, a housekeeping gene that was constitutively expressed, was used as a quantitative control. The following primers were used: MUC5AC, forward (5′-TGA TCA TCC AGC AGG GCT-3′) and reverse (5′-CCG AGC TCA GAG GAC ATA TGG G-3′); Rig/S15, forward (5′-TTC CGC AAG TTC ACC TAC C-3′) and reverse (5′-CGG GCC GGC CAT GCT TTA CG-3′). The PCR mixture was denatured at 94°C for 2 min followed by 40 cycles at 94°C for 30 s, 60°C for 30 s, and 72°C for 45 s. After PCR, 5 µL of the PCR product was subjected to 1% agarose gel electrophoresis and then visualized with ethidium bromide under a transilluminator.
Whole cell extract preparation
NCI-H292 cells (confluent in 100-mm culture dish) were pretreated for 24 h at 37°C with 1, 5, 10, or 20 μM of eriodictyol and then stimulated with PMA (50 ng/mL) in serum-free RPMI 1640 for 30 min. After eriodictyol treatment, media were aspirated and the cells were washed with cold PBS. For the cell collection, the cells were scraped and centrifuged at 3,000 rpm for 5 min. After the supernatant was discarded, the cell pellet was mixed with radioimmunoprecipitation assay buffer [25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)] for 30 min with continuous agitation. The lysate was centrifuged in a microcentrifuge at 14,000 rpm for 15 min at 4°C. The supernatant was either used or immediately stored at −80°C. The amount of protein in extract was quantified using the Bradford method.
Nuclear and cytosolic extract preparation
After treatment with eriodictyol, the cells were harvested using trypsin-EDTA solution and then centrifuged in a microcentrifuge at 1,200 rpm for 3 min at 4°C. After the supernatant was discarded, the cell pellet was washed by suspending in PBS. The cytoplasmic and nuclear protein fractions were extracted using NE-PER® nuclear and cytoplasmic extraction reagent (Thermo-Pierce Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Both extracts were stored at −20°C. The amount of protein in the extracts was quantified using the Bradford method.
Western blot analysis
Whole cell, cytosolic, and nuclear extracts containing proteins (each 50 μg) were subjected to 10% SDS polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene difluoride membrane. The blots were blocked with 5% skimmed milk and probed with the appropriate primary antibody in blocking buffer overnight at 4°C. The membrane was washed with PBS and then probed using the horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were detected using an enhanced chemiluminescence kit (Pierce ECL western blotting substrate; Thermo-Pierce Scientific).
Statistical analysis
The means of individual groups were converted to percent control and expressed as the mean ± standard error of the mean. The difference between groups was assessed using one-way analysis of variance and the Holm-Sidak test as a post-hoc test. A p-value<0.05 was considered significantly significant.
RESULTS
Effect of eriodictyol on PMA-induced MUC5AC gene expression and mucin production
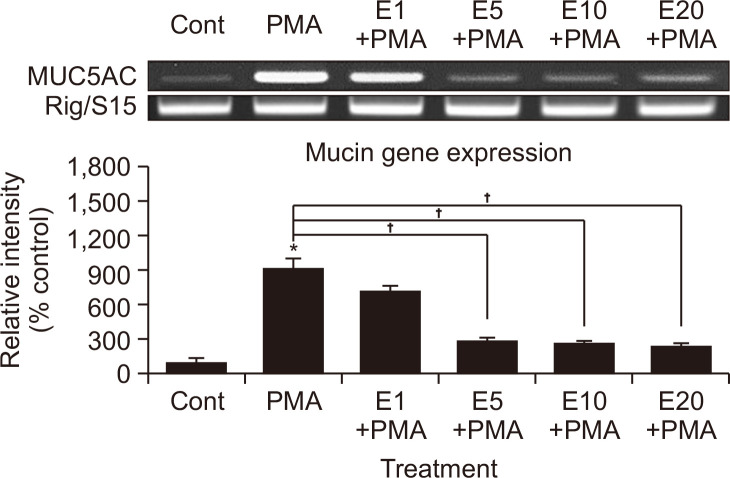
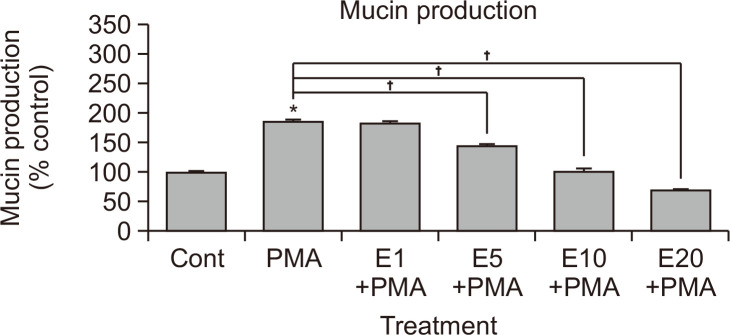
Eriodictyol suppressed PMA-induced MUC5AC gene expression (Fig. 1) and mucin production (Fig. 2), with the latter being dose dependent. As shown in Fig. 2, the amounts of MUC5AC mucin in the cells of eriodictyol-treated cultures were as follows: control, 100 ± 2%; 10 ng/mL of PMA alone, 185 ± 3%; PMA plus 1 μM eriodictyol, 182 ± 3%; PMA plus 5 μM eriodictyol, 145 ± 2%; PMA plus 10 μM eriodictyol, 102 ± 4%; PMA plus 20 μM eriodictyol, 70 ± 1%. Cell viability was assessed using the sulforhodamine B assay, which revealed that there was no cytotoxic effect of eriodictyol at concentrations of 1, 5, 10, or 20 µM (data not shown).
Fig. 1.
Effect of eriodictyol on PMA-induced MUC5AC gene expression in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations of eriodictyol for 30 min and stimulated with PMA (10 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC gene expression using RT-PCR. Three independent experiments were performed, and the representative data are shown. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05). cont, control; E, eriodictyol. Concentration unit is μM.
Fig. 2.
Effect of eriodictyol on PMA-induced MUC5AC mucin production in NCI-H292 cells. NCI-H292 cells were pretreated with varying concentrations of eriodictyol for 30 min and stimulated with PMA (10 ng/mL) for 24 h. Cell lysates were collected for measurement of MUC5AC mucin production via ELISA. Each bar represents the mean ± standard error of the mean of three culture wells compared to the control set at 100%. Three independent experiments were performed, and the representative data are shown. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05). cont, control; E, eriodictyol. Concentration unit is μM.
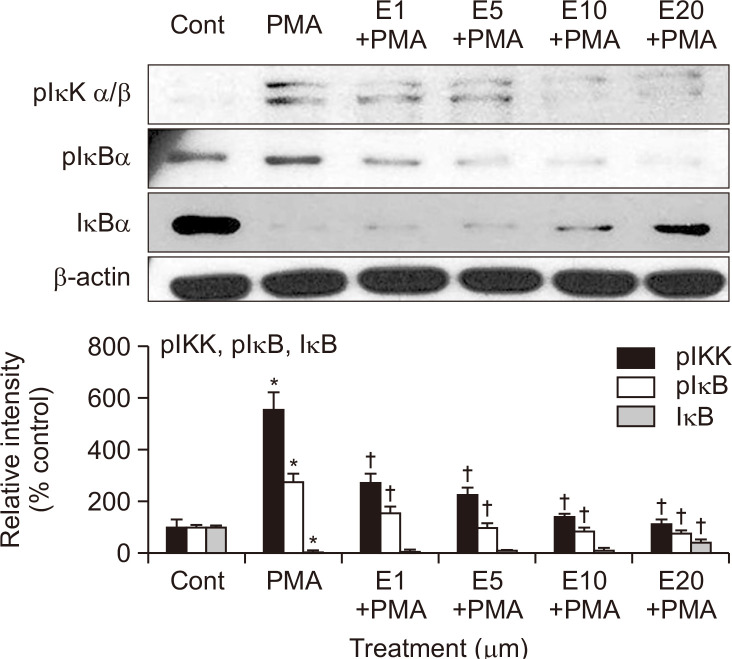
Effect of eriodictyol on PMA-induced IKKα/β and IκBα phosphorylation and IκBα degradation
To activate NF-κB, PMA induces the phosphorylation of IKK, which sequentially phosphorylates IκBα. The phosphorylated IκBα dissociates from NF-κB and is degraded. Therefore, we tested whether eriodictyol affects the PMA-induced phosphorylation of IKKα/β and IκBα and degradation of IκBα. As shown in Fig. 3, eriodictyol attenuated PMA-stimulated IKKα/β phosphorylation by controlling the phosphorylation of the serine 176/180 moiety of IKKα/β. PMA treatment increased the phosphorylation of IκBα, whereas eriodictyol treatment suppressed its phosphorylation. Moreover, PMA treatment increased the degradation of IκBα, whereas eriodictyol treatment suppressed its degradation.
Fig. 3.
Effect of eriodictyol on PMA-induced IKK phosphorylation, IκBα phosphorylation, and IκBα degradation in NCI-H292 cells. NCI-H292 cells were incubated with varying concentrations of eriodictyol for 24 h and treated with 50 ng/mL PMA for 30 min. Cytoplasmic extracts were fractionated and then subjected to western blot analysis using phospho-specific IκBα (Ser 32/36) or anti-IκBα antibodies. Whole cell lysates were prepared and then subjected to western blot analysis using phospho-specific IKKα/β (Ser 176/180) antibodies. Equal protein loading was evaluated via β-actin levels. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05). cont, control; E, eriodictyol; IκBα, inhibitory kappa B alpha; IKK, inhibitory kappa B kinase. Concentration unit is μM.
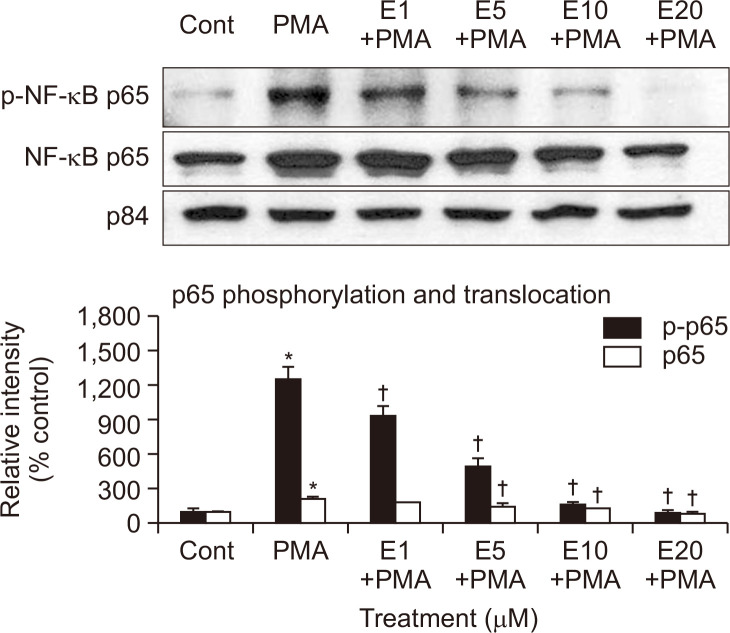
Effect of eriodictyol on PMA-induced NF-κB p65 phosphorylation and nuclear translocation
After its activation, NF-κB translocates from the cytosol to the nucleus, followed by binding to a specific DNA sequence. This NF-κB/DNA assembly recruits RNA polymerase, and the resulting mRNA is translated into the specific proteins, including MUC5AC mucins. The transcriptional activity of NF-κB p65 is dependent on its phosphorylation. As shown in Fig. 4, PMA treatment increased the phosphorylation of p65, whereas eriodictyol treatment suppressed its phosphorylation. Eventually, eriodictyol decreased the nuclear translocation of NF-κB p65 stimulated by PMA.
Fig. 4.
Effect of eriodictyol on PMA-induced NF-κB p65 phosphorylation and translocation in NCI-H292 cells. Nuclear protein extracts were prepared and subjected to western blot analysis using phospho-specific p65 (Ser 536) antibodies and antibodies against p65. p84 was used as a loading control. The results shown are the representative of three independent experiments. *Significantly different from control (p<0.05). †Significantly different from PMA alone (p<0.05). cont, control; E, eriodictyol. Concentration unit is μM.
DISCUSSION
As mentioned above, mucolytics, expectorants, and glucocorticoids have been utilized for pharmacotherapy of pulmonary diseases that exhibit hypersecretion of airway mucus; however, these drugs have limited clinical efficacy in regulating such diseases and elicit diverse side effects (Li et al., 2020). Thus, it is essential to develop novel agents that regulate production and/or secretion of mucins by controlling their biosynthesis and/or degradation. Achieving this may require investigating natural compounds (derived from diverse medicinal plants that are used empirically for alleviating inflammatory pulmonary diseases), with respect to the activity to regulate abnormal secretion and/or production of mucins. Our results showed that eriodictyol, a flavonoid with anti-inflammatory activity, decreased MUC5AC gene expression and mucin production stimulated by PMA (Fig. 1, 2). These results suggest that eriodictyol can regulate the gene and protein expression of pulmonary mucin by directly acting on airway epithelial cells. This is the first study to report about the regulatory activity of eriodictyol on airway mucin production.
Eriodictyol was reported to suppress the IL-1β-mediated inflammation of chondrocytes, by attenuating NF-κB activation, and to downregulate the IL-1β-stimulated overproduction of PGE2, nitric oxide, and COX-2 (Wang et al., 2018). In the lipopolysaccharide-mediated acute lung injury mice model, eriodictyol dramatically reduced the expression levels of IL-6, PGE2, IL-1β, and TNF-α in the bronchoalveolar lavage fluid. In addition, the activation of COX-2/NLRP3/NF-κB and Nrf2 signaling was attenuated by eriodictyol (Wang et al., 2020). Notably, a similar report confirmed that eriodictyol could function as an anti-inflammatory agent by protecting against Staphylococcus aureus-mediated lung injury (Xuewen et al., 2018). These reports indicate the need to investigate the mechanism of action of eriodictyol involved in mucin gene expression and production, focusing on the NF-κB signaling pathway.
As a multifunctional transcription regulator, NF-κB signaling commonly exists in eukaryotic cells. NF-κB activation occurs by preferentially inducing the phosphorylation of the IKKs, which then activate IĸBα phosphorylation. Consequently, the dissociated form of phosphorylated IĸBα accumulates and is degraded by the proteasome. NF-κB subunits (p65 and p50) are then released and enter the nucleus, where they bind to DNA (Nie et al., 2012; Liu et al., 2020). The NF-κB signaling pathway plays a crucial role in mediating the biological functions associated with immunity and inflammation (Huang et al., 2018; Lee et al., 2019; Shang et al., 2019). Under pathological airway conditions, NF-κB was found to be involved in mucin hypersecretion and cytokine activity regulation (Liu et al., 2020), and its abnormal activation has been observed in patients with asthma (Huang et al., 2018). Several groups of scientists reported that the gene expression and production of MUC5AC mucin might be induced by the inflammatory mediators that stimulate the activity of transcription factors including NF-κB (Fujisawa et al., 2009; Kurakula et al., 2015; Garvin et al., 2016). PMA induces airway MUC5AC gene expression and mucin production, and NF-κB signaling is involved in this activity (Ishinaga et al., 2005; Laos et al., 2006; Wu et al., 2007; Kim et al., 2012).
In our study, eriodictyol suppressed NF-κB p65 phosphorylation and nuclear translocation by acting on the steps of the phosphorylation and degradation of IκBα, in human airway epithelial cells (Fig. 3, 4). Therefore, the pharmacological effect of eriodictyol may be partly exerted on MUC5AC gene expression and mucin production by reducing IκBα degradation and NF-κB p65 nuclear translocation.
Collectively, these results indicate that the inhibitory activity of eriodictyol on airway mucin gene expression and production might be mediated by controlling PMA-stimulated IκBα degradation and NF-κB p65 nuclear translocation. Therefore, eriodictyol could possibly be used as an effective novel mucoregulator for inflammatory pulmonary diseases. It is desirable to modify the chemical structure of eriodictyol to obtain an optimal compound that will show the most effective regulatory effect on the secretion and/or production of pulmonary mucus.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2014R1A6A1029617).
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
REFERENCES
- Adler K. B., Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am. J. Respir. Cell Mol. Biol. 2001;25:397–400. doi: 10.1165/ajrcmb.25.4.f214. [DOI] [PubMed] [Google Scholar]
- Choi B. S., Kim Y. J., Choi J. S., Lee H. J., Lee C. J. Obtusifolin isolated from the seeds of Cassia obtusifolia regulates the gene expression and production of MUC5AC mucin in airway epithelial cells via affecting NF-κB pathway. Phytother. Res. 2019;33:919–928. doi: 10.1002/ptr.6284. [DOI] [PubMed] [Google Scholar]
- Deshpande R. R., Tiwari A. P., Nyayanit N., Modak M. In silico molecular docking analysis for repurposing therapeutics against multiple proteins from SARS-CoV-2. Eur. J. Pharmacol. 2020;886:173430. doi: 10.1016/j.ejphar.2020.173430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eniafe G. O., Metibemu D. S., Omotuyi O. I., Ogunleye A. J., Inyang O. K., Adelakun N. S., Adeniran Y. O., Adewumi B., Enejoh O. A., Osunmuyiwa J. O., Shodehinde S. A., Oyeneyin O. E. Agemone mexicana flavanones; apposite inverse agonists of the β2-adrenergic receptor in asthma treatment. Bioinformation. 2018;14:60–67. doi: 10.6026/97320630014060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Velichko S., Thai P., Hung L. Y., Huang F., Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappa B paradigm. J. Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin L. M., Chen Y., Damsker J. M., Rose M. C. A novel dissociative steroid VBP15 reduces MUC5AC gene expression in airway epithelial cells but lacks the GRE mediated transcriptional properties of dexamethasone. Pulm. Pharmacol. Ther. 2016;38:17–26. doi: 10.1016/j.pupt.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Huang W. C., Wu L. Y., Hu S., Wu S. J. Spilanthol inhibits COX-2 and ICAM-1 expression via suppression of NF-kappaB and MAPK signaling in interleukin-1beta-stimulated human lung epithelial cells. Inflammation. 2018;41:1934–1944. doi: 10.1007/s10753-018-0837-0. [DOI] [PubMed] [Google Scholar]
- Ishinaga H., Takeuchi K., Kishioka C., Suzuki S., Basbaum C., Majima Y. Pranlukast inhibits NF-kappaB activation and MUC2 gene expression in cultured human epithelial cells. Pharmacology. 2005;73:89–96. doi: 10.1159/000081294. [DOI] [PubMed] [Google Scholar]
- Islam A., Islam M. S., Rahman M. K., Uddin M. N., Akanda M. R. The pharmacological and biological roles of eriodictyol. Arch. Pharm. Res. 2020;43:582–592. doi: 10.1007/s12272-020-01243-0. [DOI] [PubMed] [Google Scholar]
- Kim J. O., Sikder M. A., Lee H. J., Rahman M., Kim J. H., Chang G. T., Lee C. J. Phorbol ester or epidermal growth factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by eriodictyol and wogonin. Phytother. Res. 2012;26:1784–1788. doi: 10.1002/ptr.4650. [DOI] [PubMed] [Google Scholar]
- Kurakula K., Hamers A. A., van Loenen P., de Vries C. J. 6-Mercaptopurine reduces cytokine and Muc5ac expression involving inhibition of NF-kB activation in airway epithelial cells. Respir. Res. 2015;16:73. doi: 10.1186/s12931-015-0236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laos S., Baeckstrom D., Hansson G. C. Inhibition of NF-kappaB activation and chemokine expression by the leukocyte glycoprotein, CD43, in colon cancer cells. Int. J. Oncol. 2006;28:695–704. doi: 10.3892/ijo.28.3.695. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Ryu H. W., Lee S. U., Kim M. G., Kwon O. K., Kim M. O., Oh T. K., Lee J. K., Kim T. Y., Lee S. W., Choi S., Li W. Y., Ahn K. S., Oh S. R. Pistacia weinmannifolia ameliorates cigarette smoke and lipopolysaccharide-induced pulmonary inflammation by inhibiting interleukin-8 production and NF-kappaB activation. Int. J. Mol. Med. 2019;44:949–959. doi: 10.3892/ijmm.2019.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Dohrman A. F., Gallup M., Miyata S., Gum J. R., Kim Y. S., Nadel J. A., Prince A., Basbaum C. B. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc. Natl. Acad. Sci. U.S.A. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Jin F., Lee H. J., Lee C. J. Recent advances in the development of novel drug candidates for regulating the secretion of pulmonary mucus. Biomol. Ther. (Seoul) 2020;28:293–301. doi: 10.4062/biomolther.2020.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. R., Kim K. C. Airway mucus: its components and function. Arch. Pharm. Res. 2002;25:770–780. doi: 10.1007/BF02976990. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang B., Zhang T., Wang H., Peng L., Zhou L. Effect of NF-kappaB signal pathway on mucus secretion induced by atmospheric PM 2.5 in asthmatic rats. Ecotoxicol. Environ. Saf. 2020;190:10094. doi: 10.1016/j.ecoenv.2019.110094. [DOI] [PubMed] [Google Scholar]
- Nie Y. C., Wu H., Li P. B., Xie L. M., Luo Y. L., Shen J. G., Su W. W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IkappaB-NF-kappaB signaling pathways. Eur. J. Pharmacol. 2012;690:207–213. doi: 10.1016/j.ejphar.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voynow J. A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Seo H. S., Sikder M. A., Lee H. J., Ryu J., Lee C. J. Apigenin inhibits tumor necrosis factor-α-induced production and gene expression of mucin through regulating nuclear factor-kappa B signaling pathway in airway epithelial cells. Biomol. Ther. (Seoul) 2014;22:525–531. doi: 10.4062/biomolther.2014.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Liu W., Yin C., Chu H., Zhang M. Cucurbitacin E ameliorates lipopolysaccharide-evoked injury, inflammation and MUC5AC expression in bronchial epithelial cells by restraining the HMGB1-TLR4-NF-kappaB signaling. Mol. Immunol. 2019;114:571–577. doi: 10.1016/j.molimm.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Shao M. X., Ueki I. F., Nadel J. A. TNF-alpha converting enzyme mediated MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11618–11623. doi: 10.1073/pnas.1534804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama K., Dabbagh K., Lee H., Agusti C., Lausier J. A., Ueki I. F., Grattan K. M., Nadel J. A. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Deng R., Dong J., Huang L., Li J., Zhang B. Eriodictyol ameliorates lipopolysaccharide-induced acute lung injury by suppressing the inflammatory COX-2/NLRP3/NF-kappaB pathway in mice. J. Biochem. Mol. Toxicol. 2020;34:e22434. doi: 10.1002/jbt.22434. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Chen Y., Zhou B., Shan X., Yang G. Eriodictyol inhibits IL-1 beta-induced inflammatory response in human osteoarthritis chondrocytes. Biomed. Pharmacother. 2018;107:1128–1134. doi: 10.1016/j.biopha.2018.08.103. [DOI] [PubMed] [Google Scholar]
- Wu D. Y., Wu R., Reddy S. P., Lee Y. C., Chang M. M. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am. J. Pathol. 2007;170:20–32. doi: 10.2353/ajpath.2007.060452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuewen H., Ping O., Zhongwei Y., Zhongqiong Y., Hualin F., Juchun L., Changliang H., Gang S., Zhixiang Y., Xu S., Yuanfeng Z., Lixia L., Lizi Y. Eriodictyol protects against Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. World J. Microbiol. Biotechnol. 2018;34:64. doi: 10.1007/s11274-018-2446-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang R., Ni H. Eriodictyol exerts potent anticancer activity against A549 human lung cancer cell line by inducing mitochondrial-mediated apoptosis, G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signalling pathway. Arch. Med. Sci. 2019;16:446–452. doi: 10.5114/aoms.2019.85152. [DOI] [PMC free article] [PubMed] [Google Scholar]